Visible-Light-Induced Photocatalytic Degradation of Rhodamine B Dye Using a CuS/ZnS p-n Heterojunction Nanocomposite under Visible-Light Irradiation
Abstract
:1. Introduction
2. Results and Discussion
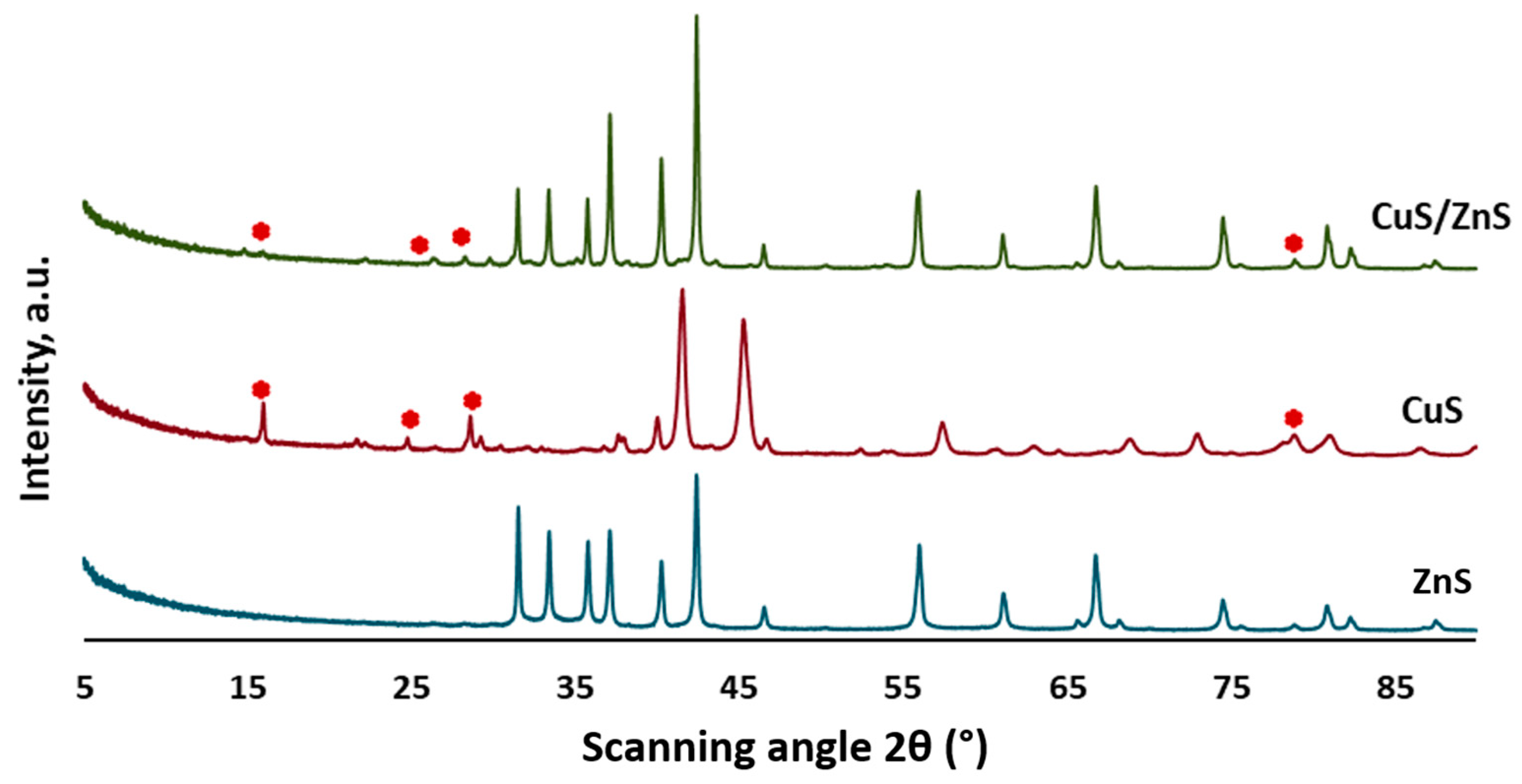
2.1. XRD Analysis
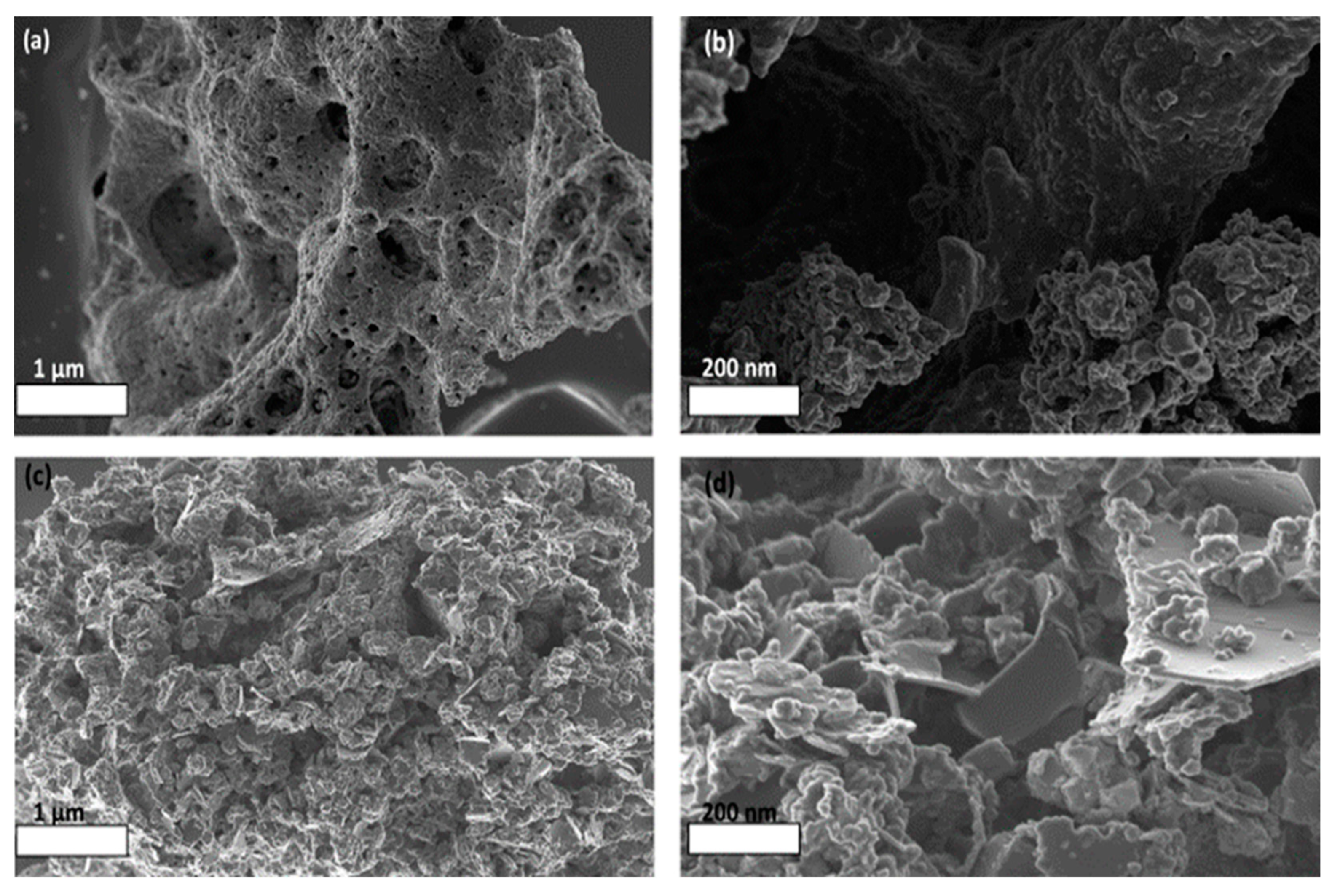
2.2. Catalyst Morphology
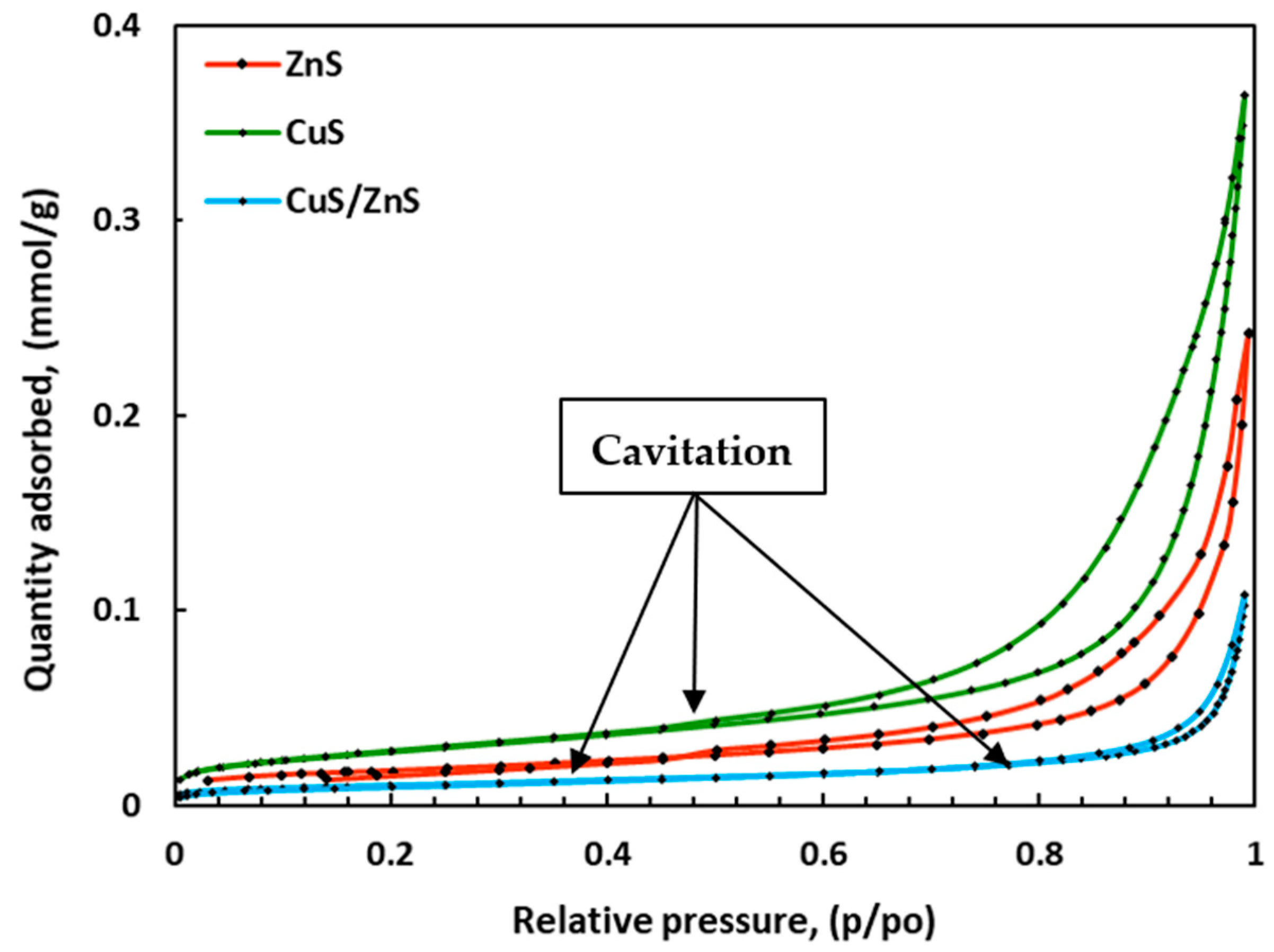
2.3. Surface Area and Pore Size Analysis
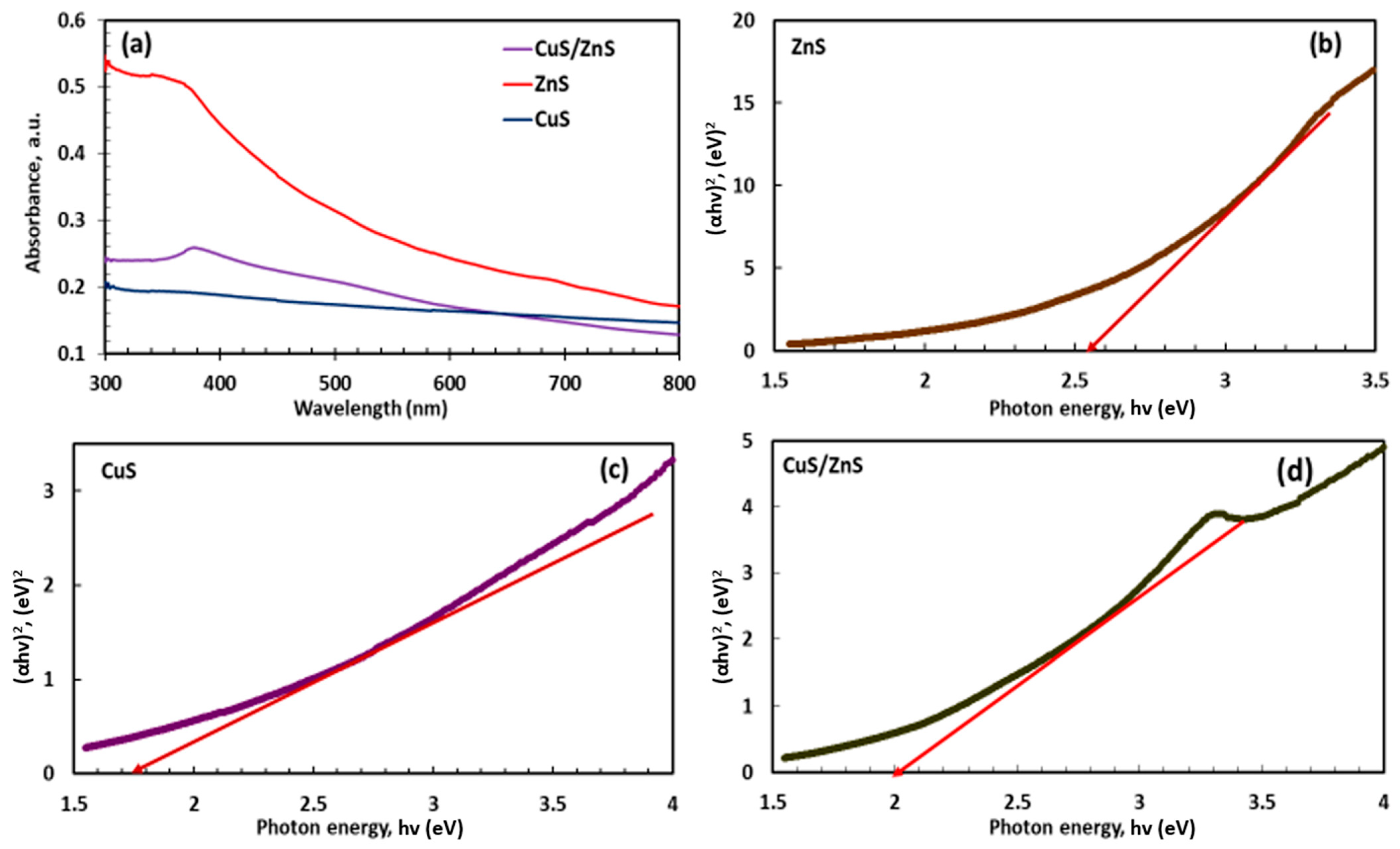
2.4. Photo-Absorption and Bandgap Analysis
2.5. Photocatalytic Performance
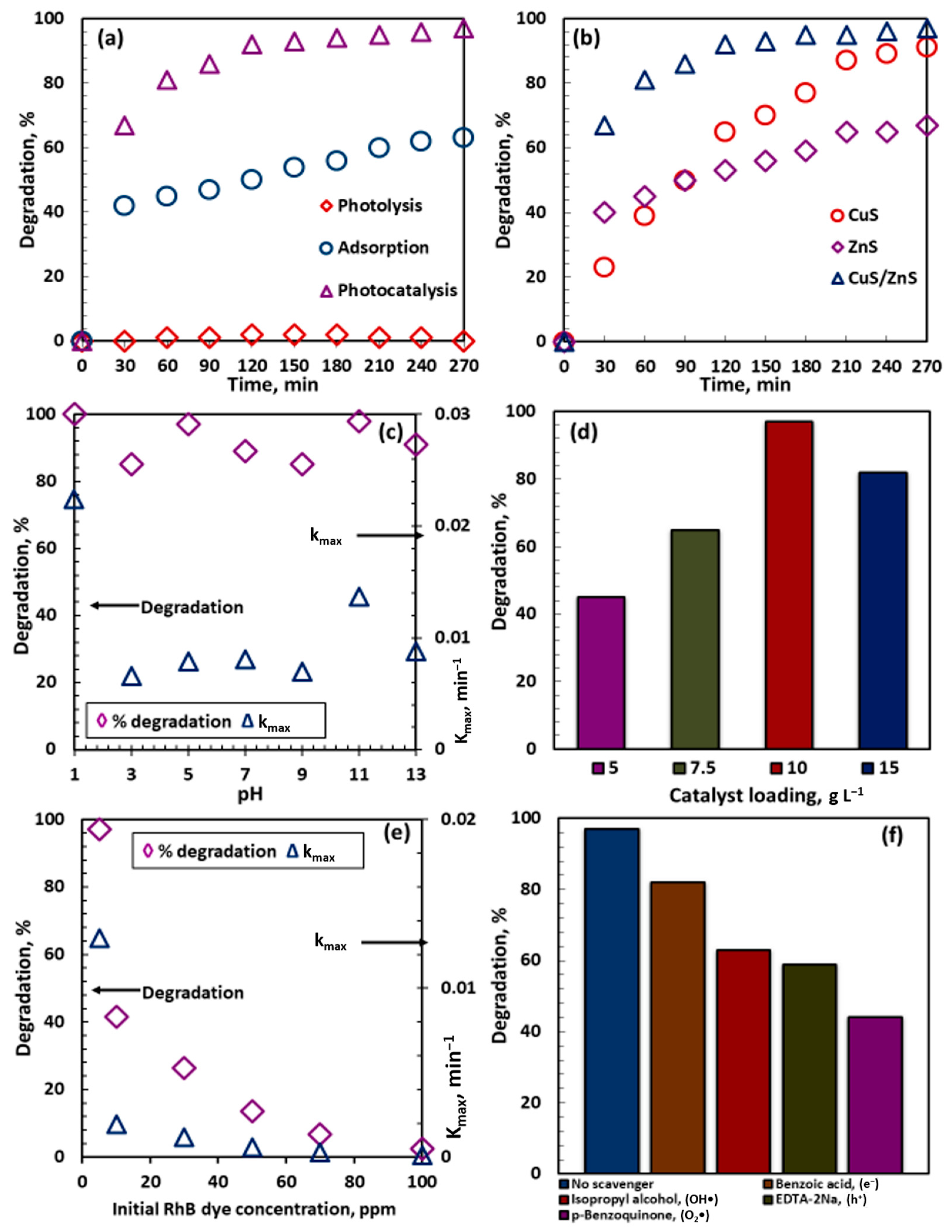
2.5.1. Effect of Individual Catalyst Composites
2.5.2. Effect of Initial pH
2.5.3. Effect of Catalyst Loading
2.5.4. Effect of Initial Rhodamine B Dye Concentration
2.6. Radical Scavenging Test
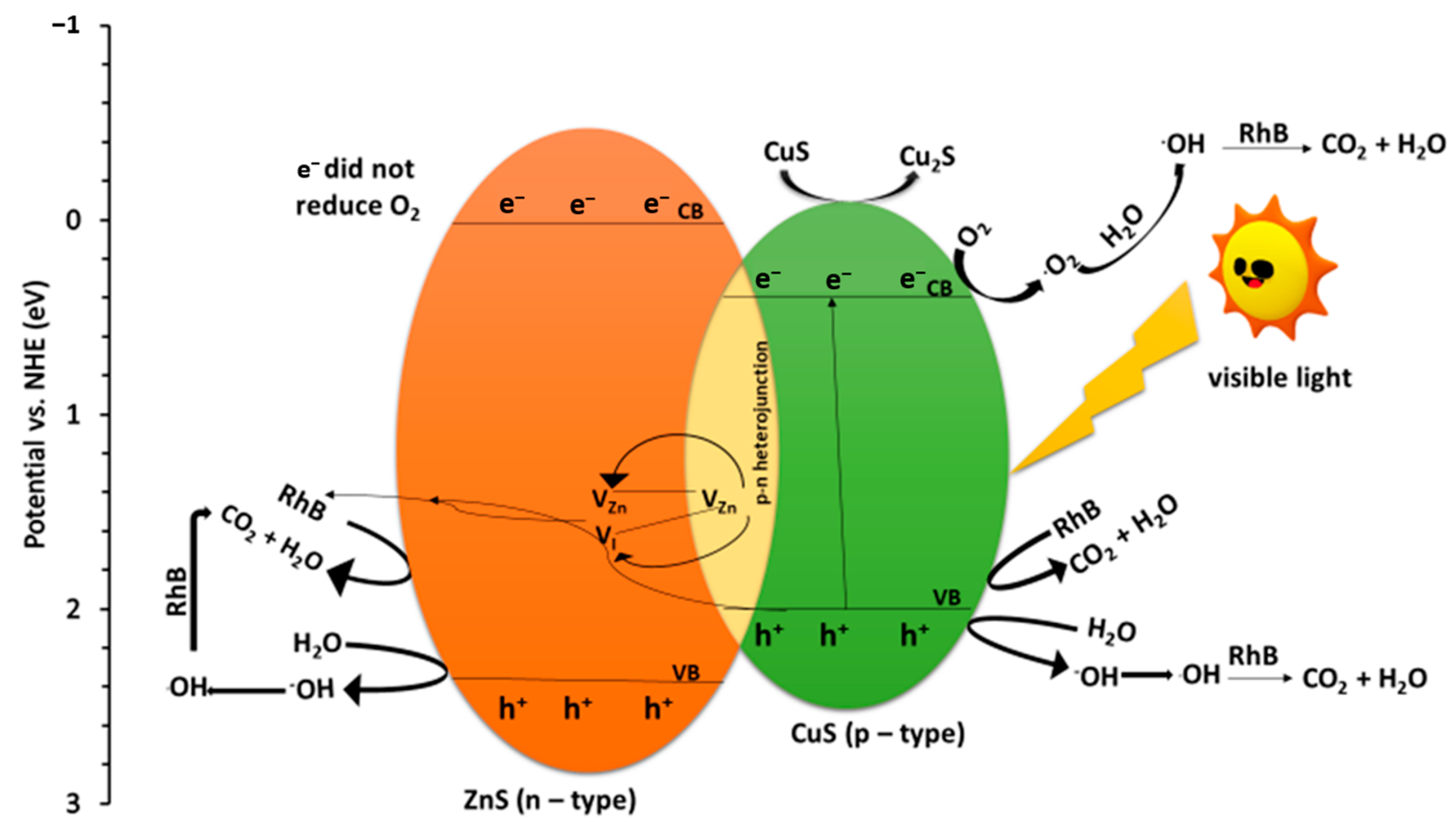
2.7. Mechanism
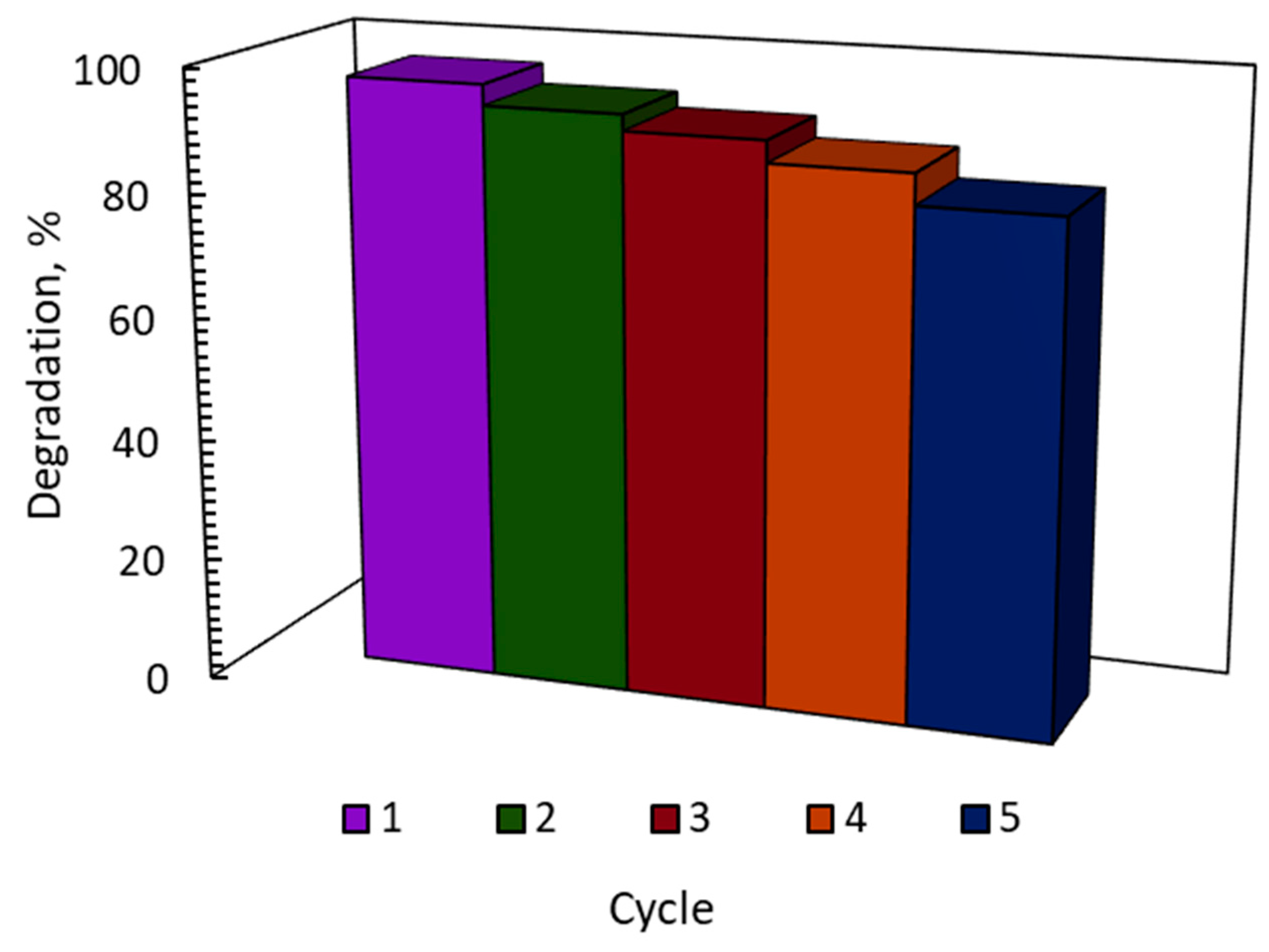
3. Catalyst Recyclability
4. Materials and Methods
4.1. Chemicals
4.2. Catalyst Synthesis
4.3. Degradation Studies
4.4. Material Characterisation
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Tang, J.Y.; McCarty, S.; Glenn, E.; Neale, P.A.; Warne, M.S.J.; Escher, B.I. Mixture effects of organic micropollutants present in water: Towards the development of effect-based water quality trigger values for baseline toxicity. Water Res. 2013, 47, 3300–3314. [Google Scholar] [CrossRef]
- Al-Buriahi, A.K.; Al-Gheethi, A.A.; Kumar, P.S.; Mohamed, R.M.S.R.; Yusof, H.; Alshalif, A.F.; Khalifa, N.A. Elimination of rhodamine B from textile wastewater using nanoparticle photocatalysts: A review for sustainable approaches. Chemosphere 2022, 287, 132162. [Google Scholar] [CrossRef]
- Carmen, Z.; Daniela, S. Textile Organic Dyes-Characteristics, Polluting Effects and Separation/Elimination Procedures from Industrial Effluents—A Critical Overview; IntechOpen: Rijeka, Croatia, 2012; Volume 3. [Google Scholar]
