Synthesis of Palladium Nanoparticles Supported over Fused Graphene-like Material for Hydrogen Evolution Reaction
Abstract
1. Introduction
2. Results
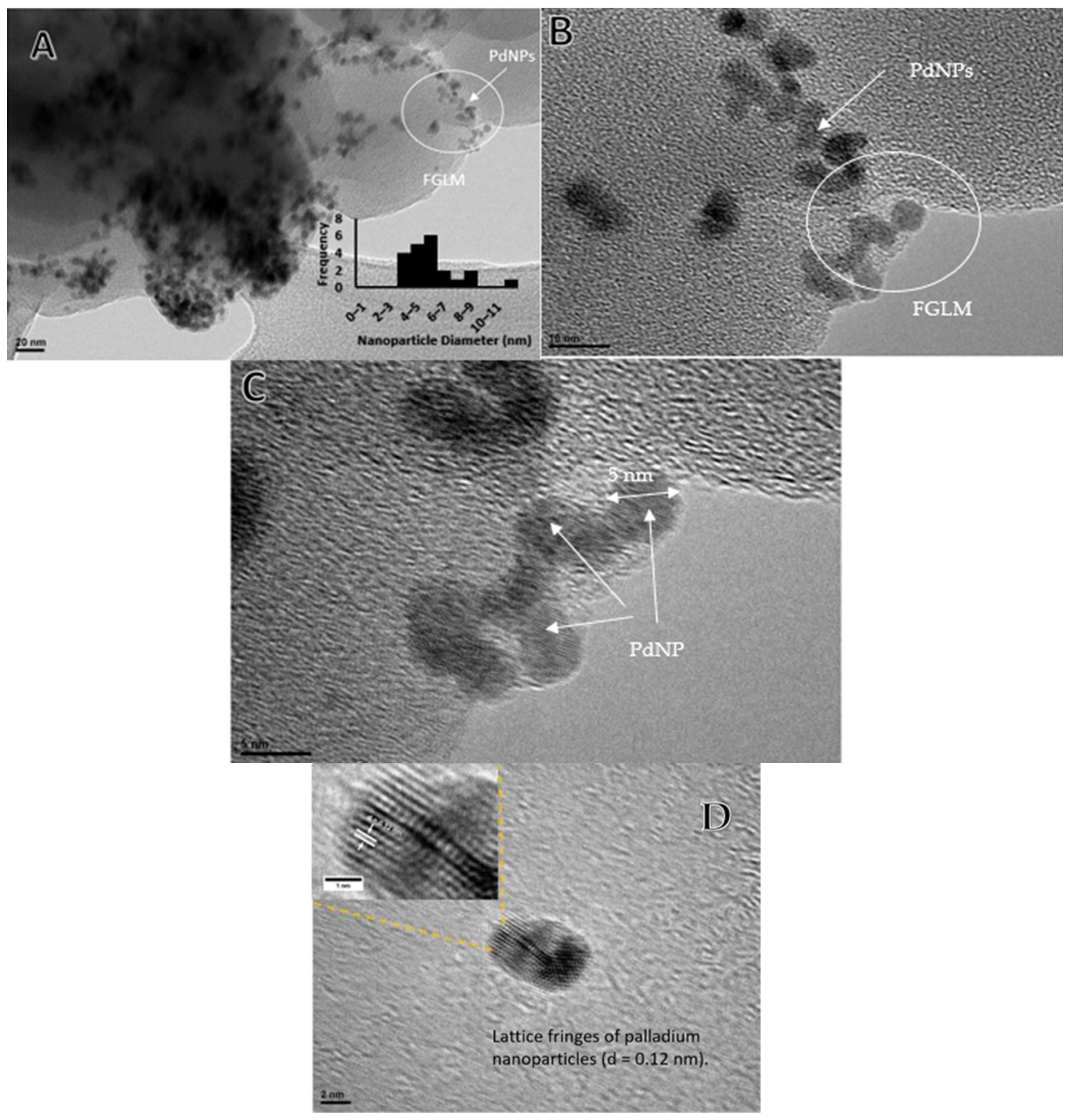
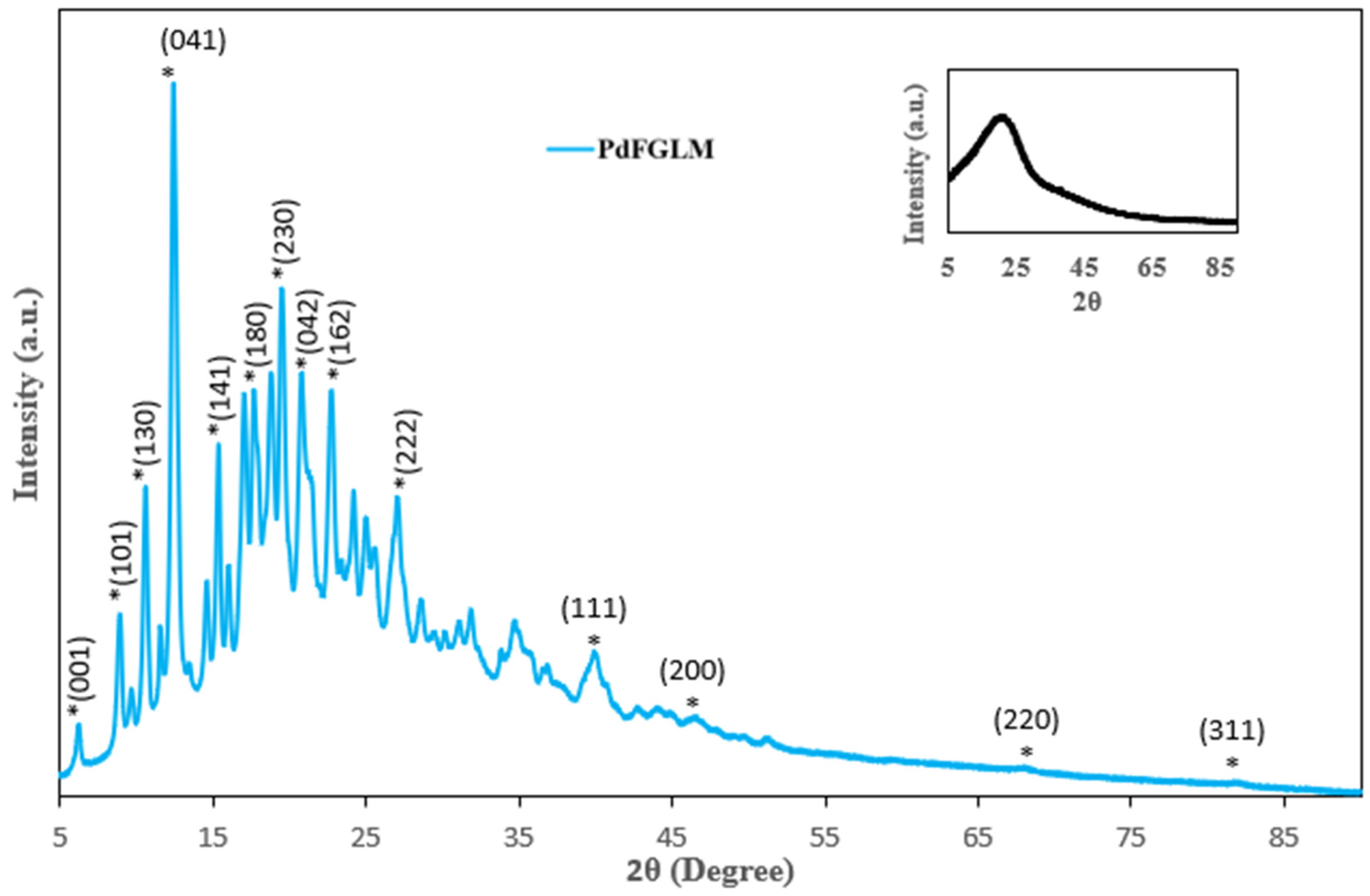
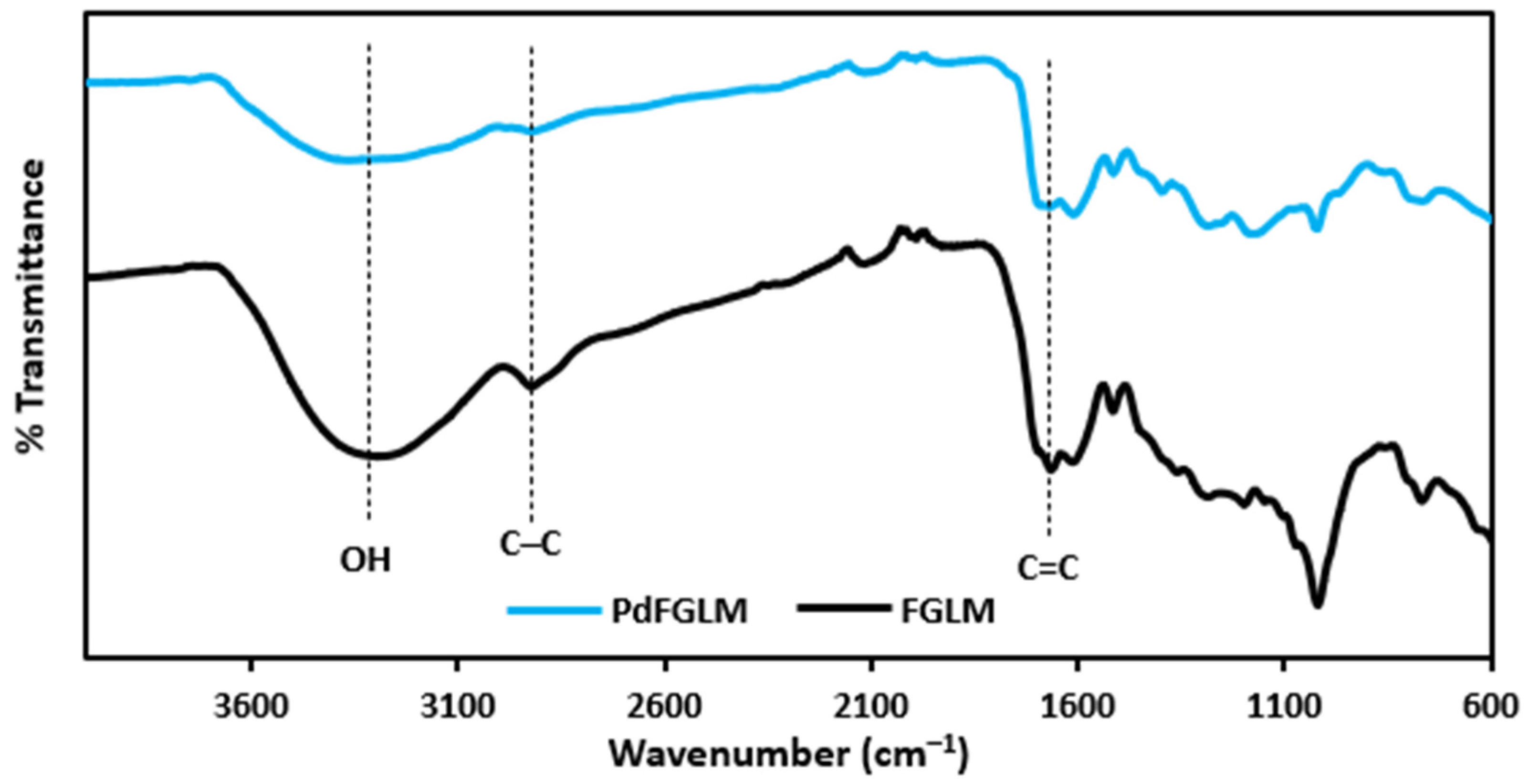
2.1. Characterization
2.2. Catalytic Evaluation with Varied Dosage of Reactants
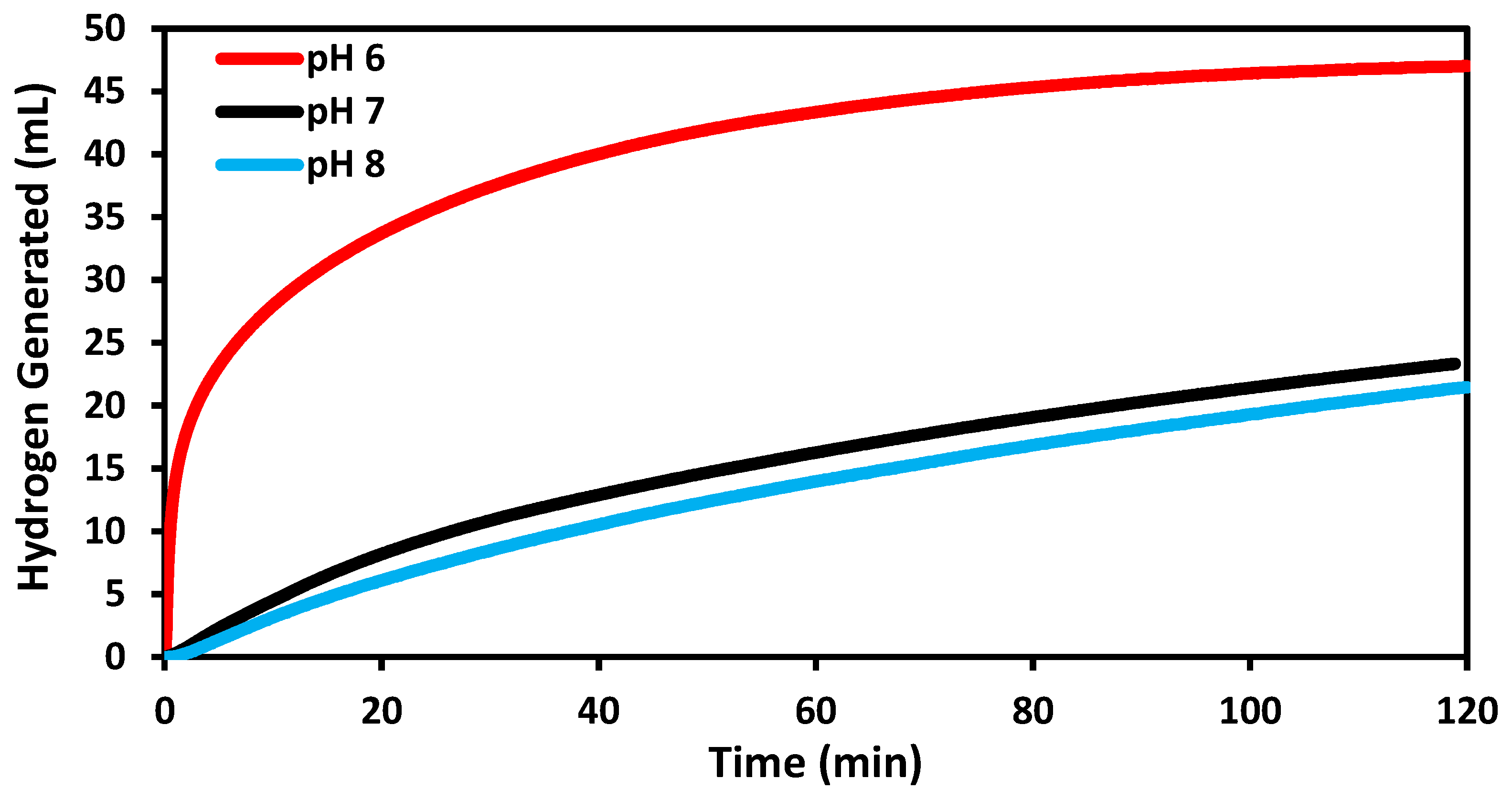
2.3. Catalytic Evaluation at Varied pH Conditions
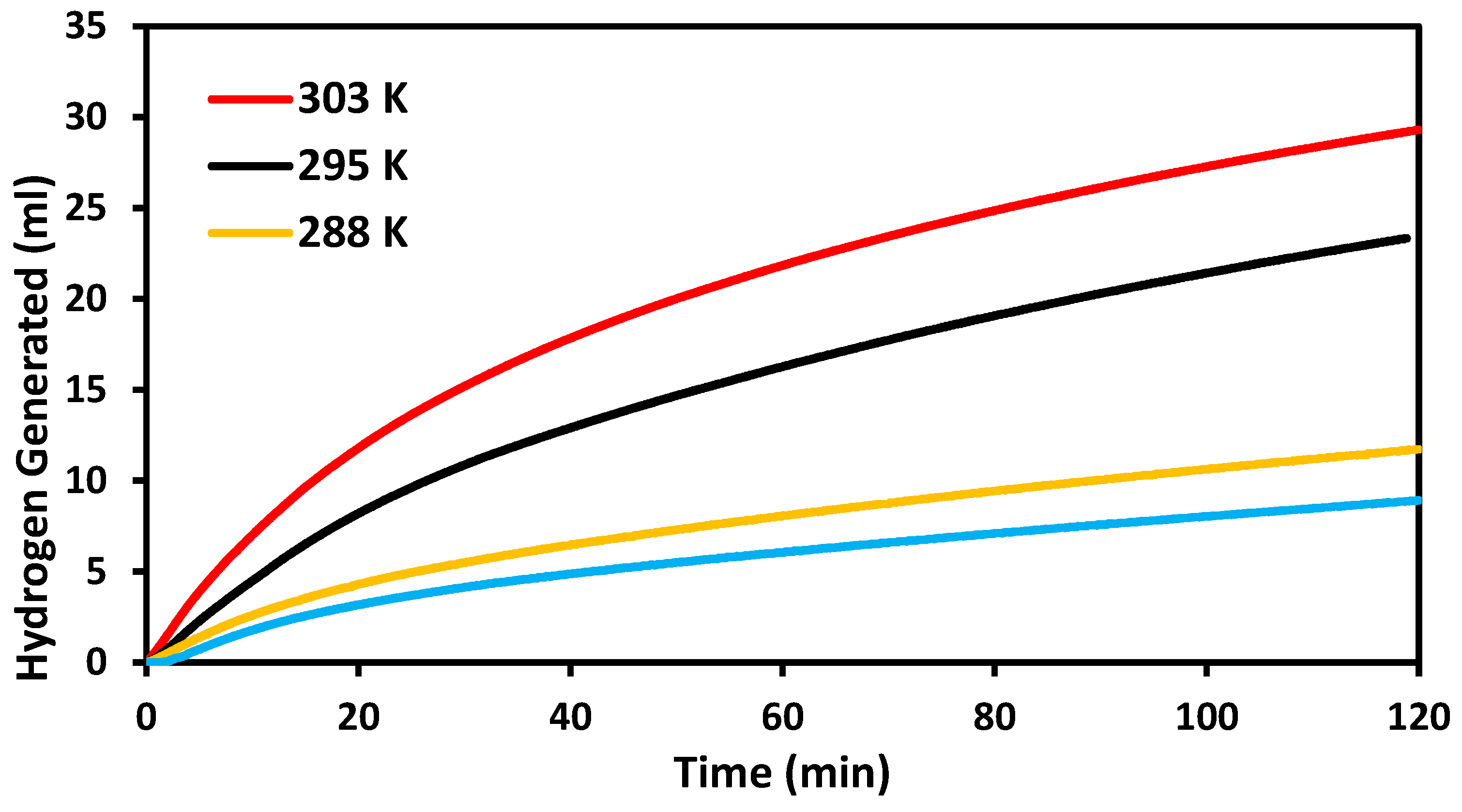
2.4. Catalytic Evaluation at Varied Temperatures
2.5. Activation Energies
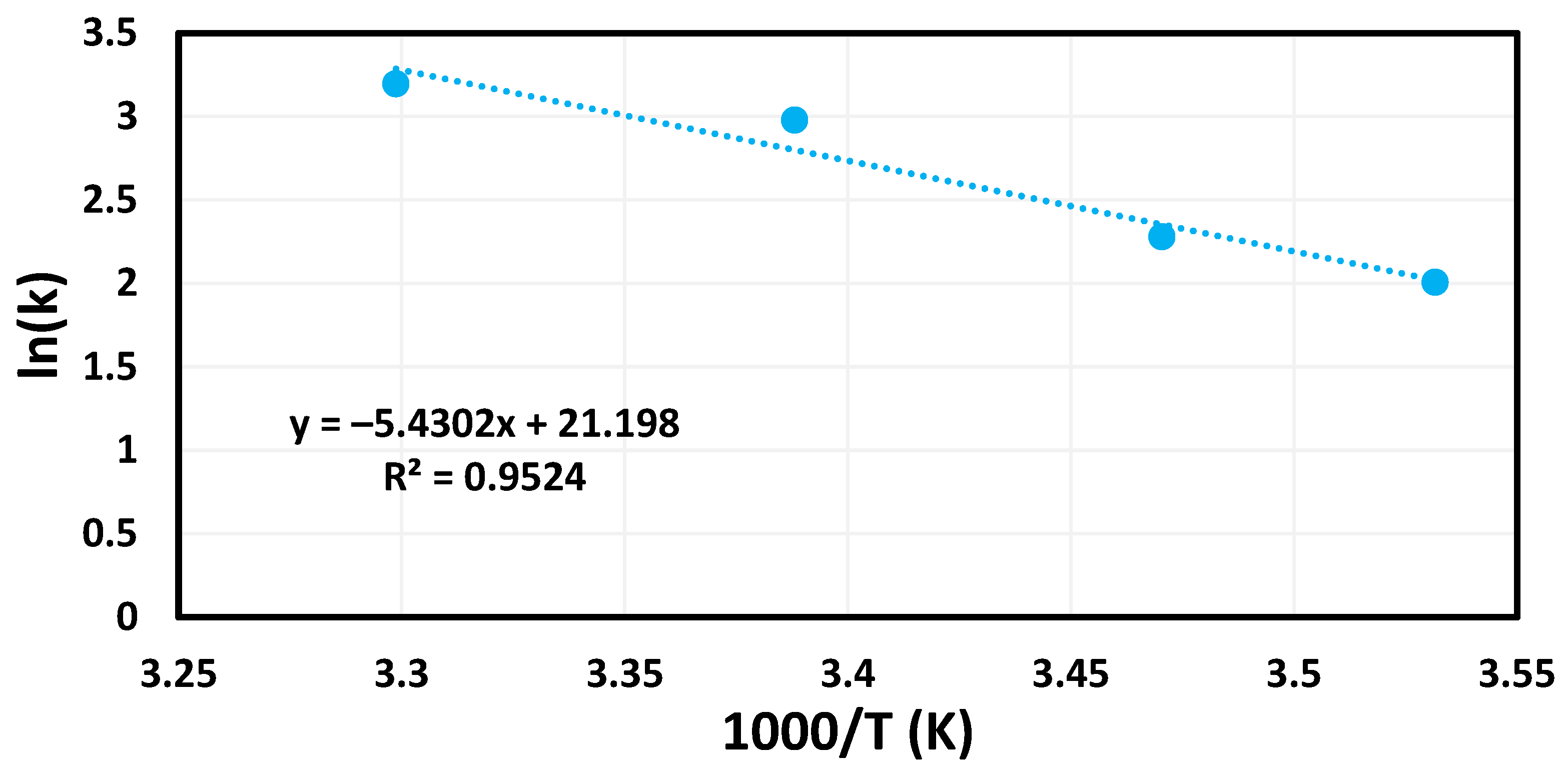
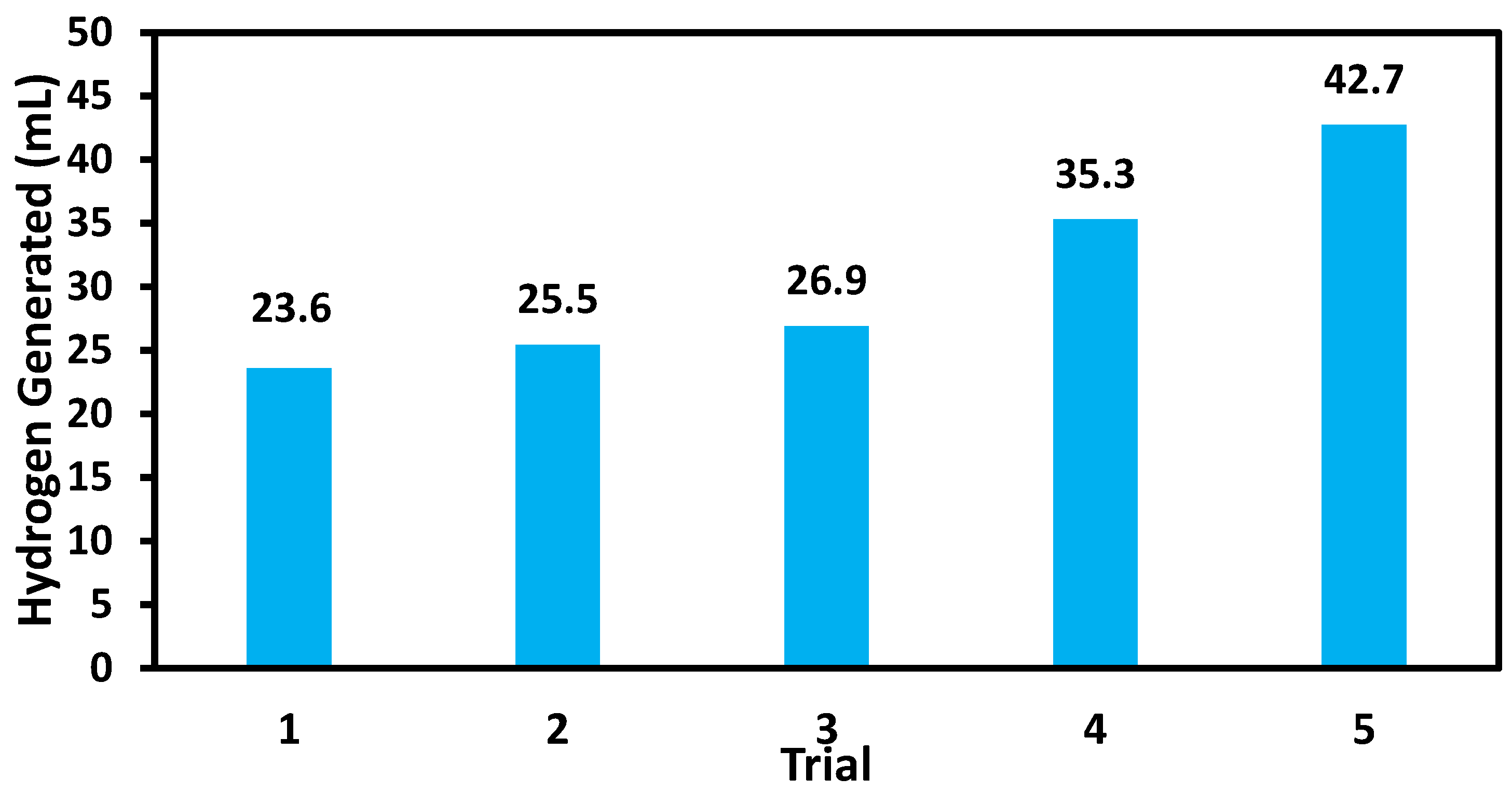
2.6. Catalytic Reusability of Palladium Composite
3. Proposed Mechanism
4. Experimental
4.1. Synthesis of Fused Graphene-like Material (FGLM)
4.2. Synthesis of Palladium Nanoparticle (PdNPs) and PdFGLM
4.3. Characterization
4.4. Catalysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hook, M.; Li, J.; Johansson, K.; Snowden, S. Growth Rates of Global Energy Systems and Future Outlooks. Nat. Resour. Res. 2011, 21, 23–41. [Google Scholar] [CrossRef]
- Hook, M.; Tang, X. Depletion of fossil fuels and anthropogenic climate change—A review. Energy Policy 2013, 52, 797–809. [Google Scholar] [CrossRef]
- Perera, F. Pollution from Fossil-Fuel Combustion is the Leading Environmental Threat to Global Pediatric Health and Equity: Solutions Exist. Int. J. Environ. Res. Public Health 2018, 15, 16. [Google Scholar] [CrossRef] [PubMed]
- Brandon, N.P.; Kurban, Z. Clean energy and the hydrogen economy. Phil. Trans. R. Soc. A 2017, 375, 20160400. [Google Scholar] [CrossRef]
- Bosnjakovic, M.; Sinaga, N. The Perspective of Large-Scale Production of Algae Biodiese. Appl. Sci. 2020, 10, 8181. [Google Scholar] [CrossRef]
- Ozawa, A.; Kudoh, Y.; Murata, A.; Honda, T.; Saita, I.; Takagi, H. Hydrogen in low-carbon energy systems in Japan by 2050: The uncertainties of technology development and implementation. Int. J. Hydrogen Energy 2018, 43, 18083–18094. [Google Scholar] [CrossRef]
