Dispersible Supertetrahedral Chalcogenide T3 Clusters: Photocatalytic Activity and Photogenerated Carrier Dynamics
Abstract
:1. Introduction
2. Results and Discussion
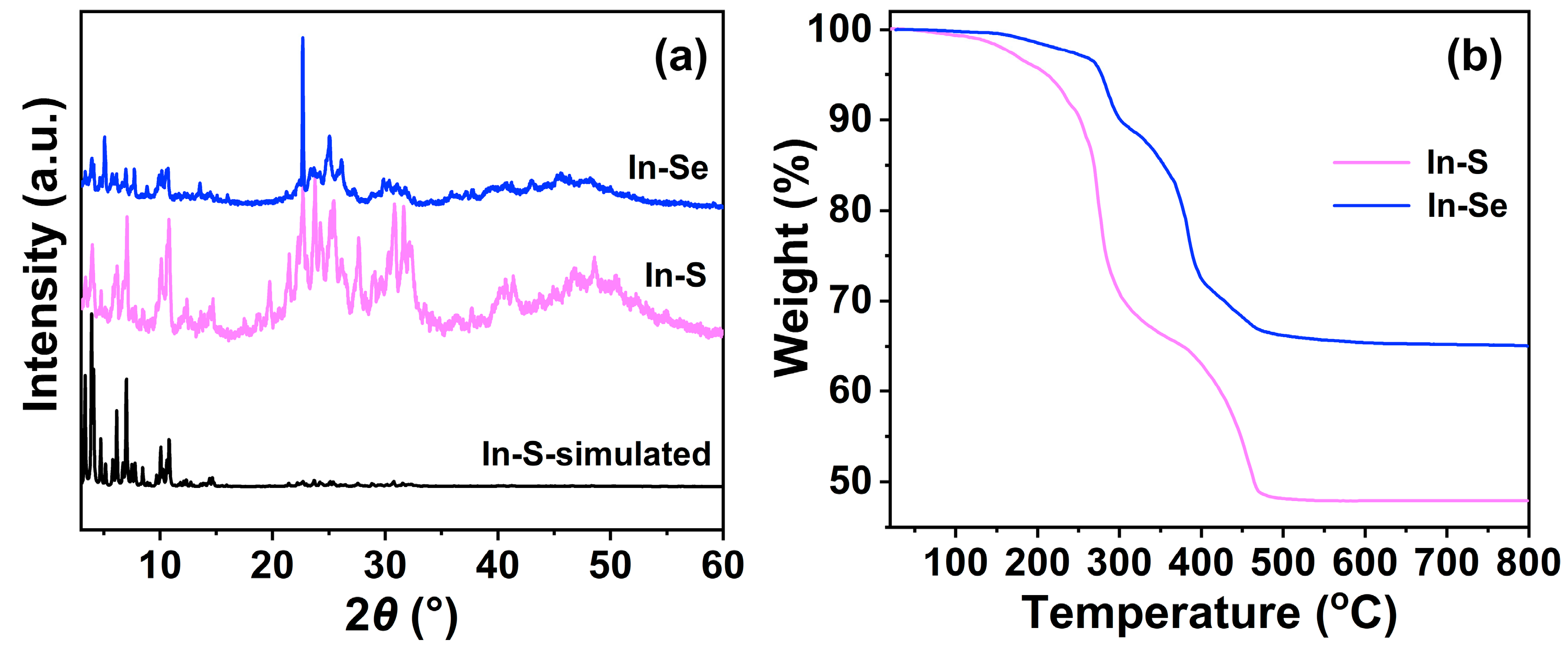
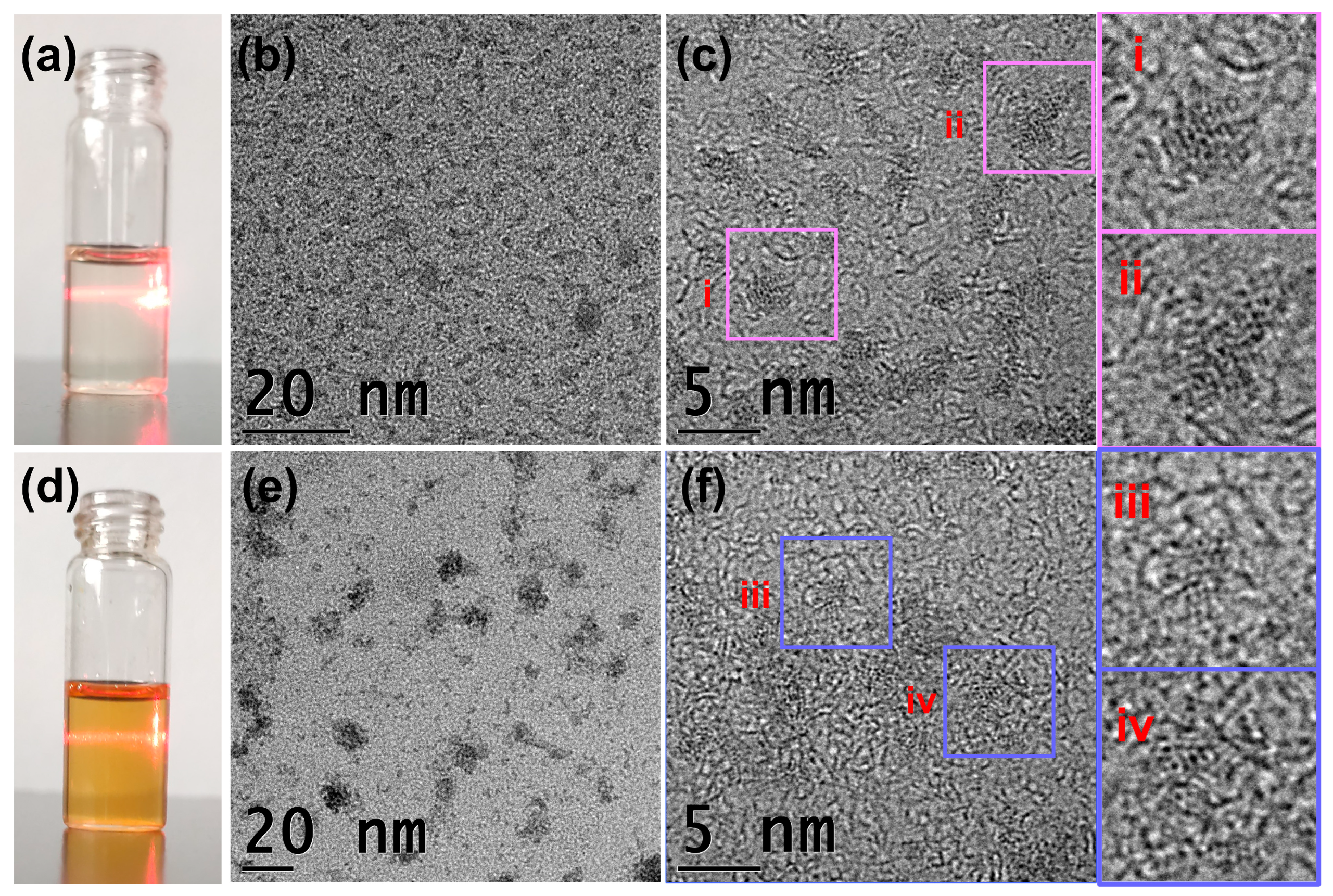
2.1. Crystal Structural Description
2.2. Crystal Structural Description
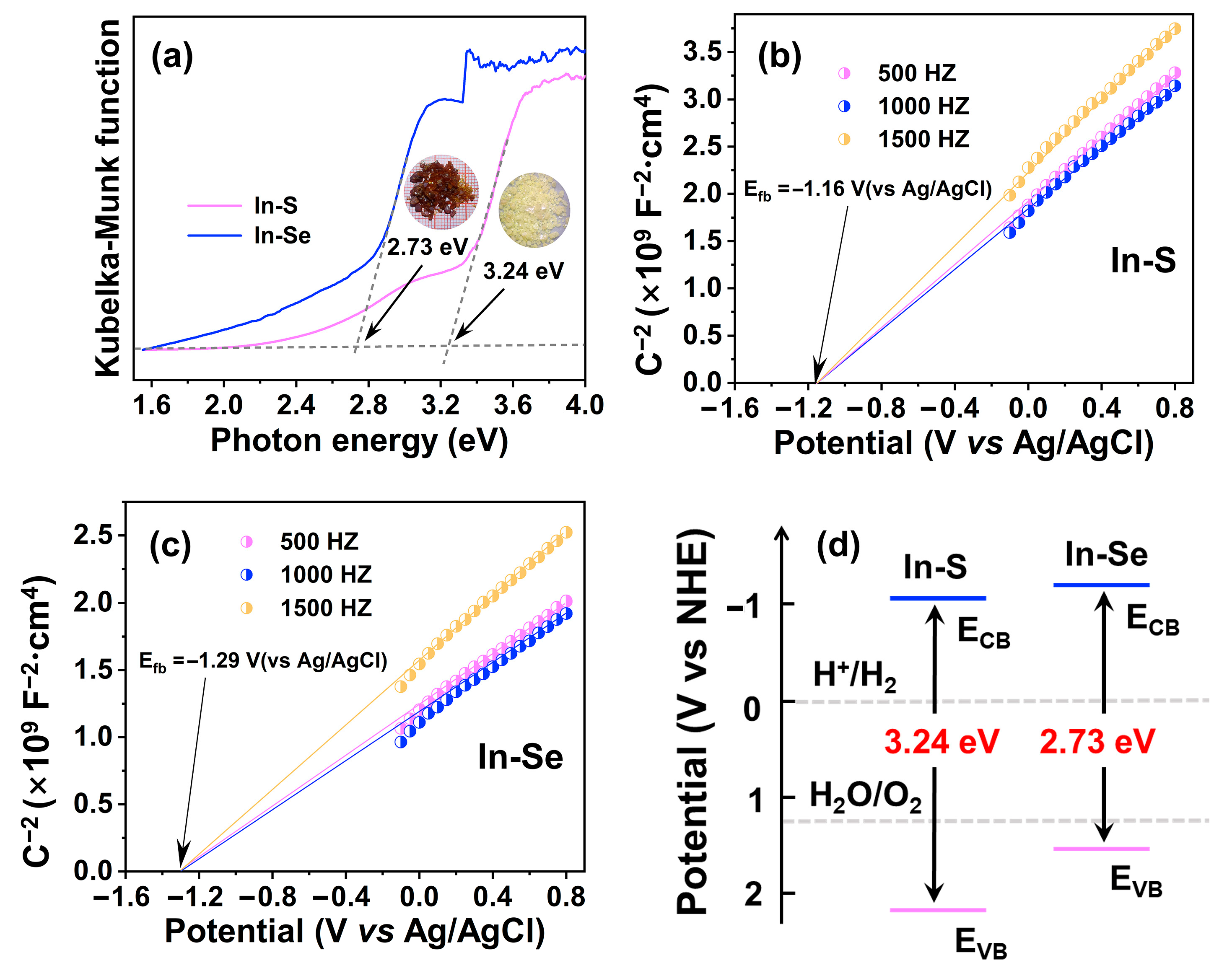
2.3. Energy Band Structure and Photocatalytic Properties
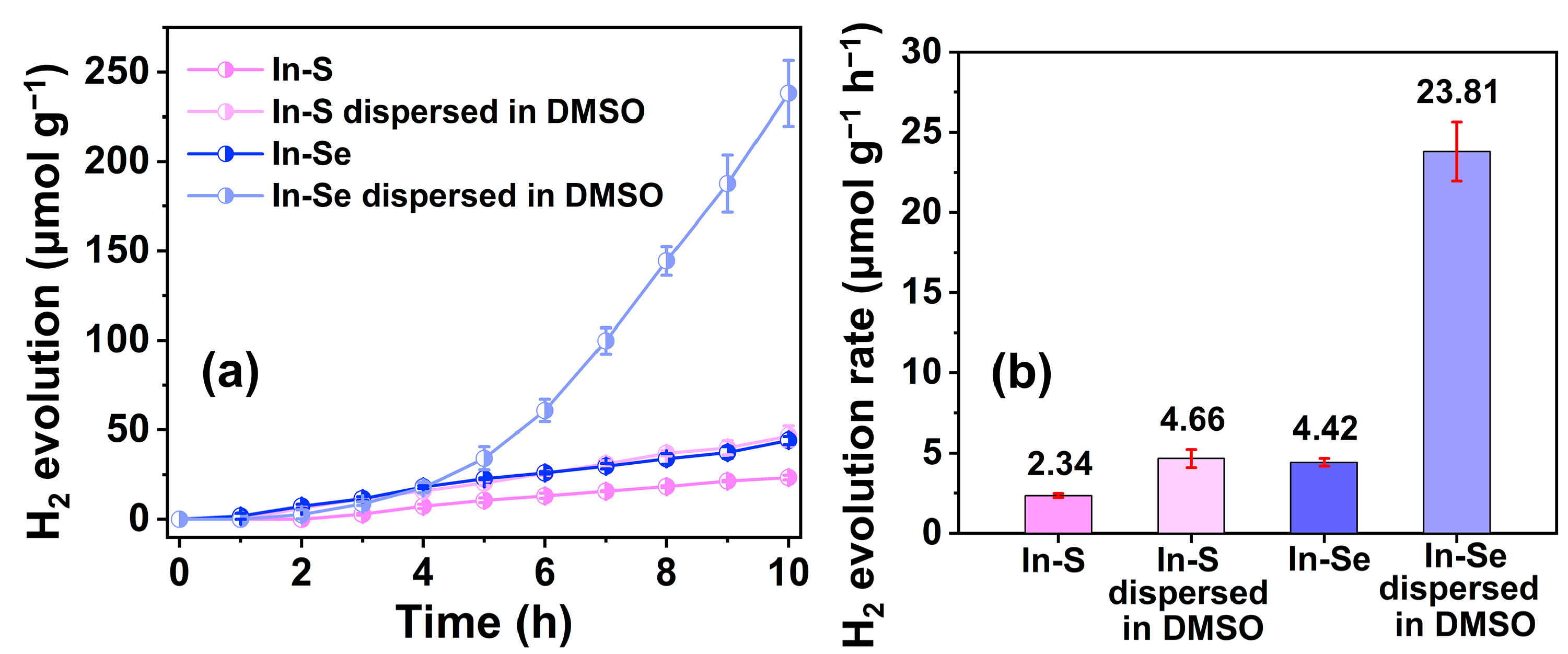
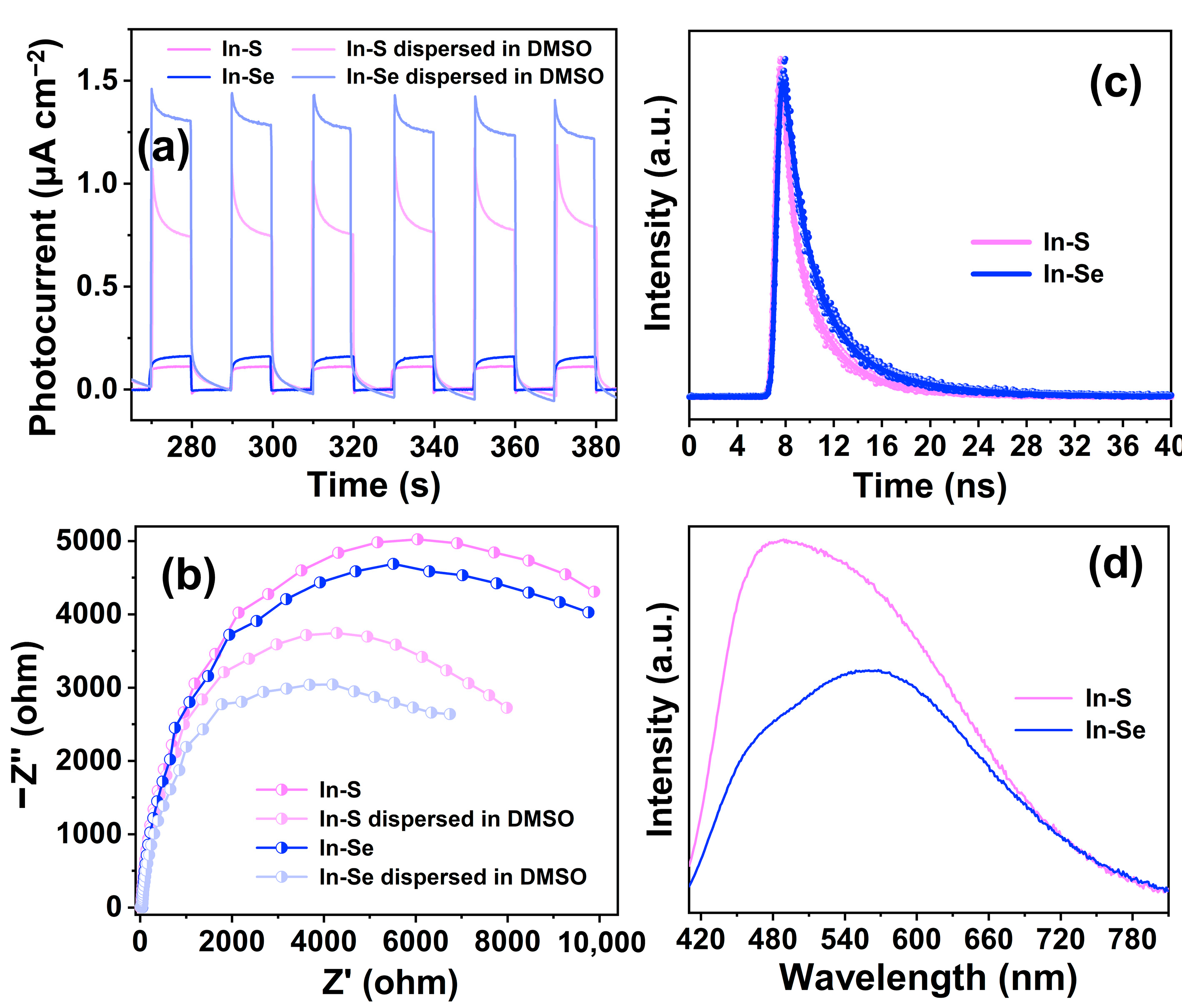
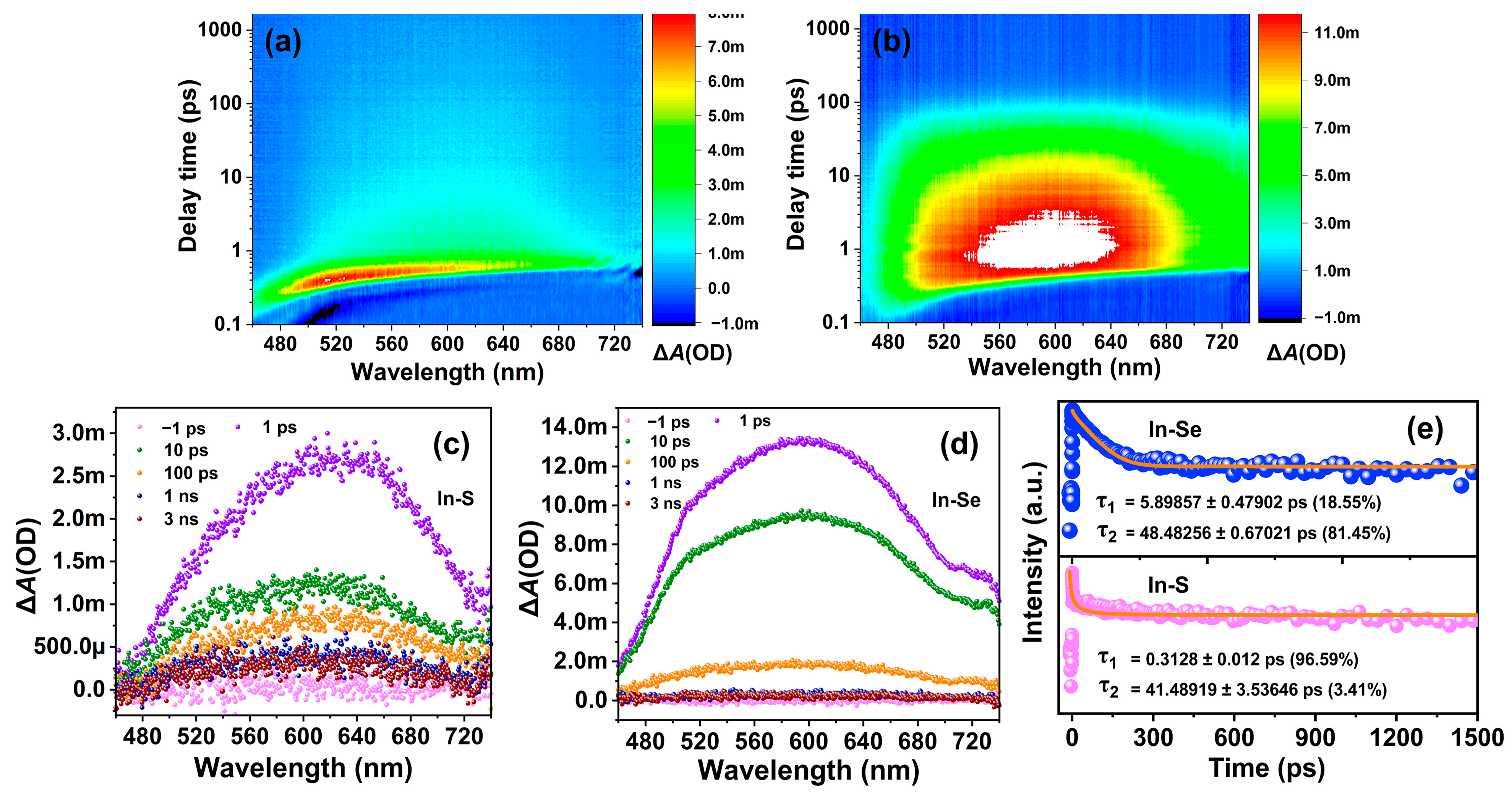
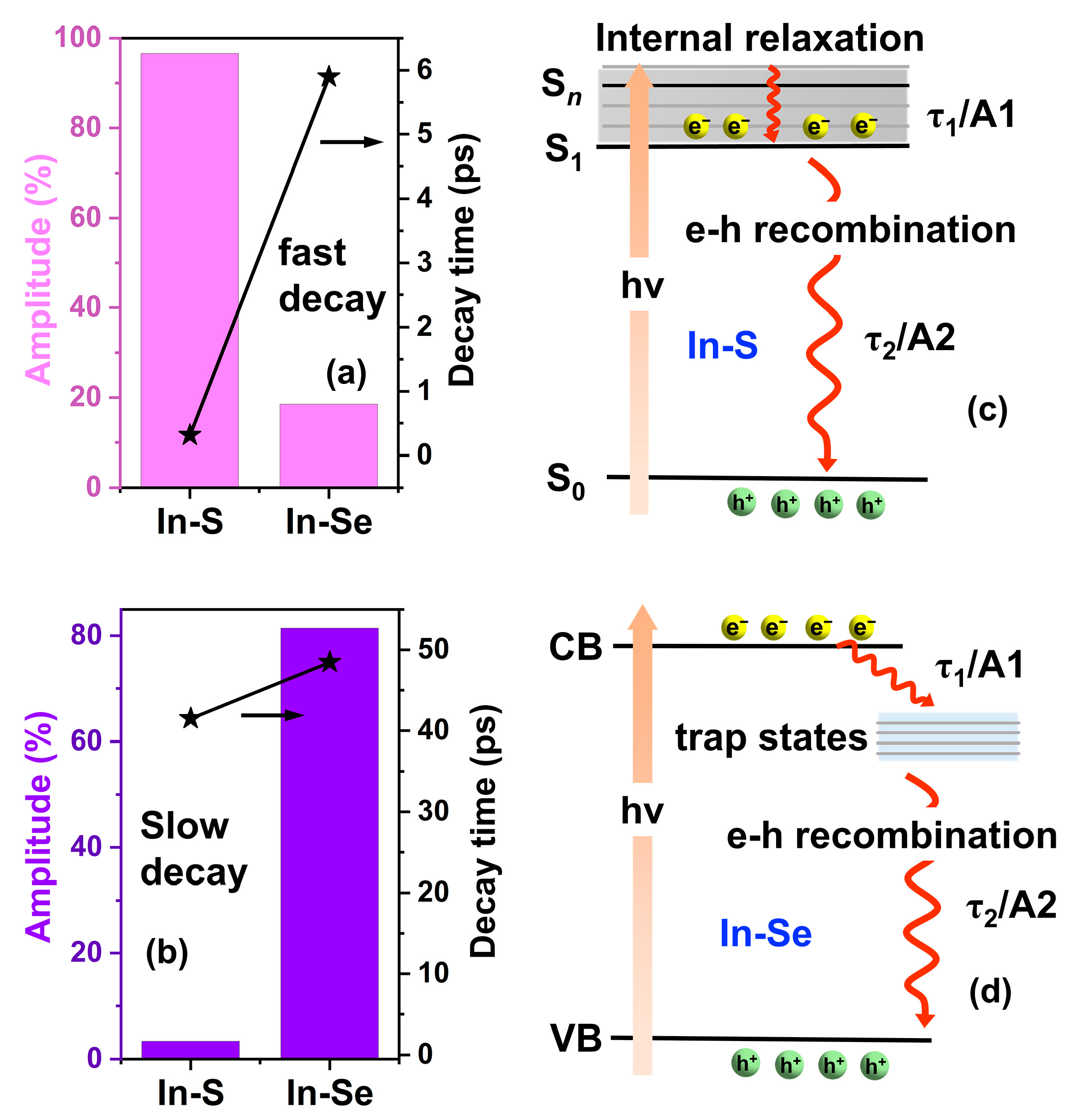
2.4. Photocatalytic Performance Analysis
3. Materials and Methods
3.1. Materials
3.2. Preparation of Catalysts
3.3. Characterizations
3.4. Photocatalytic Performance Tests
3.5. Electrochemical Measurements
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Asif, M.; Muneer, T. Energy supply, its demand and security issues for developed and emerging economies. Renew. Sustain. Energy Rev. 2007, 11, 1388–1413. [Google Scholar] [CrossRef]
- Karl, T.R.; Trenberth, K.E. Modern global climate change. Science 2003, 302, 1719–1723. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Balat, M. Potential importance of hydrogen as a future solution to environmental and transportation problems. Int. J. Hydrogen Energy 2008, 33, 4013–4029. [Google Scholar] [CrossRef]
- Fujishima, A.; Honda, K. Electrochemical Photolysis of Water at a Semiconductor Electrode. Nature 1972, 238, 37–38. [Google Scholar] [CrossRef] [PubMed]
