Dry Reforming of Methane over Ni-Supported SBA-15 Prepared with Physical Mixing Method by Complexing with Citric Acid
Abstract
:1. Introduction
2. Results and Discussion
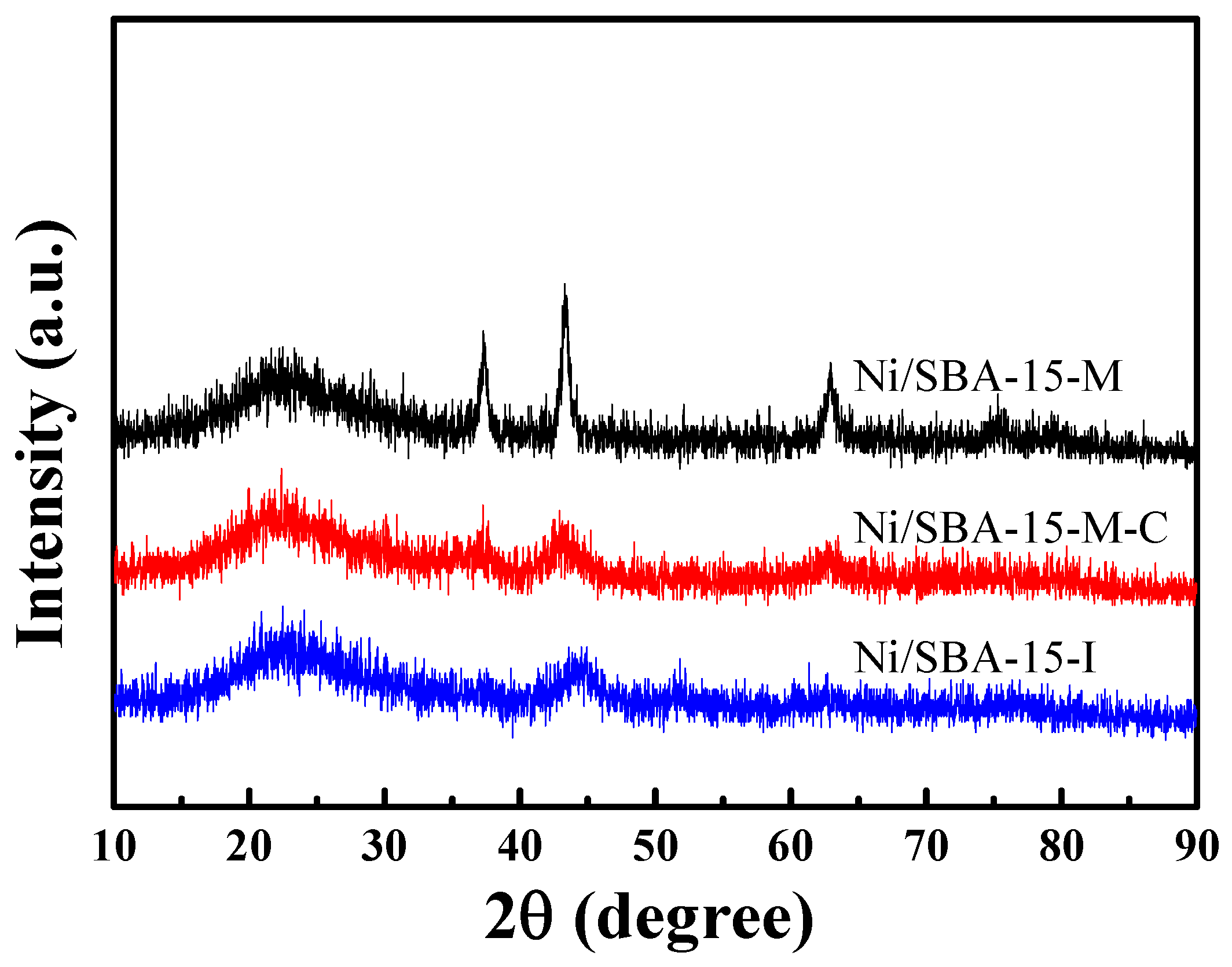
2.1. Structural Properties of Ni-Based Catalysts
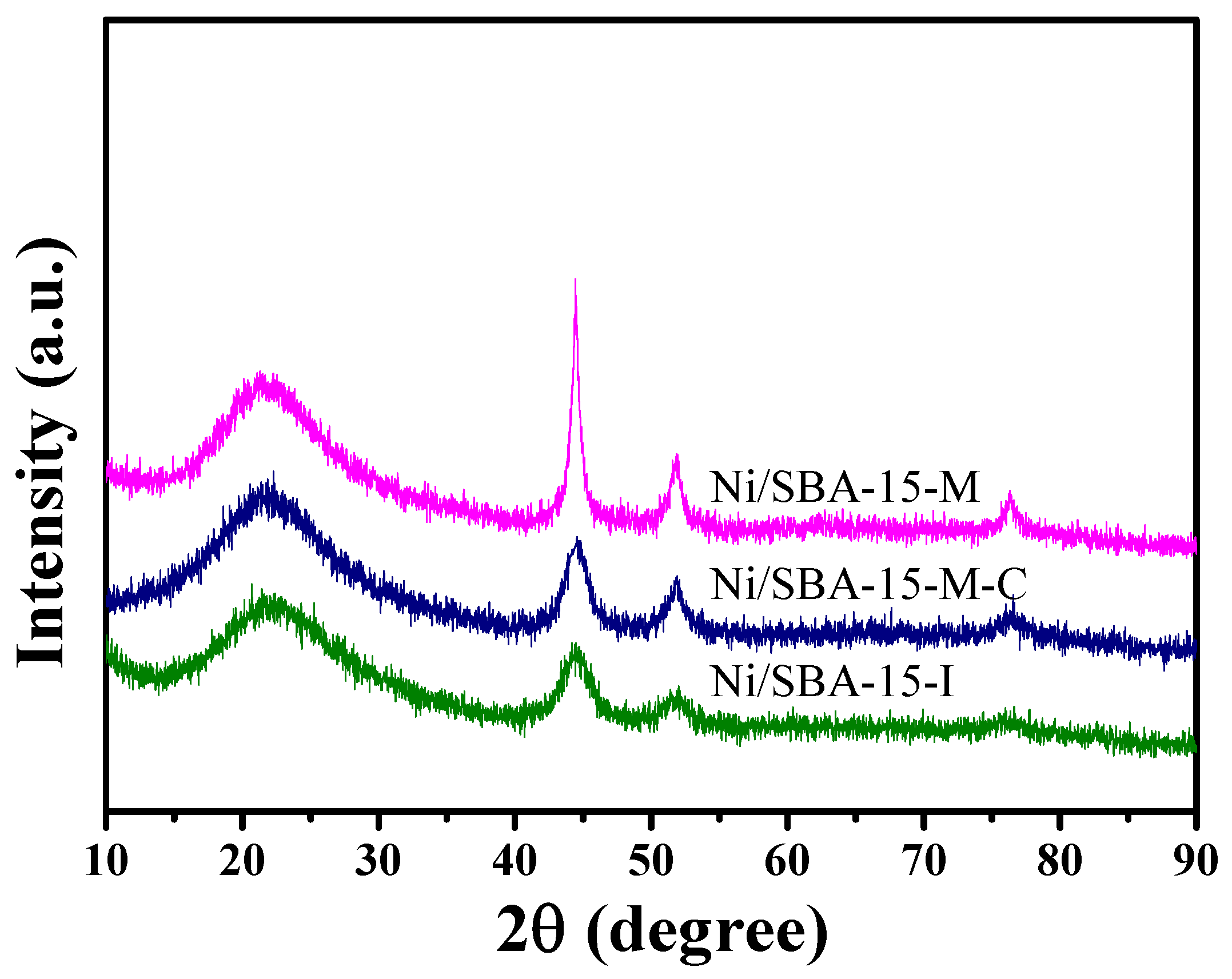
2.2. Structural Properties and Crystal Size of Reduced Ni-Based Catalysts
2.3. Textural Properties of the Ni-Based Catalysts
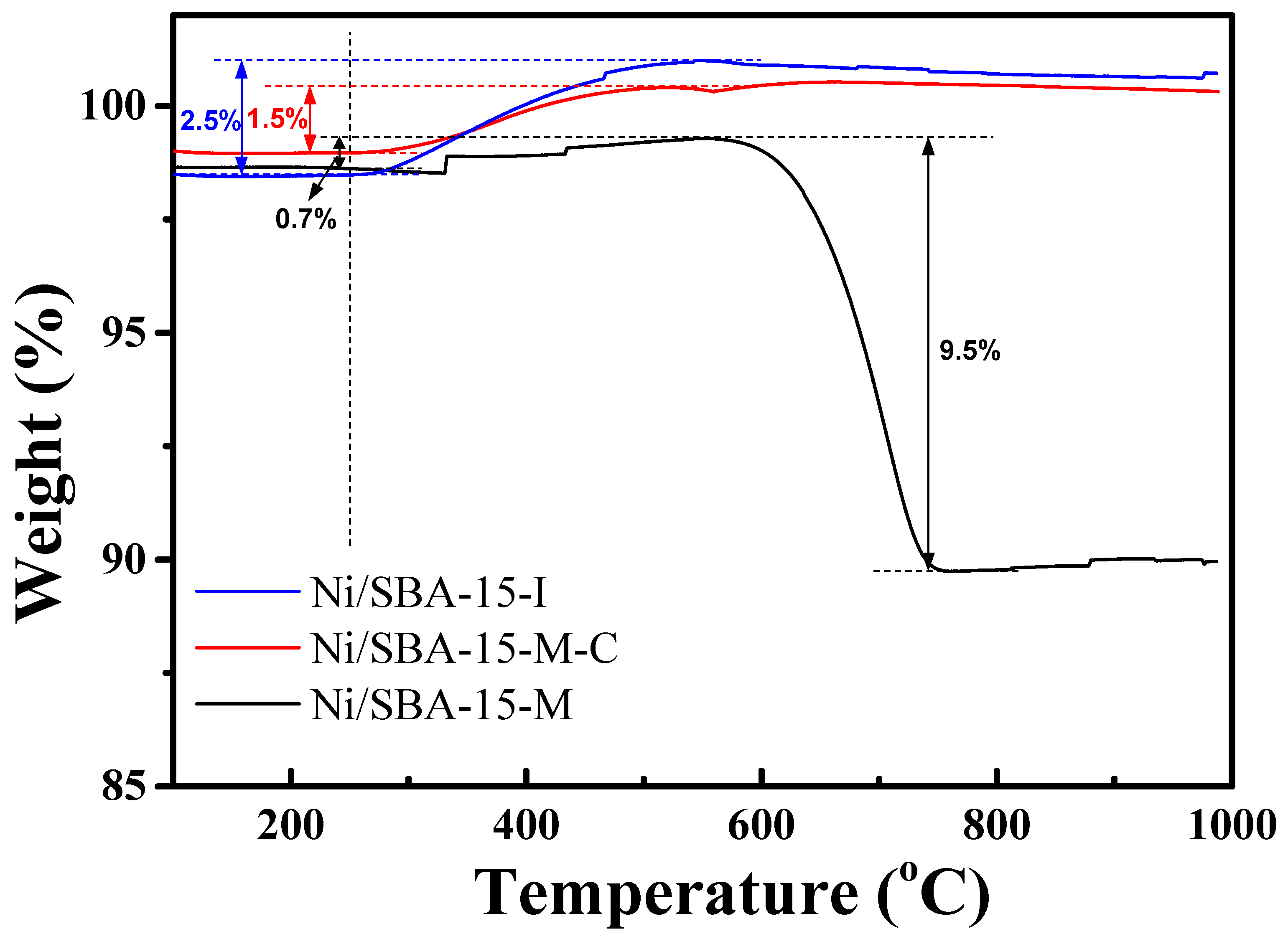
2.4. Reduction Behavior of the Ni-Based Catalysts
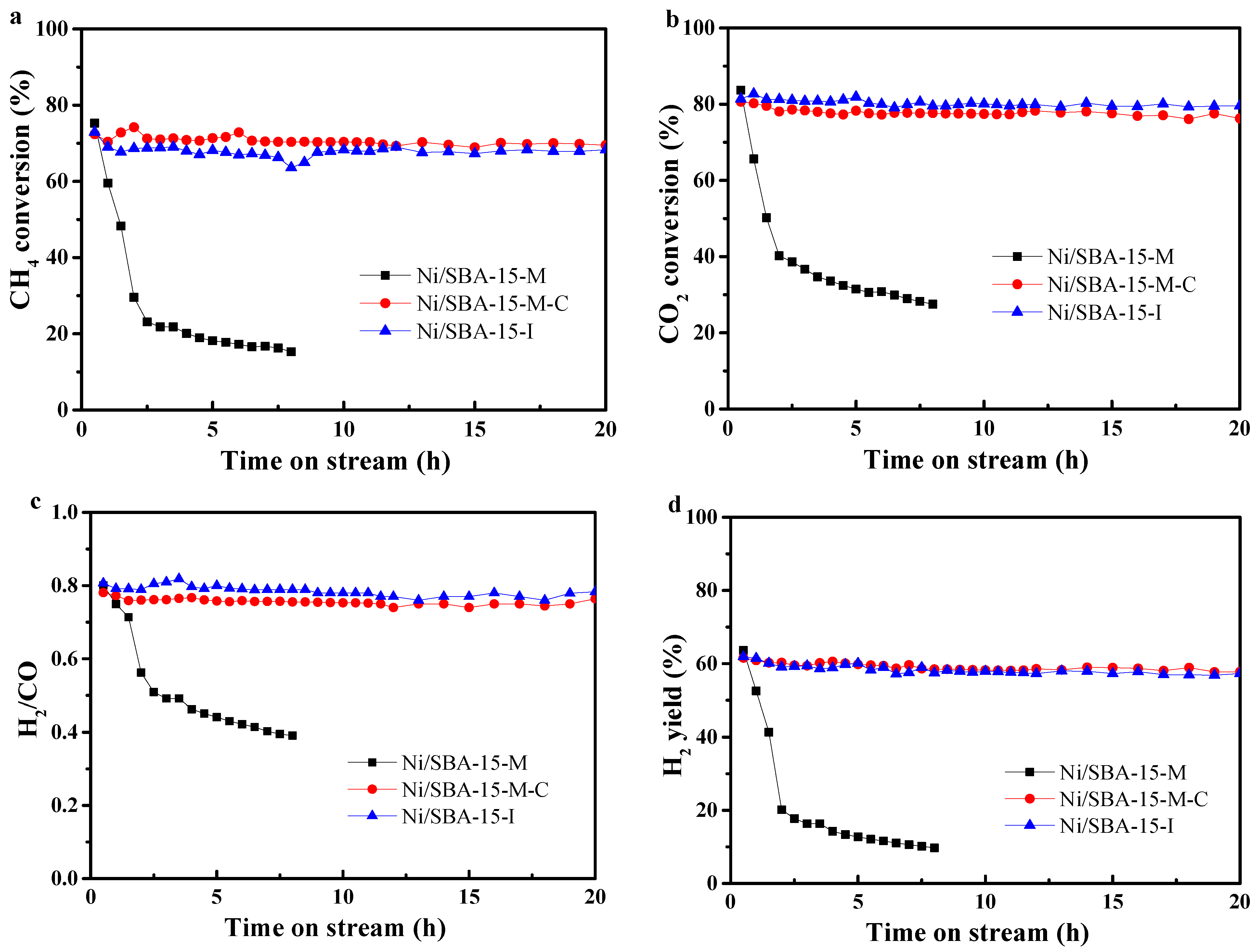
2.5. Catalytic Performance Tests
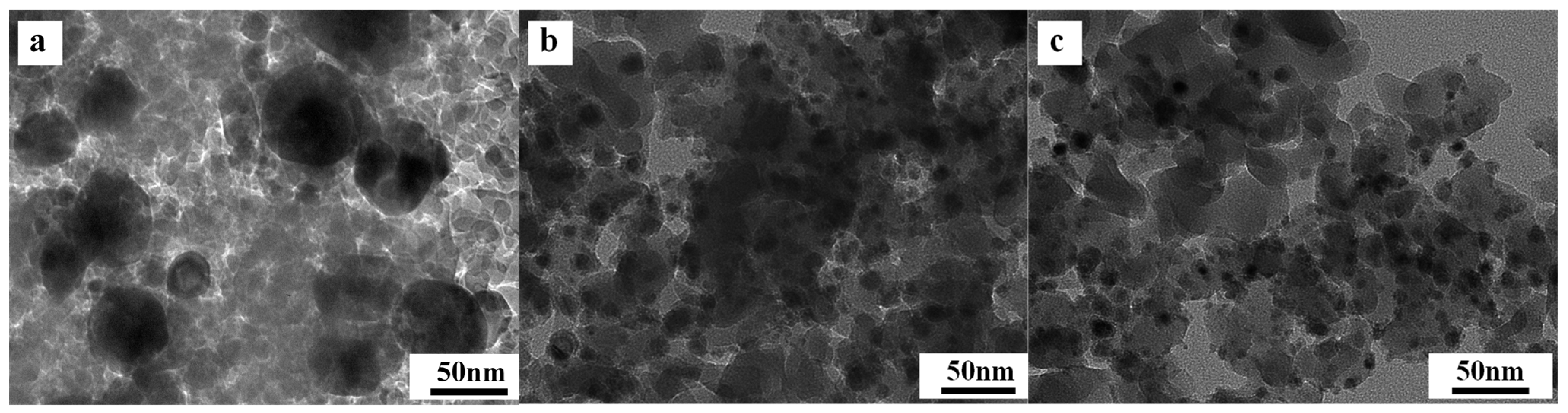
2.6. Characterization of Used Catalysts
3. Experimental Sections
3.1. Catalysts Preparation
3.2. Characterization Technique of the Catalysts
3.3. Activity Evaluation of the Catalysts
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Fan, L.; Li, C.; Biert, L.; Zhou, S.-H.; Tabish, A.N.; Mokhov, A.; Aravind, P.V.; Cai, W. Advances on methane reforming in solid oxide fuel cells. Renew. Sustain. Energy Rev. 2022, 166, 112646. [Google Scholar] [CrossRef]
- Jiang, Z.; Shi, Y.; Bai, Y.; Song, X.; Wang, J.; Lv, P.; Wu, S.; Su, W. Pyridinic N and carbon defects synergistically promote methane dry reforming to syngas catalyzed by Co/N-CNTs. Fuel 2023, 337, 27136. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, R.; Yan, B. Ni/Ce0.9Eu0.1O1.95 with enhanced coke resistance for dry reforming of methane. J. Catal. 2022, 407, 77–89. [Google Scholar] [CrossRef]
- Marinho, A.L.A.; Toniolo, F.S.; Noronha, F.B.; Epron, F.; Duprez, D.; Bion, N. Highly active and stable Ni dispersed on mesoporous CeO2-Al2O3 catalysts for production of syngas by dry reforming of methane. Appl. Catal. B 2021, 281, 119459. [Google Scholar] [CrossRef]
