Catalytic Oxidation of Volatile Organic Compounds Alone or in Mixture over Mg4Al2−xCex Mixed Oxides
Abstract
:1. Introduction
2. Results and Discussion
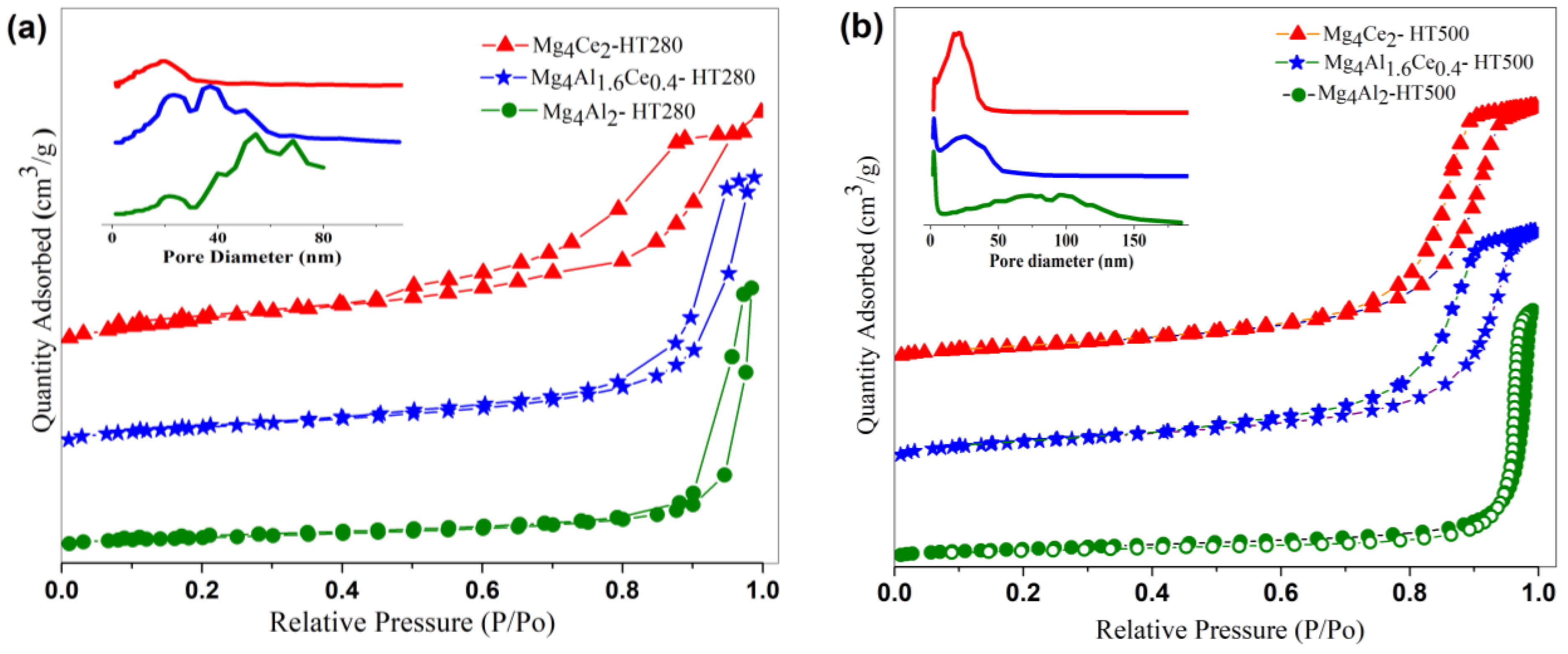
2.1. Characterization of Dried Samples
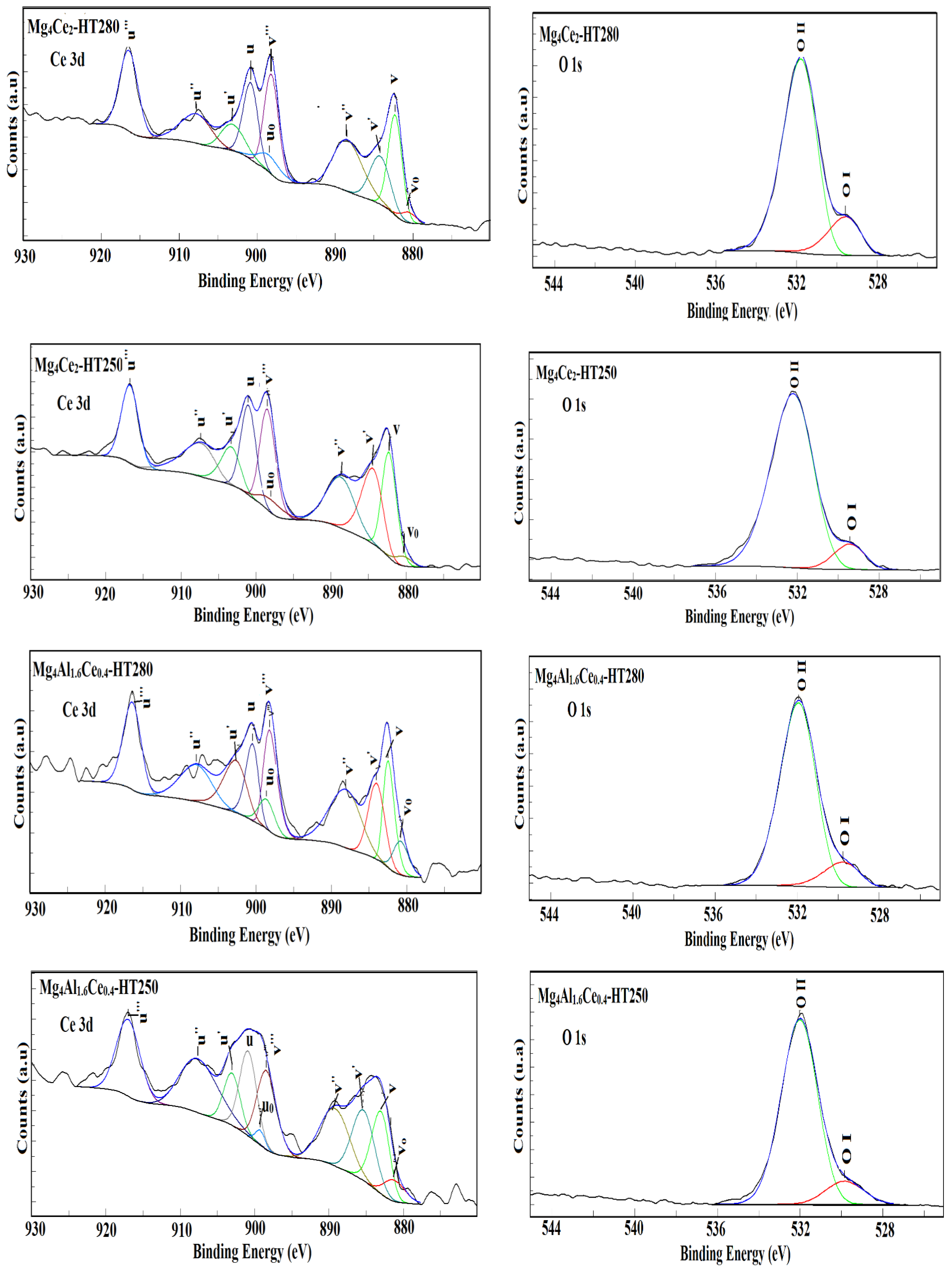
2.2. Characterization of Calcined Samples
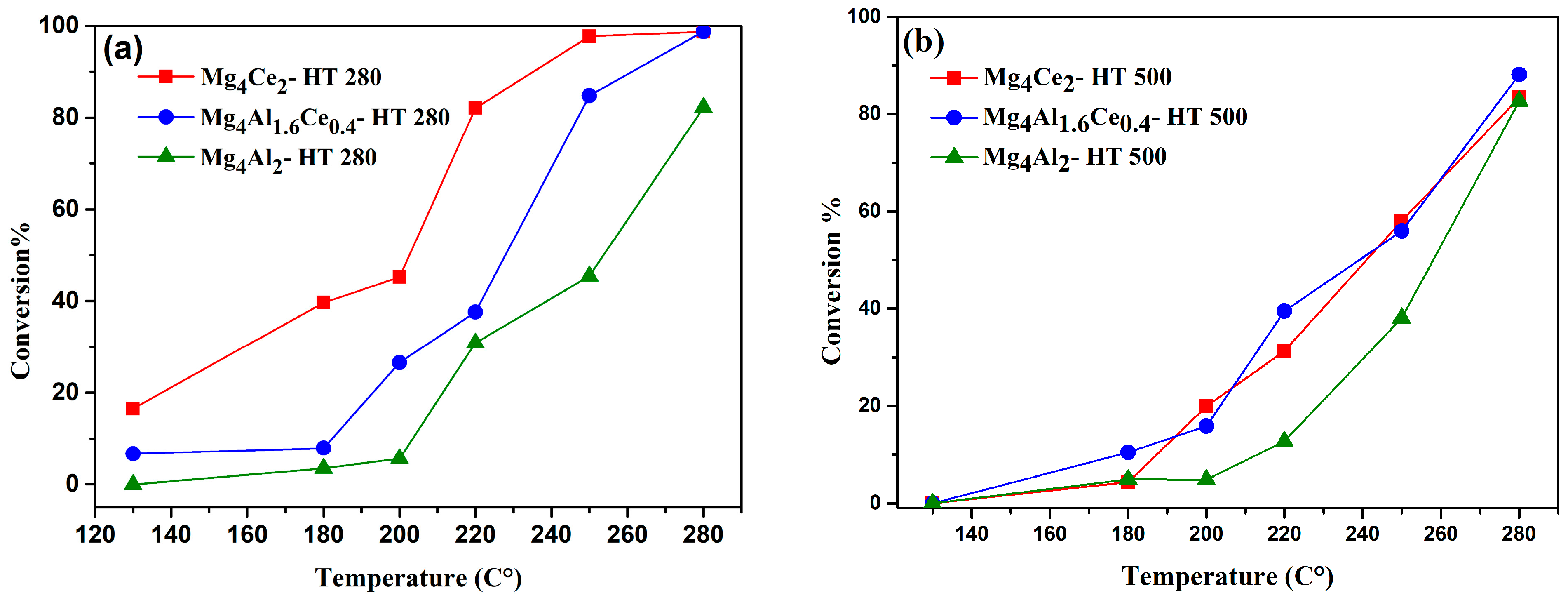
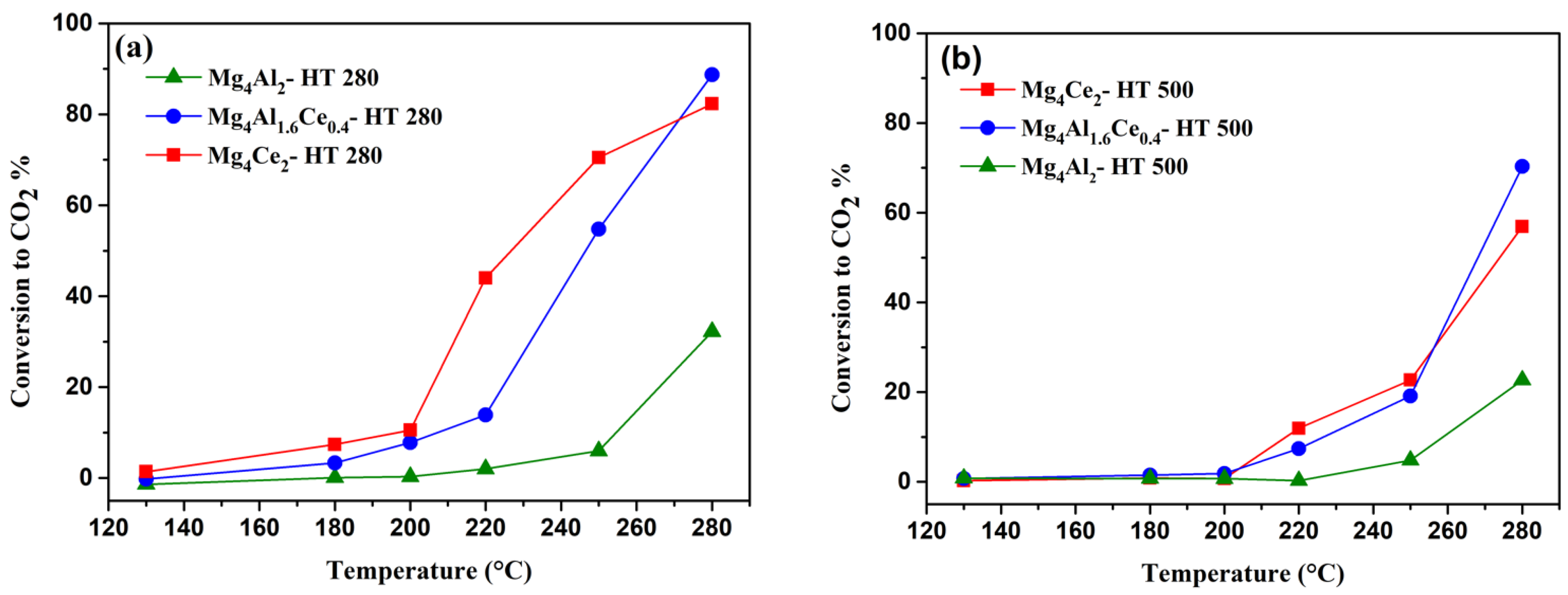
2.3. Catalytic Performance in VOC Oxidation
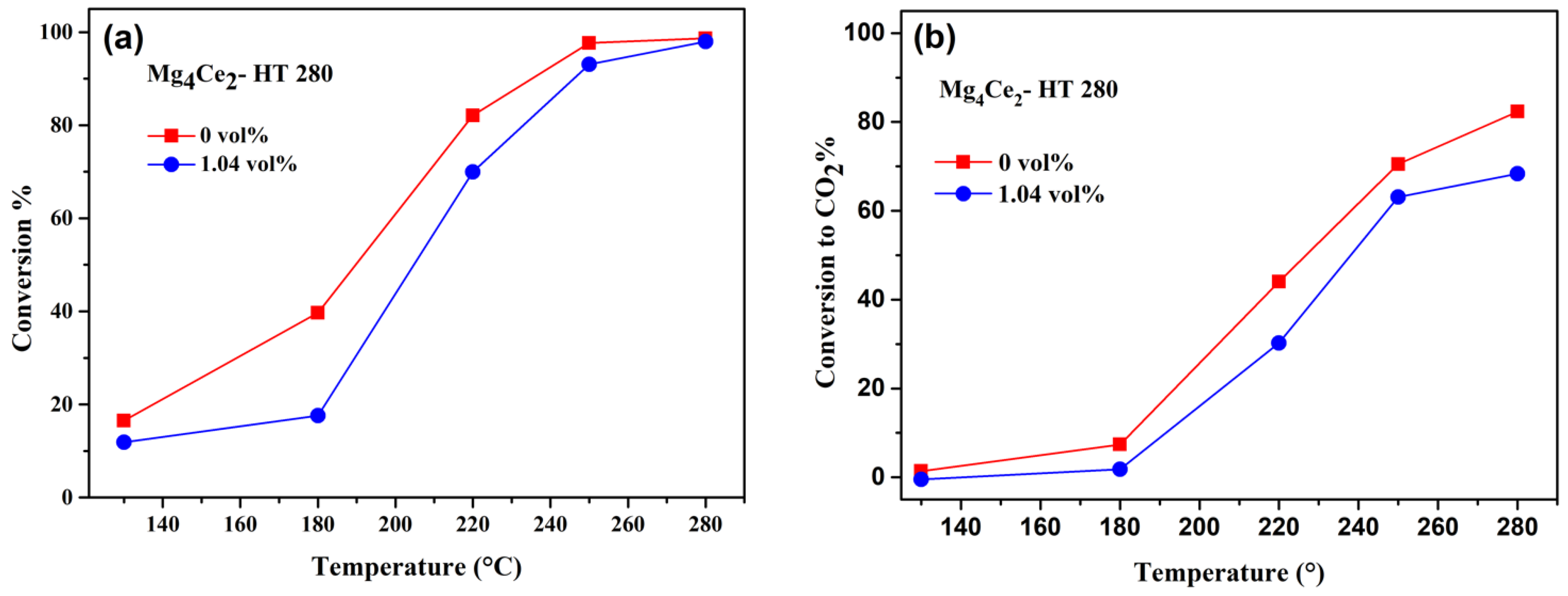
2.3.1. n-Butanol Oxidation
2.3.2. Effect of Water Vapor on the Reactivity of Mg4Ce2HT-280 Material
2.3.3. Effect of n-Butanol/Ethyl Acetate Mixture on the Reactivity of Mg4Ce2HT-280 Material
3. Experimental Section
3.1. Preparation of Mg-Al-Ce Mixed Oxides
3.2. Characterization Techniques and Methods
3.3. Catalytic Experiments
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- El Khawaja, R.; Veerapandian, S.K.P.; Rim Bitar, R.; De Geyter, N.; Rino Morent, R.; Heymans, N.; De Weireld, G.; Barakat, T.; Ding, Y.; Grêce, A.; et al. Boosting VOCs elimination by coupling different Techniques. Chem. Synth. 2022, 2, 13. [Google Scholar] [CrossRef]
- Papaefthimiou, P.; Ioannides, T.; Verykios, X.E. VOC removal: Investigation of ethylacetate oxidation over supported Pt catalysts. Catal. Today 1999, 54, 81–92. [Google Scholar] [CrossRef]
- Finol, M.F.; Rooke, J.; Su, B.L.; Trentesaux, M.; Giraudon, J.M.; Lamonier, J.F. Additional effects of Pt and Nb on hierarchically porous titania in the catalytic removal of n-butanol. Catal. Today 2012, 192, 154–159. [Google Scholar] [CrossRef]
