Boosting the Photoreactivity of g-C3N4 towards CO2 Reduction by Polymerization of Dicyandiamide in Ammonium Chloride
Abstract
:1. Introduction
2. Results and Discussion
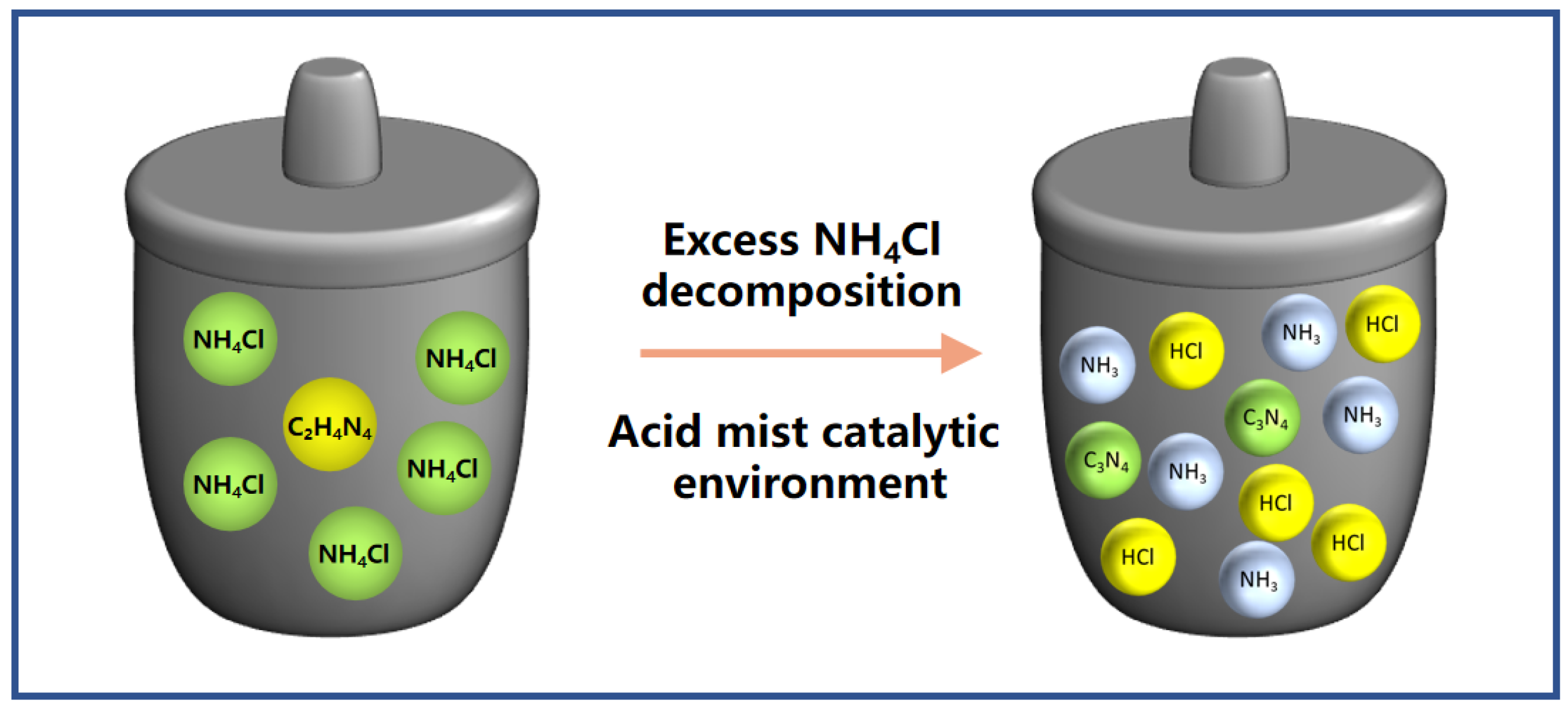
2.1. Effect of Ammonium Chloride on the Morphology of CN
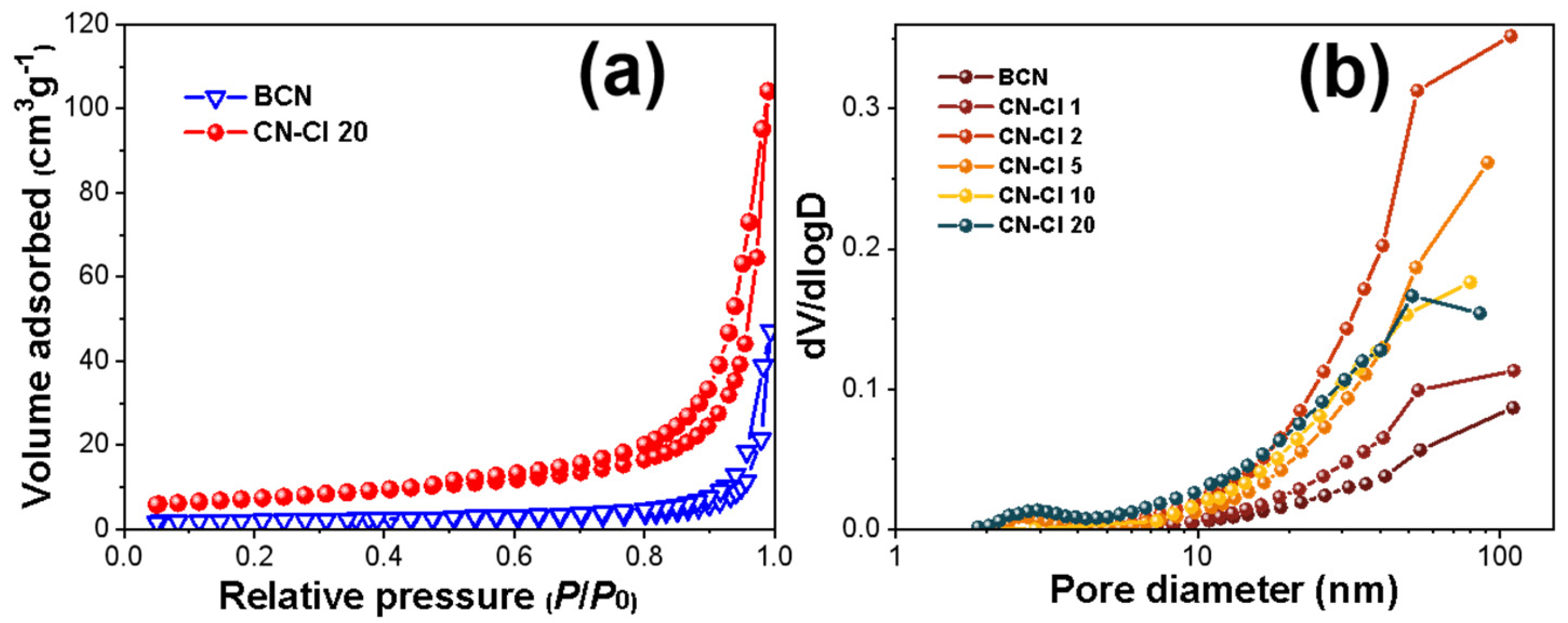
2.2. Effect of Ammonium Chloride on the Microstructures of CN
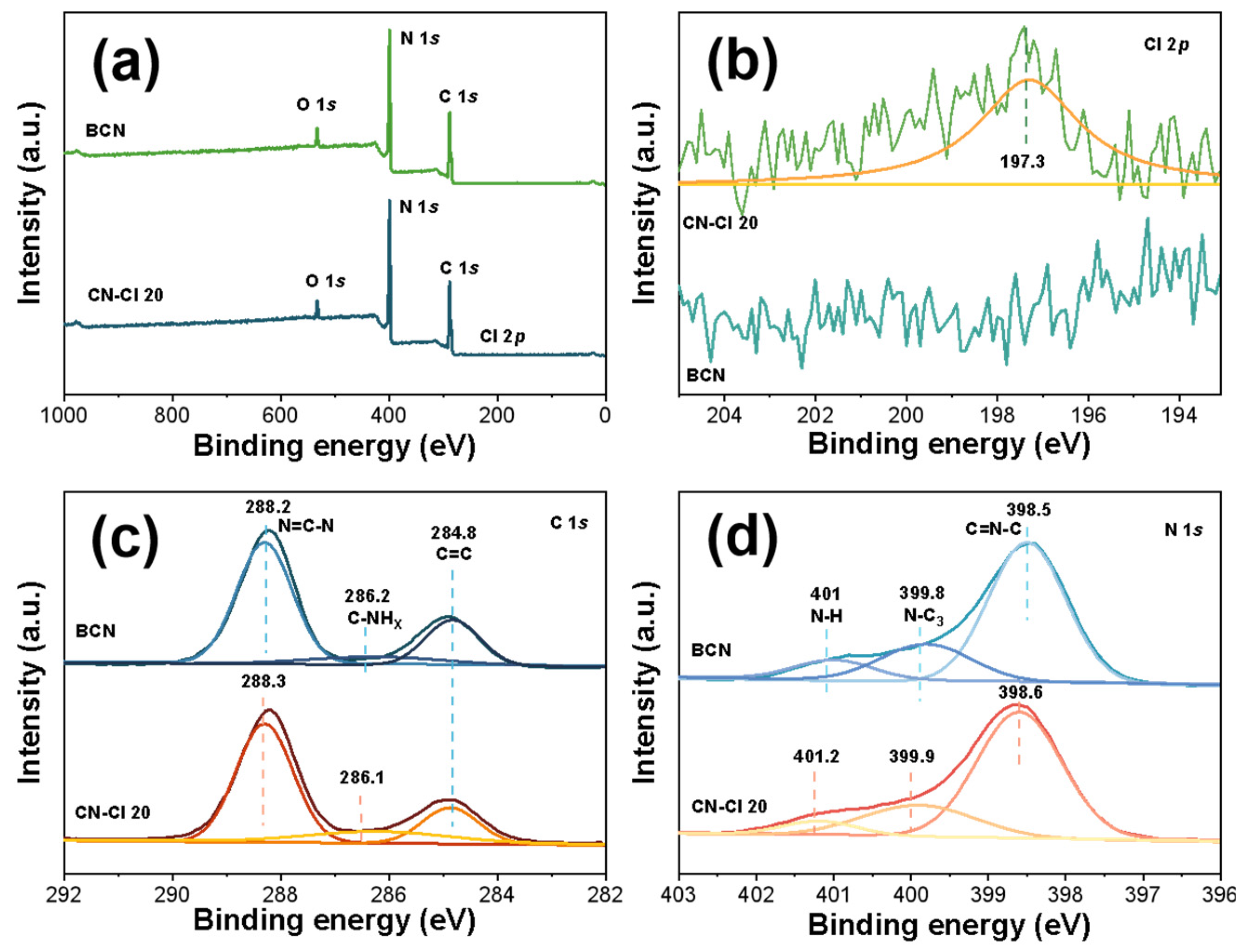
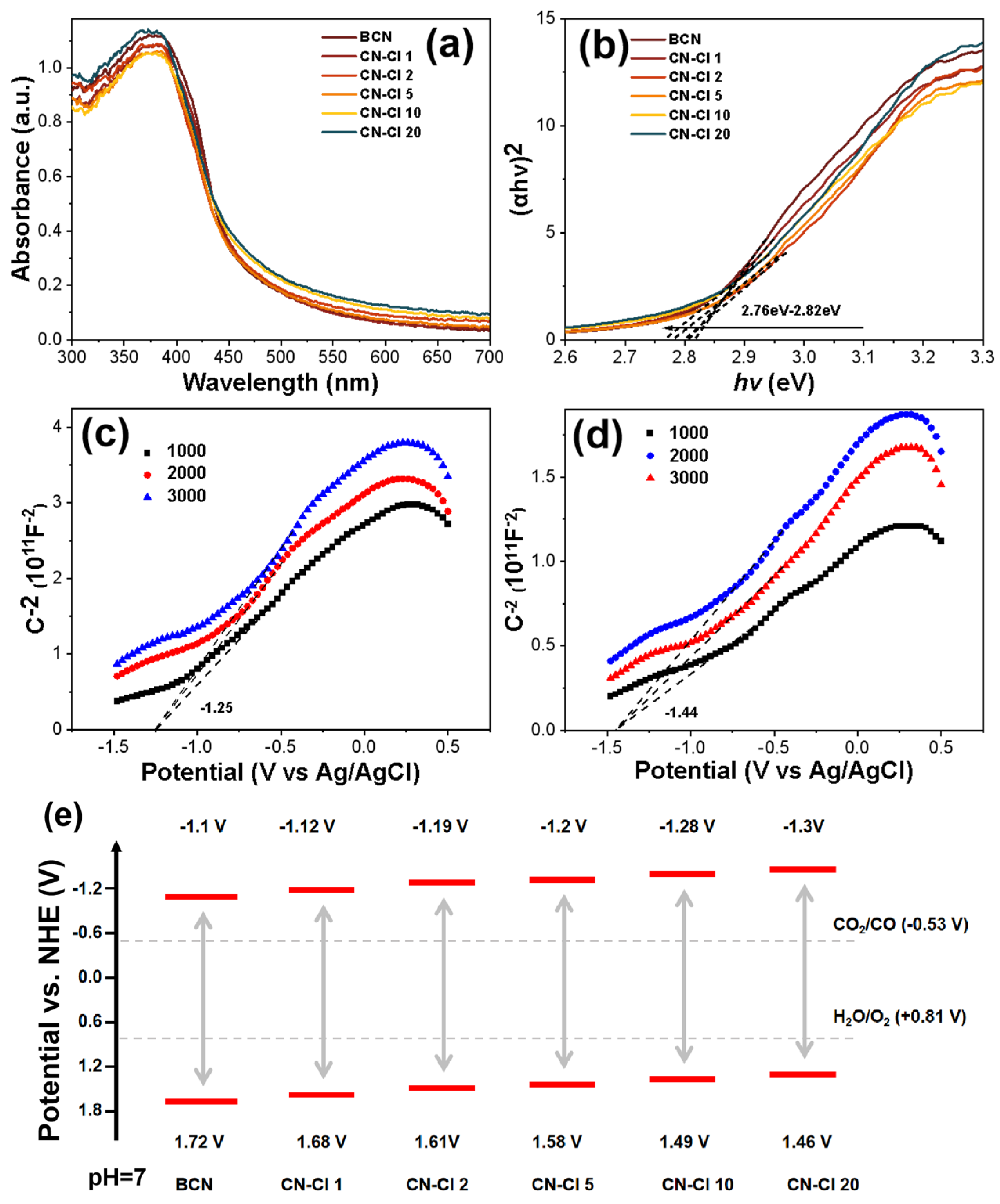
2.3. Phase Structures and Surface Chemical States
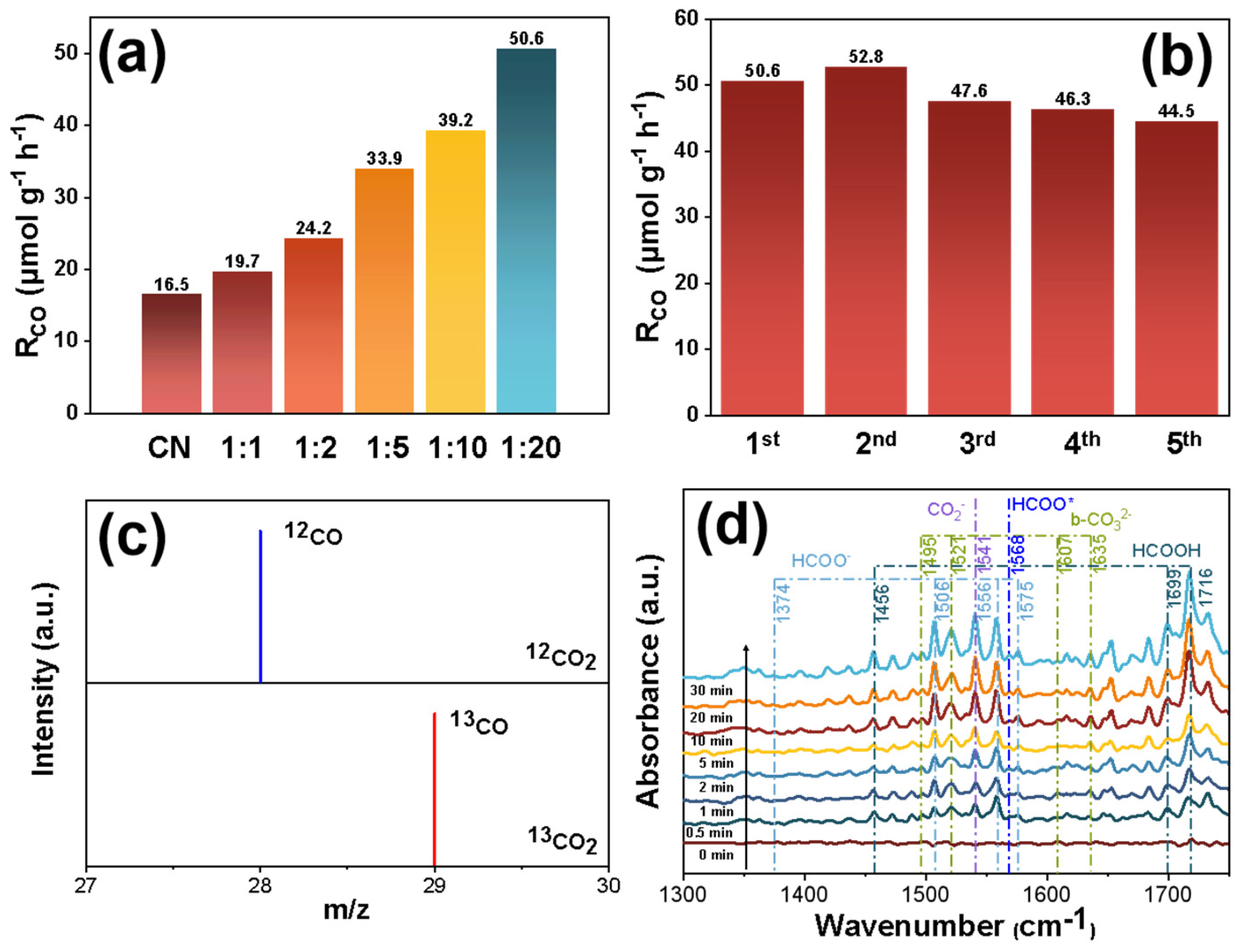
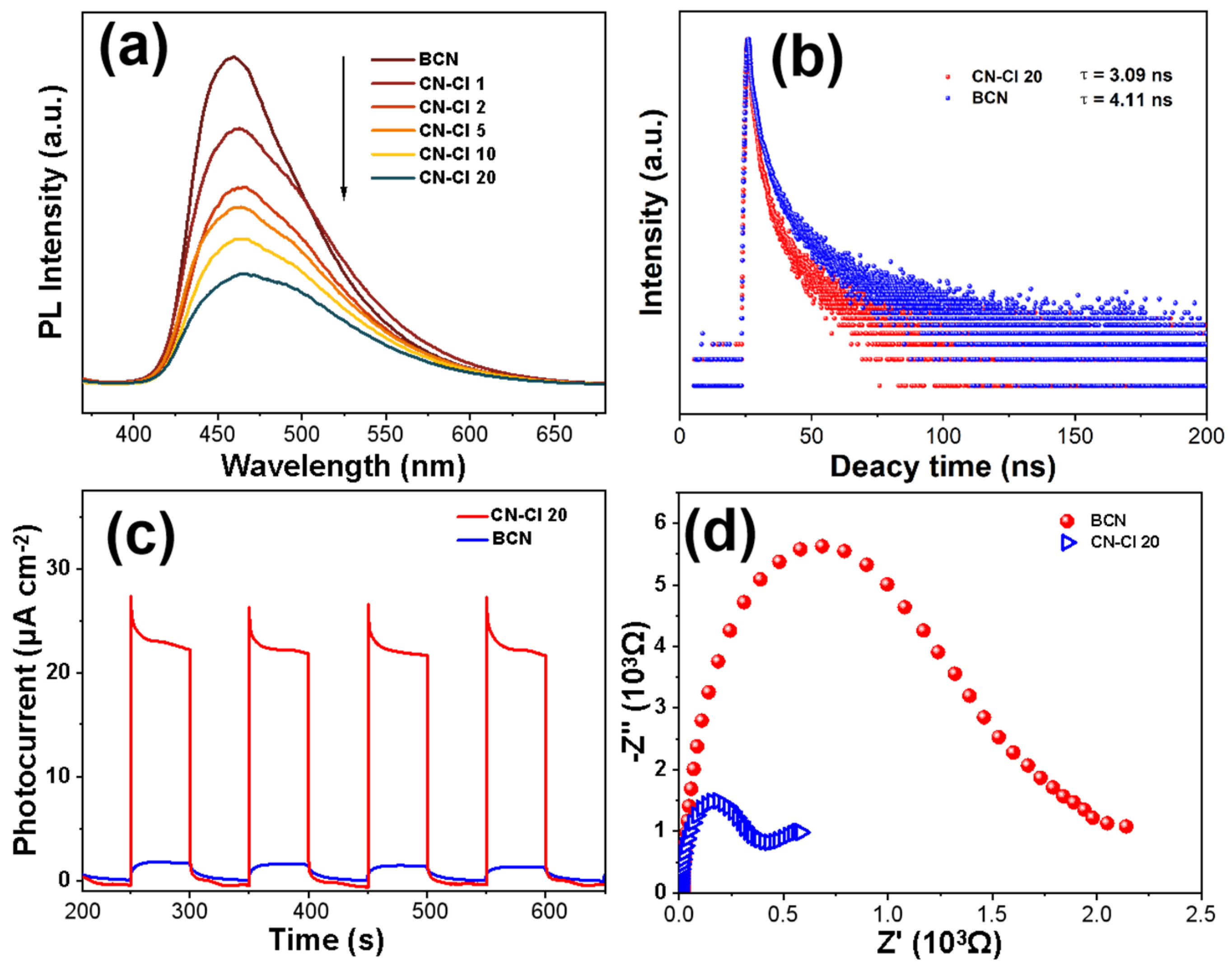
2.4. Photocatalytic Performance
2.5. Photoelectrochemical Properties
3. Experimental Section
3.1. Materials
3.2. Sample Synthesis
3.3. Sample Characterization
3.4. Photocatalytic Reduction of CO2
3.5. In Situ DRIFTs Measurement
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Lin, X.; Ng, S.-F.; Ong, W.-J. Coordinating single-atom catalysts on two-dimensional nanomaterials: A paradigm towards bolstered photocatalytic energy conversion. Coord. Chem. Rev. 2022, 471, 214743. [Google Scholar] [CrossRef]
