Effect of Ultraviolet Illumination on the Fixation of Silver Ions on Zinc Oxide Films and Their Photocatalytic Efficiency
Abstract
1. Introduction
2. Results and Discussion
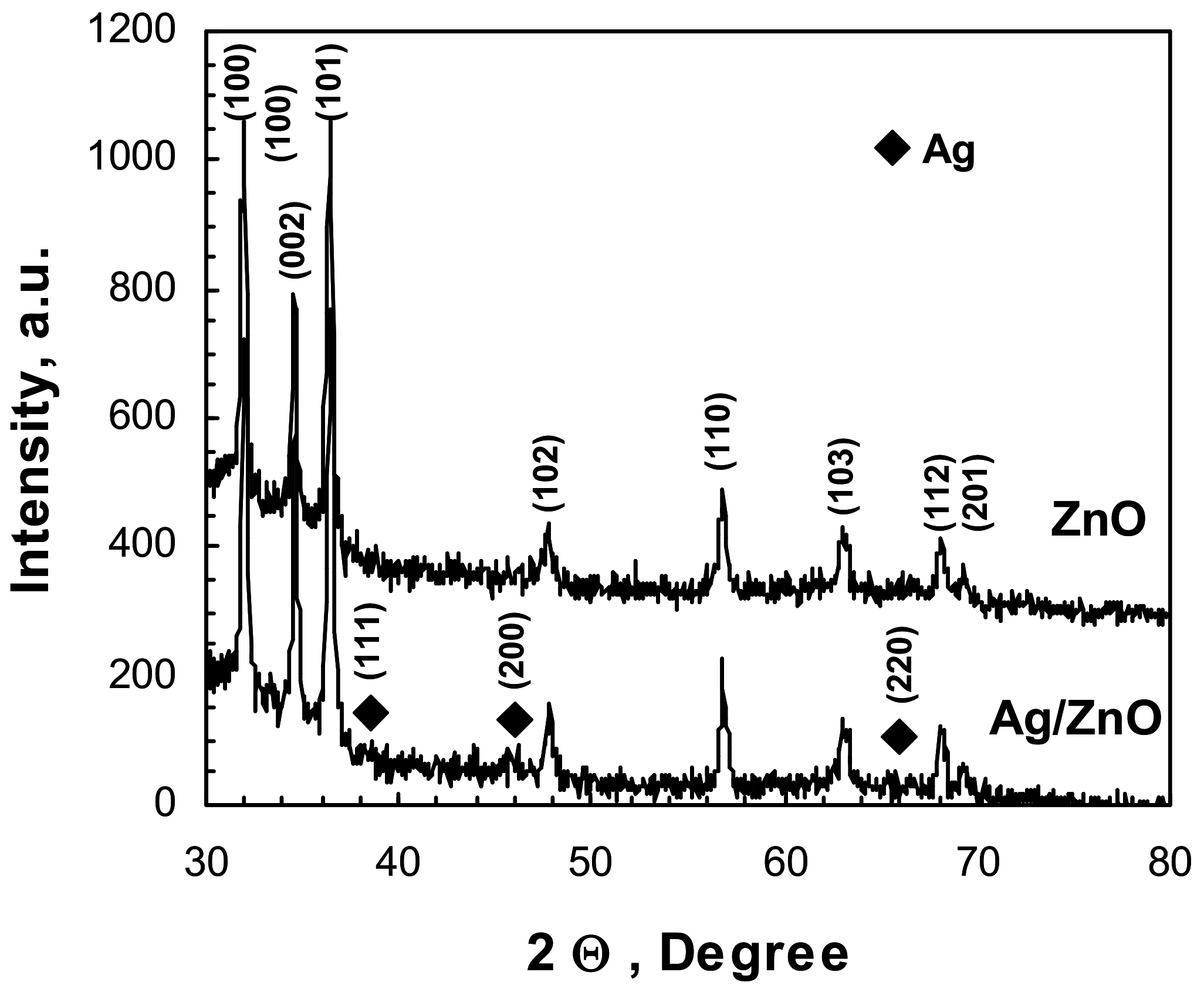
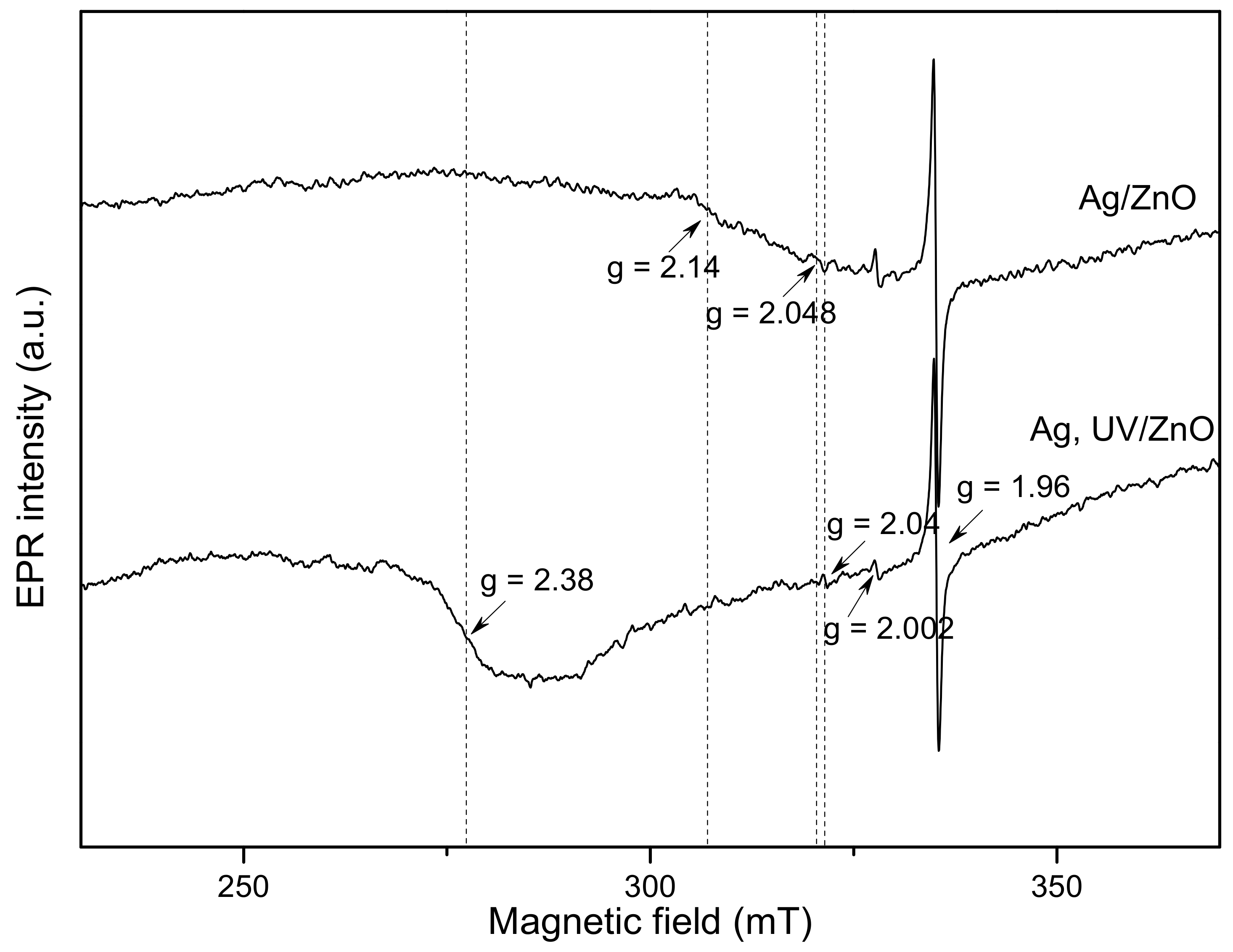
2.1. Characterization of Photocatalysts
2.2. Optical Characterization
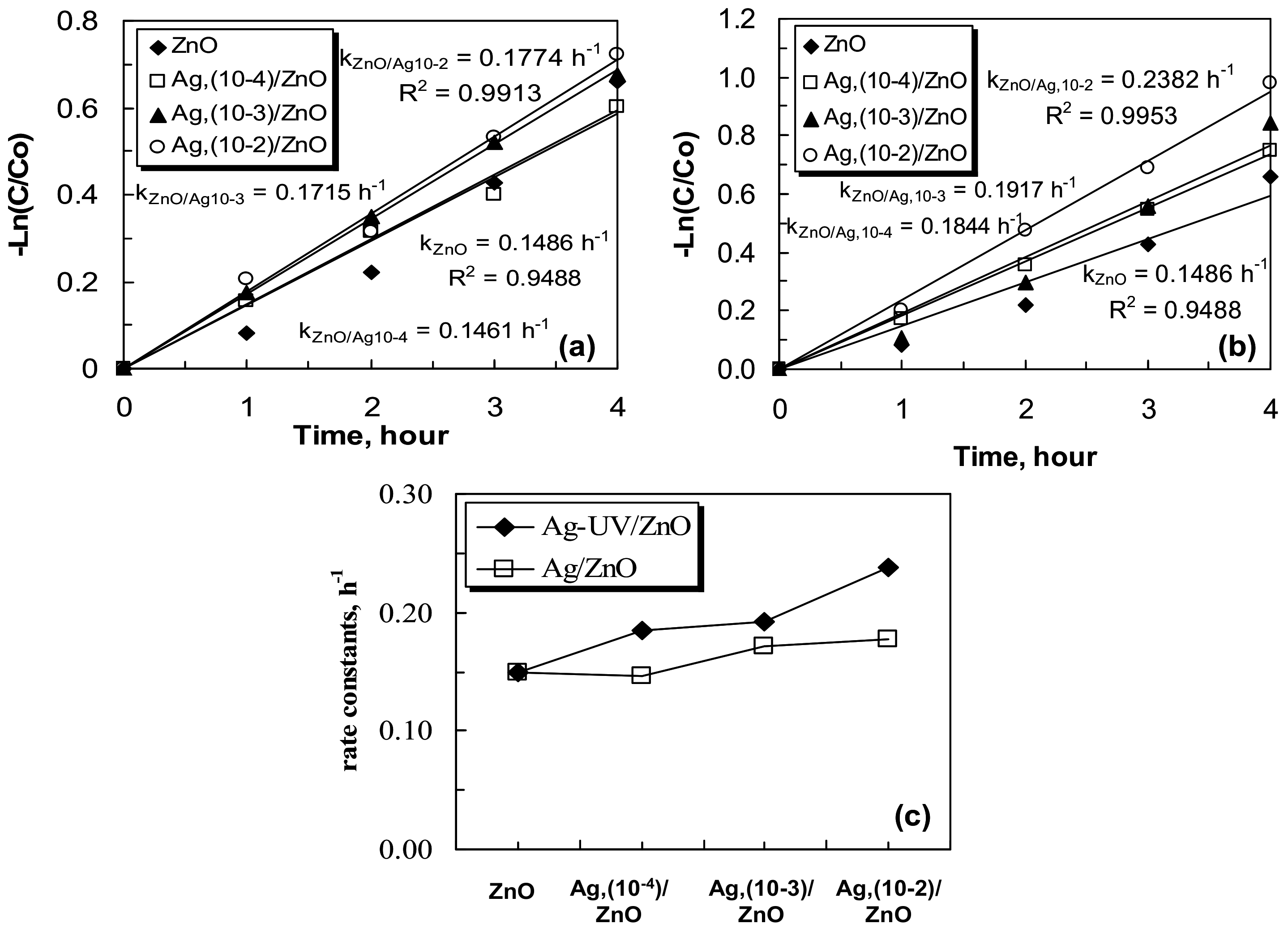
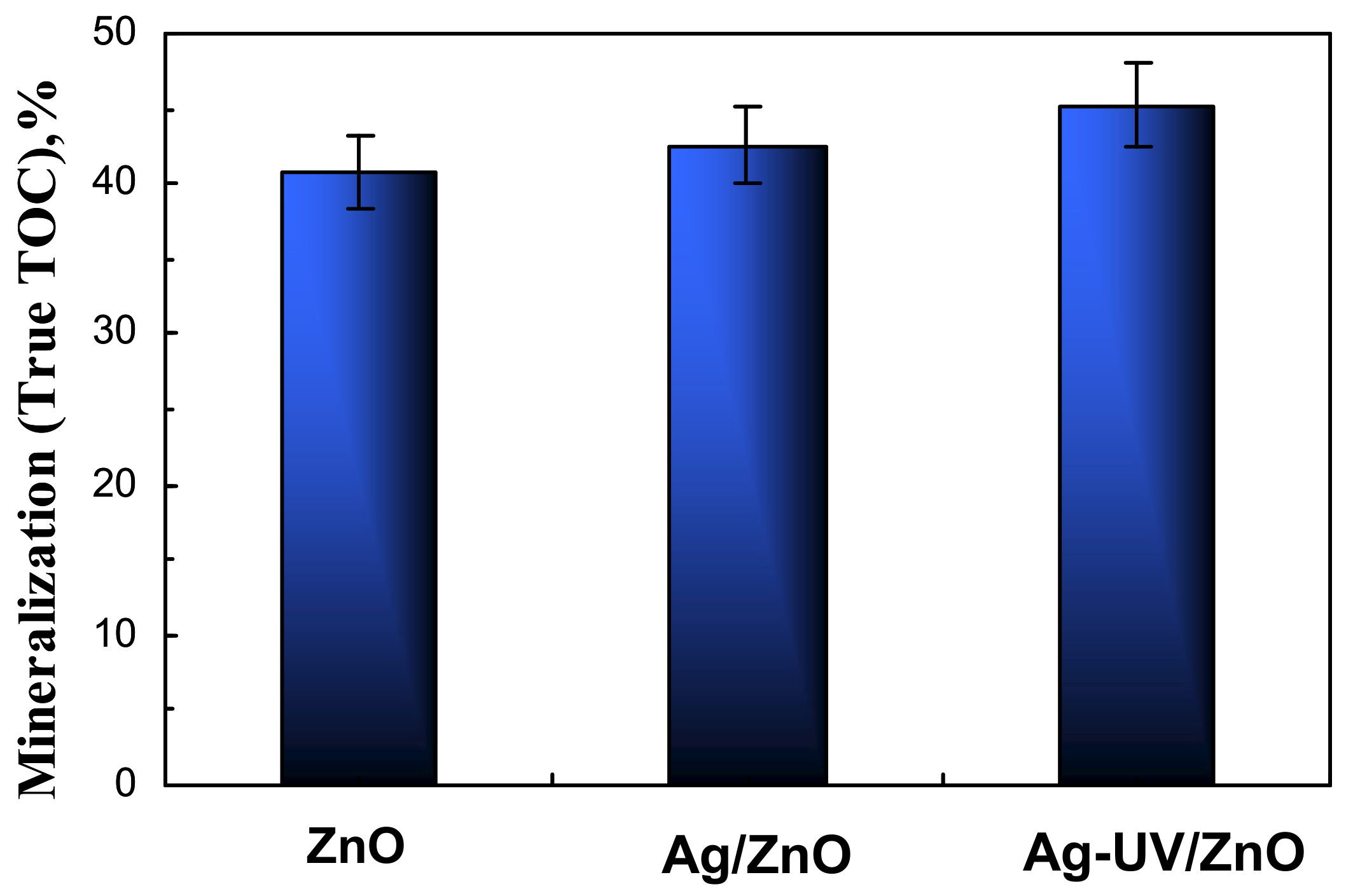
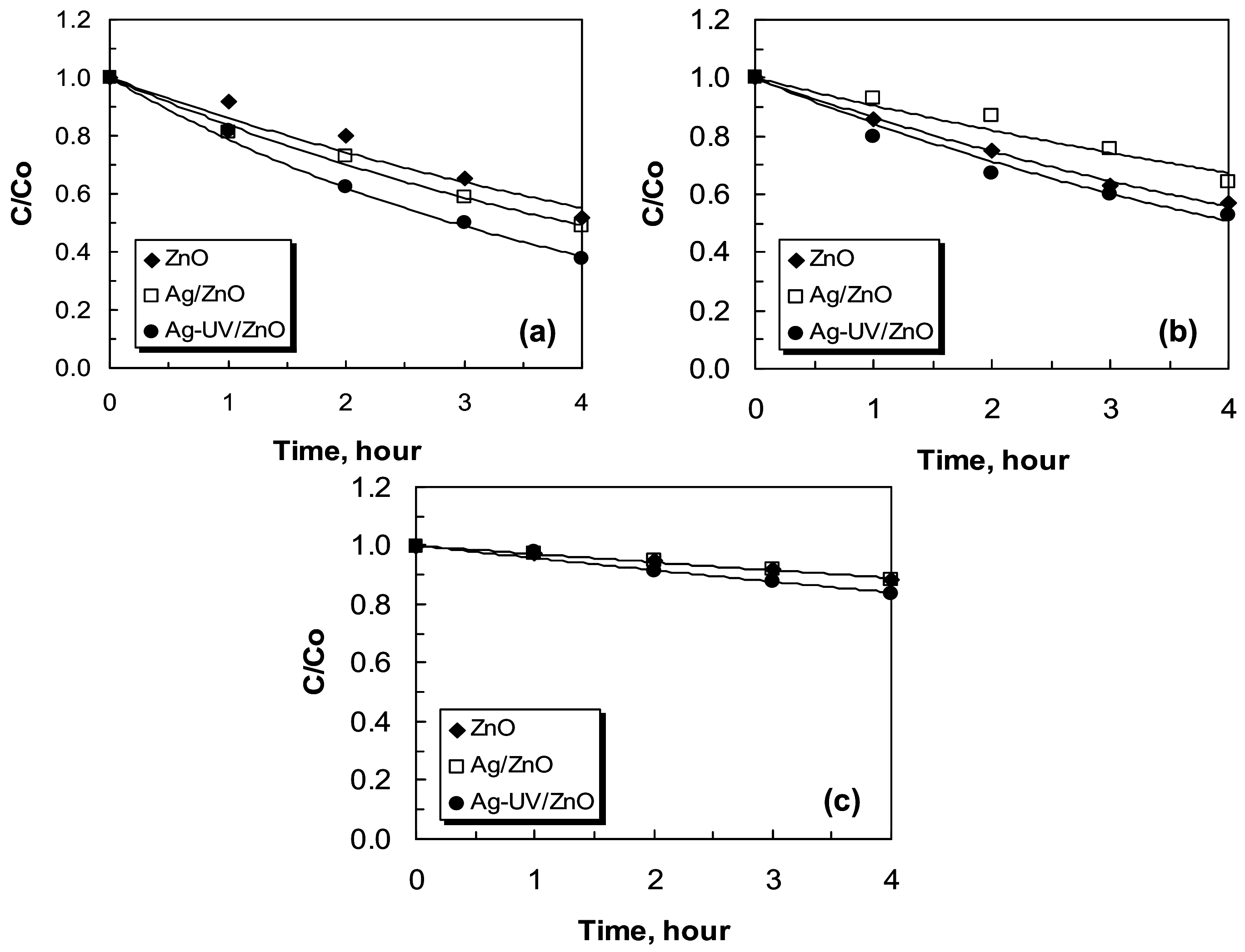
2.3. Photocatalytic Efficiency
3. Materials and Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, Q.; Xu, M.; You, B.; Zhang, Q.; Yuan, H.; Ostrikov, K. Oxygen vacancymediated ZnO nanoparticle photocatalyst for degradation of methylene blue. Appl. Sci. 2018, 8, 353. [Google Scholar]
- Ma, Y.; Manzoor, R.; Jia, P.; Bian, W.; Hamid, N.; Xie, Z.; Pei, D. Transcriptome and in silico approaches provide new insights into the mechanism of male reproductive toxicity induced by chronic exposure to DEHP. Environ. Pollut. 2021, 289, 117944. [Google Scholar] [CrossRef] [PubMed]
- Adamovsky, O.; Buerger, A.; Vespalcova, H.; Sohag, S.; Hanlon, A.; Ginn, P.; Craft, S.; Smatana, S.; Budinska, E.; Persico, M.; et al. Evaluation of Microbiome-Host Relationships in the Zebrafish Gastrointestinal System Reveals Adap-tive Immunity Is a Target of Bis(2-ethylhexyl) Phthalate (DEHP) Exposure. Environ. Sci. Technol. 2020, 54, 5719–5728. [Google Scholar] [CrossRef] [PubMed]