- Aggett, K.; Davies, T.E.; Morgan, D.J.; Hewes, D.; Taylor, S.T. The Influence of Precursor on the Preparation of CeO2 Catalysts for the Total Oxidation of the Volatile Organic Compound Propane. Catalysts 2021, 11, 1461. [Google Scholar] [CrossRef]
- Górecka, S.; Pacultová, K.; Fridrichová, D.; Górecki, K.; Bílková, T.; Žebrák, R.; Obalová, L. Catalytic Oxidation of Ammonia over Cerium-Modified Copper Aluminium Zinc Mixed Oxides. Materials 2021, 14, 6581. [Google Scholar] [CrossRef] [PubMed]
- Pande, G.; Selvakumar, S.; Batra, V.S.; Gardoll, O.; Lamonier, J.F. Unburned carbon from bagasse fly ash as a support for a VOC oxidation catalyst. Catal. Today 2012, 190, 47–53. [Google Scholar] [CrossRef]
- Valente, J.S.; Tzompantzi, F.; Prince, J. Highly efficient photocatalytic elimination of phenol and chlorinated phenols by CeO2/MgAl layered double hydroxides. Appl. Catal. B Environ. 2011, 102, 276–285. [Google Scholar] [CrossRef]
- Azalim, S.; Franco, M.; Brahmi, R.; Giraudon, J.M.; Lamonier, J.F. Removal of oxygenated volatile organic compounds by catalytic oxidation over Zr-Ce-Mn catalysts. J. Hazard. Mater. 2011, 188, 422–427. [Google Scholar] [CrossRef]
- Blin, J.-L.; Michelin, L.; Lebeau, B.; Naydenov, A.; Velinova, R.; Kolev, H.; Gaudin, P.; Vidal, L.; Dotzeva, A.; Tenchev, K.; et al. Co–Ce Oxides Supported on SBA-15 for VOCs Oxidation. Catalysts 2021, 11, 366. [Google Scholar] [CrossRef]
