Recyclable Ni-Containing Coordination Polymer as an Efficient Catalyst for the Synthesis of Oxindole and Quinoline Derivatives through the Borrowing Hydrogen Strategy
Abstract
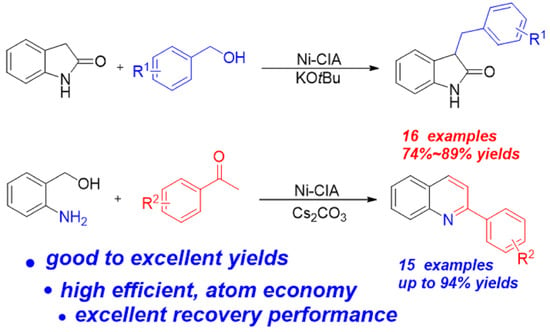
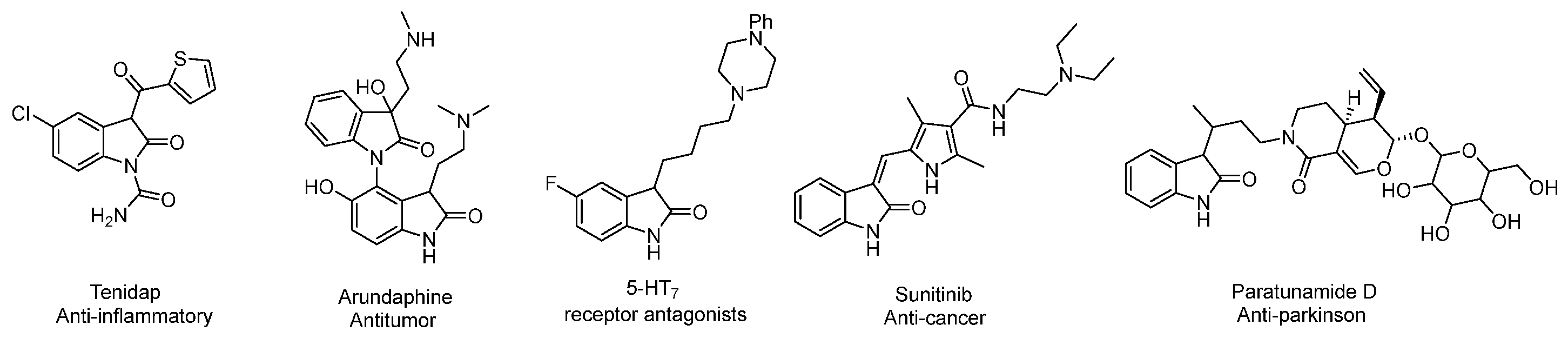
1. Introduction
2. Results and Discussion
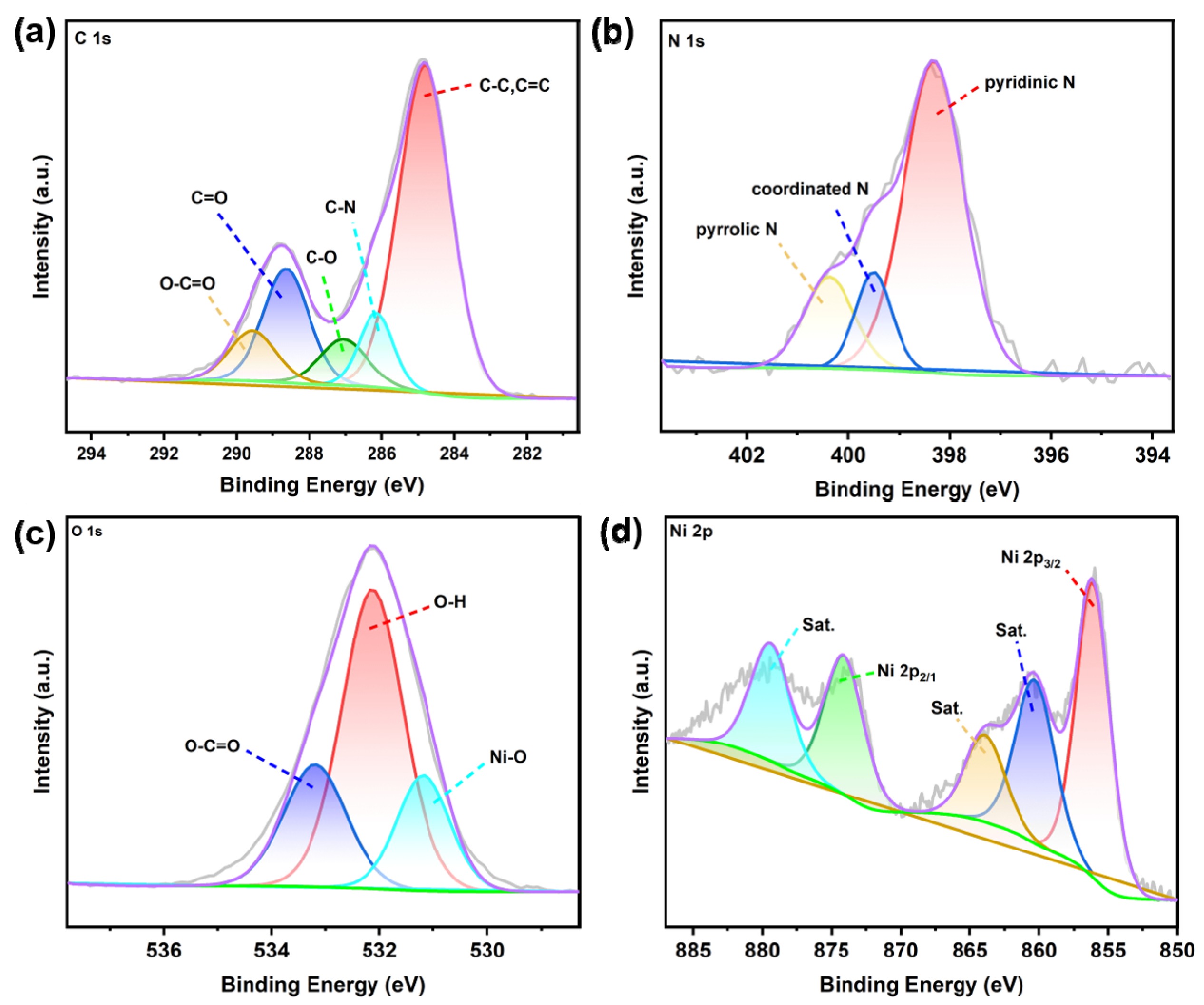
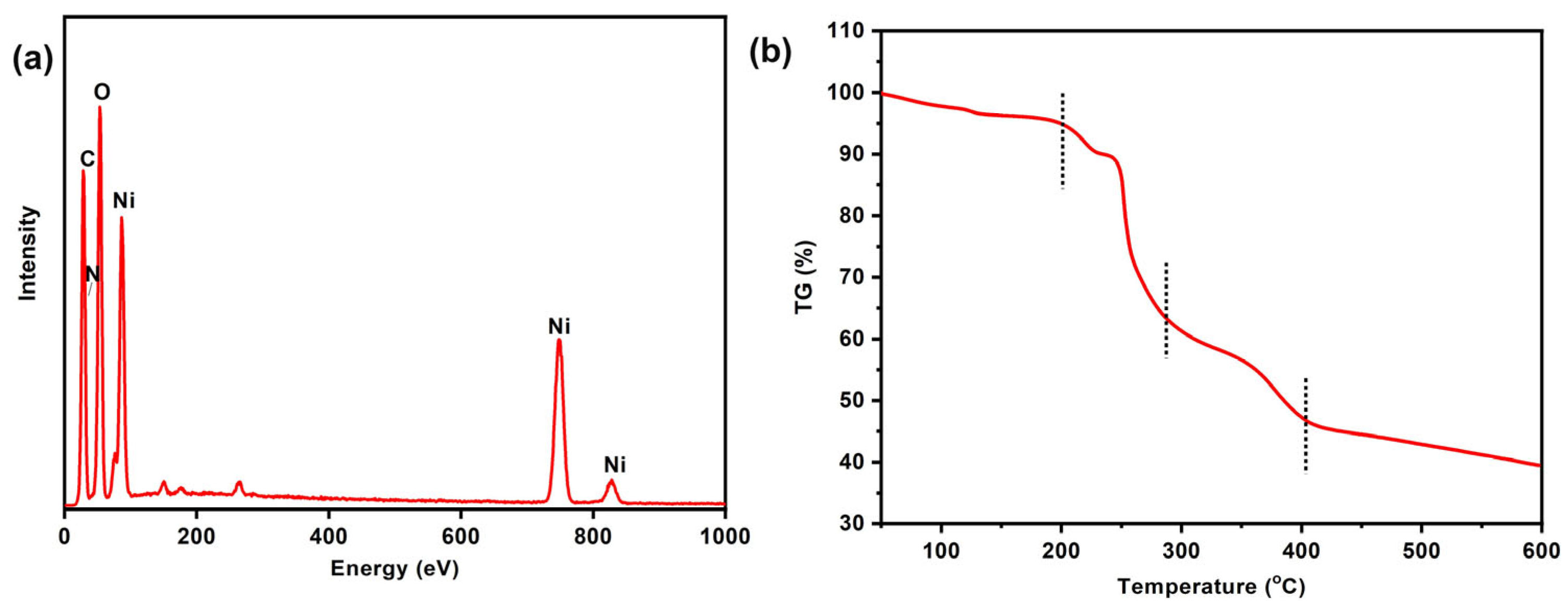
2.1. Characterization of Ni-CIA
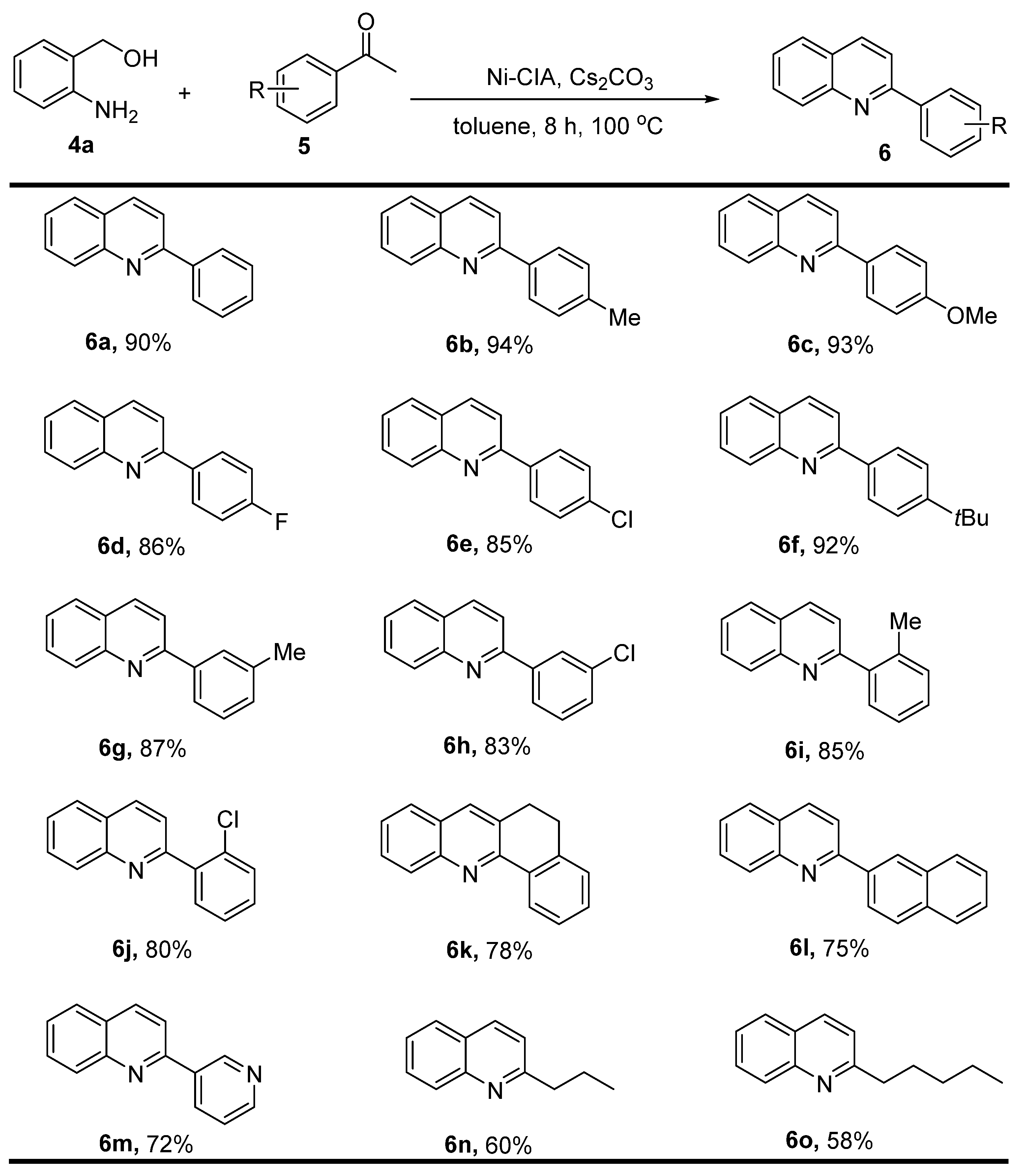
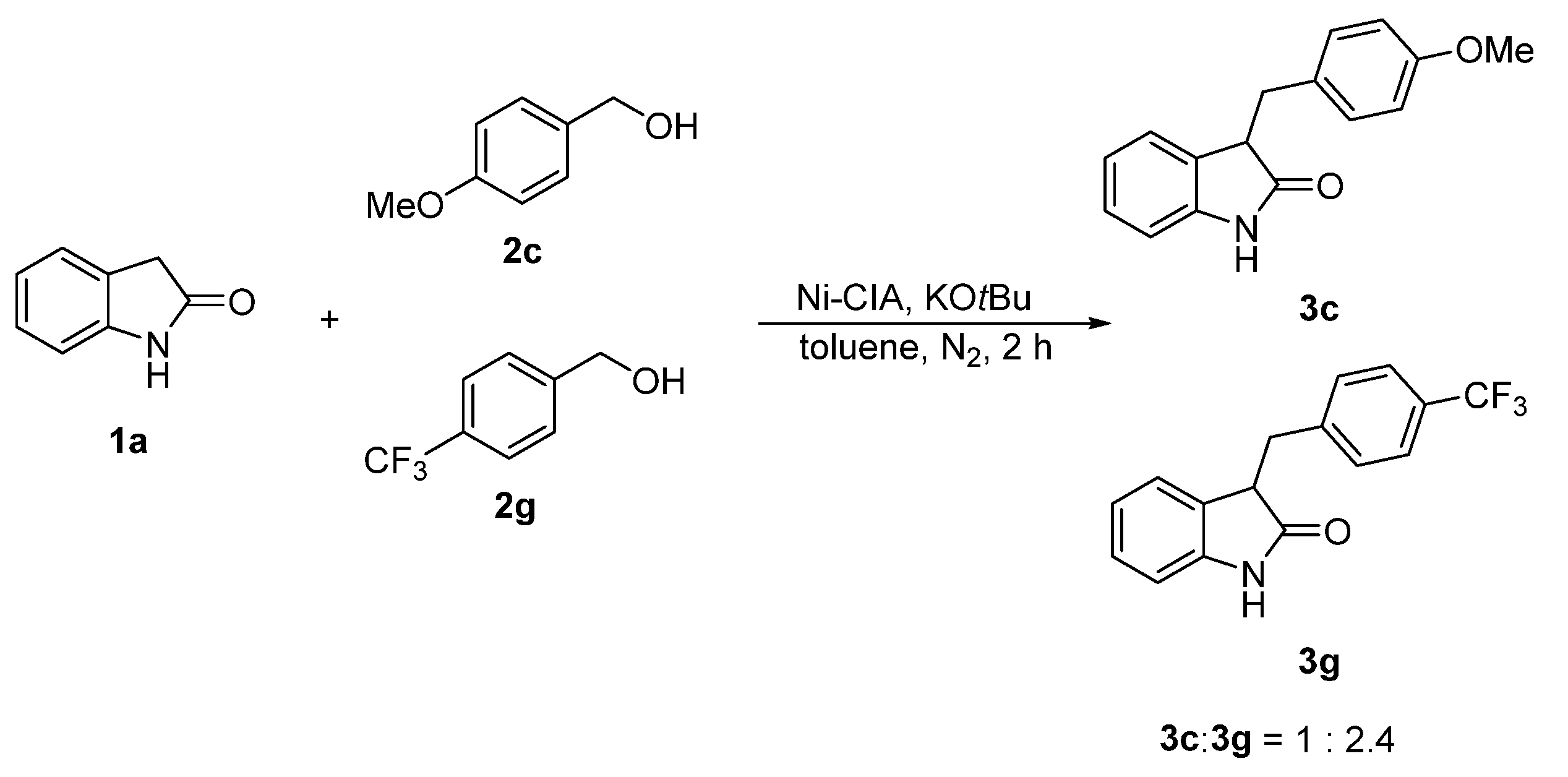
2.2. Catalytic Activity
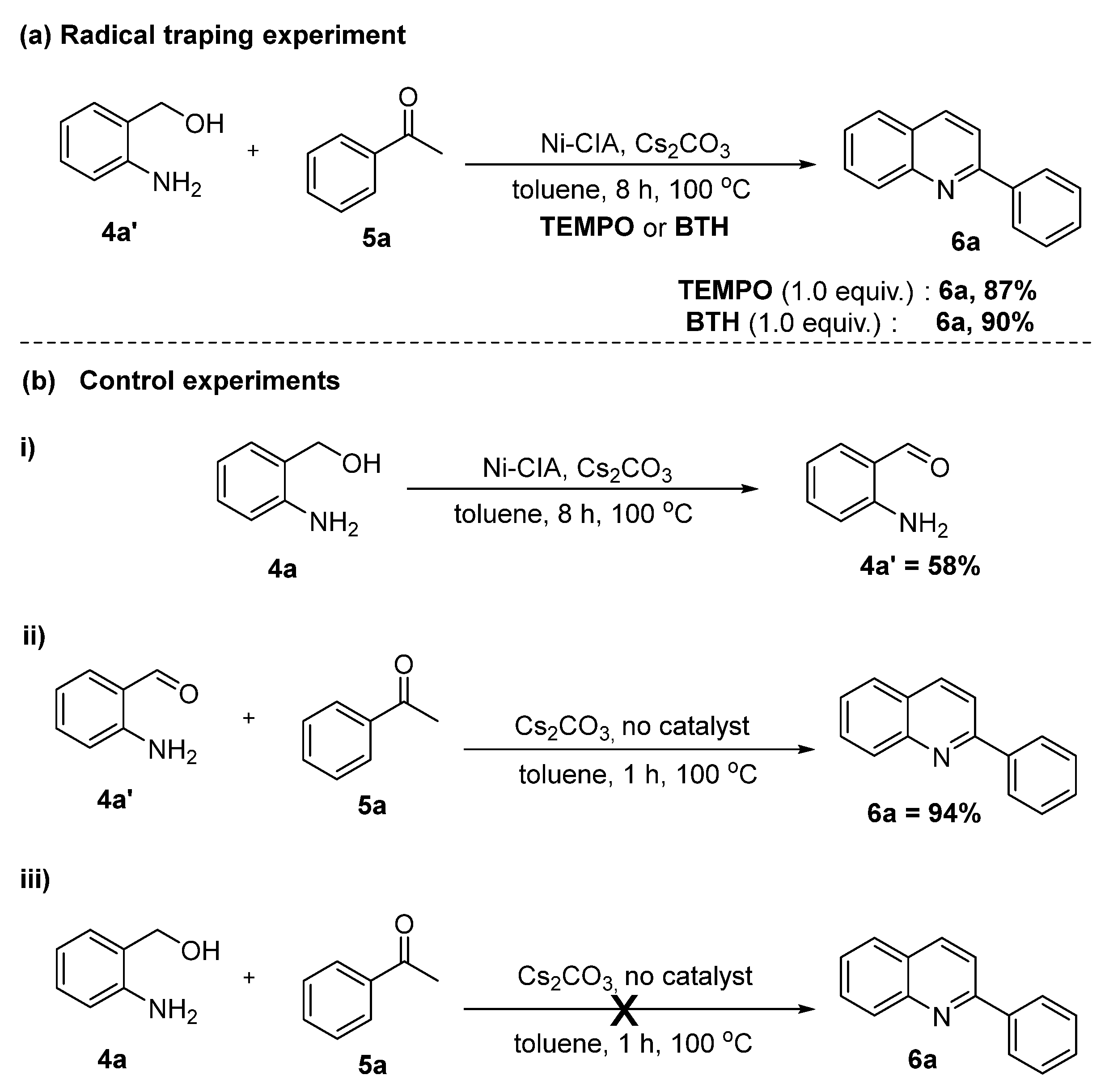
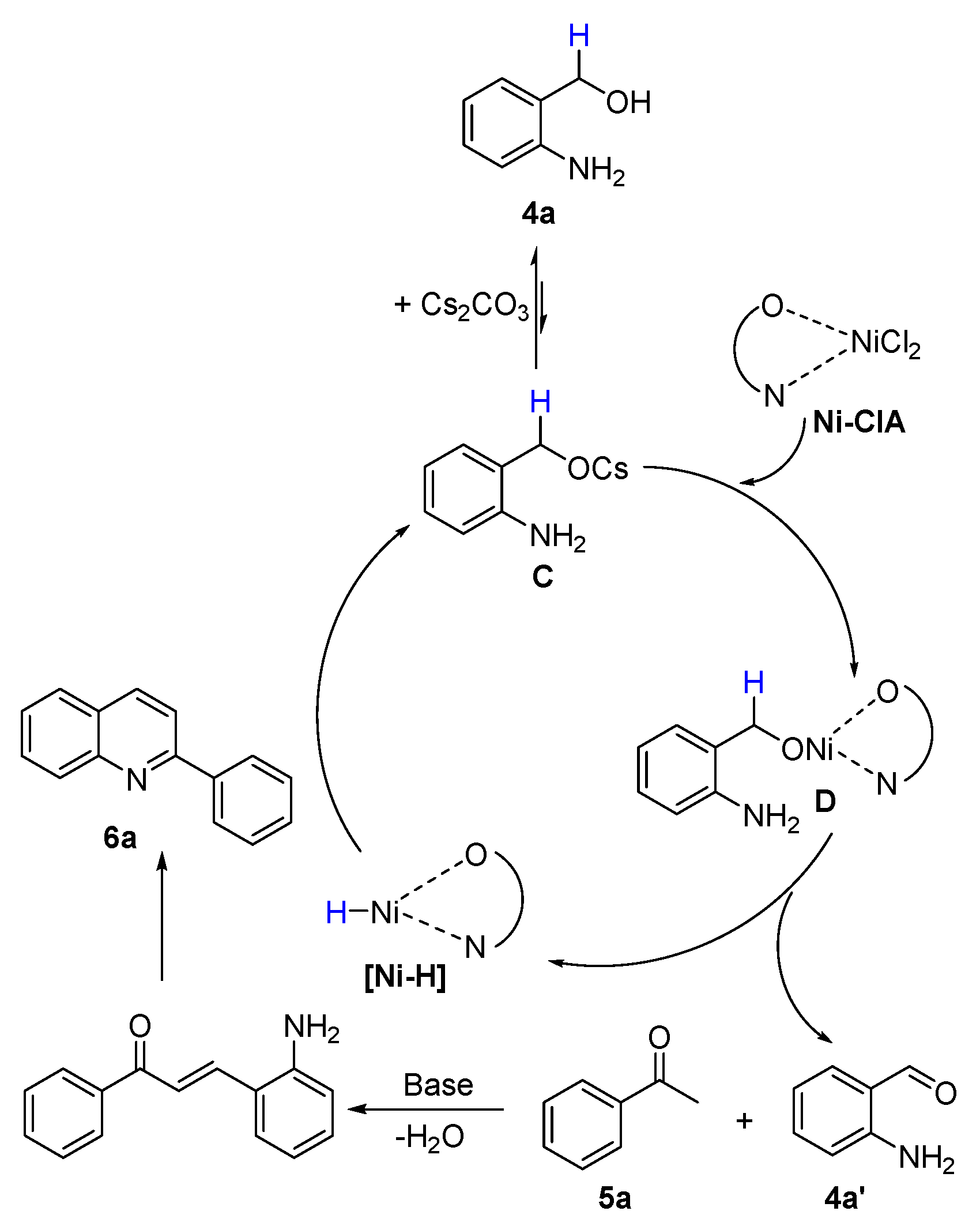
2.3. Mechanism Explorations
2.4. Reusability of the Catalyst
3. Materials and Methods
3.1. Catalyst Characterization
3.2. Chemicals
3.3. Catalyst Preparation
3.4. Alkylation of 2-Oxindole with Alcohols
3.5. Reaction of Synthesis of Quinoline Derivatives
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Jiang, T.; Kuhen, K.L.; Wolff, K.; Yin, H.; Bieza, K.; Caldwell, J.; Bursulaya, B.; Wu, T.Y.; He, Y. Design, synthesis and biological evaluations of novel oxindoles as HIV-1 non-nucleoside reverse transcriptase inhibitors. Part I. Bioorg. Med. Chem. Lett. 2006, 16, 2105–2108. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Chen, M.; Wang, L. Selective Synthesis and Utility of One Tripyrrolic Compound and Its Intermediates. Chem. Pharm. Bull. 2007, 55, 1439–1441. [Google Scholar] [CrossRef]
- Reisman, S.E.; Ready, J.M.; Weiss, M.M.; Hasuoka, A.; Hirata, M.; Tamaki, K.; Ovaska, T.V.; Smith, C.J.; Wood, J.L. Evolution of a Synthetic Strategy: Total Synthesis of (±)-Welwitindolinone A Isonitrile. J. Am. Chem. Soc. 2008, 130, 2087–2100. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, T.; Reker, D.; Schneider, P.; Schneider, G. Counting on natural products for drug design. Nat. Chem. 2016, 8, 531–541. [Google Scholar] [CrossRef]
- Huang, L.; Yang, L.; Wan, J.P.; Zhou, L.; Liu, Y.; Hao, G. Metal-free three-component assemblies of anilines, alpha-keto acids and alkyl lactates for quinoline synthesis and their anti-inflammatory activity. Org. Biomol. Chem. 2022, 20, 4385–4390. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Zeng, X.-Q.; Zuo, H.-W.; Zhang, N.; Huang, Z.-Y. Design, Synthesis and Seed Germination Inhibition Activity of Quinoline-6-sulfonamide Compounds. Chin. J. Org. Chem. 2022, 42, 2947–2953. [Google Scholar] [CrossRef]