- Song, Y.; Ozdemir, E.; Ramesh, S.; Adishev, A.; Subramanian, S.; Harale, A.; Albuali, M.; Fadhel, B.A.; Jamal, A.; Moon, D.; et al. Dry reforming of methane by stable Ni-Mo nanocatalysts on single-crystalline MgO. Science 2020, 367, 777. [Google Scholar] [CrossRef]
- Niu, J.; Wang, Y.; Liland, S.E.; Regli, S.K.; Yang, J.; Rout, K.R.; Luo, J.; Rønning, M.; Ran, J.; Chen, D. Unraveling enhanced activity, selectivity, and coke resistance of Pt-Ni bimetallic clusters in dry reforming. ACS Catal. 2021, 11, 2398–2411. [Google Scholar] [CrossRef]
- Jin, B.; Wang, K.; Yu, H.; He, X.; Liang, X. Engineering oxygen vacancy-rich CeOx overcoating onto Ni/Al2O3 by atomic layer deposition for bi-reforming of methane. Chem. Eng. J. 2023, 459, 141611. [Google Scholar] [CrossRef]
- Bakhtiari, K.; Kootenaei, A.S.; Maghsoodi, S.; Azizi, S.; Ghomsheh, S.M.T. Synthesis of high sintering-resistant Ni-modified halloysite based catalysts containing La, Ce, and Co for dry reforming of methane. Ceram. Int. 2022, 48, 37394–37402. [Google Scholar] [CrossRef]
- Song, C. Global challenges and strategies for control, conversion and utilization of CO2 for sustainable development involving energy, catalysis, adsorption and chemical processing. Catal. Today 2006, 115, 2–32. [Google Scholar] [CrossRef]
- Centi, G.; Quadrelli, E.A.; Perathoner, S. Catalysis for CO2 conversion: A key technology for rapid introduction of renewable energy in the value chain of chemical industries. Energy Environ. Sci. 2013, 6, 1711–1731. [Google Scholar] [CrossRef]
- Kawi, S.; Kathiraser, Y.; Oemar, J.U.; Li, Z.; Saw, E.T. Progress in synthesis of highly active and stable nickel-based catalysts for carbon dioxide reforming of methane. ChemSusChem 2015, 8, 3556–3575. [Google Scholar] [CrossRef]