- Hua, T.Q.; Ahluwalia, R.K. Alane hydrogen storage for automotive fuel cells—Off-board regeneration processes and efficiencies. Int. J. Hydrogen Energy 2011, 36, 15259–15265. [Google Scholar] [CrossRef]
- Witkowski, A.; Rusin, A.; Majkut, M.; Stolecka, K. Comprehensive analysis of hydrogen compression and pipeline transportation from thermodynamics and safety aspects. Energy 2017, 141, 2508–2518. [Google Scholar] [CrossRef]
- Zhang, L.; Li, L.; Liang, J.; Fan, X.; He, X.; Chen, J.; Li, J.; Li, Z.; Cai, Z.; Sun, S.; et al. Highly efficient and stable oxygen evolution from seawater enabled by a hierarchical NiMoSx microcolumn@NiFe-layered double hydroxide nanosheet array. Inorg. Chem. Front. 2023, 10, 2766–2775. [Google Scholar] [CrossRef]
- Li, L.; Zhang, L.; Gou, L.; Wei, S.; Hou, X.; Wu, L. High-performance methanol electrolysis towards energy-saving hydrogen production: Using Cu2O-Cu decorated Ni2P nanoarray as bifunctional monolithic catalyst. Chem. Eng. J. 2023, 454, 140292. [Google Scholar] [CrossRef]
- Puszkiel, J.; Garroni, S.; Milanese, C.; Gennari, F.; Klassen, T.; Dornheim, M.; Pistidda, C. Tetrahydroborates: Development and Potential as Hydrogen Storage Medium. Inorganics 2017, 54, 74. [Google Scholar] [CrossRef]
- Li, H.W.; Yan, Y.; Orimo, S.I.; Zuttel, A.; Jesen, C.M. Recent progress in metal borohydrides for hydrogen storage. Energies 2011, 4, 185–214. [Google Scholar] [CrossRef]
- Demirci, U.B. About the Technological Readiness of the H2 Generationby Hydrolysis of B(-N)-H Compounds. Energy Technol. 2018, 6, 470–486. [Google Scholar] [CrossRef]
- Schlesinger, H.I.; Brown, H.C.; Finholt, A.E.; Gilbreath, J.R.; Hoekstra, H.R.; Hyde, E.K. Sodium borohydride, its hydrolysis and its use as a reducing agent and in the generation of hydrogen1. J. Am. Chem. Soc. 1953, 75, 215–219. [Google Scholar] [CrossRef]
- Dushatinski, T.; Huff, C.; Abdel-Fattah, T.M. Characterization of electrochemically deposited films from aqueous and ionic liquid cobalt precursors toward hydrogen evolution reactions. Appl. Surf. Sci. 2016, 385, 282–288. [Google Scholar] [CrossRef]