- Moutaouakil, M.; Tighadouini, S.; Almarhoon, Z.M.; Al-Zaben, M.I.; Bacha, A.B.; Masand, V.H.; Jamaleddine, J.; Saddik, R. Quinoline Derivatives with Different Functional Groups: Evaluation of Their Catecholase Activity. Catalysts 2022, 12, 1468. [Google Scholar] [CrossRef]
- Hu, C.; Lu, L.; Wan, J.P.; Wen, C. The Pharmacological Mechanisms and Therapeutic Activities of Hydroxychloroquine in Rheumatic and Related Diseases. Curr. Med. Chem. 2017, 24, 2241–2249. [Google Scholar] [CrossRef]
- Trost, B.M.; Zhang, Y. Molybdenum-Catalyzed Asymmetric Allylation of 3-Alkyloxindoles: Application to the Formal Total Synthesis of (−)-Physostigmine. J. Am. Chem. Soc. 2006, 128, 4590–4591. [Google Scholar] [CrossRef]
- Kende, A.S.; Hodges, J.C. Regioselective C-3 Alkylations of Oxindole Dianion. Synth. Commun. 2007, 12, 1–10. [Google Scholar] [CrossRef]
- Chaudhari, M.B.; Sutar, Y.; Malpathak, S.; Hazra, A.; Gnanaprakasam, B. Transition-Metal-Free C-H Hydroxylation of Carbonyl Compounds. Org. Lett. 2017, 19, 3628–3631. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, S.; Daw, P.; David, Y.B.; Milstein, D. Manganese-Catalyzed alpha-Alkylation of Ketones, Esters, and Amides Using Alcohols. ACS Catal. 2018, 8, 10300–10305. [Google Scholar] [CrossRef]
- Duan, Y.T.; Wang, Z.X. Transition-Metal-Free α-C-Alkylation of Oxindoles with Alcohols. Asian J. Org. Chem. 2023, 12, e202300015. [Google Scholar] [CrossRef]
- Luo, X.; Tian, A.; Pei, M.; Yan, J.; Liu, X.; Wang, L. Highly Stable Univalent Copper of a Cu@Al/SBA-15 Nanocomposite Catalyzes the Synthesis of Fluorescent Aminobenzotriazoles Derivatives. Chem. Eur. J. 2022, 28, e202103361. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Xia, X.; Hu, W.; Yang, Y.; Li, J. Catalytic Synthesis of Pyrazine and Ketone Derivatives by Unsymmetrical Triazolyl-Naphthyridinyl-Pyridine Copper. Chin. J. Org. Chem. 2022, 42, 190–199. [Google Scholar] [CrossRef]
- Pena-Lopez, M.; Piehl, P.; Elangovan, S.; Neumann, H.; Beller, M. Manganese-Catalyzed Hydrogen-Autotransfer C-C Bond Formation: Alpha-Alkylation of Ketones with Primary Alcohols. Angew. Chem. Int. Ed. Engl. 2016, 55, 14967–14971. [Google Scholar] [CrossRef]
- Li, J.; Mao, A.; Yao, W.; Zhu, H.; Wang, D. Iridium supported on porous polypyridine-oxadiazole as high-activity and recyclable catalyst for the borrowing hydrogen reaction. Green Chem. 2022, 24, 2602–2612. [Google Scholar] [CrossRef]