- Lu, X.; Xie, X.; Li, S.; Zhou, J.; Sun, W.; Xu, Y.; Sun, Y. Treatment of Purified Terephthalic Acid Waste water by Ozone Catalytic Oxidation Method. Water 2021, 13, 1906. [Google Scholar] [CrossRef]
- Figueras, F.; Lopez, J.; Sanchez-Valente, J.; Vu, T.; Clacens, J.; Palomeque, J. Isophorone Isomerization as Model Reaction for the Characterization of Solid Bases: Application to the Determination of the Number of Sites. J. Catal. 2002, 211, 144–149. [Google Scholar] [CrossRef]
- Wang, C.; Humayun, M.; Debecker, D.P.; Wu, C. Electrocatalytic water oxidation with layered double hydroxides confining single atoms. Coord. Chem. Rev. 2023, 478, 214973. [Google Scholar] [CrossRef]
- Lim, A.M.H.; Yeo, J.W.; Zeng, H.C. Preparation of CuZn-Doped MgAl-Layered Double Hydroxide Catalysts through the Memory Effect of Hydrotalcite for Effective Hydrogenation of CO2 to Methanol. ACS Appl. Energy Mater. 2023, 6, 782–794. [Google Scholar] [CrossRef]
- Das, J.; Das, D.; Parida, K.M. Preparation and characterization of Mg-Al hydrotalcite-like compounds containing cerium. J. Colloid Interface Sci. 2006, 301, 569–574. [Google Scholar] [CrossRef]
- Aguero, F.N.; Barbero, B.P.; Gambaro, L.; Cadús, L.E. Catalytic combustion of volatile organic compounds in binary mixtures over MnOx/Al2O3 catalyst. Appl. Catal. B Environ. 2009, 91, 108–112. [Google Scholar] [CrossRef]
- Santos, V.P.; Pereira, M.F.R.; Órfão, J.J.M.; Figueiredo, J.L. Mixture effects during the oxidation of toluene, ethyl acetate and ethanol over a cryptomelane catalyst. J. Hazard. Mater. 2011, 185, 1236–1240. [Google Scholar] [CrossRef]