- Liang, Y.; Wu, X.; Liu, X.; Li, C.; Liu, S. Recovering solar fuels from photocatalytic CO2 reduction over W6+-incorporated crystalline g-C3N4 nanorods by synergetic modulation of active centers. Appl. Catal. B Environ. 2022, 304, 120978. [Google Scholar] [CrossRef]
- Jiang, L.; Yuan, X.; Pan, Y.; Liang, J.; Zeng, G.; Wu, Z.; Wang, H. Doping of graphitic carbon nitride for photocatalysis: A review. Appl. Catal. B Environ. 2017, 217, 388–406. [Google Scholar] [CrossRef]
- Yang, L.; Luo, Y.; Yang, L.; Luo, S.; Luo, X.; Dai, W.; Li, T.; Luo, Y. Enhanced photocatalytic activity of hierarchical titanium dioxide microspheres with combining carbon nanotubes as “e-bridge”. J. Hazard. Mater. 2019, 367, 550–558. [Google Scholar] [CrossRef]
- Bu, X.; Li, J.; Yang, S.; Sun, J.; Deng, Y.; Yang, Y.; Wang, G.; Peng, Z.; He, P.; Wang, X.; et al. Surface Modification of C3N4 through Oxygen-Plasma Treatment: A Simple Way toward Excellent Hydrophilicity. ACS Appl. Mater. Interfaces 2016, 8, 31419–31425. [Google Scholar] [CrossRef]
