Research and Application Development of Catalytic Redox Technology for Zeolite-Based Catalysts
Abstract
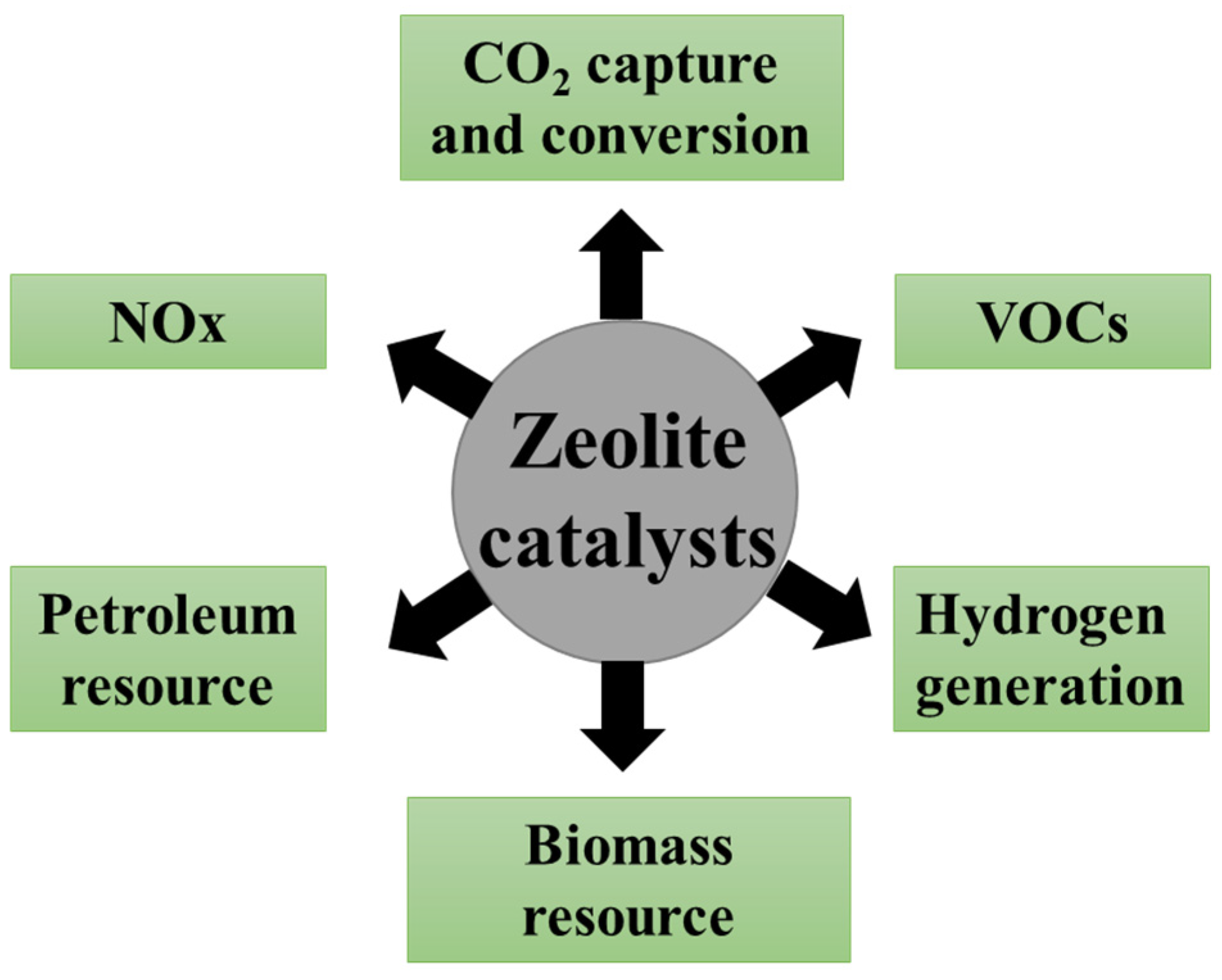
1. Introduction
2. The Application of Zeolite Catalysts
2.1. Catalytic Cracking of Petroleum Resources
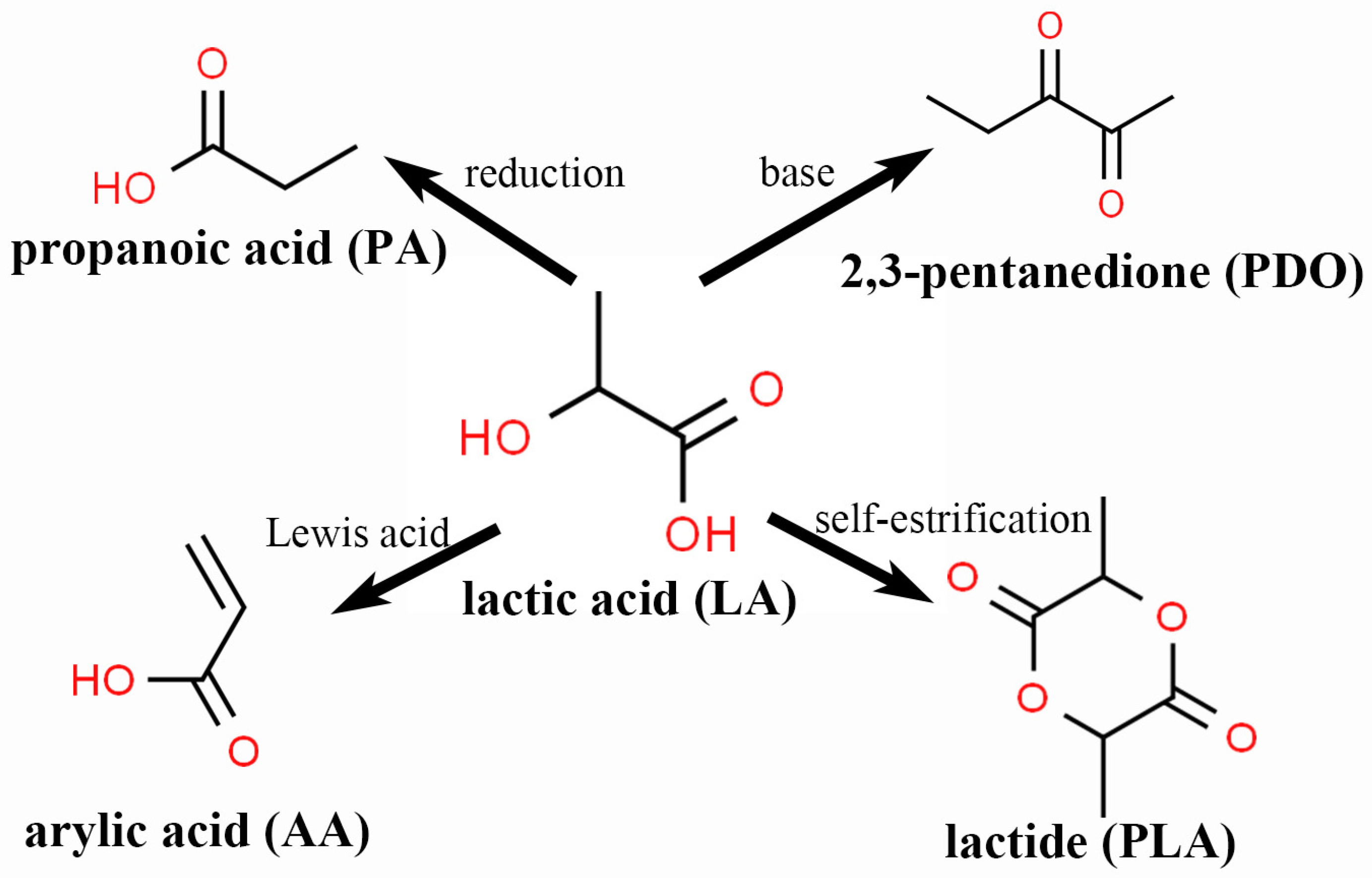
2.2. Conversion of Biomass
- (1)
- LeA via C5 and C6 route
- (2)
- HMF via the C6 route
2.3. Preparation of Propylene
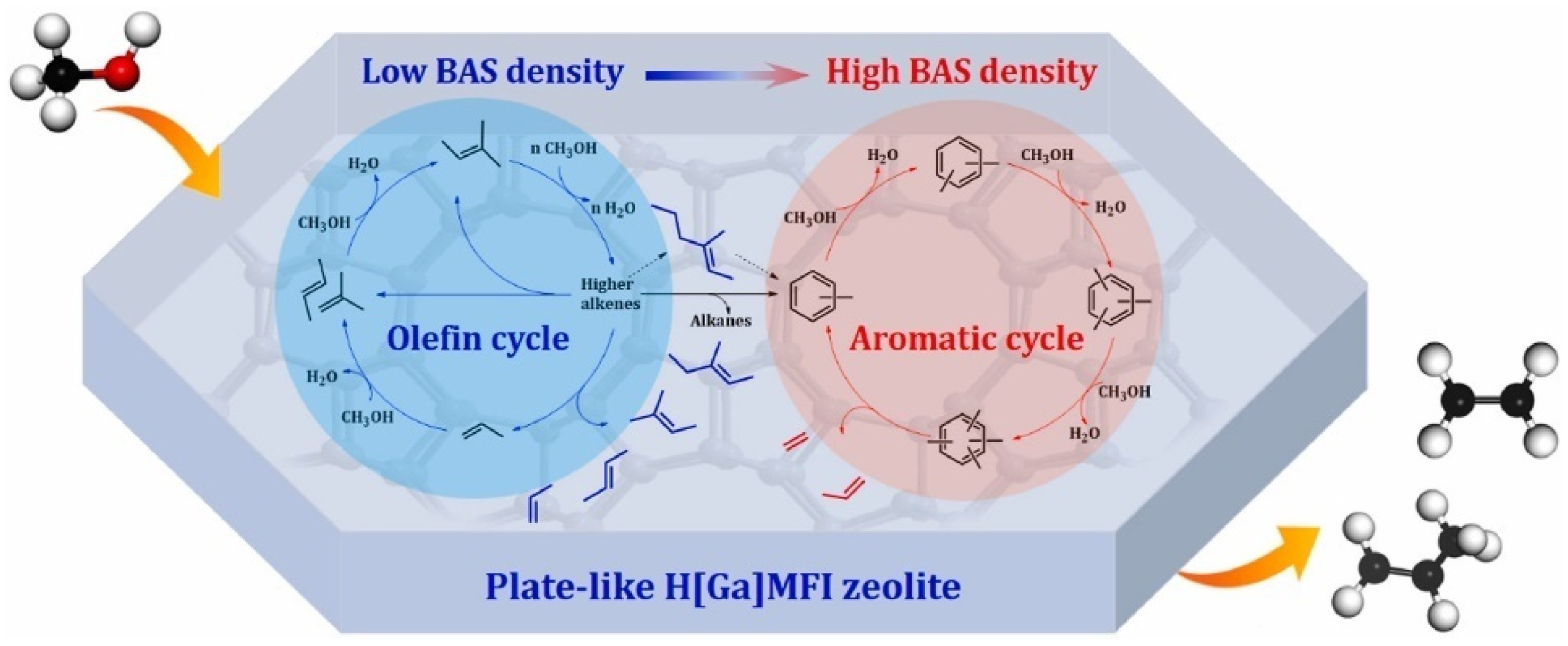
2.3.1. Methanol to Propylene (MTP)
| Catalysts | Method | Si/Al | SBET | Selectivity of Propylene | P/E | |
|---|---|---|---|---|---|---|
| HZSM-5 | two-stage crystallization | 130 130 124 139 | 361 | 50.95 48.13 49.68 50.10 | 4.40 4.44 5.89 7.48 | [32] |
| 356 | ||||||
| 352 | ||||||
| 340 | ||||||
| MFI | template | 202 | 428 | 58 | 7.9 | [34] |
| B-MFI | 205 | 404 | 66.3 | 7.9 | ||
| Cr-MFI | 201 | 408 | 59.8 | 2.7 | ||
| [Al/Ga]MFI | fluoride-assisted low-temperature crystallization | 70–73 65–275 * | 415–423 | 52% | - | [36] |
| 411–441 | ||||||
| ZSM-5 | quasi-solid-phase | 50 | 353 | 41.4% | - | [35] |
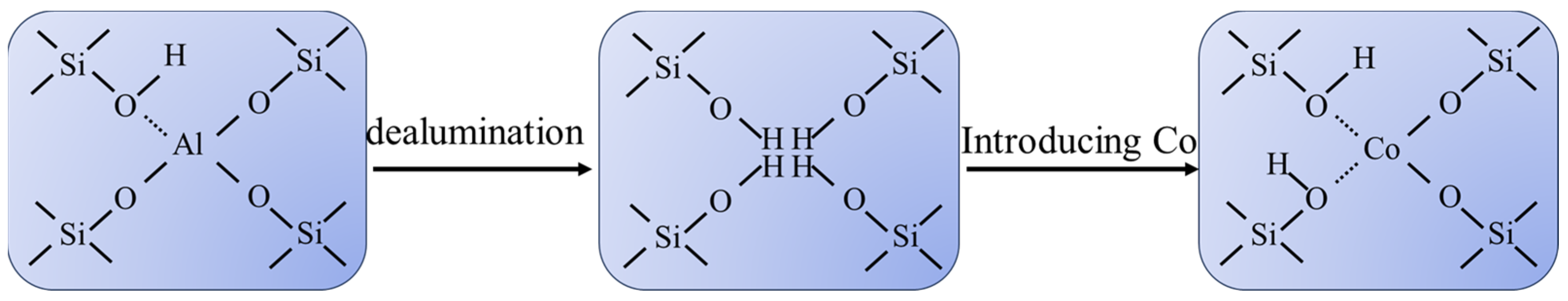
2.3.2. Propane to Propylene (PDH)
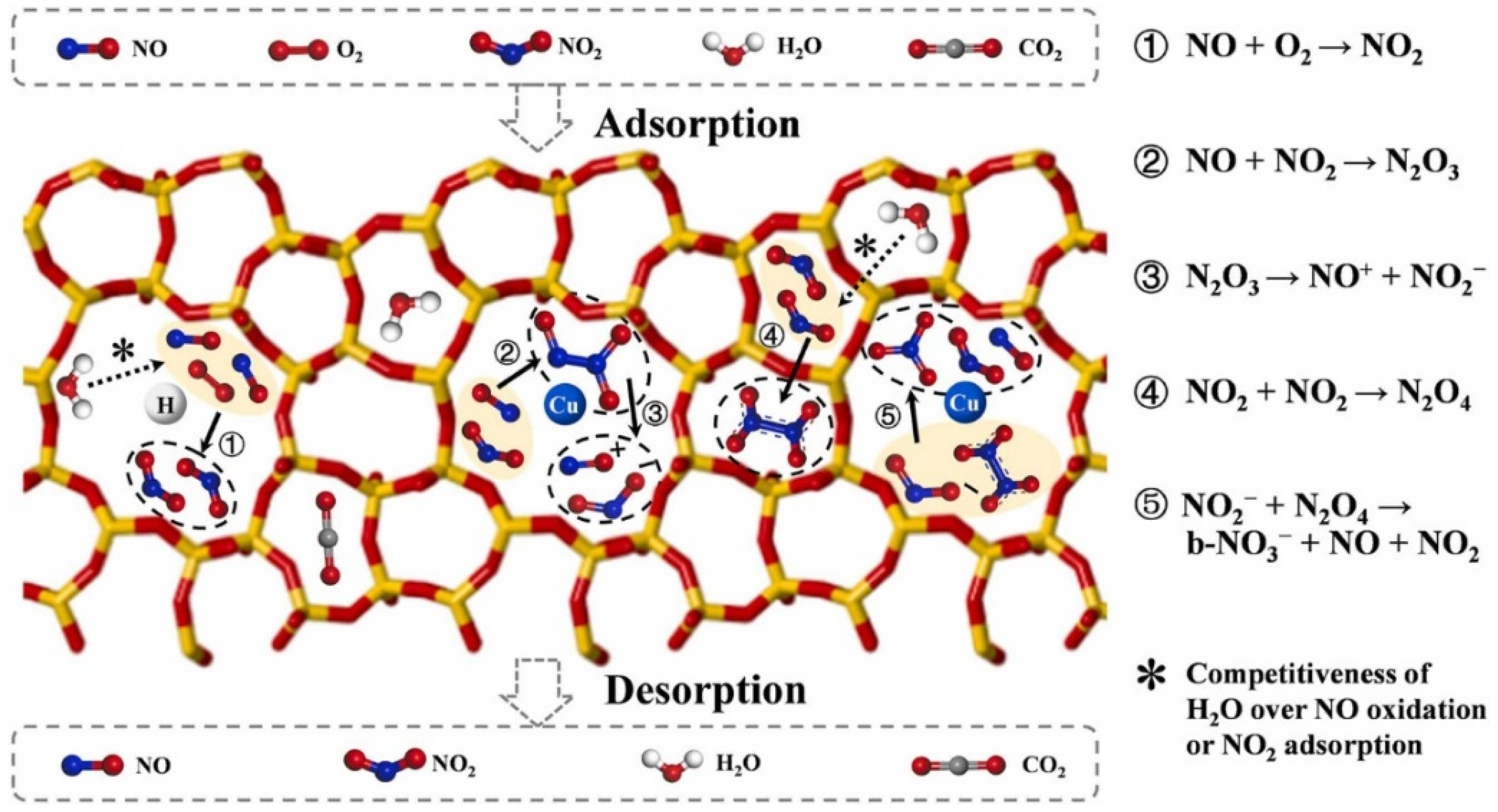
2.4. Selective Catalytic Reduction (SCR) of NOx
2.5. Hydrogen Preparation
2.6. VOCs Abatement
2.7. CO2 Capture
3. Conclusions and Future Perspectives
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Mishra, R.K.; Chistie, S.M.; Naika, S.U.; Mohanty, K. Catalytic pyrolysis of biomass over zeolites for bio-oil and chemical production: A review on their structure, porosity and acidity co-relation. Bioresour. Technol. 2022, 366, 128189. [Google Scholar] [CrossRef] [PubMed]
- Moliner, M.; Martinez, C.; Corma, A. Multipore Zeolites: Synthesis and Catalytic Applications. Angew. Chem. Int. Ed. 2015, 54, 3560–3579. [Google Scholar] [CrossRef]
- Li, Y.; Li, L.; Yu, J.H. Applications of Zeolites in Sustainable Chemistry. Chemistry 2017, 3, 928–949. [Google Scholar] [CrossRef]
- Martínez, C.; Vidal-Moya, A.; Yilmaz, B.; Kelkar, C.P.; Corma, A. Minimizing rare earth content of FCC catalysts: Understanding the fundamentals on combined P-La stabilization. Catal. Today 2023, 418, 114123. [Google Scholar] [CrossRef]
- Wang, T.; Le, T.; Ravindra, A.V.; Jue, H.; Zhang, L.; Wang, S. Enhanced regeneration of spent FCC catalyst by using oxalic acid-sulfuric acid mixture under ultrasonic irradiation. J. Mater. Res. Technol. 2021, 15, 7085–7099. [Google Scholar] [CrossRef]
- Zhang, L.; Hu, Q.; Qin, Y.; Liu, H.; Liu, H.; Cao, G.; Gao, X.; Song, L.; Zhaolin, S. Optimizing the accessibility of zeolite Y on FCC catalyst to improve heavy oil conversion capacity. Microporous Mesoporous Mater. 2023, 359, 112627. [Google Scholar] [CrossRef]
