A Cascade Synthesis of Unsymmetrical Furanized Triarylmethanes via Gold Self-Relay Catalysis
Abstract
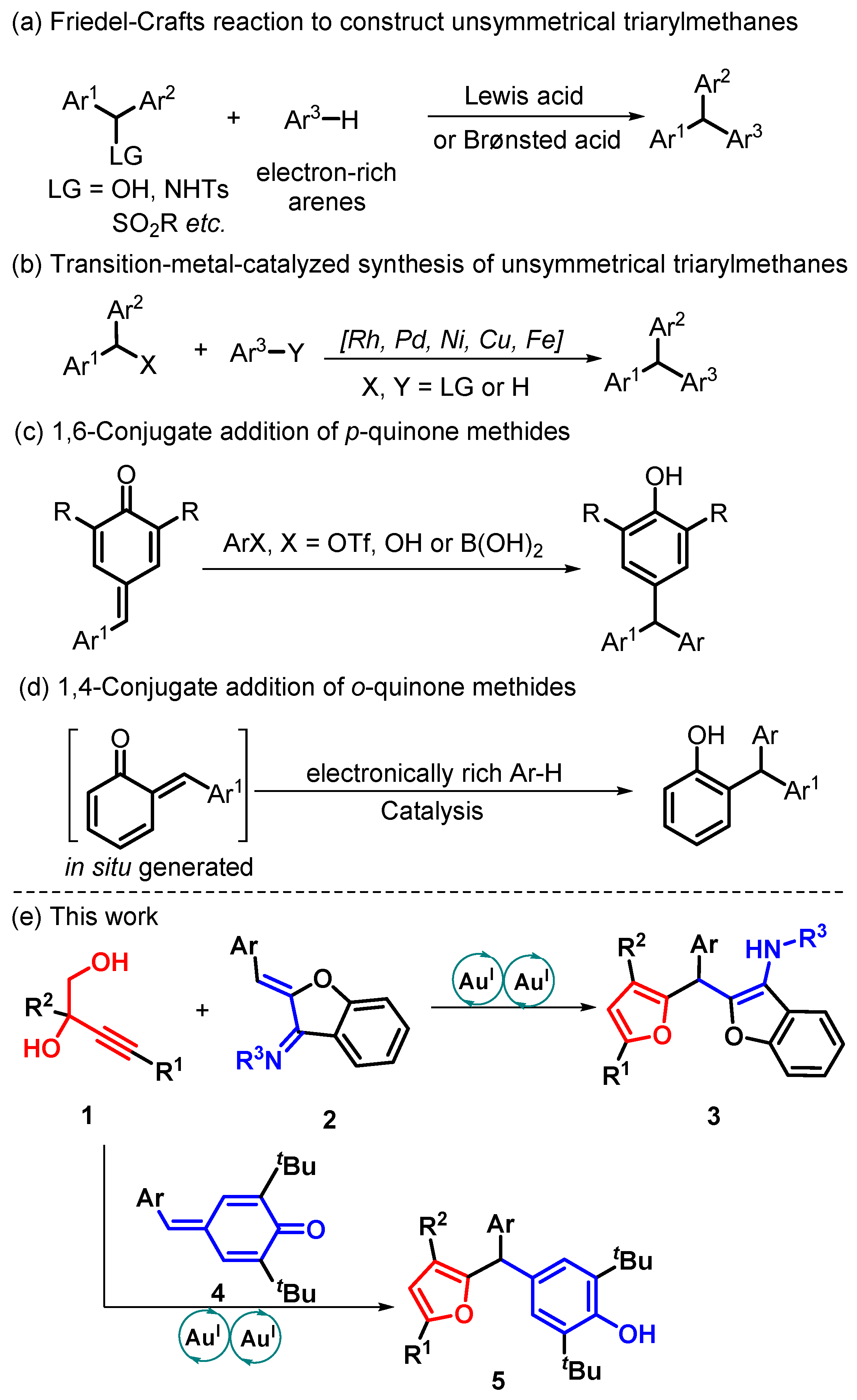
:1. Introduction
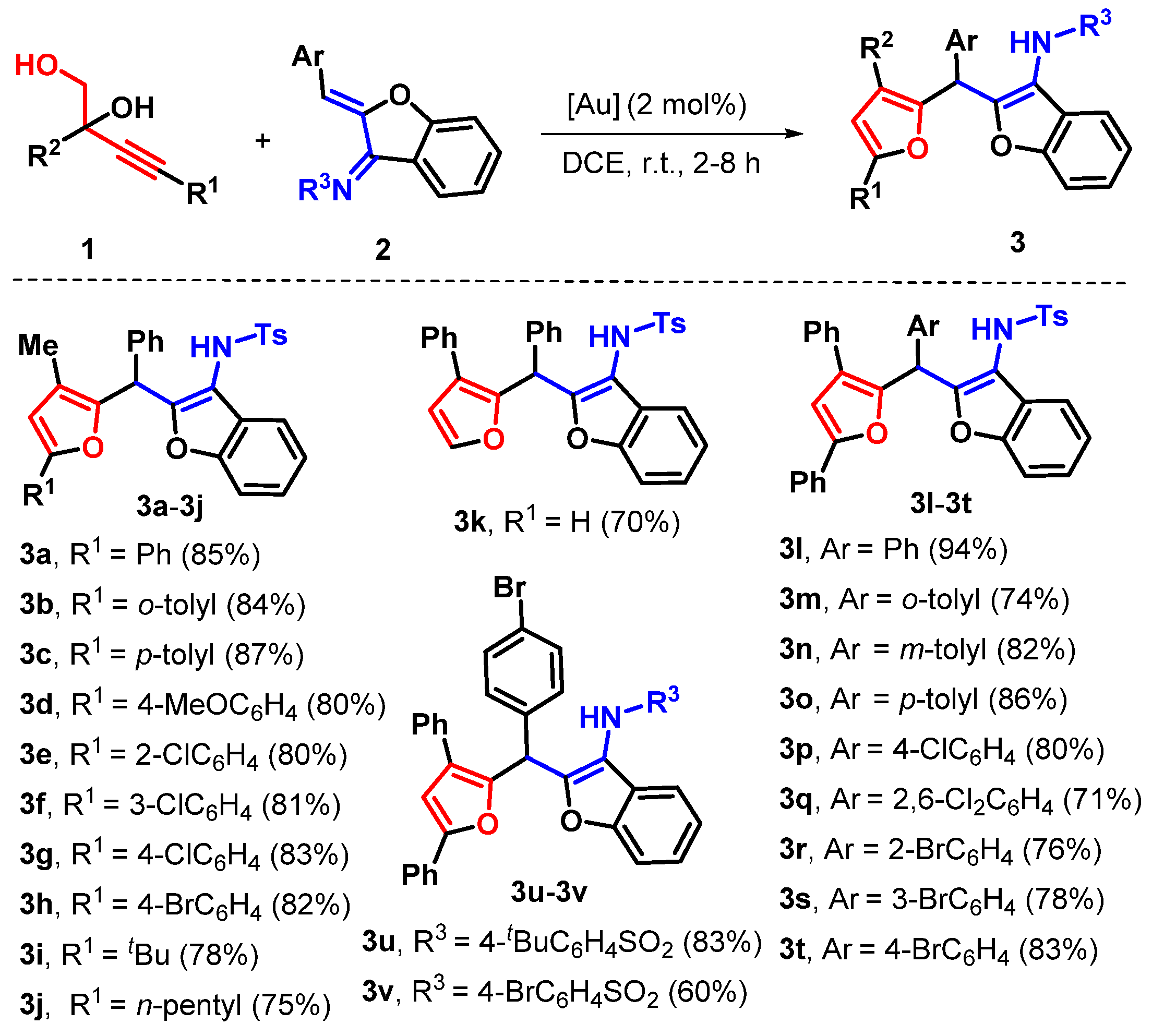
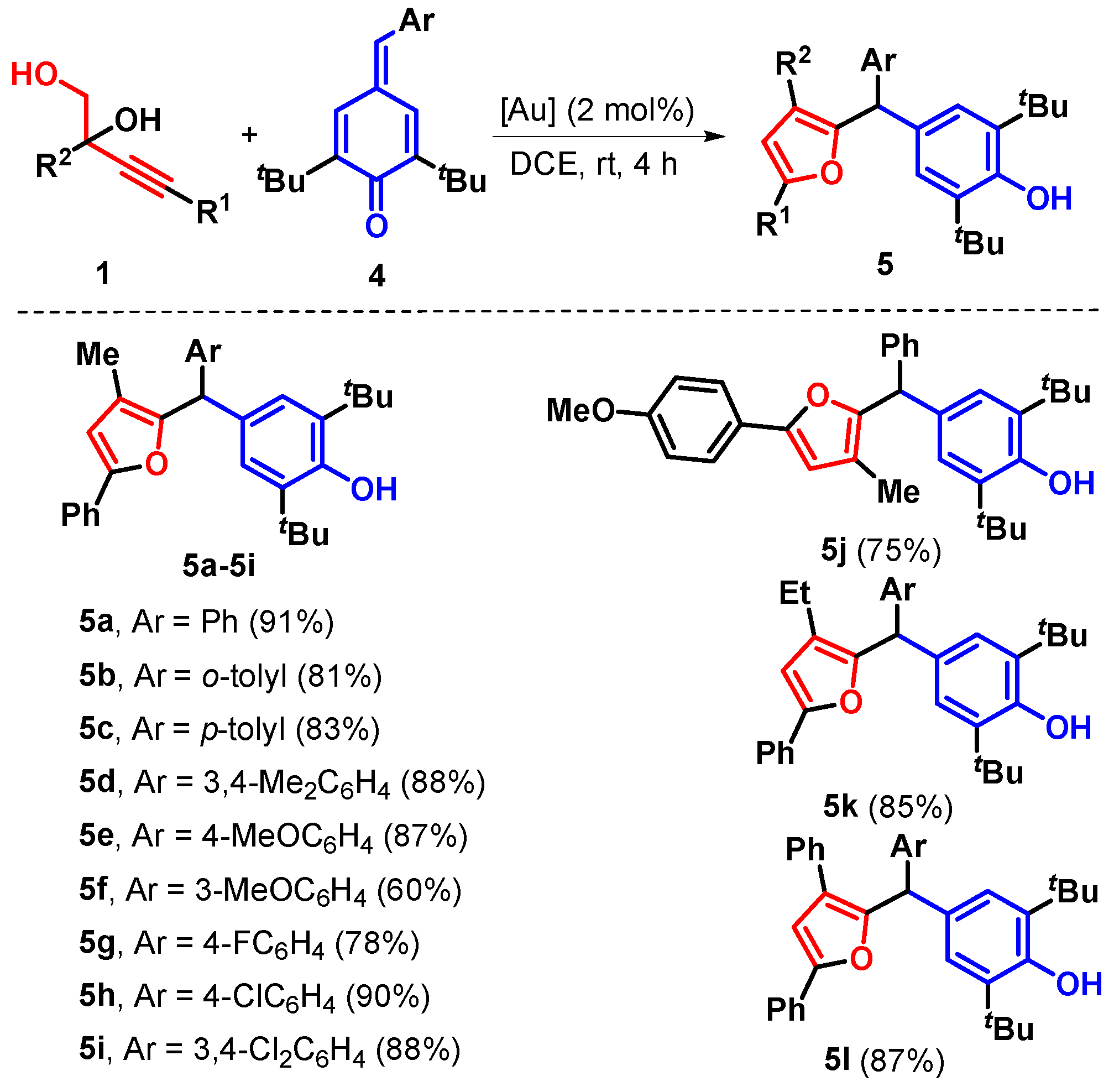
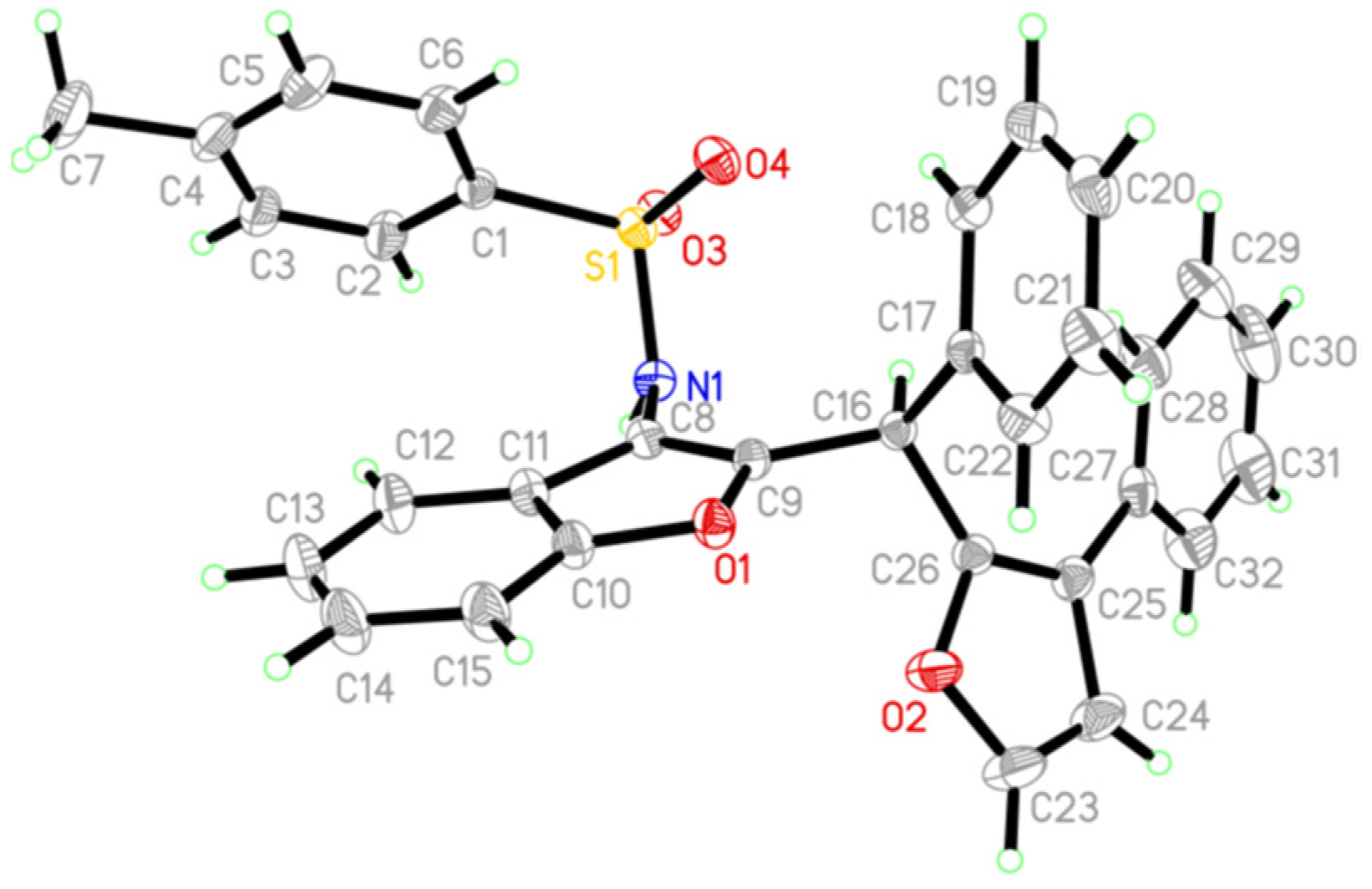
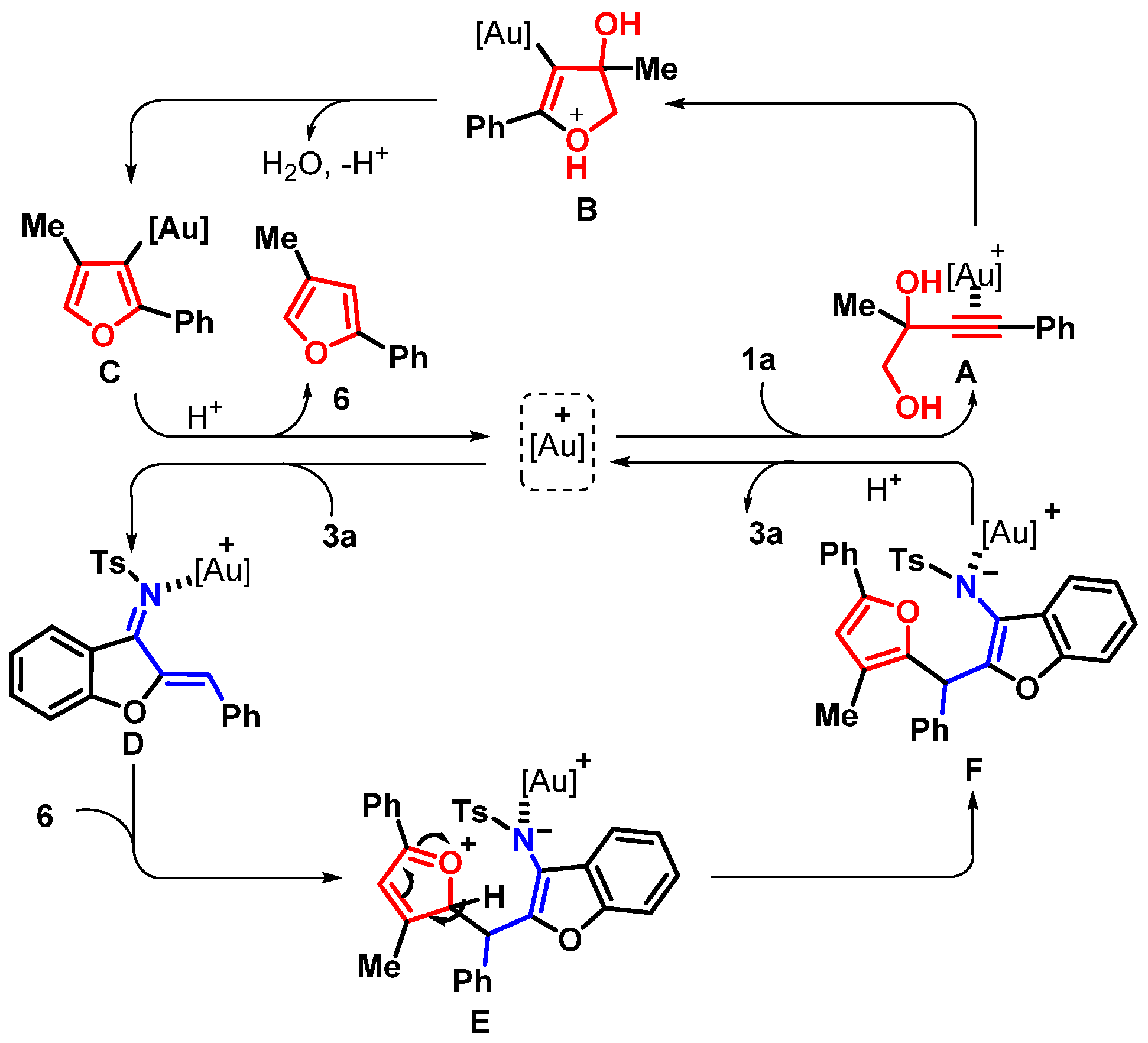
2. Results
3. Experimental Section
3.1. 4-Methyl-N-(2-((3-methyl-5-phenylfuran-2-yl)(phenyl)methyl)benzofuran-3-yl)benzenesulfonamide (3a)
3.2. 4-Methyl-N-(2-((3-methyl-5-(o-tolyl)furan-2-yl)(phenyl)methyl)benzofuran-3-yl)benzenesulfonamide (3b)
3.3. 4-Methyl-N-(2-((3-methyl-5-(p-tolyl)furan-2-yl)(phenyl)methyl)benzofuran-3-yl)benzenesulfonamide (3c)
3.4. N-(2-((5-(4-Methoxyphenyl)-3-methylfuran-2-yl)(phenyl)methyl)benzofuran-3-yl)-4-methylbenzenesulfonamide (3d)
3.5. N-(2-((5-(2-Chlorophenyl)-3-methylfuran-2-yl)(phenyl)methyl)benzofuran-3-yl)-4-methylbenzenesulfonamide (3e)
3.6. N-(2-((5-(3-Chlorophenyl)-3-methylfuran-2-yl)(phenyl)methyl)benzofuran-3-yl)-4-methylbenzenesulfonamide (3f)
3.7. N-(2-((5-(4-Chlorophenyl)-3-methylfuran-2-yl)(phenyl)methyl)benzofuran-3-yl)-4-methylbenzenesulfonamide (3g)
3.8. N-(2-((5-(4-Bromophenyl)-3-methylfuran-2-yl)(phenyl)methyl)benzofuran-3-yl)-4-methylbenzenesulfonamide (3h)
3.9. N-(2-((5-(tert-Butyl)-3-methylfuran-2-yl)(phenyl)methyl)benzofuran-3-yl)-4-methylbenzenesulfonamide (3i)
3.10. 4-Methyl-N-(2-((3-methyl-5-pentylfuran-2-yl)(phenyl)methyl)benzofuran-3-yl)benzenesulfonamide (3j)
3.11. 4-Methyl-N-(2-(phenyl(3-phenylfuran-2-yl)methyl)benzofuran-3-yl)benzenesulfonamide (3k)
3.12. N-(2-((3,5-Diphenylfuran-2-yl)(phenyl)methyl)benzofuran-3-yl)-4-methylbenzenesulfonamide (3l)
3.13. N-(2-((3,5-Diphenylfuran-2-yl)(o-tolyl)methyl)benzofuran-3-yl)-4-methylbenzenesulfonamide (3m)
3.14. N-(2-((3,5-Diphenylfuran-2-yl)(m-tolyl)methyl)benzofuran-3-yl)-4-methylbenzenesulfonamide (3n)
3.15. N-(2-((3,5-Diphenylfuran-2-yl)(p-tolyl)methyl)benzofuran-3-yl)-4-methylbenzenesulfonamide (3o)
3.16. N-(2-((3-Chlorophenyl)(3,5-diphenylfuran-2-yl)methyl)benzofuran-3-yl)-4-methylbenzenesulfonamide (3p)
3.17. N-(2-((2,6-Dichlorophenyl)(3,5-diphenylfuran-2-yl)methyl)benzofuran-3-yl)-4-methylbenzenesulfonamide (3q)
3.18. N-(2-((2-Bromophenyl)(3,5-diphenylfuran-2-yl)methyl)benzofuran-3-yl)-4-methylbenzenesulfonamide (3r)
3.19. N-(2-((3-Bromophenyl)(3,5-diphenylfuran-2-yl)methyl)benzofuran-3-yl)-4-methylbenzenesulfonamide (3s)
3.20. N-(2-((4-Bromophenyl)(3,5-diphenylfuran-2-yl)methyl)benzofuran-3-yl)-4-methylbenzenesulfonamide (3t)
