Highly Efficient RGO-Supported Pd Catalyst for Low Temperature Hydrocarbon Oxidation
Abstract
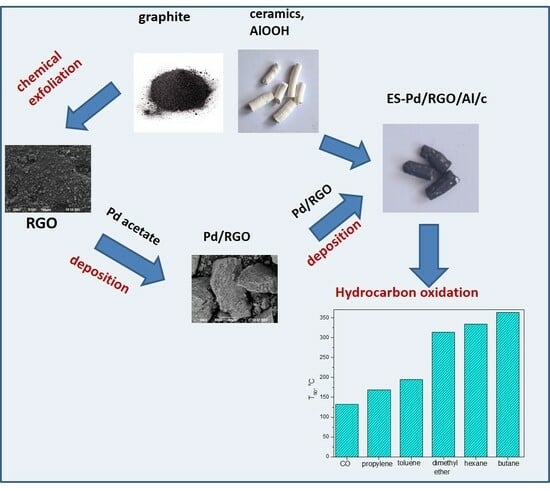
:1. Introduction
2. Results and Discussion
2.1. Scanning Electron Microscopy
2.2. Low-Temperature Nitrogen Adsorption
2.3. Powder X-ray Diffraction
2.4. Transmission Electron Microscopy
2.5. X-ray Photoelectron Spectroscopy
2.6. Catalytic Tests of Pd/RGO–Al
2.7. Catalytic Tests of ES-Pd/RGO/Al/c
2.8. CO Chemisorption on ES-Pd/RGO/Al/c
2.9. Kinetic Studies on ES-Pd/RGO/Al/c
3. Materials and Methods
3.1. Materials
3.2. Characterization
3.3. Catalytic Tests
3.4. Reactor Model for Simulation of the Process of Toluene Abatement in Waste Gases
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Nomenclature
| Cg | concentration of toluene at working conditions, mol/m3; |
| Dr | radial diffusion coefficient, m2/s; |
| effective radial dispersion coefficient for heat, m2/s; | |
| ΔHr | heat of reaction, J/mol; |
| Cp | specific heat capacity, J/K.kg; |
| ur | gas velocity at radial coordinate r, m/s; |
| R(C,T) | reaction rate, mol/m3.s; |
| r | radial coordinate, m; |
| z | axial coordinate, m; |
| effective radial thermal conductivity, W/m.K; | |
| ρ | gas density, kg/m3. |
References
- Finlayson-Pitts, B.J.; Pitts, N.J. Tropospheric air pollution: Ozone, airborne toxics, polycyclic aromatichydrocarbons, and particles. Science 1997, 276, 1045–1051. [Google Scholar] [CrossRef]
- Iranpour, R.; Cox, H.H.J.; Deshusses, M.A.; Schroeder, E.D. Literature review of air pollution control biofilters and biotrickling filters for odor and volatile organic compound removal. Environ. Prog. 2005, 24, 254–267. [Google Scholar] [CrossRef]
- Tomatis, M.; Xu, H.-H.; He, J.; Zhang, X.-D. Recent development of catalysts for removal of volatile organic compounds in flue gas by combustion: A review. J. Chem. 2016, 2016, 8324826. [Google Scholar] [CrossRef]
- Vu, V.H.; Belkouch, J.; Ould-Dris, A.; Taouk, B. Removal of hazardous chlorinated VOCs over Mn-Cu mixed oxide based catalyst. J. Hazard Mater. 2009, 169, 758–765. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Jiang, Z.; Shangguan, W. Low-temperature catalysis for VOCs removal in technology and application: A state-of-the-art review. Catal. Today. 2016, 264, 270–278. [Google Scholar] [CrossRef]
- He, C.; Cheng, L.; Zhang, X.; Douthwaite, M.; Pattisson, S.; Hao, Z. Recent Advances in the Catalytic Oxidation of Volatile Organic Compounds: A Review Based on Pollutant Sorts and Sources. Chem. Rev. 2019, 119, 4471–4568. [Google Scholar] [CrossRef] [PubMed]
- Song, S.; Zhang, S.; Zhang, X.; Verma, P.; Wen, M. Advances in Catalytic Oxidation of Volatile Organic Compounds over Pd-Supported Catalysts: Recent Trends and Challenges. Front. Mater. 2020, 7, 595667. [Google Scholar] [CrossRef]
- Liotta, L.F. Catalytic oxidation of volatile organic compounds on supported noble metals. Appl. Catal. B Environ. 2010, 100, 403–412. [Google Scholar] [CrossRef]