- Cui, W.; Zhu, D.; Tan, J.; Chen, N.; Fan, D.; Wang, J.; Han, J.; Wang, L.; Tian, P.; Liu, Z. Synthesis of mesoporous high-silica zeolite Y and their catalytic cracking performance. Chin. J. Catal. 2022, 43, 1945–1954. [Google Scholar] [CrossRef]
- Zhang, R.; Ju, Y.; Wu, P.; Chen, J.; Lv, Z.; Zhang, Y.; Song, S.; Zhang, Z.; Ma, C.; Zhang, R.; et al. Efficiently reducing olefin content of FCC gasoline over ZSM-5 zeolite based catalyst via hydro-upgrading. Catal. Today 2022, 405–406, 57–65. [Google Scholar] [CrossRef]
- Liu, P.; Cui, Y.; Wang, J.; Du, X.; Zhang, H.; Humphries, A.; Jia, M.; Yu, J. Structure stabilization of zeolite Y induced by yttrium and its role in promoting n-docosane conversion. Microporous Mesoporous Mater. 2021, 323, 111225. [Google Scholar] [CrossRef]
- Yamazaki, H.; Hasegawa, H.; Tanaka, C.; Takamiya, Y.; Mitsui, T.; Mizuno, T. Al ion-exchanged USY in FCC catalyst for high LPG yield. Catal. Commun. 2021, 159, 106354. [Google Scholar] [CrossRef]
- Miao, P.; Zhu, X.; Zhou, Z.; Feng, X.; Miao, J.; Hou, C.; Li, C. Combined dealkylation and transalkylation reaction in FCC condition for efficient conversion of light fraction light cycle oil into value-added products. Fuel 2021, 304, 121356. [Google Scholar] [CrossRef]
- Zhong, M.; Li, X.; Chu, X.; Gui, H.; Zuo, S.; Yao, C.; Li, Z.; Chen, Y. Solar driven catalytic conversion of cellulose biomass into lactic acid over copper reconstructed natural mineral. Appl. Catal. B Environ. 2022, 317, 121718. [Google Scholar] [CrossRef]
- Perego, C.; Bosetti, A. Biomass to fuels: The role of zeolite and mesoporous materials. Microporous Mesoporous Mater. 2011, 144, 28–39. [Google Scholar] [CrossRef]
- Ong, H.C.; Yu, K.L.; Chen, W.-H.; Pillejera, M.K.; Bi, X.; Tran, K.-Q.; Pétrissans, A.; Pétrissans, M. Variation of lignocellulosic biomass structure from torrefaction: A critical review. Renew. Sustain. Energy Rev. 2021, 152, 111698. [Google Scholar] [CrossRef]
- Yan, P.; Wang, H.; Liao, Y.; Wang, C. Zeolite catalysts for the valorization of biomass into platform compounds and biochemicals/biofuels: A review. Renew. Sustain. Energy Rev. 2023, 178, 113219. [Google Scholar] [CrossRef]
- Abu Zarin, M.A.; Zainol, M.M.; Ramli, N.A.S.; Amin, N.A.S. Zeolite immobilized ionic liquid as an effective catalyst for conversion of biomass derivatives to levulinic acid. Mol. Catal. 2022, 528, 112506. [Google Scholar] [CrossRef]
- Yan, P.; Wang, H.; Liao, Y.; Sun, P.; Wang, C. Introducing mesopore and regulating Al distribution for improving catalytic performances of ZSM-5 in furfuryl alcohol to levulinic acid. Fuel 2022, 329, 125213. [Google Scholar] [CrossRef]
- Chung, N.H.; Oanh, V.T.; Thoa, L.K.; Hoang, P.H. Catalytic Conversion of Glucose into 5-Hydroxymethyl Furfural Over Cu-Cr/ZSM-5 Zeolite. Catal. Lett. 2020, 150, 170–177. [Google Scholar] [CrossRef]