3.21. N-(2-((4-Bromophenyl)(3,5-diphenylfuran-2-yl)methyl)benzofuran-3-yl)-4-(tert-butyl)benzenesulfonamide (3u)
3.22. 4-Bromo-N-(2-((4-bromophenyl)(3,5-diphenylfuran-2-yl)methyl)benzofuran-3-yl)benzenesulfonamide (3v)
3.23. 2,6-Di-tert-butyl-4-((3-methyl-5-phenylfuran-2-yl)(phenyl)methyl)phenol (5a)
3.24. 2,6-Di-tert-butyl-4-((3-methyl-5-phenylfuran-2-yl)(o-tolyl)methyl)1phenol (5b)
3.25. 2,6-Di-tert-butyl-4-((3-methyl-5-phenylfuran-2-yl)(p-tolyl)methyl)phenol (5c)
3.26. 2,6-Di-tert-butyl-4-((3,4-dimethylphenyl)(3-methyl-5-phenylfuran-2-yl)methyl)phenol (5d)
3.27. 2,6-Di-tert-butyl-4-((4-methoxyphenyl)(3-methyl-5-phenylfuran-2-yl)methyl)phenol (5e)
3.28. 2,6-Di-tert-butyl-4-((3-methoxyphenyl)(3-methyl-5-phenylfuran-2-yl)methyl)phenol (5f)
3.29. 2,6-Di-tert-butyl-4-((4-fluorophenyl)(3-methyl-5-phenylfuran-2-yl)methyl)phenol (5g)
3.30. 2,6-Di-tert-butyl-4-((4-chlorophenyl)(3-methyl-5-phenylfuran-2-yl)methyl)phenol (5h)
3.31. 2,6-Di-tert-butyl-4-((3,4-dichlorophenyl)(3-methyl-5-phenylfuran-2-yl)methyl)phenol (5i)
3.32. 2,6-Di-tert-butyl-4-((3-methyl-5-(p-tolyl)furan-2-yl)(phenyl)methyl)phenol (5j)
3.33. 2,6-Di-tert-butyl-4-((3-ethyl-5-phenylfuran-2-yl)(phenyl)methyl)phenol (5k)
3.34. 2,6-Di-tert-butyl-4-((3,5-diphenylfuran-2-yl)(phenyl)methyl)phenol (5l)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Parai, M.K.; Panda, G.; Chaturvedi, V.; Manju, Y.K.; Sinha, S. Thiophene containing triarylmethanes as antitubercular agents. Bioorg. Med. Chem. Lett. 2008, 18, 289–292. [Google Scholar] [CrossRef] [PubMed]
- Al-Qawasmeh, R.A.; Lee, Y.; Cao, M.-Y.; Gu, X.; Vassilakos, A.; Wright, J.A.; Young, A. Triaryl methane derivatives as antiproliferative agents. Bioorg. Med. Chem. Lett. 2004, 14, 347–350. [Google Scholar] [CrossRef] [PubMed]
- Shchepinov, M.S.; Korshun, V.A. Recent applications of bifunctional trityl groups. Chem. Soc. Rev. 2003, 32, 170–180. [Google Scholar] [CrossRef]
- Wulff, H.; Zhorov, B.S. K+ Channel Modulators for the Treatment of Neurological Disorders and Autoimmune Diseases. Chem. Rev. 2008, 108, 1744–1773. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Esquivias, J.; Gomez Arrayas, R.; Carretero, J.C. A copper(II)-catalyzed aza-Friedel-Crafts reaction of N-(2-pyridyl)sulfonyl aldimines: Synthesis of unsymmetrical diaryl amines and triaryl methanes. Angew. Chem. Int. Ed. 2006, 45, 629–633. [Google Scholar] [CrossRef]
- Li, Z.; Duan, Z.; Kang, J.; Wang, H.; Yu, L.; Wu, Y. A simple access to triarylmethane derivatives from aromatic aldehydes and electron-rich arenes catalyzed by FeCl3. Tetrahedron 2008, 64, 1924–1930. [Google Scholar] [CrossRef]
