Metal Bi Loaded Bi2Ti2O7/CaTiO3 for Enhanced Photocatalytic Efficiency for NO Removal under Visible Light
Abstract
:1. Introduction
2. Results and Discussion
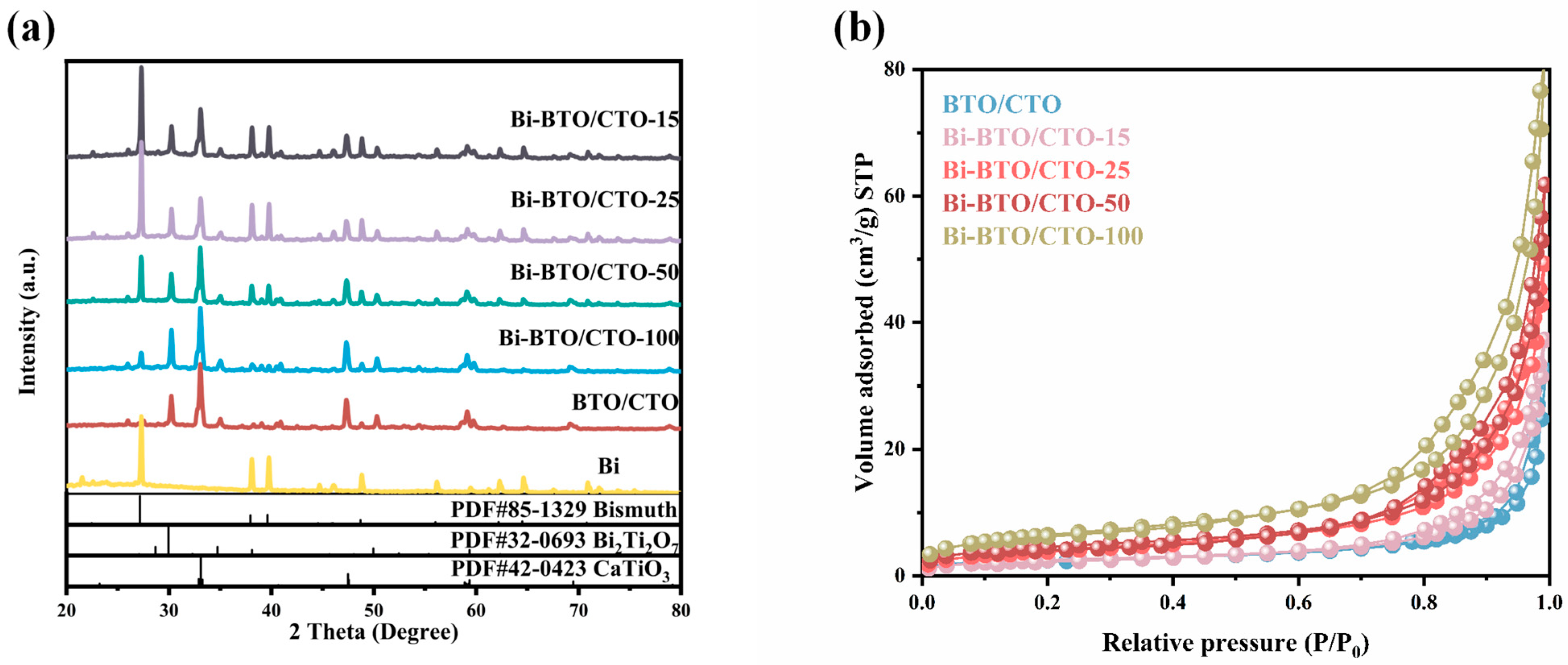
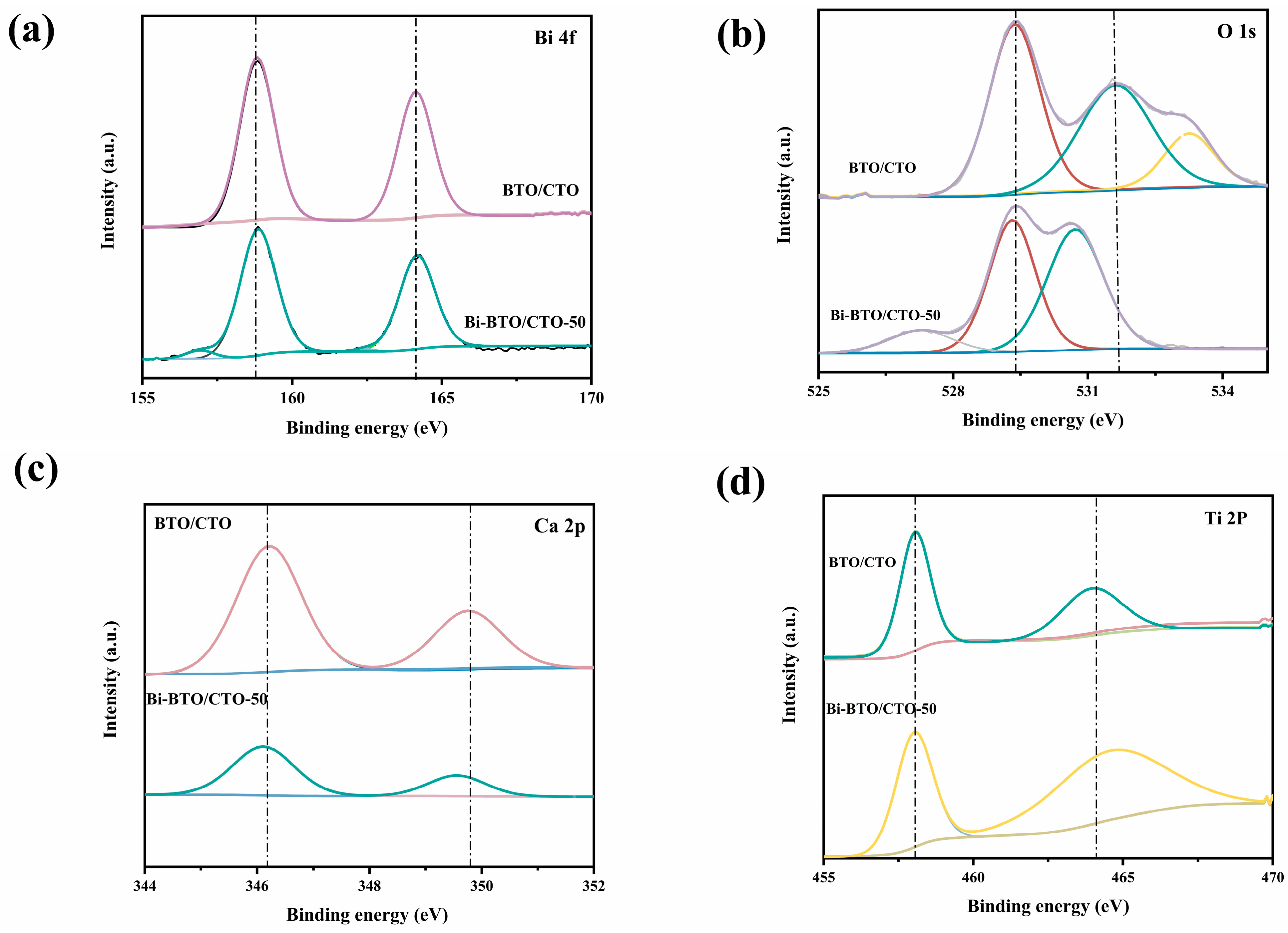
2.1. Phase and Composition
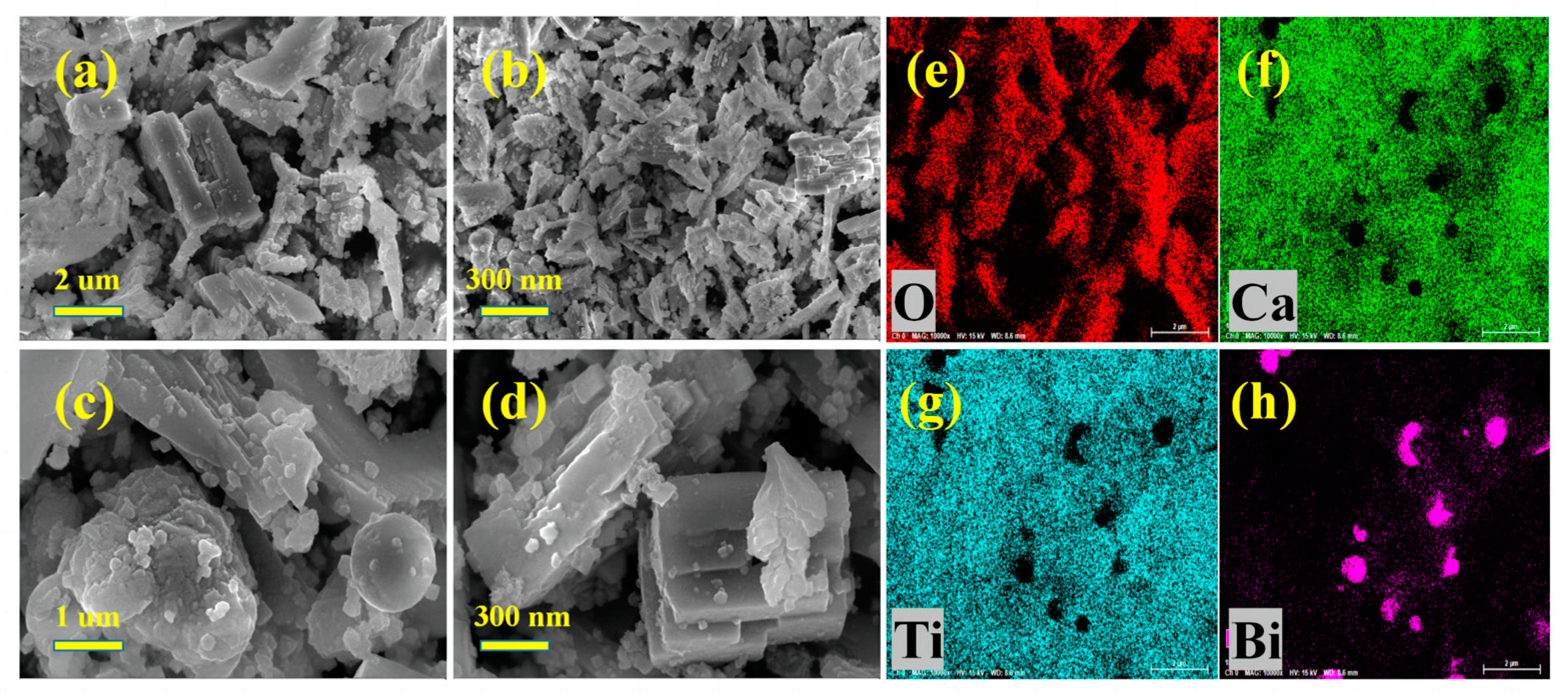
2.2. Morphology of the Photocatalysts
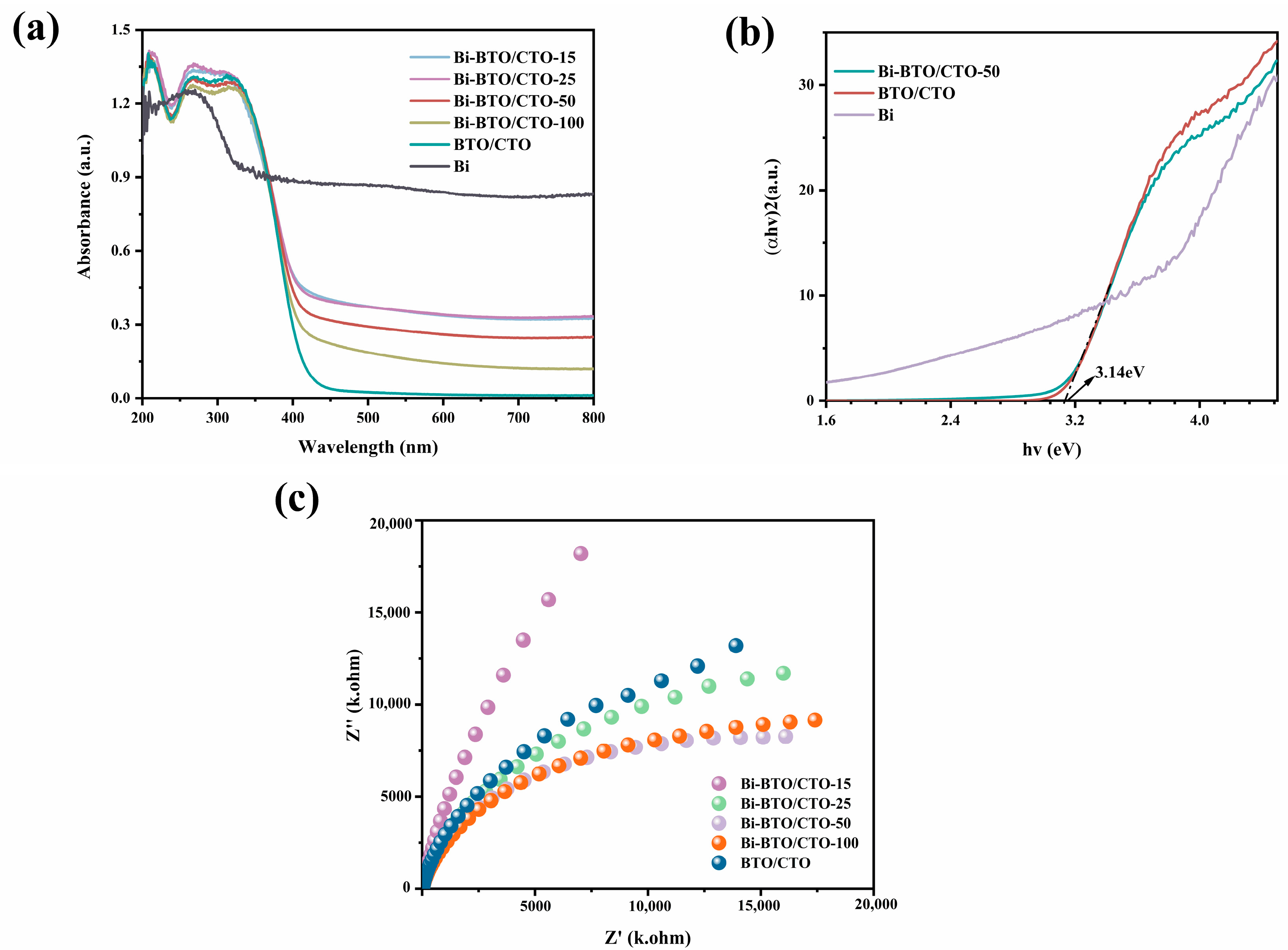
2.3. Optical and Photoelectrochemical Properties
2.4. Photocatalytic Performance of NO Removal
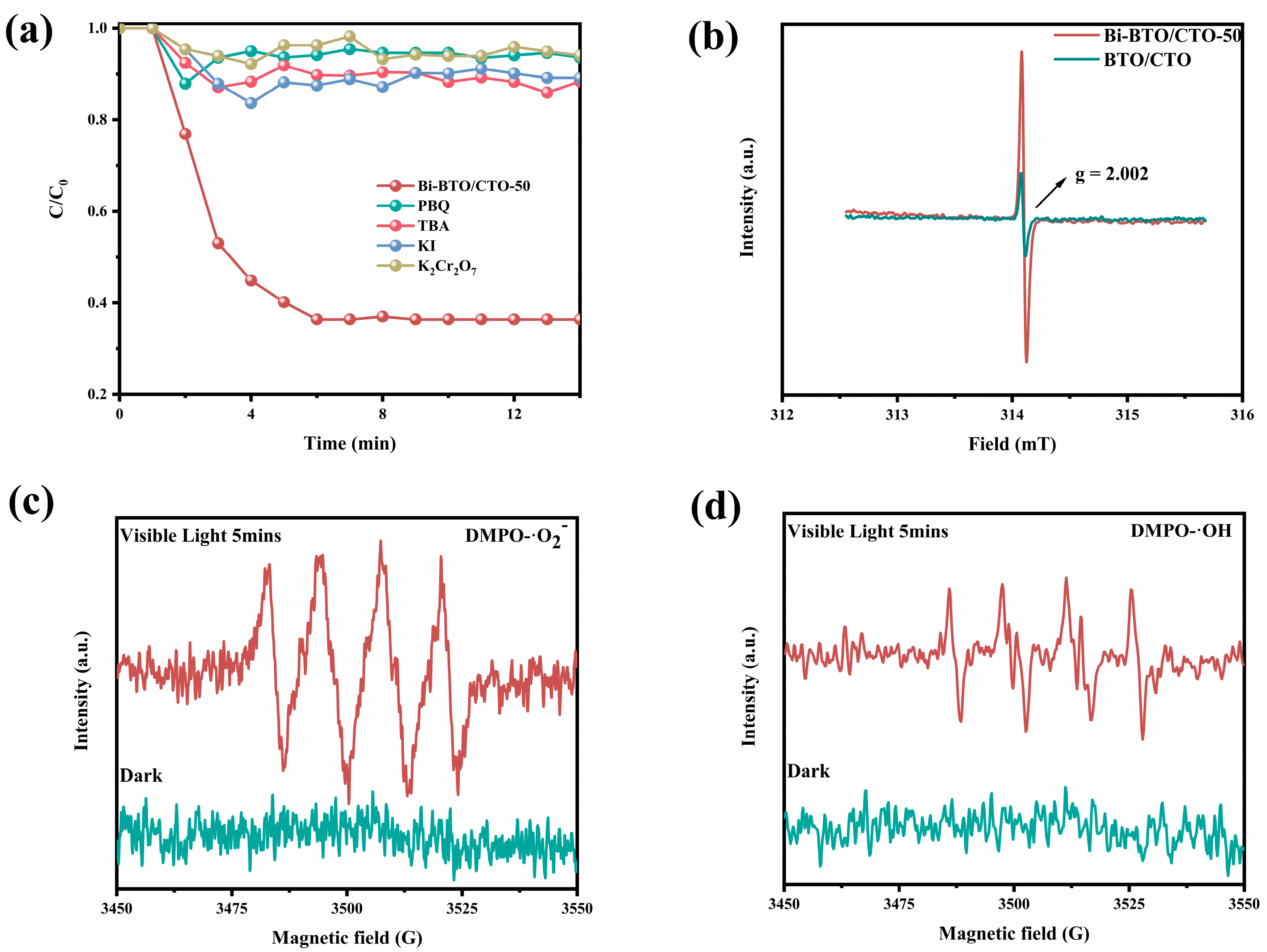
2.5. Possible Photocatalytic Mechanism
3. Experimental Section
3.1. Materials and Reagents
3.2. Synthesis of Photocatalysts
3.3. Photocatalytic NO Removal Performance Test
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Huo, W.C.; Cao, T.; Xu, W.N.; Guo, Z.Y.; Liu, X.Y.; Yao, H.C.; Zhang, Y.X.; Dong, F. Facile construction of Bi2Mo3O12@Bi2O2CO3 heterojunctions for enhanced photocatalytic efficiency toward NO removal and study of the conversion process. Chin. J. Catal. 2020, 41, 268–275. [Google Scholar] [CrossRef]
- Li, J.Y.; Yan, P.; Li, K.L.; Cen, W.L.; Yu, X.W.; Yuan, S.D.; Chu, Y.H.; Wang, Z.M. Generation and transformation of ROS on g-C3N4 for efficient photocatalytic NO removal: A combined in situ DRIFTS and DFT investigation. Chin. J. Catal. 2018, 39, 1695–1703. [Google Scholar] [CrossRef]