- Fu, Z.; Liu, L.; Song, Y.; Ye, Q.; Cheng, S.; Kang, T.; Dai, H. Catalytic oxidation of carbon monoxide, toluene, and ethyl acetate over the xPd/OMS-2 catalysts: Effect of Pd loading. Front. Chem. Sci. Eng. 2017, 11, 185–196. [Google Scholar] [CrossRef]
- Liu, R.; Wu, H.; Shi, J.; Xu, X.; Zhao, D.; Ng, Y.H.; Zhang, M.; Liu, S.; Ding, H. Recent progress on catalysts for catalytic oxidation of volatile organic compounds: A review. Catal. Sci. Technol. 2022, 12, 6945–6991. [Google Scholar] [CrossRef]
- Liu, Y.; Deng, J.; Xie, S.; Wang, Z.; Dai, H. Catalytic removal of volatile organic compounds using ordered porous transition metal oxide and supported noble metal catalysts. Chin. J. Catal. 2016, 37, 1193–1205. [Google Scholar] [CrossRef]
- Tian, Z.-Y.; Ngamou, P.H.T.; Vannier, V.; Kohse-Höinghaus, K.; Bahlawane, N. Catalytic oxidation of VOCs over mixed Co–Mn oxides. Appl. Catal. B Environ. 2012, 117–118, 125–134. [Google Scholar] [CrossRef]
- Kamal, M.S.; Razzak, S.A.; Hossain, M.M. Catalytic oxidation of volatile organic compounds (VOCs)-a review. Atmos. Environ. 2016, 140, 117–134. [Google Scholar] [CrossRef]
- Xiong, H.F.; Wiebenga, M.H.; Carrillo, C.; Gaudet, J.R.; Pham, H.N.; Kunwar, D.; Oh, S.H.; Qi, G.; Kim, C.H.; Datye, A.K. Design considerations for low-temperature hydrocarbon oxidation reactions on Pd based catalysts. Appl. Catal. B Environ. 2018, 236, 436–444. [Google Scholar] [CrossRef]
- Odoom-Wubah, T.; Li, Q.; Adilov, I.; Huang, J.; Li, Q. Towards efficient Pd/Mn3O4 catalyst with enhanced acidic sites and low temperature reducibility for benzene abatement. Mol. Catal. 2019, 477, 110558. [Google Scholar] [CrossRef]
- Bendahou, K.; Cherif, L.; Siffert, S.; Tidahy, H.L.; Benaïssa, H.; Aboukaïs, A. The effect of the use of lanthanum-doped mesoporous SBA-15 on the performance of Pt/SBA-15 and Pd/SBA-15 catalysts for total oxidation of toluene. Appl. Catal. Gen. 2008, 351, 82–87. [Google Scholar] [CrossRef]
- González-Velasco, J.R.; Aranzabal, A.; López-Fonseca, R.; Ferret, R.; González-Marcos, J.A. Enhancement of the catalytic oxidation of hydrogen-lean chlorinated VOCs in the presence of hydrogen-supplying compounds. Appl. Catal. B Environ. 2000, 33, 24–43. [Google Scholar] [CrossRef]
- Papaefthimiou, P.; Ioannides, T.; Verykios, X.E. Combustion of non-halogenated volatile organic compounds over group VIII metal catalysts. Appl. Catal. B Environ. 1997, 13, 175–184. [Google Scholar] [CrossRef]
- Kim, S.C.; Shim, W.G. Properties and performance of Pd based catalysts for catalytic oxidation of volatile organic compounds. Appl. Catal. B Environ. 2009, 92, 429–436. [Google Scholar] [CrossRef]
- Morales-Torres, S.; Maldonado-Hodar, F.J.; Perez-Cadenas, A.F.; Carrasco-Marín, F. Design of low-temperature Pt-carbon combustion catalysts for VOC’s treatments. J. Hazard. Mater. 2010, 183, 814–822. [Google Scholar] [CrossRef]
- Bell, A.T. The impact of nanoscience on heterogeneous catalysis. Science 2003, 299, 1688–1691. [Google Scholar] [CrossRef]