- Hanafi, M.F.; Sapawe, N. A review on the water problem associate with organic pollutants derived from phenol, methyl orange, and remazol brilliant blue dyes. Mater. Today Proc. 2020, 31, A141–A150. [Google Scholar] [CrossRef]
- Sharifzade, G.; Asghari, A.; Rajabi, M. Highly effective adsorption of xanthene dyes (rhodamine B and erythrosine B) from aqueous solutions onto lemon citrus peel active carbon: Characterization, resolving analysis, optimization and mechanistic studies. RSC Adv. 2017, 7, 5362–5371. [Google Scholar] [CrossRef] [Green Version]
- Ajiboye, T.O.; Oyewo, O.A.; Onwudiwe, D.C. Adsorption and photocatalytic removal of Rhodamine B from wastewater using carbon-based materials. FlatChem 2021, 29, 100277. [Google Scholar] [CrossRef]
- Saigl, Z.M. Various adsorbents for removal of rhodamine b dye: A review. Indones. J. Chem. 2021, 21, 1039–1056. [Google Scholar] [CrossRef]
- Samuel, M.S.; Savunthari, K.V.; Ethiraj, S. Synthesis of a copper (II) metal–organic framework for photocatalytic degradation of rhodamine B dye in water. Environ. Sci. Pollut. Res. 2021, 28, 40835–40843. [Google Scholar] [CrossRef]
- Crini, G.; Lichtfouse, E. Advantages and disadvantages of techniques used for wastewater treatment. Environ. Chem. Lett. 2019, 17, 145–155. [Google Scholar] [CrossRef]
- Naciri, Y.; Ahsaine, H.A.; Chennah, A.; Amedlous, A.; Taoufyq, A.; Bakiz, B.; Ezahri, M.; Villain, S.; Benlhachemi, A. Facile synthesis, characterization and photocatalytic performance of Zn3(PO4)2 platelets toward photodegradation of Rhodamine B dye. J. Environ. Chem. Eng. 2018, 6, 1840–1847. [Google Scholar] [CrossRef]
- Gedda, G.; Balakrishnan, K.; Devi, R.U.; Shah, K.J.; Gandhi, V.; Gandh, V.; Shah, K. Introduction to conventional wastewater treatment technologies: Limitations and recent advances. Mater. Res. Found 2021, 91, 1–36. [Google Scholar]
