Abstract
ZnO and TiO2 are both well-known electron transport materials; however, an exact comparison of their performance, when fabricated under the same synthesis conditions, is missing in the literature. Considering this, we introduced a viable electrospinning route for the development of highly polycrystalline TiO2 and ZnO nanofibers for an electron transport material (ETM) of perovskite solar cells and photocatalysts for textiles. Thanks to the effective tuning of band structure and morphology of TiO2, a significant improvement in performance as compared to ZnO was observed when both were used as photoanodes and photocatalysts. X-ray diffraction detected polycrystalline structural properties and showed that peaks are highly corresponding to TiO2 and ZnO. Morphological analysis was carried out with a scanning electron microscope, which revealed that nanofibers are long, uniform, and polycrystalline, having diameter in the nano regime. TiO2 nanofibers are more aligned and electron-supportive for conduction as compared to ZnO nanofibers, which are dense and agglomerated at some points. Optoelectronic properties showed that TiO2 and ZnO show absorption values in the range of ultraviolet, and visible range and band gap values for TiO2 and ZnO were 3.3 and 3.2 eV, respectively. The TiO2 band gap and semiconductor nature was more compatible for ETL as compared to ZnO. Electrical studies revealed that TiO2 nanofibers have enhanced values of conductivity and sheet carrier mobility as compared to ZnO nanofibers. Therefore, a higher photovoltaic conversion efficiency and antibacterial activity was achieved for TiO2 nanofibers (10.33%), as compared to ZnO (8.48%). In addition, the antibacterial activity of TiO2 was also recorded as better than ZnO. Similarly, compared to ZnO nanofibers, TiO2 nanofibers possess enhanced photoactivity for antimicrobial and dye degradation effects when applied to fabrics.
1. Introduction
Semiconductor nanomaterials have attracted great interest from scientists because of their unique functionalities in nanoscale devices. Specifically, their application in harvesting solar energy for photocatalysis [1,2], energy [3], and environmental remediation [4] has emerged as the center of research. As important semiconductors, TiO2 and ZnO nanostructures have been extensively studied in the field of photovoltaics, and specifically, in perovskite solar cells (PSCs) [5]. Since the one-dimensional (1D) TiO2 and ZnO have a large surface area and their 1D morphology provides a direct path for the electron flow, they are preferred for electron transport materials (ETM). Some researchers proposed that the crystalline properties of TiO2 nanostructures are more compatible with ETM, whereas others reported that ZnO as a wurtzite or hexagonal structure offers better performance [6,7]. Both TiO2 and ZnO nanofibers generally have a wide distribution range from 80 to 300 nm and have a surface area of 30–40 m2g−1. Thanks to their key feature of high electron mobility, suitable photovoltaic performance is achieved for mesoporous TiO2 and ZnO-based ETM for PSCs. Ideally, higher carrier mobility or electron mobility favors enhanced open-circuit voltage and high current densities [8]. In addition, the rate of recombination must be less, to avoid the internal losses of the PSCs [9].
Metal oxides have demonstrated special optical and electronic properties as compared to those of bulk materials. The metal oxide-based polymer nanocomposites also exhibit good electrical and optical properties and thus, have long been of interest to researchers [10]. TiO2 and ZnO metal oxides have been suggested for absorption, photocatalytic and photovoltaic applications [11]. One-dimensional (1D) nanostructures, including nanowires, nanotubes and quantum wires, have been regarded as the most promising building blocks for nanoscale electronic and optoelectronic devices. Their larger surface area provides a better platform for electron transportation in photoanodes and a better active photocatalyst area for photocatalysis [12]. Therefore, researchers working in the 1D nanomaterials area of study are constantly striving to develop new fundamental science as well as potential applications [13].
In many studies, synthesis of one-dimensional (1D) TiO2 nanomaterials is reported, which is intended for their high surface area and large fibrous dimension [14]. IMA Mohamed et al. [15] reported electrospun TiO2 nanofibers as achieving a suitable performance (5.41%) in PSCs. The choice of an electrode, its surface area, and the photoabsorption contact layer are the main factors for tuning its optoelectronic properties [16]. XL Wang et al. [17] studied functional 1D TiO2 nanostructures such as nanowires, and nanofibers for perovskite solar cell applications [18]. By tailoring the size and functional groups, tailored band gap and enhanced efficiencies of up to 5.9% were achieved using TiO2 ETM in PSCs [19]. The sol-gel electrospinning of TiO2 nanofibers was reported, which possessed a unique opportunity for high surface area with adhesive properties [20]. On the other hand, one-dimensional (1D) ZnO is also reported in perovskite solar cells due to the enhanced mobility of electrons in the oriented direction and the high current density inducing a high proportion of photoactivity [21]. ZnO nanofibers were synthesized by electrospinning and applied as ETM to obtain unique properties, such as better electron mobility and a suitable band gap (3.3 eV) [22,23]. ZnO nanofibers showed compatibility in photoanode material and perovskite solar cells to achieve efficiency of up to 8.7% [24]. ZnO nanofibers offer fewer energy trap states between the valence and conduction bands, thus reducing losses [25]. The ZnO surface modification and nanofibers’ diameter optimization result in highly efficient perovskite solar cells (PSCs) having a current density of 14.51 mA/cm2 [26]. Electrospun TiO2 and ZnO nanofibers with their 1D structures offer less quantum confinement and better photovoltaic performance, as compared to nanoparticles [27].
Photoactive textiles are of greatly emerging scientific interest after COVID-19. Antimicrobial and antibacterial materials damage the cell wall and rupture the microbe, which results in the inhibition of growth pathways in microbes. Exciton is produced due to photon absorption in semiconductor materials, which generates reactive oxygen species (ROS). Antibacterial activity of nanomaterials is due to photocatalytic degradation of microbes via the reactive oxygen species (ROS) produced by them [28]. It is mainly ROS who are responsible for antibacterial activity, as they cause the rupture of the cell wall and depolarize the cell membrane [29]. This also further inhibits nuclei acid synthesis and metabolic pathways in bacteria, which leads to degradation of bacteria and microbes [30].
Recently, there have been several reports on the synthesis of electrospun ZnO and TiO2 nanofibers synthesized at various processing conditions [31]. However, a comparison of photovoltaic performances of TiO2 and ZnO nanofibers synthesized at the same processing conditions is not reported yet. In this study, we successfully synthesized TiO2 and ZnO nanofibers under the same processing conditions and then compared them as an ETM for perovskite solar cells and as photocatalysts on textile surfaces. Both nanofibers were compared for their morphological and structural features using scanning electron microscopic analysis, X-ray diffraction, and Raman spectroscopy. The optical properties, chemical composition, and electronic properties of TiO2 and ZnO nanofibers were compared so that the underlying mechanism of better performance can be elucidated. Electrical and antibacterial activities of TiO2 and ZnO nanofibers were also investigated, for antibacterial textile applications. Similarly, compared to ZnO, TiO2 possesses enhanced photoactivity for antimicrobial and dye degradation effects when applied to fabrics.
2. Results and Discussion
2.1. Crystallographic Characterizations
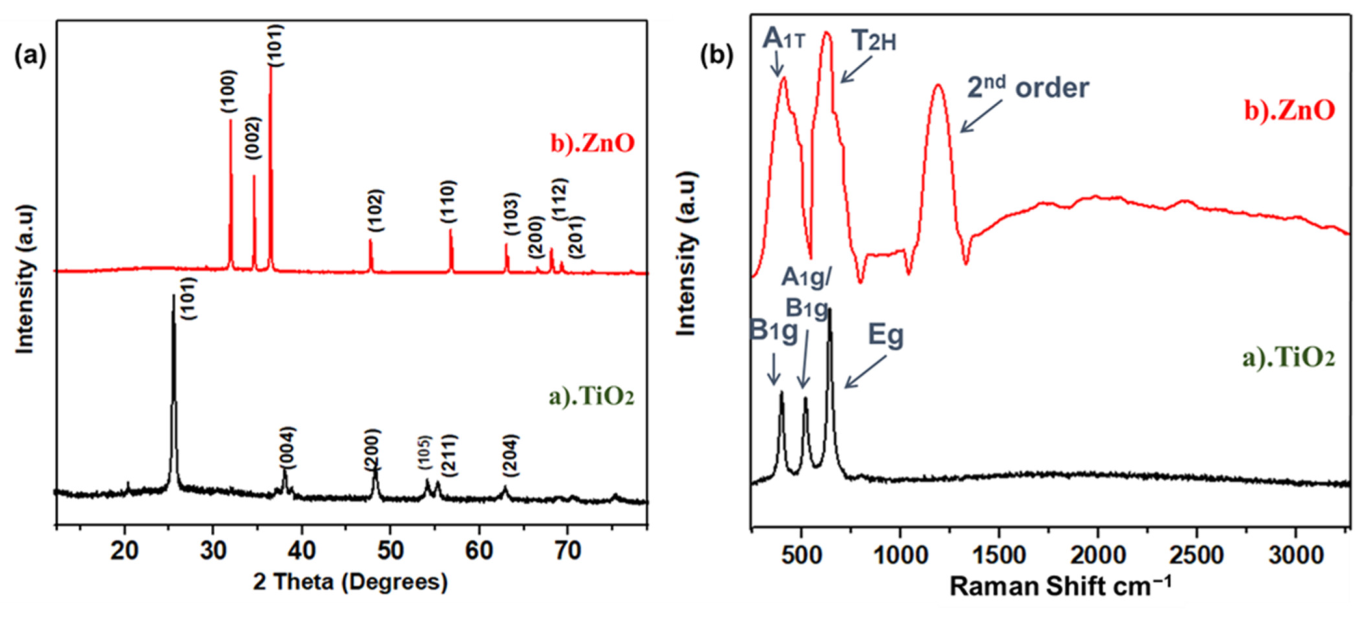
XRD analysis was performed to probe the crystal structure, crystallographic phases, and peaks at diffracted positions. This test confirms the basic structure of materials and its crystal planes. XRD spectra for TiO2 and ZnO nanofibers after calcination surveyed between 12.5° to 80° are shown in Figure 1a. This crystallinity and the defined crystalline features were achieved after annealing at 450 °C; as the binder is removed after calcination, it results in the anatase phase of Titania and wurtzite phase in zinc oxide nanofibers [31]. The XRD survey of TiO2 nanofibers showed that the peaks at 2θ of 25.5, 40.69, 38.52, 48.49, 54.09, 55.62, and 63.80° correspond to (101), (004), (200), (105), (211), and (204) planes, respectively. Peaks were indexed using JCPDS No. 21-1272, 29-1363 for a sintered sample at room temperature [32]. The peaks of (101) at (2θ = 25.5°) are much stronger than the other peaks, and no peaks of impurity were observed in the TiO2 nanofibers [33]. The maximum positions and their relative intensity are consistent with the general perturbation pattern of the anatase TiO2 with a preferential growth of (101) plane. In ZnO spectra, mainly five peaks were observed, which were related to (100), (002), (101), (102), (110), (103), (200), (112), and (201) crystallographic planes, typically representing the hexagonal wurtzite structure of ZnO [34]. This result agrees well with ZnO’s Joint Committee on Powder Diffraction Standards [JCPDS](36-1453), related to the polycrystalline ZnO nanofibers’ structure, without any peaks of impurity, as shown in Figure 1a. The sharp characteristic peaks of these nanofibers show that highly oriented nanofibers, with a suitable dislocation density and nominated planes, are formed as a result of annealing. The annealing temperature has a strong effect on the production of poly-crystalline nanofibers as the removal of the binder and the sintering of nanodomains occurs at this stage [35].