- Kobayashi, T.; Furuya, T.; Fujitsuka, H.; Tago, T. Synthesis of Birdcage-type zeolite encapsulating ultrafine Pt nanoparticles and its application in dry reforming of methane. Chem. Eng. J. 2019, 377, 120203. [Google Scholar] [CrossRef]
- Theofanidis, S.-A.; Pieterse, J.A.Z.; Poelman, H.; Longo, A.; Sabbe, M.K.; Virginie, M.; Detavernier, C.; Marin, G.B.; Galvita, V.V. Effect of Rh in Ni-based catalysts on sulfur impurities during methane reforming. Appl. Catal. B 2020, 267, 118691. [Google Scholar] [CrossRef]
- Alabdullah, M.; Ibrahim, M.; Dhawale, D.; Bau, J.A.; Harale, A.; Katikaneni, S.; Gascon, J. Rhodium nanoparticle size effects on the CO2 reforming of methane and propane. ChemCatChem 2021, 13, 2879–2886. [Google Scholar] [CrossRef]
- Egawa, C. Methane dry reforming reaction on Ru(001) surfaces. J. Catal. 2018, 358, 35–42. [Google Scholar] [CrossRef]
- Dama, S.; Ghodke, S.R.; Bobade, R.; Gurav, H.R.; Chilukuri, S. Active and durable alkaline earth metal substituted perovskite catalysts for dry reforming of methane. Appl. Catal. B 2018, 224, 146–158. [Google Scholar] [CrossRef]
- Mendoza-Nieto, J.A.; Tehuacanero-Cuapa, S.; Arenas-Alatorre, J.; Pfeiffer, H. Nickel-doped sodium zirconate catalysts for carbon dioxide storage and hydrogen production through dry methane reforming process. Appl. Catal. B 2018, 224, 80–87. [Google Scholar] [CrossRef]
- Zhang, J.; Li, F. Coke-resistant Ni@SiO2 catalyst for dry reforming of methane. Appl. Catal. B 2015, 176–177, 513–521. [Google Scholar] [CrossRef]
- Abdulghani, A.J.A.; Park, J.-H.; Kozlov, S.M.; Kang, D.-C.; AlSabban, B.; Pedireddy, S.; Aguilar-Tapia, A.; Ould-Chikh, S.; Hazemann, J.-L.; Basset, J.-M.; et al. Methane dry reforming on supported cobalt nanoparticles promoted by boron. J. Catal. 2020, 392, 126–134. [Google Scholar] [CrossRef]
- Azancot, L.; Bobadilla, L.F.; Centeno, M.A.; Odriozola, J.A. Effect of potassium loading on basic properties of Ni/MgAl2O4 catalyst for CO2 reforming of methane. J. CO2 Util. 2021, 52, 101681. [Google Scholar] [CrossRef]