- Gao, J.; Wang, Y.; Zhou, S.; Lin, W.; Kong, Y. A Facile One-Step Synthesis of Fe-Doped g-C3N4 Nanosheets and Their Improved Visible-Light Photocatalytic Performance. ChemCatChem 2017, 9, 1708–1715. [Google Scholar] [CrossRef]
- Sun, X.; Sun, L.; Li, G.; Tuo, Y.; Ye, C.; Yang, J.; Low, J.; Yu, X.; Bitter, J.H.; Lei, Y.; et al. Phosphorus Tailors the d-Band Center of Copper Atomic Sites for Efficient CO2 Photoreduction under Visible-Light Irradiation. Angew. Chem. Int. Ed. Engl. 2022, 61, e202207677. [Google Scholar] [CrossRef]
- Ryaboshapka, D.; Bargiela, P.; Piccolo, L.; Afanasiev, P. On the electronic properties and catalytic activity of MoS2–C3N4 materials prepared by one-pot reaction. Int. J. Hydrog. Energy 2022, 47, 34012–34024. [Google Scholar] [CrossRef]
- Ong, W.J.; Tan, L.L.; Ng, Y.H.; Yong, S.T.; Chai, S.P. Graphitic Carbon Nitride (g-C3N4)-Based Photocatalysts for Artificial Photosynthesis and Environmental Remediation: Are We a Step Closer To Achieving Sustainability. Chem. Rev. 2016, 116, 7159–7329. [Google Scholar] [CrossRef]
- Yang, J.; Yang, K.; Zhu, X.; Wang, Z.; Yang, Z.; Ding, X.; Zhong, K.; He, M.; Li, H.; Xu, H. Band engineering of non-metal modified polymeric carbon nitride with broad spectral response for enhancing photocatalytic CO2 reduction. Chem. Eng. J. 2023, 461, 141841. [Google Scholar] [CrossRef]