- Wang, D.; Ge, B.; Zhang, B.; Liu, H.; Li, J. Synthesis of Supported Indazolyl-Pyridyl-Quinoline Iridium Catalyst and Its Application to N-Alkylation of 2-Aminobenzothiazoles. Chin. J. Org. Chem. 2022, 42, 619–630. [Google Scholar] [CrossRef]
- Banik, A.; Datta, P.; Mandal, S.K. C-Alkylation by Phenalenyl-Based Molecule via a Borrowing Hydrogen Pathway. Org. Lett. 2023, 25, 1305–1309. [Google Scholar] [CrossRef]
- Chen, X.; Liu, D.; Yang, C.; Shi, L.; Li, F. Hexaazatrinaphthalene-Based Covalent Triazine Framework-Supported Rhodium(III) Complex: A Recyclable Heterogeneous Catalyst for the Reductive Amination of Ketones to Primary Amines. Inorg. Chem. 2023, 62, 9360–9368. [Google Scholar] [CrossRef]
- Liu, Y.; Diao, H.; Hong, G.; Edward, J.; Zhang, T.; Yang, G.; Yang, B.M.; Zhao, Y. Iridium-Catalyzed Enantioconvergent Borrowing Hydrogen Annulation of Racemic 1,4-Diols with Amines. J. Am. Chem. Soc. 2023, 145, 5007–5016. [Google Scholar] [CrossRef]
- Ng, T.W.; Tao, R.; See, W.W.L.; Poh, S.B.; Zhao, Y. Economical Access to Diverse Enantiopure Tetrahydropyridines and Piperidines Enabled by Catalytic Borrowing Hydrogen. Angew. Chem. Int. Ed. 2023, 62, e202212528. [Google Scholar] [CrossRef] [PubMed]
- Hamid, M.H.S.A.; Slatford, P.A.; Williams, J.M.J. Borrowing Hydrogen in the Activation of Alcohols. Adv. Synth. Catal. 2007, 349, 1555–1575. [Google Scholar] [CrossRef]
- Nakayama, T.; Harada, S.; Kikkawa, S.; Hikawa, H.; Azumaya, I. Palladium-Catalyzed Dehydrogenative Synthesis of Imidazoquinolines in Water. Asian J. Org. Chem. 2022, 11, e202200510. [Google Scholar] [CrossRef]
- Putra, A.E.; Takigawa, K.; Tanaka, H.; Ito, Y.; Oe, Y.; Ohta, T. Transition-Metal-Catalyzed Regioselective Alkylation of Indoles with Alcohols. Eur. J. Org. Chem. 2013, 2013, 6344–6354. [Google Scholar] [CrossRef]
- Yang, Q.; Wang, Q.; Yu, Z. Substitution of alcohols by N-nucleophiles via transition metal-catalyzed dehydrogenation. Chem. Soc. Rev. 2015, 44, 2305–2329. [Google Scholar] [CrossRef]
- Huang, F.; Liu, Z.; Yu, Z. C-Alkylation of Ketones and Related Compounds by Alcohols: Transition-Metal-Catalyzed Dehydrogenation. Angew. Chem. Int. Ed. 2016, 55, 862–875. [Google Scholar] [CrossRef]
- Corma, A.; Navas, J.; Sabater, M.J. Advances in One-Pot Synthesis through Borrowing Hydrogen Catalysis. Chem. Rev. 2018, 118, 1410–1459. [Google Scholar] [CrossRef]