- Bhapkar, A.; Prasad, R.; Jaspal, D.; Shirolkar, M.; Gheisari, K.; Bhame, S. Visible light driven photocatalytic degradation of methylene blue by ZnO nanostructures synthesized by glycine nitrate auto combustion route. Inorg. Chem. Commun. 2023, 148, 110311. [Google Scholar] [CrossRef]
- Oladoye, P.; Ajiboye, T.; Omotola, E.; Oyewola, O. Methylene blue dye: Toxicity and potential elimination technology from wastewater. Res. Eng. 2022, 16, 100678. [Google Scholar] [CrossRef]
- Vutskits, L.; Briner, A.; Klauser, P.; Gascon, E.; Dayer, A.; Kiss, J.; Muller, D.; Licker, M.; Morel, D. Adverse Effects of Methylene Blue on the Central Nervous System. Anesthesiology 2008, 108, 684–692. [Google Scholar] [CrossRef] [PubMed]
- Al-Tohamy, R.; Ali, S.; Li, F.; Okasha, K.; Mahmoud, Y.; Elsamahy, T.; Jiao, H.; Fu, Y.; Sun, J. A critical review on the treatment of dye-containing wastewater: Ecotoxicological and health concerns of textile dyes and possible remediation approaches for environmental safety. Ecotoxicol. Environ. Saf. 2022, 231, 113160. [Google Scholar] [CrossRef] [PubMed]
- Saleh, I.; Zouari, N.; Al-Ghouti, M. Removal of pesticides from water and wastewater: Chemical, physical and biological treatment approaches. Environ. Technol. Innov. 2020, 19, 101026. [Google Scholar] [CrossRef]
