Surface Modification of Zinc Ferrite with Titanium to Be a Photo-Active Catalyst in Commercial LED Light
Abstract
1. Introduction
2. Results and Discussion
2.1. X-ray Diffraction (XRD)
2.2. Diffuse Reflectance
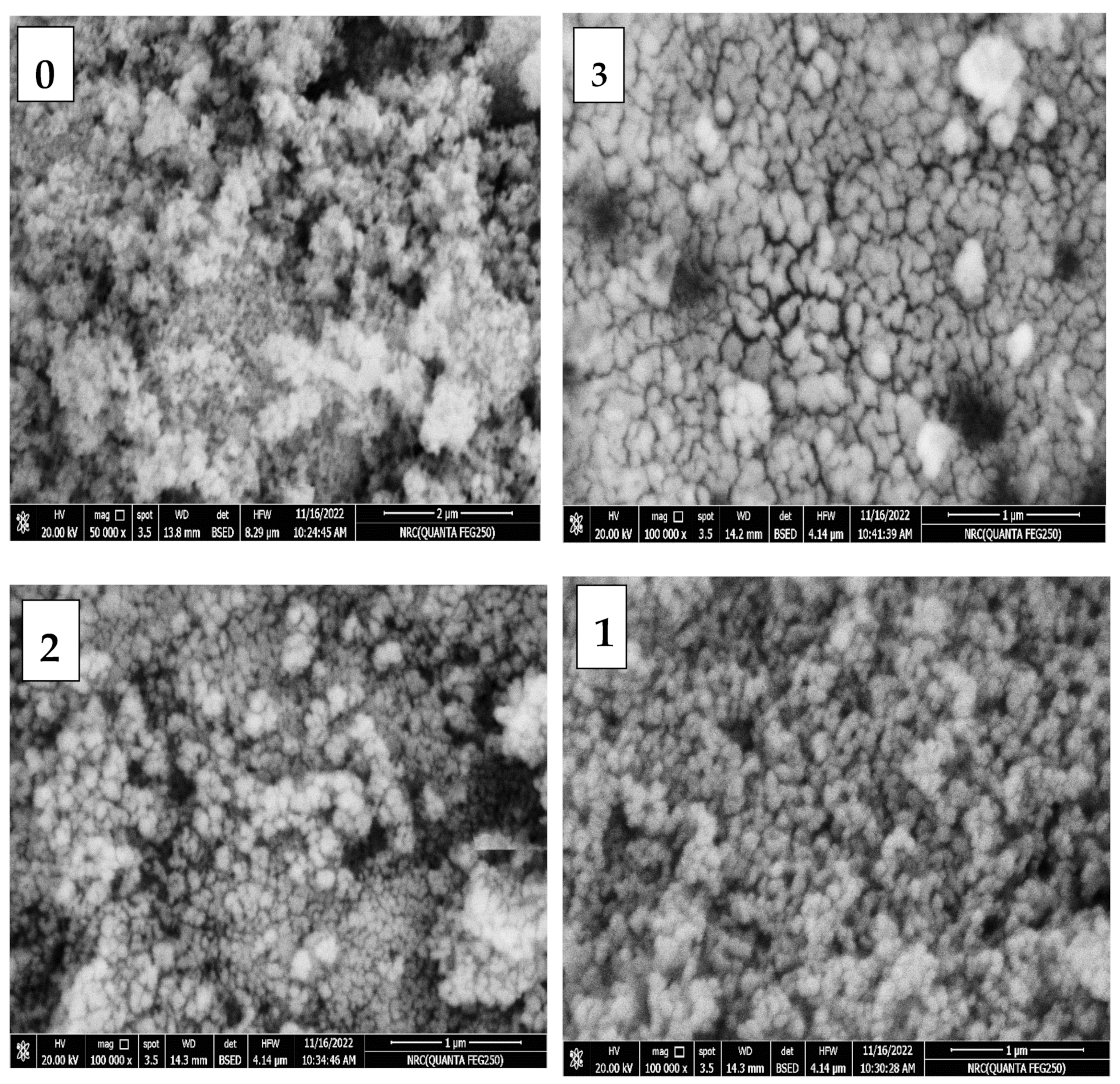
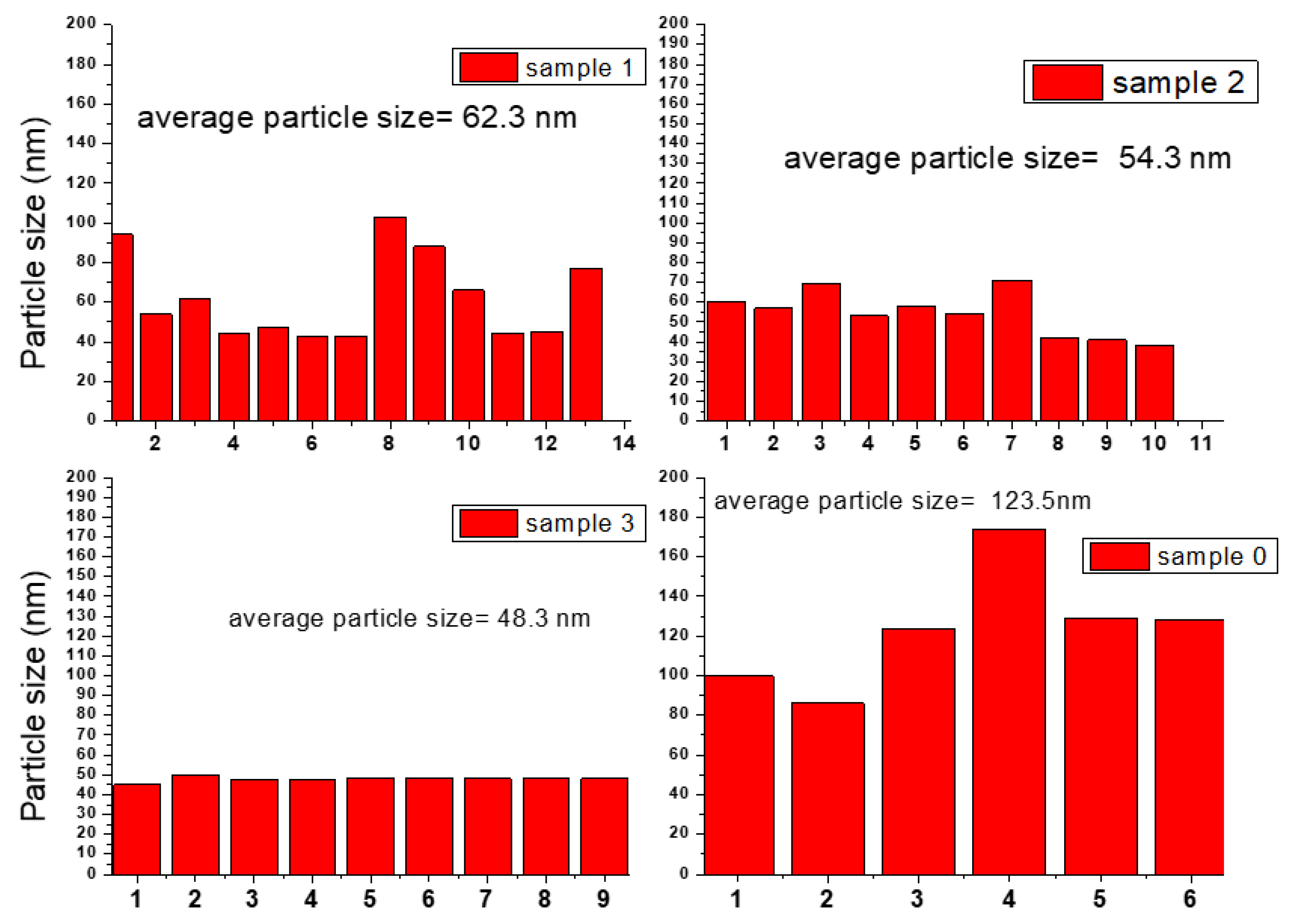
2.3. SEM Images and EDX Analysis
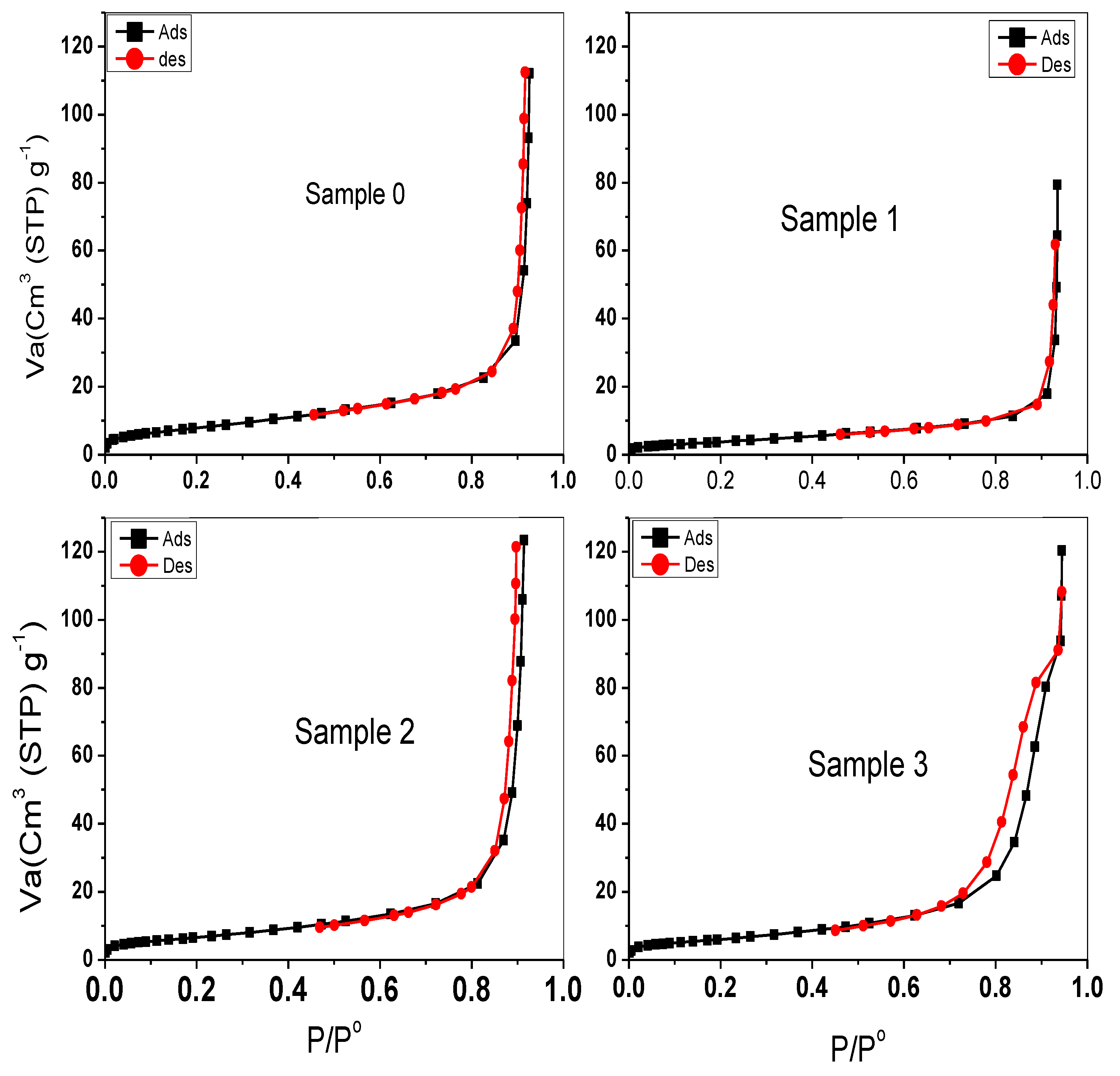
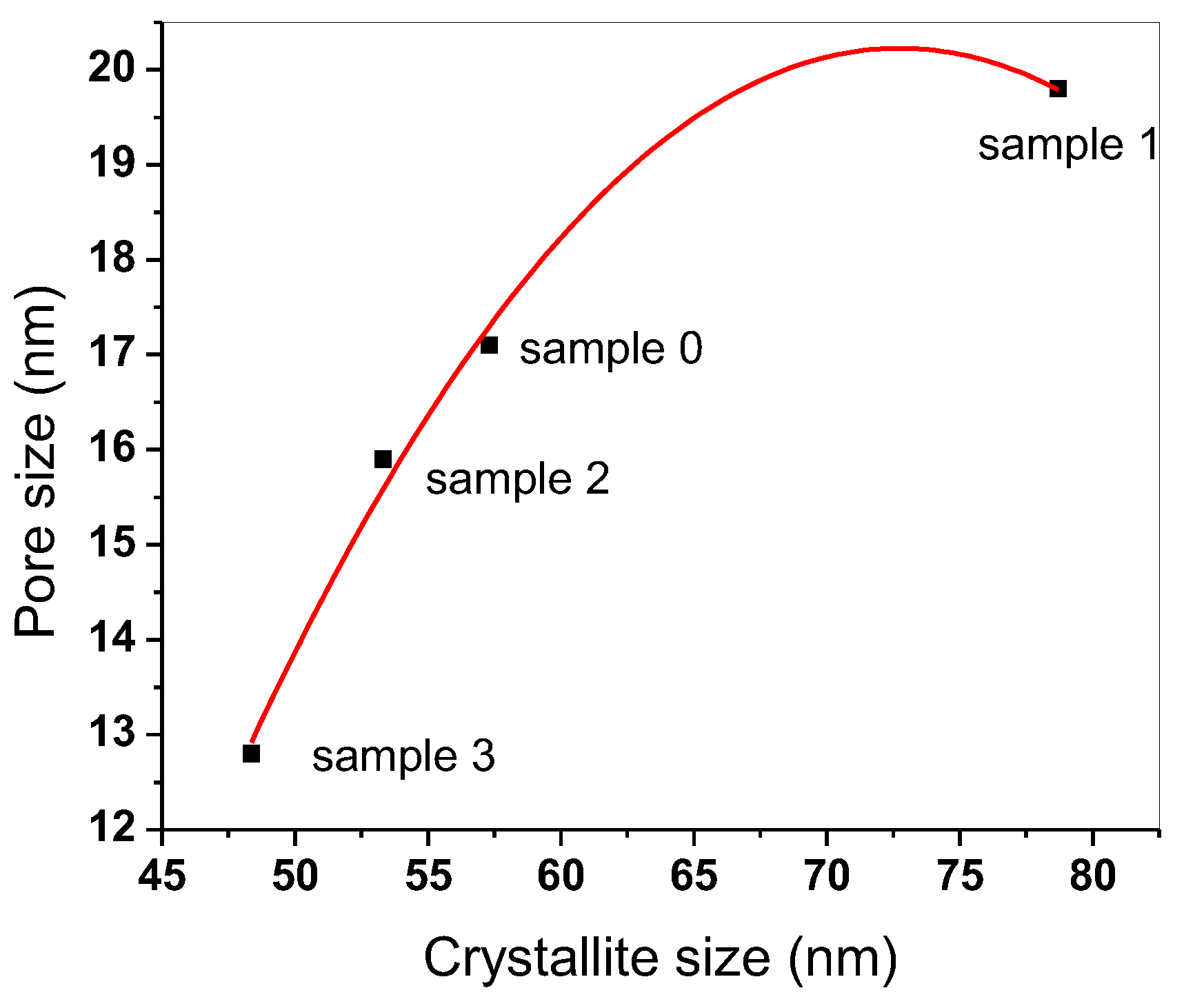
2.4. Textural Properties of Different Investigated Samples
2.5. Photo Catalytic Degradation of Methylene Blue
- Substitute Zn in tetrahedral positions by Ti showed more UV response.
- Rebelling off Zn forming nano zinc oxide, which is very active for visible irradiation.
- Higher loading of Ti, resulting in formation of agglomeration of TiO2 nanoparticles inside the crystals of zinc ferrite, which is also a UV active response.
- The evidences are arisen that for both UV and LED irradiation, there is more than one active center existing.
2.6. Kinetic Study of All Samples
3. Experimental
3.1. Materials
3.2. Preparation of Zinc Ferrite and Doped Samples
3.3. X-ray Powder Diffraction Analysis (XRD)
3.4. Scanning Electron Microscopy (SEM)
3.5. Surface Area Measurements (Brunauer Emmett Teller (BET))
3.6. Photochemical Reactor
DRUV–Vis Spectral Data
4. Mechanism of Photo Degradation over Zinc Ferrite
- The kinetic analysis showed that the degradation process follows up pseudo first-order reaction; however, some samples showed second-order ones, which enables us to conclude that there is more than one photo-active site.
- The surface modified sites are photo-active in both UV and visible irradiations.