- Thirupathi, P.; Kim, S.S. Fe(ClO4)3·xH2O-Catalyzed direct C–C bond forming reactions between secondary benzylic alcohols with different types of nucleophiles. Tetrahedron 2010, 66, 2995–3003. [Google Scholar] [CrossRef]
- Hikawa, H.; Suzuki, H.; Yokoyama, Y.; Azumaya, I. Chemoselective Benzylation of Unprotected Anthranilic Acids with Benzhydryl Alcohols by Water-Soluble Au(III)/TPPMS in Water. J. Org. Chem. 2013, 78, 6714–6720. [Google Scholar] [CrossRef]
- Singh, P.; Dinda, S.K.; Shagufta, P.G. Synthetic approach towards trisubstituted methanes and a chiral tertiary α-hydroxyaldehyde, a possible intermediate for tetrasubstituted methanes. RSC Adv. 2013, 3, 12100–12103. [Google Scholar] [CrossRef]
- Mondal, S.; Panda, G. Synthetic methodologies of achiral diarylmethanols, diaryl and triarylmethanes (TRAMs) and medicinal properties of diaryl and triarylmethanes-an overview. RSC Adv. 2014, 4, 28317–28358. [Google Scholar] [CrossRef]
- Nambo, M.; Crudden, C.M. Recent Advances in the Synthesis of Triarylmethanes by Transition Metal Catalysis. ACS Catal. 2015, 5, 4734–4742. [Google Scholar] [CrossRef]
- Yu, J.-Y.; Kuwano, R. Suzuki-Miyaura Coupling of Diarylmethyl Carbonates with Arylboronic Acids: A New Access to Triarylmethanes. Org. Lett. 2008, 10, 973–976. [Google Scholar] [CrossRef]
- Nambo, M.; Yar, M.; Smith, J.D.; Crudden, C.M. The Concise Synthesis of Unsymmetric Triarylacetonitriles via Pd-Catalyzed Sequential Arylation: A New Synthetic Approach to Tri- and Tetraarylmethanes. Org. Lett. 2015, 17, 50–53. [Google Scholar] [CrossRef]
- Tabuchi, S.; Hirano, K.; Satoh, T.; Miura, M. Synthesis of Triarylmethanes by Palladium-Catalyzed C–H/C–O Coupling of Oxazoles and Diarylmethanol Derivatives. J. Org. Chem. 2014, 79, 5401–5411. [Google Scholar] [CrossRef]
- Xia, Y.; Hu, F.; Liu, Z.; Qu, P.; Ge, R.; Ma, C.; Zhang, Y.; Wang, J. Palladium-Catalyzed Diarylmethyl C(sp3)-C(sp2) Bond Formation: A New Coupling Approach toward Triarylmethanes. Org. Lett. 2013, 15, 1784–1787. [Google Scholar] [CrossRef]
- Matthew, S.C.; Glasspoole, B.W.; Eisenberger, P.; Crudden, C.M. Synthesis of Enantiomerically Enriched Triarylmethanes by Enantiospecific Suzuki–Miyaura Cross-Coupling Reactions. J. Am. Chem. Soc. 2014, 136, 5828–5831. [Google Scholar] [CrossRef]
