Recent Advances in Synergistic Modulation of Transition-Metal-Based Electrocatalysts for Water Oxidation: A Mini Review
Abstract
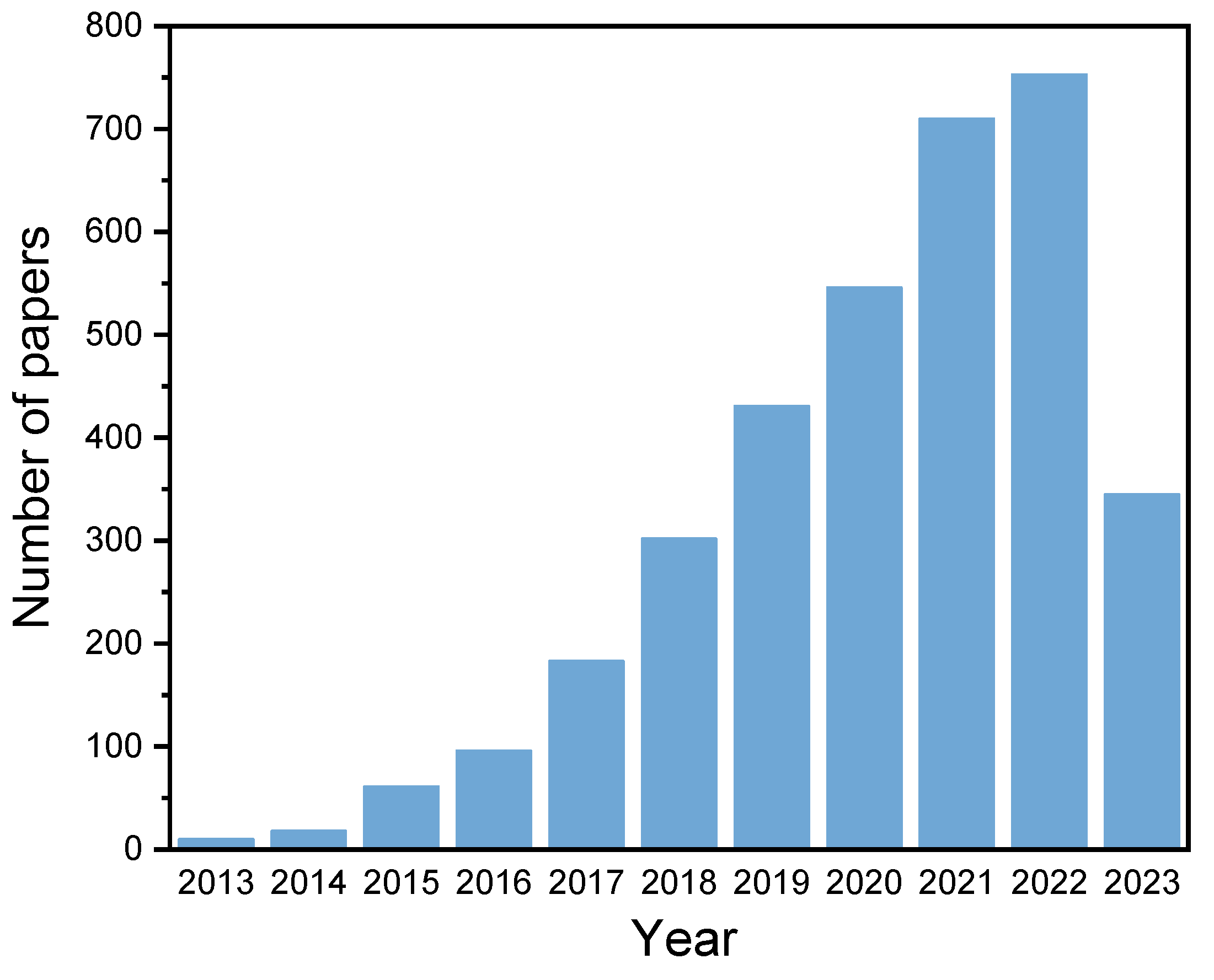
:1. Introduction
2. Development of Transition-Metal-Based OER Electrocatalysts
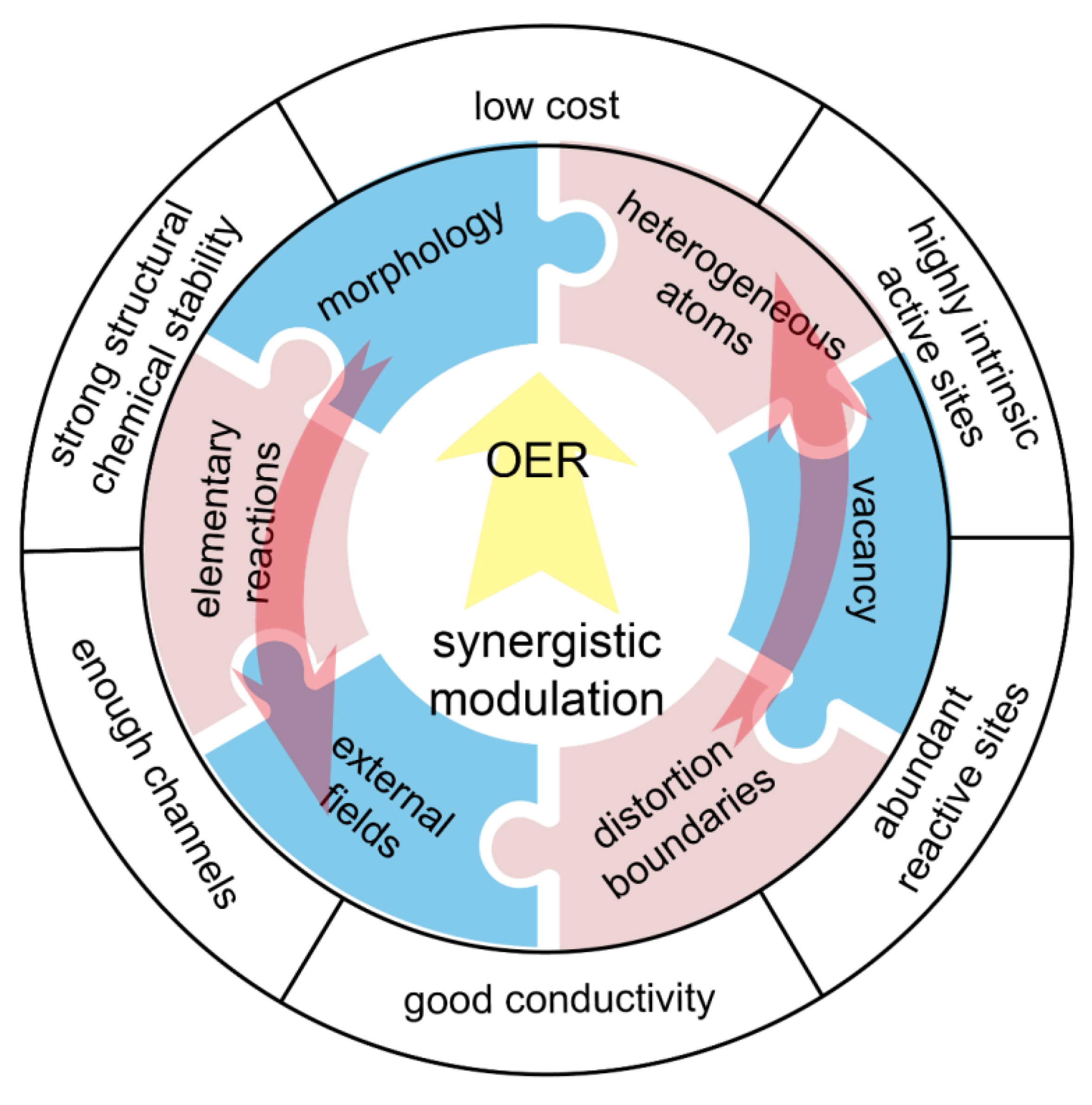
3. Synergistic Modulation on Transition-Metal-Based Electrocatalysts for OER
3.1. Metal Active Sites and Heterogeneous Atoms
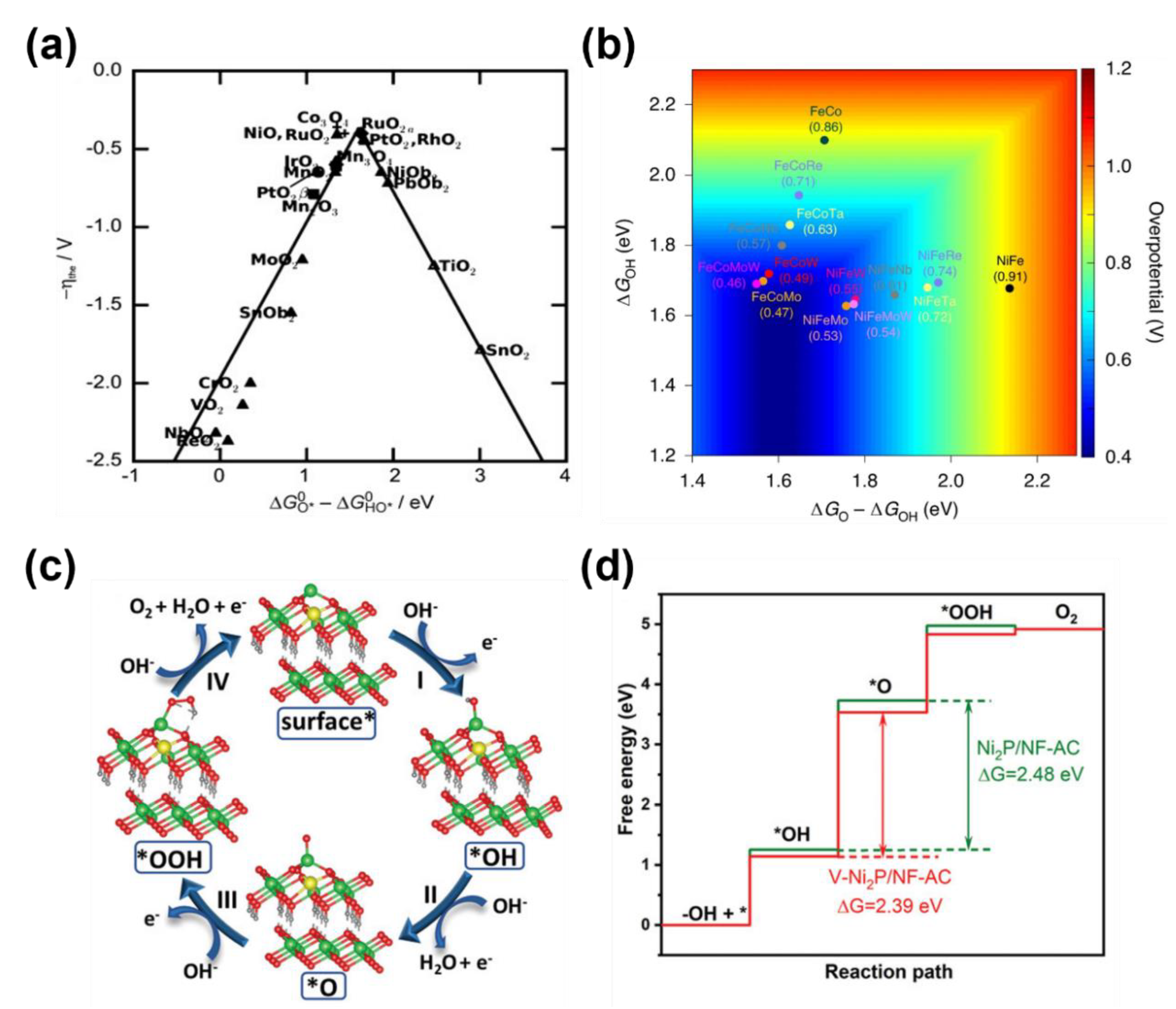
3.1.1. Metal Active Sites and Heterogeneous Metal Atoms
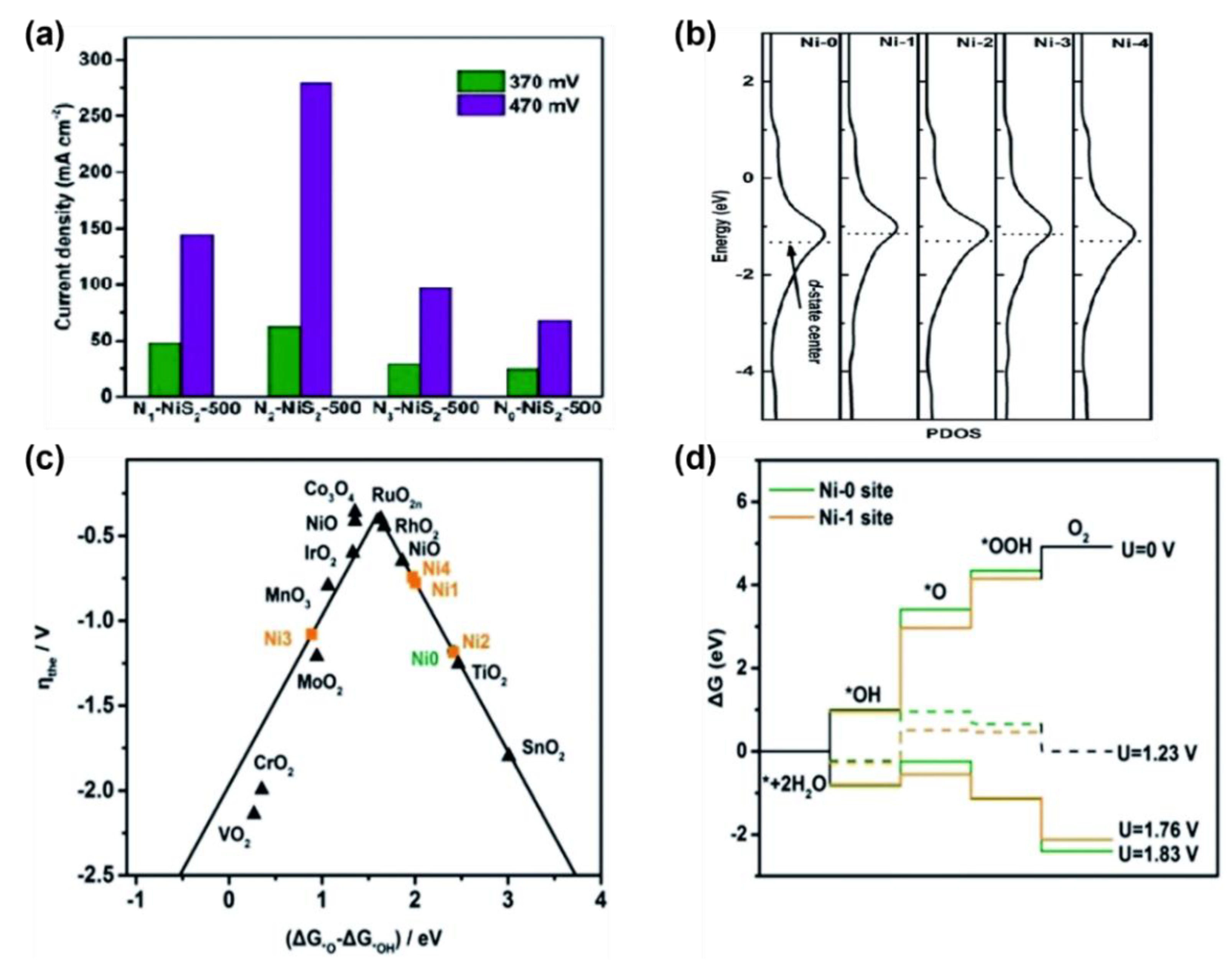
3.1.2. Metal Active Sites and Heterogeneous Non-Metal Atoms
3.2. Heterogeneous Atoms and Crystallographic Structure
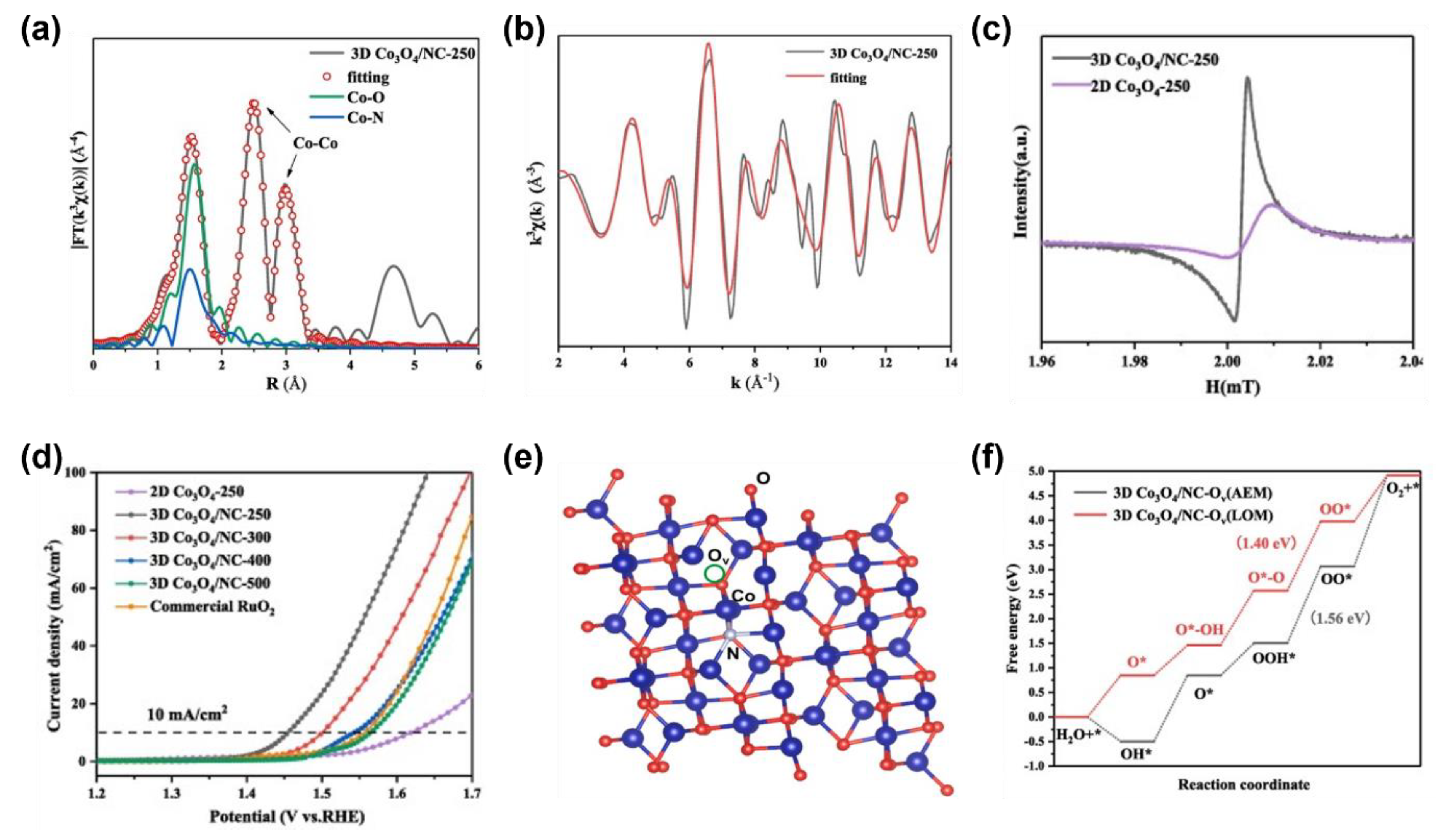
3.2.1. Synergistic Modulation on Heterogeneous Atoms and Cation/Anion Vacancies
3.2.2. Heterogeneous Atoms and Lattice Distortion/Grain Boundaries
3.3. Electronic Structure and Morphology
3.4. Synergistic Modulation on Elementary Reactions
3.5. Synergistic Modulation on External Fields
4. Conclusions and Perspective
Author Contributions
Funding
Conflicts of Interest
References
- Wu, Z.P.; Lu, X.F.; Zang, S.Q.; Lou, X.W. Non-noble-metal-based electrocatalysts toward the oxygen evolution reaction. Adv. Funct. Mater. 2020, 30, 1910274. [Google Scholar] [CrossRef]
- Chen, F.Y.; Wu, Z.Y.; Adler, Z.; Wang, H.T. Stability challenges of electrocatalytic oxygen evolution reaction: From mechanistic understanding to reactor design. Joule 2021, 5, 1704–1731. [Google Scholar] [CrossRef]
- Suen, N.T.; Hung, S.F.; Quan, Q.; Zhang, N.; Xu, Y.J.; Chen, H.M. Electrocatalysis for the oxygen evolution reaction: Recent development and future perspectives. Chem. Soc. Rev. 2017, 46, 337–365. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.C.; Han, C.D.; Gao, J.; Pan, L.; Wu, J.T.; Zhu, X.D.; Zou, J.J. NiCo-based electrocatalysts for the alkaline oxygen evolution reaction: A Review. ACS Catal. 2021, 11, 12485–12509. [Google Scholar] [CrossRef]
- Xie, X.H.; Du, L.; Yon, L.T.; Park, S.Y.; Qiu, Y.; Sokolowski, J.; Wang, W.; Shao, Y.Y. Oxygen evolution reaction in alkaline environment: Material challenges and solutions. Adv. Funct. Mater. 2022, 32, 2110036. [Google Scholar] [CrossRef]
- Yang, Y.; Dang, L.N.; Shearer, M.J.; Sheng, H.Y.; Li, W.J.; Chen, J.; Xiao, P.; Zhang, Y.H.; Hamers, R.J.; Jin, S. Highly active trimetallic NiFeCr layered double hydroxide electrocatalysts for oxygen evolution reaction. Adv. Energy Mater. 2018, 8, 1703189. [Google Scholar] [CrossRef]
- Subbaraman, R.; Tripkovic, D.; Chang, K.C.; Strmcnik, D.; Paulikas, A.P.; Hirunsit, P.; Chan, M.; Greeley, J.; Stamenkovic, V.; Markovic, N.M. Trends in activity for the water electrolyser reactions on 3d M(Ni,Co,Fe,Mn) hydr(oxy)oxide catalysts. Nat. Mater. 2012, 11, 550–557. [Google Scholar] [CrossRef]
- Wu, Z.P.; Zhang, H.B.; Zuo, S.W.; Wang, Y.; Zhang, S.L.; Zhang, J.; Zang, S.Q.; Lou, X.W. Manipulating the local coordination and electronic structures for efficient electrocatalytic oxygen evolution. Adv. Mater. 2021, 33, 2103004. [Google Scholar] [CrossRef]
- Hao, Y.M.; Li, Y.F.; Wu, J.X.; Meng, L.S.; Wang, J.L.; Jia, C.L.; Liu, T.; Yang, X.J.; Liu, Z.P.; Gong, M. Recognition of surface oxygen intermediates on NiFe oxyhydroxide oxygen-evolving catalysts by homogeneous oxidation reactivity. J. Am. Chem. Soc. 2021, 143, 1493–1502. [Google Scholar] [CrossRef]
- Betley, T.A.; Wu, Q.; Van Voorhis, T.; Nocera, D.G. Electronic design criteria for O-O bond formation via metal-oxo complexes. Inorg. Chem. 2008, 47, 1849–1861. [Google Scholar] [CrossRef]
- Hong, W.T.; Risch, M.; Stoerzinger, K.A.; Grimaud, A.; Suntivich, J.; Shao-Horn, Y. Toward the rational design of non-precious transition metal oxides for oxygen electrocatalysis. Energ. Environ. Sci. 2015, 8, 1404–1427. [Google Scholar] [CrossRef]
- Moysiadou, A.; Lee, S.; Hsu, C.S.; Chen, H.M.; Hu, X.L. Mechanism of oxygen evolution catalyzed by cobalt oxyhydroxide: Cobalt superoxide species as a key intermediate and dioxygen release as a rate-determining step. J. Am. Chem. Soc. 2020, 142, 11901–11914. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.; Zhang, Y.J.; Zhu, X.J.; Lu, S.; Long, L.L.; Chen, J.J. Nanostructured metallic FeNi2S4 with reconstruction to generate FeNi-based oxide as a highly-efficient oxygen evolution electrocatalyst. Nano Energy 2021, 81, 105619. [Google Scholar] [CrossRef]
- Liao, H.X.; Luo, T.; Tan, P.F.; Chen, K.J.; Lu, L.L.; Liu, Y.; Liu, M.; Pan, J. Unveiling role of sulfate ion in nickel-iron (oxy)hydroxide with enhanced oxygen-evolving performance. Adv. Funct. Mater. 2021, 31, 2102772. [Google Scholar] [CrossRef]
- Dai, W.J.; Bai, X.W.; Zhu, Y.A.; Zhang, Y.; Lu, T.; Pan, Y.; Wang, J.L. Surface reconstruction induced in situ phosphorus doping in nickel oxides for an enhanced oxygen evolution reaction. J. Mater. Chem. A 2021, 9, 6432–6441. [Google Scholar] [CrossRef]