- Huff, C.; Long, J.M.; Heyman, A.; Abdel-Fattah, T.M. Palladium Nanoparticle Multiwalled Carbon Nanotube Composite as Catalyst for Hydrogen Production by the Hydrolysis of Sodium Borohydride. ACS Appl. Energy Mater. 2018, 1, 4635–4640. [Google Scholar] [CrossRef]
- Huff, C.; Dushatinski, T.; Barzanji, A.; Abdel-Fattah, N.; Barzanji, K.; Abdel-Fattah, T.M. Pretreatment of Gold Nanoparticle Multi-Walled Carbon Nanotube Composites for Catalytic Activity toward Hydrogen Generation Reaction. ECS J. Solid State Sci. Technol. 2017, 6, M69–M71. [Google Scholar] [CrossRef]
- Huff, C.; Long, J.M.; Abdel-Fattah, T.M. Beta-Cyclodextrin Assisted Synthesis of Silver Nanoparticle Network and its Application in a Hydrogen Generation Reaction. Catalysts 2020, 10, 1014. [Google Scholar] [CrossRef]
- Osborne, J.; Horten, M.; Abdel-Fattah, T.M. Gold Nanoparticles Supported Over Low-Cost Supports for Hydrogen Generation from a Hydrogen Feedstock Material. ECS J. Solid State Sci. Technol. 2020, 9, 071004. [Google Scholar] [CrossRef]
- Kushch, S.D.; Kujunko, N.S.; Tarasov, B.P. Platinum nanoparticles on carbon nanomaterials with graphene structure as hydrogenation catalysts. Russ. J. Gen. Chem. 2009, 79, 706–710. [Google Scholar] [CrossRef]
- Hitrik, M.; Sasson, Y. Aggregation of catalytically active Ru nanoparticles to inactive bulk, monitored in situ during an allylic isomerization reaction. Influence of solvent, surfactant and stirring. RSC Adv. 2018, 8, 1481–1492. [Google Scholar] [CrossRef] [PubMed]
- Tang, Z.; Yin, X.; Zhang, Y.; Zhang, N.; Xu, Y. Inhibiting Pd nanoparticle aggregation and improving catalytic performance using one-dimensional CeO2 nanotubes as support. Chin. J. Catal. 2013, 34, 1123–1127. [Google Scholar] [CrossRef]
- Quach, Q.; Abdel-Fattah, T.M. Silver Nanoparticles functionalized Nanoporous Silica Nanoparticle grown over Graphene Oxide for enhancing Antibacterial effect. Nanomaterials 2022, 12, 3341. [Google Scholar] [CrossRef] [PubMed]
