Preparation and Photocatalytic CO Oxidation Performance Study of Au/Oxygen-Deficient (Anatase/B-Phase) TiO2 Heterojunction Microspheres
Abstract
:1. Introduction
2. Experimental Section
2.1. Experimental Materials and Instruments
2.2. Synthesis of B-Phase Titanium Dioxide
2.3. Preparation of Gold-Loaded B-Phase Titania Porous Microspheres
2.4. Photocatalysis Experiment Section
3. Results and Discussion
3.1. Morphology and Structural Analyses
3.1.1. Scanning Electron Microscopy
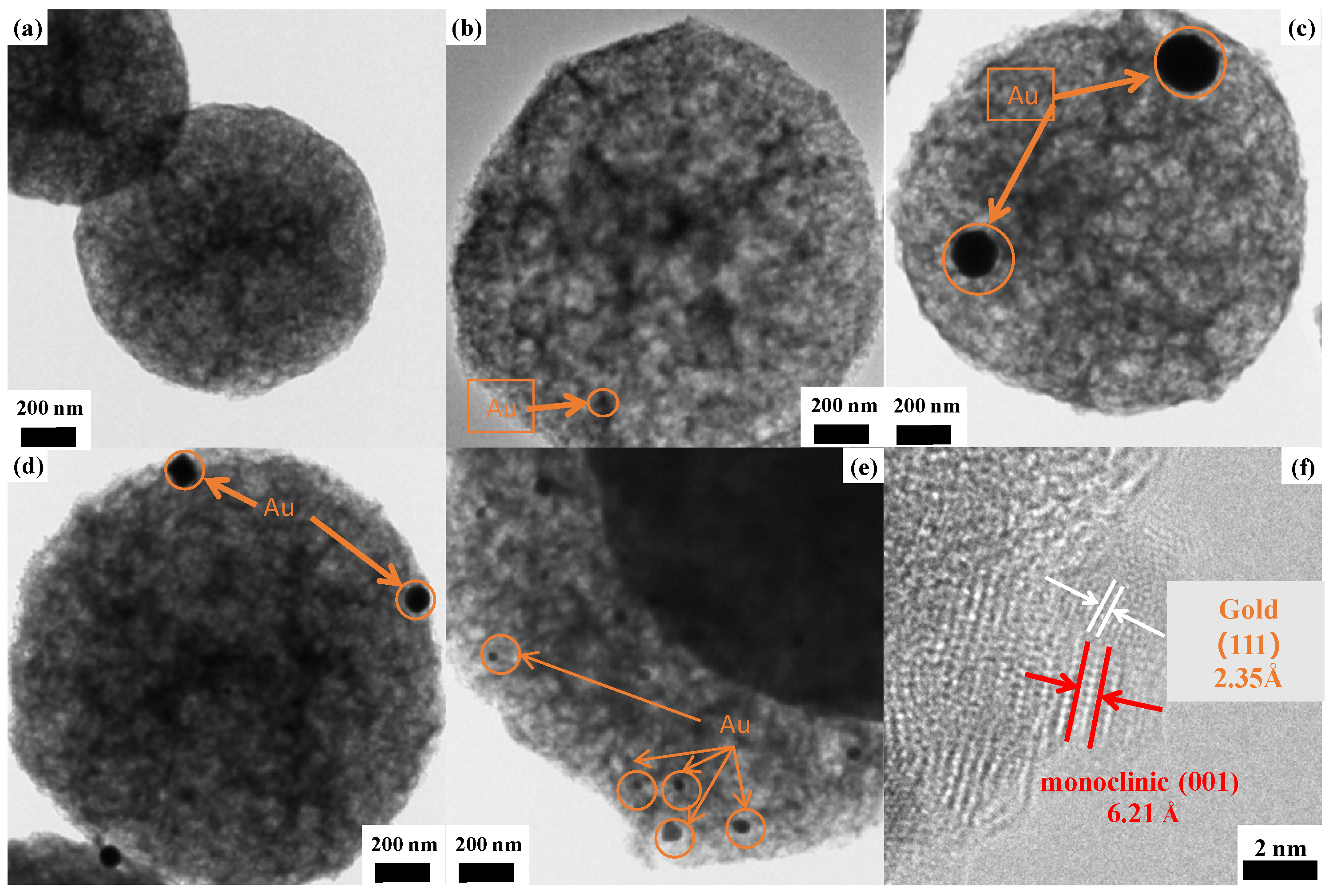
3.1.2. Transmission Electron Microscopy
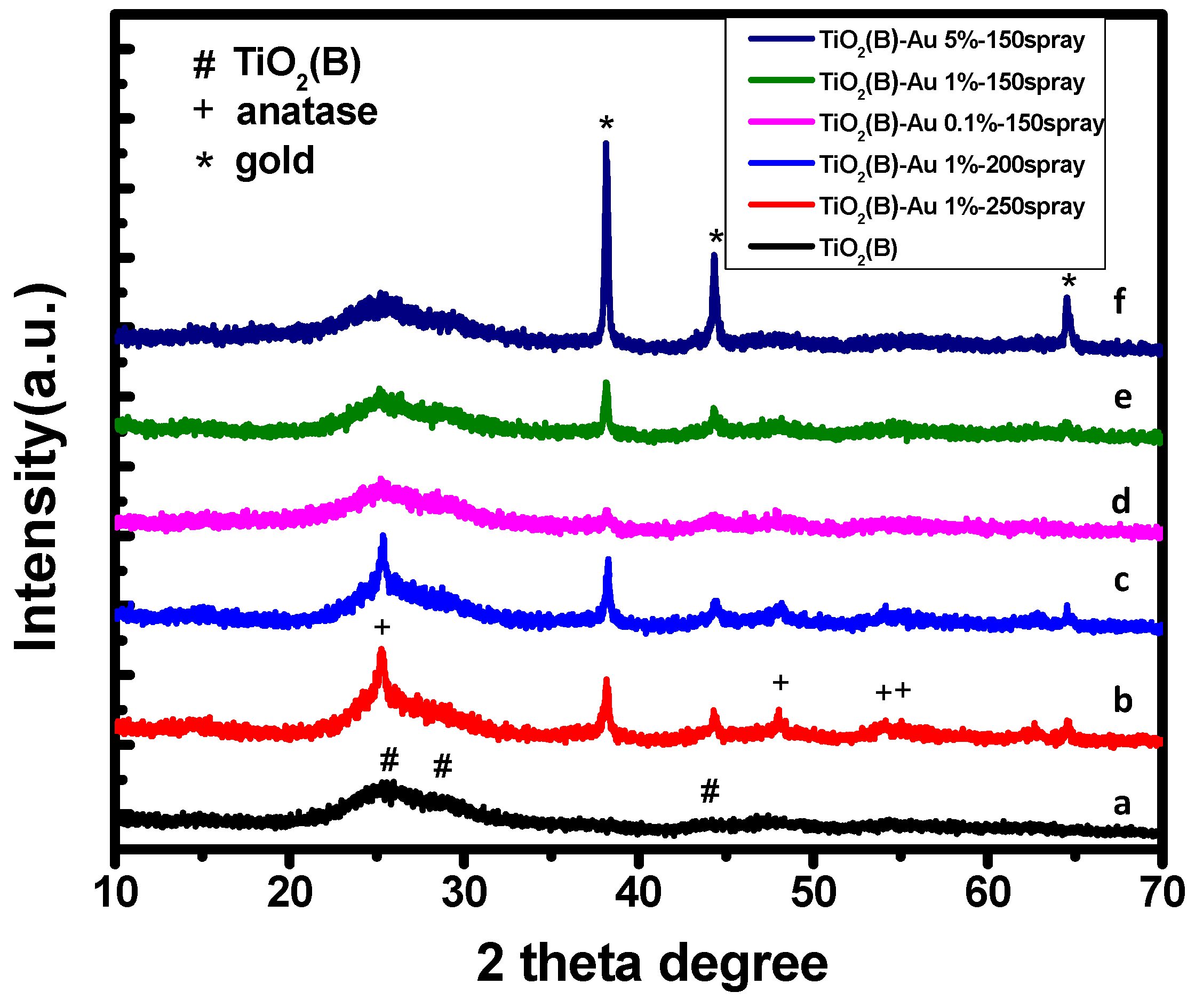
3.1.3. X-ray Diffraction Spectra
3.2. Optical Properties
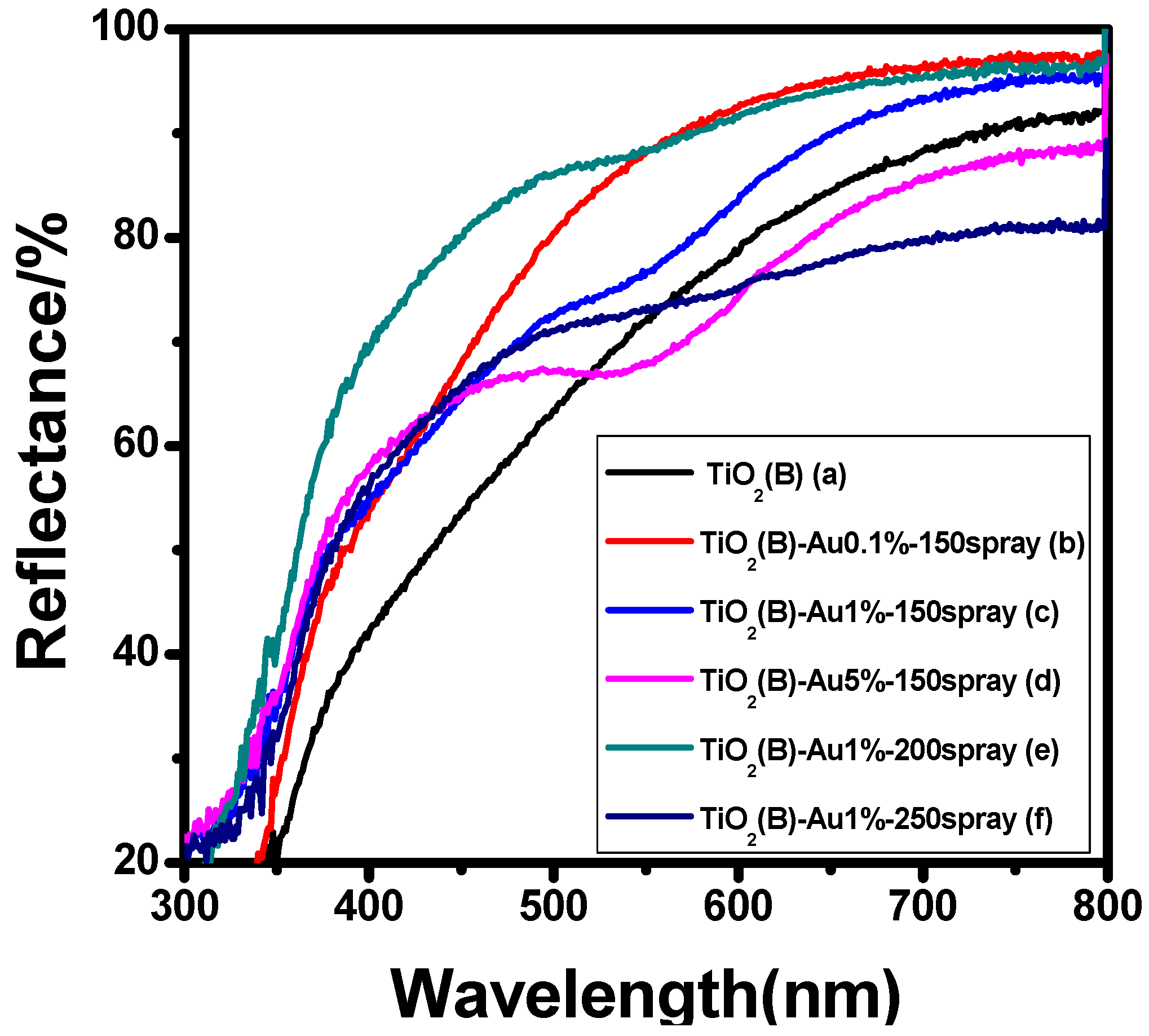
3.2.1. UV-Visible Diffuse Reflectance Spectra
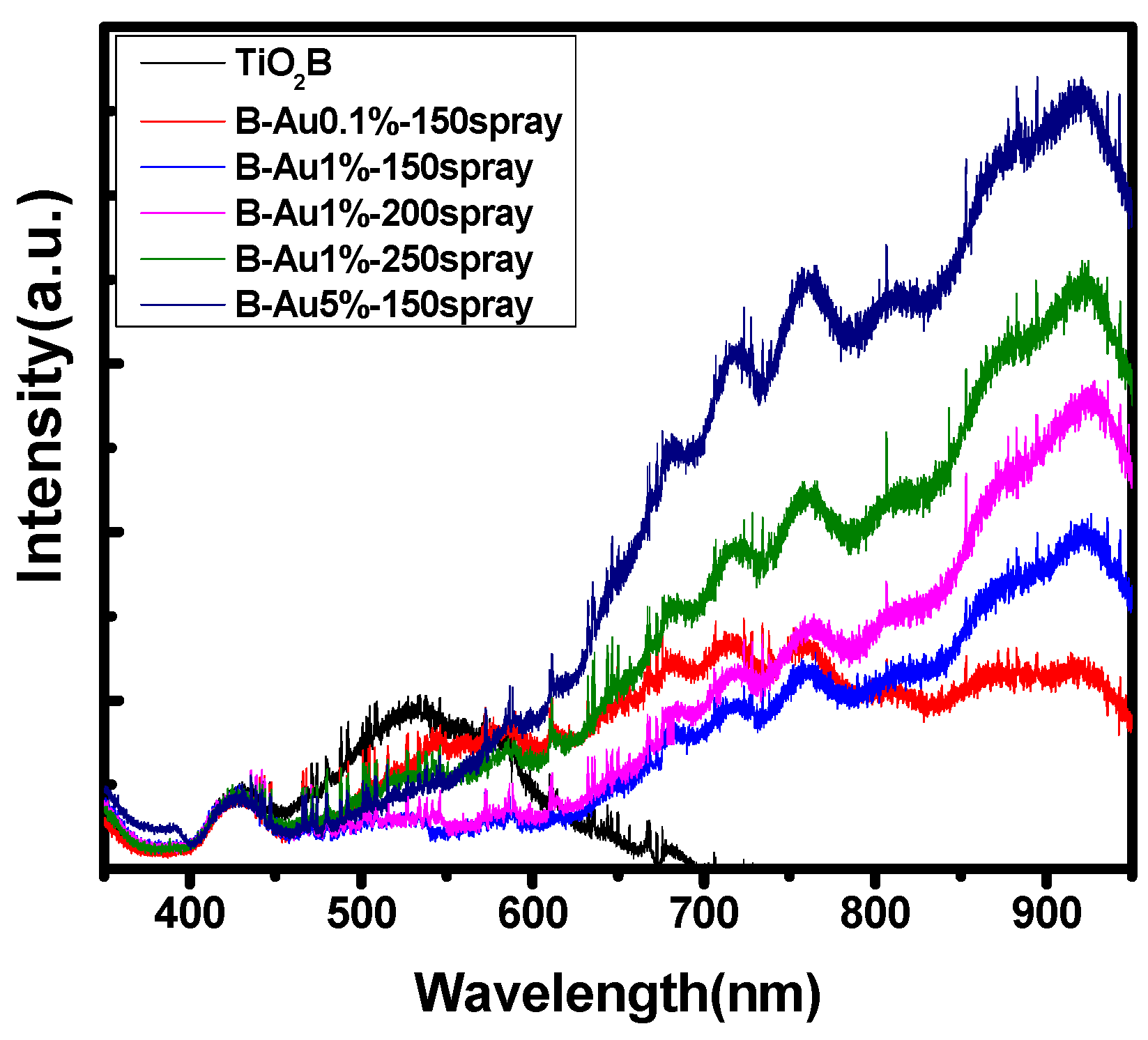
3.2.2. Photoluminescence Spectroscopy
3.3. Visible Light Photocatalytic Oxidation of Carbon Monoxide
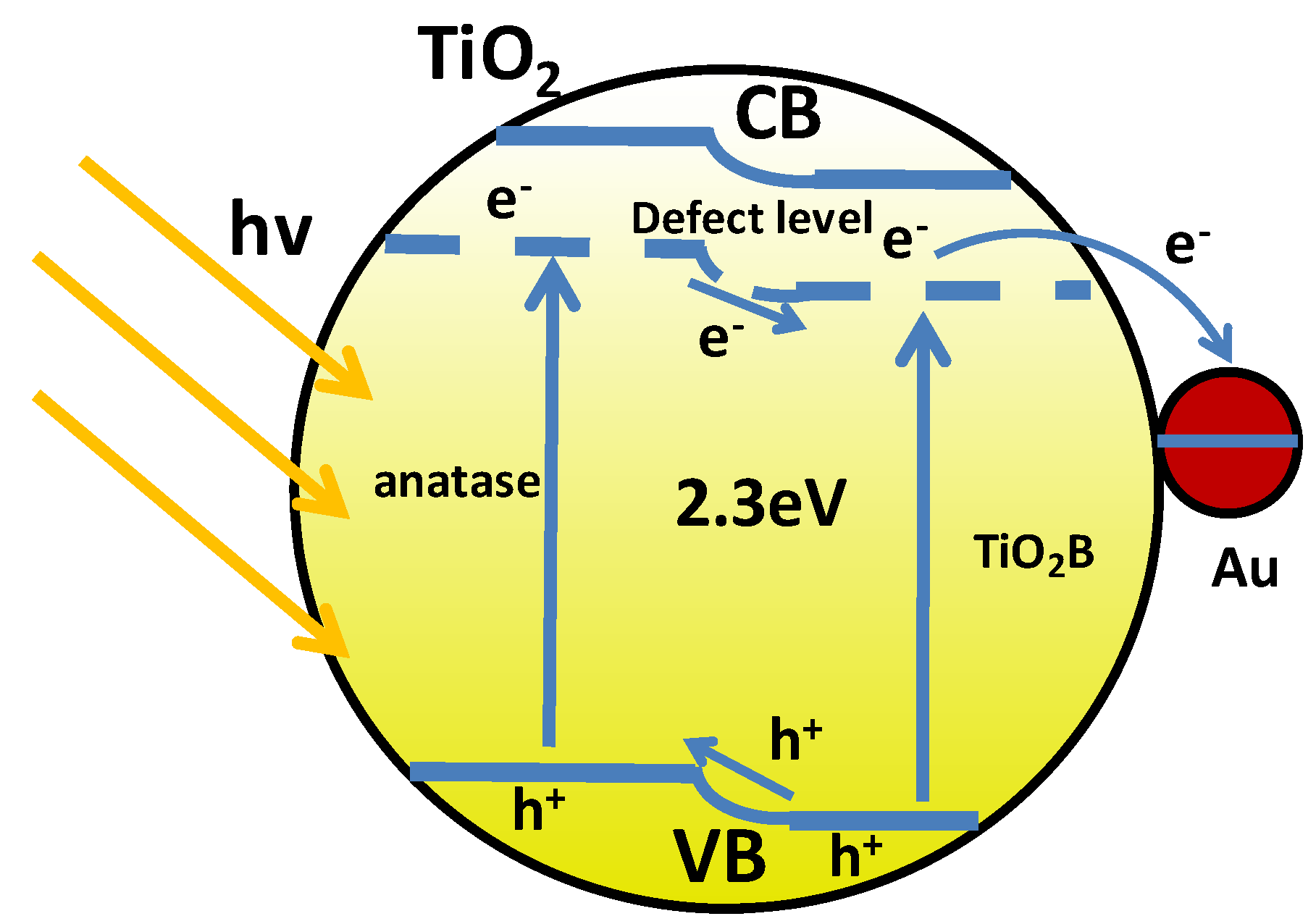
4. Mechanism Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hoffmann, M.R.; Martin, S.T.; Choi, W.; Bahnemann, D.W. Environmental applications of semiconductor photocatalysis. Chem. Rev. 1995, 95, 69–96. [Google Scholar] [CrossRef]
- Ollis, D.F.; Al-Ekabi, H. Photocatalytic purification and treatment of water and air. In Proceedings of the 1st International Conference on TiO2 Photocatalytic Purification and Treatment of Water and Air, London, ON, Canada, 8–13 November 1992; Elsevier Science Ltd.: Amsterdam, The Netherlands, 1993. [Google Scholar]
- Choi, W.; Hong, S.J. Photocatalytic Degradation of Polychlorinated Dibenzo-p-dioxins on TiO2 Film under UV or Solar Light Irradiation. Environ. Sci. Technol. 2000, 34, 4810–4815. [Google Scholar] [CrossRef]
- Cho, Y.; Choi, W. Visible Light-Induced Degradation of Carbon Tetrachloride on Dye-Sensitized TiO2. Environ. Sci. Technol. 2001, 35, 966–970. [Google Scholar] [CrossRef] [PubMed]