- The surface area measurements showed a negligible role in photo degradation and the main role was found to be due to the electronic surface modification.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Magdalane, C.M.; Priyadharsini, G.M.A.; Kaviyarasu, K.; Jothi, A.I.; Simiyon, G.G. Synthesis and characterization of TiO2 doped cobalt ferrite nanoparticles via microwave method: Investigation of photocatalytic performance of congo red degradation dye. Surf. Interfaces 2021, 25, 101296. [Google Scholar] [CrossRef]
- Van Tran, C.; La, D.D.; Hoai, P.N.T.; Ninh, H.D.; Vu, T.H.T.; Nadda, A.K.; Nguyen, X.C.; Nguyen, D.D.; Ngo, H.H. New TiO2-doped Cu–Mg spinel-ferrite-based photocatalyst for degrading highly toxic rhodamine B dye in wastewater. J. Hazard. Mater. 2021, 420, 126636. [Google Scholar] [CrossRef] [PubMed]
- Ciocarlan, R.-G.; Hoeven, N.; Irtem, E.; Van Acker, V.; Mertens, M.; Seftel, E.M.; Breugelmans, T.; Cool, P. Ferrite@TiO2-nanocomposites as Z-scheme photocatalysts for CO2 conversion: Insight into the correlation of the Co-Zn metal composition and the catalytic activity. J. CO2 Util. 2020, 36, 177–186. [Google Scholar] [CrossRef]
- Yang, Y.; Xu, X.; Zhao, J.; Wang, B.; Li, C.; Chen, X.; Xu, F. Nano-TiO2 substituted LiZnBi ferrite ceramics with low sintering temperature and enhanced magnetic properties for LTCC applications. J. Alloys Compd. 2019, 775, 1244–1250. [Google Scholar] [CrossRef]
- Beatrice, C.; Dobák, S.; Tsakaloudi, V.; Fiorillo, F.; Maniοudaki, A.; Zaspalis, V. Magnetic aging in TiO2-doped Mn-Zn ferrites. J. Magn. Magn. Mater. 2020, 502, 166576. [Google Scholar] [CrossRef]
- Yousefi-Mohammadi, S.; Movahedi, M.; Salavati, H. MnCo–Ferrite/TiO2 composite as an efficient magnetically separable photocatalyst for decolorization of dye pollutants in aqueous solution. Surf. Interfaces 2018, 11, 91–97. [Google Scholar] [CrossRef]
- Yi, S.; Bai, G.; Wang, X.; Zhang, X.; Hussain, A.; Jin, J.; Yan, M. Development of high-temperature high-permeability MnZn power ferrites for MHz application by Nb2O5 and TiO2 co-doping. Ceram. Int. 2019, 46, 8935–8941. [Google Scholar] [CrossRef]
