Efficient Conversion of Monosaccharides into 5-Hydroxymethylfurfural Using Acidic Deep Eutectic Solvents
Abstract
:1. Introduction
2. Result
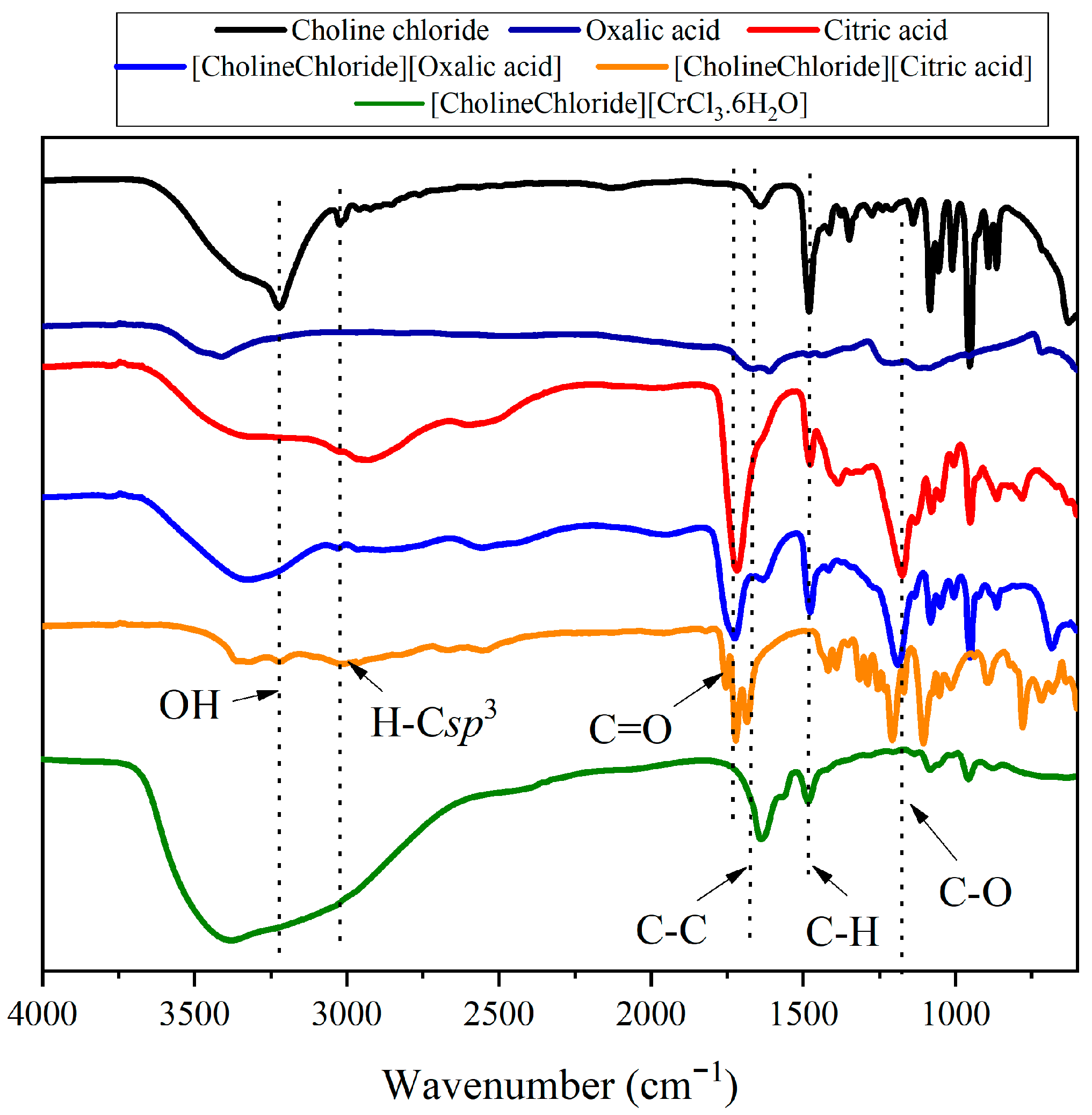
2.1. Characterization of DESs
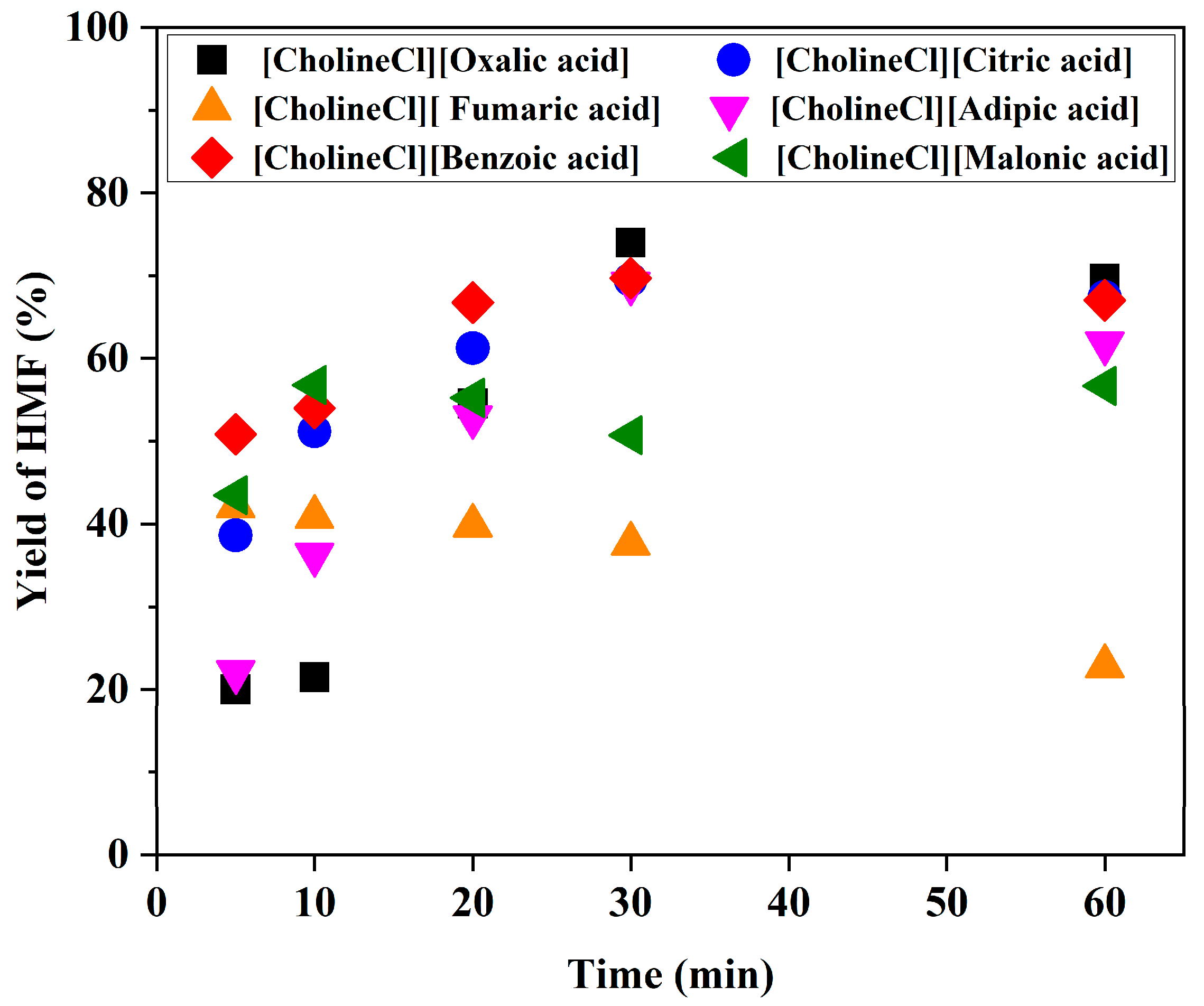
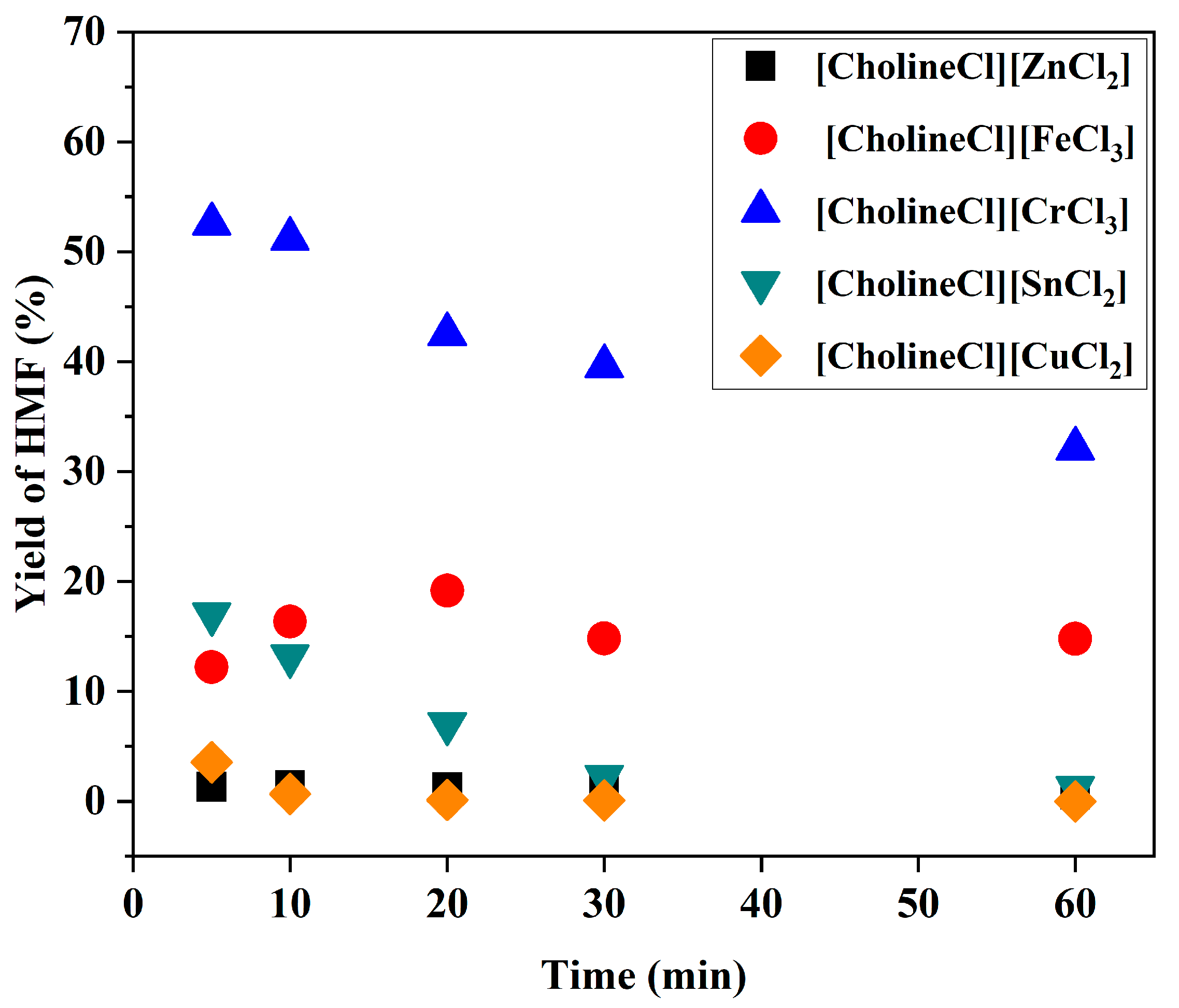
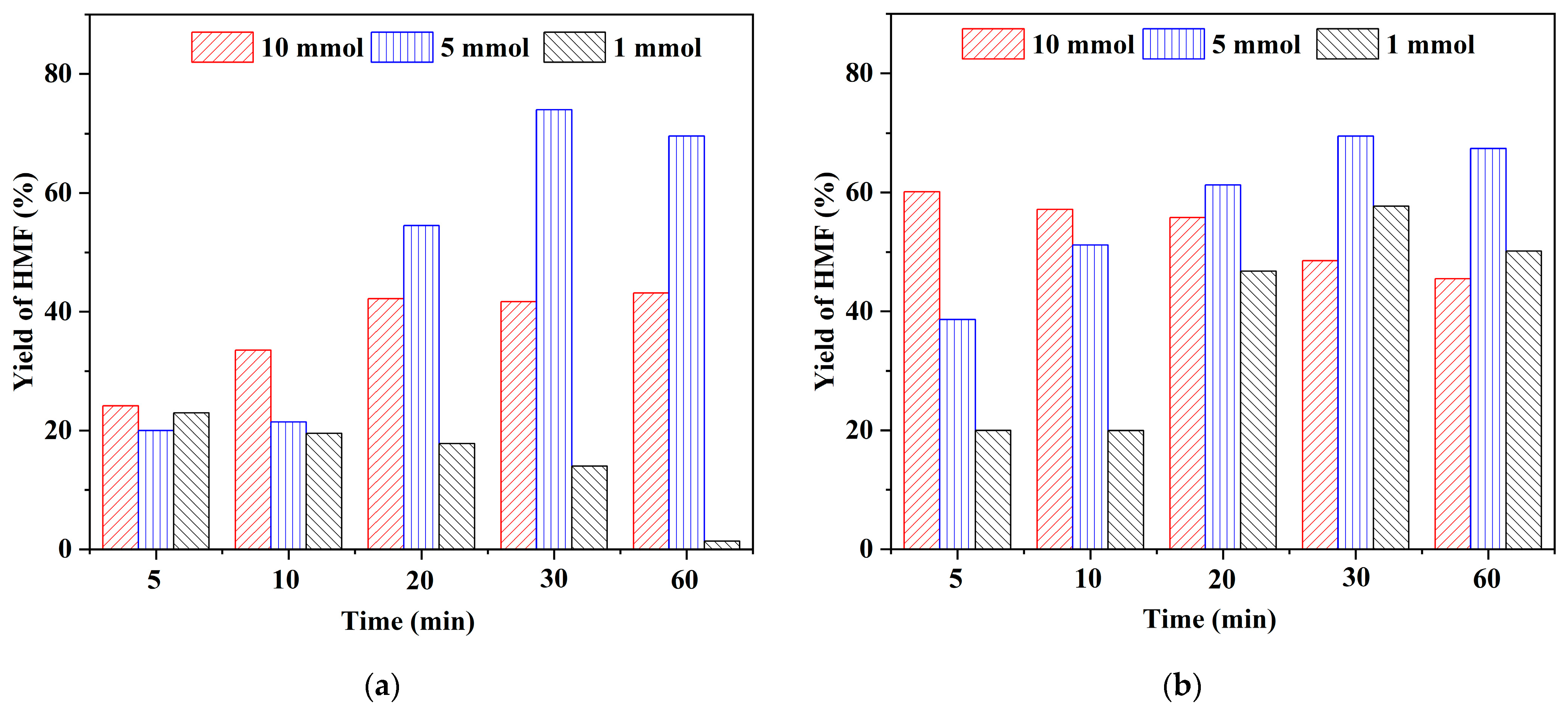
2.2. Effect of Brønsted Acidic Additives on the Conversion of Fructose into HMF
2.3. Mechanism Proposal
2.4. The Comparison of This Work with Previous Work
3. Materials and Methods
3.1. Chemical and Equipment
3.2. Synthesis of Deep Eutectic Solvents
3.3. Procedure of Synthesizing HMF
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Corma, A.; Iborra, S.; Velty, A. Chemical routes for the transformation of biomass into chemicals. Chem. Rev. 2007, 107, 2411–2502. [Google Scholar] [CrossRef] [PubMed]
- Mascal, M.; Nikitin, E.B. Direct, High-yield conversion of cellulose into biofuel. Angew. Chem. 2008, 120, 8042–8044. [Google Scholar] [CrossRef]
- Metzger, J.O. Production of liquid hydrocarbons from biomass. Angew. Chem. Int. Ed. 2006, 45, 696–698. [Google Scholar] [CrossRef] [PubMed]
- Román-Leshkov, Y.; Barrett, C.J.; Liu, Z.Y.; Dumesic, J.A. Production of dimethylfuran for liquid fuels from biomass-derived carbohydrates. Nature 2007, 447, 982–985. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Denard, C.A.; Alamillo, R.; Crisci, A.J.; Miao, Y.; Dumesic, J.A.; Scott, S.L.; Zhao, H. Tandem catalytic conversion of glucose to 5-hydroxymethylfurfural with an immobilized enzyme and a solid acid. ACS Catal. 2014, 4, 2165–2168. [Google Scholar] [CrossRef]