- Shang, Z.; Li, S.; Li, L.; Liu, G.; Liang, X. Highly active and stable alumina supported nickel nanoparticle catalysts for dry reforming of methane. Appl. Catal. B 2017, 201, 302–309. [Google Scholar] [CrossRef]
- Akri, M.; Zhao, S.; Li, X.; Zang, K.; Lee, A.F.; Isaacs, M.A.; Xi, W.; Gangarajula, Y.; Luo, J.; Ren, Y.; et al. Atomically dispersed nickel as coke-resistant active sites for methane dry reforming. Nat. Commun. 2019, 10, 5181. [Google Scholar] [CrossRef]
- Rodríguez-González, V.; Marceau, E.; Beauniera, P.; Chea, M.; Train, C. Stabilization of hexagonal close-packed metallic nickel for alumina-supported systems prepared from Ni(II) glycinate. J. Solid State Chem. 2007, 180, 22–30. [Google Scholar] [CrossRef]
- Yu, Y.-X.; Yang, J.; Zhu, K.-K.; Sui, Z.-J.; Chen, D.; Zhu, Y.-A.; Zhou, X.-G. High-throughput screening of alloy catalysts for dry methane reforming. ACS Catal. 2021, 11, 8881–8894. [Google Scholar] [CrossRef]
- Liu, H.; Li, Y.; Wu, H.; Miyake, T.; He, D. CO2 reforming of methane over Ni/SBA-15 prepared with β-cyclodextrin-Role of b-cyclodextrin in Ni dispersion and performance. Int. J. Hydrog. Energy 2013, 38, 15200–15209. [Google Scholar] [CrossRef]
- Danghyan, V.; Kumar, A.; Mukasyan, A.; Wolf, E.E. An active and stable NiOMgO solid solution based catalysts prepared by paper assisted combustion synthesis for the dry reforming of methane. Appl. Catal. B 2020, 273, 119056. [Google Scholar] [CrossRef]
- Costa, K.Ś.; Gálvez, M.E.; Motak, M.; Grzybek, T.; Rønning, M.; Costa, P.D. Dry reforming of methane over Zr- and Y-modified Ni/Mg/Al double-layered hydroxides. Catal. Commun. 2018, 117, 26–32. [Google Scholar] [CrossRef]
