Bioenergy Production from Agro-Industrial Wastewater Using Advanced Oxidation Processes as Pre-Treatment
Abstract
:1. Introduction
2. Agro-Industrial Effluents
2.1. Olive Mill Industry
- The traditional discontinuous press process involves the combined use of a crusher and hydraulic press. This process does not require the addition of water, thus the OMW generation produces between 0.5 and 0.8 m3 of wastewater per ton of olives [35]. On the other hand, the lower production efficiency and greater labor costs have led to its replacement by other extraction systems [36].
- The two-phase centrifugal system separates pomace in two stages: oil and solid waste. It requires a small amount of water for the oil extraction, reducing the processing costs and the quantity of produced solid residues. However, the wet pomace that is produced tends to have high moisture contents and is more difficult and expensive to treat [35,37].
Olive Mill Wastewater
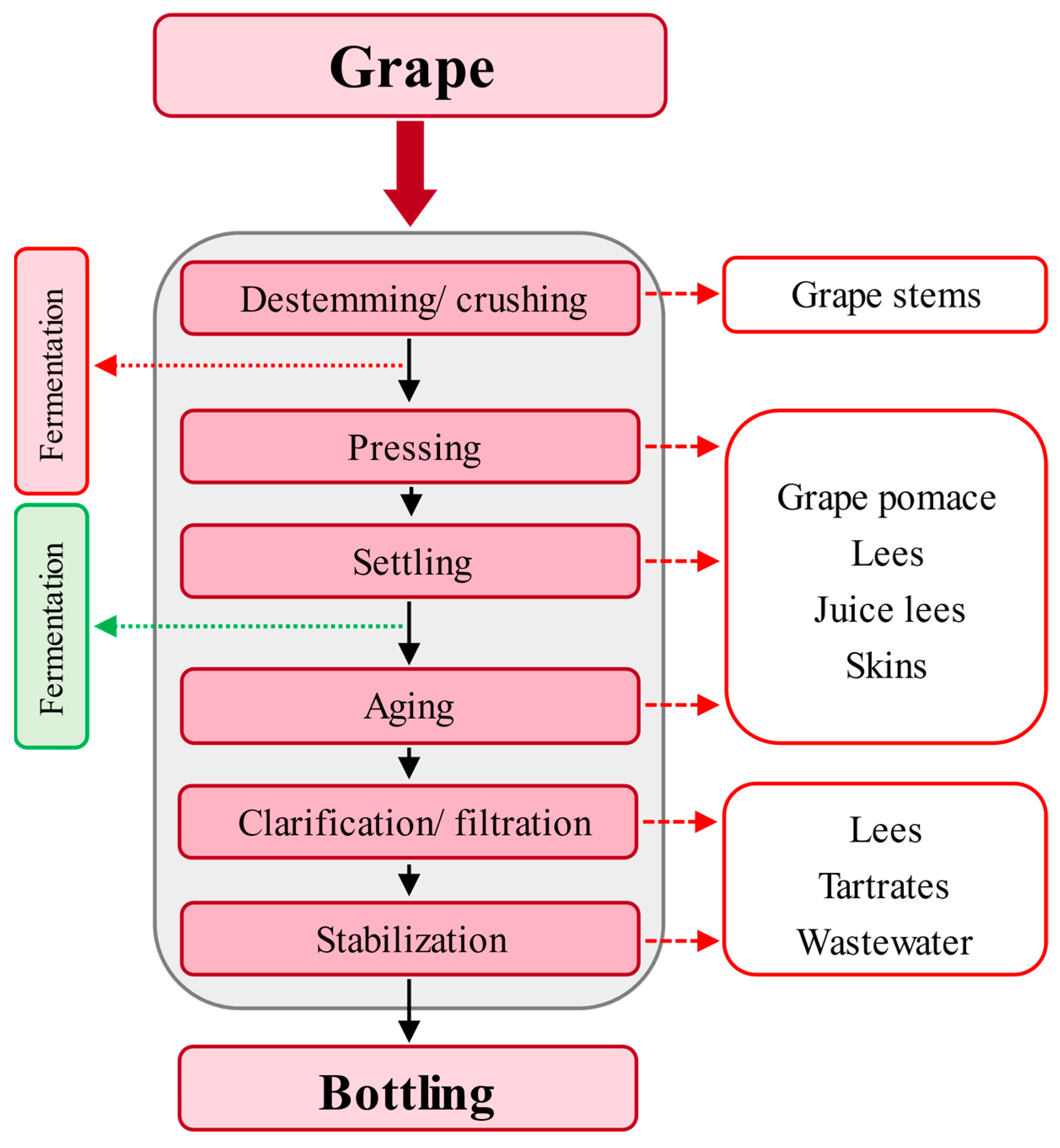
2.2. Winery Industry
Winery Wastewater
2.3. Livestock Industry
