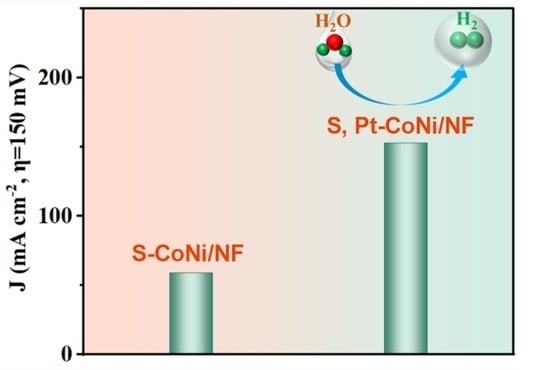
Dual-Modification Engineering of CoNi Alloy Realizing Robust Performance for Electrocatalytic Hydrogen Production
Abstract
:1. Introduction
2. Results and Discussion
2.1. The Characterizations of Structural and Morphology for the Catalysts
2.2. The Characterizations of Composition and Valence States for the Catalysts
2.3. The HER Performance of the Catalysts
2.4. The Overall Water Splitting Performance of the Catalysts
3. Experimental Section
3.1. Materials
3.2. Synthesis of FeOOH/NF
3.3. Synthesis of Pt/C/NF
3.4. Material Characterizations
3.5. Electrochemical Measurements
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yue, M.; Lambert, H.; Pahon, E.; Roche, R.; Jemei, S.; Hissel, D. Hydrogen energy systems: A critical review of technologies, applications, trends and challenges. Renew. Sustain. Energy Rev. 2021, 146, 111180. [Google Scholar] [CrossRef]
- Feng, D.; Ye, R.; Tong, Y.; Ren, X.; Chen, P. Engineering cobalt molybdate nanosheet arrays with phosphorus-modified nickel as heterogeneous electrodes for highly-active energy-saving water splitting. J. Colloid Interface Sci. 2023, 636, 425–434. [Google Scholar] [CrossRef] [PubMed]
- Sazali, N. Emerging technologies by hydrogen: A review. Int. J. Hydrogen Energy 2020, 45, 18753–18771. [Google Scholar] [CrossRef]
- Lubitz, W.; Tumas, W. Hydrogen: An Overview. Chem. Rev. 2007, 107, 3900–3903. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Liu, X.; Zang, Y.; Wu, Y.; Zhang, Q.; Wang, Z.; Liu, Y.; Zheng, Z.; Cheng, H.; Huang, B.; et al. Constructing a bifunctional MoO2/Co heterojunction for efficient electrocatalytic hydrogen evolution and hydrazine oxidation. J. Mater. Chem. A 2022, 10, 17297–17306. [Google Scholar] [CrossRef]
- Tong, S.; Fu, B.; Gan, L.; Zhang, Z. Single atom catalysts for boosting electrocatalytic and photoelectrocatalytic performances. J. Mater. Chem. A 2021, 9, 10731–10738. [Google Scholar] [CrossRef]
- Kang, Z.; Khan, M.A.; Gong, Y.; Javed, R.; Xu, Y.; Ye, D.; Zhao, H.; Zhang, J. Recent progress of MXenes and MXene-based nanomaterials for the electrocatalytic hydrogen evolution reaction. J. Mater. Chem. A 2021, 9, 6089–6108. [Google Scholar] [CrossRef]