- Mashuri, S.; Ibrahim, M.; Kasim, M.; Mastuli, M.; Rashid, U.; Abdullah, A.; Islam, A.; Mijan, N.; Tan, Y.; Mansir, N.; et al. Photocatalysis for Organic Wastewater Treatment: From the Basis to Current Challenges for Society. Catalysts 2020, 10, 1260. [Google Scholar]
- Srisai, J.; Muangnapoh, T.; Vas-Umnuay, P. Comparative study on photocatalytic degradation of methylene blue using pristine ZnO and Ni/ZnO composite films. Mat. Today Proceed. 2022, 66, 3168–3173. [Google Scholar] [CrossRef]
- Utami, F.; Rahman, D.; Sustini, E.; Abdullah, M. Immobilization of TiO2 on transparent plastic and its application in photo-catalytic wastewater treatment. J. Phys. Conf. Ser. 2019, 1171, 6–13. [Google Scholar] [CrossRef]
- Patil, S.; Mali, M.; Tamboli, M.; Patil, D.; Kulkarni, M.; Yoon, H.; Kim, H.; Al-Deyab, S.; Yoon, S.; Kolekar, S.; et al. Green approach for hierarchical nanostructured Ag-ZnO and their photocatalytic performance under sunlight. Catal. Today 2016, 260, 126–134. [Google Scholar] [CrossRef]
- Sun, F.; Qiao, X.; Tan, F.; Wang, W.; Qiu, X. One-step microwave synthesis of Ag/ZnO nanocomposites with enhanced photo-catalytic performance. J. Mater. Sci. 2012, 47, 7262–7268. [Google Scholar] [CrossRef]
- Yang, X.; Qiu, L.; Luo, X. ZIF-8 derived Ag-doped ZnO photocatalyst with enhanced photocatalytic activity. RSC Adv. 2018, 8, 4890–4894. [Google Scholar] [CrossRef]