- Xin, Y.G.; Li, J.L. Air pollution and cardiovascular diseases in young adults. Eur. Heart J. 2021, 42, 4192. [Google Scholar] [CrossRef]
- Li, N.; Wang, C.Y.; Zhang, K.; Lv, H.Q.; Yuan, M.Z.; Bahnemann, D.W. Progress and prospects of photocatalytic conversion of low-concentration NOX. Chin. J. Catal. 2022, 43, 2363–2387. [Google Scholar] [CrossRef]
- Hailili, R.; Ji, H.W.; Wang, K.W.; Dong, X.A.; Chen, C.C.; Sheng, H.; Bahnemann, D.W.; Zhao, J.C. ZnO with Controllable Oxygen Vacancies for Photocatalytic Nitrogen Oxide Removal. ACS Catal. 2022, 12, 10004–10017. [Google Scholar] [CrossRef]
- Li, K.L.; Wang, H.; Li, J.J.; Dong, F. Design and mechanism of photocatalytic oxidation for the removal of air pollutants: A review. Environ. Chem. Lett. 2022, 20, 2687–2708. [Google Scholar] [CrossRef]
- Nakata, K.; Ochiai, T.; Murakami, T.; Fujishima, A. Photoenergy conversion with TiO2 photocatalysis: New materials and recent applications. Electrochim. Acta 2012, 84, 103–111. [Google Scholar] [CrossRef]
- Liao, J.Z.; Chen, L.C.; Sun, M.L.; Lei, B.; Zeng, X.L.; Sun, Y.J.; Dong, F. Improving visible-light-driven photocatalytic NO oxidation over BiOBr nanoplates through tunable oxygen vacancies. Chin. J. Catal. 2018, 39, 779–789. [Google Scholar] [CrossRef]