- Wang, C.Z.; Wang, R.; Peng, Y.; Chen, J.J.; Chen, Z.; Yin, H.B.; Li, J.H. Nb-incorporated Fe(oxy)hydroxide derived from structural transformation for efficient oxygen evolution electrocatalysis. J. Mater. Chem. A 2020, 8, 24598–24607. [Google Scholar] [CrossRef]
- Guo, T.Q.; Li, L.D.; Wang, Z.C. Recent development and future perspectives of amorphous transition metal-based electrocatalysts for oxygen evolution reaction. Adv. Energy Mater. 2022, 12, 2200827. [Google Scholar] [CrossRef]
- Zhang, K.X.; Zou, R.Q. Advanced transition metal-based OER electrocatalysts: Current status, opportunities, and challenges. Small 2021, 17, 2100129. [Google Scholar] [CrossRef]
- Peng, X.; Yan, Y.J.; Jin, X.; Huang, C.; Jin, W.H.; Gao, B.; Chu, P.K. Recent advance and prospectives of electrocatalysts based on transition metal selenides for efficient water splitting. Nano Energy 2020, 78, 105234. [Google Scholar] [CrossRef]
- Yang, B.P.; Liu, K.; Li, H.J.W.; Liu, C.X.; Fu, J.W.; Li, H.M.; Huang, J.E.; Ou, P.F.; Alkayyali, T.; Cai, C.; et al. Accelerating CO2 electroreduction to multicarbon products via synergistic electric-thermal field on copper nanoneedles. J. Am. Chem. Soc. 2022, 144, 3039–3049. [Google Scholar] [CrossRef]
- Wang, Y.H.; Xu, A.; Wang, Z.Y.; Huang, L.S.; Li, J.; Li, F.W.; Wicks, J.; Luo, M.C.; Nam, D.H.; Tan, C.S.; et al. Enhanced nitrate-to-ammonia activity on copper-nickel alloys via tuning of intermediate adsorption. J. Am. Chem. Soc. 2020, 142, 5702–5708. [Google Scholar] [CrossRef] [PubMed]
- Koolen, C.D.; Luo, W.; Zuttel, A. From single crystal to single atom catalysts: Structural factors influencing the performance of metal catalysts for CO2 electroreduction. ACS Catal. 2023, 13, 948–973. [Google Scholar] [CrossRef]
- Zhang, M.D.; Huang, J.R.; Shi, W.; Liao, P.Q.; Chen, X.M. Synergistic effect in a metal-organic framework boosting the electrochemical CO2 overall splitting. J. Am. Chem. Soc. 2023, 145, 2439–2447. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Pham, T.H.; Ko, Y.D.; Li, M.; Yang, S.L.; Koolen, C.D.; Zhong, L.P.; Luo, W.; Zuttel, A. Tandem effect of Ag@C@Cu catalysts enhances ethanol selectivity for electrochemical CO2 reduction in flow reactors. Cell Rep. Phys. Sci. 2022, 3, 100949. [Google Scholar] [CrossRef]
- Lu, F.; Zhou, M.; Zhou, Y.X.; Zeng, X.H. First-row transition metal based catalysts for the oxygen evolution reaction under alkaline conditions: Basic principles and recent advances. Small 2017, 13, 1701931. [Google Scholar] [CrossRef]
- Feng, C.; Faheem, M.B.; Fu, J.; Xiao, Y.Q.; Li, C.L.; Li, Y.B. Fe-based electrocatalysts for oxygen evolution reaction: Progress and perspectives. ACS Catal. 2020, 10, 4019–4047. [Google Scholar] [CrossRef]
- Han, L.; Dong, S.J.; Wang, E.K. Transition-metal (Co, Ni, and Fe)-based electrocatalysts for the water oxidation reaction. Adv. Mater. 2016, 28, 9266–9291. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Park, B.J.; Paidi, V.K.; Huang, R.; Lee, Y.C.; Noh, K.J.; Lee, K.S.; Han, J.W. Precisely constructing orbital coupling-modulated dual-atom Fe pair sites for synergistic CO2 electroreduction. ACS Energy Lett. 2022, 7, 640–649. [Google Scholar] [CrossRef]
- Jia, G.; Wang, Y.; Sun, M.; Zhang, H.; Li, L.; Shi, Y.; Zhang, L.; Cui, X.; Lo, T.W.B.; Huang, B.; et al. Size effects of highly dispersed bismuth nanoparticles on electrocatalytic reduction of carbon dioxide to formic acid. J. Am. Chem. Soc. 2023, 145, 14133–14142. [Google Scholar] [CrossRef]
- Chen, M.X.; Li, H.J.; Wu, C.L.; Liang, Y.B.; Qi, J.; Li, J.; Shangguan, E.; Zhang, W.; Cao, R. Interfacial engineering of heterostructured Co(OH)2/NiPx nanosheets for enhanced oxygen evolution reaction. Adv. Funct. Mater. 2022, 32, 2206407. [Google Scholar] [CrossRef]
- Liu, Y.H.; Chen, G.Y.; Ge, R.Y.; Pei, K.; Song, C.X.; Li, W.X.; Chen, Y.Y.; Zhang, Y.; Feng, L.Y.; Che, R.C. Construction of CoNiFe trimetallic carbonate hydroxide hierarchical hollow microflowers with oxygen vacancies for electrocatalytic water oxidation. Adv. Funct. Mater. 2022, 32, 2200726. [Google Scholar] [CrossRef]
- Song, Y.Y.; Sun, M.Z.; Zhang, S.C.; Zhang, X.Y.; Yi, P.; Liu, J.Z.; Huang, B.L.; Huang, M.H.; Zhang, L.X. Alleviating the work function of vein-like CoXP by Cr doping for enhanced seawater electrolysis. Adv. Funct. Mater. 2023, 33, 2214081. [Google Scholar] [CrossRef]
- Wu, J.S.; Yang, T.; Fu, R.; Zhou, M.; Xia, L.X.; Wang, Z.Y.; Zhao, Y. Constructing electrocatalysts with composition gradient distribution by solubility product theory: Amorphous/crystalline CoNiFe-LDH hollow nanocages. Adv. Funct. Mater. 2023, 2300808. [Google Scholar] [CrossRef]
- Zhang, H.T.; Guo, H.R.; Li, Y.Y.; Zhang, Q.H.; Zheng, L.R.; Gu, L.; Song, R. The guest doping effects of Fe on bimetallic NiCo layered double hydroxide for enhanced electrochemical oxygen evolution reaction: Theoretical screening and experimental verification. Adv. Funct. Mater. 2023, 2304403. [Google Scholar] [CrossRef]
- Song, H.Q.; Yu, J.K.; Tang, Z.Y.; Yang, B.; Lu, S.Y. Halogen-doped carbon dots on amorphous cobalt phosphide as robust electrocatalysts for overall water splitting. Adv. Energy Mater. 2022, 12, 2102573. [Google Scholar] [CrossRef]
