The Role of Undecenoic Acid on the Preparation of Decorated MCM-41/Polyethylene Hybrids by In Situ Polymerization: Catalytic Aspects and Properties of the Resultant Materials
Abstract
:1. Introduction
2. Results and Discussion
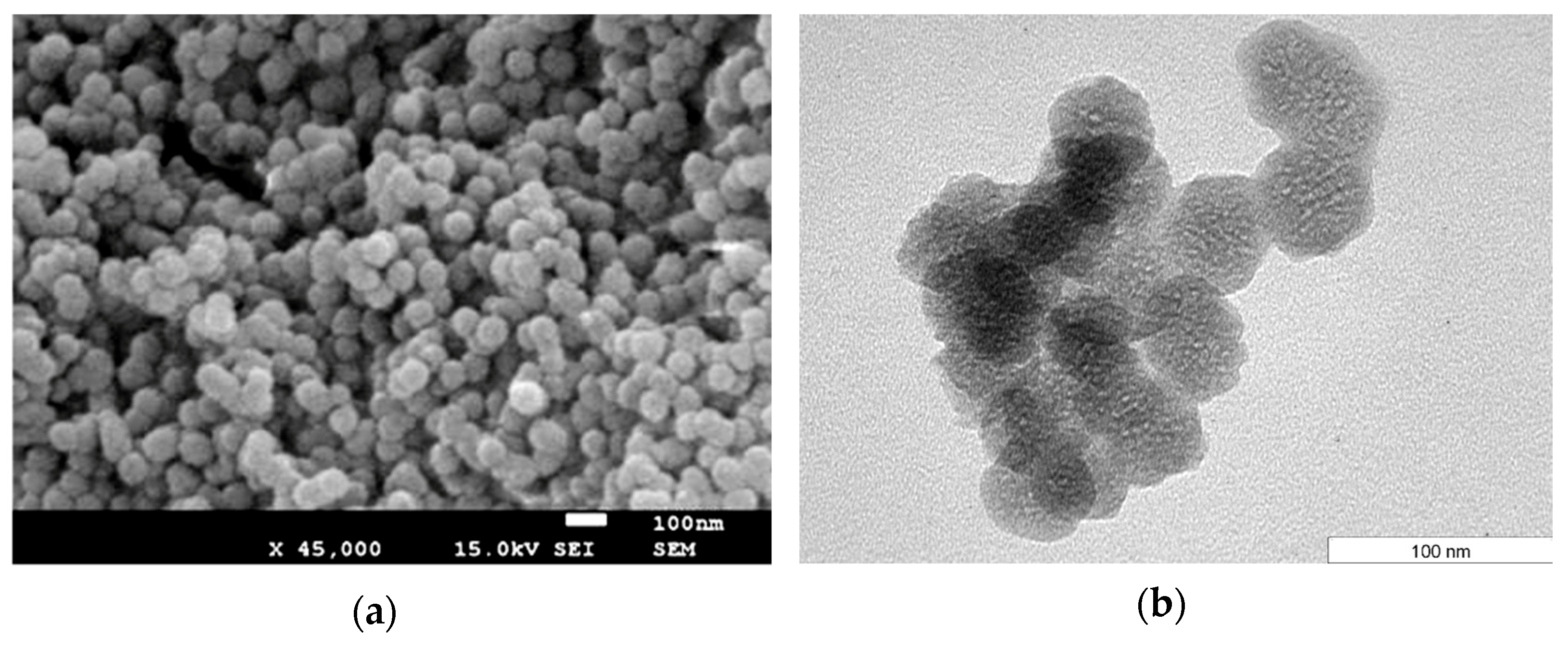
2.1. Characterization of NMCM-41
2.2. Preparation of PE_UA_NMCM-41 Nanocomposites
Polymerization Behavior
2.3. Study of PE_UA_NMCM-41 Nanocomposites
2.3.1. Thermal Stability
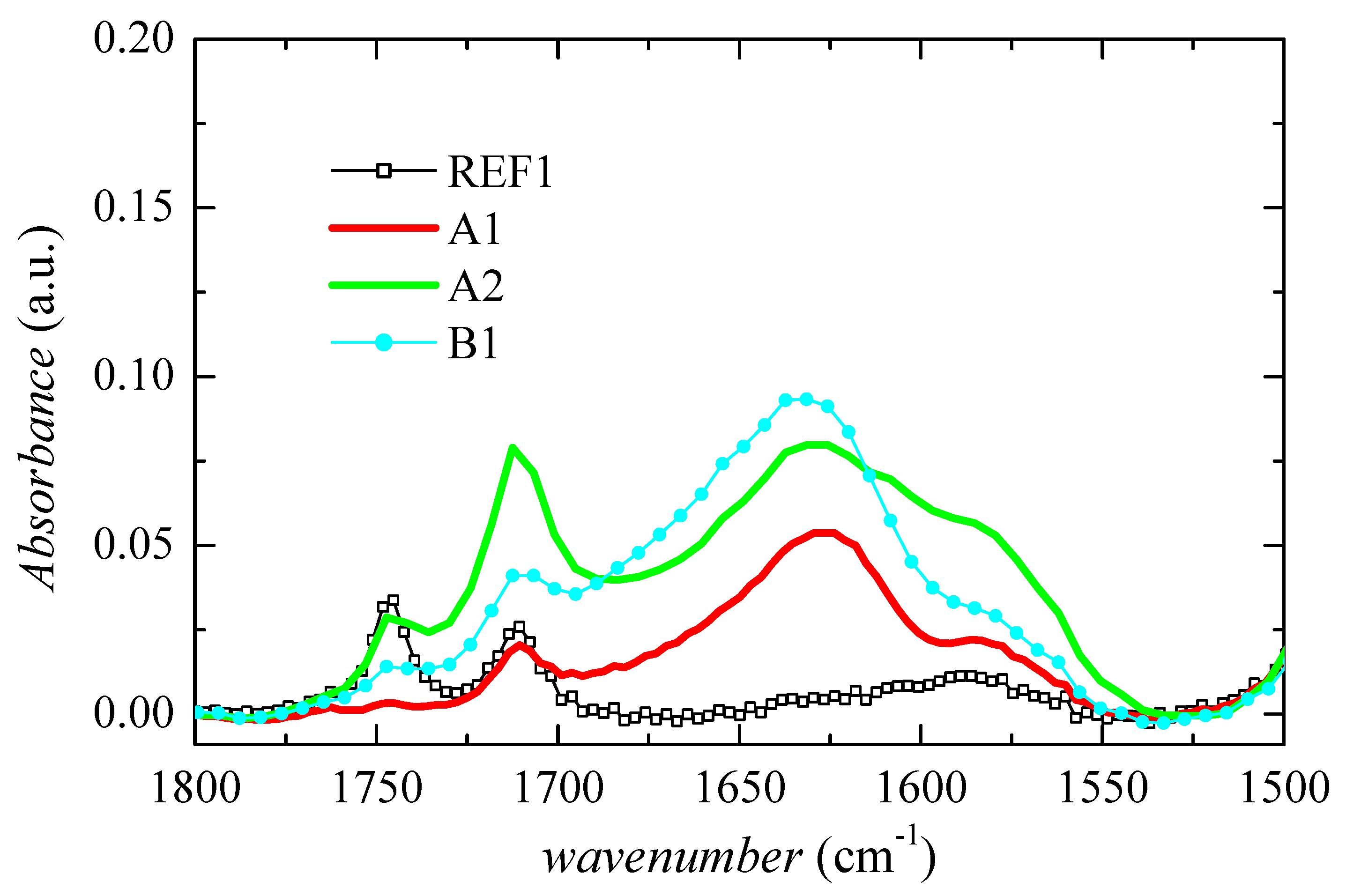
2.3.2. Structural Characterization
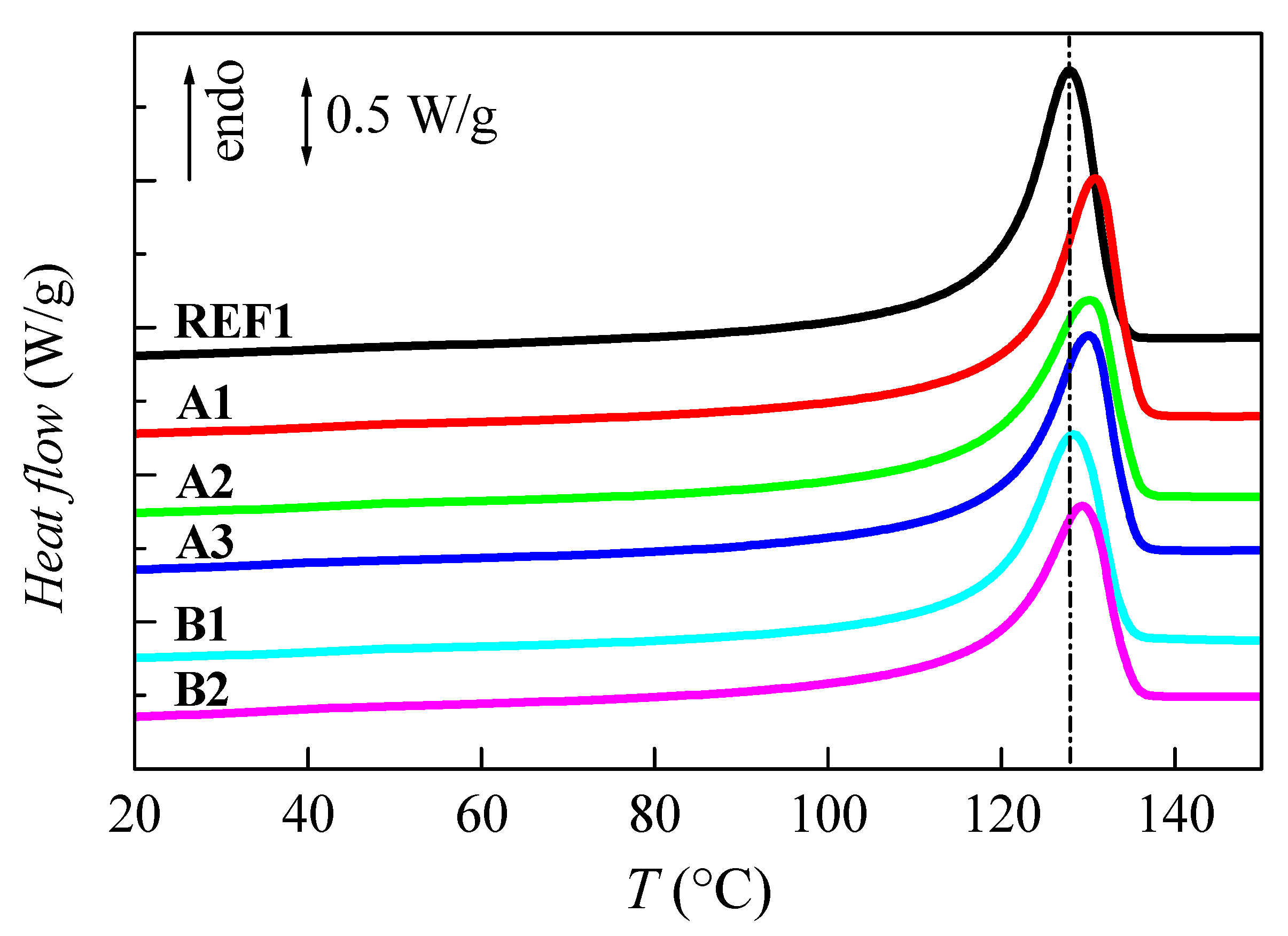
2.3.3. Phase Transitions
2.3.4. Morphological Details
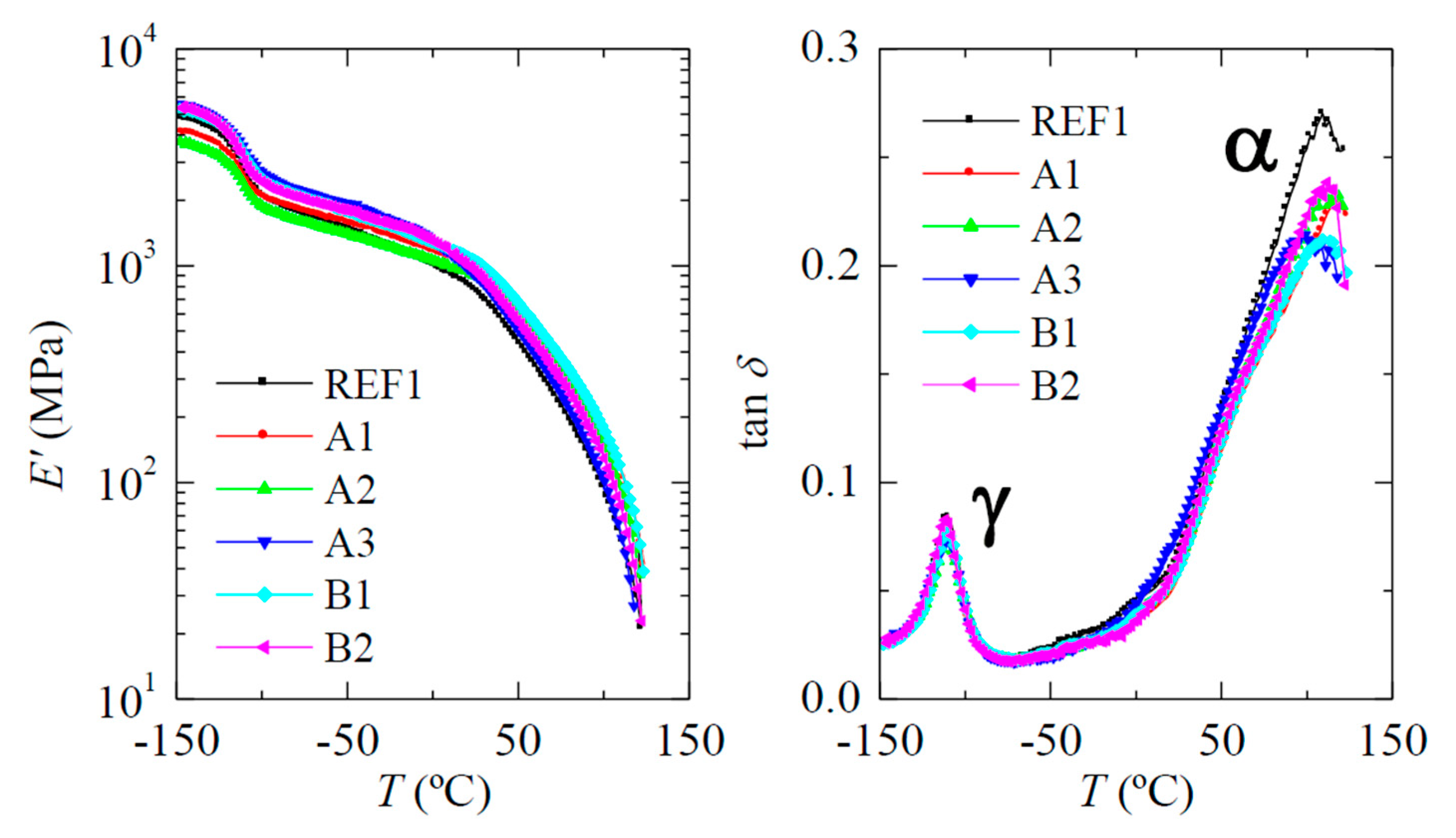
2.3.5. Viscoelastic Behavior
3. Materials and Methods
3.1. Materials
3.2. Preparation and Characterization of Neat and Modified NMCM-41
3.3. Preparation of the Supported Catalyst on the Modified NMCM-41
3.4. Catalytic Tests
3.5. Characterization of the PE_UA_NMCM-41 Nanocomposites
3.5.1. Scanning Electronic Microscopy
3.5.2. Transmission Electronic Microscopy
3.5.3. Preparation of Films
3.5.4. X-ray Diffraction Experiments
3.5.5. Thermogravimetric Experiments
3.5.6. Differential Scanning Calorimetry Measurements
3.5.7. Viscoelastic Relaxation Study
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mu, H.; Jian, Z. Stereoselective Copolymerization of Olefin with Polar Monomers to Access Stereoregular Functionalized Polyolefins. Org. Mater. 2022, 4, 178–189. [Google Scholar] [CrossRef]
- Januszewski, R.; Dutkiewicz, M.; Kownacki, I. An Efficient Methodology for the Synthesis of Unique Functional Polyolefins. Mater Des. 2021, 206, 109801. [Google Scholar] [CrossRef]
- Dong, J.Y.; Hu, Y. Design and Synthesis of Structurally Well-Defined Functional Polyolefins via Transition Metal-Mediated Olefin Polymerization Chemistry. Coord. Chem. Rev. 2006, 250, 47–65. [Google Scholar] [CrossRef]
- Boffa, L.S.; Novak, B.M. Copolymerization of Polar Monomers with Olefins Using Transition-Metal Complexes. Chem. Rev. 2000, 100, 1479–1493. [Google Scholar] [CrossRef]