- Alig, L.; Fritz, M.; Schneider, S. First-Row Transition Metal (De)Hydrogenation Catalysis Based on Functional Pincer Ligands. Chem. Rev. 2019, 119, 2681–2751. [Google Scholar] [CrossRef]
- Irrgang, T.; Kempe, R. 3d-Metal Catalyzed N- and C-Alkylation Reactions via Borrowing Hydrogen or Hydrogen Autotransfer. Chem. Rev. 2019, 119, 2524–2549. [Google Scholar] [CrossRef]
- Reed-Berendt, B.G.; Latham, D.E.; Dambatta, M.B.; Morrill, L.C. Borrowing Hydrogen for Organic Synthesis. ACS Cent. Sci. 2021, 7, 570–585. [Google Scholar] [CrossRef]
- Volk, B.; Mezei, T.; Simig, G. Raney Nickel-Induced 3-Alkylation of Oxindole with Alcohols and Diols. Synthesis 2002, 2002, 595–597. [Google Scholar] [CrossRef]
- Grigg, R.; Whitney, S.; Sridharan, V.; Keep, A.; Derrick, A. Iridium catalysed C-3 alkylation of oxindole with alcohols under solvent free thermal or microwave conditions. Tetrahedron 2009, 65, 4375–4383. [Google Scholar] [CrossRef]
- Jensen, T.; Madsen, R. Ruthenium-Catalyzed Alkylation of Oxindole with Alcohols. J. Org. Chem. 2009, 74, 3990–3992. [Google Scholar] [CrossRef]
- Bisht, G.S.; Chaudhari, M.B.; Gupte, V.S.; Gnanaprakasam, B. Ru-NHC Catalyzed Domino Reaction of Carbonyl Compounds and Alcohols: A Short Synthesis of Donaxaridine. ACS Omega 2017, 2, 8234–8252. [Google Scholar] [CrossRef]
- Dambatta, M.B.; Polidano, K.; Northey, A.D.; Williams, J.M.J.; Morrill, L.C. Iron-Catalyzed Borrowing Hydrogen C-Alkylation of Oxindoles with Alcohols. ChemSusChem 2019, 12, 2345–2349. [Google Scholar] [CrossRef]
- Saini, P.; Dolui, P.; Nair, A.; Verma, A.; Elias, A.J. A Bench-stable 8-Aminoquinoline Derived Phosphine-free Manganese (I)-Catalyst for Environmentally Benign C(alpha)-Alkylation of Oxindoles with Secondary and Primary Alcohols. Chem. Asian J. 2023, 18, e202201148. [Google Scholar] [CrossRef]
- Wu, Q.; Pan, L.; Du, G.; Zhang, C.; Wang, D. Preparation of pyridyltriazole ruthenium complexes as effective catalysts for the selective alkylation and one-pot C–H hydroxylation of 2-oxindole with alcohols and mechanism exploration. Org. Chem. Front. 2018, 5, 2668–2675. [Google Scholar] [CrossRef]
- Liu, G.; Huang, T.; Zhang, Y.; Liang, X.; Li, Y.; Li, H. C-3 alkylation of oxindole with alcohols catalyzed by an indene-functionalized mesoporous iridium catalyst. Catal. Commun. 2011, 12, 655–659. [Google Scholar] [CrossRef]