- Gielen, D.; Boshell, F.; Saygin, D.; Bazilian, M.D.; Wagner, N.; Gorini, R. The role of renewable energy in the global energy transformation. Energy Strategy Rev. 2019, 24, 38–50. [Google Scholar] [CrossRef]
- Panwar, N.L.; Kaushik, S.C.; Kothari, S. Role of renewable energy sources in environmental protection: A review. Renew. Sustain. Energy Rev. 2011, 15, 1513–1524. [Google Scholar] [CrossRef]
- Wang, J.; Shi, Y.; Sun, H.; Shi, W.; Guo, F. Fabrication of Bi4Ti3O12/ZnIn2S4 S-scheme heterojunction for achieving efficient photocatalytic hydrogen production. J. Alloys Compd. 2023, 930, 167450. [Google Scholar] [CrossRef]
- Wang, L.; Yang, G.; Wang, S.; Wang, J.; Nasir, M.S.; Wang, C.; Peng, S.; Yan, W.; Ramakrishna, S. Fabrication of hierarchically one-dimensional ZnxCd1-xS/NiTiO3 nanostructures and their enhanced photocatalytic water splitting activity. Int. J. Hydrogen Energy 2019, 44, 30974–30985. [Google Scholar] [CrossRef]
- Yadav, A.A.; Hunge, Y.M.; Dhodamani, A.G.; Kang, S.-W. Hydrothermally Synthesized Ag@MoS2 Composite for Enhanced Photocatalytic Hydrogen Production. Catalysts 2023, 13, 716. [Google Scholar] [CrossRef]
- Kundu, S.; Patra, A. Nanoscale Strategies for Light Harvesting. Chem. Rev. 2017, 117, 712–757. [Google Scholar] [CrossRef] [Green Version]
- Liu, Q.; Chen, H.; Wang, Z.; Weng, Y. Observation of the Polaron Excited State in a Single-Crystal ZnO. J. Phys. Chem. C 2021, 125, 10274–10283. [Google Scholar] [CrossRef]