- Feng, D.; Liu, X.-Y.; Ye, R.; Huang, W.; Tong, Y. Carbon-encapsulated Co2P/P-modified NiMoO4 hierarchical heterojunction as superior pH-universal electrocatalyst for hydrogen production. J. Colloid Interface Sci. 2023, 634, 693–702. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Cen, X.; He, J.; Tong, Y. Coupled W–Co2P hybrid nanosheets as a robust bifunctional electrocatalyst for hydrazine-assisted hydrogen production. Chem. Commun. 2023, 59, 5575–5578. [Google Scholar] [CrossRef]
- Hansen, J.N.; Prats, H.; Toudahl, K.K.; Mørch Secher, N.; Chan, K.; Kibsgaard, J.; Chorkendorff, I. Is There Anything Better than Pt for HER? ACS Energy Lett. 2021, 6, 1175–1180. [Google Scholar] [CrossRef]
- Koo, B.; Chu, J.; Seo, J.; Jung, G.; Baek, S.H.; Nam, S.-W.; Duah, C.; Lee, Y.K.; Jung, W.; Shin, B. Drop-casted Platinum Nanocube Catalysts for Hydrogen Evolution Reaction with Ultrahigh Mass Activity. ChemSusChem 2021, 14, 2585–2590. [Google Scholar] [CrossRef]
- Cheng, N.; Stambula, S.; Wang, D.; Banis, M.N.; Liu, J.; Riese, A.; Xiao, B.; Li, R.; Sham, T.-K.; Liu, L.-M.; et al. Platinum single-atom and cluster catalysis of the hydrogen evolution reaction. Nat. Commun. 2016, 7, 13638. [Google Scholar] [CrossRef] [Green Version]
- Ma, Z.; Tian, H.; Meng, G.; Peng, L.; Chen, Y.; Chen, C.; Chang, Z.; Cui, X.; Wang, L.; Jiang, W.; et al. Size effects of platinum particles@CNT on HER and ORR performance. Sci. China Mater. 2020, 63, 2517–2529. [Google Scholar] [CrossRef]
- Lee, D.; Kim, Y.; Kim, H.W.; Choi, M.; Park, N.; Chang, H.; Kwon, Y.; Park, J.H.; Kim, H.J. In situ electrochemically synthesized Pt-MoO3−x nanostructure catalysts for efficient hydrogen evolution reaction. J. Catal. 2020, 381, 1–13. [Google Scholar] [CrossRef]
- Zhou, S.; Yang, Y.; Yin, P.; Ren, Z.; Wang, L.; Wei, M. Metal–Support Synergistic Catalysis in Pt/MoO3–x Nanorods toward Ammonia Borane Hydrolysis with Efficient Hydrogen Generation. ACS Appl. Mater. Interfaces 2022, 14, 5275–5286. [Google Scholar] [CrossRef]
- Lin, G.; Wang, Y.; Hong, J.; Suenaga, K.; Liu, L.; Chang, L.-Y.; Pao, C.-W.; Zhang, T.; Zhao, W.; Huang, F.; et al. Nanoheterostructures of Partially Oxidized RuNi Alloy as Bifunctional Electrocatalysts for Overall Water Splitting. ChemSusChem 2020, 13, 2739–2744. [Google Scholar] [CrossRef] [PubMed]
- Ruqia, B.; Choi, S.-I. Pt and Pt–Ni(OH)2 Electrodes for the Hydrogen Evolution Reaction in Alkaline Electrolytes and Their Nanoscaled Electrocatalysts. ChemSusChem 2018, 11, 2643–2653. [Google Scholar] [CrossRef] [PubMed]