- Zhang, Y.S.; Jia, A.P.; Li, Z.R.; Yuan, Z.X.; Huang, W.X. Titania-Morphology-Dependent Pt-TiO2 Interfacial Catalysis in Water-Gas Shift Reaction. ACS Catal. 2023, 13, 392–399. [Google Scholar] [CrossRef]
- Passi, M.; Pal, B. A review on CaTiO3 photocatalyst: Activity enhancement methods and photocatalytic applications. Powder Technol. 2021, 388, 274–304. [Google Scholar] [CrossRef]
- Yan, X.; Huang, X.J.; Fang, Y.; Min, Y.H.; Wu, Z.J.; Li, W.S.; Yuan, J.M.; Tan, L.G. Synthesis of Rodlike CaTiO3 with Enhanced Charge Separation Efficiency and High Photocatalytic Activity. Int. J. Electrochem. Sci. 2014, 9, 5155–5163. [Google Scholar] [CrossRef]
- Huang, S.Q.; Guo, S.J.; Wang, Q.J.; Zhu, N.W.; Lou, Z.Y.; Li, L.; Shan, A.D.; Yuan, H.P. CaF2-Based Near-Infrared Photocatalyst Using the Multifunctional CaTiO3 Precursors as the Calcium Source. ACS Appl. Mater. Interfaces 2015, 7, 20170–20178. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.J.; Xu, D.T.; Wang, F.J.; Chen, M. Element-doped graphitic carbon nitride: Confirmation of doped elements and applications. Nanoscale Adv. 2021, 3, 4370–4387. [Google Scholar] [CrossRef]
- Kim, S.H.; Yook, K.S.; Jang, J.; Lee, Y. Correlation of memory characteristics of polymer bistable memory devices with metal deposition process. Synth. Met. 2008, 158, 861–864. [Google Scholar] [CrossRef]