- Xu, S.Q.; Pan, D.H.; Hu, F.; Wu, Y.F.; Wang, H.Z.; Chen, Y.; Yuan, H.; Gao, L.J.; Xiao, G.M. Highly efficient Cr/beta zeolite catalyst for conversion of carbohydrates into 5-hydroxymethylfurfural: Characterization and performance. Fuel Process. Technol. 2019, 190, 38–46. [Google Scholar] [CrossRef]
- Werpy, T.; Petersen, G. Top Value Added Chemicals from Biomass: Volume I—Results of Screening for Potential Candidates from Sugars and Synthesis Gas; Medium: Boston, MA, USA, 2004; 76p. [Google Scholar]
- Venschott, M.; Hoelderich, W.F.; Eisenacher, M. 2nd generation PLA. Lactide formation directly from aqueous lactic acid. Catal. Commun. 2023, 177, 106636. [Google Scholar] [CrossRef]
- Ma, H.; Tingelstad, P.; Chen, D. Lactic acid production by catalytic conversion of glucose: An experimental and techno-economic evaluation. Catal. Today 2023, 408, 2–8. [Google Scholar] [CrossRef]
- Kim, J.; Bang, J.; Choi, J.-S.; Lim, D.-H.; Guk, D.; Jae, J. Selective conversion of lactic acid to renewable acrylic acid over SDA-free Na-ZSM-5: The critical role of basic sites of sodium oxide. J. Catal. 2023, 421, 271–284. [Google Scholar] [CrossRef]
- Ye, J.; Chen, C.; Zheng, Y.; Zhou, D.; Liu, Y.; Chen, D.; Ni, L.; Xu, G.; Wang, F. Efficient conversion of cellulose to lactic acid over yttrium modified siliceous Beta zeolites. Appl. Catal. A Gen. 2021, 619, 118133. [Google Scholar] [CrossRef]
- Koyunoğlu, C.; Gündüz, F.; Karaca, H.; Çınar, T.; Soyhan, G.G. Developing an adaptive catalyst for an FCC reactor using a CFD RSM, CFD DPM, and CFD DDPM–EM approach. Fuel 2023, 334, 126550. [Google Scholar] [CrossRef]
- Silva, J.M.; Ribeiro, M.F.; Graça, I.; Fernandes, A. Bio-oils/FCC co-processing: Insights into the adsorption of guaiacol on Y zeolites with distinct acidity and textural properties. Microporous Mesoporous Mater. 2021, 323, 111170. [Google Scholar] [CrossRef]
- Le-Phuc, N.; Tran, T.V.; Phan, T.T.; Ngo, P.T.; Ha, Q.L.M.; Luong, T.N.; Tran, T.H.; Phan, T.T. High-efficient production of biofuels using spent fluid catalytic cracking (FCC) catalysts and high acid value waste cooking oils. Renew. Energy 2021, 168, 57–63. [Google Scholar] [CrossRef]
- Magrini, K.; Olstad, J.; Peterson, B.; Jackson, R.; Parent, Y.; Mukarakate, C.; Iisa, K.; Christensen, E.; Seiser, R. Feedstock and catalyst impact on bio-oil production and FCC Co-processing to fuels. Biomass Bioenergy 2022, 163, 106502. [Google Scholar] [CrossRef]
- Zabihpour, A.; Ahmadpour, J.; Yaripour, F. Strategies to control reversible and irreversible deactivation of ZSM-5 zeolite during the conversion of methanol to propylene (MTP): A review. Chem. Eng. Sci. 2023, 273, 118639. [Google Scholar] [CrossRef]
- Qiu, B.; Lu, W.-D.; Gao, X.-Q.; Sheng, J.; Ji, M.; Wang, D.; Lu, A.-H. Boosting the propylene selectivity over embryonic borosilicate zeolite catalyst for oxidative dehydrogenation of propane. J. Catal. 2023, 417, 14–21. [Google Scholar] [CrossRef]