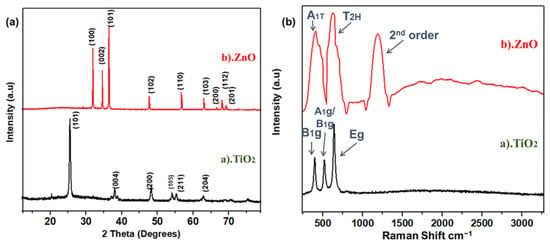
Figure 1.
(a) XRD spectra for TiO2, ZnO nanofibers; (b) Raman spectra for TiO2 and ZnO nanofibers.
Raman spectra are useful for supporting the crystallographic analysis obtained from XRD. Raman spectra of both fibers were obtained in the range of 250 to 3250 cm−1, which has good agreement with XRD results, shown in Figure 1b. For anatase TiO2 fibers, there were three peaks observed, including Eg, A1g, and B1g modes located at 648, 405 and 527 cm−1, respectively [36]. These highly corresponded to anatase titania structure. Raman spectra of ZnO showed an A1T phonon mode at 436.5 cm−1, whereas the phonetic frequency of T2H is indicating a strong mode at 638.5 cm−1. Such confined phonon frequency is only observed in wurtzite crystal structure. Compared to TiO2, the TO phonon frequency redshift in ZnO nanofibers has been previously observed by Yang et al. [37] as well. The second order phonon mode is shown in the range of 1538.5 cm−1, as highlighted in Figure 1b.
2.2. Morphological Characterizations
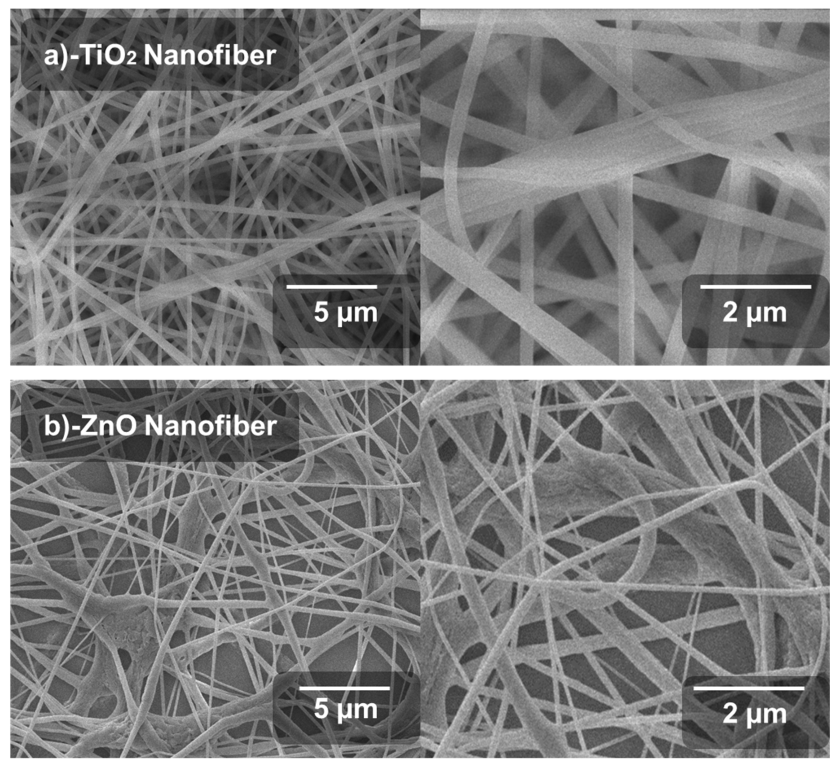
Scanning electron microscopy images were used to check the surface morphology of the porous network formed by nanofibers. Nanofibers should be uniform, elongated, fine and semi-porous, to achieve high interface for electrons conduction. These characteristics of nanofibers support better light absorption and then extraction of electrons from the conduction band of the perovskite material. The fibers with optimum shape are formed with a controlled rate of production and the optimum electrospinning setup parameters. In order to obtain a dense and optimal collection of nanofibers, the collector FTO glass was moved according to the nature of the nanofibers. Sol-gel viscosity strongly affects the production of nanofibers; otherwise, nanofibers are not formed at highly dilute concentrations. Considering the above parameters, we optimized the processing parameters to achieve significantly fine fibers, which can be used for the ETL [38].
SEM images of the synthesized TiO2 and ZnO nanofibers after the annealing process are shown in Figure 2a,b. The nanofibers were localized at various orientations, as expected due to the spontaneous fiber extrusion through the electrospinning jet. All fibers were elongated and uniformly formed without any aggregation. This results in the formation of nano-porous titania and zinc oxide nanofiber films onto the substrate. TiO2 nanofibers are more dense, elongated and uniform as compared to ZnO. The highly dense structure of TiO2 nanofibers results in the maximum conduction of photo-generated electrons from the valence band to the conduction band. This TiO2 fibrous structure also supports a more aligned path of electrons leading to the highest current densities. It also leads to efficient diffusion of electrons from the perovskite layer. In the SEM morphology, the Titania nanofibers are aligned, elongated and smooth, directly related to enhanced electron transportation as well as dye degradation, while ZnO nanofibers are dense, and agglomerated at some points. This conglutination does not support effective transportation of electrons in PSCs. Morphology of fibers was uniform, twisted at some points and uniformly porous all over the FTO-glass substrate.
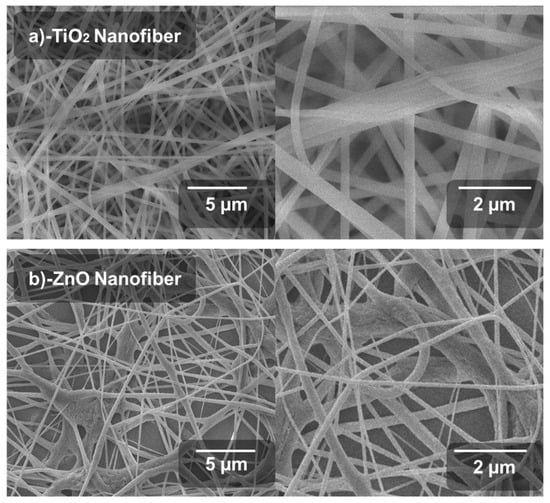
Figure 2.
Scanning electron microscopic images: (a) TiO2 nanofiber, (b) ZnO nanofiber.
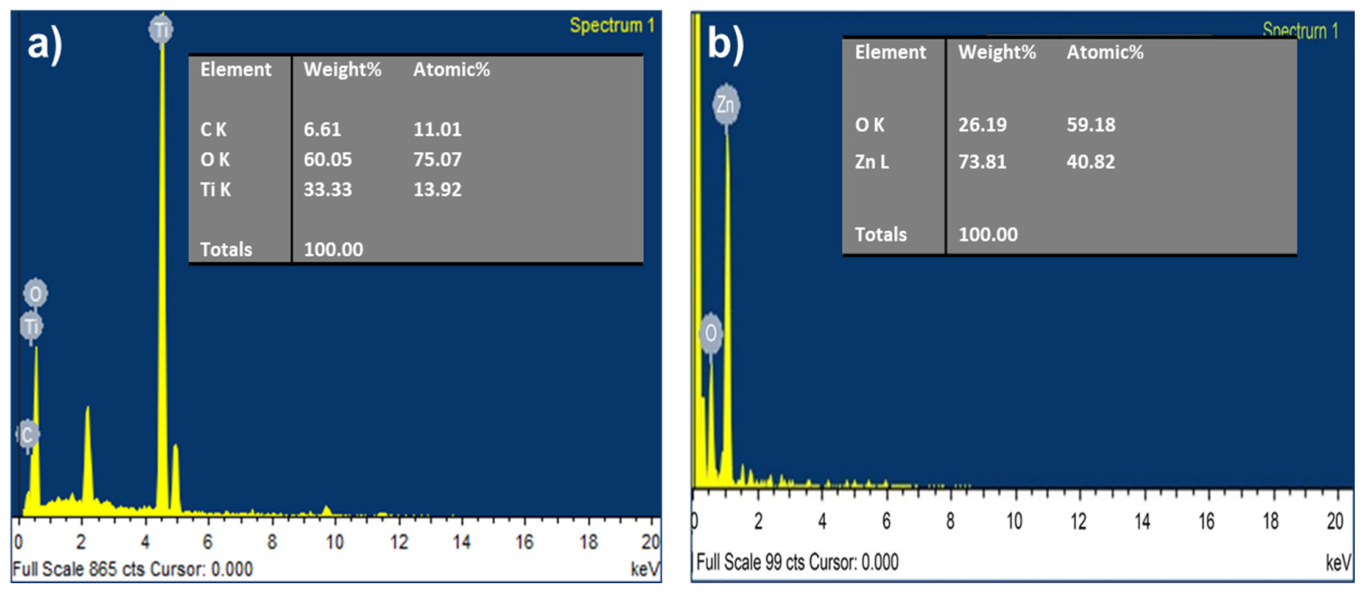
Energy dispersive X-ray spectroscopy (EDX) was characterized for both TiO2 and ZnO nanofibers as shown in Figure 3a,b. In TiO2, the major contribution is Oxygen and Titanium, while in ZnO, there is Zinc and oxygen.
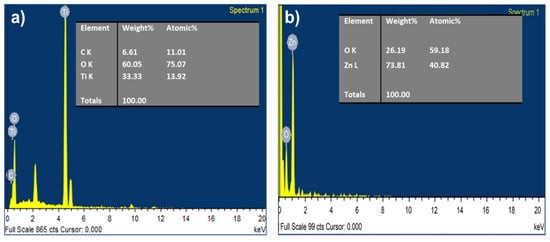
Figure 3.
EDS spectra for (a) TiO2 nanofiber, and (b) ZnO nanofiber.