- Franssen, N.M.G.; Reek, J.N.H.; de Bruin, B. Synthesis of Functional ‘Polyolefins’: State of the Art and Remaining Challenges. Chem. Soc. Rev. 2013, 42, 5809–5832. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vicente, A.I.; Campos, J.; Bordado, J.M.; Rosário Ribeiro, M. Maleic Anhydride Modified Ethylene-Diene Copolymers: Synthesis and Properties. React. Funct. Polym. 2008, 68, 519–526. [Google Scholar] [CrossRef]
- Mohanty, A.D.; Bae, C. Transition Metal-Catalyzed Functionalization of Polyolefins Containing CC, CC, and CH Bonds. Adv. Organomet. Chem. 2015, 64, 1–39. [Google Scholar] [CrossRef]
- Goretzki, R.; Fink, G. Homogeneous and Heterogeneous Metallocene/MAO-Catalyzed Polymerization of Functionalized Olefins. Macromol. Chem. Phys. 1999, 200, 881–886. [Google Scholar] [CrossRef]
- Ahjopalo, L.; Löfgren, B.; Hakala, K.; Pietilä, L.O. Molecular Modeling of Metallocene Catalyzed Copolymerization of Ethylene with Functional Comonomers. Eur. Polym. J. 1999, 35, 1519–1528. [Google Scholar] [CrossRef]
- Santos, J.M.; Ribeiro, M.R.; Portela, M.F.; Pereira, S.G.; Nunes, T.G.; Deffieux, A. Metallocene-Catalysed Copolymerisation of Ethylene with 10-Undecenoic Acid: The Effect of Experimental Conditions. Macromol. Chem. Phys. 2001, 202, 2195–2201. [Google Scholar] [CrossRef]
- Cerrada, M.L.; Benavente, R.; Pérez, E.; Moniz-Santos, J.; Campos, J.M.; Ribeiro, M.R. Ethylene/10-Undecenoic Acid Copolymers Prepared with Different Metallocene Catalysts. Macromol. Chem. Phys. 2007, 208, 841–850. [Google Scholar] [CrossRef]
- Kaminsky, W. Polyolefin-Nanocomposites with Special Properties by in-Situ Polymerization. Front. Chem. Sci. Eng. 2018, 12, 555–563. [Google Scholar] [CrossRef]
- Feldman, D. Polyolefin, Olefin Copolymers and Polyolefin Polyblend Nanocomposites. J. Macromol. Sci. Part A 2016, 53, 651–658. [Google Scholar] [CrossRef]
- Campos, J.M.; Lourenço, J.P.; Cramail, H.; Ribeiro, M.R. Nanostructured Silica Materials in Olefin Polymerisation: From Catalytic Behaviour to Polymer Characteristics. Prog. Polym. Sci. 2012, 37, 1764–1804. [Google Scholar] [CrossRef]
- Zou, H.; Wu, S.; Shen, J. Polymer/Silica Nanocomposites: Preparation, Characterization, Propertles, and Applications. Chem. Rev. 2008, 108, 3893–3957. [Google Scholar] [CrossRef]