- Zhao, Y.-N.; Fan, S.-B.; Ma, Q.-X.; Zhang, J.-L.; Zhao, T.-S. Methanol converting to propylene on weakly acidic and hierarchical porous MFI zeolite. J. Fuel Chem. Technol. 2022, 50, 210–217. [Google Scholar] [CrossRef]
- Feng, R.; Zhou, P.; Liu, B.; Yan, X.; Hu, X.; Zhou, M. Direct synthesis of HZSM-5 zeolites with enhanced catalytic performance in the methanol-to-propylene reaction. Catal. Today 2022, 405–406, 299–308. [Google Scholar] [CrossRef]
- Feng, R.; Liu, B.; Zhou, P.; Yan, X.; Hu, X.; Zhou, M.; Yan, Z. Influence of framework Al distribution in HZSM-5 channels on catalytic performance in the methanol to propylene reaction. Appl. Catal. A Gen. 2022, 629, 118422. [Google Scholar] [CrossRef]
- Kalantari, N.; Bekheet, M.F.; Delir Kheyrollahi Nezhad, P.; Back, J.O.; Farzi, A.; Penner, S.; Delibaş, N.; Schwarz, S.; Bernardi, J.; Salari, D.; et al. Effect of chromium and boron incorporation methods on structural and catalytic properties of hierarchical ZSM-5 in the methanol-to-propylene process. J. Ind. Eng. Chem. 2022, 111, 168–182. [Google Scholar] [CrossRef]
- Tuo, J.; Lv, J.; Fan, S.; Li, H.; Yang, N.; Cheng, S.; Gao, X.; Zhao, T. One-pot synthesis of [Mn,H]ZSM-5 and the role of Mn in methanol-to-propylene reaction. Fuel 2022, 308, 121995. [Google Scholar] [CrossRef]
- Zhang, L.; Yang, L.; Liu, R.; Shao, X.; Dai, W.; Wu, G.; Guan, N.; Guo, Z.; Zhu, W.; Li, L. Design of plate-like H[Ga]MFI zeolite catalysts for high-performance methanol-to-propylene reaction. Microporous Mesoporous Mater. 2022, 333, 111767. [Google Scholar] [CrossRef]
- Dai, W.; Zhang, L.; Liu, R.; Huo, Z.; Dai, W.; Guan, N. Facile fabrication of a plate-like ZSM-5 zeolite as a highly efficient and stable catalyst for methanol to propylene conversion. Mater. Today Sustain. 2023, 22, 100364. [Google Scholar] [CrossRef]
- Yang, G.; Yan, X.; Chen, Y.; Guo, X.-J.; Lang, W.-Z.; Guo, Y.-J. Improved propylene selectivity and superior catalytic performance of Ga-xMg/ZSM-5 catalysts for propane dehydrogenation (PDH) reaction. Appl. Catal. A Gen. 2022, 643, 118778. [Google Scholar] [CrossRef]
- Xiong, Y.; Tian, T.; L’Hermitte, A.; Méndez, A.S.J.; Danaci, D.; Platero-Prats, A.E.; Petit, C. Using silver exchange to achieve high uptake and selectivity for propylene/propane separation in zeolite Y. Chem. Eng. J. 2022, 446, 137104. [Google Scholar] [CrossRef]
- Moradi, H.; Azizpour, H.; Mohammadi, M. Study of adsorption of propane and propylene on CHA zeolite in different Si/Al ratios using molecular dynamics simulation. Powder Technol. 2023, 419, 118329. [Google Scholar] [CrossRef]