2.3. Optoelectronic Characterizations
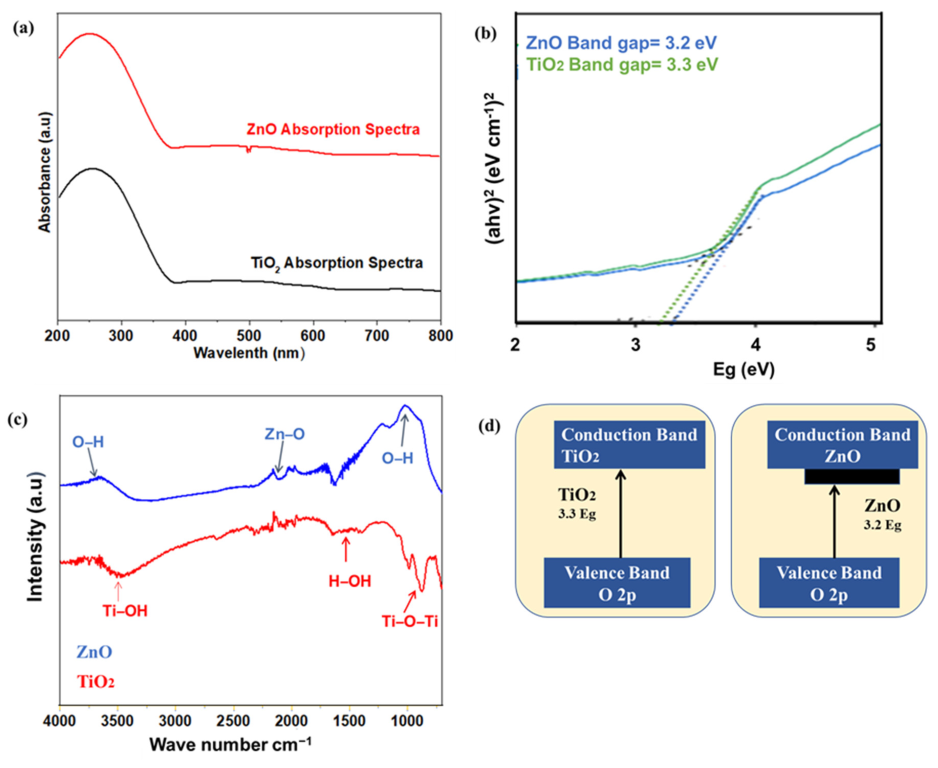
The optical characterizations of TiO2 and ZnO nanofibers were carried out to check the absorption and band gap of both samples as shown in Figure 4a,b. The semiconductor materials absorb ultraviolet (UV) or near-UV visible light, resulting in associated photoexcitation. Only a selective portion of the light is absorbed, i.e., higher than the bandgap. The exciton (electron–hole pair) formed as a result of photoexcitation is responsible for photocatalytic activity. The absorption of nanofibers was recorded from 220 to 800 cm−1 with the UV-absorbance spectrophotometer. As shown in the Figures, both TiO2 and ZnO showed absorption in a high energy spectrum, whereas low energy photons pass through, as required for the perovskite absorber layer [39,40]. From the spectra, it can be observed that a major part of absorption occurs in the ultraviolet (UV) and near-UV region; hence, a transparent ETL can be fabricated. The band gap of samples was calculated from the Tauc plot, which describes the minimum energy required for the photoexcitation. The band gap of all samples was calculated by converting the wavelength into energy, using the following Equation (2):
wherein “Eg” is the energy, “h” the Plank constant, and “α” is the absorption coefficient. Titania has an indirect bandgap around 3.3 eV. The ZnO absorption spectra show the exciton band gap (Eg = 3.2 eV). This suitable band gap provides the required Fermi energy level and transports the photoexcited electrons from the perovskite to the FTO glass. The better charge transport leads to less energy loss, hence offering high open-circuit voltage, along with higher current densities of the solar cell. The ZnO band gap was slightly less, as compared to titania, but the titania band gap is more tuned for Fermi electron conduction and transportation from the valence band towards the conduction band.
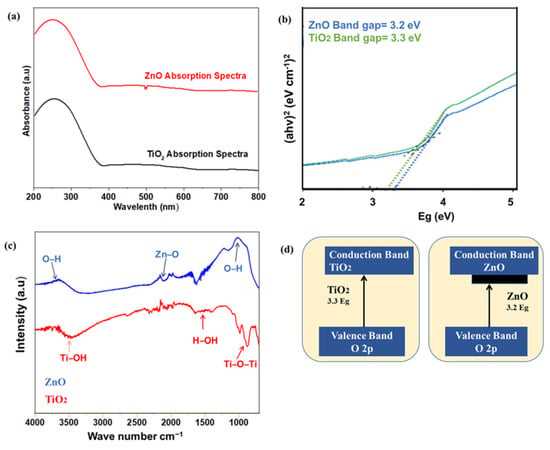
Figure 4.
(a,b) UV vis absorption spectra and band gap for TiO2 and ZnO nanofibers; (c) FTIR spectra for TiO2 and ZnO; (d) Band gap mechanism for TiO2 and ZnO.
Fourier transform infrared spectroscopy analysis was carried out to detect all the functional groups in the range of 720–4000 cm−1, as shown in Figure 4c. Both nanofibers showed a typical broad peak at 3200–3600 cm−1 related to the vibration of the OH bond, and peaks at 1630 cm−1 that correspond to the bending of the OH bond [41]. These hydroxyl groups are produced as a result of dangling bonds on the surface of nanoparticles. These dangling bonds generate defect states, responsible for the absorbance and longer dwell time of excitons. Hence, the functional surface is expected to have enhanced photocatalytic activity. It is reported that the Ti-OH bonds are formed as a result of the hydrolysis reaction of precursors, which stay pendent at the surface. The ZnO showed slightly higher hydroxyl functional groups, as compared to titania. Such a functional surface provides a stronger interface for the adhesion of the perovskite layer [42]. However, the higher moisture uptake by such hydrophilic groups reduces the solar cell life [43,44].
2.4. Electric Characterizations
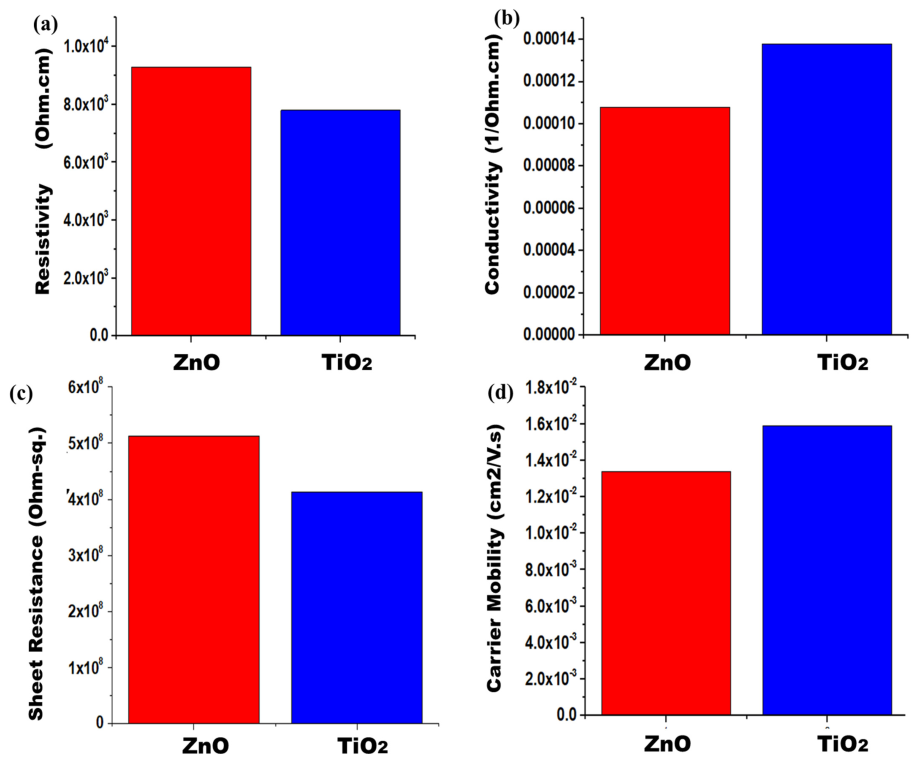
In order to characterize the values of sheet resistance, conductivity, resistivity, and sheet carrier mobility, the Hall Effect swin system was used. Four probe methods were utilized to detect the conductive parameters of nanofibers’ films. Figure 5a–d compares the resistivity, sheet resistance, carrier mobility, and conductivity of both samples. The ZnO nanofibers possess resistivity which is 9.28 × 103 Ω cm, but pure TiO2 has a resistivity of 7.8 × 103 Ω cm. Zinc oxide shows higher resistivity as compared to TiO2 because its electron transportation is slow. TiO2 provides a uniform and aligned path for electron transportation. It fastens the diffusion of photoelectrons, thereby decreasing resistivity. The pure ZnO sample has a conductivity which is 1.08 × 10−04 Ohm-cm−1, whereas pure TiO2 has a conductivity of 1.38 × 10−04 Ohm-cm−1. As conductivity is reciprocal to resistivity, so TiO2 is a more conductive material as compared to ZnO. TiO2 will facilitate in fast electrons’ mobility. Thus, the pure ZnO sample gives a sheet resistance which is 5.13 × 108 Ohm-sq. Pure TiO2 has a sheet resistance of 4.13 × 108 Ohm-sq. In the case of ZnO, the electrons will suffer more sheet resistance in their path, thereby reducing its photoactivity. The pure ZnO sample gives carrier mobility which is 1.34 × 10−2 cm2/Vs. Pure TiO2 has a carrier mobility of 1.59 × 10−2 cm2/Vs. From this, it can be concluded that TiO2 has a tuned electronic band structure, with slightly higher light absorption. These parameters will support its better electron conduction and lower resistance losses in TiO2. Better conduction of electrons provides higher current density, and thereby increases the efficiencies of solar cells. Overall, TiO2 shows better electrical properties than ZnO, along with an efficient electron transport nature [45].
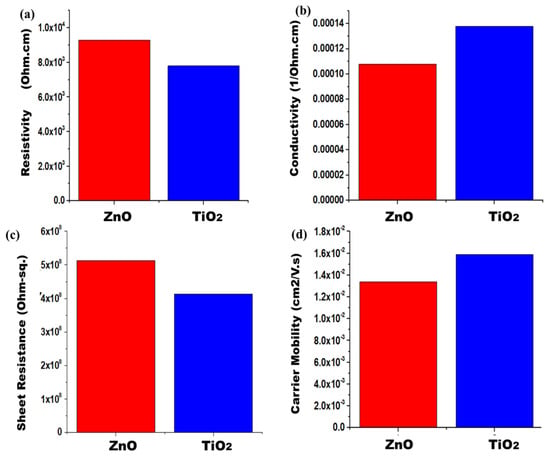
Figure 5.
Photoanodes electrical measurements (a) Conductivity, (b) resistivity, (c) sheet resistance, and (d) sheet carrier mobility for both samples.