- Rafaie, H.; Nor, R.; Azmina, M.; Ramli, N.; Mohamed, R. Decoration of ZnO microstructures with Ag nanoparticles enhanced the catalytic photodegradation of methylene blue dye. J. Environ. Chem. Eng. 2017, 5, 3963–3972. [Google Scholar] [CrossRef]
- Shaikh, A.; Patil, M.; Jagdale, B.; Adole, V. Synthesis and characterization of Ag doped ZnO nanomaterial as an effective photocatalyst for photocatalytic degradation of Eriochrome Black T dye and antimicrobial agent. Inorg. Chem. Commun. 2023, 151, 110570. [Google Scholar] [CrossRef]
- Rani, B.; Praveenkumar, A.; Ramesh, S.; Ravi, K. Ag implanted ZnO hierarchical nanoflowers for photoelectrochemical wa-ter-splitting applications. J. Mater. Sci. Mater. Electron. 2019, 30, 731–745. [Google Scholar] [CrossRef]
- Gnanaprakasam, A.; Sivakumar, V.; Thirumarimurugan, M. A study on Cu and Ag doped ZnO nanoparticles for the photo-catalytic degradation of brilliant green dye: Synthesis and characterization. Water Sci. Technol. 2016, 74, 1426–1435. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Yu, S.; Zhang, H. Preparation and performance analysis of Ag/ZnO humidity sensor. Sensors 2021, 21, 857. [Google Scholar] [CrossRef] [PubMed]