- Tanning processes are applied to the hides produced at slaughterhouses. These processes include several steps, where large amounts of wastewater and solid waste are produced [59].
- Milk is produced and stored at the farm before its collection and transportation to a processing plant. This consumes significant energy and generates wastewater and solid waste [59].
Livestock Wastewater
2.4. Pulp and Paper Mill Industry
Pulp and Paper Mill Wastewater
3. Advanced Oxidation Processes
3.1. Ozonation
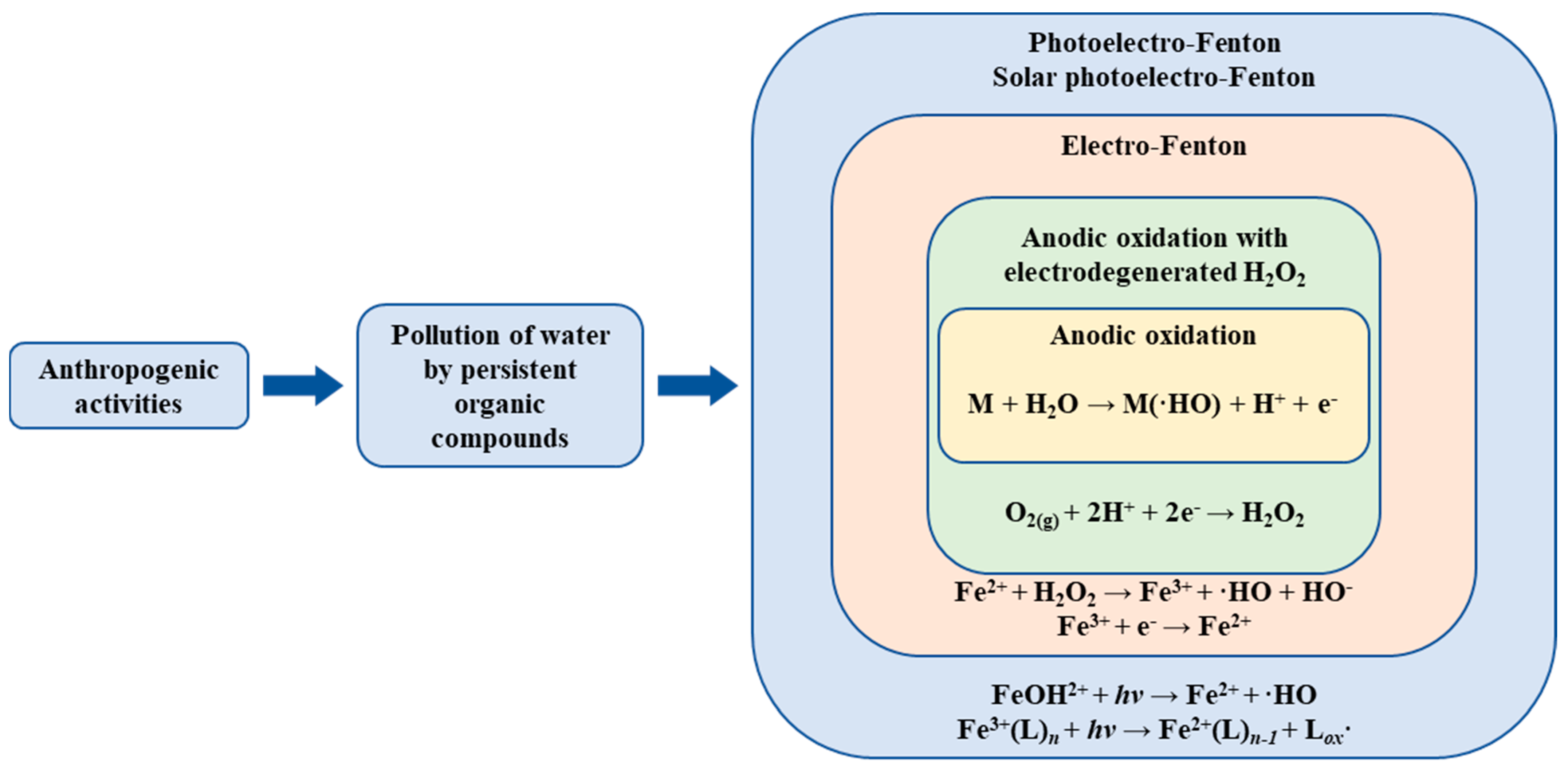
3.2. Fenton Reagent
3.3. Ultraviolet Radiation
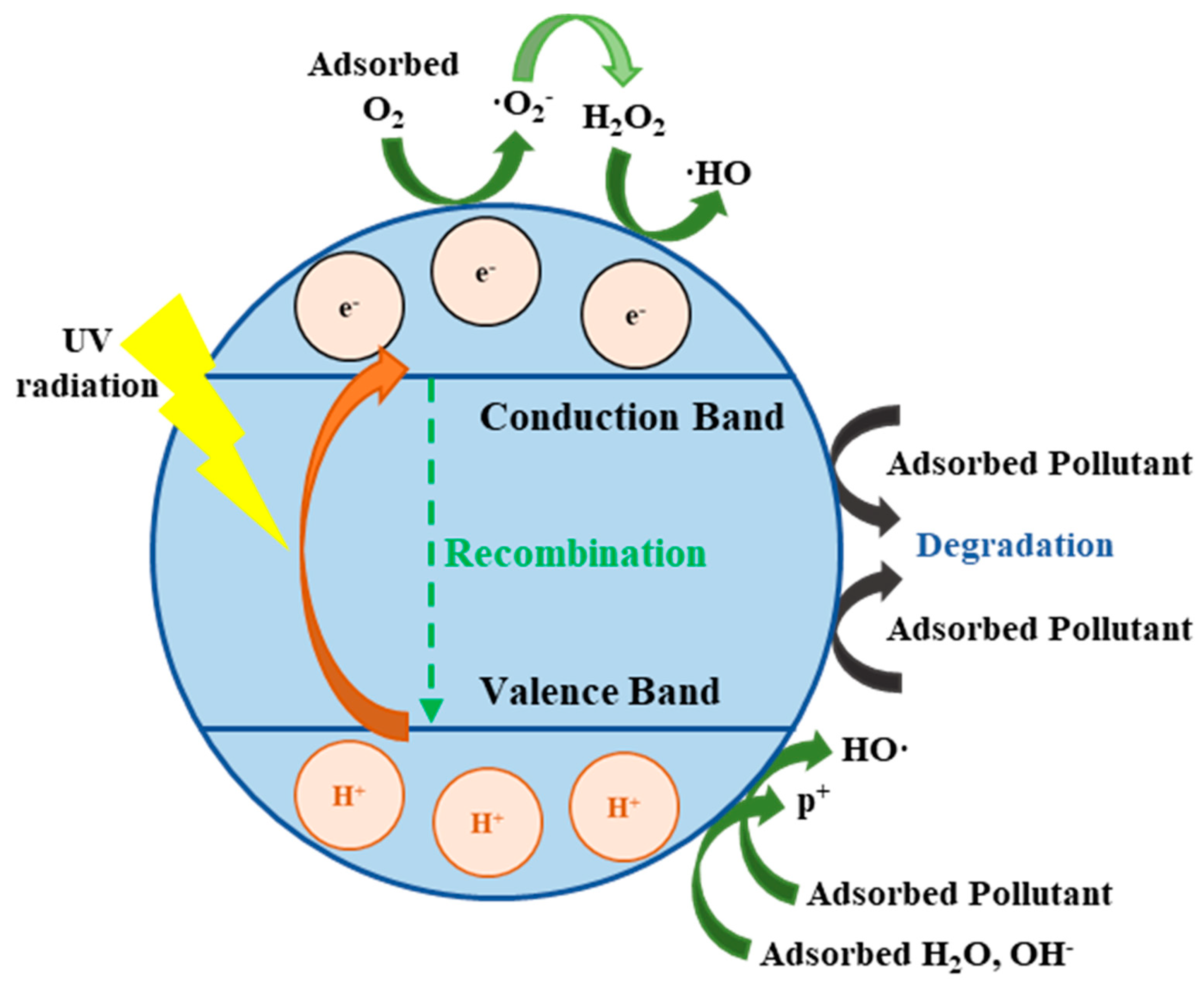
3.4. Photocatalysis
3.5. Wet Air Oxidation
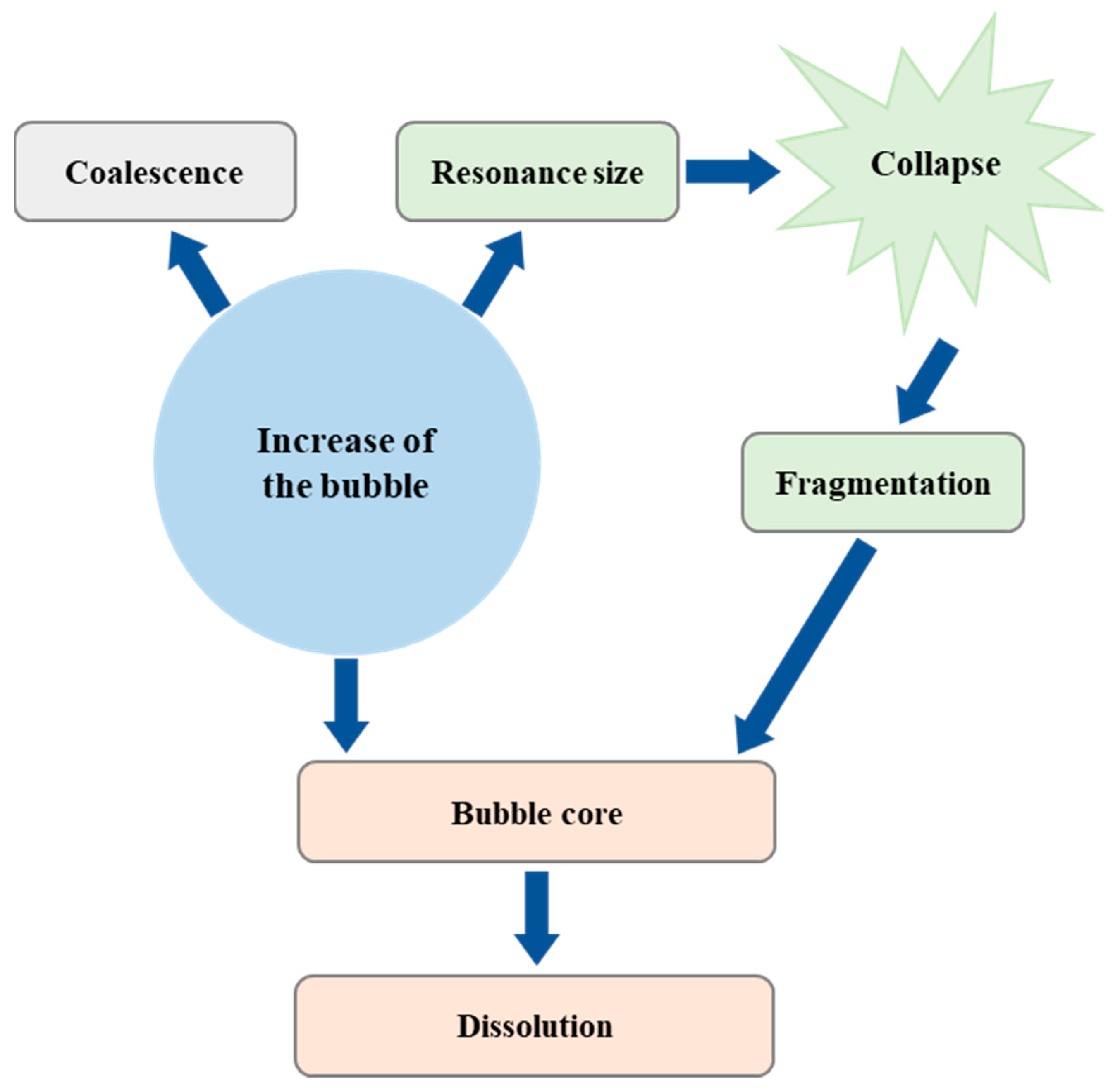
3.6. Ultrasonication
3.7. Microwave Radiation
3.8. Electrochemical Oxidation
- In direct oxidation reactions, the pollutants are adsorbed and oxidized on the anode surface, producing lower kinetic rates and a high anode electrocatalytic activity dependence with a low potential [114].
- In indirect oxidation, the intervention of precursors or oxidizing species in the pollutant oxidation occurs. These precursors are electrogenerated on the anode surface and only oxidize the pollutants in the solution media [114].
