The Role of Fe in Ni-Fe/TiO2 Catalysts for the Dry Reforming of Methane
Abstract
1. Introduction
2. Results and Discussion
2.1. Redox Properties
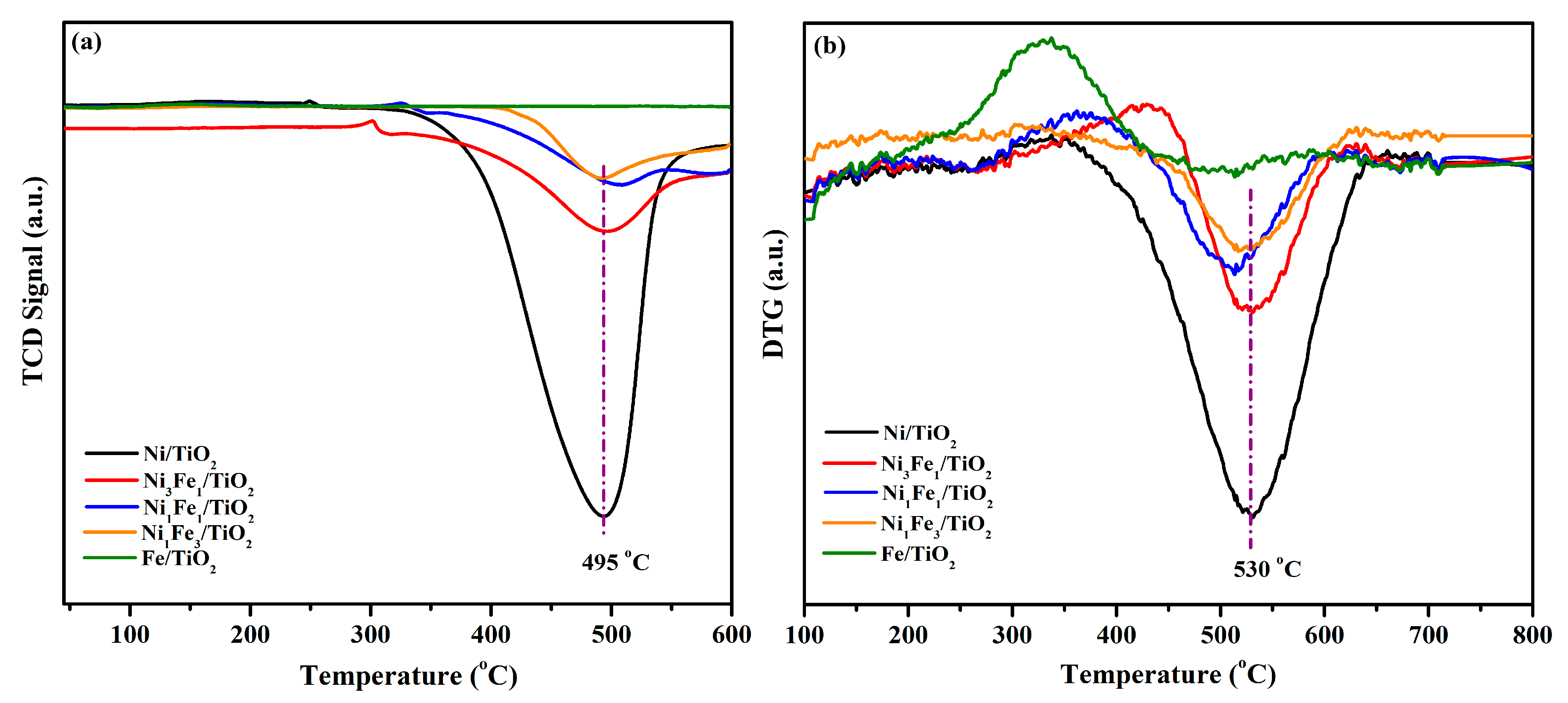
2.2. Methane–Temperature-Programmed Surface Reaction followed by Differential Thermogravimetry (CH4-TPSR/DTG)
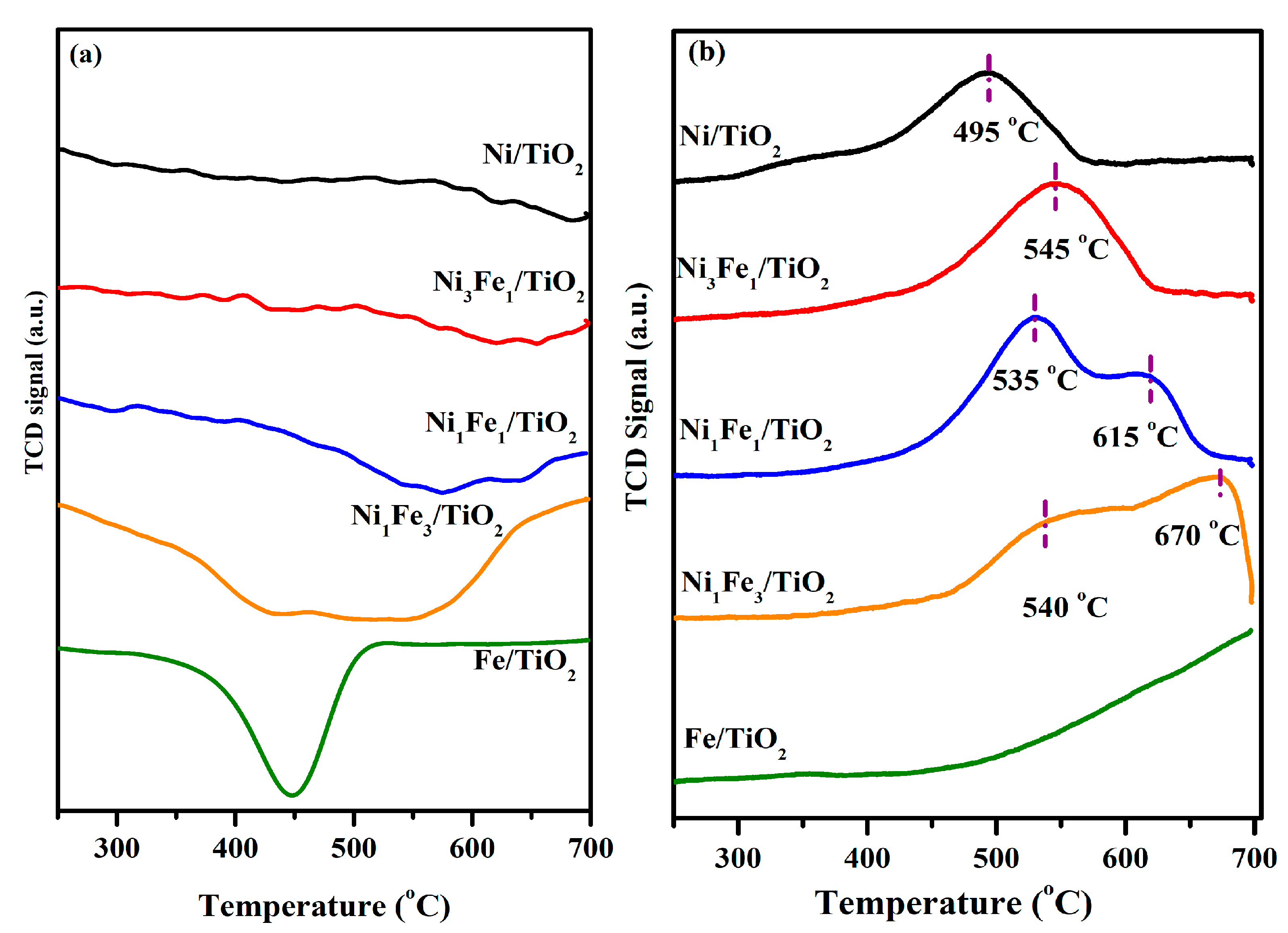
2.3. Carbon Dioxide–Temperature-Programmed Surface Reaction followed by Hydrogen–Temperature-Programmed Reduction (CO2-TPSR/H2-TPR)
2.4. Catalytic Activity Tests
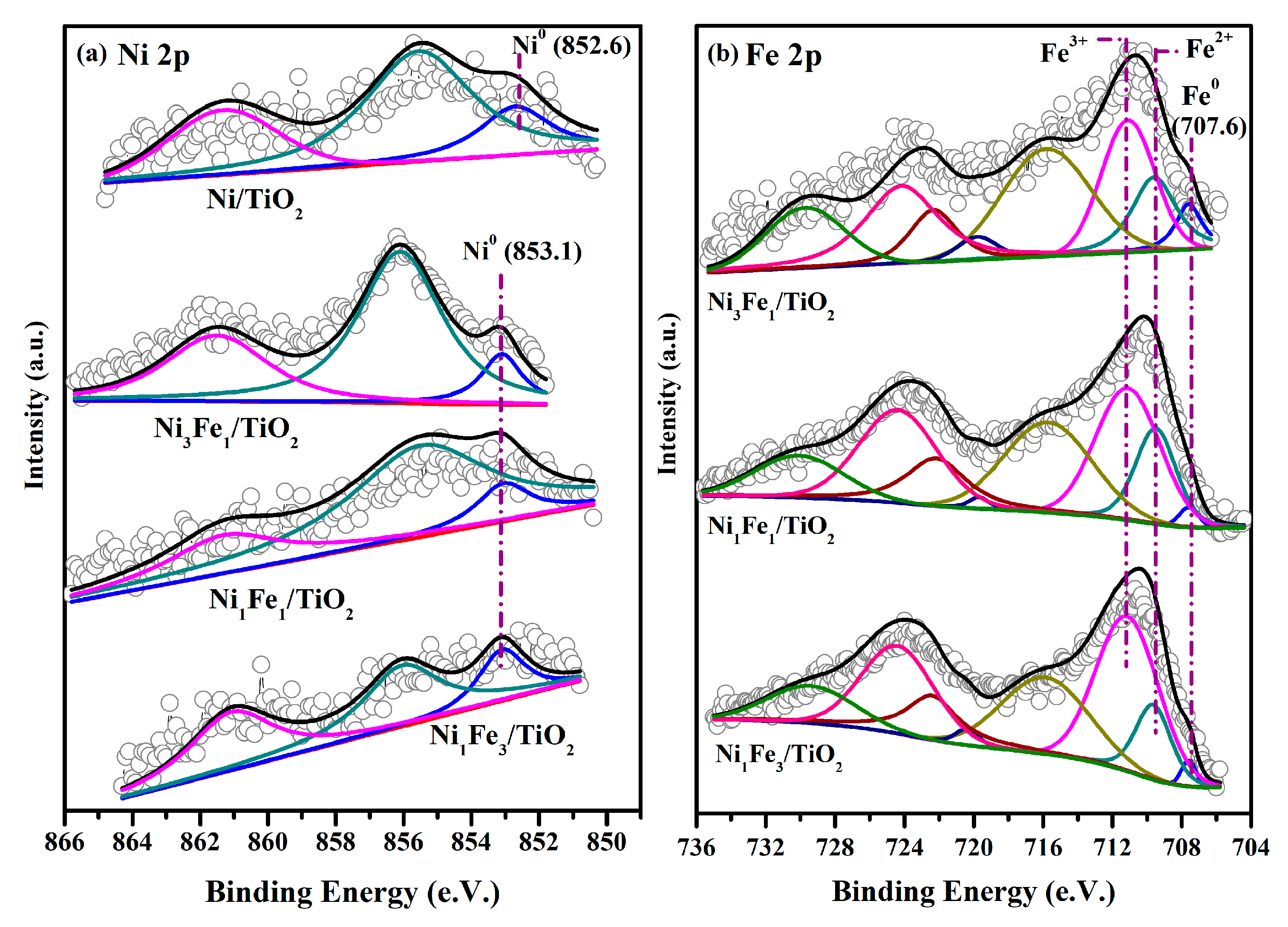
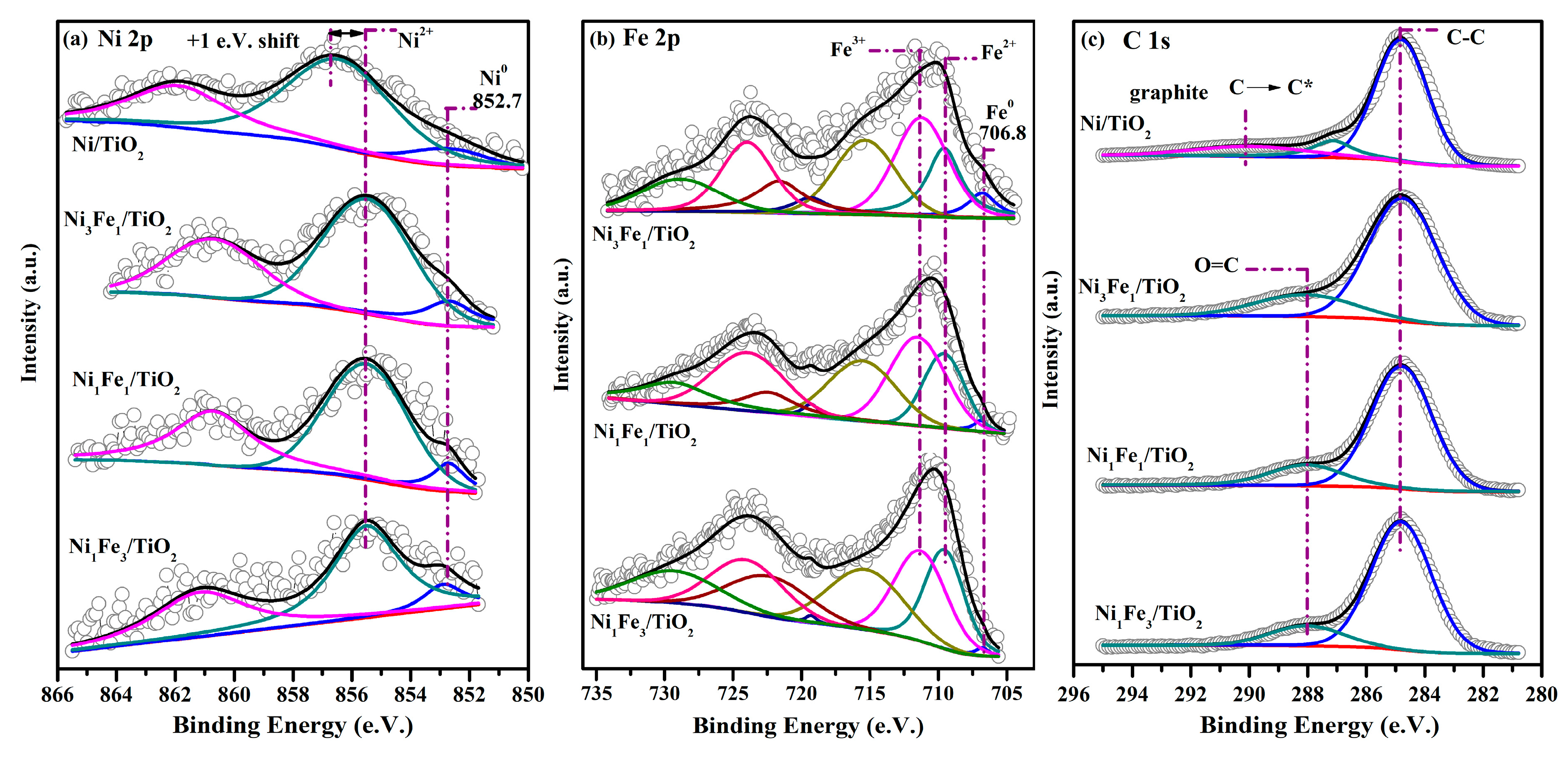
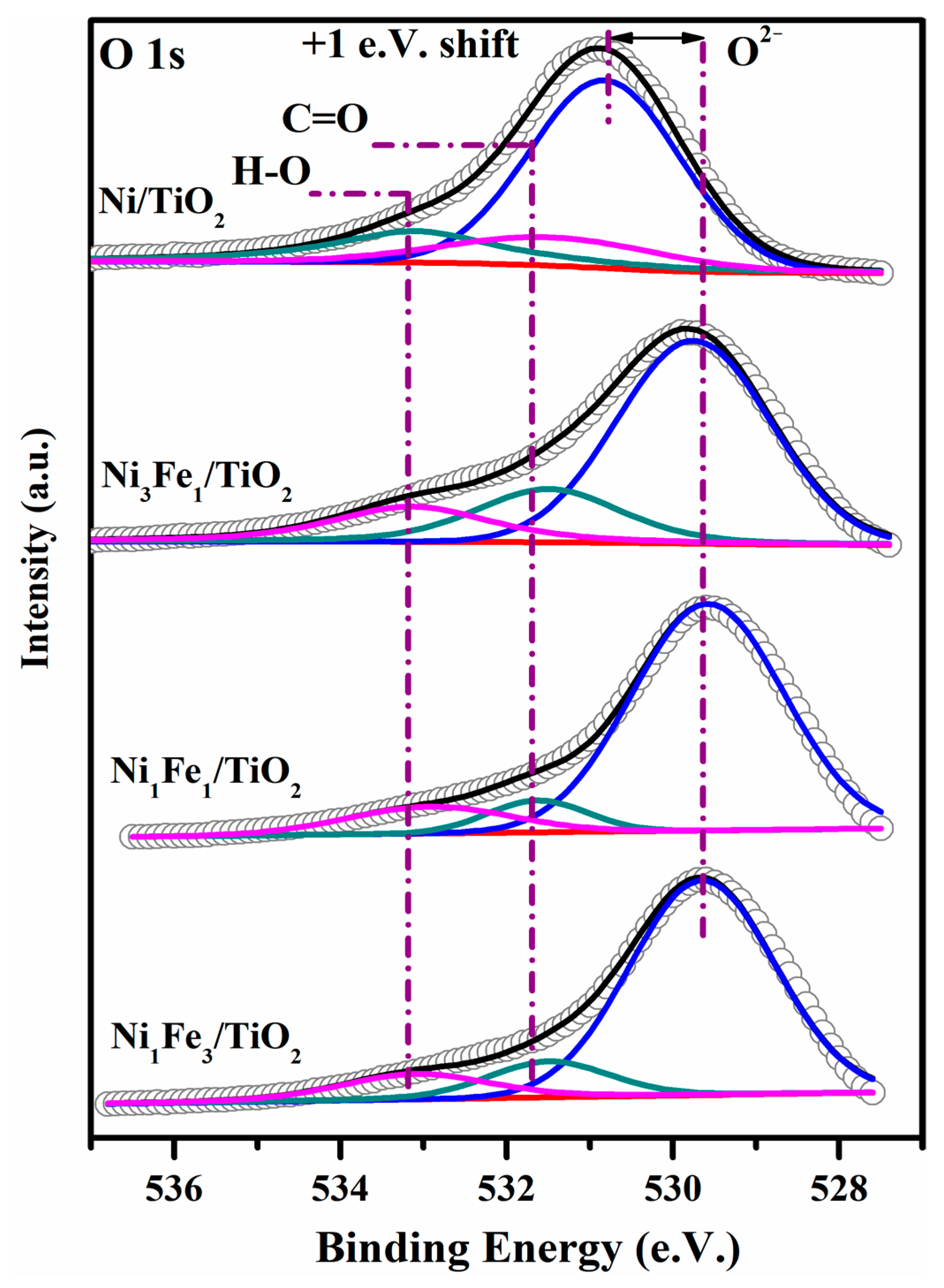
2.5. XPS Analysis
2.6. Thermogravimetric Analysis
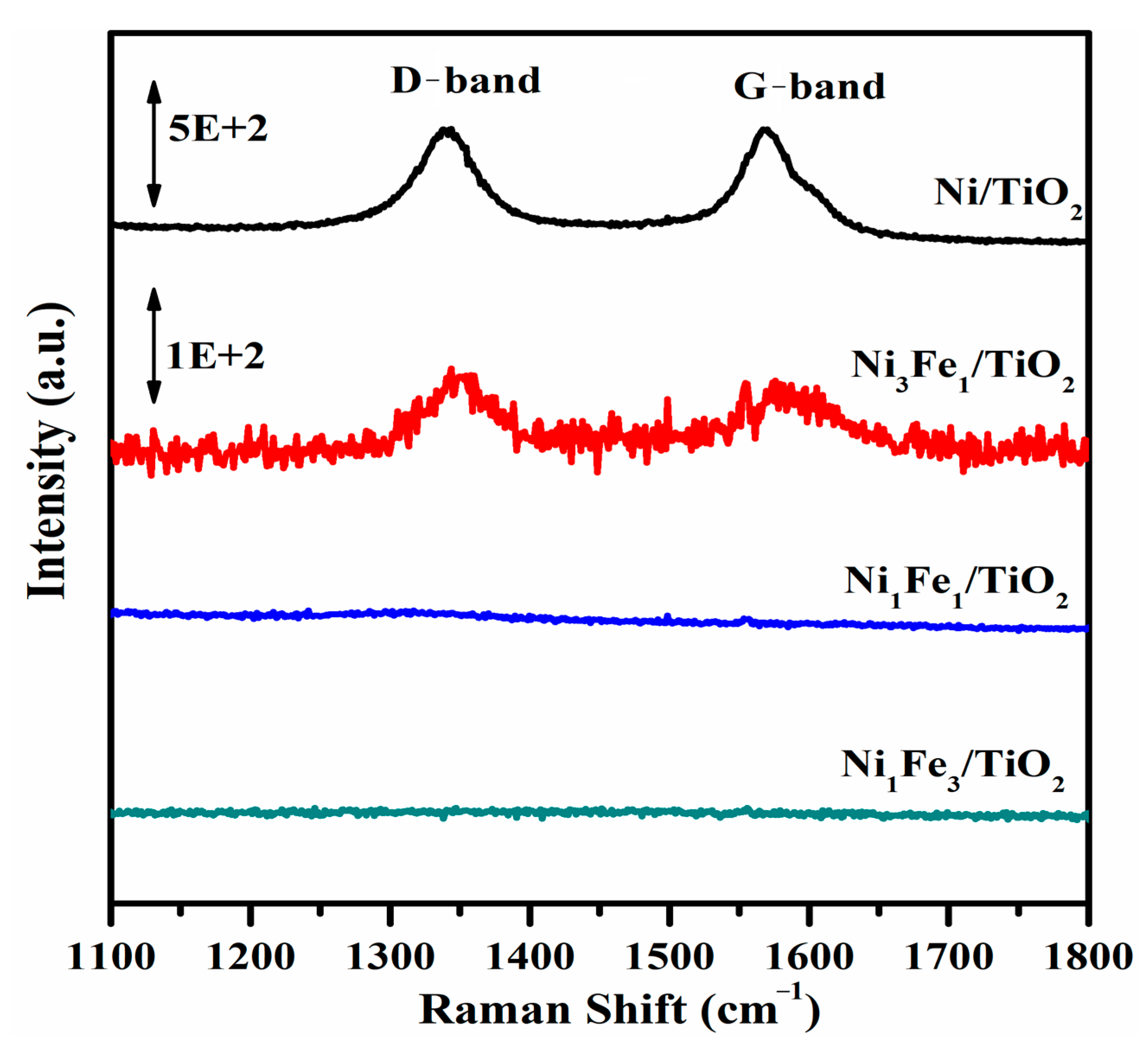
2.7. Raman Spectroscopy
2.8. In Situ DRIFTS Analysis
3. Materials and Methods
3.1. Catalyst Preparation
3.2. Catalyst Characterization
3.2.1. Inductively Coupled Plasma Atomic Emission Spectroscopy (ICP-AES)
3.2.2. Temperature-Programmed Reactions
3.2.3. Thermogravimetric Analysis/Differential Thermogravimetry (TGA-DTG)
3.2.4. X-ray Photoelectron Spectroscopy (XPS)
3.2.5. Raman Spectroscopy
3.2.6. In Situ Diffuse Reflectance Infrared Fourier Transform Spectroscopy (DRIFTS)
3.3. Catalytic Activity Performance
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Medeiros, F.G.M.D.; Lopes, F.W.B.; Vasconcelos, B.R.D. Prospects and technical challenges in hydrogen production through dry reforming of methane. Catalysts 2022, 12, 363. [Google Scholar] [CrossRef]
- Sengupta, S.; Ray, K.; Deo, G. Effects of modifying Ni/Al2O3 catalyst with cobalt on the reforming of CH4 with CO2 and cracking of CH4 reactions. Int. J. Hydrogen Energy 2014, 39, 11462–11472. [Google Scholar] [CrossRef]
- Kim, J.; Suh, D.J.; Park, T.; Kim, K. Effect of metal particle size on coking during CO2 reforming of CH4 over Ni–alumina aerogel catalysts. Appl. Catal. A Gen. 2000, 197, 191–200. [Google Scholar] [CrossRef]
- Pakhare, D.; Shaw, C.; Haynes, D.; Shekhawat, D.; Spivey, J. Effect of reaction temperature on activity of Pt-and Ru-substituted lanthanum zirconate pyrochlores (La2Zr2O7) for dry (CO2) reforming of methane (DRM). J. CO2 Util. 2013, 1, 37–42. [Google Scholar] [CrossRef]