- Biehler, E.; Whiteman, R.; Lin, P.; Zhang, K.; Baumgart, H.; Abdel-Fattah, T.M. Controlled Synthesis of ZnO Nanorods Using Different Seed Layers. ECS J. Solid State Sci. Technol. 2020, 9, 121008. [Google Scholar] [CrossRef]
- Rhazouani, A.; Gamrani, H.; Achaby, M.E.; Aziz, K.; Gebrati, L.; Uddin, M.S.; Aziz, F. Synthesis and Toxicity of Graphene Oxide Nanoparticles: A Literature Review of In Vitro and In Vivo Studies. Biomed. Res. Int. 2021, 2021, 5518999. [Google Scholar] [CrossRef]
- Mahmoud, A.M.; Desouky, E.M.; Hozayen, W.G.; Bin-Jumah, M.; El-Nahass, E.S.; Soliman, H.A.; Farghali, A.A. Mesoporous Silica Nanoparticles Trigger Lier and Kidney Injury and Fibrosis Via Altering TLR4/NF-κB, JAK2/STAT3 and Nrf2/HO-1 Signaling in Rats. Biomolecules 2019, 9, 528. [Google Scholar] [CrossRef]
- Zhang, C.; Wu, L.; Perror, M.D.; Zhao, X. Carbon Nanotubes: A Summary of Beneficial and Dangerous Aspects of an Increasingly Popular Group of Nanomaterials. Front. Oncol. 2021, 11, 693814. [Google Scholar] [CrossRef]
- Antolini, E. Palladium in fuelcell catalysis. Energy Environ. Sci. 2009, 2, 915–931. [Google Scholar] [CrossRef]
- Adams, B.D.; Chen, A. The role of palladium in a hydrogen economy. Mater. Today 2011, 14, 282–289. [Google Scholar] [CrossRef]
- Mahesh, K.N.; Balaji, R.; Dhathathreyan, K.S. Palladium nanoparticles as hydrogen evolution reaction (HER) electrocatalyst in electrochemical methanol reformer. Int. J. Hydrogen Energy 2016, 41, 46–51. [Google Scholar] [CrossRef]
- Albano, G.; Petri, A.; Aronica, A.A. Palladium Supported on Bioinspired Materials as Catalysts for C–C Coupling Reactions. Catalysts 2023, 13, 210. [Google Scholar] [CrossRef]
- Kathiresan, M. Metal-Ion/Metal Nanoparticle-Anchored Porous Organic Polymers as Efficient Catalysts for Organic Transformations—A Recent Overview. Chem. Asian J. 2023, 18, e202201299. [Google Scholar] [CrossRef]
- Cao, Y.; Ran, R.; Wu, X.; Si, Z.; Kang, F.; Weng, D. Progress on metal-support interactions in Pd-based catalysts for automobile emission control. J. Environ. Sci. 2023, 125, 401–426. [Google Scholar] [CrossRef]