- Wu, W.; Xu, W.; An, X.; Wang, L.; Zhang, J.; Li, Z.; Wu, M. Multiaspect insight into synergetic modification of carbon nitride with halide salt and water vapor. Appl. Catal. B Environ. 2018, 229, 204–210. [Google Scholar] [CrossRef]
- Shi, L.; Chang, K.; Zhang, H.; Hai, X.; Yang, L.; Wang, T.; Ye, J. Drastic Enhancement of Photocatalytic Activities over Phosphoric Acid Protonated Porous g-C3 N4 Nanosheets under Visible Light. Small 2016, 12, 4431–4439. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Lin, J.; Cheng, H.; Wei, L.; Wang, F. Simultaneous co-Photocatalytic CO2 Reduction and Ethanol Oxidation towards Synergistic Acetaldehyde Synthesis. Angew. Chem. Int. Ed. Engl. 2023, 62, e202218720. [Google Scholar] [CrossRef]
- Madhurima, V.P.; Kumari, K.; Jain, P.K. A facile single-step approach to achieve in situ expandedg-C3N4for improved photodegradation performance. Polym. Adv. Technol. 2022, 34, 578–586. [Google Scholar] [CrossRef]
- Zhang, D.; Guo, Y.; Zhao, Z. Porous defect-modified graphitic carbon nitride via a facile one-step approach with significantly enhanced photocatalytic hydrogen evolution under visible light irradiation. Appl. Catal. B Environ. 2018, 226, 1–9. [Google Scholar] [CrossRef]
- Yang, Q.; Hu, S.; Yao, Y.; Lin, X.; Du, H.; Yuan, Y. Engineering graphitic carbon nitride with expanded interlayer distance for boosting photocatalytic hydrogen evolution. Chin. J. Catal. 2021, 42, 217–224. [Google Scholar] [CrossRef]
- Wang, Y.; Li, Y.; Ju, W.; Wang, J.; Yao, H.; Zhang, L.; Wang, J.; Li, Z. Molten salt synthesis of water-dispersible polymeric carbon nitride nanoseaweeds and their application as luminescent probes. Carbon 2016, 102, 477–486. [Google Scholar] [CrossRef]
- Hou, Y.; Guan, H.; Yu, J.; Cao, S. Potassium/oxygen co-doped polymeric carbon nitride for enhanced photocatalytic CO2 reduction. Appl. Surf. Sci. 2021, 563, 150310. [Google Scholar] [CrossRef]
- Yan, J.; Han, X.; Zheng, X.; Qian, J.; Liu, J.; Dong, X.; Xi, F. One-step template/chemical blowing route to synthesize flake-like porous carbon nitride photocatalyst. Mater. Res. Bull. 2017, 94, 423–427. [Google Scholar] [CrossRef]