- Wei, S.; Dai, H.; Long, J.; Lin, H.; Gu, J.; Zong, X.; Yang, D.; Tang, Y.; Yang, Y.; Dai, Y. Nonoxidative propane dehydrogenation by isolated Co2+ in BEA zeolite: Dealumination-determined key steps of propane CH activation and propylene desorption. Chem. Eng. J. 2023, 455, 140726. [Google Scholar] [CrossRef]
- Liu, Y.; Tao, H.; Yang, X.; Wu, X.; Li, J.; Zhang, C.; Yang, R.T.; Li, Z. Adsorptive purification of NOx by HZSM-5 zeolites: Effects of Si/Al ratio, temperature, humidity, and gas composition. Microporous Mesoporous Mater. 2023, 348, 112331. [Google Scholar] [CrossRef]
- Sunil Kumar, M.; Alphin, M.S.; Manigandan, S.; Vignesh, S.; Vigneshwaran, S.; Subash, T. A review of comparison between the traditional catalyst and zeolite catalyst for ammonia-selective catalytic reduction of NOx. Fuel 2023, 344, 128125. [Google Scholar] [CrossRef]
- Zang, Y.; Bi, Y.; Liu, C.; Zhang, Y.; Li, Q.; Wang, Y.; Zhang, M.; Liu, Q.; Zhang, Z. The study on Cu-ZK-5 catalyst of copper content regulation and anti-propylene poisoning mechanism in NH3-SCR reaction. Fuel 2023, 340, 127442. [Google Scholar] [CrossRef]
- Jin, P.; Yang, L.; Sheng, Z.; Chu, X.; Chen, D. Selective catalytic reduction of NOx with NH3 and tolerance to H2O & SO2 at high temperature over zeolite supported indium-copper bimetallic catalysts for gas turbine. J. Environ. Chem. Eng. 2023, 11, 109218. [Google Scholar] [CrossRef]
- Gao, L.; Gao, W.; Wang, H.; Xu, S.; Tian, X.; Cao, J.; Chen, J.; Zhang, Q.; Ning, P.; Hao, J. Boosting low-temperature and high-temperature hydrothermal stability of Cu/SAPO-34 for NOx removal via yttrium decoration. Chem. Eng. J. 2023, 455, 140520. [Google Scholar] [CrossRef]
- Liu, Y.; Wu, X.; Yang, X.; Tao, H.; Li, J.; Zhang, C.; Yang, R.T.; Li, Z. Enhancement of NOx adsorption performance on zeolite via a facile modification strategy. J. Hazard. Mater. 2023, 443, 130225. [Google Scholar] [CrossRef]
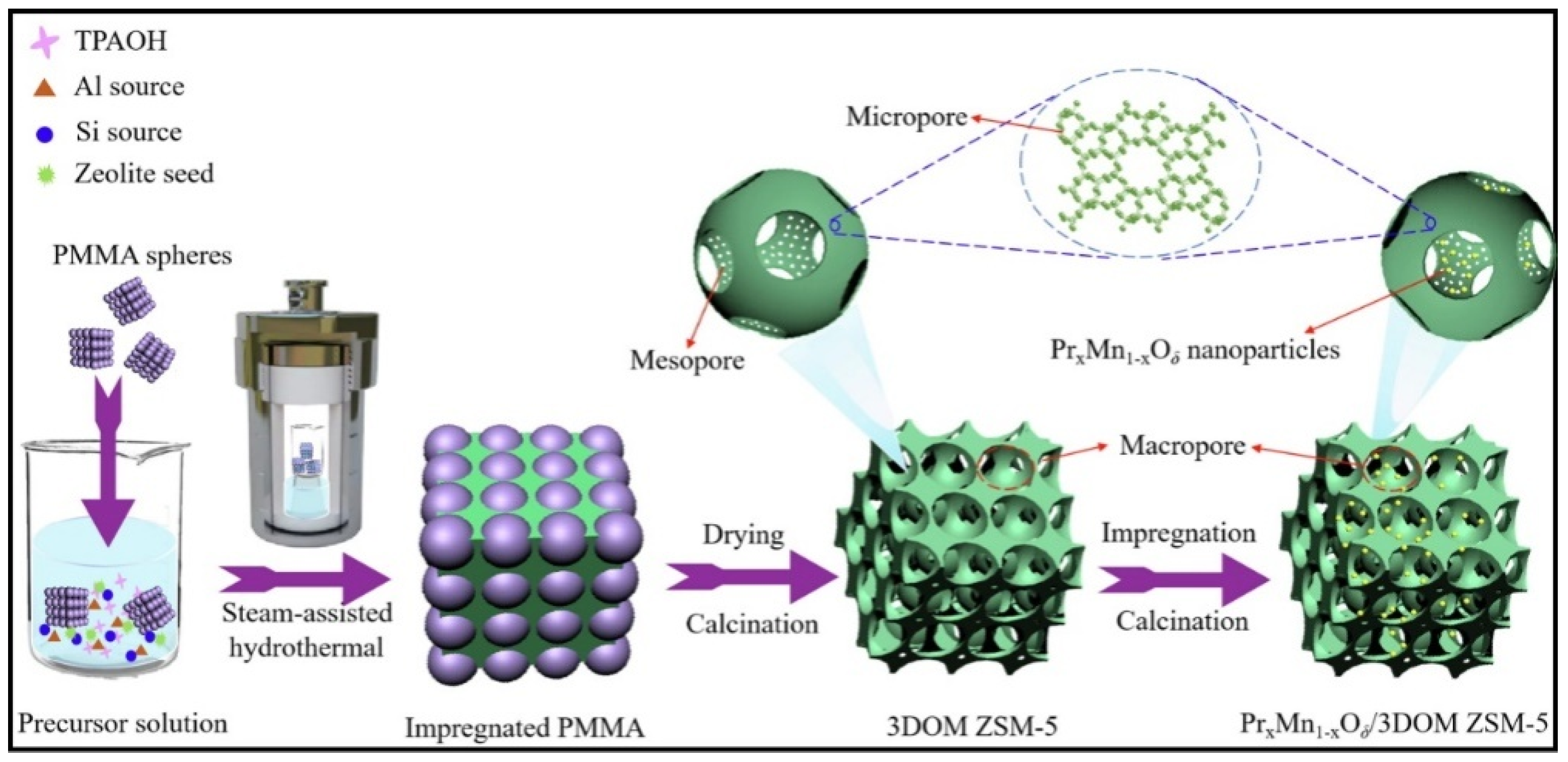
- Wang, L.; Ren, Y.; Yu, X.; Peng, C.; Yu, D.; Zhong, C.; Hou, J.; Yin, C.; Fan, X.; Zhao, Z.; et al. Novel preparation method, catalytic performance and reaction mechanisms of PrxMn1−xOδ/3DOM ZSM-5 catalysts for the simultaneous removal of soot and NOx. J. Catal. 2023, 417, 226–247. [Google Scholar] [CrossRef]
- Kang, T.H.; Kim, H.S.; Lee, H.; Kim, D.H. Synergistic effect of V2O5-WO3/TiO2 and H-ZSM-5 catalysts prepared by physical mixing on the selective catalytic reduction of NOx with NH3. Appl. Surf. Sci. 2023, 614, 156159. [Google Scholar] [CrossRef]
- Zhang, L.; Shan, Y.; Yan, Z.; Liu, Z.; Yu, Y.; He, H. Efficient Pt/KFI zeolite catalysts for the selective catalytic reduction of NOx by hydrogen. J. Environ. Sci. 2024, 138, 102–111. [Google Scholar] [CrossRef]
- Wang, S.; He, B.; Wang, Y.; Wu, X.; Duan, H.; Di, J.; Yu, Z.; Liu, Y.; Xin, Z.; Jia, L.; et al. Hydrogen production from the steam reforming of bioethanol over novel supported Ca/Ni-hierarchical Beta zeolite catalysts. Int. J. Hydrogen Energy 2021, 46, 36245–36256. [Google Scholar] [CrossRef]