- Chen, C.-C.; Jaihindh, D.; Hu, S.-H.; Fu, Y.-P. Magnetic recyclable photocatalysts of Ni-Cu-Zn ferrite@SiO2@TiO2@Ag and their photocatalytic activities. J. Photochem. Photobiol. A Chem. 2017, 334, 74–85. [Google Scholar] [CrossRef]
- Xu, S.; Shangguan, W.; Yuan, J.; Chen, M.; Shi, J. Preparation and Photocatalytic Properties of Magnetically Separable TiO2 Supported on Nickel Ferrite. Chin. J. Chem. Eng. 2007, 15, 190–195. [Google Scholar] [CrossRef]
- Gu, M.; Liu, G. Effects of MoO3 and TiO2 additions on the magnetic properties of manganese–zinc power ferrites. J. Alloys Compd. 2009, 475, 356–360. [Google Scholar] [CrossRef]
- Mirzaee, O. Influence of PbO and TiO2 additives on the microstructure development and magnetic properties of Ni–Zn soft ferrites. J. King Saud Univ.-Eng. Sci. 2014, 26, 152–158. [Google Scholar] [CrossRef]
- Gaikwad, P.; Hankare, P.; Wandre, T.; Garadkar, K.; Sasikala, R. Photocatalytic performance of magnetically separable Fe, N co-doped TiO2-cobalt ferrite nanocomposite. Mater. Sci. Eng. B 2016, 205, 40–45. [Google Scholar] [CrossRef]
- Karmakar, S.; Routray, K.L.; Panda, B.; Sahoo, B.; Behera, D. Construction of core@shell nanostructured NiFe2O4@TiO2 ferrite NAND logic gate using fluorescence quenching mechanism for TiO2 sensing. J. Alloys Compd. 2018, 765, 527–537. [Google Scholar] [CrossRef]
- Hegazy, E.Z.; Kosa, S.A.; Elmaksod, I.H.A.; Mojamami, J.T. Preparation, characterization and photocatalytic evaluation of aluminum doped metal ferrites. Ceram. Int. 2019, 45, 7318–7327. [Google Scholar] [CrossRef]
- Shih, Y.-J.; Su, C.-C.; Chen, C.-W.; Dong, C.-D. Synthesis of magnetically recoverable ferrite (MFe2O4, MCo, Ni and Fe)-supported TiO2 photocatalysts for decolorization of methylene blue. Catal. Commun. 2015, 72, 127–132. [Google Scholar] [CrossRef]
- Laohhasurayotin, K.; Pookboonmee, S.; Viboonratanasri, D.; Kangwansupamonkon, W. Preparation of magnetic photocatalyst nanoparticles—TiO2/SiO2/Mn–Zn ferrite—And its photocatalytic activity influenced by silica interlayer. Mater. Res. Bull. 2012, 47, 1500–1507. [Google Scholar] [CrossRef]