- Yun, H.J.; Paik, T.; Diroll, B.; Edley, M.E.; Baxter, J.B.; Murray, C.B. Nanocrystal Size-Dependent Efficiency of Quantum Dot Sensitized Solar Cells in the Strongly Coupled CdSe Nanocrystals/TiO2 System. ACS Appl. Mater. Interfaces 2016, 8, 14692–14700. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Gao, H.-Y.; Yang, Y.; Lin, P.-B.; Yuan, J.; Shangguan, W.-F.; Su, J.-C.; Sun, Y.-Z. Polymerizable Complex Synthesis of Protonated Form of Layered Perovskite K0.5La0.5Bi2Ta2O9 for Water Splitting into Hydrogen. Acta Phys. Chim. Sin. 2012, 28, 2911–2916. [Google Scholar]
- He, J.; Zhao, L.-D.; Zheng, J.-C.; Doak, J.W.; Wu, H.; Wang, H.-Q.; Lee, Y.; Wolverton, C.; Kanatzidis, M.G.; Dravid, V.P. Role of Sodium Doping in Lead Chalcogenide Thermoelectrics. J. Am. Chem. Soc. 2013, 135, 4624–4627. [Google Scholar] [CrossRef] [PubMed]
- Pradhan, N.; Peng, X. Efficient and color-tunable Mn-doped ZnSe nanocrystal emitters: Control of optical performance via greener synthetic chemistry. J. Am. Chem. Soc. 2007, 129, 3339–3347. [Google Scholar] [CrossRef]
- Feng, P.Y.; Bu, X.H.; Zheng, N.F. The interface chemistry between chalcogenide clusters and open framework chalcogenides. Acc. Chem. Res. 2005, 38, 293–303. [Google Scholar] [CrossRef]
- Zhang, J.; Bu, X.; Feng, P.; Wu, T. Metal Chalcogenide Supertetrahedral Clusters: Synthetic Control over Assembly, Dispersibility, and Their Functional Applications. Acc. Chem. Res. 2020, 53, 2261–2272. [Google Scholar] [CrossRef]
- Li, Z.-Q.; Mo, C.-J.; Guo, Y.; Xu, N.-N.; Zhu, Q.-Y.; Dai, J. Discrete supertetrahedral CuInS nanoclusters and their application in fabrication of cluster-sensitized TiO2 photoelectrodes. J. Mater. Chem. A 2017, 5, 8519–8525. [Google Scholar] [CrossRef]