- Mahamuni, N.N.; Adewuyi, Y.G. Advanced oxidation processes (AOPs) involving ultrasound for waste water treatment: A review with emphasis on cost estimation. Ultrason. Sonochem. 2010, 17, 990–1003. [Google Scholar] [CrossRef] [PubMed]
- Cashman, M.A.; Kirschenbaum, L.; Holowachuk, J.; Boving, T.B. Identification of hydroxyl and sulfate free radicals involved in the reaction of 1, 4-dioxane with peroxone activated persulfate oxidant. J. Hazard. Mater. 2019, 380, 120875. [Google Scholar] [CrossRef]
- Li, S.; Yu, W.; Zhang, X.; Liu, L.; Wang, H.; Peng, Y.; Bian, Z. Mo-Based Heterogeneous Interface and Sulfur Vacancy Synergistic Effect Enhances the Fenton-like Catalytic Performance for Organic Pollutant Degradation. ACS Appl. Mater. Interfaces 2022, 15, 1326–1338. [Google Scholar] [CrossRef]
- Wang, X.; Zhao, H.; Quan, X.; Zhao, Y.; Chen, S. Visible light photoelectrocatalysis with salicylic acid-modified TiO2 nanotube array electrode for p-nitrophenol degradation. J. Hazard. Mater. 2009, 166, 547–552. [Google Scholar] [CrossRef] [PubMed]
- Jourshabani, M.; Dominic, J.A.; Achari, G.; Shariatinia, Z. Synergetic photocatalytic ozonation using modified graphitic carbon nitride for treatment of emerging contaminants under UVC, UVA and visible irradiation. Chem. Eng. Sci. 2019, 209, 115181. [Google Scholar] [CrossRef]
- Isac, L.; Andronic, L.; Visa, M.; Enesca, A. Selective photocatalytic degradation of organic pollutants by CuxS/ZnO/TiO2 heterostructures. Ceram. Int. 2020, 46, 4265–4273. [Google Scholar] [CrossRef]
- Zhang, L.; Wu, L.; Feng, Z.; Meng, Q.; Li, Y.; Duan, T. Adopting sulfur-atom sharing strategy to construct CoS2/MoS2 heterostructure on three-dimensional nitrogen-doped graphene aerogels: A novel photocatalyst for wastewater treatment. J. Environ. Chem. Eng. 2021, 9, 104771. [Google Scholar] [CrossRef]
- Wang, J.; Wang, G.; Cheng, B.; Yu, J.; Fan, J. Sulfur-doped g-C3N4/TiO2 S-scheme heterojunction photocatalyst for Congo Red photodegradation. Chin. J. Catal. 2021, 42, 56–68. [Google Scholar] [CrossRef]