- Lv, G.; Wang, H.; Yang, Y.; Deng, T.; Chen, C.; Zhu, Y.; Hou, X. Direct synthesis of 2, 5-diformylfuran from fructose with graphene oxide as a bifunctional and metal-free catalyst. Green Chem. 2016, 18, 2302–2307. [Google Scholar] [CrossRef]
- Rosatella, A.A.; Simeonov, S.P.; Frade, R.F.; Afonso, C.A. 5-Hydroxymethylfurfural (HMF) as a building block platform: Biological properties, synthesis and synthetic applications. Green Chem. 2011, 13, 754–793. [Google Scholar] [CrossRef]
- Salavati-fard, T.; Caratzoulas, S.; Lobo, R.F.; Doren, D.J. Catalysis of the Diels–Alder reaction of furan and methyl acrylate in Lewis acidic zeolites. ACS Catal. 2017, 7, 2240–2246. [Google Scholar] [CrossRef]
- Tuteja, J.; Choudhary, H.; Nishimura, S.; Ebitani, K. Direct synthesis of 1, 6-hexanediol from HMF over a heterogeneous Pd/ZrP catalyst using formic acid as hydrogen source. ChemSusChem 2014, 7, 96–100. [Google Scholar] [CrossRef]
- Ly, P.D.; Phan, H.B.; Le, Y.N.T.; Tran, P.H. Continuous-Flow Synthesis of 5-Hydroxymethylfurfural, Furfural from Monosaccharides: A Simple, Fast, and Practical Method. ChemistrySelect 2021, 6, 10827–10833. [Google Scholar] [CrossRef]
- Sonsiam, C.; Kaewchada, A.; Jaree, A. Synthesis of 5-hydroxymethylfurfural (5-HMF) from fructose over cation exchange resin in a continuous flow reactor. Chem Eng Process 2019, 138, 65–72. [Google Scholar] [CrossRef]
- Hansen, B.B.; Spittle, S.; Chen, B.; Poe, D.; Zhang, Y.; Klein, J.M.; Horton, A.; Adhikari, L.; Zelovich, T.; Doherty, B.W.; et al. Deep Eutectic Solvents: A Review of Fundamentals and Applications. Chemical Rev. 2021, 121, 1232–1285. [Google Scholar] [CrossRef]
- Lang, J.; Lu, J.; Lan, P.; Wang, N.; Yang, H.; Zhang, H. Preparation of 5-HMF in a DES/Ethyl N-Butyrate Two-Phase System. Catalysts 2020, 10, 636. [Google Scholar] [CrossRef]