- Wu, J.; Chen, N.; Wu, T. Two discrete dimeric metal-chalcogenide supertetrahedral clusters. Dalton Trans. 2023, 52, 5019–5022. [Google Scholar] [CrossRef]
- Xue, C.; Zhang, L.; Wang, X.; Hu, D.; Wang, X.-L.; Zhang, J.; Zhou, R.; Li, D.-S.; Su, H.; Wu, T. Enhanced Water Dispersibility of Discrete Chalcogenide Nanoclusters with a Sodalite-Net Loose-Packing Pattern in a Crystal Lattice. Inorg. Chem. 2020, 59, 15587–15594. [Google Scholar] [CrossRef]
- Zhang, J.X.; Feng, P.Y.; Bu, X.H.; Wu, T. Atomically precise metal chalcogenide supertetrahedral clusters: Frameworks to molecules, and structure to function. Natl. Sci. Rev. 2022, 9, nwab076. [Google Scholar] [CrossRef]
- Li, X.; Yu, J.; Low, J.; Fang, Y.; Xiao, J.; Chen, X. Engineering heterogeneous semiconductors for solar water splitting. J. Mater. Chem. A 2015, 3, 2485–2534. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, J.; Wu, T.; Bu, X.; Feng, P. Three-Dimensional Open Framework Built from Cu-S Icosahedral Clusters and Its Photocatalytic Property. J. Am. Chem. Soc. 2008, 130, 15238–15239. [Google Scholar] [CrossRef] [PubMed]
- Zheng, N.; Bu, X.H.; Vu, H.; Feng, P.Y. Open-framework chalcogenides as visible-light photocatalysts for hydrogen generation from water. Angew. Chem. Int. Ed. 2005, 44, 5299–5303. [Google Scholar] [CrossRef] [PubMed]