- Husin, H.; Erdiwansyah, E.; Ahmadi, A.; Nasution, F.; Rinaldi, W.; Abnisa, F.; Mamat, R. Efficient hydrogen production by microwave-assisted catalysis for glycerol-water solutions via NiO/zeolite-CaO catalyst. South Afr. J. Chem. Eng. 2022, 41, 43–50. [Google Scholar] [CrossRef]
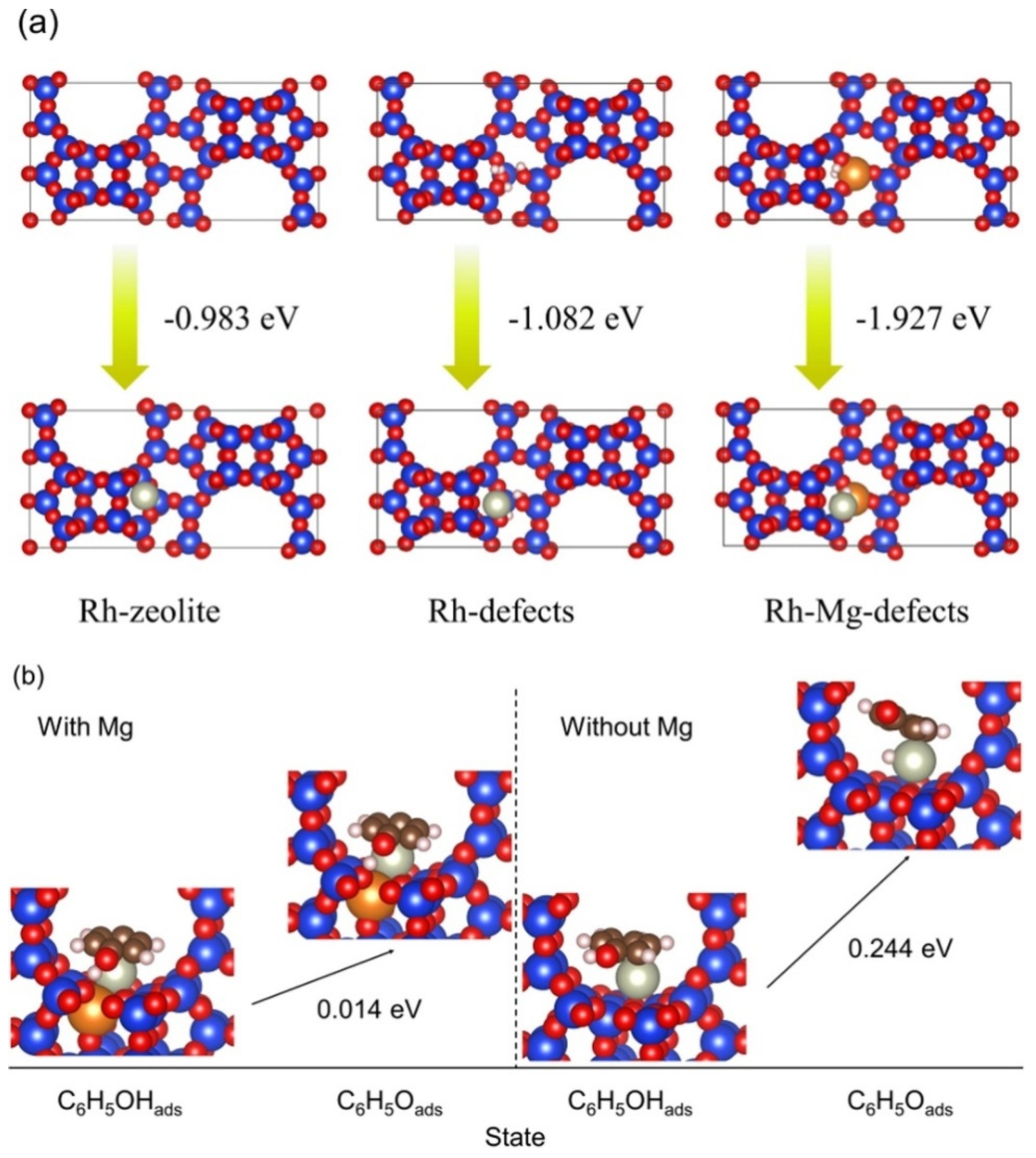
- Zhang, H.; Zhong, L.; Bin Samsudin, I.; Okumura, K.; Tan, H.-R.; Li, S.; Jaenicke, S.; Chuah, G.-K. Mg-stabilized subnanometer Rh particles in zeolite Beta as highly efficient catalysts for selective hydrogenation. J. Catal. 2022, 405, 489–498. [Google Scholar] [CrossRef]
- Da Costa-Serra, J.F.; Miralles-Martínez, A.; García-Muñoz, B.; Maestro-Cuadrado, S.; Chica, A. Ni and Co-based catalysts supported on ITQ-6 zeolite for hydrogen production by steam reforming of ethanol. Int. J. Hydrogen Energy 2022, 48, 26518–26525. [Google Scholar] [CrossRef]
- Ismaila, A.; Chen, H.; Fan, X. Nickel encapsulated in silicalite-1 zeolite catalysts for steam reforming of glycerol (SRG) towards renewable hydrogen production. Fuel Process. Technol. 2022, 233, 107306. [Google Scholar] [CrossRef]
- Yu, L.; Zhang, R.; Cao, C.; Liu, L.; Fang, J.; Jin, H. Hydrogen production from supercritical water gasification of lignin catalyzed by Ni supported on various zeolites. Fuel 2022, 319, 123744. [Google Scholar] [CrossRef]
- Chen, M.; Sun, G.; Wang, Y.; Liang, D.; Li, C.; Wang, J.; Liu, Q. Steam reforming of methanol for hydrogen production over attapulgite-based zeolite-supported Cu-Zr catalyst. Fuel 2022, 314, 122733. [Google Scholar] [CrossRef]
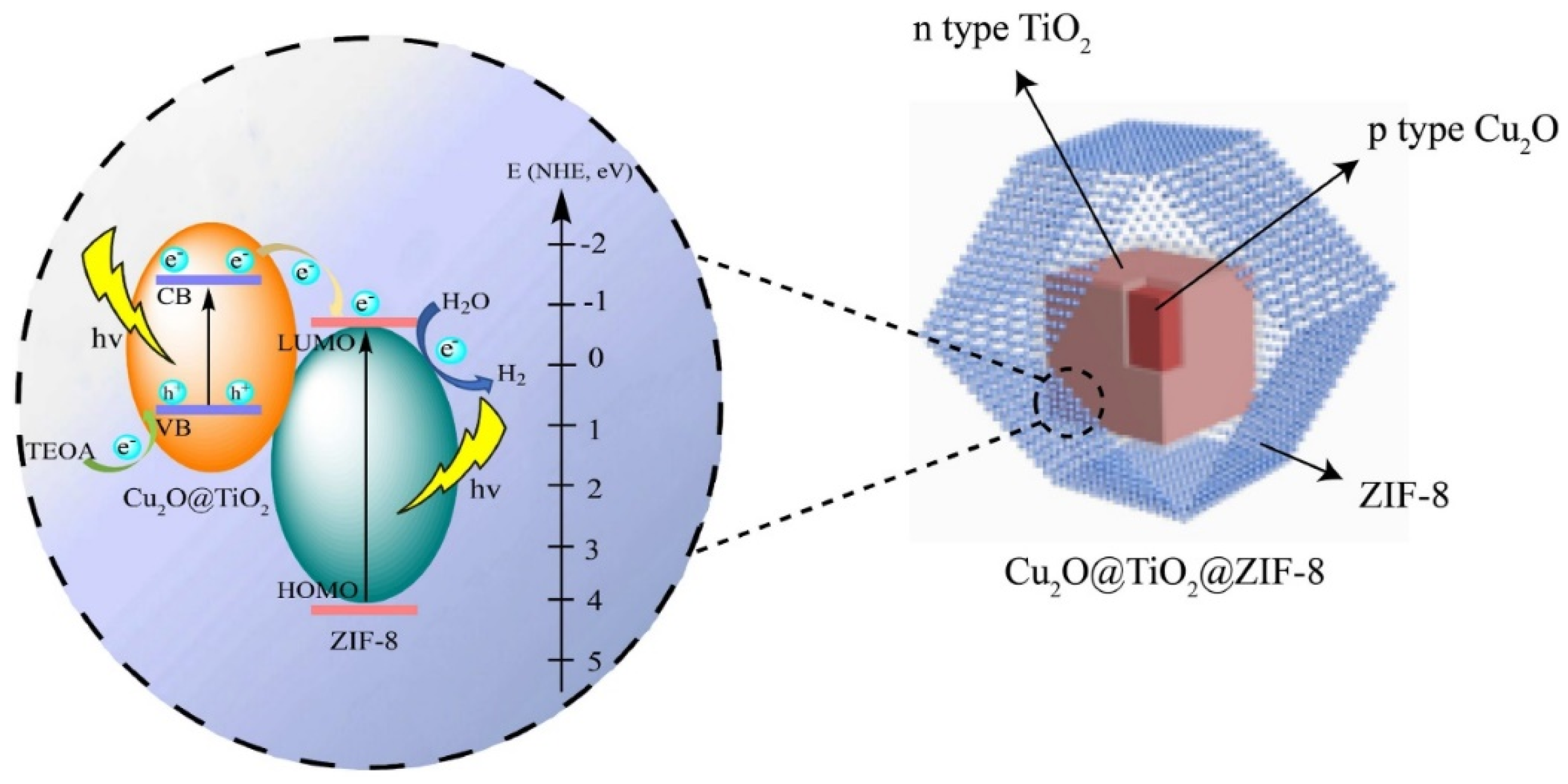
- Zhou, Y.; Wang, P.; Qin, L.; Kang, S.-Z.; Li, X. Double shell composite nanoarchitectonics of Cu2O core with TiO2/metal-organic frameworks for efficient hydrogen generation. Int. J. Hydrogen Energy 2023, 48, 629–639. [Google Scholar] [CrossRef]
- Saka, C. Highly active hydrogen generation from sodium borohydride methanolysis and ethylene glycolysis reactions using protonated chitosan-zeolite hybrid metal-free particles. Appl. Catal. B Environ. 2023, 325, 122335. [Google Scholar] [CrossRef]
- Sun, T.; Wei, J.; Zhou, C.; Wang, Y.; Shu, Z.; Zhou, J.; Chen, J. Facile preparation and enhanced photocatalytic hydrogen evolution of cation-exchanged zeolite LTA supported TiO2 photocatalysts. Int. J. Hydrogen Energy 2023, 48, 13851–13863. [Google Scholar] [CrossRef]
- Shen, Y. Biomass-derived porous carbons for sorption of Volatile organic compounds (VOCs). Fuel 2023, 336, 126801. [Google Scholar] [CrossRef]