- Tran, P.H.; Tran, P.V. A highly selective and efficient method for the production of 5-hydroxymethylfurfural from dehydration of fructose using SACS/DES catalytic system. Fuel 2019, 246, 18–23. [Google Scholar] [CrossRef]
- Marullo, S.; Rizzo, C.; D’Anna, F. Activity of a Heterogeneous Catalyst in Deep Eutectic Solvents: The Case of Carbohydrate Conversion into 5-Hydroxymethylfurfural. ACS Sustain. Chem. Eng. 2019, 7, 13359–13368. [Google Scholar] [CrossRef]
- Tao, S.; Hu, L.; Zhang, X.; Mai, Y.; Xian, X.; Zheng, X.; Lin, X. Insights into the Play of Novel Brønsted Acid-Based Deep Eutectic Solvents for the Conversion of Glucose into 5-Hydroxymethylfurfural without Additional Catalysts. Ind. Eng. Chem. Res. 2022, 61, 11645–11654. [Google Scholar] [CrossRef]
- Chen, W.-C.; Lin, Y.-C.; Chu, I.M.; Wang, L.-F.; Tsai, S.-L.; Wei, Y.-H. Feasibility of enhancing production of 5-hydroxymethylfurfural using deep eutectic solvents as reaction media in a high-pressure reactor. Biochem. Eng. J. 2020, 154, 107440. [Google Scholar] [CrossRef]
- Vigier, K.D.O.; Benguerba, A.; Barrault, J.; Jérôme, F. Conversion of fructose and inulin to 5-hydroxymethylfurfural in sustainable betaine hydrochloride-based media. Green Chem. 2012, 14, 285–289. [Google Scholar] [CrossRef]
- Delbecq, F.; Wang, Y.; Len, C. Various carbohydrate precursors dehydration to 5-HMF in an acidic biphasic system under microwave heating using betaine as a co-catalyst. J. Mol. Catal. 2017, 434, 80–85. [Google Scholar] [CrossRef]