- Raghavan, N.; Thangavel, S.; Sivalingam, Y.; Venugopal, G. Investigation of photocatalytic performances of sulfur based reduced graphene oxide-TiO2 nanohybrids. Appl. Surf. Sci. 2018, 449, 712–718. [Google Scholar] [CrossRef]
- Lin, H.-F.; Liao, S.-C.; Hung, S.-W. The dc thermal plasma synthesis of ZnO nanoparticles for visible-light photocatalyst. J. Photochem. Photobiol. A Chem. 2005, 174, 82–87. [Google Scholar] [CrossRef]
- Xu, Q.; Zhang, L.; Cheng, B.; Fan, J.; Yu, J. S-scheme heterojunction photocatalyst. Chem 2020, 6, 1543–1559. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, Z.; Liu, Y.; Fang, X.; Xu, J.; Wang, X.; Xu, X. Band-Gap engineering: A new tool for tailoring the activity of semiconducting oxide catalysts for CO oxidation. J. Phys. Chem. Lett. 2021, 12, 9188–9196. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Shen, X.; Zhu, G.; Zhou, H.; Ji, Z.; Chen, K.; Yuan, A. Synthesis of ternary Ag/ZnO/ZnFe2O4 porous and hollow nanostructures with enhanced photocatalytic activity. Appl. Catal. B Environ. 2016, 184, 328–336. [Google Scholar] [CrossRef]
- Guo, Y.; Li, J.; Gao, Z.; Zhu, X.; Liu, Y.; Wei, Z.; Zhao, W.; Sun, C. A simple and effective method for fabricating novel p–n heterojunction photocatalyst g-C3N4/Bi4Ti3O12 and its photocatalytic performances. Appl. Catal. B Environ. 2016, 192, 57–71. [Google Scholar] [CrossRef]
- Low, J.; Yu, J.; Jaroniec, M.; Wageh, S.; Al-Ghamdi, A.A. Heterojunction photocatalysts. Adv. Mater. 2017, 29, 1601694. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, L.; Chen, Z.; Hu, J.; Li, S.; Wang, Z.; Liu, J.; Wang, X. Semiconductor heterojunction photocatalysts: Design, construction, and photocatalytic performances. Chem. Soc. Rev. 2014, 43, 5234–5244. [Google Scholar] [CrossRef]