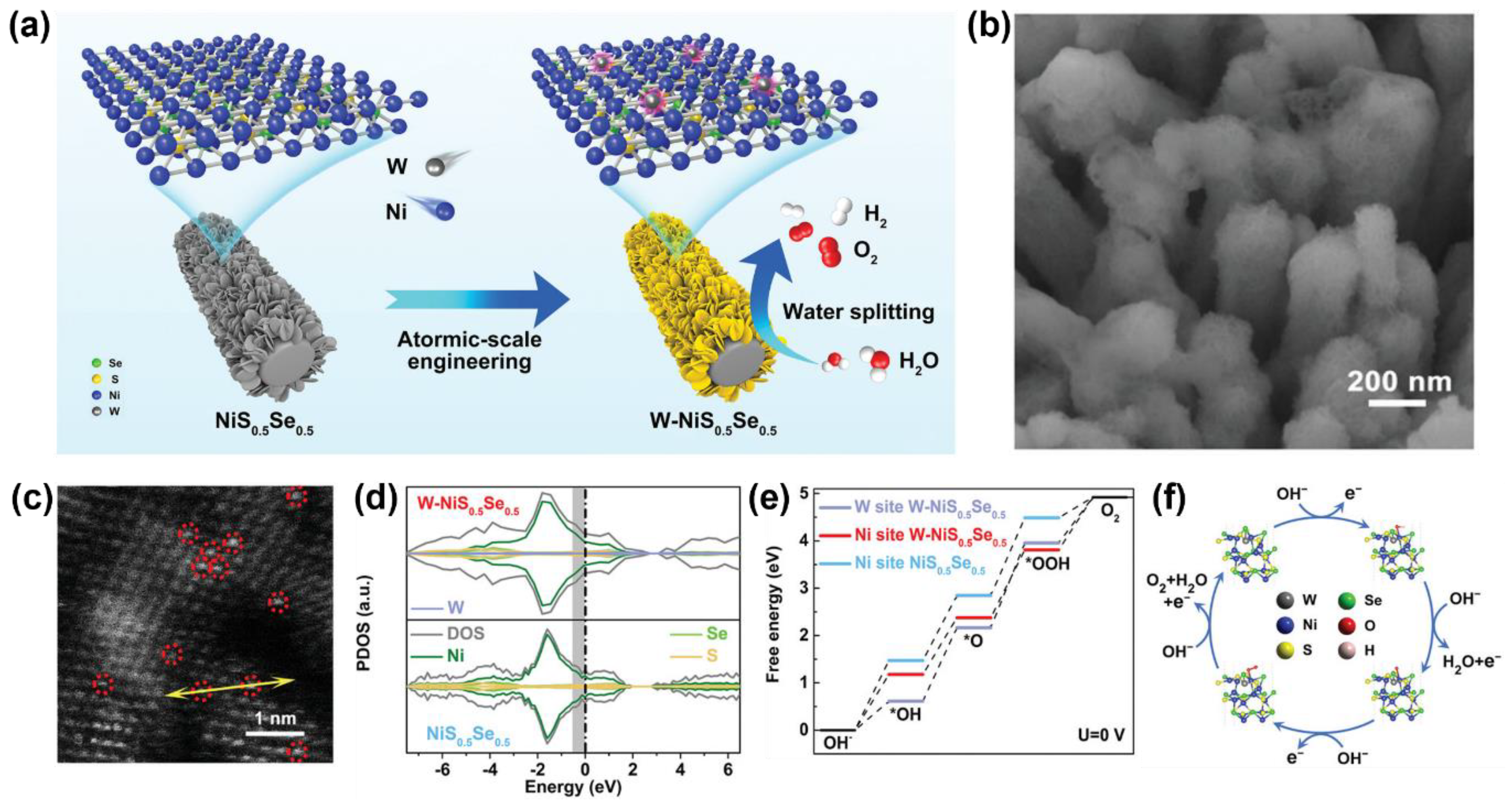
- Wang, Y.; Li, X.P.; Zhang, M.M.; Zhang, J.F.; Chen, Z.L.; Zheng, X.R.; Tian, Z.L.; Zhao, N.Q.; Han, X.P.; Zaghib, K.R.; et al. Highly active and durable single-atom tungsten-doped NiS0.5Se0.5 nanosheet@NiS0.5Se0.5 nanorod heterostructures for water splitting. Adv. Mater. 2022, 34, 2107053. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Mao, Z.L.; Mao, X.; Hu, X.M.; Gao, F.Y.; Gao, M.R.; Wu, Q.L.; Lyu, X.; Du, A.J.; Xu, X.S.; et al. Dual integrating oxygen and sulphur on surface of CoTe nanorods triggers enhanced oxygen evolution reaction. Adv. Sci. 2023, 10, 2206204. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Wu, Z.H.; Shao, W.J.; Gao, Y.; Wang, W.W.; Ma, T.; Ma, L.; Li, S.; Cheng, C.; Zhao, C.S. Interfacial atom-substitution engineered transition-metal hydroxide nanofibers with high-valence Fe for efficient electrochemical water oxidation. Angew. Chem. Int. Edit. 2022, 61, e202115331. [Google Scholar]
- Yao, N.; Wang, G.W.; Jia, H.N.; Yin, J.L.; Cong, H.J.; Chen, S.L.; Luo, W. Intermolecular energy gap-induced formation of high-valent cobalt species in CoOOH surface layer on cobalt sulfides for efficient water oxidation. Angew. Chem. Int. Edit. 2022, 61, e202117178. [Google Scholar] [CrossRef]
- Jo, S.; Park, W.B.; Lee, K.B.; Choi, H.; Lee, K.S.; Ahn, D.; Lee, Y.W.; Sohn, K.S.; Hong, J.; Sohn, J.I. Bi/BiFe(oxy)hydroxide for sustainable lattice oxygen-boosted electrocatalysis at a practical high current density. Appl. Catal. B-Environ. 2022, 317, 121685. [Google Scholar] [CrossRef]
- Meena, A.; Thangavel, P.; Jeong, D.; Singh, A.N.; Jana, A.; Im, H.; Nguyen, D.A.; Kim, K.S. Crystalline-amorphous interface of mesoporous Ni2P@FePOxHy for oxygen evolution at high current density in alkaline-anion-exchange-membrane water-electrolyzer. Appl. Catal. B-Environ. 2022, 306, 121127. [Google Scholar] [CrossRef]
- Liao, H.X.; Zhang, X.D.; Niu, S.W.; Tan, P.F.; Chen, K.J.; Liu, Y.; Wang, G.M.; Liu, M.; Pan, J. Dynamic dissolution and re-adsorption of molybdate ion in iron incorporated nickel-molybdenum oxyhydroxide for promoting oxygen evolution reaction. Appl. Catal. B-Environ. 2022, 307, 121150. [Google Scholar] [CrossRef]
- Wang, J.Q.; Tran, D.T.; Chang, K.; Prabhakaran, S.; Zhao, J.H.; Kim, D.H.; Kim, N.H.; Lee, J.H. Hierarchical Ni@CNTs-bridged MoxC/Ni2P heterostructure micro-pillars for enhanced seawater splitting and Mg/seawater battery. Nano Energy 2023, 111, 108440. [Google Scholar] [CrossRef]
- Lin, L.; Wang, Y.F.; Ye, Q.; Zhao, Y.X.; Cheng, Y.L. Rapid fabrication of FexNi2-xP4O12 and graphene hybrids as electrocatalyst for highly efficient oxygen evolution reaction. Appl. Catal. B-Environ. 2023, 334, 122834. [Google Scholar] [CrossRef]
- Xia, L.C.; Bo, L.L.; Shi, W.P.; Zhang, Y.N.; Shen, Y.X.; Ji, X.C.; Guan, X.L.; Wang, Y.X.; Tong, J.H. Defect and interface engineering of templated synthesis of hollow porous Co3O4/CoMoO4 with highly enhanced electrocatalytic activity for oxygen evolution reaction br. Chem. Eng. J. 2023, 452, 139250. [Google Scholar] [CrossRef]
- Li, H.X.; Zhang, C.Y.; Xiang, W.J.; Amin, M.A.; Na, J.; Wang, S.P.; Yu, J.X.; Yamauchi, Y. Efficient electrocatalysis for oxygen evolution: W-doped NiFe nanosheets with oxygen vacancies constructed by facile electrodeposition and corrosion. Chem. Eng. J. 2023, 452, 139104. [Google Scholar] [CrossRef]
- Wen, S.T.; Huang, J.; Li, T.T.; Chen, W.; Chen, G.L.; Zhang, Q.; Zhang, X.H.; Qian, Q.Y.; Ostrikov, K. Multiphase nanosheet-nanowire cerium oxide and nickel-cobalt phosphide for highly-efficient electrocatalytic overall water splitting. Appl. Catal. B-Environ. 2022, 316, 121678. [Google Scholar] [CrossRef]
- Zhang, L.L.; Rong, J.; Yang, Y.Q.; Zhu, H.Z.; Yu, X.H.; Chen, C.L.; Cheng, H.M.; Liu, G. Activated FeS2@NiS2 core-Shell structure boosting cascade reaction for superior electrocatalytic oxygen evolution. Small 2023, 19, 2207472. [Google Scholar] [CrossRef] [PubMed]
- Zhai, Y.Y.; Ren, X.R.; Sun, Y.; Li, D.; Wang, B.L.; Liu, S. Synergistic effect of multiple vacancies to induce lattice oxygen redox in NiFe-layered double hydroxide OER catalysts. Appl. Catal. B-Environ. 2023, 323, 122091. [Google Scholar] [CrossRef]
- Ai, T.T.; Wang, H.H.; Bao, W.W.; Feng, L.L.; Zou, X.Y.; Wei, X.L.; Liu, D.; Deng, Z.F.; Bin, R. Fe-V synergistic doping effect of hierarchical Ni3S2 oblate-nanorod arrays for efficient electrocatalytic oxygen evolution reaction. Chem. Eng. J. 2022, 450, 138358. [Google Scholar] [CrossRef]
- Bockris, J.O.; Otagawa, T. The electrocatalysis of oxygen evolution on perovskites. J. Electrochem. Soc. 1984, 131, 290–302. [Google Scholar] [CrossRef]
- Suntivich, J.; May, K.J.; Gasteiger, H.A.; Goodenough, J.B.; Shao-Horn, Y. A perovskite oxide pptimized for oxygen evolution catalysis from molecular orbital principles. Science 2011, 334, 1383–1385. [Google Scholar] [CrossRef] [PubMed]