- Phan, H.B.; Nguyen, T.H.; Le, D.D.; Nguyen, N.H.; Van Nguyen, T.; Tran, P.H. Continuous flow synthesis of HMF from glucose using gadolinium (III) trifluoromethanesulfonate in Brønsted acidic ionic liquid as a catalytic system. J. Flow Chem. 2022, 13, 121–132. [Google Scholar] [CrossRef]
- Phan, H.B.; Luong, C.M.; Nguyen, L.P.; Bui, B.T.; Nguyen, H.T.; Mai, B.V.; Mai, T.V.T.; Huynh, L.K.; Tran, P.H. Eco-Friendly Synthesis of 5-Hydroxymethylfurfural and Its Applications as a Starting Material to Synthesize Valuable Heterocyclic Compounds. ACS Sustain. Chem. Eng. 2022, 10, 8673–8684. [Google Scholar] [CrossRef]
- Phan, H.B.; Thi Nguyen, Q.B.; Luong, C.M.; Tran, K.N.; Tran, P.H. A green and highly efficient synthesis of 5-hydroxymethylfurfural from monosaccharides using a novel binary ionic liquid mixture. J. Mol. Catal. 2021, 503, 111428. [Google Scholar] [CrossRef]
- Zunita, M.; Yuan, D.M.; Syafi’ Laksono, A. Glucose conversion into hydroxymethylfurfural via ionic liquid-based processes. Adv. Chem. Eng. 2022, 11, 100307. [Google Scholar] [CrossRef]
- Nguyen, D.; Van Huynh, T.; Nguyen, V.S.; Cao, P.-L.D.; Nguyen, H.T.; Wei, T.-C.; Tran, P.H.; Nguyen, P.T. Choline chloride-based deep eutectic solvents as effective electrolytes for dye-sensitized solar cells. RSC Adv. 2021, 11, 21560–21566. [Google Scholar] [CrossRef]
- Nguyen, H.T.; Le, T.V.; Tran, P.H. AC-SO3H/[CholineCl][Urea]2 as a green catalytic system for the synthesis of pyrano[2,3-c]pyrazole scaffolds. J. Environ. Chem. Eng. 2021, 9, 105228. [Google Scholar] [CrossRef]
- Yue, J.; Zhu, Z.; Yi, J.; Li, H.; Chen, B.; Rao, J. One-step extraction of oat protein by choline chloride-alcohol deep eutectic solvents: Role of chain length of dihydric alcohol. Food Chem. 2022, 376, 131943. [Google Scholar] [CrossRef] [PubMed]