- Chung, T.-S.; Jiang, L.Y.; Li, Y.; Kulprathipanja, S. Mixed Matrix Membranes (MMMs) Comprising Organic Polymers with Dispersed Inorganic Fillers for Gas Separation. Prog. Polym. Sci. 2007, 32, 483–507. [Google Scholar] [CrossRef]
- Cecílio, D.M.; Cerrada, M.L.; Pérez, E.; Fernandes, A.; Lourenço, J.P.; McKenna, T.F.L.; Ribeiro, M.R. A Novel Approach for Preparation of Nanocomposites with an Excellent Rigidity/Deformability Balance Based on Reinforced HDPE with Halloysite. Eur. Polym. J. 2023, 184, 111765. [Google Scholar] [CrossRef]
- Cecílio, D.M.; Fernandes, A.; Lourenço, J.P.; McKenna, T.F.L.; Ribeiro, M.R. Innovative Route for the Preparation of High-Performance Polyolefin Materials Based on Unique Dendrimeric Silica Particles. Polym. Chem. 2021, 12, 4546–4556. [Google Scholar] [CrossRef]
- Bento, A.; Lourenço, J.P.; Fernandes, A.; Cerrada, M.L.; RosárioRibeiro, M. Functionalization of Mesoporous MCM-41 (Nano)Particles: Preparation Methodologies, Role on Catalytic Features, and Dispersion within Polyethylene Nanocomposites. ChemCatChem 2013, 5, 966–976. [Google Scholar] [CrossRef]
- Bento, A.; Lourenço, J.P.; Fernandes, A.; Ribeiro, M.R.; Arranz-Andrés, J.; Lorenzo, V.; Cerrada, M.L. Gas Permeability Properties of Decorated MCM-41/Polyethylene Hybrids Prepared by in-Situ Polymerization. J. Memb. Sci. 2012, 415, 702–711. [Google Scholar] [CrossRef]
- Campos, M.J.; Paulo Lourenço, J.; Pérez, E.; Cerrada, M.L.; Ribeiro, M.R. Self-Reinforced Hybrid Polyethylene/Mcm-41 Nanocomposites: In-Situ Polymerisation and Effect of MCM-41 Content on Rigidity. J. Nanosci. Nanotechnol. 2009, 9, 3966–3974. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Campos, J.M.; Ribeiro, M.R.; Lourenço, J.P.; Fernandes, A. Ethylene Polymerisation with Zirconocene Supported in Al-Modified MCM-41: Catalytic Behaviour and Polymer Properties. J. Mol. Catal. A Chem. 2007, 277, 93–101. [Google Scholar] [CrossRef]
- Ray, S.; Galgali, G.; Lele, A.; Sivaram, S. In Situ Polymerization of Ethylene with Bis(Imino)Pyridine Iron(II) Catalysts Supported on Clay: The Synthesis and Characterization of Polyethylene–Clay Nanocomposites. J. Polym. Sci. A Polym. Chem. 2005, 43, 304–318. [Google Scholar] [CrossRef]
