Abstract
The shortage of water resources has caused extensive research to be conducted in this field to develop effective, rapid, and affordable wastewater treatment methods. For the treatment of wastewater, modern oxidation techniques are desirable due to their excellent performance and simplicity of implementation. In this project, wet impregnation and the hydrothermal technique were applied to synthesize a modified catalyst. Different analysis methods were used to determine its characteristics, including XRD, BET, FT-IR, , and FE-SEM. The catalyst features a spherical shape, large surface area, high crystallinity, and uniform active phase dispersion. In order to eliminate the methylene blue dye as a modeling effluent, the catalyst’s performance was examined in a heterogeneous quasi-electro-Fenton (EF) reaction. The impact of various performance characteristics, such as catalyst concentration in the reaction medium, solution pH, and current intensity between the two electrodes, was elucidated. According to the results, the best operational circumstances included a pH level of 2, a catalyst concentration of 0.15 g/L, and a current of 150 mA, resulting in the greatest elimination efficiency of 101%. The catalyst’s performance was stable during three consecutive tests. A pseudo-first-order model for the elimination reaction’s kinetics was developed, which showed acceptable agreement with the experimental results. This study’s findings help clarify how well the heterogeneous zeolite catalyst functions in the pseudo-EF reaction. The results revealed the method’s potential to be implemented in wastewater treatment. An artificial neural network model is utilized to predict the removal percentage. The hyperparameter tuning is used to find the best model, and the model achieved an MAE of 1.26% and the was 0.99.
1. Introduction
These days, wastewater from industrial activities, such as sewage contaminated with color, pharmaceutical, and urban wastewater, has led to many environmental problems, such as water contamination. It is imperative to conduct research into novel, affordable water treatment and purification techniques. Conventional wastewater treatment techniques are very costly, require special equipment, and do not work for special wastewater treatment [1,2,3,4]. Decolorization of wastewater is an important process to remove pollutants and ensure the water is safe for use or disposal. The electro-Fenton (EF) reaction is one of the most effective methods for removing organic contaminants from wastewater [5,6,7,8]. This process involves the generation of highly reactive hydroxyl radicals () through the electrochemical oxidation of catalyzed by . However, the EF reaction suffers from limitations such as low reaction rates, high energy consumption, and the need for a complex system [9,10,11,12]. To overcome these limitations, zeolite catalysts have been introduced in the EF process. Zeolites are crystalline, microporous materials with a large surface area and high catalytic activity. Using zeolites improves the efficiency of wastewater decolorization by enhancing the EF reaction’s oxidation potential [13,14,15,16]. The zeolite catalyst provides a surface for the adsorption of pollutants, increasing the reaction’s efficiency. The zeolite surface also enhances the generation of hydroxyl radicals, which promotes the removal of pollutants. An efficient investigation of decolorization of wastewater using zeolite catalyst involves optimizing the operating parameters such as the pH, current density, and concentration of and . The selection of the appropriate zeolite catalyst is also critical in achieving high efficiency in wastewater treatment. The efficient investigation of this process involves optimizing operating parameters and selecting appropriate zeolite catalysts for maximum efficiency [17,18,19,20]. The decolorization of wastewater is a crucial step in the treatment process, as it helps to reduce the concentration of pollutants and improve the water quality before discharge. One of the methods that have gained attention in recent years is the use of zeolite catalysts with EF reactions. Zeolites are porous materials with unique adsorption and catalytic properties, making them suitable for various applications, including wastewater treatment [21,22,23,24,25,26]. The use of a zeolite catalyst in EF reactions involves the generation of hydroxyl radicals () by the reaction of with in the presence of an acidic medium. The generated can then react with the organic pollutants present in the wastewater, resulting in their degradation and decolorization [27,28,29,30]. Several studies have investigated the use of zeolite as a catalyst in EF reactions for the decolorization of wastewater. Wang et al. [31,32,33] used natural zeolite as a catalyst in the EF reaction to decolorize methyl orange. The results showed that the combination of natural zeolite and EF reaction resulted in a higher decolorization rate compared to the EF reaction alone. In another study [34,35,36], synthetic zeolite was used in an EF reaction to decolorize Reactive Black 5 dye and was investigated. The results showed that the addition of synthetic zeolite as a catalyst in the EF reaction resulted in a higher decolorization rate compared to the EF reaction alone. The authors attributed this to the increased adsorption capacity of synthetic zeolite, which led to the more efficient removal of the dye from the wastewater. Another study [37,38,39] investigated the use of modified zeolite as a catalyst in EF reaction for phenol degradation. The modified zeolite was prepared by impregnating zeolite with iron ions. The results showed that the use of modified zeolite as a catalyst in the EF reaction resulted in a higher degradation rate of phenol compared to the EF reaction alone. The investigations into the use of zeolite catalyst in EF reaction for the decolorization of wastewater have shown promising results. The addition of zeolite as a catalyst has led to a higher decolorization rate and improved removal efficiency of organic pollutants from wastewater [40,41,42,43,44]. However, further studies are needed to determine the optimal conditions for the use of zeolite catalyst in EF reaction and to evaluate its effectiveness in treating different types of wastewater.
Implementing an advanced oxidation strategy to treat wastewater offers immense potential for improving the efficiency of specialized wastewater purification that is impossible to be removed or decomposed by conventional methods [45]. The EF process ranks among the most competitive advanced oxidation techniques. In this process, the electrical current results in the continuous production of hydrogen peroxide at the electrode surface so that it is decomposed inside the solution with the help of the catalyst to the radical hydroxyl. The EF reaction can be performed using a variety of metals with high hydroxyl production rates [45]. Much work has been done to optimize the heterogeneous EF process to achieve high productivity in dye removal and be cost-effective and appropriate operational conditions. One of the most critical issues is that developing a highly productive and reusable catalyst can reduce operating costs for semi-industrial and industrial applications [45]. Oturan et al. [46] investigated the influence of the nature and transition metals concentration, including cobalt, silver, copper, and iron metals, as the catalyst of the EF operation. Their findings demonstrated that iron is the most efficient catalytic active phase, and the methomyl destruction kinetics follows the quasi-first order model. Catalysts with different supports and active sites have been used in the Fenton and pseudo-Fenton processes, such as Fe/Al2O3 [47,48] and Fe/ZSM-5 [49]. Askari et al. [50] utilized cobalt ferrite as heterogeneous catalysts to eliminate cephalexin from aqueous solutions. In this research the R2 which shows the correlation between actual values and predicted values [51] is employed to confirm the accuracy of the model. There are different parameters to test the model. Naderian et al., employed F-test, R2, and mean relative error (MRE) to confirm the accuracy of the regression model [52].
The highest percentage of elimination has been reported at 90% in the conditions of the catalyst amount at 60 mg, the concentration of the persulfate was 0.5 (g/L), and the duration was 5 min [53]. Shen et al. [54] examined the removal of methylene blue (MB) through the heterogeneous processes of the Fenton and EF employing the Fe3O4-graphene catalyst. Their findings indicated that the catalyst had a porous structure, high magnetic saturation property, and high catalytic activity (over 90% removal) under conditions of pH equal to 2, H2O2 concentration equal to 4.4 mM, 10 ppm of MB at the start of the experiment, and 0.2 (g/L) of catalyst was present. Moreover, they used this material as a cathode in the EF process. MB was eliminated under operating conditions, including an initial concentration of 10 ppm at a pH of 2 in 45 min. According to the reported articles, there has been no report on the employment of a metal other than iron on the heterogeneous support of zeolite ZSM-5 in the quasi-EF process. Therefore, to completely comprehend the quasi-EF process’s functioning and how the active iridium phase influences the procedure’s effectiveness, the synthesis and evaluation of the iridium catalyst on the zeolite base have been discussed in this research.
2. Results
2.1. Characteristics of Catalyst
The XRD patterns related to both catalysts, including the parent and modified sample, are depicted in Figure 1. The catalysts contain the MFI structure of ZSM-5 type (JCPDS 0002-044-00), which matches well with the experimental results [55]. The lack of further peaks demonstrates that amorphous did not form in the original catalyst structure.