- Tanios, C.; Bsaibes, S.; Gennequin, C.; Labaki, M.; Cazier, F.; Billet, S.; Tidahy, H.L.; Nsouli, B.; Aboukais, A.; Abi-Aad, E. Syngas production by the CO2 reforming of CH4 over Ni–Co–Mg–Al catalysts obtained from hydrotalcite precursors. Int. J. Hydrogen Energy 2017, 42, 12818–12828. [Google Scholar] [CrossRef]
- Damyanova, S.; Pawelec, B.; Arishtirova, K.; Huerta, M.V.M.; Fierro, J.L.G. The effect of CeO2 on the surface and catalytic properties of Pt/CeO2–ZrO2 catalysts for methane dry reforming. Appl. Catal. B Environ. 2009, 89, 149–159. [Google Scholar] [CrossRef]
- Liu, Z.; Zhang, F.; Rui, N.; Li, X.; Lin, L.; Betancourt, L.E.; Su, D.; Xu, W.; Cen, J.; Attenkofer, J.; et al. Highly active ceria-supported Ru catalyst for the dry reforming of methane: In Situ identification of Ruδ+–Ce3+ interactions for enhanced conversion. ACS Catal. 2019, 9, 3349–3359. [Google Scholar] [CrossRef]
- Yentekakis, I.V.; Goula, G.; Hatzisymeon, M.; Argyropoulou, I.B.; Botzolaki, G.; Kousi, K.; Kondarides, D.I.; Taylor, M.J.; Parlett, C.M.A.; Osatiashtiani, A.; et al. Effect of support oxygen storage capacity on the catalytic performance of Rh nanoparticles for CO2 reforming of methane. Appl. Catal. B Environ. 2019, 243, 490–501. [Google Scholar] [CrossRef]
- Yue, L.; Li, J.; Chen, C.; Fu, X.; Gong, Y.; Xia, X.; Hou, J.; Xiao, C.; Chen, X.; Zhao, L.; et al. Thermal-stable Pd@ mesoporous silica core-shell nanocatalysts for dry reforming of methane with good coke-resistant performance. Fuel 2018, 218, 335–341. [Google Scholar] [CrossRef]
- Maina, S.C.P.; Ballarini, A.D.; Vilella, J.I.; Miguel, S.R. Study of the performance and stability in the dry reforming of methane of doped alumina supported iridium catalysts. Catal. Today 2020, 344, 129–142. [Google Scholar] [CrossRef]
- Bradford, M.C.J.; Vannice, M.A. CO2 Reforming of CH4. Catal. Rev. 1999, 41, 1–42. [Google Scholar] [CrossRef]
- Das, S.; Sengupta, M.; Patel, J.; Bordoloi, A. A study of the synergy between support surface properties and catalyst deactivation for CO2 reforming over supported Ni nanoparticles. Appl. Catal. A Gen. 2017, 545, 113–126. [Google Scholar] [CrossRef]