- Yang, F.; Li, W.; Ou, R.; Lu, Y.; Dong, X.; Tu, W.; Zhu, W.; Wang, X.; Li, L.; Yuan, A.; et al. Superb VOCs capture engineering carbon adsorbent derived from shaddock peel owning uncompromising thermal-stability and adsorption property. Chin. J. Chem. Eng. 2022, 47, 120–133. [Google Scholar] [CrossRef]
- Zhang, Y.; Yu, Q.; Yuan, Y.; Tang, X.; Zhao, S.; Yi, H. Adsorption behavior of Mo-MEL zeolites for reducing VOCs from cooking oil fumes. Sep. Purif. Technol. 2023, 322, 124059. [Google Scholar] [CrossRef]
- Yu, Q.; Feng, Y.; Wei, J.; Tang, X.; Yi, H. Development of Mn-Si-MEL as a bi-functional adsorption-catalytic oxidation material for VOCs elimination. Chin. Chem. Lett. 2022, 33, 3087–3090. [Google Scholar] [CrossRef]
- Liu, H.; Wei, K.; Long, C. Enhancing adsorption capacities of low-concentration VOCs under humid conditions using NaY@meso-SiO2 core–shell composite. Chem. Eng. J. 2022, 442, 136108. [Google Scholar] [CrossRef]
- Yin, T.; Meng, X.; Wang, S.; Yao, X.; Liu, N.; Shi, L. Study on the adsorption of low-concentration VOCs on zeolite composites based on chemisorption of metal-oxides under dry and wet conditions. Sep. Purif. Technol. 2022, 280, 119634. [Google Scholar] [CrossRef]
- Feng, A.; Yu, Y.; Mi, L.; Cao, Y.; Yu, Y.; Song, L. Synthesis and VOCs adsorption performance of surfactant-templated USY zeolites with controllable mesopores. Chem. Phys. Lett. 2022, 798, 139578. [Google Scholar] [CrossRef]
- Lu, S.; Han, R.; Wang, H.; Song, C.; Ji, N.; Lu, X.; Ma, D.; Liu, Q. Three birds with one stone: Designing a novel binder-free monolithic zeolite pellet for wet VOC gas adsorption. Chem. Eng. J. 2022, 448, 137629. [Google Scholar] [CrossRef]
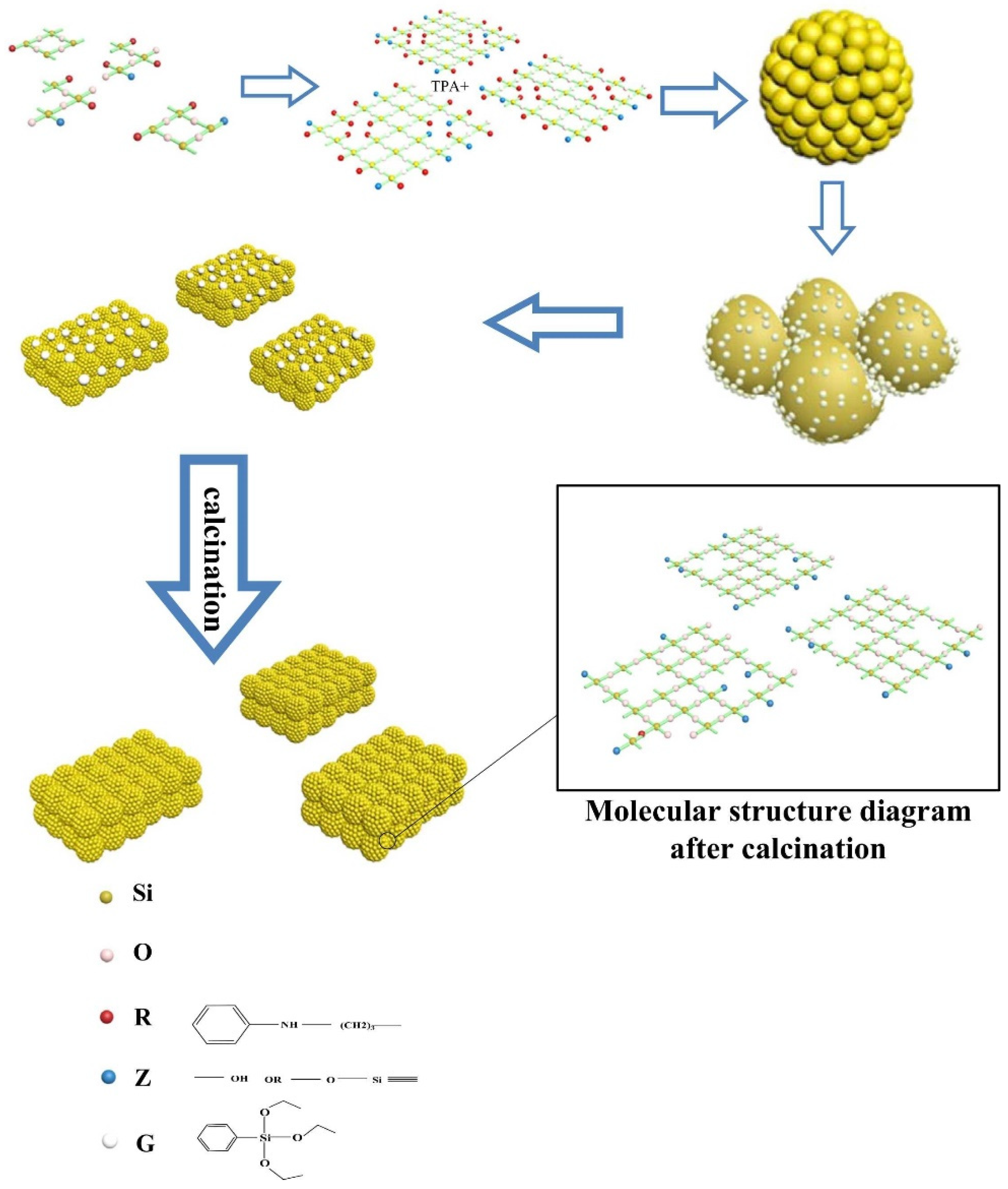
- Chen, L.H.; Sun, M.H.; Wang, Z.; Yang, W.M.; Xie, Z.K.; Su, B.L. Hierarchically Structured Zeolites: From Design to Application. Chem. Rev. 2020, 120, 11194–11294. [Google Scholar] [CrossRef]
- Ivanova, I.I.; Knyazeva, E.E. Micro-mesoporous materials obtained by zeolite recrystallization: Synthesis, characterization and catalytic applications. Chem. Soc. Rev. 2013, 42, 3671–3688. [Google Scholar] [CrossRef]
- Zhao, C.; Hu, X.; Liu, C.; Chen, D.; Yun, J.; Jiang, X.; Wei, N.; Li, M.; Chen, Z. Hierarchical architectures of ZSM-5 with controllable mesoporous and their particular adsorption/desorption performance for VOCs. J. Environ. Chem. Eng. 2022, 10, 106868. [Google Scholar] [CrossRef]
- Wang, J.; Shi, Y.; Kong, F.; Zhou, R. Low-temperature VOCs oxidation performance of Pt/zeolites catalysts with hierarchical pore structure. J. Environ. Sci. 2023, 124, 505–512. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Wang, L.; Lu, Y.; Deng, H.; Zhang, Z.; Wang, Y.; Ma, Y.; Pan, T.; Zhao, Q.; Shan, Y.; et al. New insights into the catalytic mechanism of VOCs abatement over Pt/Beta with active sites regulated by zeolite acidity. Appl. Catal. B Environ. 2023, 334, 122811. [Google Scholar] [CrossRef]