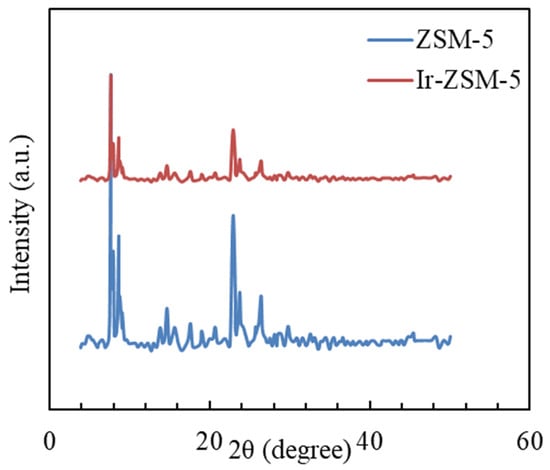
Figure 1.
XRD patterns related to original and modified catalysts.
The XRD analysis, shown in Figure 1, reveals that the relative crystallinity is determined by comparing the peak area at to that of the original nanocatalyst. To determine the total surface area (SBET) and volume (Vtotal), the BET iso-thermal equation and nitrogen adsorbed volume at were used. The micropore volume (Vmicro) was calculated using the t-plot method, whereas the mesopore volume (Vmeso) was obtained by subtracting the micropore data from the total data. According to Table 1, the relative crystallinity of the catalysts is high. The homogeneous distribution of the active site can be verified by the disappearance of further peaks (related to the promoter).

Table 1.
Crystallinity and textural properties for parent and modified catalysts.
FESEM images of the catalysts are demonstrated in Figure 2. Observation of the surface morphology of the catalysts reveals that they have a spherical shape. There is no significant morphological or structural degradation of the catalysts during the impregnating operation, as indicated by their identical surface morphology and particle size distribution, which agrees with XRD analysis results.
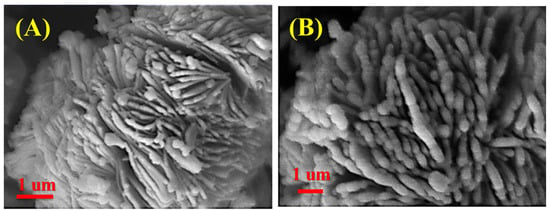
Figure 2.
FE-SEM pictures related to parent (A) and modified catalyst (B).
The adsorption–desorption isotherms for catalysts within the relative pressure range of 0–1 are depicted in Figure 3. When examining the isotherms, it should be noted that they comprise types I and IV isotherms. H4-type hysteresis circuits at moderately elevated pressures () provide evidence of mesoporous structure. The mesoporous structure is generated from the interconnection of crystals and, as a result, the space between the particles. The high nitrogen adsorption capacity at relatively reduced pressures () is indicative of the microporous structure [21]. The catalysts’ pore size spread in Figure 3 supports the mesoporous structure’s formation. The textural properties tabulated in Table 1 show the high specific surface area and the appearance of mesopores [56,57]. The destruction of micro-pores or pore blockage causes the modified catalyst’s Brunauer–Emmet–Teller (BET) surface area to be lowered. The mesopores that emerge during the impregnation procedure provide the modified catalyst with the greatest pore volume. Cabral de Menezes et al. [58] have documented how ZSM-5 impregnation causes dealumination and the creation of mesopores.
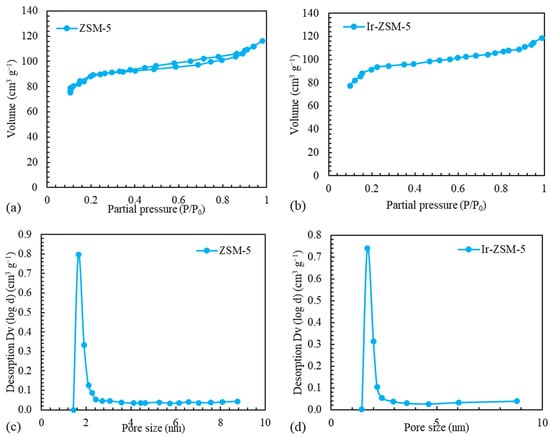
Figure 3.
The nitrogen adsorption–desorption of (a) original and (b) modified catalysts’ isotherms and distribution of pore sizes of (c) original and (d) modified catalyst.
Figure 4 displays the FT-IR spectra of two prepared samples with wave numbers between 1000 and 4000 . The absorption band within the range of (3500–3800) is ascribed to the surface functional groups of hydroxyls. The characteristic IR absorbance peaks located at approximate wavenumbers of 3610 and 3680 are attributed to the Si-OH-Al functional group’s vibrating [59]. The aluminum constituent of extra-framework (Al-OH) has led to an absorption peak at around 3680 [60,61].
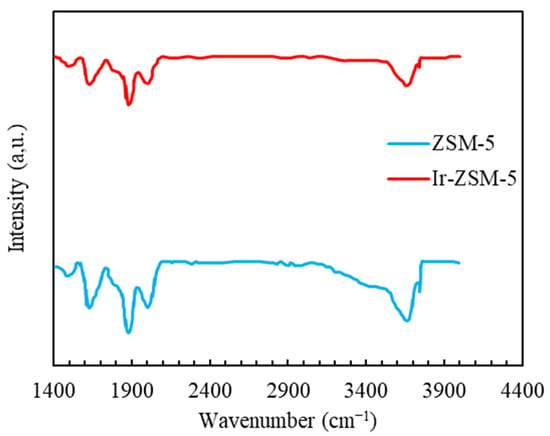
Figure 4.
FTIR spectra for both prepared samples within the wave number interval of 1000–4000 (cm−1).
The NH3-TPD analysis demonstrated a similar pattern except for the different values of acidic sites shown in Figure 5. Two peaks between 130 and 280 °C and 300 and 500 °C distinguish between the catalysts’ weak and powerful acid sites. The area of the peaks determines the concentration of acid sites, and their peak temperature indicates their intensity. The ZSM-5 catalyst contains equal amounts of powerful and weak acid sites (0.53 mmol of ammonia per gram). The results showed that the impregnation technique had caused a rise in the catalyst’s acidity, resulting from Ir molecules interacting with the zeolite framework. This behavior might be explained by closing pores and neutralizing acidic sites within the pores [62]. The smaller kinetic diameter of the Ir ion (close to 0.82 Å) allows it to enter the ZSM-5 channels (as much as 5.5 Å), leading to interactions with internal acidic sites. The acid-base titration for pristine and modified catalysts with Ir showed equal to 3.6 and 3.9, respectively. The increase in confirmed the results of NH3-TPD analysis that impregnation leads to a decrease in acidity. Ir promoter impregnation covers some acidic sites. The surface hydroxyl groups that act as Bronsted acid sites have a positive charge at and a negative charge at so that at various pH levels, they can influence the adsorption features of the produced catalysts.
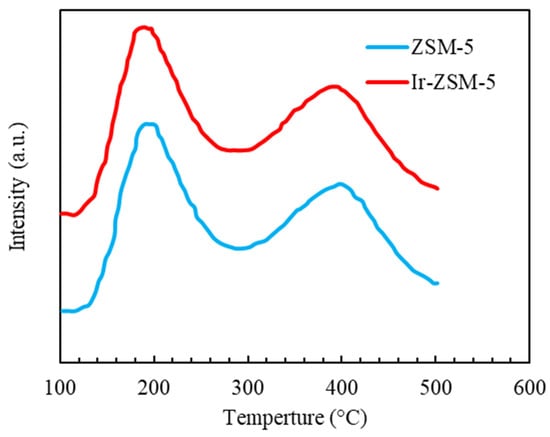
Figure 5.
NH3-TPD curves for both original and modified prepared samples.
2.2. Catalyst Performance
The experimental results of the experiments are statistically analyzed using ANOVA. The 2nd-degree model accurately models the results so that its p-value equals 0.2. The most significant effect is related to catalyst concentration and current intensity, which have F-value equal to 5.64 and 1.79, respectively. The findings revealed that the double interactions of pH parameters and catalyst concentration, current intensity, and catalyst concentration, and each parameter’s double interactions also influence the removal rate. It is essential to mention that the error rate of the findings is very low and can be ignored. Based on the results of the variance analysis, the p-value for the stated model of the regression is significant (p-value = 0.0257) and can predict results accurately. Moreover, the interaction between two parameters, including pH and the current between two electrodes, is of critical importance.