- Bao, J.; Wang, J.; Zhou, Y.; Hu, Y.; Zhang, Z.; Li, T.; Xue, Y.; Guo, C.; Zhang, Y. Anchoring ultrafine PtNi nanoparticles on N-doped graphene for highly efficient hydrogen evolution reaction. Catal. Sci. Technol. 2019, 9, 4961–4969. [Google Scholar] [CrossRef]
- Liu, C.; Pan, G.; Liang, N.; Hong, S.; Ma, J.; Liu, Y. Ir Single Atom Catalyst Loaded on Amorphous Carbon Materials with High HER Activity. Adv. Sci. 2022, 9, 2105392. [Google Scholar] [CrossRef]
- Zhu, J.; Cai, L.; Tu, Y.; Zhang, L.; Zhang, W. Emerging ruthenium single-atom catalysts for the electrocatalytic hydrogen evolution reaction. J. Mater. Chem. A 2022, 10, 15370–15389. [Google Scholar] [CrossRef]
- Yu, H.; Wang, W.; Mao, Q.; Deng, K.; Wang, Z.; Xu, Y.; Li, X.; Wang, H.; Wang, L. Pt single atom captured by oxygen vacancy-rich NiCo layered double hydroxides for coupling hydrogen evolution with selective oxidation of glycerol to formate. Appl. Catal. B Environ. 2023, 330, 122617. [Google Scholar] [CrossRef]
- Wu, Y.; Wei, W.; Yu, R.; Xia, L.; Hong, X.; Zhu, J.; Li, J.; Lv, L.; Chen, W.; Zhao, Y.; et al. Anchoring Sub-Nanometer Pt Clusters on Crumpled Paper-Like MXene Enables High Hydrogen Evolution Mass Activity. Adv. Funct. Mater. 2022, 32, 2110910. [Google Scholar] [CrossRef]
- Lin, L.; Pei, C.; Ding, R.; Li, Y.; Park, H.S.; Yu, X. Ru nanoclusters coupling with hierarchical phosphorus and oxygen dual-doped carbon nanotube architectures for effective hydrogen evolution reaction. Int. J. Hydrogen Energy 2023, 48, 20350–20358. [Google Scholar] [CrossRef]
- Lin, W.; Lu, Y.-R.; Peng, W.; Luo, M.; Chan, T.-S.; Tan, Y. Atomic bridging modulation of Ir–N, S co-doped MXene for accelerating hydrogen evolution. J. Mater. Chem. A 2022, 10, 9878–9885. [Google Scholar] [CrossRef]
- Chi, J.-Q.; Xie, J.-Y.; Zhang, W.-W.; Dong, B.; Qin, J.-F.; Zhang, X.-Y.; Lin, J.-H.; Chai, Y.-M.; Liu, C.-G. N-Doped Sandwich-Structured Mo2C@C@Pt Interface with Ultralow Pt Loading for pH-Universal Hydrogen Evolution Reaction. ACS Appl. Mater. Interfaces 2019, 11, 4047–4056. [Google Scholar] [CrossRef]
- Sun, Y.; Zang, Y.; Tian, W.; Yu, X.; Qi, J.; Chen, L.; Liu, X.; Qiu, H. Plasma-induced large-area N,Pt-doping and phase engineering of MoS2 nanosheets for alkaline hydrogen evolution. Energy Environ. Sci. 2022, 15, 1201–1210. [Google Scholar] [CrossRef]