- de la Cruz-Flores, V.G.; Martinez-Hernandez, A.; Gracia-Pinilla, M.A. Deactivation of Ni-SiO2 catalysts that are synthetized via a modified direct synthesis method during the dry reforming of methane. Appl. Catal. A Gen. 2020, 594, 117455. [Google Scholar] [CrossRef]
- Littlewood, P.; Weitz, E.; Marks, T.J.; Stair, P.C. Kinetic iso-conversion loop catalysis: A reactor operation mode to investigate slow catalyst deactivation processes, with Ni/Al2O3 for the dry reforming of methane. Ind. Eng. Chem. Res. 2019, 58, 2481–2491. [Google Scholar] [CrossRef]
- Wang, C.; Sun, N.; Zhao, N.; Wei, W.; Sun, Y.; Sun, C.; Liu, H.; Snape, C.E. Coking and deactivation of a mesoporous Ni–CaO–ZrO2 catalyst in dry reforming of methane: A study under different feeding compositions. Fuel 2015, 143, 527–535. [Google Scholar] [CrossRef]
- Wang, Z.; Cao, X.M.; Zhu, J.; Hu, P. Activity and coke formation of nickel and nickel carbide in dry reforming: A deactivation scheme from density functional theory. J. Catal. 2014, 311, 469–480. [Google Scholar] [CrossRef]
- Ay, H.; Uner, D. Dry reforming of methane over CeO2supported Ni, Co and Ni–Co catalysts. Appl. Catal. B Environ. 2015, 179, 128–138. [Google Scholar] [CrossRef]
- Sokefun, Y.O.; Trottier, J.; Yung, M.M.; Joseph, B.; Kuhn, J.N. Low temperature dry reforming of methane using Ru-Ni-Mg/ceria-zirconia catalysts: Effect of Ru loading and reduction temperature. Appl. Catal. A Gen. 2022, 645, 118842. [Google Scholar] [CrossRef]
- Cichy, M.; Panczyk, M.; Słowik, G.; Zawadzki, W.; Borowiecki, T. Ni-Re alloy catalysts on Al2O3 for methane dry reforming. Int. J. Hydrogen Energy 2022, 47, 16528–16543. [Google Scholar] [CrossRef]
- Huang, Y.; Li, X.; Zhang, Q.; Vinokurov, V.A.; Huang, W. Enhanced carbon tolerance of hydrotalcite-derived Ni-Ir/Mg(Al)O catalysts in dry reforming of methane under elevated pressures. Fuel Process. Technol. 2022, 237, 107446. [Google Scholar] [CrossRef]
- Jabbour, K.; Saad, A.; Inaty, L.; Davidson, A.; Massiani, P.; Hassan, N.E. Ordered mesoporous Fe-Al2O3 based-catalysts synthesized via a direct “one-pot” method for the dry reforming of a model biogas mixture. Int. J. Hydrogen Energy 2019, 44, 14889–14907. [Google Scholar] [CrossRef]