- Pham, P.T.; Nguyen, H.T.; Nguyen, T.T.; Nguyen, L.H.; Dang, M.-H.D.; Doan, T.L.; Pham, D.D.; Nguyen, C.T.; Tran, P.H. Rapid and Simple Microwave-Assisted Synthesis of Benzoxazoles Catalyzed by [CholineCl][Oxalic Acid]. Catalysts 2022, 12, 1394. [Google Scholar] [CrossRef]
- Nguyen, T.-H.T.; Phan, H.B.; Nguyen, T.H.; Tran, K.N.; Nguyen, L.H.T.; Doan, T.L.H.; Tran, P.H. Conversion of cellulose into valuable chemicals using sulfonated amorphous carbon in 1-ethyl-3-methylimidazolium chloride. RSC Adv. 2023, 13, 7257–7266. [Google Scholar] [CrossRef]
- Guo, X.; Zhu, H.; Si, Y.; Lyu, X.; Cheng, Y.; Zheng, L.; Wang, L.; Li, X. Conversion of Glucose to 5-Hydroxymethylfurfural in Deep Eutectic Solvent of Choline Chloride–Chromium Chloride. Ind. Eng. Chem. Res. 2022, 61, 7216–7224. [Google Scholar] [CrossRef]
- Song, X.; Wang, L.; Liu, L.; Li, J.; Wu, X. Impact of tea tree essential oil and citric acid/choline chloride on physical, structural and antibacterial properties of chitosan-based films. Food Control 2022, 141, 109186. [Google Scholar] [CrossRef]
- Zhou, L.; Liang, R.; Ma, Z.; Wu, T.; Wu, Y. Conversion of cellulose to HMF in ionic liquid catalyzed by bifunctional ionic liquids. Bioresour. Technol. 2013, 129, 450–455. [Google Scholar] [CrossRef] [PubMed]
- Olivito, F.; Algieri, V.; Tallarida, M.A.; Jiritano, A.; Costanzo, P.; Maiuolo, L.; De Nino, A. High-yield synthesis of HMF from glucose and fructose by selective catalysis with water-tolerant rare earth metal triflates assisted by choline chloride. Green Chem. 2023, 25, 1679–1689. [Google Scholar] [CrossRef]