2.5. Solar Cell Characterizations
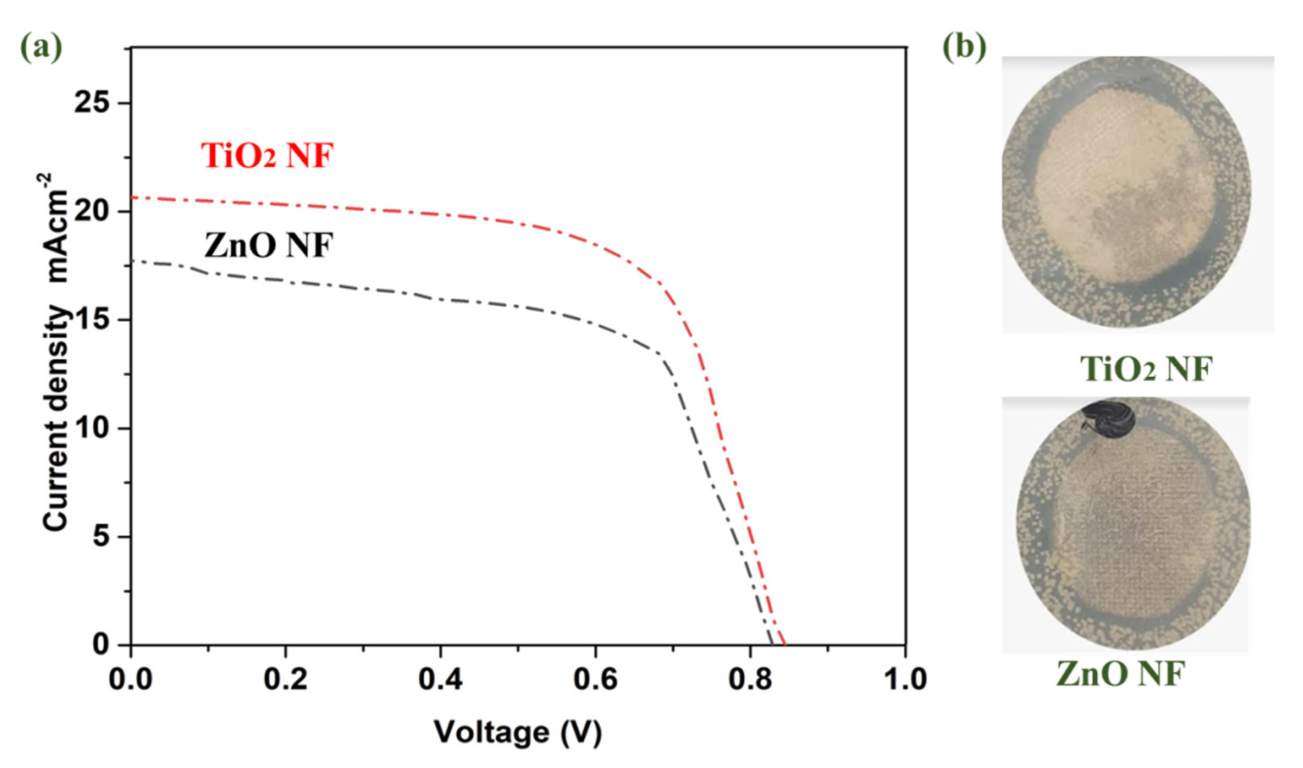
For solar cell parameters, and to detect the effect of TiO2 and ZnO nanofibers on the performance of solar cells, J-V measurements were carried out. Titania and zinc oxide nanofibers-based photoanodes showed the JV performance, as shown in Figure 6. All the performance parameters are also summarized in Table 1. ZnO nanofibers offered a current density (Jsc) of 17.55 mAcm−2, whereas TiO2 has a Jsc of 20.66 mAcm−2. This increment is due to better conductivity of the ETL via TiO2, which enables perovskite solar cells to efficiently utilize photons for harvesting energy [46]. With the TiO2 nanofibers ETL, Jsc is significantly improved, which can be due to better band alignment. Suitable band alignment favors the flow of electrons from the conduction band of perovskite to the conduction band of TiO2. The combined effect of band alignment and conduction suppresses the electron-hole recombination, and their energy barrier is maintained; hence, an effective charge separation can be achieved [47]. By contrast, a parameter like open-circuit voltage (Voc) has negligible improvement in the TiO2 ETL: 0.01 V enhancements [48,49]. Additionally, the TiO2 nanofibers-based ETL was effective in continuous electronic pathways, and thus, better electrical conductivity results in a higher FF. The increase in FF can be referred to as lowered series resistance [17,26,50,51]. ZnO shows efficiency of 8.48%, whereas TiO2 showed an efficiency of 10.33%. In the case of TiO2, electrons are transported efficiently, hence lower charge recombination is observed, resulting in a better Jsc. An optimized and tuned band gap results in enhanced electron diffusion from the valence band to the conduction band, resulting in enhanced efficiencies. In summary, the ETL fabricated using TiO2 nanofibers provides higher energy harvesting, offering better efficiency for perovskite solar cells [52].
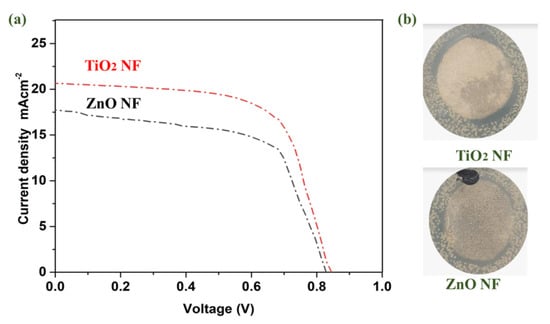
Figure 6.
(a) J ̶V characteristics of PSCs based on TiO2 and ZnO photoanodes; (b) Antibacterial comparison of TiO2 and ZnO nanofibers.

Table 1.
Photovoltaic parameters of devices fabricated with TiO2 and ZnO-based nanofibers.
2.6. Antibacterial Characterizations
Antibacterial results were determined by coating TiO2 and ZnO nanoparticles onto cotton fabrics. The qualitative antibacterial activity of ZnO and TiO2 nanofibers is compared in Figure 6b. It was observed that TiO2 nanofibers showed a superior antibacterial activity as compared to ZnO. The antibacterial activity of these nanomaterials arises from the photocatalytic effect: the light absorption generates an electron–hole pair, which produces ROS via a redox reaction. The ROS are responsible for the rupturing of the bacterial cell membrane. The higher light absorbance and longer lifetime of nanomaterials offer better photocatalytic and antibacterial activity. When the bacterial cells come into contact with the nano-coated cotton fabric, the reactive ROS are produced by the TiO2 or ZnO and degrade the bacteria. The higher ROS produced by the TiO2 are responsible for the better antibacterial activity.
2.7. EIS Measurements
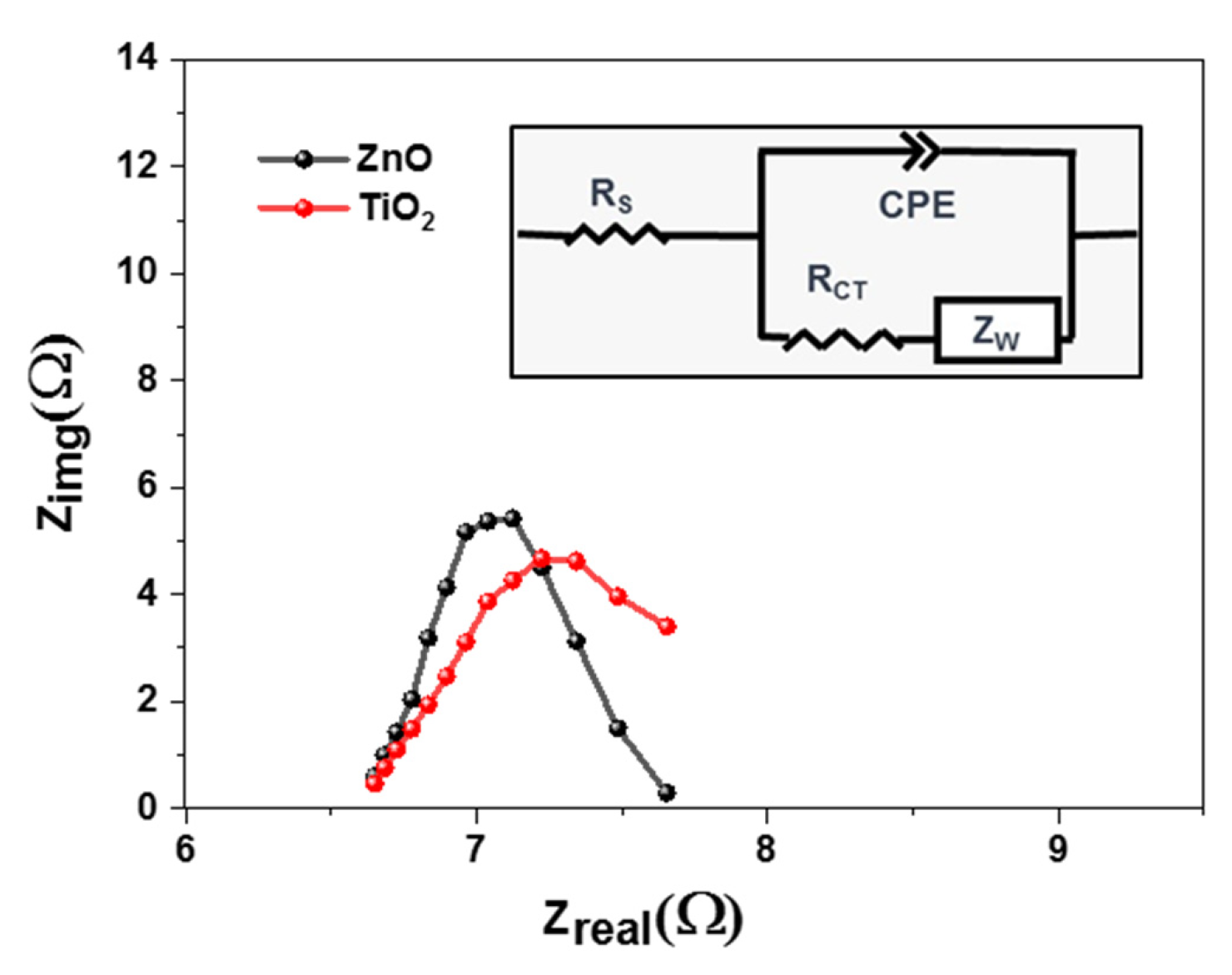
To understand the charge transfer behavior of the solar cells, an electrochemical impedance spectrum (EIS) was performed. These measurements were performed for all samples at ambient temperatures as shown in Figure 7. Charge transfer dynamics were calculated using a dummy cell structure, where both photoanodes were sandwiched face-to-face, using 60 µm surlyn. This dummy cell was filled with iodide electrolytes, as used in our previous work [53]. Using the Nyquist plot and the Randles fitting model (Figure 7(inset)), it was concluded that Titania causes a decrease in charge transfer resistance, i.e., the height of the Nyquist curve [54]. However, there was not a significant difference in series resistance, i.e., the x-intercept of the EIS curve. If the charge transfer resistance value is low, there will be an effective moment of electrons across the junction. Zinc oxide possesses higher resistance of 5.18 ohm, as compared to Titania showing 2.6 ohm.
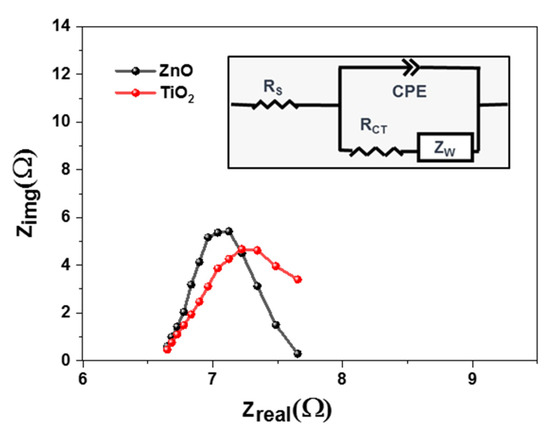
Figure 7.
EIS characteristics of PSCs based on TiO2 and ZnO photoanodes.