- Yıldırım, O.A.; Unalan, H.E.; Durucan, C. Highly efficient room temperature synthesis of silver-doped zinc oxide (ZnO: Ag) nanoparticles: Structural, optical, and photocatalytic properties. J. Am. Ceram. Soc. 2013, 96, 766–773. [Google Scholar] [CrossRef]
- Muchuweni, E.; Sathiaraj, T.; Nyakotyo, H. Synthesis and characterization of zinc oxide thin films for optoelectronic applications. Heliyon 2017, 3, e00285. [Google Scholar] [CrossRef]
- Primo, O.; Horsth, D.; Correa, S.; Das, A.; Bittencourt, C.; Umek, P.; Buzanich, A.; Radtke, M.; Yusenko, K.; Zanette, C. Synthe-sis and Characterization of Ag/ZnO Nanoparticles for Bacteria Disinfection inWater. Nanomaterials 2022, 12, 1764. [Google Scholar] [CrossRef]
- Khosravi-Gandomani, S.; Yousefi, R.; Jamali-Sheini, F.; Huang, N. Optical andelectrical properties of p-type Ag-doped ZnO nanostructures. Ceram. Int. 2014, 40, 7957–7963. [Google Scholar] [CrossRef]
- Krasil’nikov, V.; Dyachkova, T.; Tyutyunnik, A.; Gyrdasova, O.; Melkozerova, M.; Baklanova, I.; Perevozchikova, Y.A.; Emelyanova, S.M.; Weber, H.; Marchenkov, V. Magnetic and optical properties as well as EPR studies of polycrystalline ZnO synthesized from different precursors. Mat. Res. Bull. 2018, 97, 553–559. [Google Scholar] [CrossRef]
- Ammar, A.; Yildirim, I.; Aleinawi, M.; BulduAkturk, M.; Turhan, N.; Nadupalli, S.; Rostas, A.; Erdem, E. Multifrequency EPR spectroscopy study of Mn, Fe, and Cu doped nanocrystalline ZnO. Mat. Res. Bull. 2023, 160, 112117. [Google Scholar] [CrossRef]