- Kong, F.; Li, G.; Wang, J.; Shi, Y.; Zhou, R. Promoting effect of acid sites in hierarchical porous Pt/ZSM-5 catalysts for low-temperature removal of VOCs. Appl. Surf. Sci. 2022, 606, 154888. [Google Scholar] [CrossRef]
- Yu, B.; Deng, H.; Lu, Y.; Pan, T.; Shan, W.; He, H. Adsorptive interaction between typical VOCs and various topological zeolites: Mixture effect and mechanism. J. Environ. Sci. 2024, 136, 626–636. [Google Scholar] [CrossRef]
- Zheng, Y.; Han, R.; Yang, L.; Yang, J.; Shan, C.; Liu, Q. Revealing opposite behaviors of catalyst for VOCs Oxidation: Modulating electronic structure of Pt nanoparticles by Mn doping. Chem. Eng. J. 2023, 465, 142807. [Google Scholar] [CrossRef]
- Wu, R.; Xiao, Y.; Zhang, P.; Lin, J.; Cheng, G.; Chen, Z.; Yu, R. Asphalt VOCs reduction of zeolite synthesized from solid wastes of red mud and steel slag. J. Clean. Prod. 2022, 345, 131078. [Google Scholar] [CrossRef]
- Yu, C.-H.; Huang, C.-H.; Tan, C.-S. A Review of CO2 Capture by Absorption and Adsorption. Aerosol Air Qual. Res. 2012, 12, 745–769. [Google Scholar] [CrossRef]
- Choi, H.J.; Hong, S.B. Effect of framework Si/Al ratio on the mechanism of CO2 adsorption on the small-pore zeolite gismondine. Chem. Eng. J. 2022, 433, 133800. [Google Scholar] [CrossRef]
- Chen, C.; Ahn, W.-S. CO2 adsorption on LTA zeolites: Effect of mesoporosity. Appl. Surf. Sci. 2014, 311, 107–109. [Google Scholar] [CrossRef]
- Chen, S.J.; Zhu, M.; Tang, Y.C.; Fu, Y.; Li, W.L.; Xiao, B. Molecular simulation and experimental investigation of CO2 capture in a polymetallic cation-exchanged 13X zeolite. J. Mater. Chem. A 2018, 6, 19570–19583. [Google Scholar] [CrossRef]
- Liu, X.; Gao, F.; Xu, J.; Zhou, L.; Liu, H.; Hu, J. Zeolite@Mesoporous silica-supported-amine hybrids for the capture of CO2 in the presence of water. Microporous Mesoporous Mater. 2016, 222, 113–119. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, W.; Fang, D.; Huang, G.; Li, D.; Zheng, Y. Research and Application Development of Catalytic Redox Technology for Zeolite-Based Catalysts. Catalysts 2023, 13, 1197. https://doi.org/10.3390/catal13081197
Zhang W, Fang D, Huang G, Li D, Zheng Y. Research and Application Development of Catalytic Redox Technology for Zeolite-Based Catalysts. Catalysts. 2023; 13(8):1197. https://doi.org/10.3390/catal13081197
Chicago/Turabian StyleZhang, Wentao, De Fang, Guanlin Huang, Da Li, and Yun Zheng. 2023. "Research and Application Development of Catalytic Redox Technology for Zeolite-Based Catalysts" Catalysts 13, no. 8: 1197. https://doi.org/10.3390/catal13081197
APA StyleZhang, W., Fang, D., Huang, G., Li, D., & Zheng, Y. (2023). Research and Application Development of Catalytic Redox Technology for Zeolite-Based Catalysts. Catalysts, 13(8), 1197. https://doi.org/10.3390/catal13081197