- Cho, S.; Choi, W. Solid-phase photocatalytic degradation of PVC–TiO2 polymer composites. J. Photochem. Photobiol. A Chem. 2001, 143, 221–228. [Google Scholar] [CrossRef]
- Kim, S.; Choi, W. Kinetics and mechanisms of photocatalytic degradation of (CH3) n NH4-n+ (0 ≤ n ≤ 4) in TiO2 suspension: The role of OH radicals. Environ. Sci. Technol. 2002, 36, 2019–2025. [Google Scholar] [CrossRef]
- Lee, M.C.; Choi, W. Solid phase photocatalytic reaction on the soot/TiO2 interface: The role of migrating OH radicals. J. Phys. Chem. B 2002, 106, 11818–11822. [Google Scholar] [CrossRef]
- Lee, H.; Choi, W. Photocatalytic oxidation of arsenite in TiO2 suspension: Kinetics and mechanisms. Environ. Sci. Technol. 2002, 36, 3872–3878. [Google Scholar] [CrossRef]
- Choi, W.; Ko, J.Y.; Park, H.; Chung, J.S. Investigation on TiO2-coated optical fibers for gas-phase photocatalytic oxidation of acetone. Appl. Catal. B Environ. 2001, 31, 209–220. [Google Scholar] [CrossRef]
- Peral, J.; Domenech, X.; Ollis, D.F. Heterogeneous photocatalysis for purification, decontamination and deodorization of air. J. Chem. Technol. Biotechnol. 1997, 70, 117–140. [Google Scholar] [CrossRef]
- Einaga, H.; Futamura, S.; Ibusuki, T. Heterogeneous photocatalytic oxidation of benzene, toluene, cyclohexene and cyclohexane in humidified air: Comparison of decomposition behavior on photoirradiated TiO2 catalyst. Appl. Catal. B Environ. 2002, 38, 215–225. [Google Scholar] [CrossRef]
- Zou, J.; Liao, G. In-Situ Construction of Sulfur-Doped g-C3N4/defective g-C3N4 Isotype Step-Scheme Heterojunction for Boosting Photocatalytic H2 Evolution. Chin. J. Struct. Chem. 2022, 41, 2201025–2201033. [Google Scholar]
- Lu, N.; Jing, X. Effective Cascade Modulation of Charge-Carrier Kinetics in the Well-Designed Multi-Component Nanofiber System for Highly-Efficient Photocatalytic Hydrogen Generation. Acta Phys. Chim. Sin. 2023, 39, 2207045. [Google Scholar]