- Nakajima, H.; Yamada, K.; Iseki, Y.; Hosoda, S.; Hanai, A.; Oumi, Y.; Teranishi, T.; Sano, T. Preparation and Characterization of Polypropylene/Mesoporous Silica Nanocomposites with Confined Polypropylene. J. Polym. Sci. B Polym. Phys. 2003, 41, 3324–3332. [Google Scholar] [CrossRef]
- Fu, S.; Sun, Z.; Huang, P.; Li, Y.; Hu, N. Some Basic Aspects of Polymer Nanocomposites: A Critical Review. Nano Mater. Sci. 2019, 1, 2–30. [Google Scholar] [CrossRef]
- Tripathi, S.N.; Rao, G.S.S.; Mathur, A.B.; Jasra, R. Polyolefin/Graphene Nanocomposites: A Review. RSC Adv. 2017, 7, 23615–23632. [Google Scholar] [CrossRef] [Green Version]
- Kageyama, K.; Tamazawa, J.I.; Aida, T. Extrusion Polymerization: Catalyzed Synthesis of Crystalline Linear Polyethylene Nanofibers Within a Mesoporous Silica. Science 1999, 285, 2113–2115. [Google Scholar] [CrossRef]
- Ye, Z.; Zhu, S.; Wang, W.J.; Alsyouri, H.; Lin, Y.S. Morphological and Mechanical Properties of Nascent Polyethylene Fibers Produced via Ethylene Extrusion Polymerization with a Metallocene Catalyst Supported on MCM-41 Particles. J. Polym. Sci. B Polym. Phys. 2003, 41, 2433–2443. [Google Scholar] [CrossRef]
- Cerrada, M.L.; Pérez, E.; Lourenço, J.P.; Campos, J.M.; Rosário Ribeiro, M. Hybrid HDPE/MCM-41 Nanocomposites: Crystalline Structure and Viscoelastic Behaviour. Microporous Mesoporous Mater. 2010, 130, 215–223. [Google Scholar] [CrossRef]
- Barus, S.; Zanetti, M.; Lazzari, M.; Costa, L. Preparation of Polymeric Hybrid Nanocomposites Based on PE and Nanosilica. Polymer 2009, 50, 2595–2600. [Google Scholar] [CrossRef]
- Cerrada, M.L.; Bento, A.; Pérez, E.; Lorenzo, V.; Lourenço, J.P.; Ribeiro, M.R. Hybrid Materials Based on Polyethylene and MCM-41 Microparticles Functionalized with Silanes: Catalytic Aspects of in Situ Polymerization, Crystalline Features and Mechanical Properties. Microporous Mesoporous Mater. 2016, 232, 86–96. [Google Scholar] [CrossRef]
- Cerrada, M.L.; Pérez, E.; Lourenço, J.P.; Bento, A.; Ribeiro, M.R. Decorated MCM-41/Polyethylene Hybrids: Crystalline Details and Viscoelastic Behavior. Polymer 2013, 54, 2611–2620. [Google Scholar] [CrossRef] [Green Version]