- Cao, X.; Sha, S.-C.; Li, M.; Kim, B.-S.; Morgan, C.; Huang, R.; Yang, X.; Walsh, P.J. Nickel-catalyzed arylation of heteroaryl-containing diarylmethanes: Exceptional reactivity of the Ni(NIXANTPHOS)-based catalyst. Chem. Sci. 2016, 7, 611–618. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Bellomo, A.; Creamer, A.D.; Dreher, S.D.; Walsh, P.J. Palladium-Catalyzed C(sp3)-H Arylation of Diarylmethanes at Room Temperature: Synthesis of Triarylmethanes via Deprotonative-Cross-Coupling Processes. J. Am. Chem. Soc. 2012, 134, 13765–13772. [Google Scholar] [CrossRef]
- Shi, B.-F.; Maugel, N.; Zhang, Y.-H.; Yu, J.-Q. PdII-catalyzed enantioselective activation of C(sp2)-H and C(sp3)-H bonds using monoprotected amino acids as chiral ligands. Angew. Chem. Int. Ed. 2008, 47, 4882–4886. [Google Scholar] [CrossRef]
- Niwa, T.; Yorimitsu, H.; Oshima, K. Palladium-Catalyzed Direct Arylation of Aryl(azaaryl)methanes with Aryl Halides Providing Triarylmethanes. Org. Lett. 2007, 9, 2373–2375. [Google Scholar] [CrossRef]
- Nambo, M.; Crudden, C.M. Modular Synthesis of Triarylmethanes through Palladium-Catalyzed Sequential Arylation of Methyl Phenyl Sulfone. Angew. Chem. Int. Ed. 2014, 53, 742–746. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Bellomo, A.; Trongsiriwat, N.; Jia, T.; Carroll, P.J.; Dreher, S.D.; Tudge, M.T.; Yin, H.; Robinson, J.R.; Schelter, E.J.; et al. NiXantphos: A Deprotonatable Ligand for Room-Temperature Palladium-Catalyzed Cross-Couplings of Aryl Chlorides. J. Am. Chem. Soc. 2014, 136, 6276–6287. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Wang, H.; Qiu, N.; Kong, Y.; Zeng, W.; Zhang, Y.; Zhao, J. Synthesis of Triarylmethanes via Palladium-Catalyzed Suzuki Coupling of Trimethylammonium Salts and Arylboronic Acids. J. Org. Chem. 2018, 83, 8710–8715. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Wu, G.; Zhang, Y.; Wang, J. Copper-Catalyzed Direct Benzylation or Allylation of 1,3-Azoles with N-Tosylhydrazones. J. Am. Chem. Soc. 2011, 133, 3296–3299. [Google Scholar] [CrossRef]
- Rao, H.S.P.; Rao, A.V.B. Copper-mediated arylation with arylboronic acids: Facile and modular synthesis of triarylmethanes. Beilstein J. Org. Chem. 2016, 12, 496–504. [Google Scholar] [CrossRef] [Green Version]
- Tobisu, M.; Yasutome, A.; Kinuta, H.; Nakamura, K.; Chatani, N. 1,3-Dicyclohexylimidazol-2-ylidene as a Superior Ligand for the Nickel-Catalyzed Cross-Couplings of Aryl and Benzyl Methyl Ethers with Organoboron Reagents. Org. Lett. 2014, 16, 5572–5575. [Google Scholar] [CrossRef]
- Taylor, B.L.H.; Harris, M.R.; Jarvo, E.R. Synthesis of Enantioenriched Triarylmethanes by Stereospecific Cross-Coupling Reactions. Angew. Chem. Int. Ed. 2012, 51, 7790–7793. [Google Scholar] [CrossRef]