- Pidko, E.A.; Degirmenci, V.; van Santen, R.A.; Hensen, E.J.M. Coordination Properties of Ionic Liquid-Mediated Chromium(II) and Copper(II) Chlorides and Their Complexes with Glucose. Inorg. Chem. Commun. 2010, 49, 10081–10091. [Google Scholar] [CrossRef] [PubMed]
- Meilleur, F.; Snell, E.H.; van der Woerd, M.J.; Judge, R.A.; Myles, D.A.A. A quasi-Laue neutron crystallographic study of d-xylose isomerase. Eur. Biophys. J. 2006, 35, 601–609. [Google Scholar] [CrossRef]
- Fenn, T.D.; Ringe, D.; Petsko, G.A. Xylose Isomerase in Substrate and Inhibitor Michaelis States: Atomic Resolution Studies of a Metal-Mediated Hydride Shift. Biochemistry 2004, 43, 6464–6474. [Google Scholar] [CrossRef]
- Hou, Q.; Li, W.; Ju, M.; Liu, L.; Chen, Y.; Yang, Q. One-pot synthesis of sulfonated graphene oxide for efficient conversion of fructose into HMF. RSC Adv. 2016, 6, 104016–104024. [Google Scholar] [CrossRef]
- Rusanen, A.; Lahti, R.; Lappalainen, K.; Kärkkäinen, J.; Hu, T.; Romar, H.; Lassi, U. Catalytic conversion of glucose to 5-hydroxymethylfurfural over biomass-based activated carbon catalyst. Catal. 2020, 357, 94–101. [Google Scholar] [CrossRef]
- Parveen, F.; Upadhyayula, S. Efficient conversion of glucose to HMF using organocatalysts with dual acidic and basic functionalities—A mechanistic and experimental study. Fuel Process. Technol. 2017, 162, 30–36. [Google Scholar] [CrossRef]
- Marianou, A.A.; Michailof, C.M.; Pineda, A.; Iliopoulou, E.F.; Triantafyllidis, K.S.; Lappas, A.A. Effect of Lewis and Brønsted acidity on glucose conversion to 5-HMF and lactic acid in aqueous and organic media. Appl. Catal. 2018, 555, 75–87. [Google Scholar] [CrossRef]