2.8. Photocatalytic Dye Degradation Test
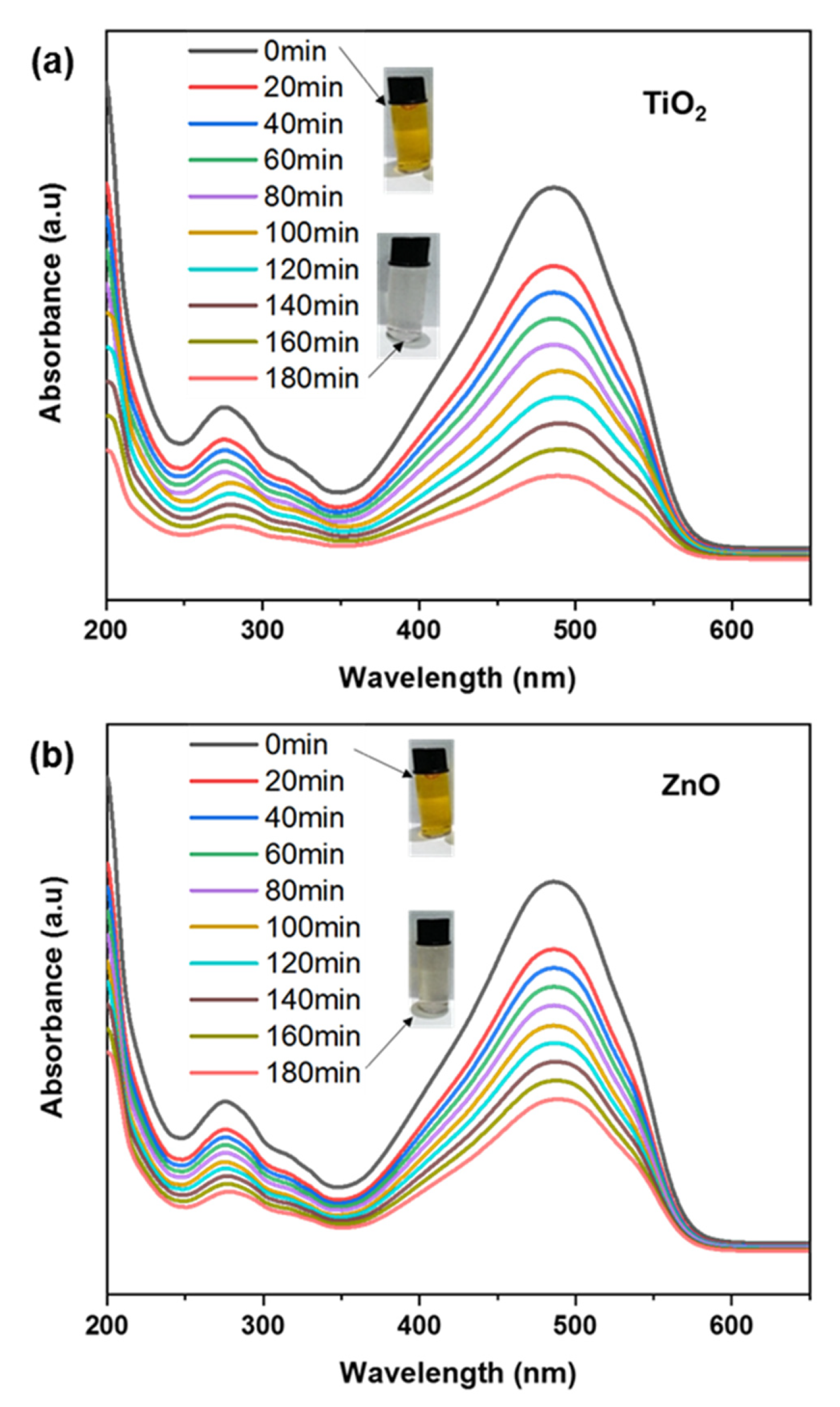
TiO2 and ZnO nanofibers-coated fabric was immersed in MO dye solution to determine their photocatalytic efficacy for dye degradation, as shown in Figure 8a,b. We conducted the degradation after immersing the fabric electrode in the dye solution and exposing it to sunlight for degradation. The MO with a concentration of 10 ppm was used for the degradation experiment. The maximum absorption intensity of MO was calculated at 464 nm, and to approximate the degradation, the absorption spectra were recorded using a UV-Visible spectrometer [55]. The adsorption of the electrodes (measured under dark conditions) is given in the negative-time to zero-time range. The photocatalysis was calculated after saturation of adsorption, after which the sample was exposed to light for photocatalytic dye degradation. By comparing the photocatalysis of all electrodes, it can be observed that the undoped ZnO electrode degraded 20% of the original concentration, whereas the TiO2 electrode degraded 80% [56]. This 60% higher photocatalytic degradation of dyes by using our proposed electrode shows that the assembled structure of the TiO2 electrode plays a significant role in the photocatalytic activity of dye [57]. In addition, a test was also performed just in the absence of a photocatalyst to analyze the dye degradation due to the light source, which was recorded to be negligible [58]. Major photodegradation of dyes by using the TiO2 electrode is due to enhanced light absorption, which generated a large number of excitons for photodegradation, as shown in Figure 8a. It serves as an electron sinker for doped semiconductors. Charge separation is also improved as a result of this effect, resulting in a longer dwell time of excitons [59]. When a photocatalyst is exposed to light, photons are absorbed, and excitons are generated. Higher excitons can enhance photocatalytic activity by increasing the probability of electron-hole separation and, thus, the production of ROS. These excitons can then be separated into their constituent electron and hole, which can participate in redox reactions with adsorbed molecules on the photocatalyst surface [60]. When a dye undergoes degradation, it typically results in changes in its molecular structure or fragmentation, leading to alterations in its absorption properties. These changes can be observed in the UV spectrum as shifts or variations in the absorption peaks. The photocatalytic properties cause degradation of the dye molecule, causing changes in molecular bonding. Specifically, the change in conjugation length of the dye molecule after interacting with the photocatalyst causes a slight shift in wavelength change. This photocatalysis in the mobile phase facilitates the generation of ROS and increases the efficiency of the process. Compared to the mobile phase, the photocatalysis is slow in the passivated state, i.e., coated on fabric. However, the easier recovery of active materials and careful design, as well as optimization of the photocatalyst’s properties with minimal secondary pollution, makes the proposed strategy closer to practical applications [61].
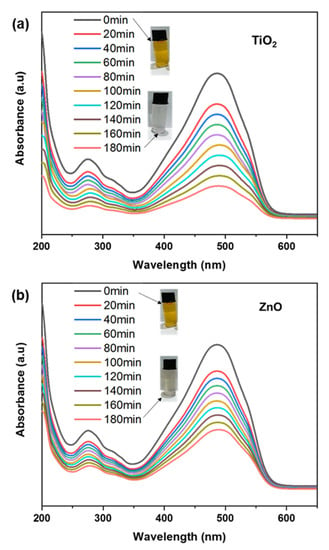
Figure 8.
(a,b) Photocatalytic dye degradation for TiO2 and ZnO nanofibers.
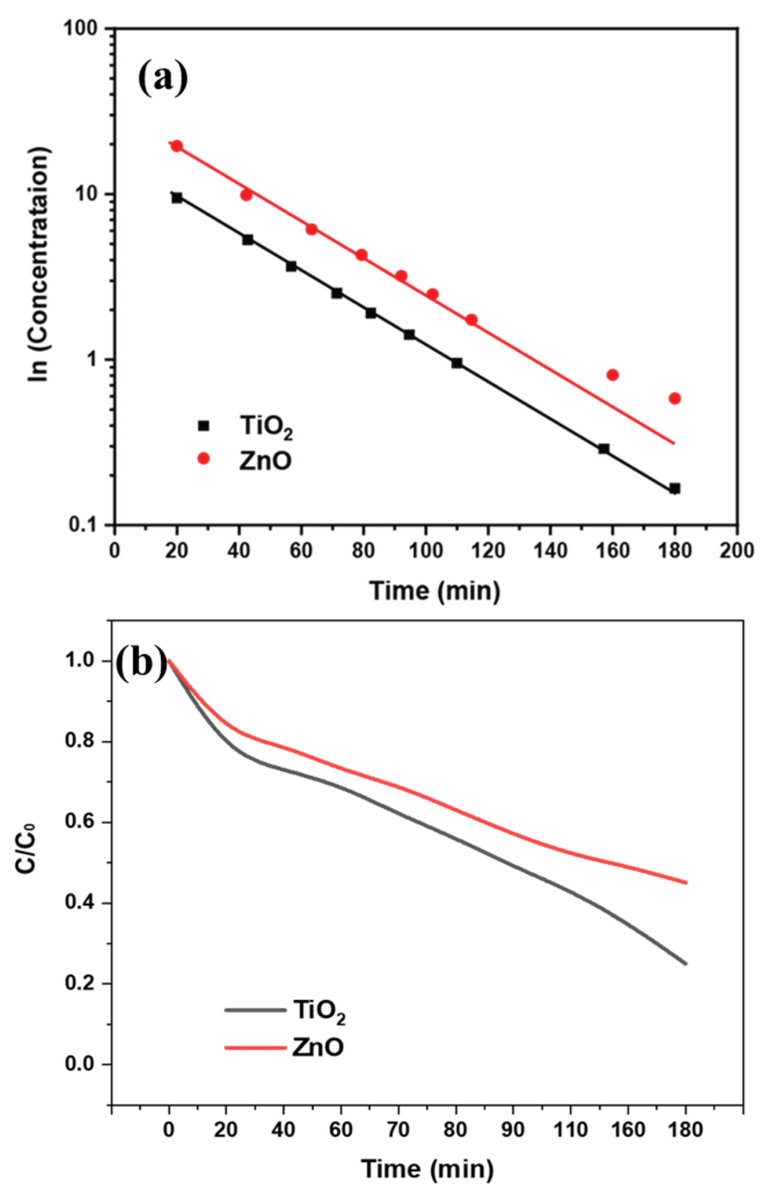
When a dye undergoes degradation, it typically results in changes in its molecular structure or fragmentation, leading to alterations in its absorption properties. These changes can be observed in the UV spectrum as shifts or variations in the absorption peaks. The photocatalytic properties cause degradation of the dye molecule, causing changes in molecular bonding. Specifically, the change in conjugation length of the dye molecule after interacting with the photocatalyst causes a slight shift in wavelength change. The difference in the charge separation leads to a variable rate of breakdown of the dye molecules. This effect is indirectly related to the variable reaction kinetics of the photocatalysis as shown in Figure 9a. The degradation rate can be observed faster for TiO2 nanofibers, with a relatively rapid decrease in dye concentration, as shown in Figure 9b. The dye degradation concentration (C/C0) graph shows the difference in the percentage of dye degradation over time. The comparison of ZnO and TiO2 showed maximum dye degradation of around 60% and 80% using TiO2 and ZnO, respectively.
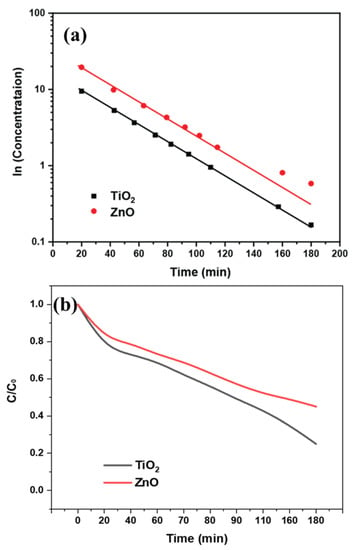
Figure 9.
(a) Kinetics study; (b) Photocatalytic dye degradation rate for TiO2 and ZnO nanofibers.