- Li, W.; Zhang, H.; Zhang, X.; Qin, G.; Li, H.; Xiong, Y.; Ye, L.; Ruan, H.; Tong, C.; Kong, C.; et al. Non-axial NO-VZn shallow acceptor complexes in nitrogen implanted p-type ZnO thin films. Appl. Surf. Sci. 2020, 529, 147168. [Google Scholar] [CrossRef]
- Stan, M.; Popa, A.; Toloman, D.; Dehelean, A.; Lung, I.; Katona, G. Enhanced photocatalytic degradation properties of zinc oxide nanoparticles synthesized by using plantex tracts. Mat. Sci. Semmicond. Proc. 2015, 39, 23–29. [Google Scholar] [CrossRef]
- Deng, R.; Zou, Y.; Tang, H. Correlation between electrical, optical properties and Ag2+ centers of ZnO:Ag thin films. Phys. B 2008, 403, 2004–2007. [Google Scholar] [CrossRef]
- Dhabekar, B.; Menon, S.; Raja, E.; Sanaye, S.; Rao, T.; Bhatt, B.; Kher, R. ESR and TL mechanism in CaSO4: Ag co-doped phosphors. J. Phys. D Appl. Phys. 2006, 39, 2488–2493. [Google Scholar] [CrossRef]
- Rini, A.; Nabilla, A.; Rati, Y. Microwave-assisted biosynthesis and characterization of ZnO film for photocatalytic application in methylene blue degradation. Commun. Sci. Technol. 2021, 6, 69–73. [Google Scholar]
- Singh, R.; Barman, P.B.; Sharma, D. Synthesis, structural and optical properties of Ag doped ZnO nanoparticles with enhanced photocatalytic properties by photo degradation of organic dyes. J. Mater. Sci. Mater. Electron. 2017, 28, 5705–5717. [Google Scholar] [CrossRef]