- Harish, S.; Archana, J.; Navaneethan, M.; Ponnusamy, S.; Singh, A.; Gupta, V.; Aswal, D.; Ikeda, H.; Hayakawa, Y. Synergetic effect of CuS@ZnS nanostructures on photocatalytic degradation of organic pollutant under visible light irradiation. RSC Adv. 2017, 7, 34366–34375. [Google Scholar] [CrossRef] [Green Version]
- Hong, Y.; Zhang, J.; Huang, F.; Zhang, J.; Wang, X.; Wu, Z.; Lin, Z.; Yu, J. Enhanced visible light photocatalytic hydrogen production activity of CuS/ZnS nanoflower spheres. J. Mater. Chem. A 2015, 3, 13913–13919. [Google Scholar] [CrossRef]
- Mondal, C.; Singh, A.; Sahoo, R.; Sasmal, A.K.; Negishi, Y.; Pal, T. Preformed ZnS nanoflower prompted evolution of CuS/ZnS p-n heterojunctions for exceptional visible-light driven photocatalytic activity. New J. Chem. 2015, 39, 5628–5635. [Google Scholar] [CrossRef]
- Guo, J.; Liang, Y.; Liu, L.; Hu, J.; Wang, H.; An, W.; Cui, W. Core-shell structure of sulphur vacancies-CdS@CuS: Enhanced photocatalytic hydrogen generation activity based on photoinduced interfacial charge transfer. J. Colloid Interface Sci. 2021, 600, 138–149. [Google Scholar]
- Li, G.; Deng, X.; Chen, P.; Wang, X.; Ma, J.; Liu, F.; Yin, S.-F. Sulphur vacancies-VS2@C3N4 drived by in situ supramolecular self-assembly for synergistic photocatalytic degradation of real wastewater and H2 production: Vacancies taming interfacial compact heterojunction and carriers transfer. Chem. Eng. J. 2022, 433, 134505. [Google Scholar] [CrossRef]
- Sitinjak, E.M.; Masmur, I.; Marbun, N.V.M.D.; Hutajulu, P.E.; Gultom, G.; Sitanggang, Y. Direct Z-scheme of n-type CuS/p-type ZnS@electrospun PVP nanofiber for the highly efficient catalytic reduction of 4-nitrophenol and mixed dyes. RSC Adv. 2022, 12, 16165–16173. [Google Scholar] [CrossRef]
- Yu, J.; Zhang, J.; Liu, S. Ion-exchange synthesis and enhanced visible-light photoactivity of CuS/ZnS nanocomposite hollow spheres. J. Phys. Chem. C 2010, 114, 13642–13649. [Google Scholar] [CrossRef]