2.2.1. The Effect of Solution pH
The catalytic activity (the type of absorption and destruction mechanism) and, consequently, the pollutant removal percentage are drastically influenced by the reaction solution’s pH value. Therefore, choosing the optimal conditions for this parameter according to the type of pollutant and the catalyst framework is particularly important. The effect of pH level on the reaction has been investigated as an operational parameter in three levels, 2, 3, 4, 5, and 6, and the results are presented in Figure 6. In this figure, D is the percentage of removal, and it is defined in the following sections.
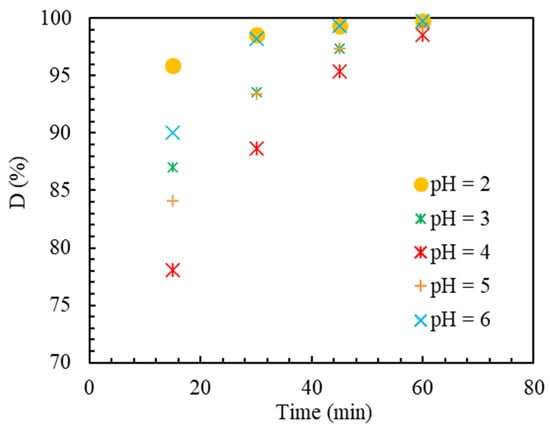
Figure 6.
Effectiveness of MB elimination at various pH values of applied current = 150 mA, Ir-ZSM-5 concentration of 0.5 (g/L).
The removal percentage at pH equal to 2 reached almost 101% in 60 min. According to the value of for MB substance which is equivalent to 3.8 [63], it can be said that at a pH level equal to 2, MB molecules are neutrally present in water. According to the results obtained from determining the characteristics of the catalyst and according to the value obtained for the Ir-ZSM-5 catalyst, which is equal to 3.9, it can be expected that around the catalyst particles, there is a state of positive charges at pH equal to 3. As a result, at this level of pH, it can be said that due to the neutrality of MB molecules and the positive charge of the surface of the particles, there is practically no electrostatic attraction and van der Waals interactions, hydrophobicity, and hydrogen bonding exist [63]. Therefore, the decrease in MB concentration can be related entirely to the occurrence of pseudo-EF reaction and anodic oxidation. In this case, molecules produced by the electrochemical method in the solution penetrate the catalyst network. After reacting with Ir components as an active site, they turn into and leave the zeolite network again, so they react with molecules of MB and destroy their structure [64]. Generally, a pH level equal to 2 is considered the most optimal level in the EF reaction [65]. This is due to various issues, such as the stability of , the stability of the catalyst’s active site on the support, and the non-formation of undesirable complexes usually formed at pH close to neutral [66]. According to Figure 6, it can be seen that the intensity of MB removal decreased with the increase of pH from 2 to 3. Considering that MB molecules have a positive charge at a pH level of 3 and catalytic particles have a negative charge (), it can be expected that electrostatic adsorption will occur, and MB molecules will be absorbed [67]. MB molecules are expected to be adsorbed on the surface of the catalyst or possibly inside the pores. In this case, some of the active sites are out of reach of molecules as a result of the absorption of MB molecules. Moreover, due to getting closer to neutral pH, molecules reach the instability threshold and decompose before reaching the active sites to produce through Equation (1). As a result, it is likely that the negative effect of decomposition overcomes the relative effect of absorption [68,69].
2.2.2. The Effect of Catalyst’s Concentration
The catalyst concentration in the pseudo-EF process is considered an operational parameter, and the effect of its different levels should be determined. As an operating parameter in the pseudo-EF reaction, one should be careful in choosing the optimal level to reduce costs and prevent the harmful effects of side reactions. To determine the impact of the catalyst in the pseudo-EF process to eliminate the MB material, it was performed using different amounts of the catalyst, and the results are demonstrated in Figure 7. It can be recognized from the graph that in the absence of the catalyst, the amount of MB removal is equal to 77.5%. This amount of removal can be related to the anodic oxidation reaction on the anode’s exterior [70]. In order to improve the removal percentage, the Ir-ZSM-5 catalyst was employed as a heterogeneous catalyst. Adding a concentration of 0.15 g/L of Ir-ZSM-5 catalyst shows a 23% increment in 60 min compared to the state without catalyst. This is because the quasi-EF reaction occurs in the reaction solution and the catalyst’s presence. The amount of elimination declines as catalyst concentration rises from 0.15 to 0.5 g/L. This can be justified by the destructive effects of the excess active phase of the catalyst on in the reaction environment [71,72]. Moreover, exposure to the catalyst’s active sites is restricted by the catalyst particles’ tendency to adhere together in high catalyst concentrations. The removal rate is slightly improved by increasing the catalyst concentration to 1 g/L. This can be interpreted by the electrostatic interaction between the catalyst surface and MB cationic molecules at pH = 3 [73]. By boosting the catalyst concentration to 1 g/L from 0.5, the positive impact of increasing the available surface, due to electrostatic or non-electrostatic interactions such as van der Waals or hydrogen, overcomes the destructive effect of the presence of excess catalytic active sites. According to the results obtained in this section, it can be said that the Ir-ZSM-5 catalyst had a significant effect in improving MB removal.
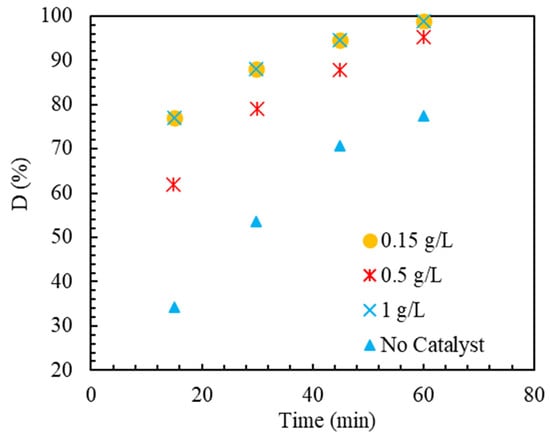
Figure 7.
MB removal performance with various concentrations of the catalyst Ir-ZSM-5. Reaction conditions: applied current = 250 mA, pH = 2.
2.2.3. The Effect of Current between Cathode and Anode
The passing current between two electrodes in the EF and quasi-EF processes is crucially important among the vital operating parameters. In this process, electrons react with air and water molecules, producing hydrogen peroxide. Finally, the hydrogen peroxide generated by the active phase of the catalyst is decomposed into hydroxyl, and the polluting substance’s oxidation process takes place. The effect of the electric current passing through two electrodes at three levels of 150, 250, and 350 mA on the amount of MB removal in the quasi-EF process was elucidated using an Ir-ZSM-5 catalyst, shown in Figure 8. By raising the current from 150 to 250 mA, the removal efficiency of MB does not change much. This is because is low due to the destructive effect of excess produced in the solution by the electrodes at high current values, as is shown in reaction 3 [74].
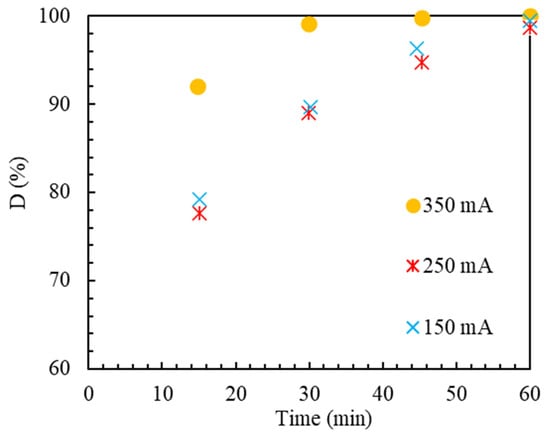
Figure 8.
MB elimination efficiency at varied applied currents. Reaction conditions: Ir-ZSM-5 concentration of 0.5 g/L, pH = 2.
However, by increasing the current from 250 to 350 mA, with respect to Figure 8, it is demonstrated that the removal efficiency has enhanced. This is a consequence of the catalyst’s active site regeneration improving with the current rise and, as a result, the increase in electrons in the solution [75]. Moreover, with the increment in the intensity of the passing current, a more considerable amount of MB is decomposed by anodic oxidation on the surface of the anode [76]. Hu et al. [77] investigated the effect of the flow rate on the removal rate. They have concluded that initially, a positive impact of the improvement in removal was observed with the enhancement in flow. However, they have stated that after a certain amount, any increase in the electric current leads to a decrease in the percentage of pollutant removal from the wastewater. It is worth noting that due to the electrode’s resistance and the anode’s erosive nature, using a very high current leads to the rapid destruction of the anode.