- Morrow, B.A. Chapter 3 Surface Groups on Oxides. Stud. Surf. Sci. Catal. 1990, 57, A161–A224. [Google Scholar] [CrossRef]
- Jentys, A.; Pham, N.H.; Vinek, H. Nature of Hydroxy Groups in MCM-41. J. Chem. Soc. Faraday Trans. 1996, 92, 3287–3291. [Google Scholar] [CrossRef]
- Landry, C.C.; Pappé, N.; Mason, M.R.; Apblett, A.W.; Tyler, A.N.; MacInnes, A.N.; Barron, A.R. From Minerals to Materials: Synthesis of Alumoxanes from the Reaction of Boehmite with Carboxylic Acids. J. Mater Chem. 1995, 5, 331–341. [Google Scholar] [CrossRef]
- Gurian, P.L.; Cheatham, L.K.; Ziller, J.W.; Barron, A.R. Aluminium Complexes of N,N’-Ethylenebis(Salicylideneimine)(H2 Salen). X-Ray Crystal Structures of [{Al(Salen)}2(µ-O)]·MeCN and [Al(OC6H2Me3-2,4,6)(Salen)]. J. Chem. Soc. Dalton. Trans. 1991, 6, 1449–1456. [Google Scholar] [CrossRef]
- Editors, G.; Milani, B.; Claver, C.; Kaminsky, W.; Funck, A.; Hähnsen, H.; Busico, V.; Trans, D.; Ciancaleoni, G.; Fraldi, N.; et al. An Investigation of the Influence of R on the Abilities of the Polar Monomers CH2CH(CH2)8OR (R = Me, PhCH2, Ph3C, Me3Si, Ph3Si) to Participate in O- Rather than H2-Coordination to Metallocene Alkene Polymerization Catalysts; an Unanticipated Role for Ether Oxygen Coordination in Promoting Polymerization. Dalton. Trans. 2009, 41, 8864–8877. [Google Scholar] [CrossRef]
- Goretzki, R.; Fink, R. Homogeneous and heterogeneous metallocene/MAO-catalyzed polymerization of trialkylsilyl-protected alcohols. Macromol. Rapid Commun. 1998, 19, 511–515. [Google Scholar] [CrossRef]
- Aaltonen, P.; Fink, G.; Löfgren, B.; Seppälä, J. Synthesis of Hydroxyl Group Containing Polyolefins with Metallocene/Methylaluminoxane Catalysts. Macromolecules 1996, 29, 5255–5260. [Google Scholar] [CrossRef]
- Turunen, J.; Pakkanen, T.T.; Lijfgren, B. NMR Studies on the Reactivity of Aluminium Compounds with an Unsaturated Alcohol. J. Mol. Catal. A Chem. 1997, 123, 35–42. [Google Scholar] [CrossRef]
- Silveira, F.; Alves, M.D.C.M.; Stedile, F.C.; Pergher, S.B.; dos Santos, J.H.Z. Microporous and Mesoporous Supports and Their Effect on the Performance of Supported Metallocene Catalysts. J. Mol. Catal. A Chem. 2010, 315, 213–220. [Google Scholar] [CrossRef]