4. Bioenergy
4.1. Bioenergy Sources
4.2. Pre-Treatment of Bioenergy Substrate
5. Application of AOPs in Bioenergy Substrate Pre-Treatment
| Substrates | Pre-Treatment Conditions | Product Target | Effect of Pre-Treatment | Effect on Output | Reference |
|---|---|---|---|---|---|
| Olive mill wastewater | Fenton: 1 to 5 g/L concentrations of H2O2 and FeSO4.7H2O at 20–35 °C for 20 min | Biogas, methane | 88.8% of the total phenolic content and a 35.4% TOC removal | 20.1 L CH4/L pre-treated olive mill wastewater | [136] |
| Olive mill waste | Microwave: 10 °C/min, 5 min | Biogas, methane | 26.2% CODsoluble and 30.3% biodegradability | Methane yields increased from 201.9 mL CH4, STP/g VSRAW to 244.5 mL CH4, STP/g VSP | [92] |
| Olive mill wastewater and olive mill solid waste | Fenton: [H2O2]/[Fe2+] = 1000:1, [Fe2+] = 1.5 mM, 120 min and pH 3 | Biogas, methane | >43.11% delignification, 32.31% hemicellulose and 82% CODsoluble degradation | Methane content increased by 24% and improved the methane yield by 15% | [44] |
| Wet olive mill wastes | 0.05 g H2O2/g COD | Biogas, methane | 28–37% COD and a 72% total phenol reduction | Methane production improve from 0.08 L CH4/g of CODremoved to 0.328 L CH4/g of CODremoved | [43] |
| Olive mill wastewater | Ultrasound: 20 kHz, 10 min | Biogas, methane | >23% CODsoluble degradation | Methane production increased by 20% | [90] |
| Olive mill wastewater | Electro-Fenton: [H2O2] = 1 g/L, 7.5 A/dm2 | Biogas, methane | 68% COD and 65.8% total polyphenols removal | Methane production improved from 0.3 L CH4/g CODintroduced to 0.35 L CH4/g CODintroduced | [135] |
| Winery waste | Microwave: 10 °C/min, 5 min | Biogas, methane | 49.7% CODsoluble and 37.1% biodegradability | Methane yields increased from 154.0 mL CH4, STP/g VSRAW to 256.8 mL CH4, STP/g VSP | [92] |
| Distillery effluent | Wet air oxidation: 6–12 bar, 150–200 °C, 15–120 min | Biogas, methane | 16–60% of COD reduction and >0.2–0.88 biodegradability | Methane increased from 4.8% to 57% | [91] |
| Vinasse | Ozonation: [O3] = 34 g/m3, [H2O2] = 4 × 10−3 mol/L, [TiO2] = 2 g/L UV light: mercury vapor lamp emitting at 200–280 nm | Biogas, methane | COD and TOC decreased from 109 g/L and 36 g/L to 74 g/L and 34 g/L, respectively | Methane yields increased from 0.87 mL CH4 /mg TOC to 1.09 mL CH4/mg TOC | [88] |
| Wood dust, sheep and cow dung and wastewater | Fenton: 0.07 g Fe2+/g H2O2, 50 g H2O2/Kg-DS Ozonation: 15.8 mg O3/g-TSS Ozonation combined with H2O2/Fe2+: 50 g H2O2 or 3.5 g Fe2+/Kg-DS | Biogas, methane | >15.2–29.5% TS and 33.6–37.5% COD removal | Cumulative methane production increased by 23–30% | [5] |
| Cattle manure | Ultrasounds: 520 kJ/kg TS | Biogas, methane | 97% COD and 92% VS removal | Methane yields increased from 0.29 m3 CH4/kg VS to 0.46 m3 CH4/kg VS and biogas production increased from 60.1% to 62.2% | [107] |
| Cattle manure and coffee pulp | Heterogeneous photocatalysis: 100 g of 10% Cu/TiO2 catalyst | Biogas | TS, VS, lignin, cellulose and hemicellulose decreased by 8.8%, 81.4%, 2.8%, 14.5% and 15.9%, respectively | Biogas production was 2.78 times higher | [89] |
| Palm oil, pulp and paper mill wastewater | Ultrasounds: 20 kHz, amplitudes 70% and 90%, 45 min | Biohydrogen | A70: 34.2% CODsoluble and 36.9% CODtotal removal A90: 34.8% CODsoluble and 36.7% CODtotal removal | A70: Biohydrogen production improved by 86.8% A90: Biohydrogen production improved by 88.4% | [20] |
| Palm oil, pulp and paper mill wastewater | Ultrasounds: 20 kHz, amplitude 20%, 10 min | Biohydrogen | 51.1% CODsoluble and 52.2% CODtotal removal | Biohydrogen production improved by 44.6% | [137] |
| Pulp and paper mill waste | Electrochemical: 15 V, 45 min | Biogas, methane | - | Methane yields increased from 0.264 mL CH4/g VS to 0.301 mL CH4/g VS | [93] |
| Juice industry waste | Microwave: 10 °C/min, 5 min | Biogas, methane | 71.4% CODsoluble and 82.0% biodegradability | Methane yields increased from 166.0 mL CH4, STP/g VSRAW to 451.5 mL CH4, STP/g VSP | [94] |
| Juice–puree industry waste and municipal sewage sludge | Electro-oxidation: 15 mA/cm2 (15 V for 120 min), pH 4 Fenton: [H2O2]/[Fe2+]:1000, [FeSO4.7H2O] = 1.5 mM, [H2O2] = 30% (v/v), pH 4. | Biogas, methane | CODsoluble removal increased from 9600 mg/L to 13,990 mg/L and 20,290 mg/L for electro-oxidation and Fenton, respectively | Biogas production, methane content and methane yield increased by 12%, 60% and 28% for electro-oxidation and 20%, 62% and 39% for Fenton. | [98] |
| Mixture of fruits, vegetables, potatoes and paper wastes | Ultrasounds: 20 kHz, during three sonication times (9, 18 and 27 min) | Biogas, methane | 58% TS removal | Biogas yield increased from 249 mL/g VSin to 396 mL/g VSin >80% methane yield | [142] |
| Fruit and vegetable waste and activated sludge | Microwave combined with H2O2: 660 W, 3 min and 80 °C, [H2O2] = 1% w/w H2O2/TS | Biogas, methane | >7.2% COD removal and 33% CODsoluble; <6% VS and 5.2% TS | Methane yield increased from 127 mL/g VS to 276 mL/g VS | [138] |
5.1. Combination of AOPs with Traditional Methods in Bioenergy Substrate Pre-Treatment
5.2. Scale-Up of AOPs in Bioenergy
5.3. Other Applications of AOPs in Bioenergy
6. Challenges, Limitations and Opportunities
7. Conclusions Remarks
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- United Nations. World Population Prospects 2022. Available online: https://population.un.org/wpp/ (accessed on 22 February 2023).
- Khalil, M.; Berawi, M.A.; Heryanto, R.; Rizalie, A. Waste to energy technology: The potential of sustainable biogas production from animal waste in Indonesia. Renew. Sust. Energ. Rev. 2019, 105, 323–331. [Google Scholar] [CrossRef]
- Abdeshahian, P.; Lim, J.S.; Ho, W.S.; Hashim, H.; Lee, C.T. Potential of biogas production from farm animal waste in Malaysia. Renew. Sust. Energ. Rev. 2016, 60, 714–723. [Google Scholar] [CrossRef]
- Eroğlu, E.; Eroğlu, İ.; Gündüz, U.; Yücel, M. Treatment of olive mill wastewater by different physicochemical methods and utilization of their liquid effluents for biological hydrogen production. Biomass Bioenerg. 2009, 33, 701–705. [Google Scholar] [CrossRef]
- Almomani, F.; Bhosale, R.R.; Khraisheh, M.A.M.; Shawaqfah, M. Enhancement of biogas production from agricultural wastes via pre-treatment with advanced oxidation processes. Fuel 2019, 253, 964–974. [Google Scholar] [CrossRef]
- Fountoulakis, M.S.; Drakopoulou, S.; Terzakis, S.; Georgaki, E.; Manios, T. Potential for methane production from typical Mediterranean agro-industrial by-products. Biomass Bioenerg. 2008, 32, 155–161. [Google Scholar] [CrossRef]
- Food and Agriculture Organization of the United States. Commodities by Country 2021. Available online: https://www.fao.org/faostat/en/#rankings/commodities_by_country (accessed on 26 September 2022).
- Martins, R.C.; Quinta-Ferreira, R.M. A Review on the applications of ozonation for the treatment of real agro-industrial wastewaters. Ozone Sci. Eng. 2014, 36, 3–35. [Google Scholar] [CrossRef]
- Idehai, I.M.; Akujieze, C.N. Estimation of landfill gas and its renewable energy potential in Lagos, Nigeria. Int. J. Energy Environ. Eng. 2015, 6, 329–343. [Google Scholar] [CrossRef] [Green Version]
- Pan, Y.; Zhang, Y.; Hou, M.; Xue, J.; Qin, R.; Zhou, M.; Zhang, Y. Properties of polyphenols and polyphenol-containing wastewaters and their treatment by Fenton/Fenton-like reactions. Sep. Purif. Technol. 2023, 317, 123905. [Google Scholar] [CrossRef]
- Farré, M.J.; Franch, M.I.; Ayllón, J.A.; Peral, J.; Domènech, X. Biodegradability of treated aqueous solutions of biorecalcitrant pesticides by means of photocatalytic ozonation. Desalination 2007, 211, 22–33. [Google Scholar] [CrossRef]
- Chamarro, E.; Marco, A.; Esplugas, S. Use of Fenton reagent to improve organic chemical biodegradability. Water Res. 2001, 35, 1047–1051. [Google Scholar] [CrossRef]
- Glaze, W.H.; Kang, J.W.; Chapin, D.H. The chemistry of water-treatment processes involving ozone, hydrogen-peroxide and ultraviolet-radiation. Ozone Sci. Eng. 1987, 9, 335–352. [Google Scholar] [CrossRef]