2.2.4. Ability to Reuse the Catalyst
From an economic standpoint, the durability and recyclability of the catalyst are crucial for expanding and boosting the color removal process’s economic effectiveness. For this purpose, the catalyst was tested consecutively in optimal conditions, including 0.1 g/L of catalyst, pH equal to 3, and current of 250 mA. After each use, centrifugation recovered the catalyst from the final solution. The catalyst was regenerated at T = 550 °C for 6 h with a temperature gradient of 3 °C/min to eliminate the absorbed organic species from the catalyst’s active sites. Figure 9 depicts both catalysts’ performances. During the regeneration process, the active sites of the catalyst are restored, which leads to the optimal performance of the catalyst in the second and third periods of use. The large total volume of the catalyst cavities as well as the specific surface area, which have facilitated the pollutant escape from the cavities and the reactivation of the active sites, can be used to clarify this phenomenon. The little decline in decolorization effectiveness for the reduced catalyst may be due to the active sites becoming poisoned by organic species adsorbing or the active sites becoming oxidized. The findings attest to the Ir-ZSM-5 catalyst’s consistent performance and reasonable capacity under multiple utilization.
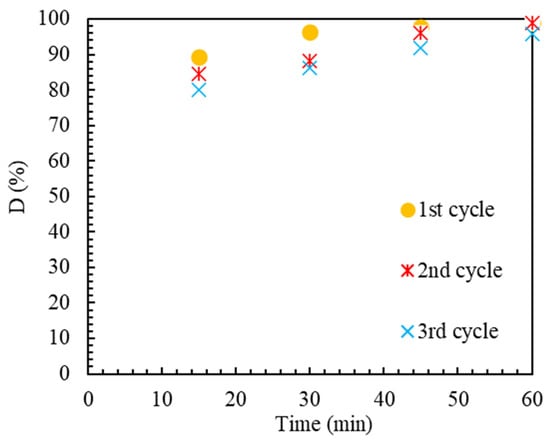
Figure 9.
Ir-ZSM-5 catalyst’s capacity for reprocessing to eliminate MB color. Reaction conditions: applied current = 150 mA, Ir-ZSM-5 concentration of 0.5 g/L, pH = 2.
3. Discussion
3.1. Kinetics of the Process
The reaction rate constant can be calculated using reaction kinetics, which is fundamental for modeling calculations and scale-ups. To obtain a kinetic model for modeling the behavior of the quasi-EF system, it is assumed that the removal of MB occurs more according to Equation (3).
Therefore, the rate of MB removal will be as follows:
In which is the concentration of MB. radicals are produced according to Equation (2), and Equations (2) and (5) are consumed by destructive properties [78]. Hence, the rate of changes in the concentration of radicals can be expressed as follows:
Many studies have considered the concentration of hydroxyl radicals. Therefore, according to Equation (6), we can write:
Therefore, by inserting Equation (7) into (4), we can write:
For high concentrations of MB, we will have the following:
which will lead to zero-order kinetics for the pseudo-EF system. However, for the effective removal of MB, the following can be assumed [78]:
Therefore, by some mathematical manipulations, we will have the following.
where is defined as Equation (13).
is equal to the apparent constant of the reaction intensity. Equation (15) obtains the pseudo-first-order kinetics of the EF system.
where is equal to the initial concentration of MB, and t is the reaction time. The results in Figure 10 indicate good cooperation between the laboratory data and the proposed model. Therefore, it can be said that the presented model is suitable for modeling the quasi-EF process catalyzed by Ir-ZSM-5.
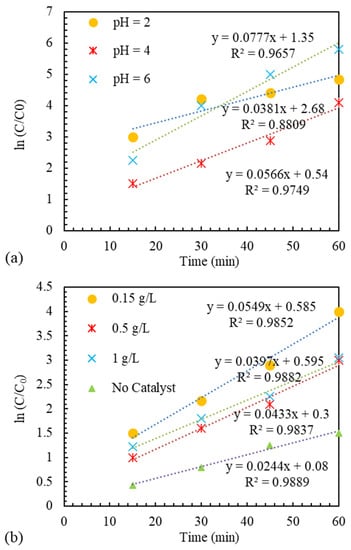
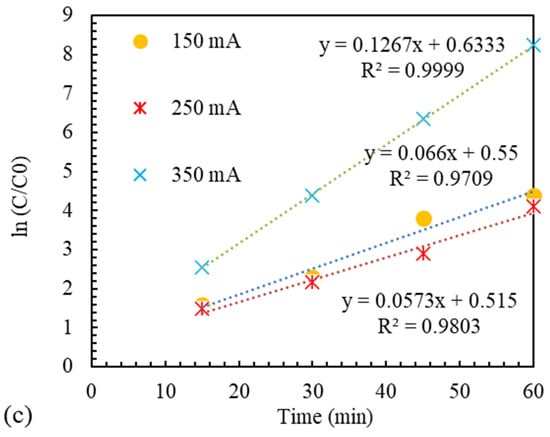
Figure 10.
Kinetics of MB color removal EF-like reaction catalyzed by Ir-ZSM-5 with various (a) pH, (b) catalyst concentration, and (c) current intensity.
3.2. Empirical Correlations and Optimization
In this section, a high-accuracy empirical correlation for the pollutant removal percentage has been presented. These models are based on pH, time, and concentration. The general polynomial equation of the models is presented as Equation (16).
The optimized values of pollutant removal percentage are presented in Figure 11 based on the correlation of Equation (16) and coefficients of Table 2.
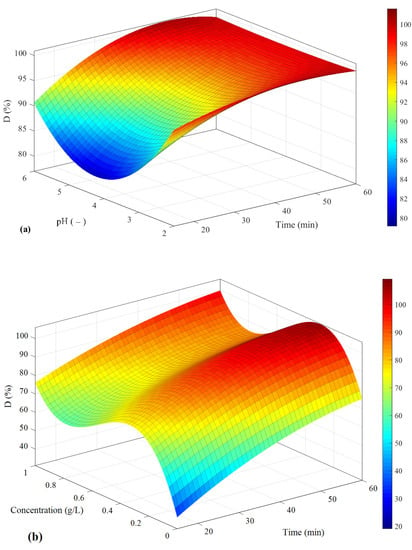
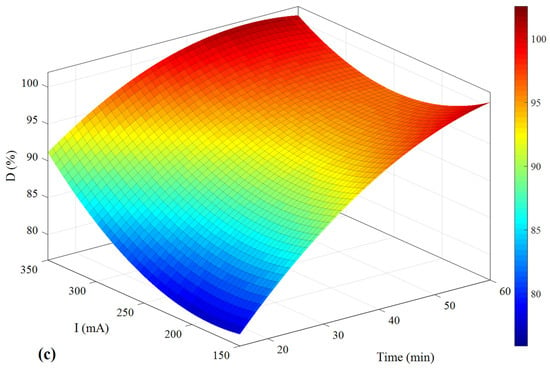
Figure 11.
The pollutant removal percentage based on (a) pH and time, (b) concentration and time, and (c) I and time.

Table 2.
The constant values for and the evaluation of the correlations using RSME and .
As can be seen in Figure 11a, in order to achieve the highest D, time should be allocated more than 50 min, but pH does not significantly affect the results. However, according to Figure 11b, in the case of concentration, the optimum value is around 0.3 g/L. Similar to the first analysis, the best results are observed within 60 min. Based on the results of Figure 11c, the highest value of D is observed when the electrical current is about 350 mA, and the time should be 50 min.
3.3. Artificial Neural Network
Carrying out experiments to determine the removal percentage could be time-consuming and costly. Therefore, researchers are always looking for new approaches to minimize the costs of experiments. As a result, the incorporation of machine learning algorithms has become prevalent. The use of the artificial neural network (ANN) in many engineering processes has recently increased. Consequently, in the present study, the percentage of removal is predicted by the ANN model. In order to achieve the most accurate ANN model, we have to test all the parameters and select the optimum one. This process is called hyperparameter tuning [79,80]. During this procedure, we used a different combination of hidden layers, batch sizes, numbers of epochs, and activation functions. The final model is presented in Table 3.

Table 3.
The settings for the final ANN model.
The total number of data gathered in the present study was 52; 36 were used to train the model, and the rest were utilized for testing. The model’s evaluation is based on mean absolute error and coefficient of determination [80]. The final model is presented in Figure 12.