- Jiang, E.H.; Song, N.; Che, G.B.; Liu, C.B.; Dong, H.J.; Yang, L.L. Construction of a Z-scheme MoS2/CaTiO3 heterostructure by the morphology-controlled strategy towards enhancing photocatalytic activity. Chem. Eng. J. 2020, 399, 125721. [Google Scholar] [CrossRef]
- Yang, J.F.; Shi, C.Y.; Dong, Y.H.; Su, H.; Sun, H.; Guo, Y.; Yin, S.Y. Efficient hydrogen generation of vector Z-scheme CaTiO3/Cu/TiO2 photocatalyst assisted by cocatalyst Cu nanoparticles. J. Colloid Interface Sci. 2022, 605, 373–384. [Google Scholar] [CrossRef]
- Zhao, J.M.; Cao, X.Y.; Bai, Y.B.; Chen, J.; Zhang, C.L. Simple synthesis of CaTiO3/g-C3N4 heterojunction for efficient photodegradation of methylene blue and levofloxacin. Opt. Mater. 2023, 135, 113239. [Google Scholar] [CrossRef]
- Shi, M.L.; Rhimi, B.; Zhang, K.; Xu, J.K.; Bahnemann, D.W.; Wang, C.A.Y. Visible light-driven novel Bi2Ti2O7/CaTiO3 composite photocatalyst with enhanced photocatalytic activity towards NO removal. Chemosphere 2021, 275, 130083. [Google Scholar] [CrossRef]
- Chen, D.D.; Fang, J.Z.; Lu, S.Y.; Zhou, G.Y.; Feng, W.H.; Yang, F.; Chen, Y.; Fang, Z.Q. Fabrication of Bi modified Bi2S3 pillared g-C3N4 photocatalyst and its efficient photocatalytic reduction and oxidation performances. Appl. Surf. Sci. 2017, 426, 427–436. [Google Scholar] [CrossRef]