- Dias, J.A.; Andrade, M.A.S.; Santos, H.L.S.; Morelli, M.R.; Mascaro, L.H. Lanthanum-based perovskites for catalytic oxygen evolution reaction. ChemElectroChem 2020, 7, 3173–3192. [Google Scholar] [CrossRef]
- Matienzo, D.D.; Kutlusoy, T.; Divanis, S.; Di Bari, C.; Instuli, E. Benchmarking perovskite electrocatalysts’ OER activity as candidate materials for industrial alkaline water electrolysis. Catalysts 2020, 10, 1387. [Google Scholar] [CrossRef]
- Liu, W.; Kamiko, M.; Yamada, I.; Yagi, S. Electrochemical deposition of amorphous cobalt oxides for oxygen evolution catalysis. RSC Adv. 2022, 12, 8731–8736. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Yuan, J.J.; He, G.Y.; Chen, H.Q. Current and future trends for spinel-type electrocatalysts in electrocatalytic oxygen evolution reaction. Coordin. Chem. Rev. 2023, 475, 214869. [Google Scholar] [CrossRef]
- Chen, S.; Qiao, S.Z. Hierarchically porous nitrogen-doped graphene-NiCo2O4 hybrid paper as an advanced electrocatalytic water-splitting material. ACS Nano 2013, 7, 10190–10196. [Google Scholar] [CrossRef]
- Harada, M.; Kotegawa, F.; Kuwa, M. Structural changes of spinel MCo2O4 (M = Mn, Fe, Co, Ni, and Zn) electrocatalysts during the oxygen evolution reaction investigated by in situ X-ray absorption spectroscopy. ACS Appl. Energ. Mater. 2022, 5, 278–294. [Google Scholar] [CrossRef]
- Yu, J.; Yu, F.; Yuen, M.F.; Wang, C.D. Two-dimensional layered double hydroxides as a platform for electrocatalytic oxygen evolution. J. Mater. Chem. A 2021, 9, 9389–9430. [Google Scholar] [CrossRef]
- Zhou, D.J.; Li, P.S.; Lin, X.; McKinley, A.; Kuang, Y.; Liu, W.; Lin, W.F.; Sun, X.M.; Duan, X. Layered double hydroxide-based electrocatalysts for the oxygen evolution reaction: Identification and tailoring of active sites, and superaerophobic nanoarray electrode assembly. Chem. Soc. Rev. 2021, 50, 8790–8817. [Google Scholar] [CrossRef]
- Zhang, X.; Zhao, Y.F.; Zhao, Y.X.; Shi, R.; Waterhouse, G.I.N.; Zhang, T.R. A simple synthetic strategy toward defect-rich porous monolayer NiFe-layered double hydroxide nanosheets for efficient electrocatalytic water oxidation. Adv. Energy Mater. 2019, 9, 1900881. [Google Scholar] [CrossRef]
- Liang, H.F.; Meng, F.; Caban-Acevedo, M.; Li, L.S.; Forticaux, A.; Xiu, L.C.; Wang, Z.C.; Jin, S. Hydrothermal continuous flow synthesis and exfoliation of NiCo layered double hydroxide nanosheets for enhanced oxygen evolution catalysis. Nano Lett. 2015, 15, 1421–1427. [Google Scholar] [CrossRef] [PubMed]
- Karmakar, A.; Karthick, K.; Kumaravel, S.; Sankar, S.S.; Kundu, S. Enabling and inducing oxygen vacancies in cobalt iron layer double hydroxide via selenization as precatalysts for electrocatalytic hydrogen and oxygen evolution reactions. Inorg. Chem. 2021, 60, 2023–2036. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.F.; Lv, C.D.; Hong, W.Z.; Zhou, X.; Wu, F.G.; Chen, G. Dual tuning of composition and nanostructure of hierarchical hollow nanopolyhedra assembled by NiCo-layered double hydroxide nanosheets for efficient electrocatalytic oxygen evolution. ACS Appl. Energ. Mater. 2019, 2, 312–319. [Google Scholar] [CrossRef]
- Pei, Y.; Ge, Y.C.; Chu, H.; Smith, W.; Dong, P.; Ajayan, P.M.; Ye, M.X.; Shen, J.F. Controlled synthesis of 3D porous structured cobalt-iron based nanosheets by electrodeposition as asymmetric electrodes for ultra-efficient water splitting. Appl. Catal. B-Environ. 2019, 244, 583–593. [Google Scholar] [CrossRef]
- Xue, Z.Q.; Li, X.; Liu, Q.L.; Cai, M.K.; Liu, K.; Liu, M.; Ke, Z.F.; Liu, X.L.; Li, G.Q. Interfacial electronic structure modulation of NiTe nanoarrays with NiS nanodots facilitates electrocatalytic oxygen evolution. Adv. Mater. 2019, 31, 1900430. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.Z.; Zhu, M.; Cao, Z.; Zhu, P.; Cao, Y.; Xu, X.Y.; Xu, C.; Yin, Z.Y. Heterogeneous bimetallic sulfides based seawater electrolysis towards stable industrial-level large current density. Appl. Catal. B-Environ. 2021, 291, 120071. [Google Scholar] [CrossRef]
- Zou, X.X.; Wu, Y.Y.; Liu, Y.P.; Liu, D.P.; Li, W.; Gu, L.; Liu, H.; Wang, P.W.; Sun, L.; Zhang, Y. In situ generation of bifunctional, efficient Fe-based catalysts from mackinawite iron sulfide for water splitting. Chem 2018, 4, 1139–1152. [Google Scholar] [CrossRef]
- Wang, Q.; Zhang, Z.; Cai, C.; Wang, M.Y.; Zhao, Z.L.; Li, M.H.; Huang, X.; Han, S.B.; Zhou, H.; Feng, Z.X.; et al. Single iridium atom doped Ni2P catalyst for optimal oxygen evolution. J. Am. Chem. Soc. 2021, 143, 13605–13615. [Google Scholar] [CrossRef]
- Chai, L.L.; Hu, Z.Y.; Wang, X.; Xu, Y.W.; Zhang, L.J.; Li, T.T.; Hu, Y.; Qian, J.J.; Huang, S.M. Stringing bimetallic metal-organic framework-derived cobalt phosphide composite for high-efficiency overall water splitting. Adv. Sci. 2020, 7, 1903195. [Google Scholar] [CrossRef]
- Li, Y.; Wu, Y.; Hao, H.; Yuan, M.; Lv, Z.; Xu, L.; Wei, B. In situ unraveling surface reconstruction of Ni5P4@FeP nanosheet array for superior alkaline oxygen evolution reaction. Appl. Catal. B: Environ. 2022, 305, 121033. [Google Scholar] [CrossRef]