- Bendova, M.; Puchberger, M.; Schubert, U. Characterization of “Cd10S4(SPh)12”, the Thermal Decomposition Product of (NMe4)4[Cd10S4(SPh)16]: Synthesis of a Neutral Cd54 Sulfide Cluster and of a Polymeric Chain of Thiolate-Bridged Cd17 Sulfide Clusters. Eur. J. Inorg. Chem. 2010, 2010, 3299–3306. [Google Scholar] [CrossRef]
- Eichhoefer, A.; Hampe, O. Investigating the Thermolysis Products of [Cd10Se4(SePh)12(PnPr3)4]—The New Cluster Ion [Cd17Se4(SePh)28]2−. J. Clust. Sci. 2007, 18, 494–504. [Google Scholar] [CrossRef]
- Eichhofer, A.; Hampe, O. Mass spectrometric study on the neutral nanocluster [Cd32Se14(SePh)36L4] (L = THF, OPPh3) upon charged ligand exchange. Chem. Phys. Lett. 2005, 407, 186–191. [Google Scholar] [CrossRef]
- Gaumet, J.J.; Khitrov, G.A.; Strouse, G.F. Mass Spectrometry Analysis of the 1.5 nm Sphalerite−CdS Core of [Cd32S14(SC6H5)36‚DMF4]. Nano Lett. 2002, 2, 375–379. [Google Scholar] [CrossRef]
- Matheis, K.; Eichhoefer, A.; Weigend, F.; Ehrler, O.T.; Hampe, O.; Kappes, M.M. Probing the Influence of Size and Composition on the Photoelectron Spectra of Cadmium Chalcogenide Cluster Dianions. J. Phys. Chem. C 2012, 116, 13800–13809. [Google Scholar] [CrossRef]
- Fan, X.B.; Yu, S.; Wang, X.; Li, Z.J.; Zhan, F.; Li, J.X.; Gao, Y.J.; Xia, A.D.; Tao, Y.; Li, X.B.; et al. Susceptible Surface Sulfide Regulates Catalytic Activity of CdSe Quantum Dots for Hydrogen Photogeneration. Adv. Mater. 2019, 31, 1804872. [Google Scholar] [CrossRef] [PubMed]