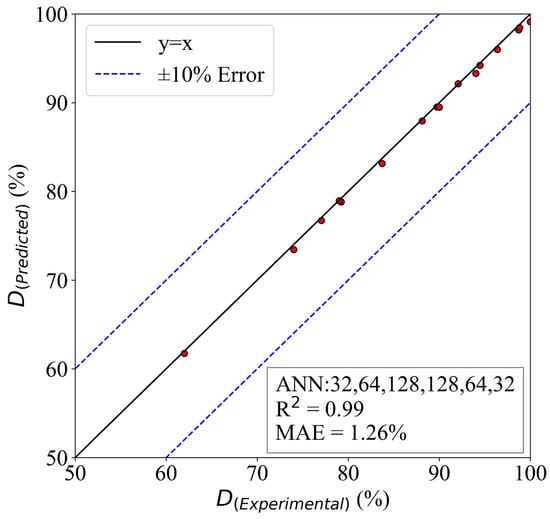
Figure 12.
The predictions of the proposed ANN model.
As can be seen, the model can predict the results with acceptable accuracy. The MAE of the model is 1.26%; this is a very reasonable error in a physically complicated problem. Moreover, the is 0.99, and this shows how capable the model is to predict the final results. Considering the accuracy and the computational costs of the deep learning model, it seems that using such algorithms to replace the conventional methods of research could be beneficial. With an abundance of data, using machine learning algorithms will be more prevalent in the future.
4. Materials and Methods
The materials utilized to construct the catalyst include tetrapropyl ammonium bromide (TPABr) (, >99 wt.%), silicic acid (, >99 wt.%), iridium chloride (, wt.% = 99%), sulfuric acid (, 98 wt.%), ammonium nitrate (, 99 wt.%), sodium hydroxide (NaOH, 99.6 wt.%), sodium aluminate (, wt.% = 55), and sodium sulfate (), all of which were purchased from Merck (Darmstadt, Germany). MB dye, as a model pollutant for use in the pseudo-EF procedure, was supplied by Merck (Darmstadt, Germany).
4.1. Catalyst Construction
The parent catalyst (HZM-5) was prepared with a ratio of Si/Al = 200 by hydrothermal strategy [60]. TPABr, sodium hydroxide, distilled water, and silicic acid were all introduced in the synthesis solution in the appropriate proportions. The solution was stirred for 3 h using a mechanical stirrer, and the solution’s pH level was regulated to 10.5 by employing the appropriate amount of sulfuric acid. The synthesis solution comprised , , , , and with the following molar ratios: 200:1.5:20:0.05:3. The crystallization occurred in a stainless-steel autoclave lined with Teflon at 180 °C for 48 h. The catalyst was filtered, washed, and dried at 105 °C overnight. The calcination procedure was performed inside an electric furnace for 12 h at a temperature gradient of 3 (°C/min). Na-ZSM-5 catalyst was exposed to an ion exchange process under vigorous agitation, with 1 M solution of at 90 °C for 10 h, and then washed three times and filtered. There were three iterations of this procedure. For the preparation of HZSM-5, powder was dried for 12 h at 105 °C, followed by calcination for 12 h at 530 °C at a rate of 3 °C/min. The modified catalyst was prepared by wet impregnating the parent catalyst with an iridium promoter. Wet impregnation was carried out in an evaporator under vacuum and during the following five steps: (1) and pressure of 300 mmHg for 10 min; (2) and for 30 min; (3) and for 30 min; (4) and for 30 min; and (5) and for 30 min. First, 75 mL of distilled water was used to dissolve a predetermined amount of iridium and introduce it into the parent catalyst. Dehydrated impregnated catalysts were calcined at 501 °C for 12 h under airflow after they had been impregnated for 12 h at 105 °C. Based on the weight of the catalyst, the modified case contained 0.5 wt.% of iridium promoter.
4.2. Characterization
Both quantitatively and qualitatively, the composition and structure of parent and modified catalysts were assessed by employing a variety of characterization strategies. To investigate the crystallographic structure of samples, utilizing a (PHILIPS PW1730, Amsterdam, The Netherlands) with Cu-K radiation (λ = 1.54 Å) as an X-ray source in the interval from 2 to 50° at a scan speed of 2° min−1, X-ray diffraction spectra of the parent and modified catalysts were obtained. The synthesized catalysts’ morphology was analyzed utilizing a field emission scanning electron microscope (FESEM) (TESCAN MIRA III, Brno, Czech Republic). In order to identify the functional groups, the Fourier transform infrared (FTIR) spectra were captured on an (EQUINOX 55, Bruker Optics, Ettlingen, Germany) employing KBr pellets as the substrate at room temperature within the wavenumber range of 1000–4000 cm−1. With a BELSORP MINI II analyzer operating at 77 K, N2 sorption isotherms were obtained (BEL Japan, Inc., Osaka, Japan). NH3-TPD was performed, respectively, to determine the total amount of primary and acidic sites on the surface of the catalysts, utilizing an instrument (BELCAT, Osaka, Japan). Helium was utilized as the carrier gas, and a system with a thermal conductivity analyzer was employed to measure TPD.
4.3. The Process of Quasi-EF
The synthesized catalyst’s performance was investigated for removing environmental pollutants during a quasi-EF discontinuous process. Experiments were determined using the Box–Behnken test design method. The investigated parameters included pH (2, 4, and 6), current intensity (150, 250, and 350 mA), and catalyst concentration (0.15, 0.5, and 1 g/L). The process container had a 50 mL capacity, and the sodium sulfate electrolyte solution concentration in the reaction medium was 0.5 M. Graphite electrodes were connected to the edge of the reaction vessel. They provided the current required for the quasi-EF reaction. According to the operating conditions, an electric current was provided between the two electrodes. The solution’s pH was modulated through sulfuric acid according to the working conditions. A magnetic stirrer continuously stirred the reaction medium. The oxygen required for the reaction was supplied by injecting ambient air. Before applying the current, the reaction environment was oxygen-saturated for five minutes. Then, an electric current was established between the electrodes, which led to hydrogen peroxide production on the cathode’s surface. Water samples were taken every 15 min and analyzed by spectrometry (maximum wavelength equal to 665 nm). The amount of light absorption obtained for each sample was converted to concentration using the calibration curve. The pollutant removal percentage can be obtained based on the following equation:
in which D is the percentage of removal. and are the initial concentration of the pollutant in the effluent and the final concentration in the sample, respectively.
5. Conclusions
The present study investigated the decolorization of wastewater containing MB using the Ir-ZSM 5 catalyst in the quasi-EF process. The ZSM-5 catalyst was prepared by hydrothermal technique and afterward impregnated with Ir metal as the active phase. Catalyst characterization confirmed high crystallinity, good distribution of active phase in the catalyst structure, and high specific surface. In a pH level of 2, catalyst concentration of 0.15 g/L, and current of 150 mA, the best MB removal efficiency (101%) was obtained. By examining the kinetics of the reaction, the pseudo-first-order model obtained was consistent with the experimental data. The reusability of the Ir-ZSM-5 catalyst can be related to the suitable structural characteristics, which resulted in high MB removal efficiency in the second and third rounds of the process. Based on a pseudo-EF technique, the findings showed the considerable potential of the synthesized catalyst for the efficient elimination of MB dye over 60 min. Moreover, by using the data from the experiments, we proposed a machine-learning-based model to predict the percentage of removal. The results of the predictions were promising. The MAE of the model is 1.26%, and the coefficient of determination is 0.99. The high capabilities of machine learning in predicting complicated problems and the time-saving they offer would definitely make them more popular in the future. As a future study, authors could gather the available data in wastewater containing MB and utilize various models of machine learning to obtain the best result. This new model could be generalized or even help avoid any other experiments with the same intent.
Author Contributions
Conceptualization, A.E.J. and M.S.; Data curation, U.R.S. and A.E.; Formal analysis, J.C.O.G.; Investigation, A.E.; Methodology, A.E.J., M.A., U.R.S., J.C.O.G., A.E. and S.S.S.; Resources, M.A.; Software, A.E.J. and M.A.; Supervision, M.S. and H.H.T.; Validation, A.E.J., J.C.O.G., M.S. and S.S.S.; Visualization, S.S.S. and H.H.T.; Writing—original draft, A.E.J.; Writing—review and editing, U.R.S. and H.H.T. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Deanship of Scientific Research at King Khalid University under grant number RGP.2/57/44.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Acknowledgments
This work was supported by King Khalid University, Abha, Saudi Arabia. The authors extend their appreciation to the Deanship of Scientific Research at King Khalid University for funding this work through Large Groups Project under grant number (R.G.P. 2/57/44).