- Yang, W.; Wang, Y. Effects of TiO2 and Co2O3 combination additions on the elemental distribution and electromagnetic properties of Mn–Zn power ferrites. J. Magn. Magn. Mater. 2015, 384, 13–17. [Google Scholar] [CrossRef]
- Sun, B.; Chen, F.; Yang, W.; Shen, H.; Xie, D. Effects of nano-TiO2 and normal size TiO2 additions on the microstructure and magnetic properties of manganese–zinc power ferrites. J. Magn. Magn. Mater. 2013, 349, 180–187. [Google Scholar] [CrossRef]
- Golshan, M.; Kakavandi, B.; Ahmadi, M.; Azizi, M. Photocatalytic activation of peroxymonosulfate by TiO2 anchored on cupper ferrite (TiO2@CuFe2O4) into 2,4-D degradation: Process feasibility, mechanism and pathway. J. Hazard. Mater. 2018, 359, 325–337. [Google Scholar] [CrossRef]
- El Maksod, I.H.A.; Al-Shehri, A.; Bawaked, S.; Mokhtar, M.; Narasimharao, K. Structural and photocatalytic properties of precious metals modified TiO2-BEA zeolite composites. Mol. Catal. 2017, 441, 140–149. [Google Scholar] [CrossRef]
- Joshi, G.P.; Saxena, N.S.; Mangal, R.; Mishra, A.; Sharma, T.P. Band gap determination of Ni–Zn ferrites. Bull. Mater. Sci. 2003, 26, 387–389. [Google Scholar] [CrossRef]
- Mikhailov, M.; Neshchimenko, V.; Li, C. Optical properties of zinc oxide powders modified by nanoparticles ZrO2, Al2O3, TiO2, SiO2, CeO2 and Y2O3 with various concentrations. Dye. Pigment. 2016, 131, 256–263. [Google Scholar] [CrossRef]
- Praveen, P.; Viruthagiri, G.; Mugundan, S.; Shanmugam, N. Structural, optical and morphological analyses of pristine titanium di-oxide nanoparticles—Synthesized via sol–gel route. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2014, 117, 622–629. [Google Scholar] [CrossRef] [PubMed]
- El Maksod, I.H.A.; Saleh, T.S. The use of nano supported nickel catalyst in reduction of p-nitrophenol using hydrazine as hydrogen donor. Green Chem. Lett. Rev. 2010, 3, 127–134. [Google Scholar] [CrossRef]
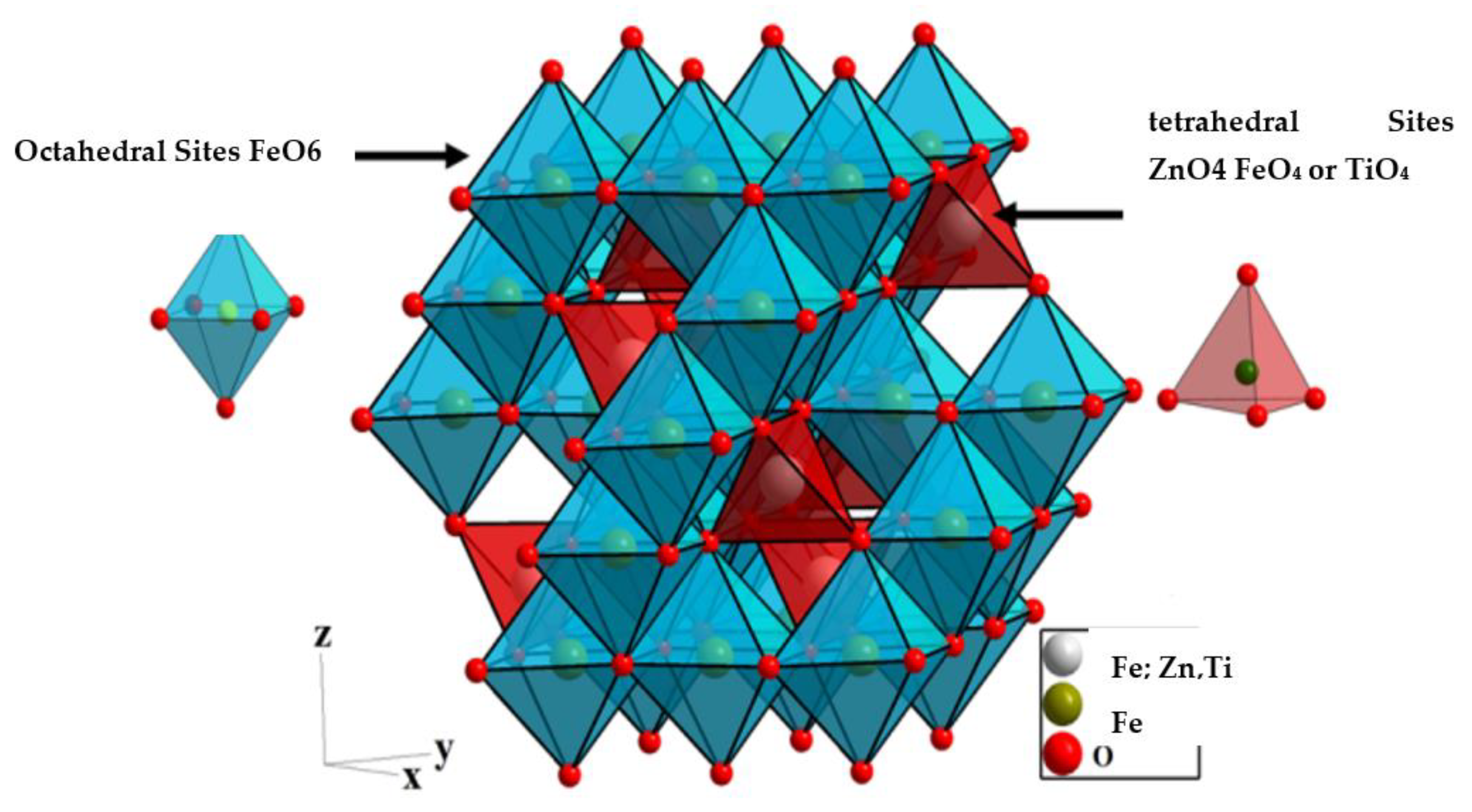
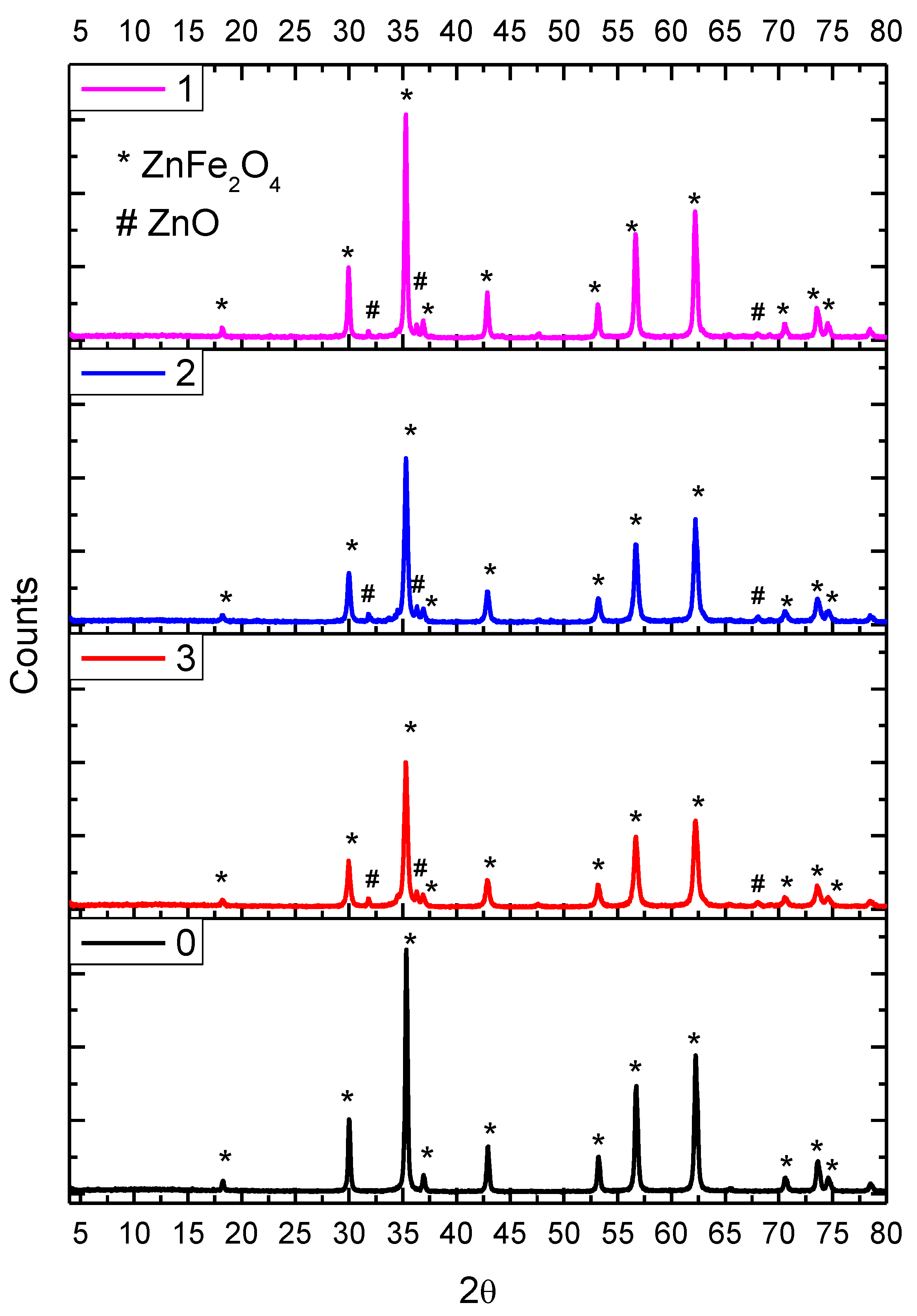
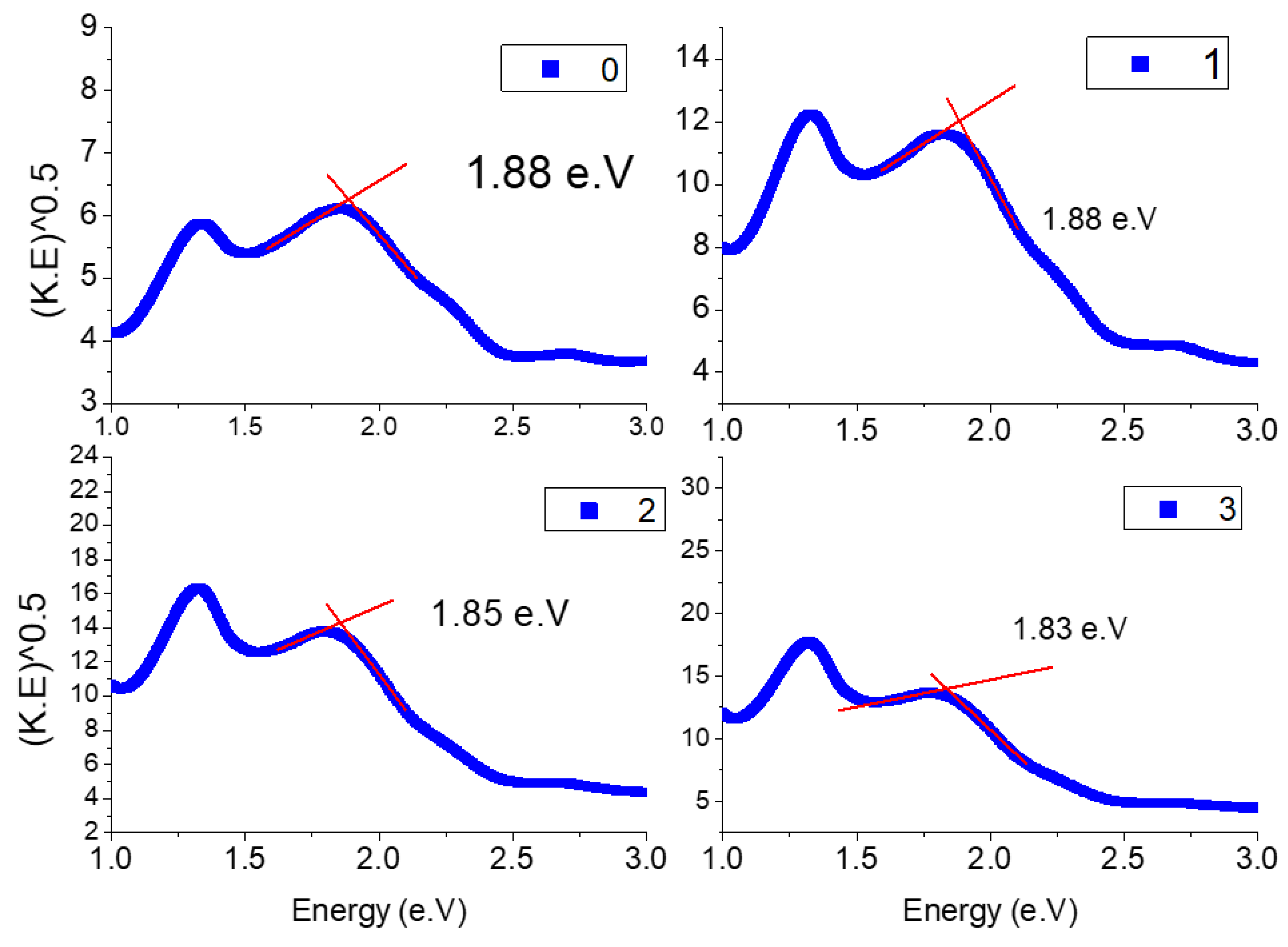
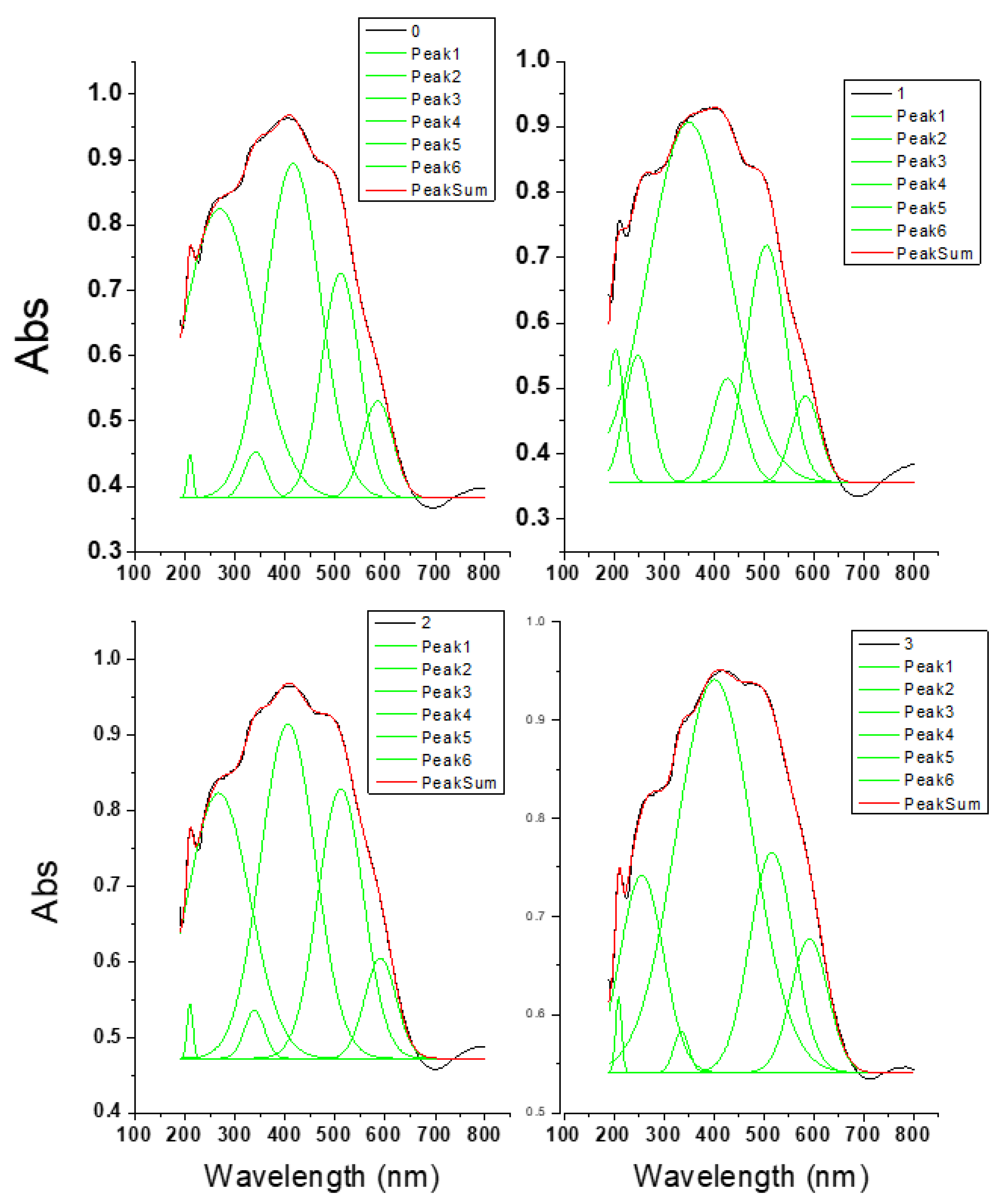



| Crystal Lattice Parameters | |||||
|---|---|---|---|---|---|