- Iftikhar, S.; Jiang, Q.; Gao, Y.; Liu, J.; Gu, H.; Neal, L.; Li, F. LaNixFe1-xO3-δ as a robust redox catalyst for CO2 splitting and methane partial oxidation. Energy Fuels 2021, 35, 13921–13929. [Google Scholar] [CrossRef]
- Daoura, O.; Fornasieri, G.; Boutros, M.; Hassan, N.E.; Beaunier, P.; Thomas, C.; Selmane, M.; Miche, A.; Sassoye, C.; Ersen, O.; et al. One-pot prepared mesoporous silica SBA-15-like monoliths with embedded Ni particles as selective and stable catalysts for methane dry reforming. Appl. Catal. B 2021, 280, 119417. [Google Scholar] [CrossRef]
- Fang, X.; Peng, C.; Peng, H.; Liu, W.; Xu, X.; Wang, X.; Li, C.; Zhou, W. Methane dry reforming over coke-resistant mesoporous Ni-Al2O3 catalysts prepared by evaporation-induced self-assembly method. ChemCatChem 2015, 7, 3753–3762. [Google Scholar] [CrossRef]
- Moura, I.P.; Reis, A.C.; Bresciani, A.E.; Alves, R.M.B. Carbon dioxide abatement by integration of methane bi-reforming process with ammonia and urea synthesis. Renew. Sustain. Energy Rev. 2021, 151, 111619. [Google Scholar] [CrossRef]
- Vakili, R.; Gholami, R.; Stere, C.E.; Chansai, S.; Chen, H.; Holmes, S.M.; Jiao, Y.; Hardacre, C.; Fan, X. Plasma-assisted catalytic dry reforming of methane (DRM) over metalorganic frameworks (MOFs)-based catalysts. Appl. Catal. B 2020, 260, 118195. [Google Scholar] [CrossRef]
- Li, X.; Li, D.; Tian, H.; Zeng, L.; Zhao, Z.-J.; Gong, J. Dry reforming of methane over Ni/La2O3 nanorod catalysts with stabilized Ni nanoparticles. Appl. Catal. B 2017, 202, 683–694. [Google Scholar] [CrossRef]
- Wang, H.; Dong, X.; Zhao, T.; Yu, H.; Li, M. Dry reforming of methane over bimetallic Ni-Co catalyst prepared from La(CoxNi1-x)0.5Fe0.5O3 perovskite precursor: Catalytic activity and coking resistance. Appl. Catal. B 2019, 245, 302–313. [Google Scholar] [CrossRef]