- Baylet, A.; Royer, S.; Marécot, P.; Tatibouët, J.M.; Duprez, D. Effect of Pd precursor salt on the activity and stability of Pd-doped hexaaluminate catalysts for the CH4 catalytic combustion. Appl. Catal. B Environ. 2008, 81, 88–96. [Google Scholar] [CrossRef]
- Mojet, B.L.; Miller, J.T.; Ramaker, D.E.; Koningsberger, D.C. A New Model Describing the Metal–Support Interaction in Noble Metal Catalysts. J. Catal. 1999, 186, 373–386. [Google Scholar] [CrossRef]
- Stakheev, A.Y.; Kustov, L.M. Effects of the support on the morphology and electronic properties of supported metal clusters: Modern concepts and progress in 1990s. Appl. Catal. A Gen. 1999, 188, 3–35. [Google Scholar] [CrossRef]
- Gelin, P.; Primet, M. Complete oxidation of methane at low temperature over noble metal based catalysts: A review. Appl. Catal. B Environ. 2002, 39, 1–39. [Google Scholar] [CrossRef]
- Mowery, D.L.; Graboski, M.S.; Ohno, T.R.; McCormick, R.L. Deactivation of PdO–Al2O3 oxidation catalyst in lean-burn natural gas engine exhaust: Aged catalyst characterization and studies of poisoning by H2O and SO2. Appl. Catal. B Environ. 1999, 21, 157–169. [Google Scholar] [CrossRef]
- Ruiz, J.A.C.; Fraga, M.A.; Pastore, H.O. Methane combustion over Pd supported on MCM-41. Appl. Catal. B Environ. 2007, 76, 115–122. [Google Scholar] [CrossRef]
- Ciuparu, D.; Lyubovsky, M.R.; Altman, E.; Pfefferle, L.D.; Datye, A. Catalytic combustion of methane over palladium-based catalysts. Catal. Rev. 2002, 44, 593–649. [Google Scholar] [CrossRef]
- Persson, K.; Pfefferle, L.D.; Schwartz, W.; Ersson, A.; Jaras, S.G. Stability of palladium based catalysts during catalytic combustion of methane: The influence of water. Appl. Catal. B Environ. 2007, 74, 242–250. [Google Scholar] [CrossRef]
- Groppi, G. Combustion of CH4 over a PdO/ZrO2 catalyst: An example of kinetic study under severe conditions. Catal. Today 2003, 77, 335–346. [Google Scholar] [CrossRef]
- Chuang, K.T.; Zhou, B.; Tong, S. Kinetics and Mechanism of Catalytic Oxidation of Formaldehyde over Hydrophobic Catalysts. Ind. Eng. Chem. Res. 1994, 33, 1680–1686. [Google Scholar] [CrossRef]
- Araya, P.; Guerrero, S.; Robertson, J.; Gracia, F.J. Methane combustion over Pd/SiO2 catalysts with different degrees of hydrophobicity. Appl. Catal. Gen. 2005, 283, 225–233. [Google Scholar] [CrossRef]
- Sharma, R.K.; Zhou, B.; Tong, S.; Chuang, K.T. Catalytic Destruction of Volatile Organic Compounds Using Supported Platinum and Palladium Hydrophobic Catalysts. Ind. Eng. Chem. Res. 1995, 34, 4310–4317. [Google Scholar] [CrossRef]
- Wu, J.C.S.; Lin, Z.A.; Tsai, F.M.; Pan, J.W. Low-temperature complete oxidation of BTX on Pt/activated carbon catalysts. Catal. Today 2000, 63, 419–426. [Google Scholar] [CrossRef]
- Bedia, J.; Rosas, J.M.; Rodríguez-Mirasol, J.; Cordero, T. Pd supported on mesoporous activated carbons with high oxidation resistance as catalysts for toluene oxidation. Appl. Catal. B Environ. 2010, 94, 8–18. [Google Scholar] [CrossRef]
- Morales-Torres, S.; Perez-Cadenas, A.F.; Kapteijn, F.; Carrasco-Marin, F.; Maldonado-Hodar, F.J.; Moulijn, J.A. Palladium and platinum catalysts supported on carbon nanofiber coated monoliths for low-temperature combustion of BTX. Appl. Catal. B Environ. 2009, 89, 411–419. [Google Scholar] [CrossRef]