- Hodges, B.C.; Cates, E.L.; Kim, J.H. Challenges and prospects of advanced oxidation water treatment processes using catalytic nanomaterials. Nat. Nanotechnol. 2018, 13, 642–650. [Google Scholar] [CrossRef]
- Amor, C.; Fernandes, J.R.; Lucas, M.S.; Peres, J.A. Hydroxyl and sulfate radical advanced oxidation processes: Application to an agro-industrial wastewater. Environ. Technol. Innov. 2021, 21, 101183. [Google Scholar] [CrossRef]
- Zhou, Z.; Liu, X.; Sun, K.; Lin, C.; Ma, J.; He, M.; Ouyang, W. Persulfate-based advanced oxidation processes (AOPs) for organic-contaminated soil remediation: A review. Chem. Eng. J. 2019, 372, 836–851. [Google Scholar] [CrossRef]
- Jallouli, N.; Pastrana-Martínez, L.M.; Ribeiro, A.R.; Moreira, N.F.F.; Faria, J.L.; Hentati, O.; Silva, A.M.T.; Ksibi, M. Heterogeneous photocatalytic degradation of ibuprofen in ultrapure water, municipal and pharmaceutical industry wastewaters using a TiO2/UV-LED system. Chem. Eng. J. 2018, 334, 976–984. [Google Scholar] [CrossRef]
- Wei, A.; Feng, H.; Jia, X.M.; Tang, H.; Liao, Y.Y.; Li, B.R. Ozone therapy ameliorates inflammation and endometrial injury in rats with pelvic inflammatory disease. Biomed. Pharmacother. 2018, 107, 1418–1425. [Google Scholar] [CrossRef]
- Michelin, C.; Hoffmann, N. Photocatalysis applied to organic synthesis—A green chemistry approach. Curr. Opin. Green Sustain. Chem. 2018, 10, 40–45. [Google Scholar] [CrossRef]
- Budiman, P.M.; Wu, T.Y. Ultrasonication pre-treatment of combined effluents from palm oil, pulp and paper mills for improving photofermentative biohydrogen production. Energy Convers. Manag. 2016, 119, 142–150. [Google Scholar] [CrossRef]
- Bhoite, G.M.; Vaidya, P.D. Fenton oxidation and adsorption pretreatment for superior biogas recovery from biomethanated spent wash. Chem. Eng. Commun. 2020, 207, 1347–1357. [Google Scholar] [CrossRef]
- Alvarado-Morales, M.; Tsapekos, P.; Awais, M.; Gulfraz, M.; Angelidaki, I. TiO2/UV based photocatalytic pretreatment of wheat straw for biogas production. Anaerobe 2017, 46, 155–161. [Google Scholar] [CrossRef] [Green Version]
- Morone, A.; Sharma, G.; Sharma, A.; Chakrabarti, T.; Pandey, R.A. Evaluation, applicability and optimization of advanced oxidation process for pretreatment of rice straw and its effect on cellulose digestibility. Renew. Energy 2018, 120, 88–97. [Google Scholar] [CrossRef]
- Malani, R.S.; Shinde, V.; Ayachit, S.; Goyal, A.; Moholkar, V.S. Ultrasound-assisted biodiesel production using heterogeneous base catalyst and mixed non-edible oils. Ultrason. Sonochem. 2019, 52, 232–243. [Google Scholar] [CrossRef] [PubMed]
- da Silva Brito, G.F.; Oliveira, R.; Grisolia, C.K.; Guirra, L.S.; Weber, I.T.; de Almeida, F.V. Evaluation of advanced oxidative processes in biodiesel wastewater treatment. J. Photochem. Photobiol. A Chem. 2019, 375, 85–90. [Google Scholar] [CrossRef]
- Martinez-Burgos, W.J.; Sydney, E.B.; Medeiros, A.B.P.; Magalhães, A.I.; de Carvalho, J.C.; Karp, S.G.; Vandenberghe, L.P.d.S.; Letti, L.A.J.; Soccol, V.T.; Pereira, G.V.d.M.; et al. Agro-industrial wastewater in a circular economy: Characteristics, impacts and applications for bioenergy and biochemicals. Bioresour. Technol. 2021, 341, 125795. [Google Scholar] [CrossRef]
- Rosete, A.R.M. Property, access, exclusion: Agribusiness venture agreements in the Philippines. J. Rural Stud. 2020, 79, 65–73. [Google Scholar] [CrossRef]
- Amaral, C.; Lucas, M.S.; Sampaio, A.; Peres, J.A.; Dias, A.A.; Peixoto, F.; Anjos, M.R.; Pais, C. Biodegradation of olive mill wastewaters by a wild isolate of Candida oleophila. Int. Biodeterior. Biodegrad. 2012, 68, 45–50. [Google Scholar] [CrossRef]
- Azman, N.F.; Abdeshahian, P.; Al-Shorgani, N.K.N.; Hamid, A.A.; Kalil, M.S. Production of hydrogen energy from dilute acid-hydrolyzed palm oil mill effluent in dark fermentation using an empirical model. Int. J. Hydrogen Energy 2016, 41, 16373–16384. [Google Scholar] [CrossRef]
- Fuess, L.T.; Garcia, M.L. Implications of stillage land disposal: A critical review on the impacts of fertigation. J. Environ. Manag. 2014, 145, 210–229. [Google Scholar] [CrossRef] [PubMed]
- International Olive Council. Available online: https://www.internationaloliveoil.org (accessed on 22 February 2023).
- Espadas-Aldana, G.; Vialle, C.; Belaud, J.P.; Vaca-Garcia, C.; Sablayrolles, C. Analysis and trends for life cycle assessment of olive oil production. Sustain. Prod. Consum. 2019, 19, 216–230. [Google Scholar] [CrossRef] [Green Version]
- Salomone, R.; Cappelletti, G.M.; Malandrino, O.; Mistretta, M.; Neri, E.; Nicoletti, G.M.; Notarnicola, B.; Pattara, C.; Russo, C.; Saija, G. Life cycle assessment in the olive oil sector. In Life Cycle Assessment in the Agri-Food Sector; Notarnicola, B., Salomone, R., Petti, L., Renzulli, P., Roma, R., Cerutti, A., Eds.; Springer: Cham, Switzerland, 2015; pp. 57–121. [Google Scholar] [CrossRef]
- Markou, G.; Georgakakis, D.; Plagou, K.; Salakou, G.; Christopoulou, N. Balanced waste management of 2-and 3-phase olive oil mills in relation to the seed oil extraction plant. Terr. Aquat. Environ. Toxicol. 2010, 4, 109–112. [Google Scholar]
- Martinez-Garcia, G.; Bachmann, R.T.; Williams, C.J.; Burgoyne, A.; Edyvean, R.G.J. Olive oil waste as a biosorbent for heavy metals. Int. Biodeterior. Biodegrad. 2006, 58, 231–238. [Google Scholar] [CrossRef]
- Kestioğlu, K.; Yonar, T.; Azbar, N. Feasibility of physico-chemical treatment and Advanced Oxidation Processes (AOPs) as a means of pretreatment of olive mill effluent (OME). Process Biochem. 2005, 40, 2409–2416. [Google Scholar] [CrossRef]
- Messineo, A.; Maniscalco, M.P.; Volpe, R. Biomethane recovery from olive mill residues through anaerobic digestion: A review of the state of the art technology. Sci. Total Environ. 2020, 703, 135508. [Google Scholar] [CrossRef] [PubMed]
- Morillo, J.A.; Antizar-Ladislao, B.; Monteoliva-Sanchez, M.; Ramos-Cormenzana, A.; Russell, N.J. Bioremediation and biovalorisation of olive-mill wastes. Appl. Microbiol. Biotechnol. 2009, 82, 25–39. [Google Scholar] [CrossRef]
- Amor, C.; Marchao, L.; Lucas, M.S.; Peres, J.A. Application of Advanced Oxidation Processes for the treatment of recalcitrant agro-industrial wastewater: A review. Water 2019, 11, 205. [Google Scholar] [CrossRef] [Green Version]
- Khdair, A.; Abu-Rumman, G. Sustainable environmental management and valorization options for olive mill byproducts in the Middle East and North Africa (MENA) Region. Processes 2020, 8, 671. [Google Scholar] [CrossRef]
- Rahmanian, N.; Jafari, S.M.; Galanakis, C.M. Recovery and removal of phenolic compounds from olive mill wastewater. J. Am. Oil Chem. Soc. 2014, 91, 1–18. [Google Scholar] [CrossRef]
- Kougias, P.G.; Kotsopoulos, T.A.; Martzopoulos, G.G. Effect of feedstock composition and organic loading rate during the mesophilic co-digestion of olive mill wastewater and swine manure. Renew. Energy 2014, 69, 202–207. [Google Scholar] [CrossRef]
- Siciliano, A.; Stillitano, M.A.; De Rosa, S. Biogas production from wet olive mill wastes pretreated with hydrogen peroxide in alkaline conditions. Renew. Energy 2016, 85, 903–916. [Google Scholar] [CrossRef]
- Maamir, W.; Ouahabi, Y.; Poncin, S.; Li, H.Z.; Bensadok, K. Effect of Fenton pretreatment on anaerobic digestion of olive mill wastewater and olive mill solid waste in mesophilic conditions. Int. J. Green Energy 2017, 14, 555–560. [Google Scholar] [CrossRef]
- El-Abbassi, A.; Kiai, H.; Hafidi, A. Phenolic profile and antioxidant activities of olive mill wastewater. Food Chem. 2012, 132, 406–412. [Google Scholar] [CrossRef]
- Domingues, E.; Fernandes, E.; Gomes, J.; Castro-Silva, S.; Martins, R.C. Olive oil extraction industry wastewater treatment by coagulation and Fenton’s process. J. Water Process Eng. 2021, 39, 101818. [Google Scholar] [CrossRef]
- Internationational Organisation of Vine and Wine. State of the World Vine and Wine Sector 2021. Available online: https://www.oiv.int/sites/default/files/documents/eng-state-of-the-world-vine-and-wine-sector-april-2022-v6_0.pdf (accessed on 14 October 2022).