- He, O.; Zhang, Y.; Wang, P.; Liu, L.; Wang, Q.; Yang, N.; Li, W.; Champagne, P.; Yu, H. Experimental and Kinetic Study on the Production of Furfural and HMF from Glucose. Catalysts 2021, 11, 11. [Google Scholar] [CrossRef]
- Huang, T.; Zhang, H.-X.; Zhang, X.-H.; Peng, D.-Y.; Nie, X.-L.; Chen, J.; Xiong, W.-M. Preparation of functionalized diallylimidazole ionic liquid and its application in conversion of D-fructose into HMF. J. Mol. Liq. 2022, 345, 118233. [Google Scholar] [CrossRef]
- Skulcova, A.; Russ, A.; Jablonsky, M.; Sima, J.J.B. The pH behavior of seventeen deep eutectic solvents. BioResources 2018, 13, 5042–5051. [Google Scholar] [CrossRef]
- Saha, S.K.; Dey, S.; Chakraborty, R. Effect of choline chloride-oxalic acid based deep eutectic solvent on the ultrasonic assisted extraction of polyphenols from Aegle marmelos. J. Mol. Liq. 2019, 287, 110956. [Google Scholar] [CrossRef]


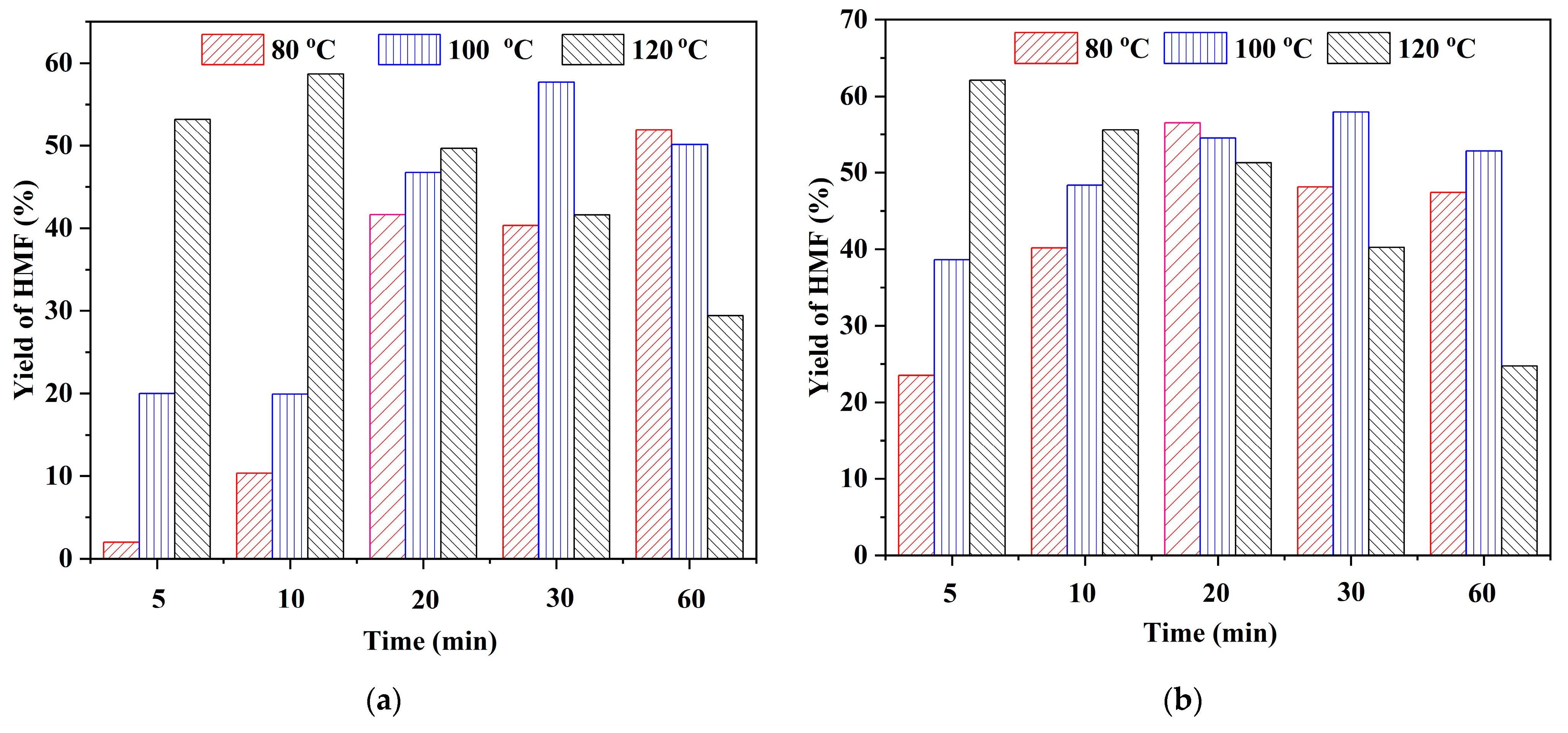

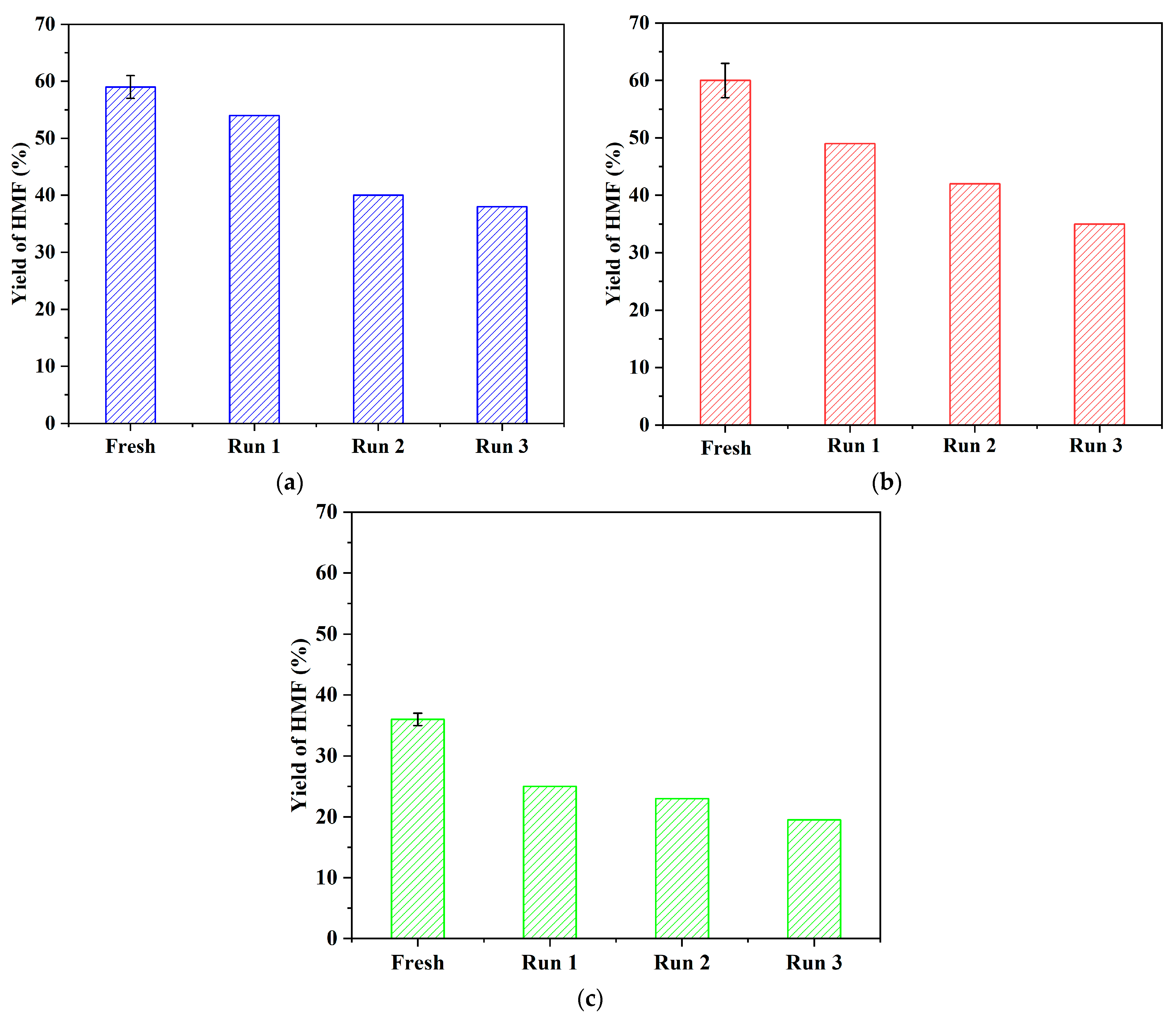
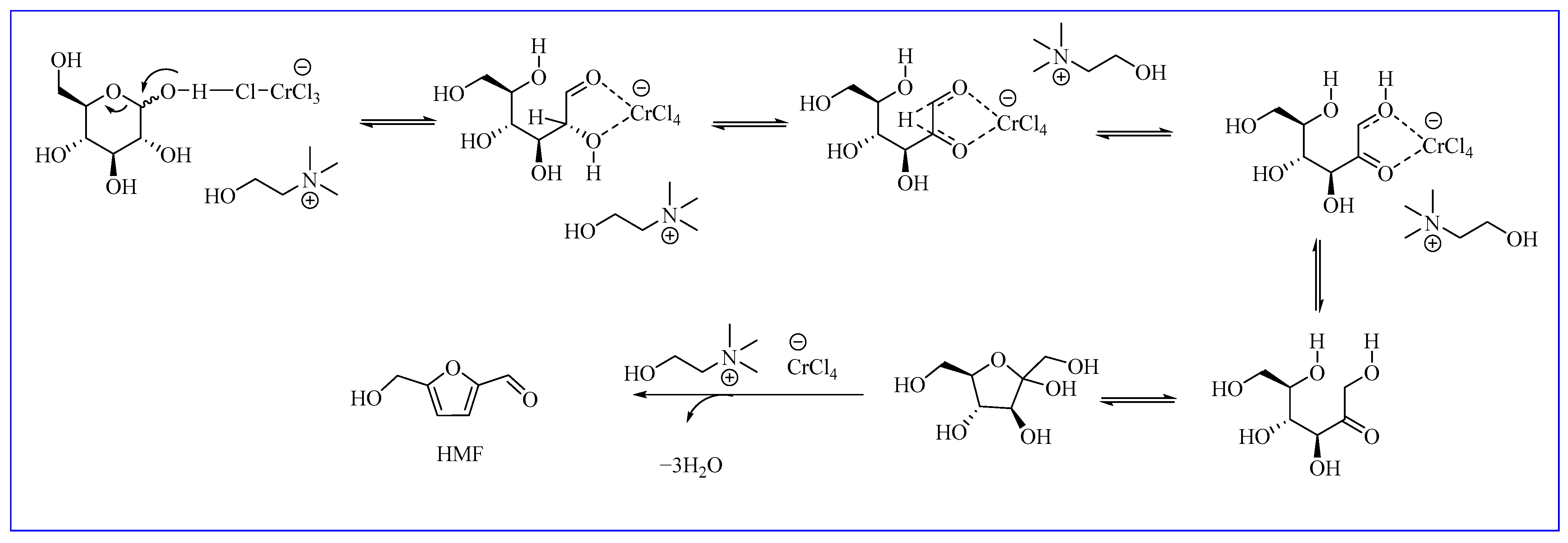
| Entry | Substrates | Reaction Conditions | Time/Temperature | Yield of HMF |
|---|---|---|---|---|
| 1 | Fructose [36] | SGO/DMSO | 60 min/120 °C | 81 |
| 2 | Glucose [37] | ACB2/THF-H2O | 8 h/160 °C | 51 |
| 3 | Glucose [38] | Sulfanilic acid/MIBK-DMSO | 30 min/160 °C | 44 |
| 4 | Glucose [39] | Sn20/γ-Al2O3/H2O-DMSO | 60 min/150 °C | 27.5 |
| 5 | Glucose [40] | SnSO4-H2SO4 | 10 min/170 °C | 34 |
| 6 | Fructose [41] | DMSO-C2DAIMF | 10 min/195 °C | 73.71 |
| 7 | Fructose (This work) | [CholineCl][Citric acid] or [CholineCl][Oxalic acid] | 5 min/120 °C | 62/59 |
| Glucose (This work) | [CholineCl][CrCl3] | 5 min/140 °C | 37 |
| Entry | HBA | HBD | Ratio | Time (min) |
|---|---|---|---|---|
| 1 | Choline Chloride | Oxalic acid | 1:1 | 5 |