- Hu, J.; Li, W.B.; Liu, R.F. Highly efficient copper-doped manganese oxide nanorod catalysts derived from CuMnO hierarchical nanowire for catalytic combustion of VOCs. Catal. Today 2018, 314, 147–153. [Google Scholar] [CrossRef]
- Azalim, S.; Brahmi, R.; Agunaou, M.; Beaurain, A.; Giraudon, J.M.; Lamonier, J.F. Washcoating of cordierite honeycomb with Ce-Zr-Mn mixed oxides for VOC catalytic oxidation. Chem. Eng. J. 2013, 223, 536–546. [Google Scholar] [CrossRef]
- Papaefthimiou, P.; Ioannides, T.; Verykios, X.E. Combustion of non-halogenated volatile organic compounds over group VIII metal catalysts. Appl. Catal. B 1997, 13, 175–184. [Google Scholar] [CrossRef]
- Papaefthimiou, P.; Ioannides, T.; Verykios, X.E. Catalytic incineration of volatile organic compounds present in industrial waste streams. Appl. Therm. Eng. 1998, 18, 1005–1012. [Google Scholar] [CrossRef]
- Yu, X.; Deng, J.; Liu, Y.; Jing, L.; Gao, R.; Hou, Z.; Zhang, Z.; Dai, H. Enhanced Water Resistance and Catalytic Performance of Ru/TiO2 by Regulating Brønsted Acid and Oxygen Vacancy for the Oxidative Removal of 1,2-Dichloroethane and Toluene. Environ. Sci. Technol. 2022, 56, 11739–11749. [Google Scholar] [CrossRef]
- Pérez, A.; Lamonier, J.F.; Giraudon, J.M.; Molina, R.; Moreno, S. Catalytic activity of Co-Mg mixed oxides in the VOC oxidation: Effects of ultrasonic assisted in the synthesis. Catal. Today 2011, 176, 286–291. [Google Scholar] [CrossRef]