Conflicts of Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Cheikh, S.; Imessaoudene, A.; Bollinger, J.C.; Hadadi, A.; Manseri, A.; Bouzaza, A.; Assadi, A.; Amrane, A.; Zamouche, M.; El Jery, A.; et al. Complete Elimination of the Ciprofloxacin Antibiotic from Water by the Combination of Adsorption–Photocatalysis Process Using Natural Hydroxyapatite and TiO2. Catalysts 2023, 13, 336. [Google Scholar] [CrossRef]
- Guo, Z.; Zhan, R.; Shi, Y.; Zhu, D.; Pan, J.; Yang, C.; Wang, Y.; Wang, J. Innovative and green utilization of zinc-bearing dust by hydrogen reduction: Recovery of zinc and lead, and synergetic preparation of Fe/C micro-electrolysis materials. Chem. Eng. J. 2023, 456, 141157. [Google Scholar] [CrossRef]
- Wang, H.; Gong, C.; Zhou, Z.; Zhou, Q.; Liu, Y.; Luo, J. Chiral 1, 2-diaminocyclohexane-α-amino ac-id-derived amidphos/AG (Ⅰ)-catalyzed divergent enantioselective 1, 3-dipolar cycloaddition of azomethine ylides. Heterocycles Int. J. Rev. Commun. Heterocycl. Chem. 2022, 104, 123–139. [Google Scholar]
- Alimoradi, H.; Shams, M.; Ashgriz, N. Enhancement in the pool boiling heat transfer of copper surface by applying electrophoretic deposited graphene oxide coatings. Int. J. Multiph. Flow 2023, 159, 104350. [Google Scholar] [CrossRef]
- Collivignarelli, M.C.; Abbà, A.; Miino, M.C.; Arab, H.; Bestetti, M.; Franz, S. Decolorization and biodegradability of a real pharmaceutical wastewater treated by H2O2-assisted photoelectrocatalysis on TiO2 meshes. J. Hazard. Mater. 2020, 387, 121668. [Google Scholar] [CrossRef]
- Li, H.; Zhao, S.; Zhang, W.; Du, H.; Yang, X.; Peng, Y.; Han, D.; Wang, B.; Li, Z. Efficient esterification over hierarchical Zr-Beta zeolite synthesized via liquid-state ion-exchange strategy. Fuel 2023, 342, 127786. [Google Scholar] [CrossRef]
- Li, G.; Huang, S.; Li, K.; Zhu, N.; Zhao, B.; Zhong, Q.; Zhang, Z.; Ge, D.; Wang, D. Near-infrared responsive Z-scheme heterojunction with strong stability and ultra-high quantum efficiency constructed by lanthanide-doped glass. Appl. Catal. B Environ. 2022, 311, 121363. [Google Scholar] [CrossRef]
- Alimoradi, H.; Shams, M.; Ashgriz, N. Bubble behavior and nucleation site density in subcooled flow boiling using a novel method for simulating the microstructure of surface roughness. Korean J. Chem. Eng. 2022, 39, 2945–2958. [Google Scholar] [CrossRef]
- Hao, O.J.; Kim, H.; Chiang, P.-C. Decolorization of Wastewater. Crit. Rev. Environ. Sci. Technol. 2000, 30, 449–505. [Google Scholar] [CrossRef]
- Liang, Y.; Li, J.; Xue, Y.; Tan, T.; Jiang, Z.; He, Y.; Shangguan, W.; Yang, J.; Pan, Y. Benzene decomposition by non-thermal plasma: A detailed mechanism study by synchrotron radiation photoionization mass spectrometry and theoretical calculations. J. Hazard. Mater. 2021, 420, 126584. [Google Scholar] [CrossRef]
- Xia, G.; Zheng, Y.; Sun, Z.; Xia, S.; Ni, Z.; Yao, J. Fabrication of ZnAl-LDH mixed metal-oxide composites for photocatalytic degradation of 4-chlorophenol. Environ. Sci. Pollut. Res. 2022, 29, 39441–39450. [Google Scholar] [CrossRef] [PubMed]
- Zaboli, S.; Alimoradi, H.; Shams, M. Numerical investigation on improvement in pool boiling heat transfer characteristics using different nanofluid concentrations. J. Therm. Anal. Calorim. 2022, 147, 10659–10676. [Google Scholar] [CrossRef]
- Zhang, L.-C.; Jia, Z.; Lyu, F.; Liang, S.-X.; Lu, J. A review of catalytic performance of metallic glasses in wastewater treatment: Recent progress and prospects. Prog. Mater. Sci. 2019, 105, 100576. [Google Scholar] [CrossRef]
- Wei, S.; Chen, T.; Hou, H.; Xu, Y. Recent advances in electrochemical sterilization. J. Electroanal. Chem. 2023, 937, 117419. [Google Scholar] [CrossRef]
- Geng, C.; Zhao, F.; Niu, H.; Zhang, J.; Dong, H.; Li, Z.; Chen, H. Enhancing the permeability, anti-biofouling performance and long-term stability of TFC nanofiltration membrane by imidazole-modified carboxylated graphene oxide/polyethersulfone substrate. J. Membr. Sci. 2022, 664, 121099. [Google Scholar] [CrossRef]
- Alimoradi, H.; Zaboli, S.; Shams, M. Numerical simulation of surface vibration effects on improvement of pool boiling heat transfer characteristics of nanofluid. Korean J. Chem. Eng. 2022, 39, 69–85. [Google Scholar] [CrossRef]
- Alvarez, L.H.; Perez-Cruz, M.A.; Rangel-Mendez, J.R.; Cervantes, F.J. Immobilized redox mediator on metal-oxides nanoparticles and its catalytic effect in a reductive decolorization process. J. Hazard. Mater. 2010, 184, 268–272. [Google Scholar] [CrossRef]
- Tang, X.; Ye, J.; Guo, L.; Pu, T.; Cheng, L.; Cao, X.; Guo, Y.; Wang, L.; Guo, Y.; Zhan, W.; et al. Atomic Insights into the Cu Species Supported on Zeolite for Direct Oxidation of Methane to Methanol via Low-Damage HAADF-STEM. Adv. Mater. 2023, 35, 2208504. [Google Scholar] [CrossRef]
- Chen, D.; Savidge, T. Comment on “Extreme electric fields power catalysis in the active site of ketosteroid isomerase”. Science 2015, 349, 936. [Google Scholar] [CrossRef]
- Alimoradi, H.; Shams, M.; Valizadeh, Z. The effects of nanoparticles in the subcooled boiling flow in the channels with different cross-sectional area and same hydraulic diameter. Modares Mech. Eng. 2017, 16, 545–554. [Google Scholar]
- Mahboub, M.J.D.; Ahmadpour, A.; Rashidi, H. Improving methane storage on wet activated carbons at various amounts of water. J. Fuel Chem. Technol. 2012, 40, 385–389. [Google Scholar] [CrossRef]
- Zuo, L.; Yu, S.; Zhang, R.; Li, H.; Wu, Y.; Abiev, R.; Sun, Z.; Sun, Z. Tunning Pd–Cu-based catalytic oxygen carrier for intensifying low-temperature methanol reforming. J. Clean. Prod. 2023, 410, 137212. [Google Scholar] [CrossRef]
- Rashid, T.; Iqbal, D.; Hazafa, A.; Hussain, S.; Sher, F.; Sher, F. Formulation of zeolite supported nano-metallic catalyst and applications in textile effluent treatment. J. Environ. Chem. Eng. 2020, 8, 104023. [Google Scholar] [CrossRef]
- Pang, S.; Zhou, C.; Sun, Y.; Zhang, K.; Ye, W.; Zhao, X.; Cai, L.; Hui, B. Natural wood-derived charcoal embedded with bimetallic iron/cobalt sites to promote ciprofloxacin degradation. J. Clean. Prod. 2023, 414, 137569. [Google Scholar] [CrossRef]
- de Souza, C.C.; Oliveira, D.; Silva, S.V.; de Souza, I.F.M.; de Melo, I.M.M.; Moreira, C.R.; da Silva, E.F.; de Oliveira, M.A.; Bezerra, A.C.D.S.; Machado, A.R.T. Activated carbon impregnated with copper to remove l-cysteine in an aqueous medium. Int. J. Environ. Sci. Technol. 2021, 18, 809–818. [Google Scholar] [CrossRef]