- Karthick, K.; Bijoy, T.K.; Sivakumaran, A.; Basha, A.B.M.; Murugan, P.; Kundu, S. Enhancing Hydrogen Evolution Reaction Activities of 2H-Phase VS2 Layers with Palladium Nanoparticles. Inorg. Chem. 2020, 59, 10197–10207. [Google Scholar] [CrossRef]
- Tang, Q.; Zhou, Z.; Chen, Z. Innovation and discovery of graphene-like materials via density-functional theory computations. Wiley Interdiscip. Rev. Comput. Mol. Sci. 2015, 5, 360–379. [Google Scholar] [CrossRef]
- Das, V.K.; Shifrina, Z.B.; Bronstein, L.M. Graphene and graphene-like materials in biomass conversion: Paving the way to the future. J. Mater. Chem. A 2017, 5, 25131–25143. [Google Scholar] [CrossRef]
- Adel, M.; El-Maghraby, A. Synthesis of few-layer graphene-like nanosheets from glucose: New facile approach for graphene-like nanosheets large-scale production. J. Mater. Res. 2016, 31, 455–467. [Google Scholar] [CrossRef]
- Zhou, Y.; Xie, C.; Su, L.; Han, X. Study of the risks of the graphene oxide preparation process by reaction calorimetry. J. Therm. Anal. Calorim. 2020, 139, 101–112. [Google Scholar] [CrossRef]
- Chen, Z.; Wang, W.; Zhang, Y.; Liang, Y.; Cui, Z.; Wang, X. Pd Nanoparticles Confined in the Porous Graphene-like Carbon Nanosheets for Olefin Hydrogenation. Langmuir 2018, 34, 12809–12814. [Google Scholar] [CrossRef]
- Klyuev, M.V.; Arbuzov, A.A.; Magdalinova, N.A.; Kalmykov, P.A.; Tarsov, B.P. Palladium-containing graphene-like material: Synthesis and catalytic activity. Russ. J. Phys. Chem. A 2016, 90, 1749–1753. [Google Scholar] [CrossRef]
- Xu, M.; Liang, T.; Shi, M.; Chen, H. Graphene-Like Two-Dimensional Materials. Chem. Rev. 2013, 113, 3766–3798. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Liu, Q.; Cheng, H.; Hu, M.; Zhang, S. Classification and Carbon Structural Transformation from Anthracite to Natural Coaly Graphite by XRD, Raman spectroscopy, and HRTEM. Spectrochim. Acta Part A 2021, 249, 119286. [Google Scholar] [CrossRef] [PubMed]