- Harris, M.R.; Hanna, L.E.; Greene, M.A.; Moore, C.E.; Jarvo, E.R. Retention or Inversion in Stereospecific Nickel-Catalyzed CrossCoupling of Benzylic Carbamates with Arylboronic Esters: Control of Absolute Stereochemistry with an Achiral Catalyst. J. Am. Chem. Soc. 2013, 135, 3303–3306. [Google Scholar] [CrossRef] [Green Version]
- Zhou, Q.; Srinivas, H.D.; Dasgupta, S.; Watson, M.P. Nickel-Catalyzed Cross-Couplings of Benzylic Pivalates with Arylboroxines: Stereospecific Formation of Diarylalkanes and Triarylmethanes. J. Am. Chem. Soc. 2013, 135, 3307–3310. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.-Z.; Li, B.-J.; Lu, X.-Y.; Lin, S.; Shi, Z.-J. Cross Dehydrogenative Arylation (CDA) of a Benzylic C−H Bond with Arenes by Iron Catalysis. Angew. Chem. Int. Ed. 2009, 48, 3817–3820. [Google Scholar] [CrossRef]
- Cao, L.-L.; Ye, Z.-S.; Jiang, G.-F.; Zhou, Y.-G. Rhodium-Catalyzed Addition of Boronic Acids to Vinylogous Imines Generated in situ from Sulfonylindoles. Adv. Synth. Catal. 2011, 353, 3352–3356. [Google Scholar] [CrossRef]
- Huang, Y.; Hayashi, T. Asymmetric Synthesis of Triarylmethanes by Rhodium-Catalyzed Enantioselective Arylation of Diarylmethylamines with Arylboroxines. J. Am. Chem. Soc. 2015, 137, 7556–7559. [Google Scholar] [CrossRef]
- Parra, A.; Tortosa, M. para-Quinone Methide: A New Player in Asymmetric Catalysis. ChemCatChem 2015, 7, 1524–1526. [Google Scholar] [CrossRef] [Green Version]
- Singh, G.; Pandey, R.; Pankhade, Y.A.; Fatma, S.; Anand, R.V. Construction of Oxygen- and Nitrogen-based Heterocycles from p-Quinone Methides. Chem. Rec. 2021, 21, 4150–4173. [Google Scholar] [CrossRef]
- Li, W.; Xu, X.; Zhang, P.; Li, P. Recent Advances in the Catalytic Enantioselective Reactions of para-Quinone Methides. Chem.-Asian J. 2018, 13, 2350–2359. [Google Scholar] [CrossRef]
- Lima, C.G.S.; Pauli, F.P.; Costa, D.C.S.; de Souza, A.S.; Forezi, L.S.M.; Ferreira, V.F.; de Carvalho da Silva, F. para-Quinone Methides as Acceptors in 1,6-Nucleophilic Conjugate Addition Reactions for the Synthesis of Structurally Diverse Molecules. Eur. J. Org. Chem. 2020, 18, 2650–2692. [Google Scholar] [CrossRef]
- Wang, J.-Y.; Hao, W.-J.; Tu, S.-J.; Jiang, B. Recent developments in 1,6-addition reactions of para-quinone methides (p-QMs). Org. Chem. Front. 2020, 7, 1743–1778. [Google Scholar] [CrossRef]
- Liu, X.; Wu, X.; Zhang, L.; Lin, X.; Huang, D. Recent Advances in Triarylmethane Synthesis. Synthesis 2020, 52, 2311–2329. [Google Scholar]