3. Materials and Methodology
For the preparation of Titania and zinc oxide nanofibers sol, absolute ethanol (C2H5OH, Merck, Darmstadt, Germany) and acetic acid (CH3CO2H, 99.7% Aldrich, St. Louis, MO, USA) were obtained from Sigma-Aldrich USA. For the binder, polyvinyl pyrrolidone (PVP, Mw~29,000 Aldrich), along with the main reagents titanium tetra isopropoxide (TTIP) and zinc acetate di-hydrate (Zn (CH3COO)2 H2O) with 97% purity for both (Aldrich, Saint Louis, MO, USA), was used as a precursor in the electrospinning process. Fluorine-doped tin oxide (FTO) glass was used as a collection source of TiO2 and ZnO nanofibers. Electrospinning was used because of its wide usage, ease of control, and fast method in the preparation of nanofibers. Solution parameters (viscosity, concentration, and surface tension), processing parameters (applied voltage, spinning distance, and nozzle radius) and environmental parameters (temperature, humidity, and atmosphere pressure) are the factors optimized to achieve nanofibers [31].
3.1. ZnO and TiO2 Nanofiber Synthesis
For ZnO nanofibers sol, a solution containing PVP (medium density of cells 1,300,000 Da) and zinc acetate di-hydrate (Zn (CH3COO)2·H2O) was used for preparation of sol. An amount of 5 g of zinc acetate was added into 40 mL of ethanol and allowed to stir for an hour. Acetic acid in the amount of 3 mL and 2.5 g of PVP were added during stirring, and again the solution was allowed to stir for 5 h at 60 °C. This was allowed to age for two days for proper gelation. For TiO2 nanofibers sol, 12 mL of TTIP was slowly added into 48 mL of ethanol. It was kept on stirring at 400 rpm for 2 h, and 2.5 g of PVP was intermediately added and allowed to stir for 5 h at 60 °C. This was allowed to age for 2 days to achieve proper gelation. Firstly, TiO2 nanofibers were fabricated and then, ZnO nanofibers were separately fabricated. The voltages, temperatures and other parameters were maintained the same.
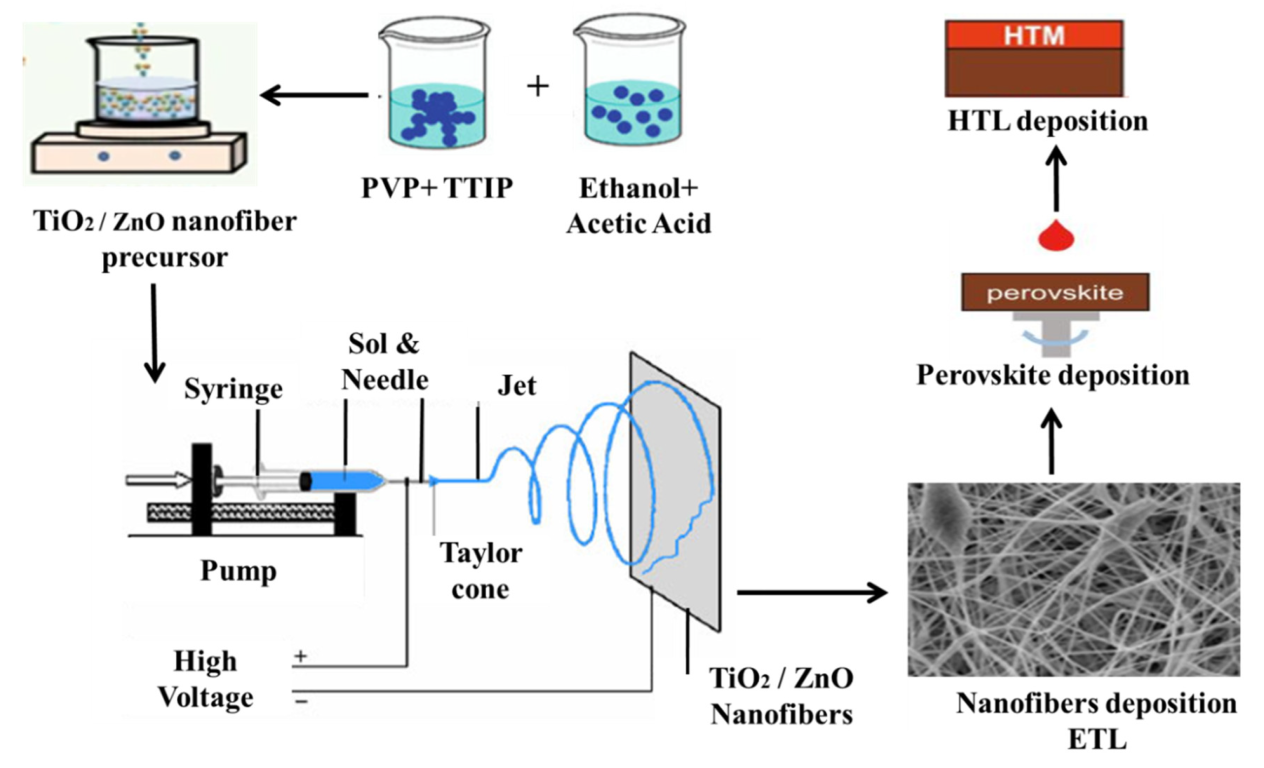
For the synthesis of TiO2 and ZnO nanofibers, the same process was carried out, except for using TiO2 and ZnO precursors. The electrospinning setup consists of the DC power supply, diffusion pumps, collector and intake stage, and a syringe with a needle tip diameter of 0.45 mm. Figure 10 is the complete setup image of the electrospinning for the synthesis of nanofibers. These mixtures were spun with a needle (21 gauge) inserting an electric field, as high as 20 kV and a pumping rate of 4000 rpm. At the time of discharge, we used a negative and direct voltage of 4 and 18 kV, respectively; the distance between the needle and the substrates was 16–20 cm, with the flow of the solutions set to 5 mL/min. Annealing of all the samples to remove the binder and remaining solvents was carried out at 450 °C, for 1 h, under ambient air conditions.
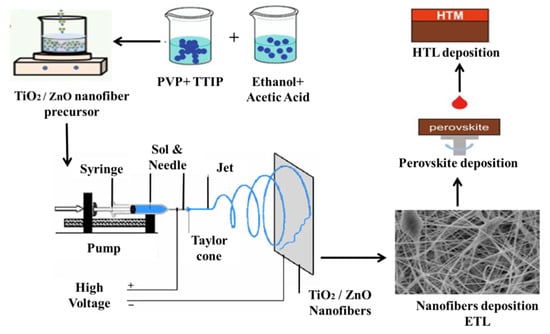
Figure 10.
Schematic illustration of electrospinning process for the ZnO/TiO2 nanofibers fabrication.
3.2. Solar Cells Fabrication
For solar cell fabrications, the fabricated ETL nanofibers layer was processed towards perovskite deposition consisting of methyl ammonium bromide (MABr, 0.2 mM). For this formamidinium iodide (FAI, 1 mM), methyl ammonium bromide (MABr, 0.2 mM), 20 μL of precursor solution containing PbBr2 (0.2 mM), and PbI2 (1.1 mM) were dissolved in dimethyl formamide (DMF, 0.8 mL) and after that, in dimethyl sulfoxide (DMSO, 0.2 mL). To deposit this, a spin coating technique was adopted, and this solution was coated onto the ETL of ZnO and TiO2 nanofibers at 4500 rpm for 7 s. Then, 90 μL of anhydrous cholorobenzene was dropped for crystallization of the uniformly deposited film. It was placed on a hot plate at 100 °C for 15 min for drying. Then, HTL was coated with solution cholorobenzene (1.094 mL) and spiro-OMeTAD (100 mg). This layer was again spin-coated on the absorber layer at 2000 rpm for 20 s. The whole fabrication process was carried out inside N2-filled glove box. Finally, gold back electrodes (80 nm) were deposited by using thermal evaporator under a vacuum of 10−7 torr.
3.3. Photocatalysis of Methyl Orange Dye
For photocatalysis measurements, fabric coated with ZnO and TiO2 nanofibers was immersed in methyl orange (MO) dye in an aqueous solution (10 ppm) to determine their dye degradation ability. Photocatalysis was performed under sunlight, with irradiance determined using a lux meter. The maximum absorption intensity of MO was reported at 464 nm in the absorption spectra recorded using a UV–Visible spectrometer. The catalyst–dye adsorption–desorption equilibrium was studied after adding 2 × 2 cm of the active-material-loaded fabric sample in 150 mL of dye solution and stirring the mixture continuously for 30 min under dark conditions to exclude the adsorption effect.
3.4. Characterizations
To determine the structural properties and to check the effect of the TiO2 and ZnO structures, XRD was carried out using D8 Advance (Bruker Advanced, Karlsruhe, Germany) furnished with Bragg–Brentano configuration and a scintillation detector with a radiation wavelength of 1.5418 Å. The samples were scanned ranging from 2θ = 10° to 80° and with a step size of 0.05°/5 s. The DIFFRAC Plus EVA Version 5.0 software was used to examine the XRD analysis. Phase composition analysis of nano fibers was confirmed using Raman spectra RENISHAW Invia 2000 Raman spectroscopy, equipped with an excitation laser of 514 nm. The morphology of the nanofiber samples was analyzed via scanning electron microscopy (SEM) JEOL JSM6490A (Jeol Ltd., Tokyo, Japan). Before the sample analysis, gold coating was carried out on all the samples for 1 min, to obtain clear images without charge accumulation. UV vis spectroscopy was performed in the spectrum range of 300–800 nm, by using Model UH4150AD UV-Vis-NIR. Fourier transform infra-red spectrophotometery (FTIR) was carried out using Cary 630 (Agilent Technologies, Santa Clara, CA, USA), in attenuated total reflection mode. The absorption of infrared spectra ranges from 4000 to 650 cm−1 with a resolution of 2 cm−1 was scanned. To conduct the electrical measurements, conductivity, resistivity, sheet resistance, and sheet carrier mobility were determined using a swin system of 5300 G magnetic field at a temperature of 300 K [10]. Current density-Voltage (J-V) and solar cells’ characteristics were measured using the power of AM 1.5 simulated light (100 mWcm−2), using a solar light simulator model (Newport 94043A). Photoelectrochemical characteristics and the electrochemical impedance spectroscopy (EIS) measurements of PSCs were recorded with a potentiostat/galvanostat (PGSTAT 30, Autolab, EcoChemie, Utrecht, the Netherlands) under 100 mW cm−2. The frequency range was explored from 10 mHz to 65 kHz. Qualitative antibacterial testing was measured by Escherichia coli (E.coli) and Staphylococcus aureus (S. aureus) with test standard AATCC 147. A single colony of S. aureus bacteria were carried out into broth. It was initially sterilized at 121 °C for 15 min and further incubated at 37 °C for 24 h. For the culture growth, it was placed under an orbital shaker (150 rpm). It was placed into a petri dish for bacterial inoculum after serial dilution. The samples were placed under UV light for half an hour and then into the bacterial lawn for 24 h at 37 °C. Photocatalysis performance was calculated after excluding the adsorption effect. For the photocatalytic dye degradation, the stable aqueous dye solution was subjected to visible light irradiation for various time intervals, along with recording the UV absorption spectra. The process was continued for a total of 180 min while keeping the ZnO-coated fabric immersed in the dye solution. To determine MO degradation due to light, (Equation (1)) was used:
wherein C0 is the concentration of the dye solution at the beginning of the photocatalytic reaction, and is the concentration of the dye solution at a given time.