- Zhang, Y.; Zheng, G.B.; Li, A.L.; Zhu, X.K.; Jiang, J.J.; Zhang, Q.; Deng, L.W.; Gao, X.H.; Ouyang, F.P. Hexagonal single-crystal CoS nanosheets: Controllable synthesis and tunable oxygen evolution performance. Inorg. Chem. 2022, 61, 7568–7578. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.H.; Qiu, F.; Yuan, W.B.; Guo, M.M.; Lu, Z.H. Nitrogen-doped carbon-decorated yolk-shell CoP@FeCoP micro-polyhedra derived from MOF for efficient overall water splitting. Chem. Eng. J. 2021, 403, 126312. [Google Scholar] [CrossRef]
- Sun, Y.H.; Zhao, Y.; Deng, X.Y.; Dai, D.M.; Gao, H.T. An efficient amorphous ternary transition metal boride (WFeNiB) electrocatalyst for oxygen evolution from water. Sustain. Energy Fuels 2022, 6, 1345–1352. [Google Scholar] [CrossRef]
- Dickens, C.F.; Kirk, C.; Norskov, J.K. Insights into the electrochemical oxygen evolution reaction with ab initio calculations and microkinetic modeling: Beyond the limiting potential volcano. J. Phys. Chem. C 2019, 123, 18960–18977. [Google Scholar] [CrossRef]
- Man, I.C.; Su, H.Y.; Calle-Vallejo, F.; Hansen, H.A.; Martinez, J.I.; Inoglu, N.G.; Kitchin, J.; Jaramillo, T.F.; Norskov, J.K.; Rossmeisl, J. Universality in oxygen evolution electrocatalysis on oxide surfaces. Chemcatchem 2011, 3, 1159–1165. [Google Scholar] [CrossRef]
- Niu, S.; Jiang, W.J.; Wei, Z.X.; Tang, T.; Ma, J.M.; Hu, J.S.; Wan, L.J. Se-doping activates FeOOH for cost-effective and efficient electrochemical water oxidation. J. Am. Chem. Soc. 2019, 141, 7005–7013. [Google Scholar] [CrossRef] [PubMed]
- Dou, S.; Tao, L.; Huo, J.; Wang, S.Y.; Dai, L.M. Etched and doped Co9S8/graphene hybrid for oxygen electrocatalysis. Energ. Environ. Sci. 2016, 9, 1320–1326. [Google Scholar] [CrossRef]
- Wang, Y.; Kong, B.; Zhao, D.Y.; Wang, H.T.; Selomulya, C. Strategies for developing transition metal phosphides as heterogeneous electrocatalysts for water splitting. Nano Today 2017, 15, 26–55. [Google Scholar] [CrossRef]
- Zhang, B.; Wang, L.; Cao, Z.; Kozlov, S.M.; de Arquer, F.P.G.; Dinh, C.T.; Li, J.; Wang, Z.Y.; Zheng, X.L.; Zhang, L.S.; et al. High-valence metals improve oxygen evolution reaction performance by modulating 3dmetal oxidation cycle energetics. Nat. Catal. 2020, 3, 985–992. [Google Scholar] [CrossRef]
- Zhao, T.W.; Shen, X.J.; Wang, Y.; Hocking, R.K.; Li, Y.B.; Rong, C.L.; Dastafkan, K.; Su, Z.; Zhao, C. In situ reconstruction of V-doped Ni2P pre-catalysts with tunable electronic structures for water oxidation. Adv. Funct. Mater. 2021, 31, 2100614. [Google Scholar] [CrossRef]
- Wang, X.Y.; Tuo, Y.X.; Zhou, Y.; Wang, D.; Wang, S.T.; Zhang, J. Ta-doping triggered electronic structural engineering and strain effect in NiFe LDH for enhanced water oxidation. Chem. Eng. J. 2021, 403, 126297. [Google Scholar] [CrossRef]
- Zhao, H.Y.; Xia, J.L.; Yin, D.D.; Luo, M.; Yan, C.H.; Du, Y.P. Rare earth incorporated electrode materials for advanced energy storage. Coordin. Chem. Rev. 2019, 390, 32–49. [Google Scholar] [CrossRef]
- Chen, P.; Han, W.; Zhao, M.; Su, J.W.; Li, Z.X.; Li, D.Y.; Pi, L.J.; Zhou, X.; Zhai, T.Y. Recent advances in 2D rare earth materials. Adv. Funct. Mater. 2021, 31, 2008790. [Google Scholar] [CrossRef]
- Sun, Y.; Li, R.; Chen, X.X.; Wu, J.; Xie, Y.; Wang, X.; Ma, K.K.; Wang, L.; Zhang, Z.; Liao, Q.L.; et al. A-site management prompts the dynamic reconstructed active phase of perovskite oxide OER catalysts. Adv. Energy Mater. 2021, 11, 2003755. [Google Scholar] [CrossRef]
- Lee, W.H.; Ko, Y.J.; Kim, J.Y.; Min, B.K.; Hwang, Y.J.; Oh, H.S. Single-atom catalysts for the oxygen evolution reaction: Recent developments and future perspectives. Chem. Commun. 2020, 56, 12687–12697. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Huang, X.; Zhao, Z.L.; Wang, M.Y.; Xiang, B.; Li, J.; Feng, Z.X.; Xu, H.; Gu, M. Ultrahigh-loading of Ir single atoms on NiO matrix to dramatically enhance oxygen evolution reaction. J. Am. Chem. Soc. 2020, 142, 7425–7433. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Wu, B.B.; Wang, Y.Y.; Zhou, W.; Li, Y.Y.; Liu, T.Y.; Xie, C.; Xu, L.T.; Du, S.Q.; Song, M.L.; et al. Deciphering the alternating synergy between interlayer Pt single-atom and NiFe layered double hydroxide for overall water splitting. Energ. Environ. Sci. 2021, 14, 6428–6440. [Google Scholar] [CrossRef]
- Li, S.S.; Wang, L.; Su, H.; Hong, A.N.; Wang, Y.X.; Yang, H.J.; Ge, L.; Song, W.Y.; Liu, J.; Ma, T.Y.; et al. Electron redistributed S-doped nickel iron phosphides derived from one-step phosphatization of MOFs for significantly boosting electrochemical water splitting. Adv. Funct. Mater. 2022, 32, 2200733. [Google Scholar] [CrossRef]
- Hao, J.H.; Yang, W.S.; Hou, J.W.; Mao, B.D.; Huang, Z.P.; Shi, W.D. Nitrogen doped NiS2 nanoarrays with enhanced electrocatalytic activity for water oxidation. J. Mater. Chem. A 2017, 5, 17811–17816. [Google Scholar] [CrossRef]