- Ye, L.-W.; Zhu, X.-Q.; Sahani, R.L.; Xu, Y.; Qian, P.-C.; Liu, R.-S. Nitrene Transfer and Carbene Transfer in Gold Catalysis. Chem. Rev. 2021, 121, 9039–9112. [Google Scholar] [CrossRef]
- Lu, Z.; Li, T.; Mudshinge, S.R.; Xu, B.; Hammond, G.B. Optimization of Catalysts and Conditions in Gold(I) Catalysis-Counterion and Additive Effects. Chem. Rev. 2021, 121, 8452–8477. [Google Scholar] [CrossRef]
- Zheng, Z.; Ma, X.; Cheng, X.; Zhao, K.; Gutman, K.; Li, T.; Zhang, L. Homogeneous Gold-Catalyzed Oxidation Reactions. Chem. Rev. 2021, 121, 8979–9038. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Zang, W.; Bird, M.J.; Hyland, C.J.T.; Shi, M. Gold-Catalyzed Conversion of Highly Strained Compounds. Chem. Rev. 2021, 121, 8685–8755. [Google Scholar] [CrossRef] [PubMed]
- Mato, M.; Franchino, A.; Garca-Morales, C.; Echavarren, A.M. Gold-Catalyzed Synthesis of Small Rings. Chem. Rev. 2021, 121, 8613–8684. [Google Scholar] [CrossRef] [PubMed]
- Reyes, R.L.; Iwai, T.; Sawamura, M. Construction of Medium-Sized Rings by Gold Catalysis. Chem. Rev. 2021, 121, 8926–8947. [Google Scholar] [CrossRef]
- Witzel, S.; Hashmi, A.S.K.; Xie, J. Light in Gold Catalysis. Chem. Rev. 2021, 121, 8868–8925. [Google Scholar] [CrossRef]
- Campeau, D.; León-Rayo, D.F.; Mansour, A.; Muratov, K.; Gagosz, F. Gold-Catalyzed Reactions of Specially Activated Alkynes, Allenes, and Alkenes. Chem. Rev. 2021, 121, 8756–8867. [Google Scholar] [CrossRef]
- Wang, T.; Hashmi, A.S.K. 1,2-Migrations onto Gold Carbene Centers. Chem. Rev. 2021, 121, 8948–8978. [Google Scholar] [CrossRef]
- Gobé, V.; Retailleau, P.; Guinchard, X. Self-Relay Gold (I)-Catalyzed Pictet-Spengler/Cyclization Cascade Reaction for the Rapid Elaboration of Pentacyclic Indole Derivatives. Chem.-Eur. J. 2015, 21, 17587–17590. [Google Scholar] [CrossRef]
- Fustero, S.; Miró, J.; Sánchez-Roselló, M.; del Pozo, C. Tandem Gold Self-Relay Catalysis for the Synthesis of 2,3-Dihydropyridin-4(1H)-ones: Combination of σ and π Lewis Acid Properties of Gold Salts. Chem.-Eur. J. 2014, 20, 14126–14131. [Google Scholar] [CrossRef]
- Shi, Z.; Mao, W.; Yang, Z.; Sun, S.; Tung, C.-H.; Xu, Z. Au-catalyzed neighboring hydroxymethyl group directed cycloaddition of alkyne with diazadienes: Synthesis of polysubstituted pyrroles. Chin. Chem. Lett. 2023, 34, 107488. [Google Scholar] [CrossRef]
- Meng, F.-T.; Wang, Y.-N.; Qin, X.-Y.; Li, S.-J.; Li, J.; Hao, W.-J.; Tu, S.-J.; Lan, Y.; Jiang, B. Azoarene activation for Schmidt-type reaction and mechanistic insights. Nat. Commun. 2022, 13, 7393. [Google Scholar] [CrossRef]