- Zhitova, E.S.; Sheveleva, R.M.; Kasatkin, A.V.; Zolotarev, A.A.; Bocharov, V.N.; Anastasia, N.; Kupchinenko, A.N.; Dmitry, I.; Belakovsky, D.I. Crystal Structure of Hydrotalcite Group Mineral—Desautelsite, Mg6MnIII2(OH)16(CO3)·4H2O, and Relationship between Cation Size and In-Plane Unit Cell Parameter. Symmetry 2023, 15, 1029. [Google Scholar] [CrossRef]
- Pérez-Ramírez, J.; Mul, G.; Kapteijn, F.; Moulijn, J.A. In situ investigation of the thermal decomposition of Co-Al hydrotalcite in different atmospheres. J. Mater. Chem. 2001, 11, 821–830. [Google Scholar] [CrossRef]
- Wagassa, A.N.; Tufa, L.T.; Lee, J.; Zereffa, E.A.; Shifa, T.A. Controllable Doping of Mn into Ni0.075−xMnxAl0.025(OH)2(CO3)0.0125·yH2O for Efficient Adsorption of Fluoride Ions. Glob. Chall. 2023, 7, 2300018. [Google Scholar] [CrossRef] [PubMed]
- Mora, M.; Jiménez-Sanchidrián, C.; Rafael Ruiz, J. Raman spectroscopy study of layered-double hydroxides containing magnesium and trivalent metals. Mater. Lett. 2014, 120, 193–195. [Google Scholar] [CrossRef]
- Pérez-Ramírez, J.; Mul, G.; Moulijn, J.A. In situ Fourier transform infrared and laser Raman spectroscopic study of the thermal decomposition of Co-Al and Ni-Al hydrotalcites. Vib. Spectrosc. 2001, 27, 75–88. [Google Scholar] [CrossRef]
- Palmer, S.J.; Frost, R.L.; Spratt, H.J. Synthesis and Raman spectroscopic study of Mg/Al, Fe hydrotalcites with variable cationic ratios. J. Raman Spectrosc. 2009, 40, 1138–1143. [Google Scholar] [CrossRef]