- Song, W.; Yu, X.; Wang, S.; Blasier, R.; Markel, D.C.; Mao, G.; Shi, T.; Ren, W. Cyclodextrin-erythromycin complexes as a drug delivery device for orthopedic application. Int. J. Nanomed. 2011, 6, 3173–3186. [Google Scholar] [CrossRef]
- Kaufman, C.M.; Sen, B. Hydrogen generation by hydrolysis of sodium tetrahydroborate: Effects of acids and transition metals and their salts. J. Chem. Soc. Dalton Trans. 1985, 2, 307–313. [Google Scholar] [CrossRef]
- Zhang, J.S.; Delgass, W.N.; Fisher, T.S.; Gore, J.P. Kinetics of Ru-catalyzed sodium borohydride hydrolysis. J. Power Sources 2007, 164, 772–781. [Google Scholar] [CrossRef]
- Liang, Y.; Dai, H.-B.; Ma, L.-P.; Wang, P.; Cheng, H.-M. Hydrogen generation from sodium borohydride solution using a ruthenium supported graphite catalyst. Int. J. Hydrogen Energy 2010, 35, 3023–3028. [Google Scholar] [CrossRef]
- Patel, N.; Patton, B.; Zanchetta, C.; Fernandes, R.; Guella, G.; Kale, A.; Miotello, A. Pd-C power and thin film catalysts for hydrogen production by hydrolysis of sodium borohydride. Int. J. Hydrogen Energy 2008, 33, 287–292. [Google Scholar] [CrossRef]
- Peña-Alonso, R.; Sicurelli, A.; Callone, E.; Carturan, G.; Raj, R. A picoscale catalyst for hydrogen generation from NaBH4 for fuel cells. J. Power Sources 2007, 165, 315–323. [Google Scholar] [CrossRef]
- Huff, C.; Dushatinski, T.; Abdel-Fattah, T.M. Gold Nanoparticle/Multi-Walled Carbon Nanotube Composite as Novel Catalyst for Hydrogen Evolution Reactions. Int. J. Hydrogen Energy 2017, 42, 18985–18990. [Google Scholar] [CrossRef]
- Huff, C.; Long, J.M.; Aboulatta, A.; Heyman, A.; Abdel-Fattah, T.M. Silver Nanoparticle/Multi-Walled Carbon Nanotube Composite as Catalyst for Hydrogen Production. ECS J. Solid State Sci. Technol. 2017, 6, M115–M118. [Google Scholar] [CrossRef]
- Huff, C.; Quach, Q.; Long, J.M.; Abdel-Fattah, T.M. Nanocomposite Catalyst Derived from Ultrafine Platinum Nanoparticles and Carbon Nanotubes for Hydrogen Generation. ECS J. Solid State Sci. Technol. 2020, 9, 101008. [Google Scholar] [CrossRef]