- Yang, C.; Zhang, S.; Huang, Y.; Lv, K.; Fang, S.; Wu, X.; Li, Q.; Fan, J. Sharply increasing the visible photoreactivity of g-C3N4 by breaking the intralayered hydrogen bonds. Appl. Surf. Sci. 2020, 505, 144654. [Google Scholar] [CrossRef]
- Lu, X.; Xu, K.; Chen, P.; Jia, K.; Liu, S.; Wu, C. Facile one step method realizing scalable production of g-C3N4 nanosheets and study of their photocatalytic H2 evolution activity. J. Mater. Chem. A 2014, 2, 18924–18928. [Google Scholar] [CrossRef]
- Yang, C.; Tan, Q.; Li, Q.; Zhou, J.; Fan, J.; Li, B.; Sun, J.; Lv, K. 2D/2D Ti3C2 MXene/g-C3N4 nanosheets heterojunction for high efficient CO2 reduction photocatalyst: Dual effects of urea. Appl. Catal. B Environ. 2020, 268, 118738. [Google Scholar] [CrossRef]
- Liu, Q.; Lu, Y.; Lin, S.; Hu, Y.; Tian, N.; Huang, H. Room-temperature controllable synthesis of Bi5O7I nanostrips for improved photocatalytic activity. Colloids Surf. A Physicochem. Eng. Asp. 2020, 594, 124642. [Google Scholar] [CrossRef]