- Wu, Z.; Yang, B.; Miao, S.; Liu, W.; Xie, J.; Lee, S.; Pellin, M.J.; Xiao, D.; Su, D.; Ma, D. Lattice strained Ni-Co alloy as a high-performance catalyst for catalytic dry reforming of methane. ACS Catal. 2019, 9, 2693–2700. [Google Scholar] [CrossRef]
- Sagar, T.V.; Padmakar, D.; Lingaiah, N.; Prasad, P.S.S. Influence of solid solution formation on the activity of CeO2 supported Ni–Cu mixed oxide catalysts in dry reforming of methane. Catal. Lett. 2019, 149, 2597–2606. [Google Scholar] [CrossRef]
- Bian, Z.; Das, S.; Wai, M.H.; Hongmanorom, P.; Kawi, S. A Review on bimetallic nickel-based catalysts for CO2 reforming of methane. ChemPhysChem 2017, 18, 3117–3134. [Google Scholar] [CrossRef]
- Kim, S.M.; Abdala, P.M.; Margossian, T.; Hosseini, D.; Foppa, L.; Armutlulu, A.; Van Beek, W.; Comas-Vives, A.; Copéret, C.; Müller, C. Cooperativity and dynamics increase the performance of NiFe dry reforming catalysts. J. Am. Chem. Soc. 2017, 139, 1937–1949. [Google Scholar] [CrossRef] [PubMed]
- Margossian, T.; Larmier, K.; Kim, S.M.; Krumeich, F.; Müller, C.; Copéret, C. Supported bimetallic NiFe nanoparticles through colloid synthesis for improved dry reforming performance. ACS Catal. 2017, 7, 6942–6948. [Google Scholar] [CrossRef]
- Theofanidis, S.A.; Galvita, V.V.; Poelman, H.; Marin, G.B. Enhanced Carbon-Resistant Dry Reforming Fe-Ni Catalyst: Role of Fe. ACS Catal. 2015, 5, 3028–3039. [Google Scholar] [CrossRef]
- Theofanidis, S.A.; Galvita, V.V.; Poelman, H.; Dharanipragada, N.V.R.A.; Longo, A.; Meledina, M.; Tendeloo, G.V.; Detavernier, C.; Marin, G.B. Fe-Containing magnesium aluminate support for stability and carbon control during methane reforming. ACS Catal. 2018, 8, 5983–5995. [Google Scholar] [CrossRef]
- Li, B.; Luo, Y.; Li, B.; Yuan, X.; Wang, X. Catalytic performance of iron-promoted nickel-based ordered mesoporous alumina FeNiAl catalysts in dry reforming of methane. Fuel Process. Technol. 2019, 193, 348–360. [Google Scholar] [CrossRef]