- Hao, M.; Hu, Q.; Zhang, Y.; Luo, M.; Wang, Y.; Hu, B.; Li, J.; Huang, X. Soluble Supertetrahedral Chalcogenido T4 Clusters: High Stability and Enhanced Hydrogen Evolution Activities. Inorg. Chem. 2019, 58, 5126–5133. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wang, Y.; Peng, Y.; Hu, Q.; Li, J.; Huang, X. Ionic-Liquid-Assisted Precursor Route Syntheses of Highly Dispersed Supertetrahedral T3 In-S/Se Clusters for Photocatalytic Hydrogen Production. ChemistrySelect 2022, 7, e202200585. [Google Scholar] [CrossRef]
- Wang, Y.; Hu, Q.; Jin, J.; Li, J.; Li, J.; Huang, X. [Bmmim]6[In10Se16Cl4]·(MIm)2: An organic-ligand free discrete T3 cluster for efficient hydrogen evolution under visible light irradiation. Dalton Trans. 2020, 49, 5020–5023. [Google Scholar] [CrossRef]
- Zhang, J.; Qin, C.; Zhong, Y.; Wang, X.; Wang, W.; Hu, D.; Liu, X.; Xue, C.; Zhou, R.; Shen, L.; et al. Atomically precise metal-chalcogenide semiconductor molecular nanoclusters with high dispersibility: Designed synthesis and intracluster photocarrier dynamics. Nano Res. 2020, 13, 2828–2836. [Google Scholar] [CrossRef]
- Liu, D.; Fan, X.; Wang, X.; Hu, D.; Xue, C.; Liu, Y.; Wang, Y.; Zhu, X.; Guo, J.; Lin, H.; et al. Cooperativity by Multi-Metals Confined in Supertetrahedral Sulfide Nanoclusters To Enhance Electrocatalytic Hydrogen Evolution. Chem. Mater. 2019, 31, 553–559. [Google Scholar] [CrossRef]
- Wang, Y.; Zhu, Z.; Sun, Z.; Hu, Q.; Li, J.; Jiang, J.; Huang, X. Discrete Supertetrahedral T5 Selenide Clusters and Their Se/S Solid Solutions: Ionic-Liquid-Assisted Precursor Route Syntheses and Photocatalytic Properties. Chem. Eur. J. 2020, 26, 1624–1632. [Google Scholar] [CrossRef]