- Sreelekha, N.; Subramanyam, K.; Reddy, D.A.; Murali, G.; Ramu, S.; Varma, K.R.; Vijayalakshmi, R. Structural, optical, magnetic and photocatalytic properties of Co doped CuS diluted magnetic semiconductor nanoparticles. Appl. Surf. Sci. 2016, 378, 330–340. [Google Scholar] [CrossRef]
- Moja, M.M.; Chirwa, E.; Tichapondwa, S.M. Visible light activated photocatalytic degradation of 2,4-dichlorophenol using silver halide photocatalysts. Chem. Eng. Trans. 2021, 86, 1411–1416. [Google Scholar]
- Varghese, J. CuS–ZnS decorated graphene nanocomposites: Synthesis and photocatalytic properties. J. Phys. Chem. Solids 2021, 156, 109911. [Google Scholar] [CrossRef]
- Rameshbabu, R.; Ravi, P.; Sathish, M. Cauliflower-like CuS/ZnS nanocomposites decorated g-C3N4 nanosheets as noble metal-free photocatalyst for superior photocatalytic water splitting. Chem. Eng. J. 2019, 360, 1277–1286. [Google Scholar] [CrossRef]
- Mzimela, N.; Tichapondwa, S.; Chirwa, E. Visible-light-activated photocatalytic degradation of rhodamine B using WO3 nanoparticles. RSC Adv. 2022, 12, 34652–34659. [Google Scholar] [CrossRef]
- Ichipi, E.O.; Tichapondwa, S.M.; Chirwa, E.M. Plasmonic effect and bandgap tailoring of Ag/Ag2S doped on ZnO nanocomposites for enhanced visible-light photocatalysis. Adv. Powder Technol. 2022, 33, 103596. [Google Scholar]
- Yu, S.; Liu, J.; Zhu, W.; Hu, Z.-T.; Lim, T.-T.; Yan, X. Facile room-temperature synthesis of carboxylated graphene oxide-copper sulfide nanocomposite with high photodegradation and disinfection activities under solar light irradiation. Sci. Rep. 2015, 5, 16369. [Google Scholar] [CrossRef] [Green Version]
- Huang, C.; Hu, J.; Cong, S.; Zhao, Z.; Qiu, X. Hierarchical BiOCl microflowers with improved visible-light-driven photocatalytic activity by Fe (III) modification. Appl. Catal. B Environ. 2015, 174, 105–112. [Google Scholar] [CrossRef]
- Adelifard, M.; Eshghi, H.; Mohagheghi, M.M.B. Synthesis and characterization of nanostructural CuS–ZnS binary compound thin films prepared by spray pyrolysis. Opt. Commun. 2012, 285, 4400–4404. [Google Scholar] [CrossRef]