- Wang, W.H.; Yang, Y.; Huan, D.M.; Wang, L.K.; Shi, N.; Xie, Y.; Xia, C.R.; Peng, R.R.; Lu, Y.L. An excellent OER electrocatalyst of cubic SrCoO3- prepared by a simple F-doping strategy. J. Mater. Chem. A 2019, 7, 12538–12546. [Google Scholar] [CrossRef]
- Zhu, Y.L.; Lin, Q.; Wang, Z.B.; Qi, D.C.; Yin, Y.C.; Liu, Y.; Zhang, X.W.; Shao, Z.P.; Wang, H.T. Chlorine-anion doping induced multi-factor optimization in perovskties for boosting intrinsic oxygen evolution. J. Energy Chem. 2021, 52, 115–120. [Google Scholar] [CrossRef]
- Zhao, Z.B.; Chang, H.N.; Wang, R.Y.; Du, P.; He, X.; Yang, J.K.; Zhang, X.L.; Huang, K.; Fan, D.Y.; Wang, Y.G.; et al. Activity origin and catalyst design principles for electrocatalytic oxygen evolution on layered transition metal oxide with halogen doping. Small Struct. 2021, 2, 2100069. [Google Scholar] [CrossRef]
- Xu, T.Y.; Jiao, D.X.; Zhang, L.; Zhang, H.Y.; Zheng, L.R.; Singh, D.J.; Zhao, J.X.; Zheng, W.T.; Cui, X.Q. Br-induced P-poor defective nickel phosphide for highly efficient overall water splitting. Appl. Catal. B-Environ. 2022, 316, 121686. [Google Scholar] [CrossRef]
- Ghanem, M.A.; Amer, M.S.; Al-Mayouf, A.M.; Arunachalam, P.; Weller, M.T. Halide-doping effect of strontium cobalt oxide electrocatalyst and the induced activity for oxygen evolution in an alkaline solution. Catalysts 2021, 11, 1408. [Google Scholar] [CrossRef]
- Yang, X.; Cheng, J.; Li, H.; Xu, Y.; Tu, W.F.; Zhou, J.H. Self-supported N-doped hierarchical Co3O4 electrocatalyst with abundant oxygen vacancies for acidic water oxidation. Chem. Eng. J. 2023, 465, 142745. [Google Scholar] [CrossRef]
- Li, C.F.; Zhao, J.W.; Xie, L.J.; Wu, J.Q.; Li, G.R. Fe doping and oxygen vacancy modulated Fe-Ni5P4/NiFeOH nanosheets as bifunctional electrocatalysts for efficient overall water splitting. Appl. Catal. B-Environ. 2021, 291, 119987. [Google Scholar] [CrossRef]
- Yuan, G.J.; Bai, J.L.; Zhang, L.; Chen, X.; Ren, L.L. The effect of P vacancies on the activity of cobalt phosphide nanorods as oxygen evolution electrocatalyst in alkali. Appl. Catal. B-Environ. 2021, 284, 119693. [Google Scholar] [CrossRef]
- Gao, Q.; Luo, W.; Ma, X.Y.; Ma, Z.M.; Li, S.J.; Gou, F.L.; Shen, W.; Jiang, Y.M.; He, R.X.; Li, M. Electronic modulation and vacancy engineering of Ni9S8 to synergistically boost efficient water splitting: Active vacancy-metal pairs. Appl. Catal. B-Environ. 2022, 310, 121356. [Google Scholar] [CrossRef]
- Tian, L.; Chen, Z.Y.; Wang, T.J.; Cao, M.; Lu, X.H.; Cheng, W.J.; He, C.C.; Wang, J.; Li, Z. Mo doping and Se vacancy engineering for boosting electrocatalytic water oxidation by regulating the electronic structure of self-supported Co9Se8@NiSe. Nanoscale 2022, 15, 259–265. [Google Scholar] [CrossRef]
- Zhu, J.Q.; Ren, Z.Y.; Du, S.C.; Xie, Y.; Wu, J.; Meng, H.Y.; Xue, Y.Z.; Fu, H.G. Co-vacancy-rich Co1-xS nanosheets anchored on rGO for high-efficiency oxygen evolution. Nano Res. 2017, 10, 1819–1831. [Google Scholar] [CrossRef]
- Liu, Y.W.; Cheng, H.; Lyu, M.J.; Fan, S.J.; Liu, Q.H.; Zhang, W.S.; Zhi, Y.D.; Wang, C.M.; Xiao, C.; Wei, S.Q.; et al. Low overpotential in vacancy-rich ultrathin CoSe2 nanosheets for water oxidation. J. Am. Chem. Soc. 2014, 136, 15670–15675. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.W.; Qiao, M.; Lu, Y.R.; Hao, L.; Liu, D.D.; Dong, C.L.; Li, Y.F.; Wang, S.Y. Preferential cation vacancies in perovskite hydroxide for the oxygen evolution reaction. Angew. Chem. Int. Edit. 2018, 57, 8691–8696. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.W.; Zhang, H.; Li, C.F.; Zhou, X.; Wu, J.Q.; Zeng, F.; Zhang, J.W.; Li, G.R. Key roles of surface Fe sites and Sr vacancies in the perovskite for an efficient oxygen evolution reaction via lattice oxygen oxidation. Energ. Environ. Sci. 2022, 15, 3912–3922. [Google Scholar] [CrossRef]
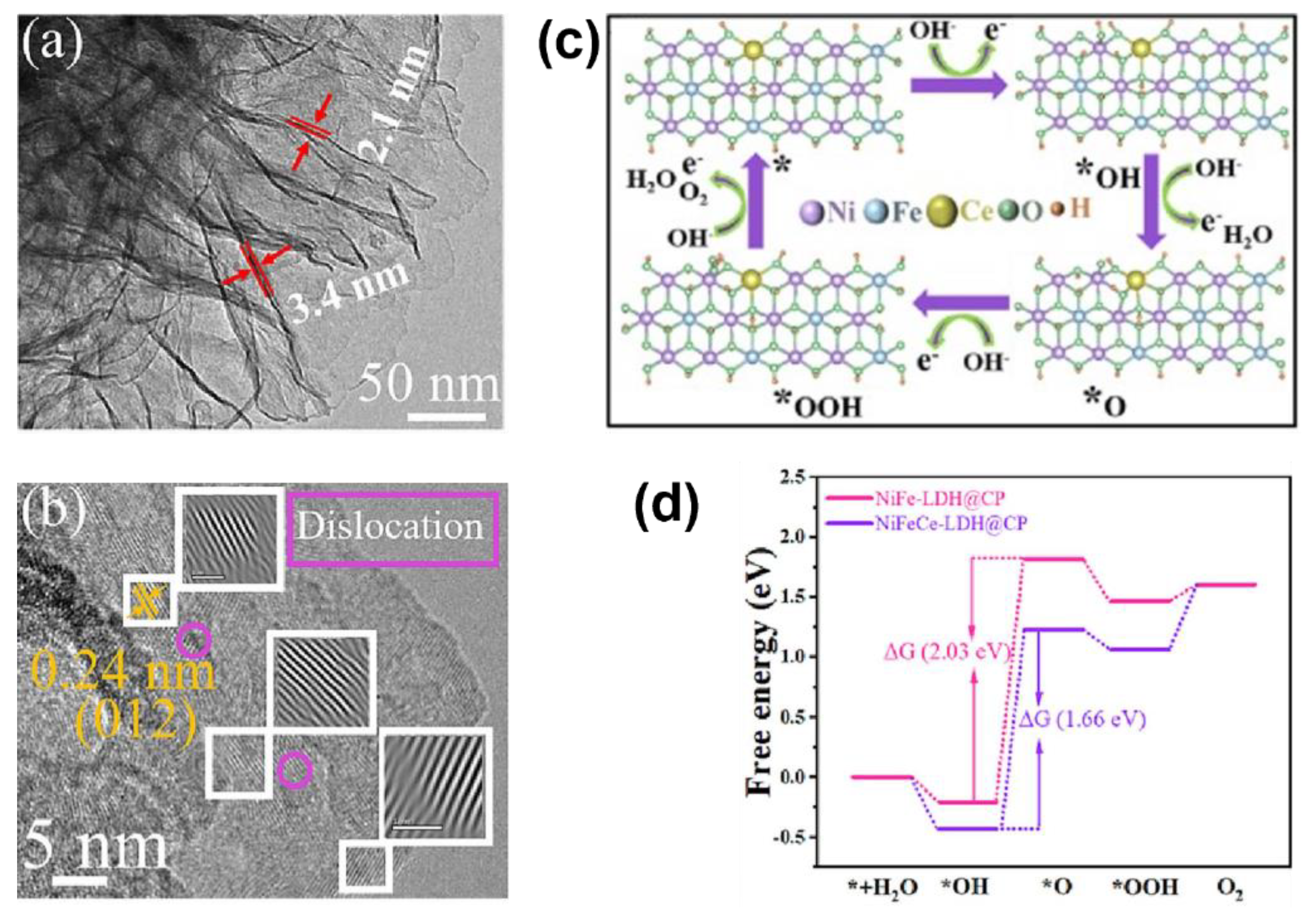
- Liao, Y.Y.; He, R.C.; Pan, W.H.; Li, Y.; Wang, Y.Y.; Li, J.; Li, Y.X. Lattice distortion induced Ce-doped NiFe-LDH for efficient oxygen evolution. Chem. Eng. J. 2023, 464, 142669. [Google Scholar] [CrossRef]
- He, Y.M.; Tang, P.Y.; Hu, Z.L.; He, Q.Y.; Zhu, C.; Wang, L.Q.; Zeng, Q.S.; Golani, P.; Gao, G.H.; Fu, W.; et al. Engineering grain boundaries at the 2D limit for the hydrogen evolution reaction. Nat. Commun. 2020, 11, 57. [Google Scholar] [CrossRef] [PubMed]
- Gu, Y.H.; Wang, X.Y.; Bao, A.T.; Dong, L.; Zhang, X.Y.; Pan, H.J.; Cui, W.Q.; Qi, X.W. Enhancing electrical conductivity of single-atom doped Co3O4 nanosheet arrays at grain boundary by phosphor doping strategy for efficient water splitting. Nano Res. 2022, 15, 9511–9519. [Google Scholar] [CrossRef]
- Qiao, X.S.; Kang, H.J.; Li, Y.; Cui, K.; Jia, X.; Wu, X.H.; Qin, W. Grain boundary density and electronic dual modulation of intermetallic Co2B by Fe doping toward efficient catalyst for oxygen evolution reaction. Appl. Catal. B-Environ. 2022, 305, 121034. [Google Scholar] [CrossRef]
- Khorshidi, A.; Violet, J.; Hashemi, J.; Peterson, A.A. How strain can break the scaling relations of catalysis. Nat. Catal. 2018, 1, 263–268. [Google Scholar] [CrossRef]