| Sample | a | b | c | Crystal System | Crytallite Size (nm) |
| Refrence (ZnFe2O4) PDf no p221012 | 8.441 | 8.441 | 8.441 | Cubic (Fd-3m) | |
| Sample 0 | 8.4357 | 8.4357 | 8.4357 | Cubic (Fd-3m) | 57.33 |
| Sample 3 | 9.739 | 9.739 | 9.377 | Tetragonal (I 41/a c d) | 48.36 |
| Sample 2 | 7.776 | 7.776 | 10.436 | Tetragonal (I 41/a c d) | 53.31 |
| Sample 1 | 8.701 | 8.701 | 7.974 | Tetragonal (I 41/a c d) | 78.7 |
| Sample 0 | Sample 2 | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Area | Center | Width | Height | Area | Center | Width | Height | ||
| 1.00 | 0.93 | 209.65 | 11.01 | 0.07 | 1.00 | 1.03 | 209.53 | 11.36 | 0.07 |
| 2.00 | 80.33 | 268.71 | 144.74 | 0.44 | 2.00 | 55.17 | 266.76 | 125.36 | 0.35 |
| 3.00 | 3.92 | 340.65 | 44.02 | 0.07 | 3.00 | 3.24 | 338.42 | 40.57 | 0.06 |
| 4.00 | 70.70 | 415.44 | 110.29 | 0.51 | 4.00 | 60.35 | 405.54 | 108.78 | 0.44 |
| 5.00 | 32.92 | 511.08 | 76.25 | 0.34 | 5.00 | 40.57 | 511.27 | 90.64 | 0.36 |
| 6.00 | 11.42 | 585.27 | 61.11 | 0.15 | 6.00 | 10.43 | 590.73 | 62.79 | 0.13 |
| sample 1 | sample 3 | ||||||||
| Area | Center | Width | Height | Area | Center | Width | Height | ||
| 1.00 | 8.56 | 203.53 | 33.48 | 0.20 | 1.00 | 1.17 | 209.48 | 12.12 | 0.08 |
| 2.00 | 12.98 | 247.85 | 53.20 | 0.19 | 2.00 | 21.75 | 255.89 | 86.22 | 0.20 |
| 3.00 | 110.81 | 349.55 | 160.49 | 0.55 | 3.00 | 1.61 | 334.66 | 30.35 | 0.04 |
| 4.00 | 12.74 | 427.08 | 63.77 | 0.16 | 4.00 | 76.74 | 401.16 | 152.98 | 0.40 |
| 5.00 | 35.78 | 505.44 | 78.76 | 0.36 | 5.00 | 24.82 | 516.24 | 88.30 | 0.22 |
| 6.00 | 9.40 | 583.75 | 56.56 | 0.13 | 6.00 | 12.63 | 591.40 | 73.84 | 0.14 |
| Theoretical | ||||
|---|---|---|---|---|
| Ti | Zn | Fe | O | |
| 0 | 0 | 14.28 | 28.5 | 57.1 |
| 1 | 1.759 | 13.53 | 27.06 | 57.6 |
| 2 | 1.11 | 13.8 | 27.6 | 57.4 |
| 3 | 0.699 | 13.98 | 27.97 | 57.3 |
| Actual | ||||
| Ti | Zn | Fe | O | |
| 0 | 0 | 12.63 | 27.52 | 59.85 |
| 1 | 1.9 | 13.53 | 23.97 | 60.59 |
| 2 | 2.09 | 14.69 | 26.06 | 57.15 |
| 3 | 1.1 | 14.42 | 28.37 | 56.1 |
| Surface excess % | ||||
| Ti | Zn | Fe | O | |
| 0 | 0 | −11.5546 | −3.4386 | 4.816112 |