- Cwele, T.; Mahadevaiah, N.; Singh, S.; Friedrich, H.B. Effect of Cu additives on the performance of a cobalt substituted ceria (Ce0.90Co0.10O2−δ) catalyst in total and preferential CO oxidation. Appl. Catal. B Environ. 2016, 182, 1–14. [Google Scholar] [CrossRef]
- Sudarsanam, P.; Mallesham, B.; Reddy, P.S.; Großmann, D.; Grünert, W.; Reddy, B.M. Nano-Au/CeO2 catalysts for CO oxidation: Influence of dopants (Fe, La and Zr) on the physicochemical properties and catalytic activity. Appl. Catal. B Environ. 2014, 144, 900–908. [Google Scholar] [CrossRef]
- Kloprogge, J.T.; Frost, R.L. Infrared emission spectroscopic study of the thermal transformation of Mg-, Ni- and Co-hydrotalcite catalysts. Appl. Catal. A Gen. 1999, 184, 61–71. [Google Scholar] [CrossRef]
- Millange, F.; Walton, R.I.; O’Hare, D. Time-resolved in situ X-ray diffraction study of the liquid-phase reconstructitm of Mg-Al-carboaate hydrotalcite-like compounds. J. Mater. Chem. 2000, 10, 1713–1720. [Google Scholar] [CrossRef]
- Lamonier, J.F.; Boutoundou, A.B.; Gennequin, C.; Pérez-Zurita, M.J.; Siffert, S.; Aboukais, A. Catalytic removal of toluene in air over Co-Mn-Al nano-oxides synthesized by hydrotalcite route. Catal. Lett. 2007, 118, 165–172. [Google Scholar] [CrossRef]
- Zhang, W.H.; Guo, X.D.; He, J.; Qian, Z.Y. Preparation of Ni(II)/Ti(IV) Layered Double Hydroxide at High Supersaturation. J. Eur. Ceram. Soc. 2008, 28, 1623–1629. [Google Scholar] [CrossRef]