- Li, X.; Li, K.; Ding, D.; Yan, J.; Wang, C.; Carabineiro, S.A.C.; Liu, Y.; Lv, K. Effect of oxygen vacancies on the photocatalytic activity of flower-like BiOBr microspheres towards NO oxidation and CO2 reduction. Sep. Purif. Technol. 2023, 309, 123054. [Google Scholar] [CrossRef]
- Qin, D.; Xie, D.; Zheng, H.; Li, Z.; Tang, J.; Wei, Z. In-Situ FTIR Study of CO2 Adsorption and Methanation Mechanism Over Bimetallic Catalyst at Low Temperature. Catal. Lett. 2021, 151, 2894–2905. [Google Scholar] [CrossRef]
- Liu, Z.; Ma, J.; Hong, M.; Sun, R. Potassium and Sulfur Dual Sites on Highly Crystalline Carbon Nitride for Photocatalytic Biorefinery and CO2 Reduction. ACS Catal. 2023, 13, 2106–2117. [Google Scholar] [CrossRef]
- Duan, Y.; Li, X.; Lv, K.; Zhao, L.; Liu, Y. Flower-like g-C3N4 assembly from holy nanosheets with nitrogen vacancies for efficient NO abatement. Appl. Surf. Sci. 2019, 492, 166–176. [Google Scholar] [CrossRef]
- Li, Y.; Gu, M.; Zhang, M.; Zhang, X.; Lv, K.; Liu, Y.; Ho, W.; Dong, F. C3N4 with engineered three coordinated (N3C) nitrogen vacancy boosts the production of 1O2 for Efficient and stable NO photo-oxidation. Chem. Eng. J. 2020, 389, 124421. [Google Scholar] [CrossRef]