- Xie, D.; Chen, Y.; Yu, D.; Han, S.; Song, J.; Xie, Y.; Hu, F.; Li, L.; Peng, S. Single-layer carbon-coated FeCo alloy nanoparticles embedded in single-walled carbon nanotubes for high oxygen electrocatalysis. Chem. Commun. 2020, 56, 6842–6845. [Google Scholar] [CrossRef] [PubMed]
- Fan, A.; Qin, C.; Zhang, X.; Yang, J.; Ge, J.; Wang, S.; Yuan, X.; Wang, S.; Dai, X. Engineering FeNi alloy nanoparticles via synergistic ultralow Pt doping and nanocarbon capsulation for efficient hydrogen evolution. J. Mater. Chem. A 2019, 7, 24347–24355. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, D.; Wang, S. High-Entropy Alloys for Electrocatalysis: Design, Characterization, and Applications. Small 2022, 18, 2104339. [Google Scholar] [CrossRef]
- Yang, C.; Gao, Y.; Ma, T.; Bai, M.; He, C.; Ren, X.; Luo, X.; Wu, C.; Li, S.; Cheng, C. Metal Alloys-Structured Electrocatalysts: Metal-Metal Interactions, Coordination Microenvironments, and Structural Property-Reactivity Relationships. Adv. Mater. 2023, 2301836. [Google Scholar] [CrossRef]
- Li, X.; Cai, W.; Li, D.-S.; Xu, J.; Tao, H.; Liu, B. Amorphous alloys for electrocatalysis: The significant role of the amorphous alloy structure. Nano Res. 2023, 16, 4277–4288. [Google Scholar] [CrossRef]
- Qi, B.; Chang, W.; Xu, Q.; Jiang, L.; An, S.; Chu, J.-F.; Song, Y.-F. Regulating Hollow Carbon Cage Supported NiCo Alloy Nanoparticles for Efficient Electrocatalytic Hydrogen Evolution Reaction. ACS Appl. Mater. Interfaces 2023, 15, 12078–12087. [Google Scholar] [CrossRef]
- Yamada, T.; Kojima, T.; Abe, E.; Kameoka, S.; Murakami, Y.; Gille, P.; Tsai, A.P. Probing Single Pt Atoms in Complex Intermetallic Al13Fe4. J. Am. Chem. Soc. 2018, 140, 3838–3841. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, N.; Choi, H.W.; Kumar, M.; Yoon, D.H. Nanoarchitectonics Pt/NiCo in a carbon matrix as highly efficient electrocatalyst for hydrogen evolution reaction. Electrochim. Acta 2023, 460, 142634. [Google Scholar] [CrossRef]
- Li, L.; Qiu, H.; Zhu, Y.; Chen, G.; She, S.; Guo, X.; Li, H.; Liu, T.; Lin, Z.; Zhou, H.; et al. Atomic ruthenium modification of nickel-cobalt alloy for enhanced alkaline hydrogen evolution. Appl. Catal. B Environ. 2023, 331, 122710. [Google Scholar] [CrossRef]