- Quach, Q.; Biehler, E.; Elzamzami, A.; Huff, C.; Long, J.M.; Abdel-Fattah, T.M. Catalytic Activity of Beta-Cyclodextrin-Gold Nanoparticles Network in Hydrogen Evolution Reaction. Catalysts 2021, 11, 118. [Google Scholar] [CrossRef]
- Huff, C.; Biehler, E.; Quach, Q.; Long, J.M.; Abdel-Fattah, T.M. Synthesis of Highly Dispersive Platinum Nanoparticles and their Application in a Hydrogen Generation Reaction. Colloids Surf. A. 2021, 610, 125734. [Google Scholar] [CrossRef]
- Biehler, E.; Quach, Q.; Huff, C.; Abdel-Fattah, T.M. Organo-Nanocups Assist the Formation of Ultra-Small Palladium Nanoparticle Catalysts for Hydrogen Evolution Reaction. Materials 2022, 15, 2692. [Google Scholar] [CrossRef]
- Quach, Q.; Biehler, E.; Abdel-Fattah, T.M. Synthesis of Copper Nanoparticles Supported over Graphene-like Material Composite as a Catalyst for Hydrogen Evolution. J. Compos. Sci. 2023, 7, 279. [Google Scholar] [CrossRef]
- Biehler, E.; Quach, Q.; Abdel-Fattah, T.M. Gold Nanoparticles AuNP Decorated on Fused Graphene-like Materials for Application in a Hydrogen Generation. Materials 2023, 16, 4779. [Google Scholar] [CrossRef]
- Biehler, E.; Quach, Q.; Abdel-Fattah, T.M. Synthesis of Platinum Nanoparticles Supported on Fused Nanosized Carbon Spheres Derived from Sustainable Source for Application in a Hydrogen Generation Reaction. Nanomaterials 2023, 13, 1994. [Google Scholar] [CrossRef]
- Biehler, E.; Quach, Q.; Abdel-Fattah, T.M. Silver-Nanoparticle-Decorated Fused Carbon Sphere Composite as a Catalyst for Hydrogen Generation. Energies 2023, 16, 5053. [Google Scholar] [CrossRef]
- Deraedt, C.; Salmon, L.; Gatard, S.; Ciganda, R.; Hernandez, E.; Ruiz, J.; Astruc, D. Sodium borohydride stabilizes very active gold nanoparticle catalysts. Chem. Commun. 2014, 50, 14194–14196. [Google Scholar] [CrossRef]
- Guella, G.; Zanchetta, C.; Patton, B.; Miotello, A. New Insights on the Mechanism of Palladium-Catalyzed Hydrolysis of Sodium Borohydride from 11B NMR Measurements. J. Phys. Chem. B 2006, 110, 17024–17033. [Google Scholar] [CrossRef]