- Musee, N.; Lorenzen, L.; Aldrich, C. Decision support for waste minimization in wine-making processes. Environ. Prog. 2006, 25, 56–63. [Google Scholar] [CrossRef]
- Domínguez, C.M.; Quintanilla, A.; Casas, J.A.; Rodriguez, J.J. Treatment of real winery wastewater by wet oxidation at mild temperature. Sep. Purif. Technol. 2014, 129, 121–128. [Google Scholar] [CrossRef]
- Lucas, M.S.; Peres, J.A.; Puma, G.L. Treatment of winery wastewater by ozone-based advanced oxidation processes (O3, O3/UV and O3/UV/H2O2) in a pilot-scale bubble column reactor and process economics. Sep. Purif. Technol. 2010, 72, 235–241. [Google Scholar] [CrossRef]
- Lucas, M.S.; Mouta, M.; Pirra, A.; Peres, J.A. Winery wastewater treatment by a combined process: Long term aerated storage and Fenton’s reagent. Water Sci. Technol. 2009, 60, 1089–1095. [Google Scholar] [CrossRef] [PubMed]
- Oller, I.; Malato, S.; Sanchez-Perez, J.A. Combination of Advanced Oxidation Processes and biological treatments for wastewater decontamination: A review. Sci. Total Environ. 2011, 409, 4141–4166. [Google Scholar] [CrossRef]
- Jorge, N.; Teixeira, A.R.; Matos, C.C.; Lucas, M.S.; Peres, J.A. Combination of Coagulation-Flocculation-Decantation and Ozonation Processes for winery wastewater treatment. Int. J. Environ. Res. Public Health 2021, 18, 8882. [Google Scholar] [CrossRef]
- Jorge, N.; Teixeira, A.; Guimarães, V.; Lucas, M.; Peres, J. Treatment of winery wastewater with a combination of adsorption and thermocatalytic processes. Processes 2022, 10, 75. [Google Scholar] [CrossRef]
- Rodríguez-Chueca, J.; Amor, C.; Silva, T.; Dionysiou, D.D.; Puma, G.L.; Lucas, M.S.; Peres, J.A. Treatment of winery wastewater by sulphate radicals: HSO5−/transition metal/UV-A LEDs. Chem. Eng. J. 2017, 310, 473–483. [Google Scholar] [CrossRef] [Green Version]
- Braz, R.; Pirra, A.; Lucas, M.S.; Peres, J.A. Combination of long term aerated storage and chemical coagulation/flocculation to winery wastewater treatment. Desalination 2010, 263, 226–232. [Google Scholar] [CrossRef]
- Jorge, N.; Teixeira, A.R.; Lucas, M.S.; Peres, J.A. Agro-Industrial Wastewater Treatment with Acacia dealbata Coagulation/Flocculation and Photo-Fenton-Based Processes. Recycling 2022, 7, 54. [Google Scholar] [CrossRef]
- Brucculeri, M.; Bolzonella, D.; Battistoni, P.; Cecchi, F. Treatment of mixed municipal and winery wastewaters in a conventional activated sludge process: A case study. Water Sci. Technol. 2005, 51, 89–98. [Google Scholar] [CrossRef] [Green Version]
- Food and Agriculture Organization of the United Nations. Available online: https://www.fao.org/home/en/ (accessed on 12 December 2022).
- Bodirsky, B.L.; Rolinski, S.; Biewald, A.; Weindl, I.; Popp, A.; Lotze-Campen, H. Global food demand scenarios for the 21st century. PLoS ONE 2015, 10, e0139201. [Google Scholar] [CrossRef] [PubMed]
- Ozdemir, S.; Yetilmezsoy, K. A mini literature review on sustainable management of poultry abattoir wastes. J. Mater. Cycles Waste Manag. 2019, 22, 11–21. [Google Scholar] [CrossRef]
- Weindl, I.; Bodirsky, B.L.; Rolinski, S.; Biewald, A.; Lotze-Campen, H.; Muller, C.; Dietrich, J.P.; Humpenoder, F.; Stevanovic, M.; Schaphoff, S.; et al. Livestock production and the water challenge of future food supply: Implications of agricultural management and dietary choices. Glob. Environ. Chang. 2017, 47, 121–132. [Google Scholar] [CrossRef]
- Mekonnen, M.M.; Hoekstra, A.Y. A Global assessment of the water footprint of farm animal products. Ecosystems 2012, 15, 401–415. [Google Scholar] [CrossRef] [Green Version]
- Sakadevan, K.; Nguyen, M.L. Livestock production and its impact on nutrient pollution and greenhouse gas emissions. In Advances in Agronomy; Sparks, D.L., Ed.; Academic Press: Newark, DE, USA, 2017; Volume 141, Chapter 4; pp. 147–184. [Google Scholar] [CrossRef]
- Cheng, D.L.; Ngo, H.H.; Guo, W.S.; Liu, Y.W.; Zhou, J.L.; Chang, S.W.; Nguyen, D.D.; Bui, X.T.; Zhang, X.B. Bioprocessing for elimination antibiotics and hormones from swine wastewater. Sci. Total Environ. 2018, 621, 1664–1682. [Google Scholar] [CrossRef]
- Garcia, B.B.; Lourinho, G.; Romano, P.; Brito, P.S.D. Photocatalytic degradation of swine wastewater on aqueous TiO2 suspensions: Optimization and modeling via Box-Behnken design. Heliyon 2020, 6, e03293. [Google Scholar] [CrossRef]
- Lv, J.; Liu, Y.; Feng, J.; Liu, Q.; Nan, F.; Xie, S. Nutrients removal from undiluted cattle farm wastewater by the two-stage process of microalgae-based wastewater treatment. Bioresour. Technol. 2018, 264, 311–318. [Google Scholar] [CrossRef]
- Van Boeckel, T.P.; Brower, C.; Gilbert, M.; Grenfell, B.T.; Levin, S.A.; Robinson, T.P.; Teillant, A.; Laxminarayan, R. Global trends in antimicrobial use in food animals. Proc. Natl. Acad. Sci. USA 2015, 112, 5649–5654. [Google Scholar] [CrossRef]
- Han, X.Y.; Qu, Y.P.; Li, D.; Dong, Y.; Chen, D.H.; Yu, Y.L.; Ren, N.Q.; Feng, Y.J. Combined microbial electrolysis cell-iron-air battery system for hydrogen production and swine wastewater treatment. Process Biochem. 2021, 101, 104–110. [Google Scholar] [CrossRef]
- Ungureanu, N.; Vlăduţ, V.; Istrate, I.-A.; Zăbavă, B.Ș.; Tociu, C.; Ferdeș, M.; Dincă, M. Advanced electrochemical treatment of the wastewater from cattle farm. In Proceedings of the 47th International Symposium, Actual Tasks on Agricultural Engineering, Opatija, Croatia, 5–7 March 2019; pp. 147–157. [Google Scholar]
- Zheng, T.; Li, P.; Ma, X.; Sun, X.; Wu, C.; Wang, Q.; Gao, M. Pilot-scale experiments on multilevel contact oxidation treatment of poultry farm wastewater using saran lock carriers under different operation model. J. Environ. Sci. 2019, 77, 336–345. [Google Scholar] [CrossRef] [PubMed]
- Coskun, T.; Debik, E.; Kabuk, H.A.; Demir, N.M.; Basturk, I.; Yildirim, B.; Temizel, D.; Kucuk, S. Treatment of poultry slaughterhouse wastewater using a membrane process, water reuse, and economic analysis. Desalin. Water Treat. 2016, 57, 4944–4951. [Google Scholar] [CrossRef]
- Ziara, R.M.M.; Li, S.B.; Subbiah, J.; Dvorak, B.I. Characterization of wastewater in two US cattle slaughterhouses. Water Environ. Res. 2018, 90, 851–863. [Google Scholar] [CrossRef] [PubMed]
- Rajab, A.R.; Salim, M.R.; Sohaili, J.; Anuar, A.N.; Salmiati; Lakkaboyana, S.K. Performance of integrated anaerobic/aerobic sequencing batch reactor treating poultry slaughterhouse wastewater. Chem. Eng. J. 2017, 313, 967–974. [Google Scholar] [CrossRef]
- João, J.J.; Silva, C.S.d.; Vieira, J.L.; Silveira, M.F.d. Treatment of swine wastewater using the Fenton process with ultrasound and recycled iron. Rev. Ambient. Água 2020, 15, 1. [Google Scholar] [CrossRef]
- Hossain, K.; Ismail, N. Bioremediation and detoxification of pulp and paper mill effluent: A review. Res. J. Environ. Toxicol. 2015, 9, 113–134. [Google Scholar] [CrossRef] [Green Version]
- Food and Agriculture Organization of the United Stations. FAO Yearbook of Forest Products 2019. Available online: https://www.fao.org/forestry/statistics/80570/en/ (accessed on 12 December 2022).