- Bonmassar, N.; Bekheet, M.F.; Schlicker, L.; Gili, A.; Gurlo, A.; Doran, A.; Gao, Y.; Heggen, M.; Bernardi, J.; Klötzer, B.; et al. In situ-determined catalytically active state of LaNiO3 in methane dry reforming. ACS Catal. 2020, 10, 1102–1112. [Google Scholar] [CrossRef]
- Wang, M.; Zhao, T.; Dong, X.; Li, M.; Wang, H. Effects of Ce substitution at the A-site of LaNi0.5Fe0.5O3 perovskite on the enhanced catalytic activity for dry reforming of methane. Appl. Catal. B Environ. 2018, 224, 214–221. [Google Scholar] [CrossRef]
- Lima, S.M.; Assaf, J.M. Ni-Fe catalysts based on perovskite-type oxides for dry reforming of methane to syngas. Catal. Lett. 2006, 108, 63–70. [Google Scholar] [CrossRef]
- Song, X.; Dong, X.; Yin, S.; Wang, M.; Li, M.; Wang, H. Effects of Fe partial substitution of La2NiO4/LaNiO3 catalyst precursors prepared by wet impregnation method for the dry reforming of methane. Appl. Catal. A Gen. 2016, 526, 132–138. [Google Scholar] [CrossRef]
- Zhang, T.; Liu, Z.; Zhu, Y.; Liu, Z.; Sui, Z.; Zhu, K.; Zhou, X. Dry reforming of methane on Ni-Fe-MgO catalysts: Influence of Fe on carbon-resistant property and kinetics. Appl. Catal. B Environ. 2020, 264, 118497. [Google Scholar] [CrossRef]
- Nikoo, M.K.; Amin, N.A.S. Thermodynamic analysis of carbon dioxide reforming of methane in view of solid carbon formation. Fuel Process. Technol. 2011, 92, 678–691. [Google Scholar] [CrossRef]
- Dhillon, G.S. Nick-Iron Catalysts for Low Temperature Dry Reformation of Methane. Ph.D. Thesis, University of New Hampshire, Durham, NH, USA, 2021. [Google Scholar]
- Xie, T.; Zhang, Z.Y.; Zheng, H.Y.; Xu, K.D.; Hu, Z.; Lei, Y. Enhanced photothermal catalytic performance of dry reforming of methane over Ni/mesoporous TiO2 composite catalyst. Chem. Eng. J. 2022, 429, 132507. [Google Scholar] [CrossRef]
- Yan, Q.G.; Weng, W.Z.; Wan, H.L.; Toghiani, H.; Toghiani, R.K.; Pittman, C.U. Activation of methane to syngas over a Ni/TiO2 catalyst. Appl. Catal. A Gen. 2003, 239, 43–58. [Google Scholar] [CrossRef]
- Ray, K.; Sengupta, S.; Deo, G. Reforming and cracking of CH4 over Al2O3supported Ni, Ni-Fe and Ni-Co catalysts. Fuel Process. Technol. 2017, 156, 195–203. [Google Scholar] [CrossRef]