- Gao, X.C.; Song, M.Y.; Sun, D.W.; Guan, R.Q.; Zhai, H.J.; Zhao, Z.; Zhang, Q.; Li, X.H. A Facile In Situ Hydrothermal Etching Method to CaTiO3/TiO2 Heterostructure for Efficient Photocatalytic N2 Reduction. Catal. Lett. 2022, 152, 1990–1998. [Google Scholar] [CrossRef]
- Shen, T.; Shi, X.K.; Guo, J.X.; Li, J.; Yuan, S.D. Photocatalytic removal of NO by light-driven Mn3O4/BiOCl heterojunction photocatalyst: Optimization and mechanism. Chem. Eng. J. 2021, 408, 14. [Google Scholar] [CrossRef]
- Han, C.; Liu, J.J.; Yang, W.J.; Wu, Q.Q.; Yang, H.; Xue, X.X. Photocatalytic activity of CaTiO3 synthesized by solid state, sol-gel and hydrothermal methods. J. Sol-Gel Sci. Technol. 2017, 81, 806–813. [Google Scholar] [CrossRef]
- Lalan, V.; Pillai, V.P.M.; Gopchandran, K.G. Enhanced electron transfer due to rGO makes Ag-CaTiO3@rGO a promising plasmonic photocatalyst. J. Sci. 2022, 7, 13. [Google Scholar] [CrossRef]
- Yan, Y.X.; Yang, H.; Yi, Z.; Xian, T.; Wang, X.X. Direct Z-scheme CaTiO3@BiOBr composite photocatalysts with enhanced photodegradation of dyes. Environ. Sci. Pollut. Res. 2019, 26, 29020–29031. [Google Scholar] [CrossRef]
- Pflaum, C.; Rahimi, Z. An iterative solver for the finite-difference frequency-domain (FDFD) method for the simulation of materials with negative permittivity. Numer. Linear Algebra Appl. 2011, 18, 653–670. [Google Scholar] [CrossRef]