- Sajjadi, S.M.; Haghighi, M.; Eshghi, J. Synergic influence of potassium loading and plasma-treatment on anti-coke property of K-Promoted bimetallic NiCoNiAl2O4 nanocatalyst applied in O2-Enhanced dry reforming of CH4. Int. J. Hydrog. Energy 2019, 44, 13397–13414. [Google Scholar] [CrossRef]
- Liu, H.; Li, Y.; Wu, H.; Yang, W.; He, D. Promoting effect of glucose and β-cyclodextrin on Ni dispersion of Ni/MCM-41 catalysts for carbon dioxide reforming of methane to syngas. Fuel 2014, 136, 19–24. [Google Scholar] [CrossRef]
- Lizama, L.; Klimova, T. Highly active deep HDS catalysts prepared using Mo and W heteropoly acids supported on SBA-15. Appl. Catal. B 2008, 82, 139–150. [Google Scholar] [CrossRef]
- Calderón-Magdaleno, M.Á.; Mendoza-Nieto, J.A.; Klimova, T.E. Effect of the amount of citric acid used in the preparation of NiMo/SBA-15 catalysts on their performance in HDS of dibenzothiophene-type compounds. Catal. Today 2014, 220–222, 78–88. [Google Scholar] [CrossRef]
- Tao, M.; Zhou, C.; Shi, Y.; Meng, X.; Gu, J.; Gao, W.; Xin, Z. Enhanced sintering resistance of bimetal/SBA-15 catalysts with promising activity under a low temperature for CO methanation. RSC Adv. 2020, 10, 20852–20861. [Google Scholar] [CrossRef]
- Waris, M.; Ra, H.; Yoon, S.; Kim, M.-J.; Lee, K. Efficient and Stable Ni/SBA-15 Catalyst for Dry Reforming of Methane: Effect of Citric Acid Concentration. Catalysts 2023, 13, 916. [Google Scholar] [CrossRef]
- Ren, H.-P.; Hao, Q.-Q.; Wang, W.; Song, Y.-H.; Cheng, J.; Liu, Z.-W.; Liu, Z.-T.; Lu, J.; Hao, Z. High-performance Ni-SiO2 for pressurized carbon dioxide reforming of methane. Int. J. Hydrog. Energy 2014, 39, 11592–11605. [Google Scholar] [CrossRef]
- Zhang, C.; Yue, H.; Huang, Z.; Li, S.; Wu, G.; Ma, X.; Gong, J. Hydrogen production via steam reforming of ethanol on phyllosilicate-derived Ni/SiO2: Enhanced metal-support interaction and catalytic stability. ACS Sustain. Chem. Eng. 2013, 1, 161–173. [Google Scholar] [CrossRef]