- Kotlhao, K.; Mtunzi, F.M.; Pakade, V.; Laloo, N.; Ejidike, I.P.; Modise, S.J.; Moutloali, R.M.; Klink, M.J. Enhancing the photo-catalytic degradation of selected chlorophenols using Ag/ZnO nanocomposites. MRS Adv. 2018, 3, 2129–2136. [Google Scholar] [CrossRef]
- Haq, S.; Ali, M.; Mezni, A.; Hedfi, A.; Rehman, W.; Sarwar, G. Fabrication and characterization of zinc oxide nanoparticles for photocatalytic application. Dig. J. Nanomat. Biostr. 2022, 17, 499–505. [Google Scholar] [CrossRef]
- Azfar, A.; Kasim, M.; Lokman, I.; Rafaie, H.; Mastuli, M. Comparative study on photocatalytic activity of transition metals (Ag and Ni)-doped ZnO nanomaterials synthesized via sol-gel method. R. Soc. Open Sci. 2020, 7, 191590. [Google Scholar] [CrossRef]
- Yazdani, O.; Irandoust, M.; Ghasemi, J.; Hooshmand, S. Thermodynamic study of the dimerization equilibrium of methylene blue, methylene green and thiazole orange at various surfactant concentrations and different ionic strengths and in mixed solvents by spectral titration and chemometric analysis. Dye Pigment. 2012, 92, 1031–1041. [Google Scholar] [CrossRef]
- Nayak, S.; Das, K.; Parida, K. Indulgent of the physiochemical features of MgCr-LDH nanosheets towards photodegradation process of methylene blue. J. Colloid Interface Sci. 2023, 634, 121–137. [Google Scholar] [CrossRef] [PubMed]