- Wu, J.C.S.; Chang, T.Y. VOC deep oxidation over Pt catalysts using hydrophobic supports. Catal. Today 1998, 44, 111–118. [Google Scholar] [CrossRef]
- Thommes, M.; Kaneko, K.; Neimark, A.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S.W. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef]
- De Pedro, Z.M.; Gomez-Sainero, L.M.; Gonzalez-Serrano, E.; Rodriguez, J.J. Gas-Phase Hydrodechlorination of Dichloromethane at Low Concentrations with Palladium/Carbon Catalysts. Ind. Eng. Chem. Res. 2006, 45, 7760–7766. [Google Scholar] [CrossRef]
- Radkevich, V.Z.; Senko, T.L.; Wilson, K.; Grishenko, L.M.; Zaderko, A.N.; Diyuk, V.Y. The influence of surface functionalization of activated carbon on palladium dispersion and catalytic activity in hydrogen oxidation. Appl. Catal. A 2008, 335, 241–251. [Google Scholar] [CrossRef]
- Moulder, J.F.; Stickle, W.F.; Sobol, P.E.; Bomben, K.D. Handbook of X-ray Photoelectron Spectroscopy; Chastain, J., King, R.C., Jr., Eds.; Physical Electronics, Inc.: Eden Prairie, MN, USA, 1995. [Google Scholar]
- Kim, S.C. The catalytic oxidation of aromatic hydrocarbons over supported metal oxide. J. Hazard. Mater. 2002, 91, 285–299. [Google Scholar] [CrossRef] [PubMed]
- Zboray, M.; Bell, A.T.; Iglesia, E. Role of C-H bond strength in the rate and selectivity of oxidative dehydrogenation of alkanes. J. Phys. Chem. C 2009, 113, 12380–12386. [Google Scholar] [CrossRef]
- Deshlahra, P.; Iglesia, E. Reactivity and selectivity descriptors for the activation of C-H bonds in hydrocarbons and oxygenates on metal oxides. J. Phys. Chem. C 2016, 120, 16741–16760. [Google Scholar] [CrossRef]
- Duprat, F. Light-off curve of catalytic reaction and kinetics. Chem. Eng. Sci. 2002, 57, 901–911. [Google Scholar] [CrossRef]
- Markova-Velichkova, M.; Lazarova, T.; Tumbalev, V.; Ivanov, G.; Kovacheva, D.; Stefanov, P.; Naydenov, A. Complete oxidation of hydrocarbons on YFeO3 and LaFeO3 catalysts. Chem. Eng. J. 2013, 231, 236–244. [Google Scholar] [CrossRef]
- Mars, P.; van Krevelen, D.W. Oxidations carried out by means of vanadium oxide catalysts. Spec. Suppl. Chem. Eng. Sci. 1954, 3, 41–59. [Google Scholar] [CrossRef]
- Hurtado, P.; Ordóñez, S.; Sastre, H.; Diez, F.V. Development of a kinetic model for the oxidation of methane over Pd/Al2O3 at dry and wet conditions. Appl. Catal. B Environ. 2004, 51, 229–238. [Google Scholar] [CrossRef]
- Heynderickx, P.; Thybaut, J.; Poelman, H.; Poelman, D.; Marin, G. Kinetic modeling of the total oxidation of propane over anatase and vanadia sputter deposited catalysts. Appl. Catal. B Environ. 2009, 90, 295–306. [Google Scholar] [CrossRef]
- Trung, B.C.; Tu, L.N.Q.; Tri, N.T.M.; An, N.T.; Long, N.Q. Granular-carbon supported nano noble-metal (Au, Pd, Au-Pd): New dual-functional adsorbent/catalysts for effective removal of toluene at low-temperature and humid condition. Environ Technol. 2021, 42, 1772–1786. [Google Scholar] [CrossRef]