- Han, N.; Zhang, J.H.; Hoang, M.; Gray, S.; Xie, Z.L. A review of process and wastewater reuse in the recycled paper industry. Environ. Tech. Innov. 2021, 24, 101860. [Google Scholar] [CrossRef]
- Bajpai, P. Environmentally Friendly Production of Pulp and Paper; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2011; pp. 5–107. ISBN 978-0-470-52810-5. [Google Scholar]
- Ordóñez, R.; Hermosilla, D.; Merayo, N.; Gascó, A.; Negro, C.; Blanco, Á. Application of multi-barrier membrane filtration technologies to reclaim municipal wastewater for industrial use. Sep. Purif. Rev. 2014, 43, 263–310. [Google Scholar] [CrossRef] [Green Version]
- Bajpai, P. Basic overview of pulp and paper manufacturing process. In Green Chemistry and Sustainability in Pulp and Paper Industry; Springer: Cham, Switzerland, 2015; pp. 11–39. ISBN 978-3-319-18744-0. [Google Scholar]
- Pokhrel, D.; Viraraghavan, T. Treatment of pulp and paper mill wastewater: A review. Sci. Total Environ. 2004, 333, 37–58. [Google Scholar] [CrossRef]
- Ali, M.; Sreekrishnan, T.R. Aquatic toxicity from pulp and paper mill effluents: A review. Adv. Environ. Res. 2001, 5, 175–196. [Google Scholar] [CrossRef]
- Fernandes, L.; Lucas, M.S.; Maldonado, M.I.; Oller, I.; Sampaio, A. Treatment of pulp mill wastewater by Cryptococcus podzolicus and solar photo-Fenton: A case study. Chem. Eng. J. 2014, 245, 158–165. [Google Scholar] [CrossRef]
- Merayo, N.; Hermosilla, D.; Blanco, L.; Cortijo, L.; Blanco, A. Assessing the application of advanced oxidation processes, and their combination with biological treatment, to effluents from pulp and paper industry. J. Hazard. Mater. 2013, 262, 420–427. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baycan Parilti, N.; Akten, D. Optimization of TiO2/Fe(III)/solar UV conditions for the removal of organic contaminants in pulp mill effluents. Desalination 2011, 265, 37–42. [Google Scholar] [CrossRef]
- Lucas, M.S.; Peres, J.A.; Amor, C.; Prieto-Rodriguez, L.; Maldonado, M.I.; Malato, S. Tertiary treatment of pulp mill wastewater by solar photo-Fenton. J. Hazard. Mater. 2012, 225–226, 173–181. [Google Scholar] [CrossRef]
- Martín, M.A.; Raposo, F.; Borja, R.; Martín, A. Kinetic study of the anaerobic digestion of vinasse pretreated with ozone, ozone plus ultraviolet light, and ozone plus ultraviolet light in the presence of titanium dioxide. Process Biochem. 2002, 37, 699–706. [Google Scholar] [CrossRef]
- Corro, G.; Pal, U.; Cebada, S. Enhanced biogas production from coffee pulp through deligninocellulosic photocatalytic pretreatment. Energy Sci. Eng. 2014, 2, 177–187. [Google Scholar] [CrossRef]
- Oz, N.A.; Uzun, A.C. Ultrasound pretreatment for enhanced biogas production from olive mill wastewater. Ultrason. Sonochem. 2015, 22, 565–572. [Google Scholar] [CrossRef] [PubMed]
- Malik, S.N.; Saratchandra, T.; Tembhekar, P.D.; Padoley, K.V.; Mudliar, S.L.; Mudliar, S.N. Wet air oxidation induced enhanced biodegradability of distillery effluent. J. Environ. Manag. 2014, 136, 132–138. [Google Scholar] [CrossRef]
- Pellera, F.M.; Gidarakos, E. Microwave pretreatment of lignocellulosic agroindustrial waste for methane production. J. Environ. Chem. Eng. 2017, 5, 352–365. [Google Scholar] [CrossRef]
- Veluchamy, C.; Raju, V.W.; Kalamdhad, A.S. Electrohydrolysis pretreatment for enhanced methane production from lignocellulose waste pulp and paper mill sludge and its kinetics. Bioresour. Technol. 2018, 252, 52–58. [Google Scholar] [CrossRef]
- Staehelin, J.; Hoigne, J. Decomposition of ozone in water—Rate of initiation by hydroxide ions and hydrogen-peroxide. Environ. Sci. Technol. 1982, 16, 676–681. [Google Scholar] [CrossRef]
- Titchou, F.E.; Zazou, H.; Afanga, H.; El Gaayda, J.; Akbour, R.A.; Nidheesh, P.V.; Hamdani, M. Removal of organic pollutants from wastewater by advanced oxidation processes and its combination with membrane processes. Chem. Eng. Process. 2021, 169, 108631. [Google Scholar] [CrossRef]
- Wang, J.; Chen, H. Catalytic ozonation for water and wastewater treatment: Recent advances and perspective. Sci. Total Environ. 2020, 704, 135249. [Google Scholar] [CrossRef] [PubMed]
- Lipczynska-Kochany, E. Degradation of aqueous nitrophenols and nitrobenzene by means of the Fenton reaction. Chemosphere 1991, 22, 529–536. [Google Scholar] [CrossRef]
- Akbay, H.E.G.; Dizge, N.; Kumbur, H. Evaluation of electro-oxidation and Fenton pretreatments on industrial fruit waste and municipal sewage sludge to enhance biogas production by anaerobic co-digestion. J. Environ. Manag. 2022, 319, 115711. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, L.C.; Salmerón, I.; Peres, J.A.; Tavares, P.B.; Lucas, M.S.; Malato, S. Advanced Oxidation Processes as sustainable technologies for the reduction of elderberry agro-industrial water impact. Water Resour. Ind. 2020, 24, 100137. [Google Scholar] [CrossRef]
- Lucas, M.S.; Peres, J.A. Treatment of olive mill wastewater by a combined process: Fenton’s reagent and chemical coagulation. J. Environ. Sci. Health A 2009, 44, 198–205. [Google Scholar] [CrossRef]
- Sharma, A.; Ahmad, J.; Flora, S.J.S. Application of advanced oxidation processes and toxicity assessment of transformation products. Environ. Res. 2018, 167, 223–233. [Google Scholar] [CrossRef]
- Hoffmann, M.R.; Martin, S.T.; Choi, W.Y.; Bahnemann, D.W. Environmental applications of semiconductor photocatalysis. Chem. Rev. 1995, 95, 69–96. [Google Scholar] [CrossRef]
- Chen, J.; Rulkens, W.H.; Bruning, H. Photochemical elimination of phenols and cod in industrial wastewaters. Water Sci. Technol. 1997, 35, 231–238. [Google Scholar] [CrossRef]
- Bjerre, A.B.; Olesen, A.B.; Fernqvist, T.; Ploger, A.; Schmidt, A.S. Pretreatment of wheat straw using combined wet oxidation and alkaline hydrolysis resulting in convertible cellulose and hemicellulose. Biotechnol. Bioeng. 1996, 49, 568–577. [Google Scholar] [CrossRef]
- Chung, J.; Lee, M.; Ahn, J.; Bae, W.; Lee, Y.W.; Shim, H. Effects of operational conditions on sludge degradation and organic acids formation in low-critical wet air oxidation. J. Hazard. Mater. 2009, 162, 10–16. [Google Scholar] [CrossRef]
- Kidak, R.; Ince, N.H. Ultrasonic destruction of phenol and substituted phenols: A review of current research. Ultrason. Sonochem. 2006, 13, 195–199. [Google Scholar] [CrossRef]
- Ormaechea, P.; Castrillón, L.; Suárez-Peña, B.; Megido, L.; Fernández-Nava, Y.; Negral, L.; Marañón, E.; Rodríguez-Iglesias, J. Enhancement of biogas production from cattle manure pretreated and/or co-digested at pilot-plant scale. Characterization by SEM. Renew. Energ. 2018, 126, 897–904. [Google Scholar] [CrossRef]
- Xuan, X.; Wang, M.; You, W.; Manickam, S.; Tao, Y.; Yoon, J.Y.; Sun, X. Hydrodynamic cavitation-assisted preparation of porous carbon from garlic peels for supercapacitors. Ultrason. Sonochem. 2023, 94, 106333. [Google Scholar] [CrossRef]
- Sun, X.; You, W.; Xuan, X.; Ji, L.; Xu, X.; Wang, G.; Zhao, S.; Boczkaj, G.; Yoon, J.Y.; Chen, S. Effect of the cavitation generation unit structure on the performance of an advanced hydrodynamic cavitation reactor for process intensifications. Chem. Eng. J. 2021, 412, 128600. [Google Scholar] [CrossRef]
- Tang, B.; Yu, L.; Huang, S.; Luo, J.; Zhuo, Y. Energy efficiency of pre-treating excess sewage sludge with microwave irradiation. Bioresour. Technol. 2010, 101, 5092–5097. [Google Scholar] [CrossRef]
- Bozkurt, Y.C.; Apul, O.G. Critical review for microwave pretreatment of waste-activated sludge prior to anaerobic digestion. Curr. Opin. Environ. Sci. Health 2020, 14, 1–9. [Google Scholar] [CrossRef]
- Remya, N.; Lin, J.G. Current status of microwave application in wastewater treatment: A review. Chem. Eng. J. 2011, 166, 797–813. [Google Scholar] [CrossRef]
- Sires, I.; Brillas, E.; Oturan, M.A.; Rodrigo, M.A.; Panizza, M. Electrochemical advanced oxidation processes: Today and tomorrow: A review. Environ. Sci. Pollut. Res. Int. 2014, 21, 8336–8367. [Google Scholar] [CrossRef]
- Panizza, M.; Cerisola, G. Direct and mediated anodic oxidation of organic pollutants. Chem. Rev. 2009, 109, 6541–6569. [Google Scholar] [CrossRef]
- Moreira, F.C.; Boaventura, R.A.R.; Brillas, E.; Vilar, V.J.P. Electrochemical advanced oxidation processes: A review on their application to synthetic and real wastewaters. Appl. Catal. B-Environ. 2017, 202, 217–261. [Google Scholar] [CrossRef]
- Yadav, M.; Singh, G.; Karwadiya, J.; Chengatt, A.P.; Sebastian, D.P.; Jadeja, R. Waste to Bioenergy: A Sustainable Approach. In Bioenergy Crops, 1st ed.; Puthur, J.T., Dhankher, O.P., Eds.; CRC Press: Boca Raton, FL, USA, 2022; pp. 164–186. ISBN 978-1-0030-4352-2. [Google Scholar]
- Bioenergy Europe. Biogas: Flexible, Renewable, Enabler of Decarbonization. Available online: https://bioenergyeurope.org/article/104-biogas-flexible-renewable-enabler-of-decarbonization.html (accessed on 2 January 2023).