| 2 | Citric acid | 1:1 | 10 | |
| 3 | Fumaric acid | 2:1 | 25 | |
| 4 | Adipic acid | 1:1 | 15 | |
| 5 | Benzoic acid | 1:1 | 15 | |
| 6 | Malonic acid | 1:1 | 20 | |
| 7 | Zinc chloride | 1:3 | 15 | |
| 8 | Iron (III) chloride | 1:1 | 15 | |
| 9 | Chromium (III) chloride hexahydrate | 1:1 | 15 | |
| 10 | Tin (II) chloride dihydrate | 1:2 | 20 | |
| 11 | Copper dichloride dihydrate | 1:1 | 20 | |
| 12 | Tin (II) chloride dihydrate | 1:1 | 5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
To, L.N.; Nguyen, T.H.; Nguyen, T.P.; Phan, H.B.; Nguyen, L.H.T.; Doan, T.L.H.; Dang, C.V.; Tran, P.H. Efficient Conversion of Monosaccharides into 5-Hydroxymethylfurfural Using Acidic Deep Eutectic Solvents. Catalysts 2023, 13, 1216. https://doi.org/10.3390/catal13081216
To LN, Nguyen TH, Nguyen TP, Phan HB, Nguyen LHT, Doan TLH, Dang CV, Tran PH. Efficient Conversion of Monosaccharides into 5-Hydroxymethylfurfural Using Acidic Deep Eutectic Solvents. Catalysts. 2023; 13(8):1216. https://doi.org/10.3390/catal13081216
Chicago/Turabian StyleTo, Linh Ngoc, Trinh Hao Nguyen, Thien Phuoc Nguyen, Ha Bich Phan, Linh Ho Thuy Nguyen, Tan Le Hoang Doan, Chinh Van Dang, and Phuong Hoang Tran. 2023. "Efficient Conversion of Monosaccharides into 5-Hydroxymethylfurfural Using Acidic Deep Eutectic Solvents" Catalysts 13, no. 8: 1216. https://doi.org/10.3390/catal13081216
APA StyleTo, L. N., Nguyen, T. H., Nguyen, T. P., Phan, H. B., Nguyen, L. H. T., Doan, T. L. H., Dang, C. V., & Tran, P. H. (2023). Efficient Conversion of Monosaccharides into 5-Hydroxymethylfurfural Using Acidic Deep Eutectic Solvents. Catalysts, 13(8), 1216. https://doi.org/10.3390/catal13081216