- Yazawa, Y.; Yoshida, H.; Takagi, N.; Komai, S.; Satsuma, A.; Hattori, T. Oxidation state of palladium as a factor controlling catalytic activity of Pd/SiO2–Al2O3 in propane combustion. Appl. Catal. B Environ. 1998, 19, 261–266. [Google Scholar] [CrossRef]
- Burch, R.; Loader, P.K.; Urbano, F.J. Some aspects of hydrocarbon activation on platinum group metal combustion catalysts. Catal. Today 1996, 27, 243–248. [Google Scholar] [CrossRef]
- Kim, S.C.; Nahm, S.W.; Shim, W.G.; Lee, J.W.; Moon, H. Influence of physicochemical treatments on spent palladium based catalyst for catalytic oxidation of VOCs. J. Hazard. Mater. 2007, 141, 305–314. [Google Scholar] [CrossRef] [PubMed]
- Velinova, R.; Todorova, S.; Drenchev, B.; Ivanov, G.; Shipochka, M.; Markov, P.; Nihtianova, D.; Kovacheva, D.; Larin, A.V.; Naydenov, A. Complex study of the activity, stability and sulfur resistance of Pd/La2O3-CeO2-Al2O3 system as monolithic catalyst for abatement of methane. Chem. Eng. J. 2019, 368, 865–876. [Google Scholar] [CrossRef]
- Dury, F.; Gaigneaux, E.M.; Ruiz, P. The active role of CO2 at low temperature in oxidation processes: The case of the oxidative dehydrogenation of propane on NiMoO4 catalysts. Appl. Catal. A 2003, 242, 187–203. [Google Scholar] [CrossRef]
- Demoulin, O.; Navez, M.; Mugabo, J.-L.; Ruiz, P. The oxidizing role of CO2 at mild temperature on ceria-based catalysts. Appl. Catal. B Environ. 2007, 70, 284–293. [Google Scholar] [CrossRef]
- Marcano, D.C.; Kosynkin, D.V.; Berlin, J.M.; Sinitskii, A.; Sun, Z.; Slesarev, A.; Alemany, L.B.; Lu, W.; Tour, J.M. Improved synthesis of graphene oxide. ACS Nano 2010, 4, 4806–4814. [Google Scholar] [CrossRef] [PubMed]
- Yoldas, B.E. Alumina gels that form porous transparent Al2O3. J. Mater. Sci. 1975, 10, 1856–1860. [Google Scholar] [CrossRef]
- Bruker, A.X.S. TOPAS V4: General Profile and Structure Analysis Software for Powder Diffraction Data—User’s Manual; Bruker AXS: Karlsruhe, Germany, 2008; Available online: http://algol.fis.uc.pt/jap/TOPAS%204-2%20Users%20Manual.pdf (accessed on 7 July 2023).
- Belfiore, L.A. Transport Phenomena for Chemical Reactor Design; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2003. [Google Scholar]
- Nauman, E.B. Chemical Reactor Design, Optimization, and Scaleup; McGraw-Hill Companies: New York, NY, USA, 2002. [Google Scholar]









| Sample | S 1 m2/g | V 2 cm3/g | Dav 3 nm | Smi 4 m2/g | Sext 4 m2/g | Vmi 4 cm3/g |
|---|---|---|---|---|---|---|
| RGO | 158 | 0.11 | 2.7 | 123 | 35 | 0.05 |
| Pd/RGO | 134 | 0.17 | 5.2 | 28 | 106 | 0.01 |
| RGO-Al | 191 | 0.50 | 10.0 | - | 191 | - |
| Pd/RGO-Al | 189 | 0.47 | 9.5 | - | 189 | - |
| Sample | RGO | PdO (XRD) | PdO (TEM) PDF#41-1107 | Pd (XRD) | Pd (TEM) PDF#46-1043 | γ-Al2O3 |
|---|---|---|---|---|---|---|
| RGO | 100% 13 nm P63/mmc a = 2.447 Å c = 6.676 Å | - | - | - | ||
| Pd/RGO–Al fresh | 5% 12 nm P63/mmc a = 2.478 Å c = 6.725 Å | Not detected <2 nm | (112)-1.70 Å | Not detected <2 nm | (200)-1.98 Å | 95% 6 nm Fd-3 m a = 7.919 Å |