- Beig, B.; Riaz, M.; Naqvi, S.R.; Hassan, M.; Zheng, Z.F.; Karimi, K.; Pugazhendhi, A.; Atabani, A.E.; Chi, N.T.L. Current challenges and innovative developments in pretreatment of lignocellulosic residues for biofuel production: A review. Fuel 2021, 287, 119670. [Google Scholar] [CrossRef]
- Sanchez, O.J.; Cardona, C.A. Trends in biotechnological production of fuel ethanol from different feedstocks. Bioresour. Technol. 2008, 99, 5270–5295. [Google Scholar] [CrossRef]
- Perrone, O.M.; Colombari, F.M.; Rossi, J.S.; Moretti, M.M.; Bordignon, S.E.; Nunes Cda, C.; Gomes, E.; Boscolo, M.; Da-Silva, R. Ozonolysis combined with ultrasound as a pretreatment of sugarcane bagasse: Effect on the enzymatic saccharification and the physical and chemical characteristics of the substrate. Bioresour. Technol. 2016, 218, 69–76. [Google Scholar] [CrossRef]
- Sun, Y.; Cheng, J. Hydrolysis of lignocellulosic materials for ethanol production: A review. Bioresour. Technol. 2002, 83, 1–11. [Google Scholar] [CrossRef]
- Fukuda, H.; Kondo, A.; Noda, H. Biodiesel fuel production by transesterification of oils. J. Biosci. Bioeng. 2001, 92, 405–416. [Google Scholar] [CrossRef]
- Ma, F.; Hanna, M.A. Biodiesel production: A review. Bioresour. Technol. 1999, 70, 1–15. [Google Scholar] [CrossRef]
- Rawat, I.; Kumar, R.R.; Mutanda, T.; Bux, F. Dual role of microalgae: Phycoremediation of domestic wastewater and biomass production for sustainable biofuels production. Appl. Energy 2011, 88, 3411–3424. [Google Scholar] [CrossRef]
- Li, Y.; Horsman, M.; Wu, N.; Lan, C.Q.; Dubois-Calero, N. Biofuels from microalgae. Biotechnol. Prog. 2008, 24, 815–820. [Google Scholar] [CrossRef]
- Rezania, S.; Oryani, B.; Cho, J.; Talaiekhozani, A.; Sabbagh, F.; Hashemi, B.; Rupani, P.F.; Mohammadi, A.A. Different pretreatment technologies of lignocellulosic biomass for bioethanol production: An overview. Energy 2020, 199, 117457. [Google Scholar] [CrossRef]
- Zheng, Y.; Zhao, J.; Xu, F.Q.; Li, Y.B. Pretreatment of lignocellulosic biomass for enhanced biogas production. Prog. Energy Combust. Sci. 2014, 42, 35–53. [Google Scholar] [CrossRef]
- Seidl, P.R.; Goulart, A.K. Pretreatment processes for lignocellulosic biomass conversion to biofuels and bioproducts. Curr. Opin. Green Sustain. Chem. 2016, 2, 48–53. [Google Scholar] [CrossRef]
- Fernandes, T.V.; Klaasse, B.G.J.; Zeeman, G.; Sanders, J.P.M.; Lier, J.B.V. Effects of thermo-chemical pretreatment on anaerobic biodegradability and hydrolysis of lignocellulosic biomass. Bioresour. Technol. 2009, 100, 257–259. [Google Scholar] [CrossRef]
- Bali, G.; Meng, X.; Deneff, J.I.; Sun, Q.; Ragauskas, A.J. The effect of alkaline pretreatment methods on cellulose structure and accessibility. Chem. Sus. Chem. 2015, 8, 275–279. [Google Scholar] [CrossRef]
- Brandt, A.; Grasvik, J.; Hallett, J.P.; Welton, T. Deconstruction of lignocellulosic biomass with ionic liquids. Green Chem. 2013, 15, 550–583. [Google Scholar] [CrossRef] [Green Version]
- Muthangya, M.; Manoni Mshandete, A.; Kajumulo Kivaisi, A. Two-stage fungal pre-treatment for improved biogas production from sisal leaf decortication residues. Int. J. Mol. Sci. 2009, 10, 4805–4815. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Q.; He, J.; Tian, M.; Mao, Z.; Tang, L.; Zhang, J.; Zhang, H. Enhancement of methane production from cassava residues by biological pretreatment using a constructed microbial consortium. Bioresour. Technol. 2011, 102, 8899–8906. [Google Scholar] [CrossRef]
- Eriksson, T.; Karlsson, J.; Tjerneld, F. A model explaining declining rate in hydrolysis of lignocellulose substrates with cellobiohydrolase I (cel7A) and endoglucanase I (cel7B) of Trichoderma reesei. Appl. Biochem. Biotechnol. 2002, 101, 41–60. [Google Scholar] [CrossRef]
- Khoufi, S.; Aloui, F.; Sayadi, S. Treatment of olive oil mill wastewater by combined process electro-Fenton reaction and anaerobic digestion. Water Res. 2006, 40, 2007–2016. [Google Scholar] [CrossRef]
- Bampalioutas, K.; Vlysidis, A.; Lyberatos, G.; Vlyssides, A. Detoxification and methane production kinetics from three-phase olive mill wastewater using Fenton’s reagent followed by anaerobic digestion. J. Chem. Technol. Biot. 2019, 94, 265–275. [Google Scholar] [CrossRef] [Green Version]
- Budiman, P.M.; Wu, T.Y.; Ramanan, R.N.; Jahim, J.M. Improving photofermentative biohydrogen production by using intermittent ultrasonication and combined industrial effluents from palm oil, pulp and paper mills. Energy Convers. Manag. 2017, 132, 110–118. [Google Scholar] [CrossRef]
- Ambrose, H.W.; Philip, L.; Suraishkumar, G.K.; Karthikaichamy, A.; Sen, T.K. Anaerobic co-digestion of activated sludge and fruit and vegetable waste: Evaluation of mixing ratio and impact of hybrid (microwave and hydrogen peroxide) sludge pre- treatment on two-stage digester stability and biogas yield. J. Water Process Eng. 2020, 37, 101498. [Google Scholar] [CrossRef]
- Haghighi Mood, S.; Hossein Golfeshan, A.; Tabatabaei, M.; Salehi Jouzani, G.; Najafi, G.H.; Gholami, M.; Ardjmand, M. Lignocellulosic biomass to bioethanol, a comprehensive review with a focus on pretreatment. Renew. Sust. Energy Rev. 2013, 27, 77–93. [Google Scholar] [CrossRef]
- Zeng, Z.; Zou, H.; Li, X.; Sun, B.; Chen, J.; Shao, L. Ozonation of phenol with O3/Fe(II) in acidic environment in a rotating packed bed. Ind. Eng. Chem. Res. 2012, 51, 10509–10516. [Google Scholar] [CrossRef]
- Saldanha, L.A.S.; Santos, N.T.d.G.; Tomaz, E. Photocatalytic ethylbenzene degradation associated with ozone (TiO2/UV/O3) under different percentages of catalytic coating area: Evaluation of process parameters. Sep. Purif. Technol. 2021, 263, 118344. [Google Scholar] [CrossRef]
- Zeynali, R.; Khojastehpour, M.; Ebrahimi-Nik, M. Effect of ultrasonic pre-treatment on biogas yield and specific energy in anaerobic digestion of fruit and vegetable wholesale market wastes. Sustain. Environ. Res. 2017, 27, 259–264. [Google Scholar] [CrossRef]
- Behera, S.; Arora, R.; Nandhagopal, N.; Kumar, S. Importance of chemical pretreatment for bioconversion of lignocellulosic biomass. Renew. Sust. Energ. Rev. 2014, 36, 91–106. [Google Scholar] [CrossRef]
- M’Arimi, M.M.; Mecha, C.A.; Kiprop, A.K.; Ramkat, R. Recent trends in applications of advanced oxidation processes (AOPs) in bioenergy production: Review. Renew. Sust. Energ. Rev. 2020, 121, 109669. [Google Scholar] [CrossRef]
- Paraskeva, P.; Diamadopoulos, E. Technologies for olive mill wastewater (OMW) treatment: A review. J. Chem. Technol. Biotechnol. 2006, 81, 1475–1485. [Google Scholar] [CrossRef]
- Benitez, F.J.; Torregrosa, J.B.; Acero, J.L. Improvement of the anaerobic biodegradation of olive mill wastewaters by prior ozonation pretreatment. Bioprocess Eng. 1997, 17, 169–175. [Google Scholar] [CrossRef]
- Lucas, M.S.; Reis, N.M.; Li Puma, G. Intensification of ozonation processes in a novel, compact, multi-orifice oscillatory baffled column. Chem. Eng. J. 2016, 296, 335–339. [Google Scholar] [CrossRef] [Green Version]
- Feki, E.; Khoufi, S.; Loukil, S.; Sayadi, S. Improvement of anaerobic digestion of waste-activated sludge by using H2O2 oxidation, electrolysis, electro-oxidation and thermo-alkaline pretreatments. Environ. Sci. Pollut. Res. 2015, 22, 14717–14726. [Google Scholar] [CrossRef] [PubMed]
- Dahadha, S.; Amin, Z.; Lakeh, A.A.B.; Elbeshbishy, E. Evaluation of different pretreatment processes of lignocellulosic biomass for enhanced biomethane production. Energy Fuels 2017, 31, 10335–10347. [Google Scholar] [CrossRef]