- Alimoradi, H.; Shams, M. Numerical simulation of the effects of surface roughness on nucleation site density of nanofluid boiling. Modares Mech. Eng. 2019, 19, 1613–1622. [Google Scholar]
- Ejhieh, A.N.; Khorsandi, M. Photodecolorization of Eriochrome Black T using NiS–P zeolite as a heterogeneous catalyst. J. Hazard. Mater. 2010, 176, 629–637. [Google Scholar] [CrossRef]
- Ge, D.; Yuan, H.; Xiao, J.; Zhu, N. Insight into the enhanced sludge dewaterability by tannic acid conditioning and pH regulation. Sci. Total Environ. 2019, 679, 298–306. [Google Scholar] [CrossRef]
- Bai, B.; Bai, F.; Nie, Q.; Jia, X. A high-strength red mud–fly ash geopolymer and the implications of curing temperature. Powder Technol. 2023, 416, 118242. [Google Scholar] [CrossRef]
- Chamgordani, M.A. The Entanglement Properties of Superposition of Fermionic Coherent States. Int. J. Theor. Phys. 2022, 61, 33. [Google Scholar] [CrossRef]
- Wang, G.; Huang, R.; Zhou, A.; Xu, Q. Degradation of methyl orange by a new catalyst glyphosate ferrous. Solid State Sci. 2019, 95, 105933. [Google Scholar] [CrossRef]
- Wang, Z.; Hu, L.; Zhao, M.; Dai, L.; Hrynsphan, D.; Tatsiana, S.; Chen, J. Bamboo charcoal fused with polyurethane foam for efficiently removing organic solvents from wastewater: Experimental and simulation. Biochar 2022, 4, 28. [Google Scholar] [CrossRef]
- Bordbar, M.; Naderi, N.; Chamgordani, M.A. The relation of entanglement to the number of qubits and interactions between them for different graph states. Indian J. Phys. 2021, 95, 901–909. [Google Scholar] [CrossRef]
- Hammood, Z.A.; Chyad, T.F.; Al-Saedi, R. Adsorption Performance of Dyes Over Zeolite for Textile Wastewater Treatment. Ecol. Chem. Eng. S 2021, 28, 329–337. [Google Scholar] [CrossRef]
- Wang, Z.; Dai, L.; Yao, J.; Guo, T.; Hrynsphan, D.; Tatsiana, S.; Chen, J. Enhanced adsorption and reduction performance of nitrate by Fe–Pd–Fe3O4 embedded multi-walled carbon nanotubes. Chemosphere 2021, 281, 130718. [Google Scholar] [CrossRef] [PubMed]
- Naderi, N.; Bordbar, M.; Hasanvand, F.K.; Chamgordani, M.A. Influence of inhomogeneous magnetic field on the qutrit teleportation via XXZ Heisenberg chain under intrinsic decoherence. Optik 2021, 247, 167948. [Google Scholar] [CrossRef]
- Yu, F.; Wang, L.; Ma, H.; Pan, Y. Zeolitic imidazolate framework-8 modified active carbon fiber as an efficient cathode in electro-Fenton for tetracycline degradation. Sep. Purif. Technol. 2020, 237, 116342. [Google Scholar] [CrossRef]
- Wang, H.-C.; Liu, Y.; Yang, Y.-M.; Fang, Y.-K.; Luo, S.; Cheng, H.-Y.; Wang, A.-J. Element sulfur-based autotrophic denitrification constructed wetland as an efficient approach for nitrogen removal from low C/N wastewater. Water Res. 2022, 226, 119258. [Google Scholar] [CrossRef]
- Wang, Z.; Chen, C.; Liu, H.; Hrynshpan, D.; Savitskaya, T.; Chen, J.; Chen, J. Enhanced denitrification performance of Alcaligenes sp. TB by Pd stimulating to produce membrane adaptation mechanism coupled with nanoscale zero-valent iron. Sci. Total Environ. 2020, 708, 135063. [Google Scholar] [CrossRef]
- Sapawe, N. Effective solar-based iron oxide supported HY zeolite catalyst for the decolorization of organic and simulated dyes. New J. Chem. 2015, 39, 6377–6387. [Google Scholar] [CrossRef]
- Liu, W.; Huang, F.; Liao, Y.; Zhang, J.; Ren, G.; Zhuang, Z.; Zhen, J.; Lin, Z.; Wang, C. Treatment of CrVI -Containing Mg(OH)2 Nanowaste. Angew. Chem. 2008, 47, 5619–5622. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Liu, X.; Ni, S.-Q.; Zhuang, X.; Lee, T. Nano zero-valent iron improves anammox activity by promoting the activity of quorum sensing system. Water Res. 2021, 202, 117491. [Google Scholar] [CrossRef] [PubMed]
- Dükkancı, M.; Gündüz, G.; Yılmaz, S.; Prihod’ko, R. Heterogeneous Fenton-like degradation of Rhodamine 6G in water using CuFeZSM-5 zeolite catalyst prepared by hydrothermal synthesis. J. Hazard. Mater. 2010, 181, 343–350. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Li, Q.; Cui, Q.; Ni, S.-Q. Nitrogen recovery through fermentative dissimilatory nitrate reduction to ammonium (DNRA): Carbon source comparison and metabolic pathway. Chem. Eng. J. 2022, 441, 135938. [Google Scholar] [CrossRef]
- Le, T.X.H.; Drobek, M.; Bechelany, M.; Motuzas, J.; Julbe, A.; Cretin, M. Application of Fe-MFI zeolite catalyst in heterogeneous electro-Fenton process for water pollutants abatement. Microporous Mesoporous Mater. 2019, 278, 64–69. [Google Scholar] [CrossRef]
- Oturan, N.; Zhou, M.; Oturan, M.A. Metomyl Degradation by Electro-Fenton and Electro-Fenton-Like Processes: A Kinetics Study of the Effect of the Nature and Concentration of Some Transition Metal Ions As Catalyst. J. Phys. Chem. A 2010, 114, 10605–10611. [Google Scholar] [CrossRef]
- Zhou, H.; Su, Y.; Liao, W.; Deng, W.; Zhong, F. NO reduction by propane over monolithic cordierite-based Fe/Al2O3 catalyst: Reaction mechanism and effect of H2O/SO2. Fuel 2016, 182, 352–360. [Google Scholar] [CrossRef]
- Li, S. Efficient algorithms for scheduling equal-length jobs with processing set restrictions on uniform parallel batch machines. Math. Biosci. Eng. 2022, 19, 10731–10740. [Google Scholar] [CrossRef]
- Xie, R.; Liu, G.; Liu, D.; Liang, S.; Lei, D.; Dong, H.; Huang, H.; Leung, D.Y. Wet scrubber coupled with heterogeneous UV/Fenton for enhanced VOCs oxidation over Fe/ZSM-5 catalyst. Chemosphere 2019, 227, 401–408. [Google Scholar] [CrossRef]
- Askari, R.; Afshin, S.; Rashtbari, Y.; Moharrami, A.; Mohammadi, F.; Vosuoghi, M.; Dargahi, A. Synthesis of activated carbon from walnut wood and magnetized with cobalt ferrite (CoFe2O4) and its application in removal of cephalexin from aqueous solutions. J. Dispers. Sci. Technol. 2021, 44, 1183–1194. [Google Scholar] [CrossRef]
- Aghakhani, M.; Naderian, P. Modeling and optimization of dilution in SAW in the presence of Cr2O3 nano-particles. Int. J. Adv. Manuf. Technol. 2015, 78, 1665–1676. [Google Scholar] [CrossRef]
- Naderian, P.; Aghakhani, M.; Khoshboo, S. Modelling the hardness of weld metal in the submerged arc welding of low carbon steel plates: Addition of CR2O3 nanoparticles. Adv. Mater. Process. Technol. 2022, 9, 221–236. [Google Scholar] [CrossRef]
- Sun, S.; Liu, H.; Zhang, J.; Wang, W.; Xu, P.; Zhu, X.; Wang, Y.; Wan, S. Application of a novel coagulant in reservoir water treatment in Qingdao. Desalination Water Treat. 2023, 284, 49–60. [Google Scholar] [CrossRef]
- Shen, J.; Li, Y.; Zhu, Y.; Hu, Y.; Li, C. Aerosol synthesis of Graphene-Fe3O4 hollow hybrid microspheres for heterogeneous Fenton and electro-Fenton reaction. J. Environ. Chem. Eng. 2016, 4, 2469–2476. [Google Scholar] [CrossRef]