- Ashok, J.; Kawi, S. Steam reforming of biomass tar model compound at relatively low steam-to-carbon condition over CaO-doped nickel–iron alloy supported over iron–alumina catalysts. Appl. Catal. A Gen. 2015, 490, 24–35. [Google Scholar] [CrossRef]
- Pandey, D.; Deo, G. Effect of support on the catalytic activity of supported Ni–Fe catalysts for the CO2 methanation reaction. J. Ind. Eng. Chem. 2016, 33, 99–107. [Google Scholar] [CrossRef]
- Song, K.; Lu, M.; Xu, S.; Chen, C.; Zhan, Y.; Li, D.; Au, C.; Jiang, L.; Tomishige, K. Effect of alloy composition on catalytic performance and coke-resistance property of Ni-Cu/Mg(Al)O catalysts for dry reforming of methane. Appl. Catal. B Environ. 2018, 239, 324–333. [Google Scholar] [CrossRef]
- Li, D.; Xu, S.; Song, K.; Chen, C.; Zhan, Y.; Jiang, L. Hydrotalcite-derived Co/Mg(Al)O as a stable and coke-resistant catalyst for low-temperature carbon dioxide reforming of methane. Appl. Catal. A Gen. 2018, 552, 21–29. [Google Scholar] [CrossRef]
- Shah, M.; Das, S.; Nayak, A.K.; Mondal, P.; Bordoloi, A. Smart designing of metal-support interface for imperishable dry reforming catalyst. Appl. Catal. A Gen. 2018, 556, 137–154. [Google Scholar] [CrossRef]
- Ashok, J.; Kawi, S. Nickel–Iron alloy supported over Iron–Alumina catalysts for steam reforming of biomass tar model compound. ACS Catal. 2014, 4, 289–301. [Google Scholar] [CrossRef]
- Dȩbek, R.; Motak, M.; Duraczyska, D.; Launay, F.; Galvez, M.E.; Grzybek, T.; Da Costa, P. Methane dry reforming over hydrotalcite-derived Ni–Mg–Al mixed oxides: The influence of Ni content on catalytic activity, selectivity and stability. Catal. Sci. Technol. 2016, 6, 6705–6715. [Google Scholar] [CrossRef]
- Dębek, R.; Motak, M.; Galvez, M.E.; Grzybek, T.; Da Costa, P. Promotion effect of zirconia on Mg(Ni,Al)O mixed oxides derived from hydrotalcites in CO2 methane reforming. Appl. Catal. B Environ. 2018, 223, 36–46. [Google Scholar] [CrossRef]
- Yamashita, T.; Hayes, P. Analysis of XPS spectra of Fe2+ and Fe3+ ions in oxide materials. Appl. Surf. Sci. 2008, 254, 2441–2449. [Google Scholar] [CrossRef]