- Shen, R.; Hao, L. P-Doped g-C3N4 Nanosheets with Highly Dispersed Co0.2Ni1.6Fe0.2P Cocatalyst for Efficient Photocatalytic Hydrogen Evolution. Acta Phys. Chim. Sin. 2022, 38, 2110014. [Google Scholar]
- Yu, J.; Li, X. Preface to Solar Photocatalysis. Chin. J. Struct. Chem. 2022, 41, 2206001–2206002. [Google Scholar]
- Driessen, M.D.; Goodman, A.L.; Miller, T.M.; Zaharias, G.A.; Grassian, V.H. Gas-phase photooxidation of trichloroethylene on TiO2 and ZnO: Influence of trichloroethylene pressure, oxygen pressure, and the photocatalyst surface on the product distribution. J. Phys. Chem. B 1998, 102, 549–556. [Google Scholar] [CrossRef]
- Du, H.; Fu, J.; Liu, L.-X.; Ding, S.; Lyu, Z.; Chang, Y.-C.; Jin, X.; Kengara, F.O.; Song, B.; Min, Q.; et al. Recent progress in electrochemical reduction of carbon monoxide toward multi-carbon products. Mater. Today 2022, 59, 182–199. [Google Scholar] [CrossRef]
- Hwang, S.; Lee, M.C.; Choi, W. Highly enhanced photocatalytic oxidation of CO on titania deposited with Pt nanoparticles: Kinetics and mechanism. Appl. Catal. B Environ. 2003, 46, 49–63. [Google Scholar] [CrossRef]
- Milt, V.G.; Ivanova, S.; Sanz, O.; Domínguez, M.I.; Corrales, A.; Odriozola, J.A.; Centeno, M.A. Au/TiO2 supported on ferritic stainless steel monoliths as CO oxidation catalysts. Appl. Surf. Sci. 2013, 270, 169–177. [Google Scholar] [CrossRef]
- Roy, S.; Hegde, M.S.; Ravishankar, N.; Madras, G. Creation of redox adsorption sites by Pd2+ ion substitution in nanoTiO2 for high photocatalytic activity of CO oxidation, NO reduction, and NO decomposition. J. Phys. Chem. C 2007, 111, 8153–8160. [Google Scholar] [CrossRef]
- Li, Q.; Wang, K.; Zhang, S.; Zhang, M.; Yang, J.; Jin, Z. Effect of photocatalytic activity of CO oxidation on Pt/TiO2 by strong interaction between Pt and TiO2 under oxidizing atmosphere. J. Mol. Catal. A Chem. 2006, 258, 83–88. [Google Scholar] [CrossRef]