- Wang, L.; Zeng, Z.H.; Gao, W.P.; Maxson, T.; Raciti, D.; Giroux, M.; Pan, X.Q.; Wang, C.; Greeley, J. Tunable intrinsic strain in two-dimensional transition metal electrocatalysts. Science 2019, 363, 870–874. [Google Scholar] [CrossRef]
- Wang, H.T.; Xu, S.C.; Tsai, C.; Li, Y.Z.; Liu, C.; Zhao, J.; Liu, Y.Y.; Yuan, H.Y.; Abild-Pedersen, F.; Prinz, F.B.; et al. Direct and continuous strain control of catalysts with tunable battery electrode materials. Science 2016, 354, 1031–1036. [Google Scholar] [CrossRef] [PubMed]
- Ma, K.W.; Chang, X.R.; Wang, Z.H.; Deng, R.C.; Wu, X.; Yang, H. Tunable d-band center of a NiFeMo alloy with enlarged lattice strain enhancing the intrinsic catalytic activity for overall water-splitting. Nanoscale 2023, 15, 5843–5854. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Zhang, L.; Zheng, Y.; Guo, Z.; Zhu, Y.M.; Chen, H.J.; Li, F.; Liu, P.P.; Yu, B.; Wang, X.W.; et al. Uncovering the effect of lattice strain and oxygen deficiency on electrocatalytic activity of perovskite cobaltite thin films. Adv. Sci. 2019, 6, 1801898. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.H.; Li, W.Q.; Guo, H.A.; Yue, M.Y.; Wang, Y.J. Surface synergistic effect of sub-2 nm NiFeCr hydroxide nanodots yielding high oxygen evolution mass activities. Chem. Eng. J. 2023, 461, 141917. [Google Scholar] [CrossRef]
- Wan, K.; Luo, J.S.; Zhou, C.; Zhang, T.; Arbiol, J.; Lu, X.H.; Mao, B.W.; Zhang, X.; Fransaer, J. Hierarchical porous Ni3S4 with enriched high-valence Ni sites as a robust electrocatalyst for efficient oxygen evolution reaction. Adv. Funct. Mater. 2019, 29, 1900315. [Google Scholar] [CrossRef]
- Rossmeisl, J.; Logadottir, A.; Norskov, J.K. Electrolysis of water on (oxidized) metal surfaces. Chem. Phys. 2005, 319, 178–184. [Google Scholar] [CrossRef]
- Montoya, J.H.; Seitz, L.C.; Chakthranont, P.; Vojvodic, A.; Jaramillo, T.F.; Norskov, J.K. Materials for solar fuels and chemicals. Nat. Mater. 2017, 16, 70–81. [Google Scholar] [CrossRef]
- Koper, M.T.M. Theory of multiple proton-electron transfer reactions and its implications for electrocatalysis. Chem. Sci. 2013, 4, 2710–2723. [Google Scholar] [CrossRef]
- Liu, J.Y.; Liu, X.; Shi, H.; Luo, J.H.; Wang, L.; Liang, J.S.; Li, S.Z.; Yang, L.M.; Wang, T.Y.; Huang, Y.H.; et al. Breaking the scaling relations of oxygen evolution reaction on amorphous NiFeP nanostructures with enhanced activity for overall seawater splitting. Appl. Catal. B-Environ. 2022, 302, 120862. [Google Scholar] [CrossRef]
- Liu, H.J.; Luan, R.N.; Li, L.Y.; Lv, R.Q.; Chai, Y.M.; Dong, B. Sulphur-dopant induced breaking of the scaling relation on low-valence Ni sites in nickel ferrite nanocones for water oxidation with industrial-level current density. Chem. Eng. J. 2023, 461, 141714. [Google Scholar] [CrossRef]
- Xu, Q.C.; Zhang, J.H.; Zhang, H.X.; Zhang, L.Y.; Chen, L.; Hu, Y.J.; Jiang, H.; Li, C.Z. Atomic heterointerface engineering overcomes the activity limitation of electrocatalysts and promises highly-efficient alkaline water splitting. Energ. Environ. Sci. 2021, 14, 5228–5259. [Google Scholar] [CrossRef]
- Li, Y.Q.; Yin, Z.H.; Cui, M.; Liu, X.; Xiong, J.B.; Chen, S.R.; Ma, T.L. Interface engineering of transitional metal sulfide-MoS2 heterostructure composites as effective electrocatalysts for water-splitting. J. Mater. Chem. A 2021, 9, 2070–2092. [Google Scholar] [CrossRef]
- Zhang, H.J.; Maijenburg, A.W.; Li, X.P.; Schweizer, S.L.; Wehrspohn, R.B. Bifunctional heterostructured transition metal phosphides for efficient electrochemical water splitting. Adv. Funct. Mater. 2020, 30, 2003261. [Google Scholar] [CrossRef]
- Strasser, P.; Koh, S.; Anniyev, T.; Greeley, J.; More, K.; Yu, C.F.; Liu, Z.C.; Kaya, S.; Nordlund, D.; Ogasawara, H.; et al. Lattice-strain control of the activity in dealloyed core-shell fuel cell catalysts. Nat. Chem. 2010, 2, 454–460. [Google Scholar] [CrossRef] [PubMed]
- Du, X.C.; Huang, J.W.; Zhang, J.J.; Yan, Y.C.; Wu, C.Y.; Hu, Y.; Yan, C.Y.; Lei, T.Y.; Chen, W.; Fan, C.; et al. Modulating electronic structures of inorganic nanomaterials for efficient electrocatalytic water splitting. Angew. Chem. Int. Edit. 2019, 58, 4484–4502. [Google Scholar] [CrossRef]
- Zhang, Y.P.; Gao, F.; Wang, D.Q.; Li, Z.L.; Wang, X.M.; Wang, C.Q.; Zhang, K.W.; Du, Y.K. Amorphous/crystalline heterostructure transition-metal-based catalysts for high-performance water splitting. Coordin. Chem. Rev. 2023, 475, 214916. [Google Scholar] [CrossRef]
- Zhao, G.Q.; Li, P.; Cheng, N.Y.; Dou, S.X.; Sun, W.P. An Ir/Ni(OH)2 heterostructured electrocatalyst for the oxygen evolution reaction: Breaking the scaling relation, stabilizing iridium (V), and beyond. Adv. Mater. 2020, 32, 2000872. [Google Scholar] [CrossRef] [PubMed]
- Van Deelen, T.W.; Mejia, C.H.; de Jong, K.P. Control of metal-support interactions in heterogeneous catalysts to enhance activity and selectivity. Nat. Catal. 2019, 2, 955–970. [Google Scholar] [CrossRef]