- Dong, X.; Wang, L.; Jiang, G.; Zhao, Z.; Sun, T.; Yu, H.; Wang, W. MCM-41 and SBA-15 Supported Cp2ZrCl2 Catalysts for the Preparation of Nano-Polyethylene Fibres via in Situ Ethylene Extrusion Polymerization. J. Mol. Catal. A Chem. 2005, 240, 239–244. [Google Scholar] [CrossRef]
- Fischer, D.; Mülhaupt, R. Reversible and Irreversible Deactivation of Propene Polymerization Using Homogeneous Cp2ZrCl2/Methylaluminoxane Ziegler—Natta Catalysts. J. Organomet. Chem. 1991, 417, C7–C11. [Google Scholar] [CrossRef]
- Marcilla, A.; Gómez-Siurana, A.; Berenguer, D. Study of the Influence of the Characteristics of Different Acid Solids in the Catalytic Pyrolysis of Different Polymers. Appl. Catal. A Gen. 2006, 301, 222–231. [Google Scholar] [CrossRef]
- Sinfrônio, F.S.M.; Souza, A.G.; Santos, I.M.G.; Fernandes, V.J.; Novák, C.; Éhen, Z. Influence of H-ZSM-5, Al-MCM-41 and Acid Hybrid ZSM-5/MCM-41 on Polyethylene Decomposition. J. Therm. Anal. Calorim. 2006, 85, 391–399. [Google Scholar] [CrossRef]
- Shirayama, K.; Kita, S.-I.; Watabe, H. Effects of Branching on Some Properties of Ethylene/α-Olefin Copolymers. Die. Makromol. Chem. 1972, 151, 97–120. [Google Scholar] [CrossRef]
- Bunn, C.W. The Crystal Structure of Ethylene. Trans. Faraday Soc. 1944, 40, 23–25. [Google Scholar] [CrossRef]
- Beck, J.S.; Vartuli, J.C.; Roth, W.J.; Leonowicz, M.E.; Kresge, C.T.; Schmitt, K.D.; Chu, C.T.W.; Olson, D.H.; Sheppard, E.W.; McCullen, S.B.; et al. A New Family of Mesoporous Molecular Sieves Prepared with Liquid Crystal Templates. J. Am. Chem. Soc. 1992, 114, 10834–10843. [Google Scholar] [CrossRef]
- Popli, R.; Mandelkern, L. The Transition in Ethylene Copolymers: The β-Transition. Polym. Bull. 1983, 9, 260–267. [Google Scholar] [CrossRef]
- Popli, R.; Glotin, M.; Mandelkern, L.; Benson, R.S. Dynamic Mechanical Studies of α and β Relaxations of Polyethylenes. J. Polym. Sci. Polym. Phys. Ed. 1984, 22, 407–448. [Google Scholar] [CrossRef]
- Cerrada, M.L.; Benavente, R.; Pérez, E. Influence of Thermal History on Morphology and Viscoelastic Behavior of Ethylene-1-Octene Copolymers Synthesized with Metallocene Catalysts. J. Mater. Res. 2001, 16, 1103–1111. [Google Scholar] [CrossRef]