- Vorontsov, A.V.; Savinov, E.N.; Jin, Z.-S. Influence of the form of photodeposited platinum on titania upon its photocatalytic activity in CO and acetone oxidation. J. Photochem. Photobiol. A Chem. 1999, 125, 113–117. [Google Scholar] [CrossRef]
- Bosc, F.; Ayral, A.; Keller, N.; Keller, V. Room temperature visible light oxidation of CO by high surface area rutile TiO2-supported metal photocatalyst. Appl. Catal. B Environ. 2007, 69, 133–137. [Google Scholar] [CrossRef]
- Li, X.Z.; Li, F.B. Study of Au/Au3+-TiO2 photocatalysts toward visible photooxidation for water and wastewater treatment. Environ. Sci. Technol. 2001, 35, 2381–2387. [Google Scholar] [CrossRef] [PubMed]
- Bahnemann, D.W.; Moenig, J.; Chapman, R. Efficient photocatalysis of the irreversible one-electron and two-electron reduction of halothane on platinized colloidal titanium dioxide in aqueous suspension. J. Phys. Chem. 1987, 91, 3782–3788. [Google Scholar] [CrossRef]
- Ogata, A.; Shintani, N. Decomposition of benzene using a nonthermal plasma reactor packed with ferroelectric pellets. IEEE Trans. Ind. Appl. 1999, 35, 753–759. [Google Scholar] [CrossRef]
- Disdier, J.; Herrmann, J.M.; Pichat, P. Platinum/titanium dioxide catalysts. A photoconductivity study of electron transfer from the ultraviolet-illuminated support to the metal and of the influence of hydrogen. J. Chem. Soc. Faraday Trans. 1 Phys. Chem. Condens. Phases 1983, 79, 651–660. [Google Scholar] [CrossRef]
- Yang, K.; Zhang, Y.; Li, Y.; Huang, P.; Chen, X.; Dai, W.; Fu, X. Insight into the function of alkaline earth metal oxides as electron promoters for Au/TiO2 catalysts used in CO oxidation. Appl. Catal. B Environ. 2016, 183, 206–215. [Google Scholar] [CrossRef]
- Deng, X.; Zhu, B.; Li, X.; Liu, J.; Zhu, X.; Zhu, A. Visible-light photocatalytic oxidation of CO over plasmonic Au/TiO2: Unusual features of oxygen plasma activation. Appl. Catal. B Environ. 2016, 188, 48–55. [Google Scholar] [CrossRef]