- Dong, C.Y.; Li, Y.L.; Cheng, D.Y.; Zhang, M.T.; Liu, J.J.; Wang, Y.G.; Xiao, D.Q.; Ma, D. Supported metal clusters: Fabrication and application in heterogeneous catalysis. ACS Catal. 2020, 10, 11011–11045. [Google Scholar] [CrossRef]
- Serna, P.; Gates, B.C. Molecular metal catalysts on supports: Organometallic chemistry meets surface science. Acc. Chem. Res. 2014, 47, 2612–2620. [Google Scholar] [CrossRef]
- Gorlin, Y.; Chung, C.J.; Benck, J.D.; Nordlund, D.; Seitz, L.; Weng, T.C.; Sokaras, D.; Clemens, B.M.; Jaramillo, T.F. Understanding interactions between manganese oxide and gold that lead to enhanced activity for electrocatalytic water oxidation. J. Am. Chem. Soc. 2014, 136, 4920–4926. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Li, X.P.; Zhong, C.; Zhao, N.Q.; Deng, Y.D.; Han, X.P.; Hu, W.B. Spontaneous synthesis of silver-nanoparticle-decorated transition-metal hydroxides for enhanced oxygen evolution reaction. Angew. Chem. Int. Edit. 2020, 59, 7245–7250. [Google Scholar] [CrossRef] [PubMed]
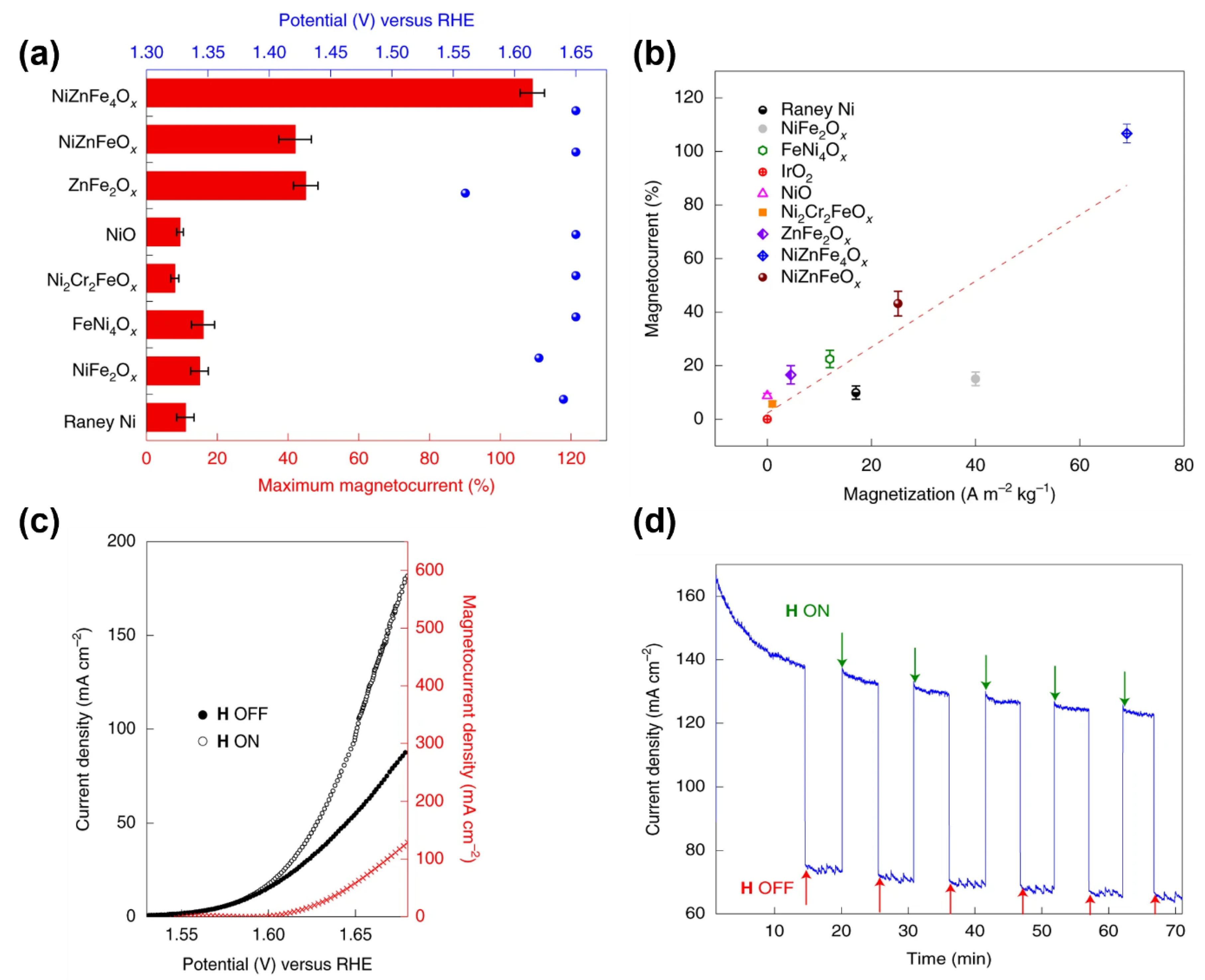
- Garces-Pineda, F.A.; Blasco-Ahicart, M.; Nieto-Castro, D.; Lopez, N.; Galan-Mascaros, J.R. Direct magnetic enhancement of electrocatalytic water oxidation in alkaline media. Nat. Energy 2019, 4, 519–525. [Google Scholar] [CrossRef]
- Meng, H.Y.; Xi, W.; Ren, Z.Y.; Du, S.C.; Wu, J.; Zhao, L.; Liu, B.W.; Fu, H.G. Solar-boosted electrocatalytic oxygen evolution via catalytic site remodelling of CoCr layered double hydroxide. Appl. Catal. B-Environ. 2021, 284, 119707. [Google Scholar] [CrossRef]
- Tian, L.; Chen, H.Y.; Lu, X.H.; Liu, D.S.; Cheng, W.J.; Liu, Y.Y.; Li, J.; Li, Z. Local photothermal and photoelectric effect synergistically boost hollow CeO2/CoS2 heterostructure electrocatalytic oxygen evolution reaction. J. Colloid Interf. Sci. 2022, 628, 663–672. [Google Scholar] [CrossRef] [PubMed]
- Bai, L.; Jia, S.J.; Gao, Y.D.; Li, C.; Chen, X.; Zhou, S.; Han, J.W.; Yang, F.C.; Zhang, X.; Lu, S.Y. A p-n WO3/SnSe2 heterojunction for efficient photo-assisted electrocatalysis of the oxygen evolution reaction. Energy Environ. Mater. 2022, e12456. [Google Scholar] [CrossRef]
- Zhang, Y.J.; Wang, Y.C.; Jiang, H.Q.; Huang, M.H. Multifunctional nickel sulfide nanosheet arrays for solar-intensified oxygen evolution reaction. Small 2020, 16, 2002550. [Google Scholar] [CrossRef]
- Zhai, H.C.; Xu, G.S.; Zhu, C.H.; Yuan, Y.P. Understanding the photothermal contribution to electrocatalysis: A case study of carbon supported NiFe layered double hydroxide. Int. J. Hydrogen Energy 2022, 47, 23971–23979. [Google Scholar] [CrossRef]
- Jin, B.J.; Li, Y.C.; Wang, J.N.; Meng, F.Y.; Cao, S.S.; He, B.; Jia, S.R.; Wang, Y.; Li, Z.; Liu, X.Q. Promoting oxygen evolution reaction of Co-based catalysts (Co3O4, CoS, CoP, and CoN) through photothermal effect. Small 2019, 15, 1903847. [Google Scholar] [CrossRef]
- Liang, Y.G.; Zhang, Y.J.; Wang, X.K.; Zhou, J.; Cao, Z.W.; Huang, M.H.; Jiang, H.Q. Multifunctional reduced graphene oxide film as electrocatalysts and photothermal layer for broad spectrum solar-enhanced oxygen evolution reaction. Mater. Today Energy 2022, 25, 100966. [Google Scholar] [CrossRef]


| Catalysts | η10 (mV) | η100 (mV) | Tafel Slope (mV dec−1) | References |
|---|---|---|---|---|
| Co(OH)2/NiPx | 236 | 304 | 52 | [30] |
| CoNiFe carbonate hydroxide | 258 | / | 48.7 | [31] |
| Vein-like Cr-doping CoxP | / | 325 | 79.2 | [32] |
| CoNiFe-LDH nanocages | 257 | / | 31.4 | [33] |
| Fe-NiCo-LDH | / | 262 | 51.9 | [34] |
| F-CDs/CoP/NF | / | 328 | 96 | [35] |
| W-NiS0.5Se0.5 | 171 | 239 | 41 | [36] |
| CoO@S-CoTe | 246 | 362 | 56 | [37] |
| NiFeV nanofibers/carbon cloth | 181 | 269 | 47 | [38] |
| CoOOH/Co9S8 | 240 | / | 86.4 | [39] |
| Bi/BiFe(oxy)hydroxide | 232 | / | 34 | [40] |
| Ni2P@FePOxHy | 220 | 260 | 43 | [41] |
| NiMo-Fe | 217 | 264 | 30 | [42] |
| Ni@CNTs-MoxC/Ni2P | 228 | 297 | 43 | [43] |
| FexNi2-xP4O12/RGO | / | 277 | 43.8 | [44] |
| Co3O4/CoMoO4 | 217 | 342 | 72 | [45] |
| NiFeW3-LDHs | 211 | 256 | 36.4 | [46] |
| CeO2-NiCoPx/NCF | 260 | / | 72 | [47] |
| FeS2@NiS2 | 237 | / | 31.4 | [48] |
| Ni0.3Fe0.7-LDH@NF | 184 | 256 | 56.7 | [49] |
| Fe-V-doped Ni3S2/NF | / | 259 | 22.4 | [50] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Z.; Wang, Y.; Lee, L.Y.S. Recent Advances in Synergistic Modulation of Transition-Metal-Based Electrocatalysts for Water Oxidation: A Mini Review. Catalysts 2023, 13, 1230. https://doi.org/10.3390/catal13091230
Li Z, Wang Y, Lee LYS. Recent Advances in Synergistic Modulation of Transition-Metal-Based Electrocatalysts for Water Oxidation: A Mini Review. Catalysts. 2023; 13(9):1230. https://doi.org/10.3390/catal13091230
Chicago/Turabian StyleLi, Zhen, Ying Wang, and Lawrence Yoon Suk Lee. 2023. "Recent Advances in Synergistic Modulation of Transition-Metal-Based Electrocatalysts for Water Oxidation: A Mini Review" Catalysts 13, no. 9: 1230. https://doi.org/10.3390/catal13091230