- Cao, G.; Yi, N. Pretreatment effect on copper-titanium dioxide catalysts in CO oxidation. Catal. Commun. 2022, 170, 106484. [Google Scholar] [CrossRef]
- Cao, G.; Deskins, N.A.; Yi, N. Carbon monoxide oxidation over copper and nitrogen modified titanium dioxide. Appl. Catal. B Environ. 2021, 285, 119748. [Google Scholar] [CrossRef]
- Theofanidis, S.A.; Batchu, R.; Galvita, V.V.; Poelman, H.; Marin, G.B. Carbon gasification from Fe–Ni catalysts after methane dry reforming. Appl. Catal. B Environ. 2016, 185, 42–55. [Google Scholar] [CrossRef]
- Zhang, M.; Zhang, J.; Zhou, Z.; Chen, S.; Zhang, T.; Song, F.; Zhang, Q.; Tsubaki, N.; Tan, Y.; Han, Y. Effects of the surface adsorbed oxygen species tuned by rare-earth metal doping on dry reforming of methane over Ni/ZrO2 catalyst. Appl. Catal. B Environ. 2020, 264, 118522. [Google Scholar] [CrossRef]
- Damyanova, S.; Shtereva, I.; Pawelec, B.; Mihaylov, L.; Fierro, J.L.G. Characterization of none and yttrium-modified Ni-based catalysts for dry reforming of methane. Appl. Catal. B Environ. 2020, 278, 119335. [Google Scholar] [CrossRef]
- Damaskinos, C.M.; Vasiliades, M.A.; Efsthathiou, A.M. The effect of Ti4+ dopant in the 5 wt% Ni/Ce1-xTixO2-δ catalyst on the carbon pathways of dry reforming of methane studied by various transient and isotopic techniques. Appl. Catal. A Gen. 2019, 579, 116–129. [Google Scholar] [CrossRef]
- Amin, A.M.; Croiset, E.; Epling, W. Review of methane catalytic cracking for hydrogen production. Int. J. Hydrogen Energy 2011, 36, 2904–2935. [Google Scholar] [CrossRef]
- Cao, K.; Gong, M.; Yang, J.; Cai, J.; Chu, S.; Chen, Z.; Shan, B.; Chen, R. Nickel catalyst with atomically-thin meshed cobalt coating for improved durability in dry reforming of methane. J. Catal. 2019, 373, 351–360. [Google Scholar] [CrossRef]
- Das, S.; Ashok, J.; Bian, Z.; Dewangan, N.; Wai, M.H.; Du, Y.; Borgna, A.; Hidajat, K.; Kawi, S. Silica–Ceria sandwiched Ni core–shell catalyst for low temperature dry reforming of biogas: Coke resistance and mechanistic insights. Appl. Catal. B Environ. 2018, 230, 220–236. [Google Scholar] [CrossRef]