D% = 1 − CtC0 × 100
4. Conclusions
In conclusion, TiO2 and ZnO nanofibers were successfully synthesized with an electrospinning technique and their annealing at 450 °C. XRD spectra revealed that both TiO2 and ZnO polycrystalline nanofibers are achieved after the removal of PVP in the annealing process. Raman spectra also have good agreement with XRD results. Scanning electron microscopic images have clearly shown long, fibrous and continuous TiO2 and ZnO nanofibers. A preliminary characterization for photoactivity revealed that both ZnO and TiO2 nanofibers show promising absorption spectra, with a suitable band gap of 3.2 and 3.3 eV, respectively. Hall Effect measurements show TiO2 nanofibers have a higher conductivity of 1.38 × 10−04 Ohm.cm−1, as compared to ZnO nanofibers which offered 1.08 × 10−04 Ohm.cm−1. Based on the better conductivity of TiO2, the better conversion efficiency was achieved for TiO2 nanofibers, i.e., 10.33%, as compared to ZnO, which was 8.48%. As a result, if both ZnO and TiO2 are synthesized at the same processing conditions, the TiO2 will have better photoactivity for energy harvesting, antibacterial effect and photocatalytic degradation.
Author Contributions
Conceptualization, Z.A. and M.A. (Mumtaz Ali); methodology, Z.A., R.S. and K.S.; software, R.S., K.S.; validation, M.A. (Mubark Alshareef), R.S. and K.S.; formal analysis, Z.A, M.A. (Mumtaz Ali) and E.-J.L.; investigation, M.A. (Mumtaz Ali), E.-J.L. and K.S. resources, K.H.L. and M.M.A.; writing—original Z.A. draft preparation, Z.A., M.A. (Mubark Alshareef); writing—review and editing, M.A. (Mumtaz Ali); supervision, M.A. (Mumtaz Ali); project administration, K.H.L.; funding acquisition, K.H.L., M.A. (Mubark Alshareef). All authors have read and agreed to the published version of the manuscript.
Funding
This project was funded by Deanship of Scientific Research at Umm Al-Qura University by Grant Code: (23UQU4320745DSR004). This project was also funded by Korea Environment Industry and Technology Institute (KEITI) through Environmental R&D Project on the Disaster Prevention of Environmental Facilities Project, funded by Korea Ministry of Environment (MOE) (2020002870004).
Data Availability Statement
All data are original and optimized.
Acknowledgments
The authors would like to thank all lab fellows and Higher Education Pakistan under the umbrella of project GCF-63.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Riaz, R.; Ali, M.; Sahito, I.A.; Ayoub Arbab, A.; Maiyalagan, T.; Anjum, A.S.; Ko, M.J.; Jeong, S.H. Self-assembled nitrogen-doped graphene quantum dots (N-GQDs) over graphene sheets for superb electro-photocatalytic activity. Appl. Surf. Sci. 2019, 480, 1035–1046. [Google Scholar] [CrossRef]
- Riaz, R.; Ali, M.; Answer, H.; Ko, M.J.; Jeong, S.H. Highly porous self-assembly of nitrogen-doped graphene quantum dots over reduced graphene sheets for photo-electrocatalytic electrode. J. Colloid Interface Sci. 2019, 557, 174–184. [Google Scholar] [CrossRef]
- Patil, J.V.; Mali, S.S.; Patil, A.P.; Patil, P.S.; Hong, C.K. Highly efficient mixed-halide mixed-cation perovskite solar cells based on rGO-TiO2 composite nanofibers. Energy 2019, 189, 116396. [Google Scholar] [CrossRef]
- Anwer, H.; Ali, M.; Lee, S.; Jeong, S.H.; Park, J.-W. Simulating alveoli-inspired air pockets in a ZnO/NiMoO4/C3N4 catalyst filter for toluene entrapment and photodecomposition. J. Hazard. Mater. 2021, 409, 124497. [Google Scholar] [CrossRef] [PubMed]
- Mali, S.S.; Shim, C.S.; Kim, H.; Patil, P.S.; Hong, C.K. In situ processed gold nanoparticle-embedded TiO2 nanofibers enabling plasmonic perovskite solar cells to exceed 14% conversion efficiency. Nanoscale 2016, 8, 2664–2677. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Wang, C.; Hou, J.; Zhang, C.; He, H.; Wang, N.; Wu, H.; Cao, G. Enhanced electron collection in perovskite solar cells employing thermoelectric NaCo2O4/TiO2 coaxial nanofibers. Small 2016, 12, 5146–5152. [Google Scholar] [CrossRef]
- Arshad, Z.; Wageh, S.; Maiyalagan, T.; Ali, M.; Arshad, U.; Ain, N.-U.; Qadir, M.B.; Mateen, F.; Al-Sehemi, A.G. Enhanced charge transport characteristics in zinc oxide nanofibers via Mg2+ doping for electron transport layer in perovskite solar cells and antibacterial textiles. Ceram. Int. 2022, 48, 24363–24371. [Google Scholar] [CrossRef]
- Valadi, K.; Gharibi, S.; Taheri-Ledari, R.; Akin, S.; Maleki, A.; Shalan, A.E. Metal oxide electron transport materials for perovskite solar cells:a review. Environ. Chem. Lett. 2021, 19, 2185–2207. [Google Scholar] [CrossRef]
- Son, D.-Y.; Im, J.-H.; Kim, H.-S.; Park, N.-G. 11% efficient perovskite solar cell based on ZnO nanorods: An effective charge collection system. J. Phys. Chem. C 2014, 118, 16567–16573. [Google Scholar] [CrossRef]
- Poudel, M.B.; Awasthi, G.P.; Kim, H.J. Novel insight into the adsorption of Cr (VI) and Pb (II) ions by MOF derived Co-Al layered double hydroxide@ hematite nanorods on 3D porous carbon nanofiber network. Chem. Eng. J. 2021, 417, 129312. [Google Scholar] [CrossRef]
- Poudel, M.B.; Kim, A.A. Silver nanoparticles decorated TiO2 nanoflakes for antibacterial properties. Inorg. Chem. Commun. 2023, 152, 110675. [Google Scholar] [CrossRef]
- Wang, W.; Niu, Q.; Zeng, G.; Zhang, C.; Huang, D.; Shao, B.; Zhou, C.; Yang, Y.; Liu, Y.; Guo, H.; et al. 1D porous tubular g-C3N4 capture black phosphorus quantum dots as 1D/0D metal-free photocatalysts for oxytetracycline hydrochloride degradation and hexavalent chromium reduction. Appl. Catal. B Environ. 2020, 273, 119051. [Google Scholar] [CrossRef]
- Guo, H.; Niu, H.-Y.; Wang, W.-J.; Wu, Y.; Xiong, T.; Chen, Y.-R.; Su, C.-Q.; Niu, C.-G. Schottky barrier height mediated Ti3C2 MXene based heterostructure for rapid photocatalytic water disinfection: Antibacterial efficiency and reaction mechanism. Sep. Purif. Technol. 2023, 312, 123412. [Google Scholar] [CrossRef]
- Chandrasekar, R.; Zhang, L.; Howe, J.Y.; Hedin, N.E.; Zhang, Y.; Fong, H. Fabrication and characterization of electrospun titania nanofibers. J. Mater. Sci. 2009, 44, 1198–1205. [Google Scholar] [CrossRef]
- Amna, T.; Hassan, M.S.; Barakat, N.A.M.; Pandeya, D.R.; Hong, S.T.; Khil, M.-S.; Kim, H.Y. Antibacterial activity and interaction mechanism of electrospun zinc-doped titania nanofibers. Appl. Microbiol. Biotechnol. 2012, 93, 743–751. [Google Scholar] [CrossRef]
- Amos, O.; Mehl, G.H.; Pashameah, R.A. The plasmonic behaviours of gold nanoparticles with different thiol (n = 6, 10, 12) capping agents. J. Umm Al-Qura Univ. Appl. Sci. 2023, 1–6. [Google Scholar] [CrossRef]
- Zhen, C.; Wu, T.; Chen, R.; Wang, L.; Liu, G.; Cheng, H.-M. Strategies for modifying TiO2 based electron transport layers to boost perovskite solar cells. ACS Sustain. Chem. Eng. 2019, 7, 4586–4618. [Google Scholar] [CrossRef]
- Qiu, L.; Zhuang, Z.; Yang, S.; Chen, W.; Song, L.; Ding, M.; Xian, G.; Du, P.; Xiong, J. Fabrication of high efficiency perovskite solar cells based on mesoporous TiO2 nanofibrous film under high humidity conditions. Mater. Res. Bull. 2018, 106, 439–445. [Google Scholar] [CrossRef]
- Aryal, S.; Kim, C.K.; Kim, K.-W.; Khil, M.S.; Kim, H.Y. Multi-walled carbon nanotubes/TiO2 composite nanofiber by electrospinning. Mater. Sci. Eng. C 2008, 28, 75–79. [Google Scholar] [CrossRef]
- Park, J.Y.; Kim, S.S. Growth of nanograins in electrospun ZnO nanofibers. J. Am. Ceram. Soc. 2009, 92, 1691–1694. [Google Scholar] [CrossRef]
- Imran, M.; Haider, S.; Ahmad, K.; Mahmood, A.; Al-masry, W.A. Fabrication and characterization of zinc oxide nanofibers for renewable energy applications. Arab. J. Chem. 2017, 10, S1067–S1072. [Google Scholar] [CrossRef]
- Pantò, F.; Dahrouch, Z.; Saha, A.; Patanè, S.; Santangelo, S.; Triolo, C. Photocatalytic degradation of methylene blue dye by porous zinc oxide nanofibers prepared via electrospinning: When defects become merits. Appl. Surf. Sci. 2021, 557, 149830. [Google Scholar] [CrossRef]
- Pant, B.; Ojha, G.P.; Kim, H.-Y.; Park, M.; Park, S.-J. Fly-ash-incorporated electrospun zinc oxide nanofibers: Potential material for environmental remediation. Environ. Pollut. 2019, 245, 163–172. [Google Scholar] [CrossRef] [PubMed]
- Ruankham, P.; Wongratanaphisan, D.; Gardchareon, A.; Phadungdhitidhada, S.; Choopun, S.; Sagawa, T. Full coverage of perovskite layer onto ZnO nanorods via a modified sequential two-step deposition method for efficiency enhancement in perovskite solar cells. Appl. Surf. Sci. 2017, 410, 393–400. [Google Scholar] [CrossRef]
- Bian, J.; Su, R.; Yao, Y.; Wang, J.; Zhou, J.; Li, F.; Wang, Z.L.; Sun, C. Mg doped perovskite LaNiO3 nanofibers as an efficient bifunctional catalyst for rechargeable zinc–air batteries. ACS Appl. Energy Mater. 2019, 2, 923–931. [Google Scholar] [CrossRef]