- Cai, C.; Liu, K.; Zhu, Y.; Li, P.; Wang, Q.; Liu, B.; Chen, S.; Li, H.; Zhu, L.; Li, H.; et al. Optimizing Hydrogen Binding on Ru Sites with RuCo Alloy Nanosheets for Efficient Alkaline Hydrogen Evolution. Angew. Chem. Int. Ed. 2022, 61, e202113664. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Song, J.; Li, Y.; Tian, Q.; Chen, J.; Yang, L. High lithium storage performance of CoO with a distinctive dual-carbon-confined nanoarchitecture. Nanoscale 2021, 13, 12938–12950. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Song, J.; Hong, X.; Tian, Q.; Sui, Z.; Yang, L. Boosting the lithium storage performance of tin dioxide by carbon nanotubes supporting and surface engineering. J. Colloid Interface Sci. 2021, 602, 789–798. [Google Scholar] [CrossRef]
- Huang, W.; Tong, Y.; Feng, D.; Guo, Z.; Ye, R.; Chen, P. Rational Design of Molybdenum-Doped Cobalt Nitride Nanowire Arrays for Robust Overall Water Splitting. ChemSusChem 2023, 16, e202202078. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Tong, Y.; Li, K.; Chen, P. Heterostructure engineering of iridium species on nickel/molybdenum nitride for highly-efficient anion exchange membrane water electrolyzer. J. Colloid Interface Sci. 2022, 628, 306–314. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Tong, Y.; Feng, D.; Chen, P. Electronic regulation of platinum species on metal nitrides realizes superior mass activity for hydrogen production. J. Colloid Interface Sci. 2022, 622, 410–418. [Google Scholar] [CrossRef]
- Cheng, X.; Tong, Y. Interface Coupling of Cobalt Hydroxide/Molybdenum Disulfide Heterostructured Nanosheet Arrays for Highly Efficient Hydrazine-Assisted Hydrogen Generation. ACS Sustain. Chem. Eng. 2023, 11, 3219–3227. [Google Scholar] [CrossRef]
- Feng, D.; Ren, X.; Tong, Y. Rational design of tungsten-doped cobalt molybdate nanosheet arrays for highly active ethanol-assisted hydrogen production. Int. J. Hydrogen Energy 2023. [Google Scholar] [CrossRef]
- Ye, R.; Tong, Y.; Feng, D.; Chen, P. A topological chemical transition strategy of bismuth-based materials for high-efficiency electrocatalytic carbon dioxide conversion to formate. J. Mater. Chem. A 2023, 11, 4691–4702. [Google Scholar] [CrossRef]
- Ye, R.; Zhu, J.; Tong, Y.; Feng, D.; Chen, P. Metal oxides heterojunction derived Bi-In hybrid electrocatalyst for robust electroreduction of CO2 to formate. J. Energy Chem. 2023, 83, 180–188. [Google Scholar] [CrossRef]