- Chaudhari, C.; Siddiki, S.M.A.H.; Kon, K.; Tomita, A.; Tai, Y.; Shimizu, K. C-3 alkylation of oxindole with alcohols by Pt/CeO2 catalyst in additive-free conditions. Catal. Sci. Technol. 2014, 4, 1064–1069. [Google Scholar] [CrossRef]
- Putra, A.E.; Oe, Y.; Ohta, T. Pd/C-Catalyzed Alkylation of Heterocyclic Nucleophiles with Alcohols through the “Borrowing Hydrogen” Process. Eur. J. Org. Chem. 2015, 2015, 7799–7805. [Google Scholar] [CrossRef]
- Fujita, S.; Imagawa, K.; Yamaguchi, S.; Yamasaki, J.; Yamazoe, S.; Mizugaki, T.; Mitsudome, T. A nickel phosphide nanoalloy catalyst for the C-3 alkylation of oxindoles with alcohols. Sci. Rep. 2021, 11, 10673. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Yu, M.; Duan, Z.-C.; Zhu, H.; Yao, W.; Wang, D. Porous cross-linked polymer copper and iridium catalyzed the synthesis of quinoxalines and functionalized ketones under solvent-free conditions. Mater. Chem. Front. 2021, 5, 7861–7872. [Google Scholar] [CrossRef]
- Zhao, S.; Wang, Y.; Dong, J.; He, C.-T.; Yin, H.; An, P.; Zhao, K.; Zhang, X.; Gao, C.; Zhang, L.; et al. Ultrathin metal–organic framework nanosheets for electrocatalytic oxygen evolution. Nat. Energy 2016, 1, 16184. [Google Scholar] [CrossRef]
- Stoica, A.-C.; Damoc, M.; Zaltariov, M.-F.; Racles, C.; Cazacu, M. Two-dimensional coordination polymers containing permethylated motifs-promising candidates for 2D emerging materials. Structural, behavioral and functional particularities. React. Funct. Polym. 2021, 168, 105039. [Google Scholar] [CrossRef]
- Farid, S.; Mao, Q.; Ren, S.; Hao, C.; Dong, X. Promoting the Oxygen Evolution Reaction via Morphological Manipulation of a Lamellar Nanorod-Assembled Ni(II)-Pyrazolate Superstructure. ACS Appl. Mater. Interfaces 2022, 14, 47775–47787. [Google Scholar] [CrossRef]
- Duan, J.; Jin, W.; Kitagawa, S. Water-resistant porous coordination polymers for gas separation. Coord. Chem. Rev. 2017, 332, 48–74. [Google Scholar] [CrossRef]
- Griffin, S.E.; Domecus, G.P.; Flores, C.E.; Sikma, R.E.; Benz, L.; Cohen, S.M. A Coordination Polymer of Vaska’s Complex as a Heterogeneous Catalyst for the Reductive Formation of Enamines from Amides. Angew. Chem. Int. Ed. 2023, 62, e202301611. [Google Scholar] [CrossRef]
- Gui, S.; Tang, W.; Huang, Z.; Wang, X.; Gui, S.; Gao, X.; Xiao, D.; Tao, L.; Jiang, Z.; Wang, X. Ultrasmall Coordination Polymer Nanodots Fe-Quer Nanozymes for Preventing and Delaying the Development and Progression of Diabetic Retinopathy. Adv. Electron. Mater. 2023, 300261. [Google Scholar] [CrossRef]