- Li, P.; He, Y.; Guang, J.; Weng, L.; Zhao, J.C.-G.; Xiang, S.; Chen, B. A Homochiral Microporous Hydrogen-Bonded Organic Framework for Highly Enantioselective Separation of Secondary Alcohols. J. Am. Chem. Soc. 2014, 136, 547–549. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Zhang, H.; Dong, J.; Qiu, M.; Kong, J.; Zhang, Y.; Li, Y.; Xu, G.; Zhang, J.; Ye, J. Surface step decoration of isolated atom as electron pumping: Atomic-level insights into visible-light hydrogen evolution. Nano Energy 2018, 45, 109–117. [Google Scholar] [CrossRef]
- Wheeler, D.A.; Zhang, J.Z. Exciton Dynamics in Semiconductor Nanocrystals. Adv. Mater. 2013, 25, 2878–2896. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.-Q.; Hu, J.; Wang, D.; Li, Z.; Zhang, Q.; Luo, Y.; Yu, S.-H.; Jiang, H.-L. Visible-Light Photoreduction of CO2 in a Metal-Organic Framework: Boosting Electron-Hole Separation via Electron Trap States. J. Am. Chem. Soc. 2015, 137, 13440–13443. [Google Scholar] [CrossRef]
- Chia, C.-H.; Fan, W.-C.; Lin, Y.-C.; Chou, W.-C. Radiative recombination of indirect exciton in type-II ZnSeTe/ZnSe multiple quantum wells. J. Lumin. 2011, 131, 956–959. [Google Scholar] [CrossRef]
- Dahan, P.; Fleurov, V.; Thurian, P.; Heitz, R.; Hoffmann, A.; Broser, I. Properties of the intermediately bound alpha-, beta- and gamma-excitons in ZnO:Cu. J. Phys. Condens. Matter 1998, 10, 2007–2019. [Google Scholar] [CrossRef]
- Jiao, X.; Chen, Z.; Li, X.; Sun, Y.; Gao, S.; Yan, W.; Wang, C.; Zhang, Q.; Lin, Y.; Luo, Y.; et al. Defect-Mediated Electron-Hole Separation in One-Unit-Cell ZnIn2S4 Layers for Boosted Solar-Driven CO2 Reduction. J. Am. Chem. Soc. 2017, 139, 7586–7594. [Google Scholar] [CrossRef] [PubMed]