- Chen, P.; Li, K.; Ye, Y.; Wu, D.; Tong, Y. Coupled MoO3−x@CoP heterostructure as a pH-universal electrode for hydrogen generation at a high current density. Dalton Trans. 2023, 52, 2262–2271. [Google Scholar] [CrossRef]
- Jeong, S.; Mai, H.D.; Nguyen, T.K.; Youn, J.-S.; Nam, K.-H.; Park, C.-M.; Jeon, K.-J. Atomic interactions of two-dimensional PtS2 quantum dots/TiC heterostructures for hydrogen evolution reaction. Appl. Catal. B Environ. 2021, 293, 120227. [Google Scholar] [CrossRef]
- Liu, J.; Xu, H.; Yan, J.; Huang, J.; Song, Y.; Deng, J.; Wu, J.; Ding, C.; Wu, X.; Yuan, S.; et al. Efficient photocatalytic hydrogen evolution mediated by defect-rich 1T-PtS2 atomic layer nanosheet modified mesoporous graphitic carbon nitride. J. Mater. Chem. A 2019, 7, 18906–18914. [Google Scholar] [CrossRef]
- Huang, W.; Guo, Y.; Cen, X.; Tong, Y.; Chen, L.; Chen, P. Sulfur-Modified Nickel-Based Hybrid Nanosheet as a Robust Bifunctional Electrode for Hydrogen Generation via Formaldehyde Reforming. ACS Appl. Energy Mater. 2023. [Google Scholar] [CrossRef]
- Li, K.; He, J.; Guan, X.; Tong, Y.; Ye, Y.; Chen, L.; Chen, P. Phosphorus-Modified Amorphous High-Entropy CoFeNiCrMn Compound as High-Performance Electrocatalyst for Hydrazine-Assisted Water Electrolysis. Small 2023, e2302130. [Google Scholar] [CrossRef]



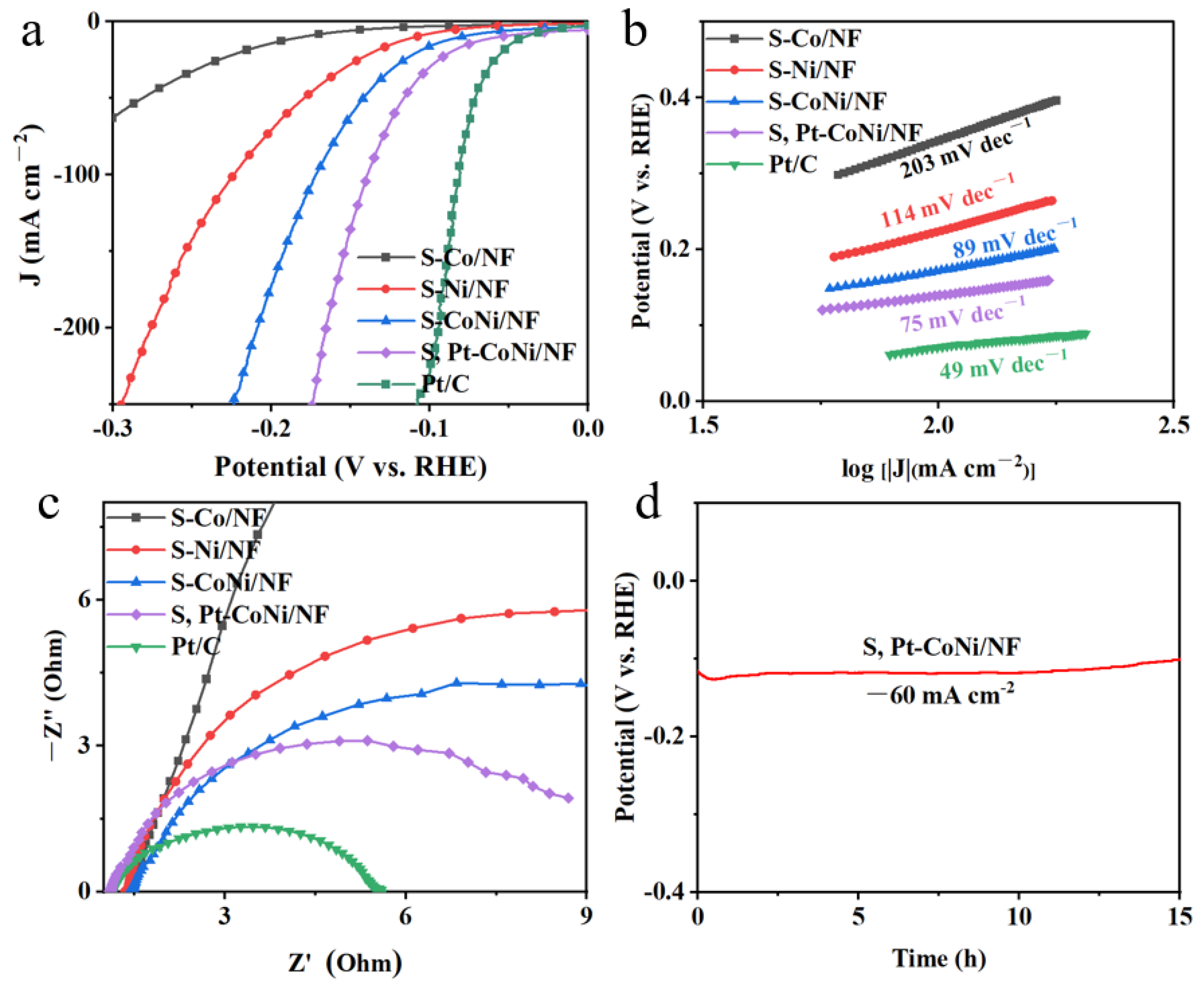


| Catalysts | Tafel Slope (mV dec−1) | Cdl (mF cm−1) | ECSA | Rct (Ω) | η50 (mV) |
|---|---|---|---|---|---|
| S-Co/NF | 203 | 3.0 | 5 | 10.4 | 281 |
| S-Ni/NF | 114 | 5.0 | 3 | 54 | 178 |
| S-CoNi/NF | 89 | 6.0 | 6 | 8 | 143 |
| S, Pt-CoNi/NF | 75 | 6.2 | 6.2 | 4.2 | 116 |
| Pt/C | 49 | / | / | 2.2 | 72 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ye, Y.; Zhou, G.; Li, K.; Tong, Y. Dual-Modification Engineering of CoNi Alloy Realizing Robust Performance for Electrocatalytic Hydrogen Production. Catalysts 2023, 13, 1064. https://doi.org/10.3390/catal13071064
Ye Y, Zhou G, Li K, Tong Y. Dual-Modification Engineering of CoNi Alloy Realizing Robust Performance for Electrocatalytic Hydrogen Production. Catalysts. 2023; 13(7):1064. https://doi.org/10.3390/catal13071064
Chicago/Turabian StyleYe, Yutong, Guorong Zhou, Kaixun Li, and Yun Tong. 2023. "Dual-Modification Engineering of CoNi Alloy Realizing Robust Performance for Electrocatalytic Hydrogen Production" Catalysts 13, no. 7: 1064. https://doi.org/10.3390/catal13071064