- Zhang, L.; Yang, C.; Lv, K.L.; Lu, Y.C.; Li, Q.; Wu, X.F.; Li, Y.H.; Li, X.F.; Fan, J.J.; Li, M. SPR effect of bismuth enhanced visible photoreactivity of Bi2WO6 for NO abatement. Chin. J. Catal. 2019, 40, 755–764. [Google Scholar] [CrossRef]
- Liu, Z.X.; Yu, Q.Q.; Liu, J.; Li, T.; Huang, F.T. Enhanced visible photocatalytic activity in flower-like CuO-WO3-Bi2WO6 ternary hybrid through the cascadal electron transfer. Micro Nano Lett. 2017, 12, 195–200. [Google Scholar] [CrossRef]
- Chen, F.; Huang, H.W.; Ye, L.Q.; Zhang, T.R.; Zhang, Y.H.; Han, X.P.; Ma, T.Y. Thickness-Dependent Facet Junction Control of Layered BiOIO3 Single Crystals for Highly Efficient CO2 Photoreduction. Adv. Funct. Mater. 2018, 28, 11. [Google Scholar] [CrossRef]
- Wang, J.Y.; Han, F.M.; Rao, Y.F.; Hu, T.F.; Huang, Y.; Cao, J.J.; Lee, S.C. Visible-Light-Driven Nitrogen-Doped Carbon Quantum Dots/CaTiO3 Composite Catalyst with Enhanced NO Adsorption for NO Removal. Ind. Eng. Chem. Res. 2018, 57, 10226–10233. [Google Scholar] [CrossRef]
- Wang, B.B.; Chen, D.Y.; Li, N.J.; Xu, Q.F.; Li, H.; He, J.H.; Lu, J.M. Z-scheme photocatalytic NO removal on a 2D/2D iodine doped BiOIO3/g-C3N4 under visible-light irradiation. J. Colloid Interface Sci. 2020, 576, 426–434. [Google Scholar] [CrossRef]