- Alshamsi, H.A.; Beshkar, F.; Amiri, O.; Salavati-Niasari, M. Porous hollow Ag/Ag2S/Ag3PO4 nanocomposites as highly efficient heterojunction photocatalysts for the removal of antibiotics under simulated sunlight irradiation. Chemosphere 2021, 274, 129765. [Google Scholar] [CrossRef] [PubMed]
- Mohanty, L.; Pattanayak, D.S.; Pradhan, D.; Dash, S.K. Synthesis of novel p-n heterojunction g-C3N4/Bi4Ti3O12 photocatalyst with improved solar-light-driven photocatalytic degradation of organic dyes. Environ. Qual. Manag. 2022, 32, 45–59. [Google Scholar] [CrossRef]
- Feng, C.; Meng, X.; Song, X.; Feng, X.; Zhao, Y.; Liu, G. Controllable synthesis of hierarchical CuS/ZnS hetero-nanowires as high-performance visible-light photocatalysts. RSC Adv. 2016, 6, 110266–110273. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.; Zhao, J.; Liu, H.; Huang, J. Design, modification and application of semiconductor photocatalysts. J. Taiwan Inst. Chem. Eng. 2018, 93, 590–602. [Google Scholar] [CrossRef]

| AOP Technique | S-Based Photocatalyst | Synthesis Method | Target Pollutant | Photocatalytic Activity | Reference |
|---|---|---|---|---|---|
| Photo-Fenton | MoS2/Mo2N | hydrothermal | 20 mg L−1 paracetamols | 71% degradation in 120 min under UV light | [14] |
| Electrochemical | SA/TiO2 | immersion | 5 mg L−1 p-nitrophenol | 5% degradation in 120 min under UV light and 5% in 180 min under visible light | [15] |
| Ozonation | Fe2O3/S-C3N4 | one-pot in situ | 10 mg L−1 methylene blue (MB) dye | 96% removal in 240 min under UV light | [16] |
| Sonolysis | CuxS/ZnO/TiO2 | spray pyrolysis | 0.58 mg L−1 phenol | 72% degradation in 600 min under UV light | [17] |
| Photocatalysis | CoS2/MoS2 | hydrothermal | 10 mg L−1 rhodamine B (RhB) dye | 78% degradation in 60 min under visible light | [18] |