- Marinho, A.L.A.; Toniolo, F.S.; Noronha, F.B.; Epron, F.; Duprez, D.; Bion, N. Highly active and stable Ni dispersed on mesoporous CeO2-Al2O3 catalysts for production of syngas by dry reforming of methane. Appl. Catal. B Environ. 2021, 281, 119459. [Google Scholar] [CrossRef]
- Bu, K.; Deng, J.; Zhang, X.; Kuboon, S.; Yan, T.; Li, H.; Shi, L.; Zhang, D. Promotional effects of B-terminated defective edges of Ni/boron nitride catalysts for coking- and sintering-resistant dry reforming of methane. Appl. Catal. B Environ. 2020, 267, 118692. [Google Scholar] [CrossRef]
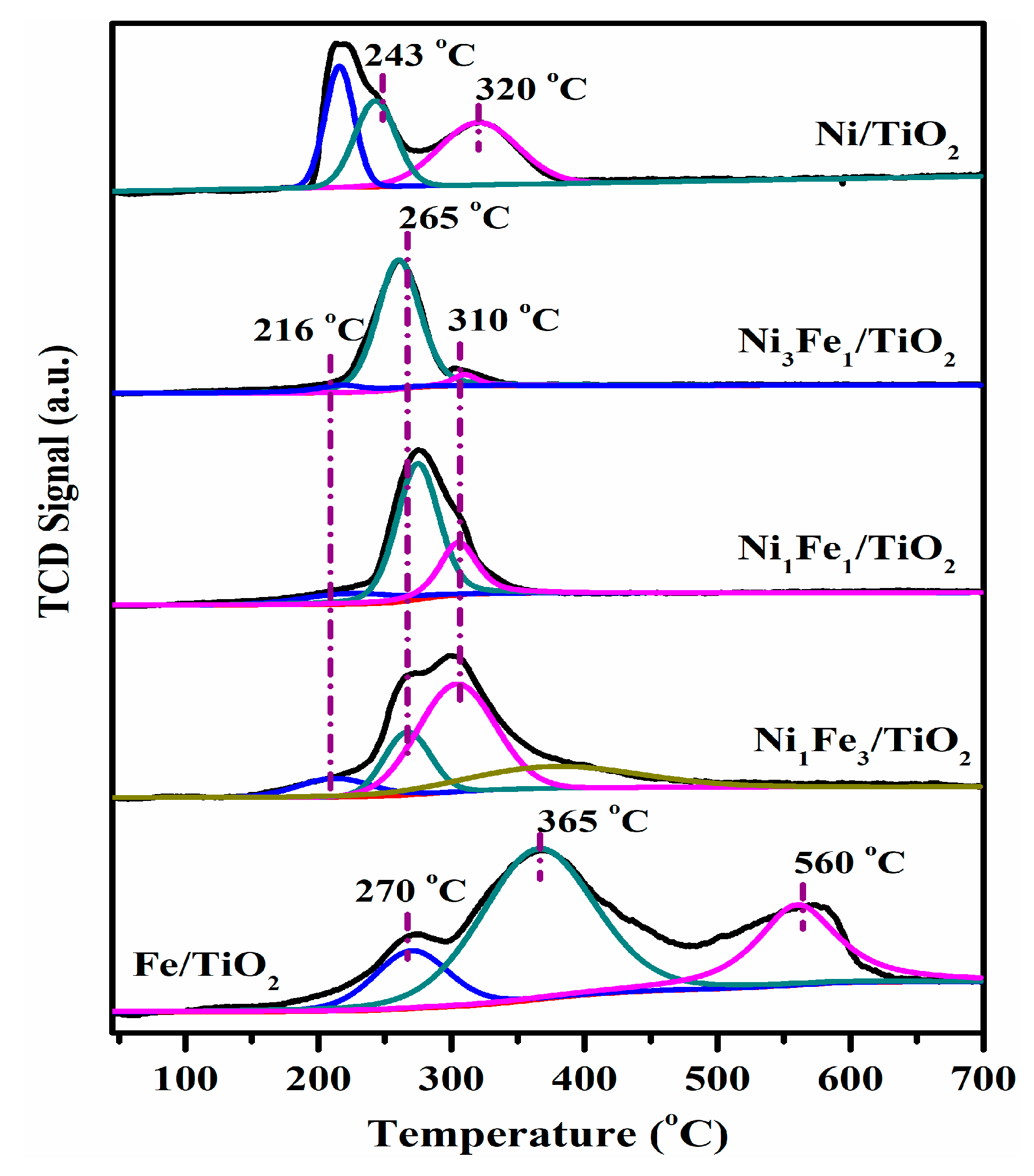




| Peak Temperature (°C) and H2-Consumption (mmol H2/gcatalyst) | |||||
|---|---|---|---|---|---|
| Ni/TiO2 | 216 (0.46) | 243 (0.46) | 320 (0.63) | - | |
| Fe/TiO2 | 270 (0.38) | 365 (1.53) | 560 (0.73) | ||
| Peak 1 | Peak 2 | Peak 3 | |||
| Ni3Fe1/TiO2 | 216 (0.14) | 265 (1.6) | 310 (0.09) | - | |
| Ni1Fe1/TiO2 | 216 (0.14) | 265 (1.22) | 310 (0.54) | - | |
| Ni1Fe3/TiO2 | 216 (0.14) | 265 (0.37) | 310 (0.88) | 365 (0.63) | |
| Catalysts | Ni0 | Ni2+ | Fe0 | Fe2+ | Fe3+ | O/Ti | Fe/(Ni+Fe) |
|---|---|---|---|---|---|---|---|
| Ni/TiO2 | 0.70 | 2.27 | 1.00 | - | |||
| Ni3Fe1/TiO2 | 0.57 | 3.29 | 0.48 | 1.25 | 2.05 | 0.74 | 0.49 |
| Ni1Fe1/TiO2 | 0.32 | 2.21 | 0.21 | 1.76 | 3.55 | 1.07 | 0.68 |
| Ni1Fe3/TiO2 | 0.29 | 1.07 | 0.19 | 1.84 | 3.69 | 1.12 | 0.80 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dhillon, G.S.; Cao, G.; Yi, N. The Role of Fe in Ni-Fe/TiO2 Catalysts for the Dry Reforming of Methane. Catalysts 2023, 13, 1171. https://doi.org/10.3390/catal13081171
Dhillon GS, Cao G, Yi N. The Role of Fe in Ni-Fe/TiO2 Catalysts for the Dry Reforming of Methane. Catalysts. 2023; 13(8):1171. https://doi.org/10.3390/catal13081171
Chicago/Turabian StyleDhillon, Gagandeep Singh, Guoqiang Cao, and Nan Yi. 2023. "The Role of Fe in Ni-Fe/TiO2 Catalysts for the Dry Reforming of Methane" Catalysts 13, no. 8: 1171. https://doi.org/10.3390/catal13081171
APA StyleDhillon, G. S., Cao, G., & Yi, N. (2023). The Role of Fe in Ni-Fe/TiO2 Catalysts for the Dry Reforming of Methane. Catalysts, 13(8), 1171. https://doi.org/10.3390/catal13081171