- Hu, X.; Zhu, H.; Sang, X.; Wang, D. Design and Synthesis of Zirconium-Containing Coordination Polymer Based on Unsymmetric Indolyl Dicarboxylic Acid and Catalytic Application on Borrowing Hydrogen Reaction. Adv. Synth. Catal. 2018, 360, 4293–4300. [Google Scholar] [CrossRef]
- Sang, X.; Hu, X.; Tao, R.; Zhang, Y.; Zhu, H.; Wang, D. A Zirconium Indazole Carboxylate Coordination Polymer as an Efficient Catalyst for Dehydrogenation-Cyclization and Oxidative Coupling Reactions. ChemPlusChem 2020, 85, 123–129. [Google Scholar] [CrossRef]
- Tao, R.; Yang, Y.; Zhu, H.; Hu, X.; Wang, D. Ligand-tuned cobalt-containing coordination polymers and applications in water. Green Chem. 2020, 22, 8452–8461. [Google Scholar] [CrossRef]
- Chang, S.; Liu, H.; Shi, G.; Xia, X.-F.; Wang, D.; Duan, Z.-C. Copper–cobalt coordination polymers and catalytic applications on borrowing hydrogen reactions. New J. Chem. 2022, 46, 15929–15936. [Google Scholar] [CrossRef]
- Han, M.-R.; Kong, X.-J.; Feng, S.-S.; Lu, L.-P. Synthesis, Structure and Magnetic Studies of a Ni(II) Coordination Polymer Constructed from Asymmetrical Tetracarboxylic Acid and N-Donor Ligand. J. Clust. Sci. 2021, 33, 809–814. [Google Scholar] [CrossRef]
- Xu, J.; He, S.; Zhang, H.; Huang, J.; Lin, H.; Wang, X.; Long, J. Layered metal–organic framework/graphene nanoarchitectures for organic photosynthesis under visible light. J. Mater. Chem. A 2015, 3, 24261–24271. [Google Scholar] [CrossRef]
- Osadchii, D.Y.; Olivos-Suarez, A.I.; Bavykina, A.V.; Gascon, J. Revisiting Nitrogen Species in Covalent Triazine Frameworks. Langmuir 2017, 33, 14278–14285. [Google Scholar] [CrossRef]
- Jia, X.; Zhang, B.; Chen, C.; Fu, X.; Huang, Q. Immobilization of chitosan grafted carboxylic Zr-MOF to porous starch for sulfanilamide adsorption. Carbohydr. Polym. 2021, 253, 117305. [Google Scholar] [CrossRef]
- Wu, H.; Li, S.; Liu, Y.; Liu, M.; Shi, Y. Metal–Organic Framework-Derived Hollow Carbon Nanosphere@Ni/Reduced Graphene Oxide Composites for Supercapacitor Electrodes with Enhanced Performance. ACS Appl. Nano Mater. 2023, 6, 1582–1591. [Google Scholar] [CrossRef]
- Shang, X.F.; Morris-Natschke, S.L.; Yang, G.Z.; Liu, Y.Q.; Guo, X.; Xu, X.S.; Goto, M.; Li, J.C.; Zhang, J.Y.; Lee, K.H. Biologically active quinoline and quinazoline alkaloids part II. Med. Res. Rev. 2018, 38, 1614–1660. [Google Scholar] [CrossRef]