- Zhang, M.; Jin, Z.; Zhang, J.; Zhang, Z.; Dang, H. Effect of calcination and reduction treatment on the photocatalytic activity of CO oxidation on Pt/TiO2. J. Mol. Catal. A Chem. 2005, 225, 59–63. [Google Scholar] [CrossRef]
- Grätzel, M. Heterogeneous Photochemical Electron Transfer; CRC Press: Boca Raton, FL, USA, 1989. [Google Scholar]
- Wei, S.; Fu, X.-P. Au/TiO2 Catalysts for CO Oxidation: Effect of Gold State to Reactivity. J. Phys. Chem. C 2018, 122, 4928–4936. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, Q. The promoted effect of a metal-organic frameworks (ZIF-8) on Au/TiO2 for CO oxidation at room temperature both in dark and under visible light irradiation. Appl. Catal. B Environ. 2018, 224, 283–294. [Google Scholar] [CrossRef]
- Wang, Y.-G.; Cantu, D.C. CO Oxidation on Au/TiO2: Condition-dependent active sites and mechanistic pathways. J. Am. Chem. Soc. 2016, 138, 10467–10476. [Google Scholar] [CrossRef] [PubMed]
- Pennington, A.M.; Pitman, C.L. Photocatalytic CO Oxidation over Nanoparticulate Au-Modified TiO2 Aerogels: The Importance of Size and Intimacy. ACS Catal. 2020, 10, 14834–14846. [Google Scholar] [CrossRef]
- Xiao, Q.; Wei, S. The Effect of Hydrogenated TiO2 to the Au/TiO2 Catalyst in Catalyzing CO Oxidation. Langmuir 2021, 37, 3270–3280. [Google Scholar] [CrossRef]
- Jiang, X.; Huang, J. Plasmonic Active “Hot Spots”-Confined Photocatalytic CO2 Reduction with High Selectivity for CH4 Production. Adv. Mater. 2022, 34, 2109330. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, L. Molecular-Level Engineering of S-scheme Heterojunction: The Site-Specific Role for Directional Charge Transfer. Chin. J. Struct. Chem. 2022, 41, 2206003–2206005. [Google Scholar]
- Zhu, B.; Hong, X. Enhanced Photocatalytic CO2 Reduction over 2D/1D BiOBr0.5Cl0.5/WO3 S-Scheme Heterostructure. Acta Phys. Chim. Sin. 2022, 38, 2111008. [Google Scholar]