- Machida, M.; Uto, M.; Kurogi, D.; Kijima, T. MnO(x)-CeO2 binary oxides for catalytic NO(x) sorption at low temperatures. Sorptive removal of NO(x). Chem. Mater. 2000, 12, 3158–3164. [Google Scholar] [CrossRef]
- Larachi, F.; Pierre, J.; Adnot, A.; Bernis, A. Ce 3d XPS study of composite CexMn1−xO2−y wet oxidation catalysts. Appl. Surf. Sci. 2002, 195, 236–250. [Google Scholar] [CrossRef]
- Shyu, J.Z.; Weber, W.H.; Gandhi, H.S. Surface characterization of alumina-supported ceria. J. Phys. Chem. 1988, 92, 4964–4970. [Google Scholar] [CrossRef]
- Martin, D.; Duprez, D. Mobility of surface species on oxides. 1. isotopic exchange of 18O2 with 16O of SiO2, AI2O3, ZrO2, MgO, CeO2, and CeO2-Al2O3. activation by noble metals. correlation with oxide basicity. J. Phys. Chem. 1996, 100, 9429–9438. [Google Scholar] [CrossRef]
- Melang Me Nze, V.; Fontaine, C.; Barbier, J. Synthèse et caractérisation d’oxydes mixtes de type MgAlCe pour l’oxydation catalytique de l’acide acétique. C. R. Chim. 2017, 20, 67–77. [Google Scholar] [CrossRef]
- Lavalley, J.C. Infrared spectrometric studies of the surface basicity of metal oxides and zeolites using adsorbed probe molecules. Catal. Today 1996, 27, 377–401. [Google Scholar] [CrossRef]
- Bordes-Richard, É. Les défis en catalyse hétérogène. L’actual. Chim. 2002, 5–6, 38–44. [Google Scholar]
- Knözinger, H.; Ratnasamy, P. Science and Engineering, Catalytic Aluminas: Surface Models and Characterization of surface sites. Catal. Rev. 1978, 17, 31–70. [Google Scholar] [CrossRef]