- Juvale, K.; Gallus, J.; Wiese, M. Investigation of quinazolines as inhibitors of breast cancer resistance protein (ABCG2). Bioorg. Med. Chem. 2013, 21, 7858–7873. [Google Scholar] [CrossRef]
- Michael, J.P. Quinoline, quinazoline and acridone alkaloids. Nat. Prod. Rep. 2002, 19, 742–760. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Song, J.; Peng, X.; Xi, S.; Liu, J.; Zhou, W.; Li, R.; Ge, R.; Liu, C.; Xu, H.; et al. Iron Single Atom Catalyzed Quinoline Synthesis. Adv. Mater. 2021, 33, e2101382. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Ai, Y.; Wang, R.; Liu, L.; Yang, J.; Li, F. Ruthenium-catalyzed acceptorless dehydrogenative coupling of o-aminobenzyl alcohols with ketones to quinolines in the presence of carbonate salt. J. Catal. 2021, 395, 340–349. [Google Scholar] [CrossRef]
- Yu, K.; Chen, Q.; Liu, W. Iron-catalysed quinoline synthesis via acceptorless dehydrogenative coupling. Org. Chem. Front. 2022, 9, 6573–6578. [Google Scholar] [CrossRef]
- Hao, Z.; Zhou, X.; Ma, Z.; Zhang, C.; Han, Z.; Lin, J.; Lu, G.L. Dehydrogenative Synthesis of Quinolines and Quinazolines via Ligand-Free Cobalt-Catalyzed Cyclization of 2-Aminoaryl Alcohols with Ketones or Nitriles. J. Org. Chem. 2022, 87, 12596–12607. [Google Scholar] [CrossRef]
- Chen, X.; Ai, Y.; Liu, D.; Liu, P.; Xu, X.; Yang, J.; Li, F. A recyclable covalent triazine framework-supported iridium(iii) terpyridine complex for the acceptorless dehydrogenative coupling of o-aminobenzyl alcohols with ketones to form quinolines. Mater. Chem. Front. 2022, 6, 1228–1235. [Google Scholar] [CrossRef]
- Yang, L.; Wan, J.-P. Ethyl lactate-involved three-component dehydrogenative reactions: Biomass feedstock in diversity-oriented quinoline synthesis. Green Chem. 2020, 22, 3074–3078. [Google Scholar] [CrossRef]
- Li, R.; Jiang, S.; Zheng, H.; Lei, H.; Huang, Z.; Chen, S.; Deng, G.-J. Iron-catalyzed indolo[2,3-c]quinoline synthesis from nitroarenes and benzylic alcohols/aldehydes promoted by elemental sulfur. Green Synth. Catal. 2022, 3, 95–101. [Google Scholar] [CrossRef]
- Ghosh, A.; Bera, A.; Banerjee, D. Nickel Catalyzed Alkylation of Oxindoles with Alkyl Alcohols. ChemCatChem 2023, 15, e202201433. [Google Scholar] [CrossRef]
- Jana, A.; Kumar, A.; Maji, B. Manganese catalyzed C-alkylation of methyl N-heteroarenes with primary alcohols. Chem. Commun. 2021, 57, 3026–3029. [Google Scholar] [CrossRef]