- Song, J.; Luo, Z.; Britt, D.K.; Furukawa, H.; Yaghi, O.M.; Hardcastle, K.I.; Hill, C.L. A Multiunit Catalyst with Synergistic Stability and Reactivity: A Polyoxometalate-Metal Organic Framework for Aerobic Decontamination. J. Am. Chem. Soc. 2011, 133, 16839–16846. [Google Scholar] [CrossRef] [PubMed]
- Leong, K.H.; Gan, B.L.; Ibrahim, S.; Saravanan, P. Synthesis of surface plasmon resonance (SPR) triggered Ag/TiO2 photocatalyst for degradation of endocrine disturbing compound. Appl. Surf. Sci. 2014, 319, 128–135. [Google Scholar] [CrossRef]
- Sun, M.L.; Zhang, W.D.; Sun, Y.J.; Zhang, Y.X.; Dong, F. Synergistic integration of metallic Bi and defects on BiOI: Enhanced photocatalytic NO removal and conversion pathway. Chin. J. Catal. 2019, 40, 826–836. [Google Scholar] [CrossRef]
- Jin, S.; Dong, G.H.; Luo, J.M.; Ma, F.Y.; Wang, C.Y. Improved photocatalytic NO removal activity of SrTiO3 by using SrCO3 as a new co-catalyst. Appl. Catal. B-Environ. 2018, 227, 24–34. [Google Scholar] [CrossRef]
- Ding, X.; Ho, W.K.; Shang, J.; Zhang, L.Z. Self doping promoted photocatalytic removal of no under visible light with bi2moo6: Indispensable role of superoxide ions. Appl. Catal. B-Environ. 2016, 182, 316–325. [Google Scholar] [CrossRef]
- Bi, W.T.; Ye, C.M.; Xiao, C.; Tong, W.; Zhang, X.D.; Shao, W.; Xie, Y. Spatial Location Engineering of Oxygen Vacancies for Optimized Photocatalytic H-2 Evolution Activity. Small 2014, 10, 2820–2825. [Google Scholar] [CrossRef]