- Wen, D.; Liu, W.; Haubold, D.; Zhu, C.; Oschatz, M.; Holzschuh, M.; Wolf, A.; Simon, F.; Kaskel, S. Gold Aerogels: Three-Dimensional Assembly of Nanoparticles and Their Use as Electrocatalytic Interfaces. ACS Nano 2016, 10, 2559–2567. [Google Scholar] [CrossRef] [PubMed]



| Catalyst | Ea (kJ mol−1) | Temperature (K) | Reference |
|---|---|---|---|
| Ni | 71 | 273–308 | [44] |
| Raney-Nickel | 63 | 273–308 | [44] |
| Co | 75 | 273–308 | [44] |
| Ru/C | 67 | 298–358 | [45] |
| Ru/Graphite | 61.1 | 398–318 | [46] |
| Pd/C | 28 | 298–328 | [47] |
| Pt–Pd/CNTs | 19 | 302–332 | [48] |
| Au/MWCNTs | 21.1 | 273–303 | [49] |
| Ag/MWCNTs | 44.5 | 273–303 | [50] |
| Pd/MWCNTs | 62.7 | 273–303 | [16] |
| Pt/MWCNTs | 46.2 | 273–303 | [51] |
| BCD-AuNP | 54.7 | 283–303 | [52] |
| PtNPs | 39.2 | 283–303 | [53] |
| PdNPs | 58.9 | 273–303 | [54] |
| CuGLM | 46.8 | 283–303 | [55] |
| AuFGLM | 45.5 | 283–303 | [56] |
| PtFCS | 53.0 | 283–303 | [57] |
| AgNP-FCS | 37.0 | 283-303 | [58] |
| PdFGLM | 45.1 | 283–303 | This Work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Quach, Q.; Biehler, E.; Abdel-Fattah, T.M. Synthesis of Palladium Nanoparticles Supported over Fused Graphene-like Material for Hydrogen Evolution Reaction. Catalysts 2023, 13, 1117. https://doi.org/10.3390/catal13071117
Quach Q, Biehler E, Abdel-Fattah TM. Synthesis of Palladium Nanoparticles Supported over Fused Graphene-like Material for Hydrogen Evolution Reaction. Catalysts. 2023; 13(7):1117. https://doi.org/10.3390/catal13071117
Chicago/Turabian StyleQuach, Qui, Erik Biehler, and Tarek M. Abdel-Fattah. 2023. "Synthesis of Palladium Nanoparticles Supported over Fused Graphene-like Material for Hydrogen Evolution Reaction" Catalysts 13, no. 7: 1117. https://doi.org/10.3390/catal13071117
APA StyleQuach, Q., Biehler, E., & Abdel-Fattah, T. M. (2023). Synthesis of Palladium Nanoparticles Supported over Fused Graphene-like Material for Hydrogen Evolution Reaction. Catalysts, 13(7), 1117. https://doi.org/10.3390/catal13071117