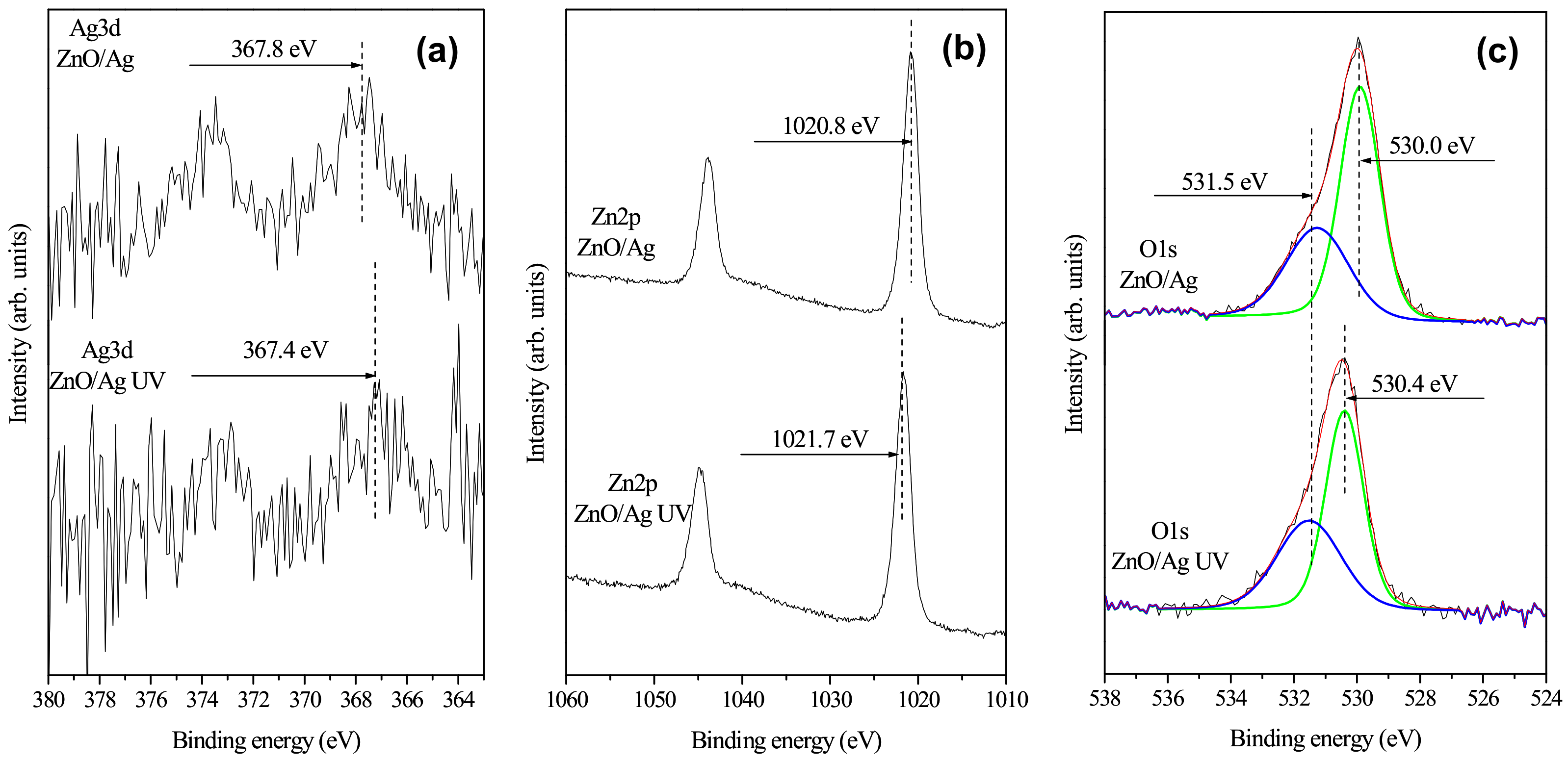


| Sample | O1s | Zn2p | Ag3d |
|---|---|---|---|
| Ag/ZnO | 48.36 | 51.57 | 0.07 |
| Ag-UV/ZnO | 49.51 | 50.41 | 0.08 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ivanova, D.; Mladenova, R.; Kolev, H.; Kaneva, N. Effect of Ultraviolet Illumination on the Fixation of Silver Ions on Zinc Oxide Films and Their Photocatalytic Efficiency. Catalysts 2023, 13, 1121. https://doi.org/10.3390/catal13071121
Ivanova D, Mladenova R, Kolev H, Kaneva N. Effect of Ultraviolet Illumination on the Fixation of Silver Ions on Zinc Oxide Films and Their Photocatalytic Efficiency. Catalysts. 2023; 13(7):1121. https://doi.org/10.3390/catal13071121
Chicago/Turabian StyleIvanova, Dobrina, Ralitsa Mladenova, Hristo Kolev, and Nina Kaneva. 2023. "Effect of Ultraviolet Illumination on the Fixation of Silver Ions on Zinc Oxide Films and Their Photocatalytic Efficiency" Catalysts 13, no. 7: 1121. https://doi.org/10.3390/catal13071121
APA StyleIvanova, D., Mladenova, R., Kolev, H., & Kaneva, N. (2023). Effect of Ultraviolet Illumination on the Fixation of Silver Ions on Zinc Oxide Films and Their Photocatalytic Efficiency. Catalysts, 13(7), 1121. https://doi.org/10.3390/catal13071121