- Xie, T.; Shi, L.; Zhang, J.; Zhang, D. Immobilizing Ni nanoparticles to mesoporous silica with size and location control via a polyolassisted route for coking- and sintering-resistant dry reforming of methane. Chem. Commun. 2014, 50, 7250–7253. [Google Scholar] [CrossRef]
- Wang, N.; Shen, K.; Yu, X.; Qian, W.; Chu, W. Preparation and characterization of a plasma treated NiMgSBA-15 catalyst for methane reforming with CO2 to produce syngas. Catal. Sci. Technol. 2013, 3, 2278–2287. [Google Scholar] [CrossRef]
- Pantaleo, G.; Parola, V.L.; Testa, M.L.; Venezia, A.M. CO2 reforming of CH4 over SiO2-supported Ni catalyst: Effect of Sn as support and metal promoter. Ind. Eng. Chem. Res. 2021, 60, 18684–18694. [Google Scholar] [CrossRef]
- Shi, Y.; Wang, S.; Li, Y.; Yang, F.; Yu, H.; Chu, Y.; Li, T.; Yin, H. Improving anti-coking properties of Ni/Al2O3 catalysts via synergistic effect of metallic nickel and nickel phosphides in dry methane reforming. Materials 2022, 15, 3044. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Quek, X.Y.; Cheo, W.N.E.; Lau, R.; Borgna, A.; Yang, Y. MCM-41 supported nickel-based bimetallic catalysts with superior stability during carbon dioxide reforming of methane: Effect of strong metal-support interaction. J. Catal. 2009, 266, 380–390. [Google Scholar] [CrossRef]
- Ren, H.-P.; Ding, S.-Y.; Ma, Q.; Song, W.-Q.; Zhao, Y.-Z.; Liu, J.; He, Y.-M.; Tian, S.-P. The effect of preparation method of Ni-supported SiO2 catalysts for carbon dioxide reforming of methane. Catalysts 2021, 11, 1221. [Google Scholar] [CrossRef]
- Song, Q.; Ran, R.; Wu, X.; Si, Z.; Weng, D. Dry reforming of methane over Ni catalysts supported on micro- and mesoporous silica. J. CO2 Util. 2023, 68, 102387. [Google Scholar] [CrossRef]