| 1 | 8.015918 | 0 | −11.4191 | 5.190972 |
| 2 | 88.28829 | 6.449275 | −5.57971 | −0.43554 |
| 3 | 57.36767 | 3.147353 | 1.430104 | −2.09424 |
| Sample | Surface Area (m2/g) | Pore Size (nm) |
|---|---|---|
| 0 | 30.1 | 17.1 |
| 1 | 15.39 | 19.8 |
| 2 | 25.3 | 15.9 |
| 3 | 23.27 | 12.8 |
| UV Irradiation | ||||||
|---|---|---|---|---|---|---|
| Sample | 1st Order | 2nd Order | 3rd Order | |||
| k | R2 | k | R2 | k | R2 | |
| Sample 0 | 0.13 | 0.99 | 0.0355 | 0.519 | 0.0245 | 0.51 |
| Sample 1 | 0.224 | 0.97 | 0.0194 | 0.998 | 0.00581 | 0.75 |
| Sample 2 | 0.216 | 0.96 | 0.0042 | 0.566 | 0.00127 | 0.4122 |
| Sample3 | 0.28 | 0.94 | 0.0047 | 0.907 | 0.0006 | 0.808 |
| Visible LED Irradiation | ||||||
| Sample | 1st Order | 2nd Order | 3rd Order | |||
| k | R2 | k | R2 | k | R2 | |
| Sample 0 | 0.04 | 0.957 | 0.0838 | 0.87 | 0.6208 | 0.775 |
| Sample 1 | 0.305 | 0.964 | 0.1378 | 0.80 | 0.3713 | 0.65 |
| Sample 2 | 0.32 | 0.974 | 0.0011 | 0.961 | 0.0002 | 0.918 |
| Sample 3 | 0.31 | 0.972 | 0.0011 | 0.957 | 0.0001 | 0.9389 |
| Mole % | |||
|---|---|---|---|
| Sample | Ti | Zn | Fe |
| 0 | 0 | 14.28 | 28.57 |
| 1 | 1.75 | 13.53 | 27.06 |
| 2 | 1.12 | 13.8 | 27.61 |
| 3 | 0.685 | 13.99 | 27.98 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baamer, D.F.; Abd El Maksod, I.H. Surface Modification of Zinc Ferrite with Titanium to Be a Photo-Active Catalyst in Commercial LED Light. Catalysts 2023, 13, 1082. https://doi.org/10.3390/catal13071082
Baamer DF, Abd El Maksod IH. Surface Modification of Zinc Ferrite with Titanium to Be a Photo-Active Catalyst in Commercial LED Light. Catalysts. 2023; 13(7):1082. https://doi.org/10.3390/catal13071082
Chicago/Turabian StyleBaamer, Doaa F., and Islam Hamdy Abd El Maksod. 2023. "Surface Modification of Zinc Ferrite with Titanium to Be a Photo-Active Catalyst in Commercial LED Light" Catalysts 13, no. 7: 1082. https://doi.org/10.3390/catal13071082
APA StyleBaamer, D. F., & Abd El Maksod, I. H. (2023). Surface Modification of Zinc Ferrite with Titanium to Be a Photo-Active Catalyst in Commercial LED Light. Catalysts, 13(7), 1082. https://doi.org/10.3390/catal13071082