| Pd/RGO–Al used | 5% 12 nm P63/mmc a = 2.455 Å c = 6.767 Å | Not detected <2 nm | (110)-2.16 Å | Not detected <2 nm | (200)-1.97 Å | 95% 6 nm Fd-3 m a = 7.932 Å |
| ES-Pd/RGO/Al/c fresh | 87% 22 nm P63/mmc a = 2.455 Å c = 6.705 Å | 12.5% 10 nm P42/mmc a = 3.040 Å c = 5.308 Å | (002)-2.70 Å (110)-2.17 Å (112)-1.69 Å (211)-1.30 Å | 0.5% 21 nm Fm-3 m a = 3.885 Å | (311)-1.17 Å (222)-1.10 Å | Traces |
| ES-Pd/RGO/Al/c used | 90% 25 nm P63/mmc a = 2.457 Å c = 6.714 Å | 8%, 15 nm P42/mmc a = 3.038 Å c = 5.315 Å | (002)-2.69 Å (110)-2.15 Å (112)-1.70 Å (103)-1.55 Å (004)-1.35 Å | 2%, 38 nm Fm-3 m a = 3.886 Å | (200)-1.98 Å (220)-1.40 Å (311)-1.20 Å | Traces |
| PWL | |||||||
|---|---|---|---|---|---|---|---|
| Ea | ko | m (C7H8) | n (O2) | p (H2O) | RSS | R2 | |
| ES-Pd/RGO/Al/c | 114.9 ± 1.7 | (1.69 ± 0.03)1012 | 0.220 ± 0.003 | 0.490 ± 0.007 | −0.03 ± −0.05 × 10−2 | 2.3 | 0.99 |
| Parameters | Models | ||
|---|---|---|---|
| ER: Water Competes with Toluene | MVK: Water Adsorbs on Oxidized and Reduced Sites, Slow Desorption of Products | LH: Adsorption on Different Sites, Water Competes with Oxygen and Toluene | |
| Ea | 98.6 ± 1.5 | n/a | 122.2 ± 1.8 |
| ko | (5.37 ± 0.08) × 1011 | n/a | (1.01 ± 0.02) × 1013 |
| −ΔHvoc | 68.0 ± 1.0 | n/a | 61.5 ± 0.9 |
| ko.voc | (1.56 ± 0.02) × 10−6 | n/a | (2.14 ± 0.03) × 10−4 |
| −ΔHwat | 51.2 ± 0.8 | n/a | n/a |
| ko.wat | (2.30 ± 0.03) × 10−6 | n/a | n/a |
| Ea.ox | n/a | 132.4 ± 2.0 | n/a |
| ko.ox | n/a | (3.73 ± 0.06) × 1014 | (7.76 ± 0.12) × 10−6 |
| Ea.red | n/a | 41.6 ± 0.62 | n/a |
| ko.red. | n/a | (8.42 ± 0.13) × 106 | n/a |
| −ΔHwat.ox | n/a | 1.13 ± 0.02 | 51.0 ± 0.8 |
| ko.wat. ox | n/a | 3.76 ± 0.06 | (1.10 ± 0.02) × 10−8 |
| −ΔHwat.red | n/a | 14.7 ± 0.2 | 43.2 ± 0.6 |
| ko.wat. red | n/a | (1.93 ± 0.03) × 10−3 | (7.24 ± 0.11) × 10−5 |
| Ea.des | n/a | 46.0 ± 0.7 | n/a |
| −ΔHox | n/a | n/a | 44.2 ± 0.7 |
| RSS | 6.4 | 10.7 | 2 |
| R2 | 0.98 | 0.99 | 0.99 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Velinova, R.; Naydenov, A.; Kichukova, D.; Tumbalev, V.; Atanasova, G.; Kovacheva, D.; Spassova, I. Highly Efficient RGO-Supported Pd Catalyst for Low Temperature Hydrocarbon Oxidation. Catalysts 2023, 13, 1224. https://doi.org/10.3390/catal13081224
Velinova R, Naydenov A, Kichukova D, Tumbalev V, Atanasova G, Kovacheva D, Spassova I. Highly Efficient RGO-Supported Pd Catalyst for Low Temperature Hydrocarbon Oxidation. Catalysts. 2023; 13(8):1224. https://doi.org/10.3390/catal13081224
Chicago/Turabian StyleVelinova, Ralitsa, Anton Naydenov, Diana Kichukova, Ventsislav Tumbalev, Genoveva Atanasova, Daniela Kovacheva, and Ivanka Spassova. 2023. "Highly Efficient RGO-Supported Pd Catalyst for Low Temperature Hydrocarbon Oxidation" Catalysts 13, no. 8: 1224. https://doi.org/10.3390/catal13081224
APA StyleVelinova, R., Naydenov, A., Kichukova, D., Tumbalev, V., Atanasova, G., Kovacheva, D., & Spassova, I. (2023). Highly Efficient RGO-Supported Pd Catalyst for Low Temperature Hydrocarbon Oxidation. Catalysts, 13(8), 1224. https://doi.org/10.3390/catal13081224