- Rostamizadeh, M.; Yaripour, F.; Hazrati, H. Ni-doped high silica HZSM-5 zeolite (Si/Al = 200) nanocatalyst for the selective production of olefins from methanol. J. Anal. Appl. Pyrolysis 2018, 132, 1–10. [Google Scholar] [CrossRef]
- Horikawa, T.; Katoh, M.; Tomida, T. Preparation and characterization of nitrogen-doped mesoporous titania with high specific surface area. Microporous Mesoporous Mater. 2008, 110, 397–404. [Google Scholar] [CrossRef]
- Kruk, M.; Kohlhaas, K.M.; Dufour, B.; Celer, E.B.; Jaroniec, M.; Matyjaszewski, K.; Ruoff, R.S.; Kowalewski, T. Partially graphitic, high-surface-area mesoporous carbons from polyacrylonitrile templated by ordered and disordered mesoporous silicas. Microporous Mesoporous Mater. 2007, 102, 178–187. [Google Scholar] [CrossRef]
- Cabral de Menezes, S.M.; Lam, Y.; Damodaran, K.; Pruski, M. Modification of H-ZSM-5 zeolites with phosphorus. 1. Identification of aluminum species by 27Al solid-state NMR and characterization of their catalytic properties. Microporous Mesoporous Mater. 2006, 95, 286–295. [Google Scholar] [CrossRef]
- Jentys, A.; Warecka, G.; Derewinski, M.; Lercher, J.A. Adsorption of water on ZSM 5 zeolites. J. Phys. Chem. 1989, 93, 4837–4843. [Google Scholar] [CrossRef]
- Gorzin, F.; Darian, J.T.; Yaripour, F.; Mousavi, S.M. Preparation of hierarchical HZSM-5 zeolites with combined desilication with NaAlO2/tetrapropylammonium hydroxide and acid modification for converting methanol to propylene. RSC Adv. 2018, 8, 41131–41142. [Google Scholar] [CrossRef]
- Campbell, S.M.; Jiang, X.-Z.; Howe, R.F. Methanol to hydrocarbons: Spectroscopic studies and the significance of extra-framework aluminium. Microporous Mesoporous Mater. 1999, 29, 91–108. [Google Scholar] [CrossRef]
- Shah, A.K.; Shah, G.T.; Shah, A.A.; Park, Y.H.; Shah, A.A.; Choi, M.; Ahmed, S.; Bukhari, S.N.S.; Chandio, A.D.; Mahar, M.A.; et al. Design of Nickel Supported Hierarchical ZSM-5/USY Zeolite Bifunctional Catalysts for One-Pot Menthol Synthesis via Liquid-Phase Citral Hydrogenation. Molecules 2023, 28, 743. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.R.; Santiano, B.; Kim, H.; Kan, E. Heterogeneous Oxidation of Methylene Blue with Surface-Modified Iron-Amended Activated Carbon. Am. J. Anal. Chem. 2013, 4, 115–122. [Google Scholar] [CrossRef]
- Spagnoli, A.A.; Giannakoudakis, D.A.; Bashkova, S. Adsorption of methylene blue on cashew nut shell based carbons activated with zinc chloride: The role of surface and structural parameters. J. Mol. Liq. 2017, 229, 465–471. [Google Scholar] [CrossRef]
- Basaleh, A.A.; Al-Malack, M.H.; Saleh, T.A. Methylene Blue removal using polyamide-vermiculite nanocomposites: Kinetics, equilibrium and thermodynamic study. J. Environ. Chem. Eng. 2019, 7, 103107. [Google Scholar] [CrossRef]
- Nidheesh, P.V.; Olvera-Vargas, H.; Oturan, N.; Oturan, M.A. Heterogeneous Electro-Fenton Process: Principles and Applications. In Electro-Fenton Process; Springer: Singapore, 2017; pp. 85–110. [Google Scholar] [CrossRef]
- Neamţu, M.; Catrinescu, C.; Kettrup, A. Effect of dealumination of iron(III)—Exchanged Y zeolites on oxidation of Reactive Yellow 84 azo dye in the presence of hydrogen peroxide. Appl. Catal. B Environ. 2004, 51, 149–157. [Google Scholar] [CrossRef]
- Nidheesh, P.; Zhou, M.; Oturan, M.A. An overview on the removal of synthetic dyes from water by electrochemical advanced oxidation processes. Chemosphere 2018, 197, 210–227. [Google Scholar] [CrossRef] [PubMed]
- Nidheesh, P.; Gandhimathi, R. Trends in electro-Fenton process for water and wastewater treatment: An overview. Desalination 2012, 299, 1–15. [Google Scholar] [CrossRef]
- Zazou, H.; Oturan, N.; Zhang, H.; Hamdani, M.; Oturan, M.A. Comparative study of electrochemical oxidation of herbicide 2,4,5-T: Kinetics, parametric optimization and mineralization pathway. Sustain. Environ. Res. 2017, 27, 15–23. [Google Scholar] [CrossRef]
- Ganiyu, S.O.; Zhou, M.; Martínez-Huitle, C.A. Heterogeneous electro-Fenton and photoelectro-Fenton processes: A critical review of fundamental principles and application for water/wastewater treatment. Appl. Catal. B Environ. 2018, 235, 103–129. [Google Scholar] [CrossRef]
- Gogoi, A.; Navgire, M.; Sarma, K.C.; Gogoi, P. Fe3O4-CeO2 metal oxide nanocomposite as a Fenton-like heterogeneous catalyst for degradation of catechol. Chem. Eng. J. 2017, 311, 153–162. [Google Scholar] [CrossRef]
- Salem Attia, T.M.; Hu, X.L.; Yin, D.Q. Synthesized magnetic nanoparticles coated zeolite for the adsorption of pharmaceutical compounds from aqueous solution using batch and column studies. Chemosphere 2013, 93, 2076–2085. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Zheng, T.; Zhang, G.; Wang, P. A review on Fenton-like processes for organic wastewater treatment. J. Environ. Chem. Eng. 2016, 4, 762–787. [Google Scholar] [CrossRef]
- Qiang, Z.; Chang, J.-H.; Huang, C.-P. Electrochemical regeneration of Fe2+ in Fenton oxidation processes. Water Res. 2003, 37, 1308–1319. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Tan, Y.; Pan, Y.; Zhen, G.; Lu, X.; Song, Y.; Zhao, Y.; Ushani, U.K. Altering Extracellular Biopolymers and Water Distribution of Waste Activated Sludge by Fe(II) Persulfate Oxidation with Natural Zeolite and Polyelectrolyte as Skeleton Builders for Positive Feedbacks to Dewaterability. ACS Sustain. Chem. Eng. 2019, 7, 16549–16559. [Google Scholar] [CrossRef]
- Hou, B.; Han, H.; Jia, S.; Zhuang, H.; Xu, P.; Wang, D. Heterogeneous electro-Fenton oxidation of catechol catalyzed by nano-Fe3O4: Kinetics with the Fermi’s equation. J. Taiwan Inst. Chem. Eng. 2015, 56, 138–147. [Google Scholar] [CrossRef]
- Babuponnusami, A.; Muthukumar, K. Advanced oxidation of phenol: A comparison between Fenton, electro-Fenton, sono-electro-Fenton and photo-electro-Fenton processes. Chem. Eng. J. 2012, 183, 1–9. [Google Scholar] [CrossRef]
- Alimoradi, H.; Eskandari, E.; Pourbagian, M.; Shams, M. A parametric study of subcooled flow boiling of Al2O3/water nanofluid using numerical simulation and artificial neural networks. Nanoscale Microscale Thermophys. Eng. 2022, 26, 129–159. [Google Scholar] [CrossRef]
- Eskandari, E.; Alimoradi, H.; Pourbagian, M.; Shams, M. Numerical investigation and deep learning-based prediction of heat transfer characteristics and bubble dynamics of subcooled flow boiling in a vertical tube. Korean J. Chem. Eng. 2022, 39, 3227–3245. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).