- Wang, W.; Lu, C.; Ni, Y.; Su, M.; Xu, Z. A new sight on hydrogenation of F and NF doped {001} facets dominated anatase TiO2 for efficient visible light photocatalyst. Appl. Catal. B Environ. 2012, 127, 28–35. [Google Scholar] [CrossRef]
- Zheng, Z.; Huang, B.; Meng, X.; Wang, J.; Wang, S.; Lou, Z.; Wang, Z.; Qin, X.; Zhang, X.; Dai, Y. Metallic zinc-assisted synthesis of Ti3+ self-doped TiO2 with tunable phase composition and visible-light photocatalytic activity. Chem. Commun. 2013, 49, 868–870. [Google Scholar] [CrossRef]
- Yang, C.; Wang, Z.; Lin, T.; Yin, H.; Lü, X.; Wan, D.; Xu, T.; Zheng, C.; Lin, J.; Huang, F.; et al. Core-shell nanostructured “black” rutile titania as excellent catalyst for hydrogen production enhanced by sulfur doping. J. Am. Chem. Soc. 2013, 135, 17831–17838. [Google Scholar] [CrossRef]
- Ihara, T.; Miyoshi, M.; Ando, M.; Sugihara, S.; Iriyama, Y. Preparation of a visible-light-active TiO2 photocatalyst by RF plasma treatment. J. Mater. Sci. 2001, 36, 4201–4207. [Google Scholar] [CrossRef]
- Xing, M.; Zhang, J.; Chen, F.; Tian, B. An economic method to prepare vacuum activated photocatalysts with high photo-activities and photosensitivities. Chem. Commun. 2011, 47, 4947–4949. [Google Scholar] [CrossRef]
- Grabstanowicz, L.R.; Gao, S.; Li, T.; Rickard, R.M.; Rajh, T.; Liu, D.J.; Xu, T. Facile oxidative conversion of TiH2 to high-concentration Ti3+-self-doped rutile TiO2 with visible-light photoactivity. Inorg. Chem. 2013, 52, 3884–3890. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Gao, S.; Xu, H.; Lou, Z.; Wang, W.; Huang, B.; Dai, Y. Green synthetic approach for Ti3+ self-doped TiO2−x nanoparticles with efficient visible light photocatalytic activity. Nanoscale 2013, 5, 1870–1875. [Google Scholar] [CrossRef] [PubMed]
- Majeed, M.H.; Aycibin, M. Influence of annealing process on structural, optical and electronic properties of nano-structured ZnO films synthesized by hydrothermal technique: Supported by DFT study. Mater. Sci. Eng. B 2022, 282, 115793. [Google Scholar] [CrossRef]