- Cerrada, M.L.; Benavente, R.; Peña, B.; Pérez, E. The Effect of Thermal Treatment on the Structure and Relaxation Processes of Olefinic Polymers Synthesized with Metallocene Catalysts. Polymer 2000, 41, 5957–5965. [Google Scholar] [CrossRef]
- Schatzki, T.F. Statistical Computation of Distribution Functions of Dimensions of Macromolecules. J. Polym. Sci. 1962, 57, 337–356. [Google Scholar] [CrossRef]
- Boyd, R.H. Relaxation Processes in Crystalline Polymers: Experimental Behaviour—A Review. Polymer 1985, 26, 323–347. [Google Scholar] [CrossRef]
- Ward, I. Mechanical Properties of Solid Polymers, 2nd ed.; John Wiley and Sons: Chichester, UK, 1983. [Google Scholar]
- McCrum, N.; Read, B.; Williams, G. Anelastic and Dielectric Effects in Polymeric Solids; Dover Publications: New York, NT, USA, 1991. [Google Scholar]
- Qiao, Z.A.; Zhang, L.; Guo, M.; Liu, Y.; Huo, Q. Synthesis of Mesoporous Silica Nanoparticles via Controlled Hydrolysis and Condensation of Silicon Alkoxide. Chem. Mater. 2009, 21, 3823–3829. [Google Scholar] [CrossRef]
- Galarneau, A.; Villemot, F.; Rodriguez, J.; Fajula, F.; Coasne, B. Validity of the T-Plot Method to Assess Microporosity in Hierarchical Micro/Mesoporous Materials. Langmuir 2014, 30, 13266–13274. [Google Scholar] [CrossRef]
- Quinn, F.A.; Mandelkern, L. Thermodynamics of Crystallization in High Polymers: Poly-(Ethylene). J. Am. Chem. Soc. 1958, 80, 3178–3182. [Google Scholar] [CrossRef]
- Wunderlich, B. Macromolecular Physics; Academic Press: New York, NT, USA, 1980. [Google Scholar]
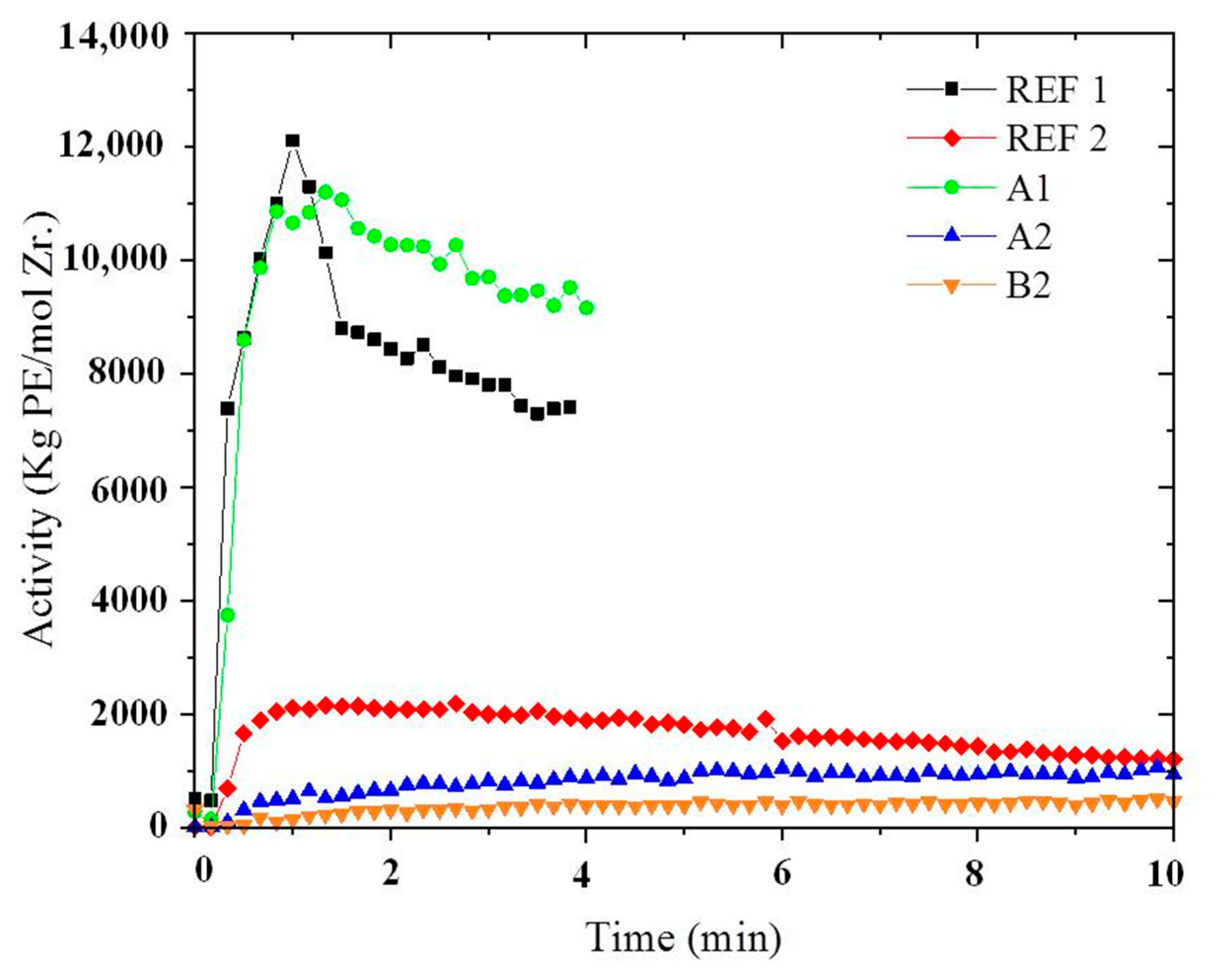





| Method and Sample Name | NMCM-41 Particles | Catalytic System | [UA] (10−5 M) | [Zr] (10−5 M) | Activity kgPE/molZr/h | UA mol% | NMCM-41 (% m/m) |
|---|---|---|---|---|---|---|---|
| REF1 | - | Homog. | 3.8 | 3.8 | 9100 | 0.7 | 0 |
| REF2 | - | Homog. | 30.5 | 6.4 | 1200 | n.d. | 0 |
| A1 1 | Neat | Homog. | 3.8 | 3.8 | 8560 | n.d. | 10 |
| A2 1 | Neat | Homog. | 30.5 | 6.4 | 780 | 1.7 | 9 |
| A3 2 | Neat | Homog. | 30.5 | 6.4 | 1000 | n.d. | 10 |