- Meng, F.-T.; Chen, J.-L.; Qin, X.-Y.; Zhang, T.-S.; Tu, S.-J.; Jiang, B.; Hao, W.-J. Gold self-relay catalysis for accessing functionalized cyclopentenones bearing an all-carbon quaternary stereocenter. Org. Chem. Front. 2022, 9, 140–146. [Google Scholar] [CrossRef]
- Meng, F.-T.; Qin, X.-Y.; Li, J.; Zhang, T.-S.; Tu, S.-J.; Jiang, B.; Hao, W.-J. Gold Self-Relay Catalysis Enabling [3,3]-Sigmatropic Rearrangement/Nazarov Cyclization and Allylic Alkylation Cascade for Constructing All-Carbon Quaternary Stereocenters. Chin. J. Chem. 2022, 40, 687–692. [Google Scholar] [CrossRef]
- Hao, M.-Y.; Zhang, Y.; Lin, N.; Fu, R.; Ji, X.-S.; Jiang, B.; Tu, S.-J.; Hao, W.-J. Gold self-relay catalysis enabling annulative oxygenation of propargylic alcohols with O-nucleophiles. Chem. Commun. 2023, 59, 4032–4035. [Google Scholar] [CrossRef]
- Egi, M.; Azechi, K.; Akai, S. Cationic Gold(I)-Mediated Intramolecular Cyclization of 3-Alkyne-1,2-diols and 1-Amino-3-alkyn-2-ols: A Practical Route to Furans and Pyrroles. Org. Lett. 2009, 11, 5002–5005. [Google Scholar] [CrossRef]
- Aponick, A.; Li, C.-Y.; Malinge, J.; Marques, E.F. An Extremely Facile Synthesis of Furans, Pyrroles, and Thiophenes by the Dehydrative Cyclization of Propargyl Alcohols. Org. Lett. 2009, 11, 4624–4627. [Google Scholar] [CrossRef]
- Liu, W.-T.; Xu, Z.-L.; Mou, X.-Q.; Zhang, B.-H.; Bao, W.; Wang, S.-H.; Lee, D.; Lei, L.-S.; Zhang, K. The synthesis of cycloalka[b]furans via an Au(I)-catalyzed tandem reaction of 3-yne-1,2-diols. Org. Biomol. Chem. 2017, 15, 6333–6337. [Google Scholar] [CrossRef]
- Wang, D.; Wang, S.-C.; Hao, W.-J.; Tu, S.-J.; Jiang, B. Dual Palladium/Scandium Catalysis toward Rotationally Hindered C3-Naphthylated Indoles from β-Alkynyl Ketones and o-Alkynyl Anilines. Chin. J. Chem. 2021, 39, 106–114. [Google Scholar] [CrossRef]
- Chen, L.-Q.; Zhu, C.-F.; Zhang, S.; Liu, B.-Y.; Tu, S.-J.; Hao, W.-J.; Jiang, B. Palladium-catalyzed annulative allylic alkylation for regioselective construction of indole-fused medium-sized cyclic ethers. Chin. Chem. Lett. 2023, 34, 108398. [Google Scholar] [CrossRef]
- Wang, D.; Wang, S.-C.; Hao, W.-J.; Tu, S.-J.; Jiang, B. Selective Syntheses of Benzo[b]carbazoles and C3-Substituted Indoles via Tunable Catalytic Annulations of β-Alkynyl Ketones with Indoles. Adv. Synth. Catal. 2020, 362, 3416–3422. [Google Scholar] [CrossRef]
- Li, X.; Villar-Yanez, A.; Tounzoua, C.N.; Benet-Buchholz, J.; Grignard, B.; Bo, C.; Detrembleur, C.; Kleij, A.W. Cascade Transformation of Carbon Dioxide and Alkyne-1,n-diols into Densely Substituted Cyclic Carbonates. ACS Catal. 2022, 12, 2854–2860. [Google Scholar] [CrossRef]