- Teesetsopon, P.; Treewut, P. Effect of pyrazine in PEDOT: PSS thin films: Structural, optical, optoelectrical, and electrical analysis. Opt. Mater. 2023, 136, 113465. [Google Scholar] [CrossRef]
- Chainiwetwattana, C.; Wongrat, E. Annealing time dependence on the structural, optical, optoelectrical, and electrical properties of copper antimony sulfide thin film synthesized using the dip coating method. Opt. Mater. 2023, 138, 113640. [Google Scholar] [CrossRef]
- Chen, X.; Liu, L.; Yu, P.Y.; Mao, S.S. Increasing Solar Absorption for Photocatalysis with Black Hydrogenated Titanium Dioxide Nanocrystals. Science 2011, 331, 746–750. [Google Scholar] [CrossRef]
- Cho, I.S.; Logar, M.; Lee, C.H.; Cai, L.; Prinz, F.B.; Zheng, X. Rapid and Controllable Flame Reduction of TiO2 Nanowires for Enhanced Solar Water-splitting. Nano Lett. 2013, 14, 24–31. [Google Scholar] [CrossRef]
- Sang, L.-X.; Zhang, Z.-Y.; Bai, G.-M.; Du, C.-X.; Ma, C.-F. A Photoelectrochemical Investigation of the Hydrogen-evolving Doped TiO2 Nanotube Arrays Electrode. Int. J. Hydrog. Energy 2012, 37, 854–859. [Google Scholar] [CrossRef]
- Ahmed, S.; Fonseca, S.M.; Kemp, T.J.; Unwin, P.R. Kinetics of O2 Reduction at Illuminated TiO2 Films. J. Phys. Chem. B 2003, 107, 5892–5900. [Google Scholar] [CrossRef]
- Tian, B.; Li, C.; Gu, F.; Jiang, H. Synergetic effects of nitrogen doping and Au loading on enhancing the visible-light photocatalytic activity of nano-TiO2. Catal. Commun. 2009, 10, 925–929. [Google Scholar] [CrossRef]
- Yin, Y.; Liu, E.; Li, H.; Wan, J.; Fan, J.; Hu, X.; Li, J.; Tang, C.; Pu, C. Fabrication of plasmonic Au/TiO2 nanotube arrays with enhanced photoelectrocatalytic activities. Ceram. Int. 2016, 42, 9387–9395. [Google Scholar] [CrossRef]