- Silverstein, R.A.; Chen, Y.; Sharma-Shivappa, R.R.; Boyette, M.D.; Osborne, J. A comparison of chemical pretreatment methods for improving saccharification of cotton stalks. Bioresour. Technol. 2007, 98, 3000–3011. [Google Scholar] [CrossRef] [PubMed]
- Mantzavinos, D.; Psillakis, E. Enhancement of biodegradability of industrial wastewaters by chemical oxidation pre-treatment. J. Chem. Technol. Biotechnol. 2004, 79, 431–454. [Google Scholar] [CrossRef]
- Pekin, G.; Haskok, S.; Sargin, S.; Gezgin, Y.; Eltem, R.; Ikizoglu, E.; Azbar, N.; Sukan, F.V. Anaerobic digestion of Aegean olive mill effluents with and without pretreatment. J. Chem. Technol. Biot. 2010, 85, 976–982. [Google Scholar] [CrossRef]
- Ruggeri, B.; Battista, F.; Bernardi, M.; Fino, D.; Mancini, G. The selection of pretreatment options for anaerobic digestion (AD): A case study in olive oil waste production. Chem. Eng. J. 2015, 259, 630–639. [Google Scholar] [CrossRef]
- Park, N.D.; Helle, S.S.; Thring, R.W. Combined alkaline and ultrasound pre-treatment of thickened pulp mill waste activated sludge for improved anaerobic digestion. Biomass. Bioenerg. 2012, 46, 750–756. [Google Scholar] [CrossRef]
- Tyagi, V.K.; Lo, S.-L.; Rajpal, A. Chemically coupled microwave and ultrasonic pre-hydrolysis of pulp and paper mill waste-activated sludge: Effect on sludge solubilisation and anaerobic digestion. Environ. Sci. Pollut. Res. 2014, 21, 6205–6217. [Google Scholar] [CrossRef]
- Amaral-Silva, N.; Martins, R.C.; Nunes, P.; Castro-Silva, S.; Quinta-Ferreira, R.M. From a lab test to industrial application: Scale-up of Fenton process for real olive mill wastewater treatment. J. Chem. Technol. Biotechnol. 2017, 92, 1336–1344. [Google Scholar] [CrossRef]
- Souza, B.S.; Moreira, F.C.; Dezotti, M.W.; Vilar, V.J.; Boaventura, R.A. Application of biological oxidation and solar driven advanced oxidation processes to remediation of winery wastewater. Catal. Today 2013, 209, 201–208. [Google Scholar] [CrossRef]
- Hu, X.; Zhou, J.T.; Liu, B.J. Effect of algal species and light intensity on the performance of an air-lift-type microbial carbon capture cell with an algae-assisted cathode. RSC Adv. 2016, 6, 25094–25100. [Google Scholar] [CrossRef]
- Selvarajan, R.; Felfoldi, T.; Tauber, T.; Sanniyasi, E.; Sibanda, T.; Tekere, M. Screening and evaluation of some green algal strains (Chlorophyceae) isolated from freshwater and soda lakes for biofuel production. Energies 2015, 8, 7502–7521. [Google Scholar] [CrossRef] [Green Version]
- Beigbeder, J.B.; Boboescu, I.Z.; Lavoie, J.M. Treatment and valorization of municipal solid waste gasification effluent through a combined advanced oxidation—Microalgal phytoremediation approach. J. Clean. Prod. 2021, 299, 126926. [Google Scholar] [CrossRef]
- Quan, X.J.; Hu, R.; Chang, H.X.; Tang, X.Y.; Huang, X.X.; Cheng, C.; Zhong, N.B.; Yang, L. Enhancing microalgae growth and landfill leachate treatment through ozonization. J. Clean. Prod. 2020, 248, 119182. [Google Scholar] [CrossRef]
- Yang, K.; Lu, J.L.; Jiang, W.L.; Jiang, C.Y.; Chen, J.Q.; Wang, Z.L.; Guo, R.X. An integrated view of the intimate coupling UV irradiation and algal treatment on antibiotic: Compatibility, efficiency and microbic impact assessment. J. Environ. Chem. Eng. 2017, 5, 4262–4268. [Google Scholar] [CrossRef]
- Marchao, L.; Fernandes, J.R.; Sampaio, A.; Peres, J.A.; Tavares, P.B.; Lucas, M.S. Microalgae and immobilized TiO2/UV-A LEDs as a sustainable alternative for winery wastewater treatment. Water Res. 2021, 203, 117464. [Google Scholar] [CrossRef]
- Cui, H.; Yu, J.; Zhu, X.; Cui, Y.; Ji, C.; Zhang, C.; Xue, J.; Jia, X.; Qin, S.; Li, R. Advanced treatment of chicken farm flushing wastewater by integrating Fenton oxidation and algal cultivation process for algal growth and nutrients removal. J. Environ. Manag. 2021, 298, 113543. [Google Scholar] [CrossRef] [PubMed]
- Komolafe, O.; Velasquez Orta, S.B.; Monje-Ramirez, I.; Noguez, I.Y.; Harvey, A.P.; Ledesma, M.O.T. Biodiesel production from indigenous microalgae grown in wastewater. Bioresour. Technol. 2014, 154, 297–304. [Google Scholar] [CrossRef] [PubMed]
- Costa, N.M.; Silva, V.M.; Damaceno, G.; Sousa, R.M.F.; Richter, E.M.; Machado, A.E.H.; Trovo, A.G. Integrating coagulation-flocculation and UV-C or H2O2/UV-C as alternatives for pre- or complete treatment of biodiesel effluents. J. Environ. Manag. 2017, 203, 229–236. [Google Scholar] [CrossRef] [PubMed]

| Parameter | Range Values | Reference |
|---|---|---|
| pH | 4.6–5.5 | [6,42,43,44,45] |
| Conductivity (mS/cm) | 6.5–43.0 | [43,45] |
| BOD5 (g O2/L) | 8.0–30.0 | [44,46] |
| COD (g O2/L) | 51.0–243.0 | [6,42,43,44,45] |
| TOC (g C/L) | 14.0–31.0 | [45] |
| Total suspended solids (g/L) | 24.7–215.0 | [6,42,43,44,45] |
| Total polyphenols (g/L) | 4.9–9.8 | [6,43,45] |
| Total nitrogen (g/L) | 0.4–2.5 | [6,42,43] |
| Total phosphorus (g/L) | 0.2–0.4 | [6,43] |
| Parameter | Range Values | Reference |
|---|---|---|
| pH | 3.6–4.6 | [53,54,55,56,57] |
| Conductivity (mS/cm) | 0.06–0.48 | [53,54,55,57] |
| BOD5 (g O2/L) | 0.55–6.5 | [53,54,57] |
| COD (g O2/L) | 0.5–38.4 | [53,54,55,56,57] |
| TOC (g C/L) | 0.14–1.96 | [53,54,55,57] |
| Total suspended solids (g/L) | 0.75–7.66 | [53,54,56,57] |
| Total polyphenols (g/L) | 0.03–1.35 | [53,54,55,56,57] |
| Total nitrogen (g/L) | 0.01–0.38 | [56,57] |
| Total phosphorus (g/L) | 0.04–0.05 | [56] |
| Animal Products | Average Annual Water Footprint (m3/Year/Animal) | Water Footprint (%) |
|---|---|---|
| Cattle | 2686 | 52 |
| Swine | 520 | 19 |
| Poultry | 59 | 18 |
| Horse | 1599 | 7 |
| Sheep | 68 | 3 |
| Goat | 32 | 1 |
| Parameter | Swine Wastewater | Cattle Wastewater | Poultry Wastewater | |||
|---|---|---|---|---|---|---|
| pH | 7.9–8.6 | [66,69] | 7.7–7.8 | [67,70] | 6.6–8.0 | [71,72] |
| Conductivity (mS/cm) | 12.0–25.4 | [66,69] | 1.80–3.92 | [70,73] | 0.8–2.8 | [72,74] |
| BOD5 (g O2/L) | 3.0–5.4 | [69,75] | 0.2–1.49 | [70,73] | 0.2–0.9 | [71,74] |
| COD (g O2/L) | 4.8–15.0 | [65,66,69,75] | 1.5–9.9 | [67,70] | 0.3–0.9 | [71,72] |
| TSS (g/L) | 0.4–0.6 | [69,75] | 1.22–4.97 | [70,73] | 0.002–0.05 | [71,72] |
| Total nitrogen (g/L) | 0.15–2.10 | [65,75] | 0.11–0.23 | [70,73] | 0.05–0.15 | [71,74] |
| Total phosphorus (g/L) | 0.02–0.25 | [65,75] | 0.04–0.08 | [67,70] | 0.008–0.05 | [71,74] |
| Parameter | Range of Values | Reference |
|---|---|---|
| pH | 5.0–8.5 | [20,84,85,86,87] |
| Conductivity (mS/cm) | 2.3–3.6 | [85,87] |
| BOD5 (g O2/L) | 0.1–0.9 | [84,85,86,87] |
| COD (g O2/L) | 0.6–2.7 | [20,84,85,86,87] |
| TOC (g C/L) | 0.2–0.4 | [86,87] |
| Total suspended solids (g/L) | 0.02–0.84 | [20,84,85,87] |
| Total polyphenols (g/L) | 0.20–0.22 | [84,87] |
| Total nitrogen (g/L) | 0.004–0.013 | [20,84,85,87] |
| Total phosphorus (g/L) | 0.0009–0.014 | [84,85,87] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gomes, A.; Borges, A.; Peres, J.A.; Lucas, M.S. Bioenergy Production from Agro-Industrial Wastewater Using Advanced Oxidation Processes as Pre-Treatment. Catalysts 2023, 13, 1186. https://doi.org/10.3390/catal13081186
Gomes A, Borges A, Peres JA, Lucas MS. Bioenergy Production from Agro-Industrial Wastewater Using Advanced Oxidation Processes as Pre-Treatment. Catalysts. 2023; 13(8):1186. https://doi.org/10.3390/catal13081186
Chicago/Turabian StyleGomes, Ana, Amadeu Borges, José A. Peres, and Marco S. Lucas. 2023. "Bioenergy Production from Agro-Industrial Wastewater Using Advanced Oxidation Processes as Pre-Treatment" Catalysts 13, no. 8: 1186. https://doi.org/10.3390/catal13081186