- Gabriele, B.; Veltri, L.; Plastina, P.; Mancuso, R.; Vetere, M.V.; Maltese, V. Copper-Catalyzed Synthesis of Substituted Furans and Pyrroles by Heterocyclodehydration and Tandem Heterocyclodehydration–Hydration of 3-Yne-1,2-diols and 1-Amino-3-yn-2-ol Derivatives. J. Org. Chem. 2013, 78, 4919–4928. [Google Scholar] [CrossRef] [PubMed]
- Minkler, S.R.K.; Isley, N.A.; Lippincott, D.J.; Krause, N.; Lipshutz, B.H. Leveraging the Micellar Effect: Gold-Catalyzed Dehydrative Cyclizations in Water at Room Temperature. Org. Lett. 2014, 16, 724–726. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, C.-F.; Chen, L.-Q.; Hao, W.-J.; Cui, C.-C.; Tu, S.-J.; Jiang, B. Diastereoselective Synthesis of 1,2-Dihydrobenzofuro[3,2-b]pyridines via a Carbon-Carbon Double Bond Cleavage–Rearrangement Cascade. Org. Lett. 2021, 23, 2654–2658. [Google Scholar] [CrossRef]
- Yang, M.; Han, H.; Jiang, H.; Ye, S.; Fan, X.; Wu, J. Photoinduced reaction of potassium alkyltrifluoroborates, sulfur dioxide and para-quinone methides via radical 1,6-addition. Chin. Chem. Lett. 2021, 32, 3535–3538. [Google Scholar] [CrossRef]
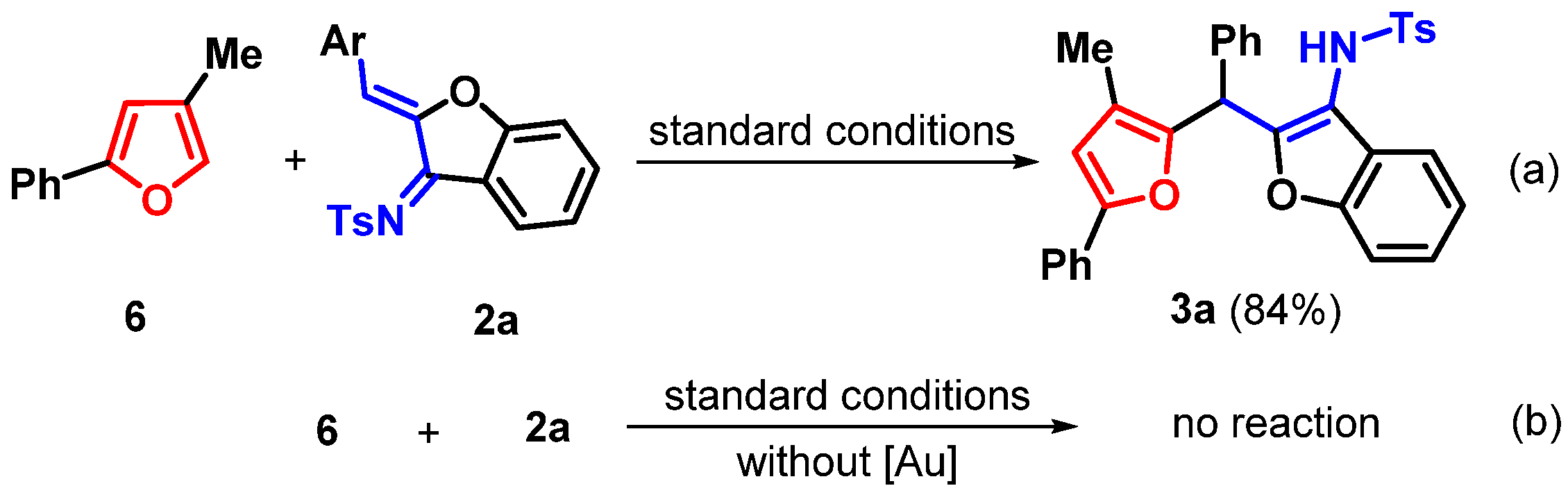
 | |||
|---|---|---|---|
| Entry | [Au] (2 mol%) | Solvent | Yield (%) b |
| 1 | JohnPhosAu(MeCN)SbF6 | DCE | 85 |
| 2 | IPrAuNTf2 | DCE | 60 |
| 3 | AuCl3 | DCE | trace |
| 4 | AgNO3 | DCE | trace |
| 5 | PbCl2 | DCE | trace |
| 6 | CuCl2 | DCE | trace |
| 7 | JohnPhosAu(MeCN)SbF6 | THF | trace |
| 8 | JohnPhosAu(MeCN)SbF6 | CH3CN | trace |
| 9 | JohnPhosAu(MeCN)SbF6 | toluene | trace |
| 10 | JohnPhosAu(MeCN)SbF6 | 1,4-dioxane | 55 |
| 11 | JohnPhosAu(MeCN)SbF6 | DCM | 60 |
| 12 | - | DCE | NR |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rao, Q.; Zhang, Y.; Gu, X.-Y.; Liu, Y.-P.; Jiang, B.; Hao, W.-J. A Cascade Synthesis of Unsymmetrical Furanized Triarylmethanes via Gold Self-Relay Catalysis. Catalysts 2023, 13, 1051. https://doi.org/10.3390/catal13071051
Rao Q, Zhang Y, Gu X-Y, Liu Y-P, Jiang B, Hao W-J. A Cascade Synthesis of Unsymmetrical Furanized Triarylmethanes via Gold Self-Relay Catalysis. Catalysts. 2023; 13(7):1051. https://doi.org/10.3390/catal13071051
Chicago/Turabian StyleRao, Qian, Yan Zhang, Xin-Yu Gu, Yin-Ping Liu, Bo Jiang, and Wen-Juan Hao. 2023. "A Cascade Synthesis of Unsymmetrical Furanized Triarylmethanes via Gold Self-Relay Catalysis" Catalysts 13, no. 7: 1051. https://doi.org/10.3390/catal13071051