| Sample | BCN | CN-Cl 1 | CN-Cl 2 | CN-Cl 5 | CN-Cl 10 | CN-Cl 20 |
|---|---|---|---|---|---|---|
| SBET (m2 g−1) | 6.8 | 10.7 | 30.7 | 21.3 | 20.6 | 21.3 |
| Total pore volume (cm3g−1) | 0.07 | 0.09 | 0.29 | 0.20 | 0.13 | 0.16 |
| Average particle size (nm) | 42.6 | 35.2 | 38.1 | 39.2 | 26.6 | 26.9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Z.; Chang, S.; Yu, M.; Zhao, Z.; Li, Q.; Lv, K. Boosting the Photoreactivity of g-C3N4 towards CO2 Reduction by Polymerization of Dicyandiamide in Ammonium Chloride. Catalysts 2023, 13, 1260. https://doi.org/10.3390/catal13091260
Wang Z, Chang S, Yu M, Zhao Z, Li Q, Lv K. Boosting the Photoreactivity of g-C3N4 towards CO2 Reduction by Polymerization of Dicyandiamide in Ammonium Chloride. Catalysts. 2023; 13(9):1260. https://doi.org/10.3390/catal13091260
Chicago/Turabian StyleWang, Zhi, Shixin Chang, Mengxue Yu, Zaiwang Zhao, Qin Li, and Kangle Lv. 2023. "Boosting the Photoreactivity of g-C3N4 towards CO2 Reduction by Polymerization of Dicyandiamide in Ammonium Chloride" Catalysts 13, no. 9: 1260. https://doi.org/10.3390/catal13091260