- Dong, F.; Zhao, Z.W.; Sun, Y.J.; Zhang, Y.X.; Yan, S.; Wu, Z.B. An Advanced Semimetal-Organic Bi Spheres-g-C3N4 Nanohybrid with SPR-Enhanced Visible-Light Photocatalytic Performance for NO Purification. Environ. Sci. Technol. 2015, 49, 12432–12440. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Du, D.; Shi, M.; Guo, Q.; Zhang, Y.; Allam, A.A.; Rady, A.; Wang, C. Metal Bi Loaded Bi2Ti2O7/CaTiO3 for Enhanced Photocatalytic Efficiency for NO Removal under Visible Light. Catalysts 2023, 13, 1169. https://doi.org/10.3390/catal13081169
Du D, Shi M, Guo Q, Zhang Y, Allam AA, Rady A, Wang C. Metal Bi Loaded Bi2Ti2O7/CaTiO3 for Enhanced Photocatalytic Efficiency for NO Removal under Visible Light. Catalysts. 2023; 13(8):1169. https://doi.org/10.3390/catal13081169
Chicago/Turabian StyleDu, Diyuan, Menglin Shi, Qingqing Guo, Yanqin Zhang, Ahmed A. Allam, Ahmed Rady, and Chuanyi Wang. 2023. "Metal Bi Loaded Bi2Ti2O7/CaTiO3 for Enhanced Photocatalytic Efficiency for NO Removal under Visible Light" Catalysts 13, no. 8: 1169. https://doi.org/10.3390/catal13081169
APA StyleDu, D., Shi, M., Guo, Q., Zhang, Y., Allam, A. A., Rady, A., & Wang, C. (2023). Metal Bi Loaded Bi2Ti2O7/CaTiO3 for Enhanced Photocatalytic Efficiency for NO Removal under Visible Light. Catalysts, 13(8), 1169. https://doi.org/10.3390/catal13081169