| B1 1 | Decorated | Supported | 3.8 | 3.8 | 620 | 1.3 | 11 |
| B2 2 | Decorated | Supported | 3.8 | 3.8 | 600 | n.d. | 9 |
| Sample Name | NMCM-41 Content by TGA | Tm (°C) | fcF1 | Tc (°C) | fcC |
|---|---|---|---|---|---|
| REF1 | 0 | 127.5 | 0.54 | 117.0 | 0.55 |
| A1 | 10 | 131.0 | 0.54 | 118.0 | 0.52 |
| A2 | 9 | 130.5 | 0.54 | 117.0 | 0.51 |
| A3 | 10 | 130.0 | 0.53 | 116.0 | 0.50 |
| B1 | 11 | 128.5 | 0.52 | 117.5 | 0.50 |
| B2 | 9 | 129.5 | 0.51 | 118.0 | 0.49 |
| Sample Name | NMCM-41 Content by TGA | fcF1 | E’ (MPa) | T (°C) | |
|---|---|---|---|---|---|
| γ | α | ||||
| REF1 | 0 | 0.54 | 825 | −110.5 | 109.0 |
| A1 | 10 | 0.54 | 1020 | −110.0 | 117.5 |
| A2 | 9 | 0.54 | 940 | −110.0 | 113.5 |
| A3 | 10 | 0.53 | 980 | −110.0 | 104.0 |
| B1 | 11 | 0.52 | 1110 | −110.0 | 112.0 |
| B2 | 9 | 0.51 | 1025 | −111.5 | 111.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cerrada, M.L.; Bento, A.; Pérez, E.; Lourenço, J.P.; Ribeiro, M.R. The Role of Undecenoic Acid on the Preparation of Decorated MCM-41/Polyethylene Hybrids by In Situ Polymerization: Catalytic Aspects and Properties of the Resultant Materials. Catalysts 2023, 13, 1182. https://doi.org/10.3390/catal13081182
Cerrada ML, Bento A, Pérez E, Lourenço JP, Ribeiro MR. The Role of Undecenoic Acid on the Preparation of Decorated MCM-41/Polyethylene Hybrids by In Situ Polymerization: Catalytic Aspects and Properties of the Resultant Materials. Catalysts. 2023; 13(8):1182. https://doi.org/10.3390/catal13081182
Chicago/Turabian StyleCerrada, María L., Artur Bento, Ernesto Pérez, João P. Lourenço, and M. Rosário Ribeiro. 2023. "The Role of Undecenoic Acid on the Preparation of Decorated MCM-41/Polyethylene Hybrids by In Situ Polymerization: Catalytic Aspects and Properties of the Resultant Materials" Catalysts 13, no. 8: 1182. https://doi.org/10.3390/catal13081182
APA StyleCerrada, M. L., Bento, A., Pérez, E., Lourenço, J. P., & Ribeiro, M. R. (2023). The Role of Undecenoic Acid on the Preparation of Decorated MCM-41/Polyethylene Hybrids by In Situ Polymerization: Catalytic Aspects and Properties of the Resultant Materials. Catalysts, 13(8), 1182. https://doi.org/10.3390/catal13081182