- Cao, F.; Tian, W.; Gu, B.; Ma, Y.; Lu, H.; Li, L. High-performance UV–vis photodetectors based on electrospun ZnO nanofiber-solution processed perovskite hybrid structures. Nano Res. 2017, 10, 2244–2256. [Google Scholar] [CrossRef]
- Mahmood, K.; Swain, B.S.; Amassian, A. Highly Efficient Hybrid Photovoltaics Based on Hyperbranched Three-Dimensional TiO2 Electron Transporting Materials. Adv. Mater. 2015, 27, 2859–2865. [Google Scholar] [CrossRef]
- Alsulami, Q.A.; Arshad, Z.; Ali, M.; Wageh, S. Efficient Tuning of the Opto-Electronic Properties of Sol–Gel-Synthesized Al-Doped Titania Nanoparticles for Perovskite Solar Cells and Functional Textiles. Gels 2023, 78, 173–371. [Google Scholar] [CrossRef]
- Riaz, R.; Ali, M.; Maiyalagan, T.; Arbab, A.A.; Anjum, A.S.; Lee, S.; Ko, M.J.; Jeong, S.H. Activated charcoal and reduced graphene sheets composite structure for highly electro-catalytically active counter electrode material and water treatment. Int. J. Hydrog. Energy 2020, 45, 7751–7763. [Google Scholar] [CrossRef]
- Alqahtani, A.M.; Abumelha, H.M.; Alnoman, R.B.; Abualnaja, M.M.; Alsharief, H.H.; Hameed, A.; Almontshery, A.M.; El-Metwaly, N.M. Copper (I)-catalysed synthesis of symmetrical perfluoroterphenyl analogues; fluorescence, antioxidant and molecular docking studies. Lumin. J. Biol. Chem. Lumin. 2023. [Google Scholar] [CrossRef]
- Siddheswaran, R.; Sankar, R.; Ramesh Babu, M.; Rathnakumari, M.; Jayavel, R.; Murugakoothan, P.; Sureshkumar, P. Preparation and characterization of ZnO nanofibers by electrospinning. Cryst. Res. Technol. J. Exp. Ind. Crystallogr. 2006, 41, 446–449. [Google Scholar] [CrossRef]
- Xu, A.-W.; Gao, Y.; Liu, H.-Q. The preparation, characterization, and their photocatalytic activities of rare-earth-doped TiO2 nanoparticles. J. Catal. 2002, 207, 151–157. [Google Scholar] [CrossRef]
- Liao, D.L.; Liao, B.Q. Shape, size and photocatalytic activity control of TiO2 nanoparticles with surfactants. J. Photochem. Photobiol. A Chem. 2007, 187, 363–369. [Google Scholar] [CrossRef]
- Liu, L.; Li, S.; Zhuang, J.; Wang, L.; Zhang, J.; Li, H.; Liu, Z.; Han, Y.; Jiang, X.; Zhang, P. Improved selective acetone sensing properties of Co-doped ZnO nanofibers by electrospinning. Sens. Actuators B Chem. 2011, 155, 782–788. [Google Scholar] [CrossRef]
- Tai, M.; Zhao, X.; Shen, H.; Guo, Y.; Zhang, M.; Zhou, Y.; Li, X.; Yao, Z.; Yin, X.; Han, J.; et al. Ultrathin Zn2SnO4 (ZTO) passivated ZnO nanocone arrays for efficient and stable perovskite solar cells. Chem. Eng. J. 2019, 361, 60–66. [Google Scholar] [CrossRef]
- Choi, H.C.; Jung, Y.M.; Kim, S.B. Size effects in the Raman spectra of TiO2 nanoparticles. Vib. Spectrosc. 2005, 37, 33–38. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, H.; An, X.; Gao, C.; Zhang, Z.; Zhou, J.; Zhou, M.; Xie, E. Effect of Al doping on the visible photoluminescence of ZnO nanofibers. J. Alloys Compd. 2010, 506, 772–776. [Google Scholar] [CrossRef]
- Zhang, X.; Thavasi, V.; Mhaisalkar, S.G.; Ramakrishna, S. Novel hollow mesoporous 1D TiO2 nanofibers as photovoltaic and photocatalytic materials. Nanoscale 2012, 4, 1707–1716. [Google Scholar] [CrossRef]
- Wang, W.; Zhang, Z.; Cai, Y.; Chan, J.; Wang, J.; Huang, R.; Lu, X.; Gao, X.; Shui, L.; Wu, S.; et al. Enhanced performance of CH3NH3PbI3−x Cl x perovskite solar cells by CH3NH3I modification of TiO2-perovskite layer interface. Nanoscale Res. Lett. 2016, 11, 316. [Google Scholar] [CrossRef]
- Mahmood, K.; Khalid, A.; Mehran, M.T. Nanostructured ZnO electron transporting materials for hysteresis-free perovskite solar cells. Sol. Energy 2018, 173, 496–503. [Google Scholar] [CrossRef]
- Onozuka, K.; Ding, B.; Tsuge, Y.; Naka, T.; Yamazaki, M.; Sugi, S.; Ohno, S.; Yoshikawa, M.; Shiratori, S. Electrospinning processed nanofibrous TiO2 membranes for photovoltaic applications. Nanotechnology 2006, 17, 1026. [Google Scholar] [CrossRef]
- Tanveer, M.; Habib, A.; Khan, M.B. Improved efficiency of organic/inorganic photovoltaic devices by electrospun ZnO nanofibers. Mater. Sci. Eng. B 2012, 177, 1144–1148. [Google Scholar] [CrossRef]
- Anjum, A.S.; Ali, M.; Sun, K.C.; Riaz, R.; Jeong, S.H. Self-assembled nanomanipulation of silica nanoparticles enable mechanochemically robust super hydrophobic and oleophilic textile. J. Colloid Interface Sci. 2020, 563, 62–73. [Google Scholar] [CrossRef]
- Anjum, A.S.; Sun, K.C.; Ali, M.; Riaz, R.; Jeong, S.H. Fabrication of coral-reef structured nano silica for self-cleaning and super-hydrophobic textile applications. Chem. Eng. J. 2020, 401, 125859. [Google Scholar] [CrossRef]
- Yakuphanoglu, F. Electrical and photovoltaic properties of cobalt doped zinc oxide nanofiber/n-silicon diode. J. Alloys Compd. 2010, 494, 451–455. [Google Scholar] [CrossRef]
- Son, D.-Y.; Bae, H.-H.; Kim, H.-S.; Park, N.-G. Effects of seed layer on growth of ZnO nanorod and performance of perovskite solar cell. J. Phys. Chem. C 2015, 119, 10321–10328. [Google Scholar] [CrossRef]
- Laila, I.K.; Mufti, N.; Maryam, S.; Fuad, A.; Taufiq, A. Sunaryono Synthesis and characterization of ZnO nanorods by hydrothermal methods and its application on perovskite solar cells. In Proceedings of the The 2017 International Conference on Mathematics, Science, and Education, Malang, Indonesia, 29–30 August 2017. [Google Scholar]
- Ali, M.; Riaz, R.; Anjum, A.S.; Sun, K.C.; Li, H.; Jeong, S.H.; Ko, M.J. Graphene quantum dots induced porous orientation of holey graphene nanosheets for improved electrocatalytic activity. Carbon 2021, 171, 493–506. [Google Scholar] [CrossRef]
- Ali, M.; Riaz, R.; Anjum, A.S.; Sun, K.C.; Li, H.; Ahn, S.; Jeong, S.H.; Ko, M.J. Microwave-assisted ultrafast in-situ growth of N-doped carbon quantum dots on multiwalled carbon nanotubes as an efficient electrocatalyst for photovoltaics. J. Colloid Interface Sci. 2021, 586, 349–361. [Google Scholar] [CrossRef] [PubMed]
- Dharani, S.; Mulmudi, H.K.; Yantara, N.; Thu Trang, P.T.; Park, N.G.; Graetzel, M.; Mhaisalkar, S.; Mathews; Boix, P.P. High efficiency electrospun TiO2 nanofiber based hybrid organic–inorganic perovskite solar cell. Nanoscale 2014, 6, 1675–1679. [Google Scholar] [CrossRef]
- Chen, D.; Zhu, Y. Electrospun perovskite nanofibers. Nanoscale Res. Lett. 2017, 12, 114. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Kwon, H.-C.; Ma, S.; Kim, K.; Yun, S.-C.; Jang, G.; Park, J.; Lee, H.; Goh, S.; Moon, J. Energy level-graded Al-doped ZnO protection layers for copper nanowire-based window electrodes for efficient flexible perovskite solar cells. ACS Appl. Mater. Interfaces 2020, 12, 13824–13835. [Google Scholar] [CrossRef]
- Ali, M.; Anjum, A.S.; Bibi, A.; Wageh, S.; Sun, K.C.; Jeong, S.H. Gradient heating-induced bi-phase synthesis of carbon quantum dots (CQDs) on graphene-coated carbon cloth for efficient photoelectrocatalysis. Carbon 2022, 196, 649–662. [Google Scholar] [CrossRef]
- Poudel, M.B.; Kim, A.A.; Lohani, P.C.; Yoo, D.J.; Kim, H.J. Assembling zinc cobalt hydroxide/ternary sulfides heterostructure and iron oxide nanorods on three-dimensional hollow porous carbon nanofiber as high energy density hybrid supercapacitor. J. Energy Storage 2023, 60, 106713. [Google Scholar] [CrossRef]
- Baylan, E.; Yildirim, O.A. Highly efficient photocatalytic activity of stable manganese-doped zinc oxide (Mn: ZnO) nanofibers via electrospinning method. Mater. Sci. Semicond. Process. 2019, 103, 104621. [Google Scholar] [CrossRef]
- Yang, P.; Bai, W.; Zou, Y.; Zhang, X.; Yang, Y.; Duan, G.; Wu, J.; Xu, Y.; Li, Y. A melanin-inspired robust aerogel for multifunctional water remediation. Mater. Horiz. 2023, 10, 1020–1029. [Google Scholar] [CrossRef]
- Wang, Y.; Hao, X.; Wang, Z.; Dong, M.; Ciu, L. Facile fabrication of Mn2+-doped ZnO photocatalysts by electrospinning. R. Soc. Open Sci. 2020, 7, 191050. [Google Scholar] [CrossRef]
- González-González, R.B.; Parra-Saldívar, R.; Alsanie, W.F.; Iqbal, H.M.N. Nanohybrid catalysts with porous structures for environmental remediation through photocatalytic degradation of emerging pollutants. Environ. Res. 2022, 214, 113955. [Google Scholar] [CrossRef]
- Xu, Y.; Hu, J.; Zhang, X.; Yuan, D.; Duan, G.; Li, Y. Robust and multifunctional natural polyphenolic composites for water remediation. Mater. Horiz. 2022, 9, 2496–2517. [Google Scholar] [CrossRef] [PubMed]
- González-González, R.B.; Flores-Contreras, E.A.; Parra-Saldívar, R.; Iqbal, H.M.N. Bio-removal of emerging pollutants by advanced bioremediation techniques. Environ. Res. 2022, 214, 113936. [Google Scholar] [CrossRef]
- Zhang, P.; Wu, J.; Wang, Y.; Sarvari, H.; Liu, D.; Chen, Z.D.; Li, S. Enhanced efficiency and environmental stability of planar perovskite solar cells by suppressing photocatalytic decomposition. J. Mater. Chem. A 2017, 5, 17368–17378. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).