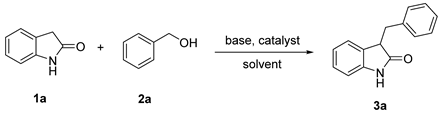 | |||||
|---|---|---|---|---|---|
| Entry | Catalyst | Base | Time [h] | Solvent | Yield [%] b |
| 1 | - | KOtBu | 6 | toluene | <5 |
| 2 | Ni-CIA | NEt3 | 6 | toluene | 17 |
| 3 | Ni-CIA | K2CO3 | 6 | toluene | 34 |
| 4 | Ni-CIA | Cs2CO3 | 6 | toluene | 65 |
| 5 | Ni-CIA | KOtBu | 6 | toluene | 84 |
| 6 | Ni-CIA | KOH | 6 | toluene | 72 |
| 7 | Ni-CIA | K3PO4 | 6 | toluene | 56 |
| 8 | Ni-CIA | KOtBu | 6 | H2O | <5 |
| 9 | Ni-CIA | KOtBu | 6 | 1,4-dioxane | 76 |
| 10 | Ni-CIA | KOtBu | 6 | xylene | 80 |
| 11 | Ni-CIA | KOtBu | 8 | DMF | 12 |
| 12 | Ni-CIA | KOtBu | 2 | toluene | 63 |
| 13 | Ni-CIA | KOtBu | 6 | toluene | 82 |
| 14 c | Ni-CIA | KOtBu | 6 | toluene | 88 |
| 15 d | Ni-CIA | KOtBu | 6 | toluene | 43 |
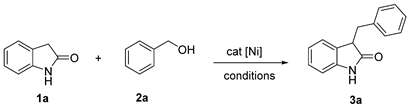 | |||||
|---|---|---|---|---|---|
| Entry | Catalyst | Yield [%] b | Entry | Catalyst | Yield [%] b |
| 1 | NiCl2 · 6H2O | 15 | 5 | NiCl2(bipy)2 | 32 |
| 2 | NiBr2 · 3H2O | <5 | 6 | NiCl2(phen)2 | 45 |
| 3 | Ni(NO3)2 · 6H2O | <5 | 7 | Ni-CIA | 88 |
| 4 | Ni(OAc)2 · 6H2O | <5 | 8 | none | <5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, J.; Tang, J.; Wang, L.; Wang, D.; Duan, Z.-C. Recyclable Ni-Containing Coordination Polymer as an Efficient Catalyst for the Synthesis of Oxindole and Quinoline Derivatives through the Borrowing Hydrogen Strategy. Catalysts 2023, 13, 1195. https://doi.org/10.3390/catal13081195
Li J, Tang J, Wang L, Wang D, Duan Z-C. Recyclable Ni-Containing Coordination Polymer as an Efficient Catalyst for the Synthesis of Oxindole and Quinoline Derivatives through the Borrowing Hydrogen Strategy. Catalysts. 2023; 13(8):1195. https://doi.org/10.3390/catal13081195
Chicago/Turabian StyleLi, Jiahao, Jiajie Tang, Likui Wang, Dawei Wang, and Zheng-Chao Duan. 2023. "Recyclable Ni-Containing Coordination Polymer as an Efficient Catalyst for the Synthesis of Oxindole and Quinoline Derivatives through the Borrowing Hydrogen Strategy" Catalysts 13, no. 8: 1195. https://doi.org/10.3390/catal13081195
APA StyleLi, J., Tang, J., Wang, L., Wang, D., & Duan, Z.-C. (2023). Recyclable Ni-Containing Coordination Polymer as an Efficient Catalyst for the Synthesis of Oxindole and Quinoline Derivatives through the Borrowing Hydrogen Strategy. Catalysts, 13(8), 1195. https://doi.org/10.3390/catal13081195