- Ren, H.-P.; Hao, Q.-Q.; Ding, S.-Y.; Zhao, Y.-Z.; Zhu, M.; Tian, S.-P.; Ma, Q.; Song, W.-Q.; Miao, Z.; Liu, Z.-T. A high-performance Ni/SiO2 prepared by the complexed-impregnation method with citric acid for carbon dioxide reforming of methane. Ind. Eng. Chem. Res. 2018, 57, 16257–16263. [Google Scholar] [CrossRef]
- Alipour, Z.; Borugadda, V.B.; Wang, H.; Dalai, A.K. Syngas production through dry reforming: A review on catalysts and their materials, preparation methods and reactor type. Chem. Eng. J. 2023, 452, 139416. [Google Scholar] [CrossRef]

| Samples | SBET (m2·g−1) a | Vp (cm3·g−1) b | Dp (nm) c | d(NiO) d | D(Ni) d |
|---|---|---|---|---|---|
| SBA-15 | 470.7 | 0.86 | 7.32 | - | - |
| Ni/SBA-15-M | 434.6 | 0.72 | 6.89 | 16.1 | 17.2 |
| Ni/SBA-15-M-C | 439.0 | 0.77 | 7.06 | 6.5 | 8.1 |
| Ni/SBA-15-I | 435.7 | 0.73 | 6.76 | 6.1 | 7.8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ren, H.-P.; Tian, S.-P.; Ding, S.-Y.; Ma, Q.; Song, W.-Q.; Zhao, Y.-Z.; Zhang, Z.; Miao, Z.; Wang, W. Dry Reforming of Methane over Ni-Supported SBA-15 Prepared with Physical Mixing Method by Complexing with Citric Acid. Catalysts 2023, 13, 1252. https://doi.org/10.3390/catal13091252
Ren H-P, Tian S-P, Ding S-Y, Ma Q, Song W-Q, Zhao Y-Z, Zhang Z, Miao Z, Wang W. Dry Reforming of Methane over Ni-Supported SBA-15 Prepared with Physical Mixing Method by Complexing with Citric Acid. Catalysts. 2023; 13(9):1252. https://doi.org/10.3390/catal13091252
Chicago/Turabian StyleRen, Hua-Ping, Shao-Peng Tian, Si-Yi Ding, Qiang Ma, Wen-Qi Song, Yu-Zhen Zhao, Zhe Zhang, Zongcheng Miao, and Wei Wang. 2023. "Dry Reforming of Methane over Ni-Supported SBA-15 Prepared with Physical Mixing Method by Complexing with Citric Acid" Catalysts 13, no. 9: 1252. https://doi.org/10.3390/catal13091252