- Chi, Z.; Chen, H.; Chen, Z.; Zhao, Q.; Chen, H.; Weng, Y.-X. Ultrafast Energy Dissipation via Coupling with Internal and External Phonons in Two-Dimensional MoS2. ACS Nano 2018, 12, 8961–8969. [Google Scholar] [CrossRef]
- Wang, B.; Yang, S.Z.; Chen, H.L.; Gao, Q.; Weng, Y.X.; Zhu, W.S.; Liu, G.P.; Zhang, Y.; Ye, Y.Z.; Zhu, H.Y.; et al. Revealing the role of oxygen vacancies in bimetallic PbBiO2Br atomic layers for boosting photocatalytic CO2 conversion. Appl. Catal. B Environ. 2020, 277, 52–54. [Google Scholar] [CrossRef]
- Tauc, J. Absorption edge and internal electric fields in amorphous semiconductors. Mater. Res. Bull. 1970, 5, 721–729. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yin, H.; Liu, Y.; Ablez, A.; Wang, Y.; Hu, Q.; Huang, X. Dispersible Supertetrahedral Chalcogenide T3 Clusters: Photocatalytic Activity and Photogenerated Carrier Dynamics. Catalysts 2023, 13, 1160. https://doi.org/10.3390/catal13081160
Yin H, Liu Y, Ablez A, Wang Y, Hu Q, Huang X. Dispersible Supertetrahedral Chalcogenide T3 Clusters: Photocatalytic Activity and Photogenerated Carrier Dynamics. Catalysts. 2023; 13(8):1160. https://doi.org/10.3390/catal13081160
Chicago/Turabian StyleYin, Haiyan, Yifan Liu, Abdusalam Ablez, Yanqi Wang, Qianqian Hu, and Xiaoying Huang. 2023. "Dispersible Supertetrahedral Chalcogenide T3 Clusters: Photocatalytic Activity and Photogenerated Carrier Dynamics" Catalysts 13, no. 8: 1160. https://doi.org/10.3390/catal13081160