- Yang, D.; Liu, H.; Zheng, Z.; Yuan, Y.; Zhao, J.; Waclawik, E.R.; Ke, X.; Zhu, H. An Efficient Photocatalyst Structure: TiO2(B) Nanofibers with a Shell of Anatase Nanocrystals. J. Am. Chem. Soc. 2009, 131, 17885–17893. [Google Scholar] [CrossRef]
- Subramanian, V.; Wolf, E.E.; Kamat, P.V. Catalysis with TiO2/gold nanocomposites. Effect of metal particle size on the Fermi level equilibration. J. Am. Chem. Soc. 2004, 126, 4943–4950. [Google Scholar] [CrossRef]
- Li, F.B.; Li, X.Z. Photocatalytic properties of gold/gold ion-modified titanium dioxide for wastewater treatment. Appl. Catal. A Gen. 2002, 228, 15–27. [Google Scholar] [CrossRef]
- Grabowska, E.; Marchelek, M.; Klimczuk, T.; Trykowski, G.; Zaleska-Medynska, A. Noble metal modified TiO2 microspheres: Surface properties and photocatalytic activity under UV–vis and visible light. J. Mol. Catal. A Chem. 2016, 423, 191–206. [Google Scholar] [CrossRef]
- Wang, C.; Zhang, X.; Wei, Y.; Kong, L.; Chang, F.; Zheng, H.; Wu, L.; Zhic, J.; Liu, Y. Correlation between band alignment and enhanced photocatalysis: A case study with anatase/TiO2(B) nanotube heterojunction. Dalton Trans. 2015, 44, 13331–13339. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hong, Z.; Ouyang, J.; Li, J.; Zheng, H.; Liu, Y. Preparation and Photocatalytic CO Oxidation Performance Study of Au/Oxygen-Deficient (Anatase/B-Phase) TiO2 Heterojunction Microspheres. Catalysts 2023, 13, 1078. https://doi.org/10.3390/catal13071078
Hong Z, Ouyang J, Li J, Zheng H, Liu Y. Preparation and Photocatalytic CO Oxidation Performance Study of Au/Oxygen-Deficient (Anatase/B-Phase) TiO2 Heterojunction Microspheres. Catalysts. 2023; 13(7):1078. https://doi.org/10.3390/catal13071078
Chicago/Turabian StyleHong, Ze, Jingying Ouyang, Jiaxin Li, Han Zheng, and Ying Liu. 2023. "Preparation and Photocatalytic CO Oxidation Performance Study of Au/Oxygen-Deficient (Anatase/B-Phase) TiO2 Heterojunction Microspheres" Catalysts 13, no. 7: 1078. https://doi.org/10.3390/catal13071078