| Photocatalysis | g-C3N4(SCN)/TiO2 | electrospinning | 50 mg L−1 congo red (CR) dye | 100% degradation in 60 min under UV light | [19] |
| Photocatalysis | S@GO/TiO2 | ultrasonication | 0.3 mg L−1 methylene blue (MB) dye | 93% degradation in 120 min under UV light | [20] |
| Materials | Surface Area (m2 g−1) | Average Pore Size (nm) | BJH Adsorption (4 V/Å) * | BJH Desorption (4 V/Å) * |
|---|---|---|---|---|
| ZnS | 4.06 | 12.03 | 73.47 | 75.61 |
| CuS | 8.73 | 11.22 | 60.68 | 82.43 |
| CuS/ZnS | 2.72 | 8.57 | 51.35 | 54.38 |
| Catalyst Loading (g L−1) | Linear Regression (R2) | Kmax (min−1) |
|---|---|---|
| 0 | 0.097 | 0.0000 |
| 5 | 0.897 | 0.0034 |
| 7.5 | 0.947 | 0.0058 |
| 10 | 0.986 | 0.0186 |
| 15 | 0.905 | 0.0094 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mugumo, R.; Ichipi, E.; Tichapondwa, S.M.; Chirwa, E.M.N. Visible-Light-Induced Photocatalytic Degradation of Rhodamine B Dye Using a CuS/ZnS p-n Heterojunction Nanocomposite under Visible-Light Irradiation. Catalysts 2023, 13, 1184. https://doi.org/10.3390/catal13081184
Mugumo R, Ichipi E, Tichapondwa SM, Chirwa EMN. Visible-Light-Induced Photocatalytic Degradation of Rhodamine B Dye Using a CuS/ZnS p-n Heterojunction Nanocomposite under Visible-Light Irradiation. Catalysts. 2023; 13(8):1184. https://doi.org/10.3390/catal13081184
Chicago/Turabian StyleMugumo, Rachel, Emmanuel Ichipi, Shepherd M. Tichapondwa, and Evans M. Nkhalambayausi Chirwa. 2023. "Visible-Light-Induced Photocatalytic Degradation of Rhodamine B Dye Using a CuS/ZnS p-n Heterojunction Nanocomposite under Visible-Light Irradiation" Catalysts 13, no. 8: 1184. https://doi.org/10.3390/catal13081184
APA StyleMugumo, R., Ichipi, E., Tichapondwa, S. M., & Chirwa, E. M. N. (2023). Visible-Light-Induced Photocatalytic Degradation of Rhodamine B Dye Using a CuS/ZnS p-n Heterojunction Nanocomposite under Visible-Light Irradiation. Catalysts, 13(8), 1184. https://doi.org/10.3390/catal13081184