- Sun, H.; Chen, S.; Wang, P.; Quan, X. Catalytic oxidation of toluene over manganese oxide octahedral molecular sieves (OMS-2) synthesized by different methods. Chem. Eng. J. 2011, 178, 191–196. [Google Scholar] [CrossRef]
- Pan, H.; Xu, M.; Li, Z.; Huang, S.; He, C. Catalytic combustion of styrene over copper based catalyst: Inhibitory effect of water vapor. Chemosphere 2009, 76, 721–726. [Google Scholar] [CrossRef]
- Kikuchi, R.; Maeda, S.; Sasaki, K.; Wennerström, S.; Eguchi, K. Low-temperature methane oxidation over oxide-supported Pd catalysts: Inhibitory effect of water vapor. Appl. Catal. A Gen. 2002, 232, 23–28. [Google Scholar] [CrossRef]
- Li, X.; Wang, L.; Xia, Q.; Liu, Z.; Li, Z. Catalytic oxidation of toluene over copper and manganese based catalysts: Effect of water vapor. Catal. Commun. 2011, 14, 15–19. [Google Scholar]
- Musialik-Piotrowska, A.; Syczewska, K. Combustion of volatile organic compounds in two-component mixtures over monolithic perovskite catalysts. Catal. Today 2000, 59, 269–278. [Google Scholar]
- Blasin-Aubé, V.; Belkouch, J.; Monceaux, L. General study of catalytic oxidation of various VOCs over La0.8Sr0.2MnO3+x perovskite catalyst—Influence of mixture. Appl. Catal. B Environ. 2003, 43, 175–186. [Google Scholar]
- Mazzarino, I.; Barresi, A.A. Catalytic combustion of voc mixtures in a monolithic reactor. Catal. Today 1993, 17, 335–347. [Google Scholar] [CrossRef]
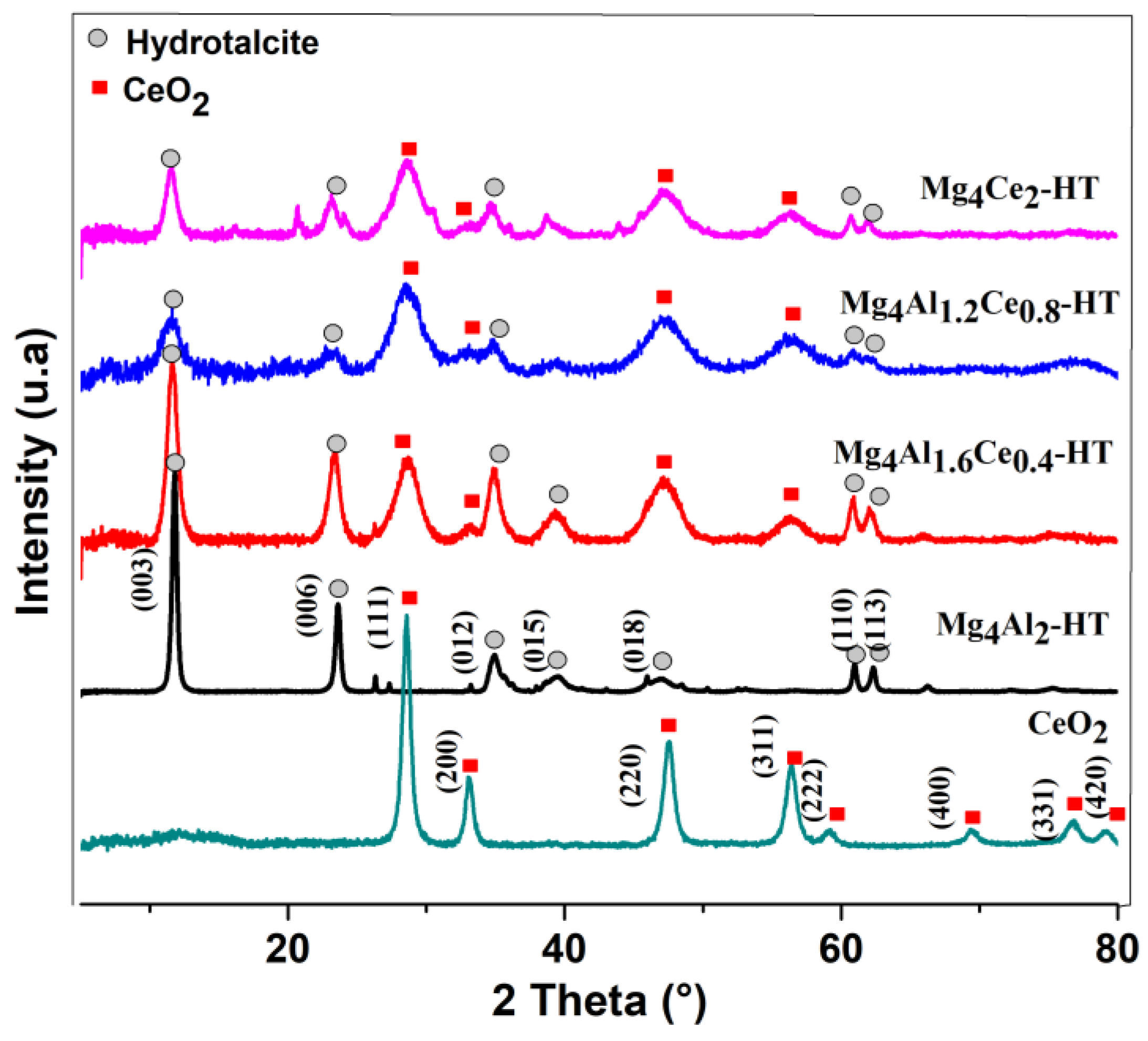
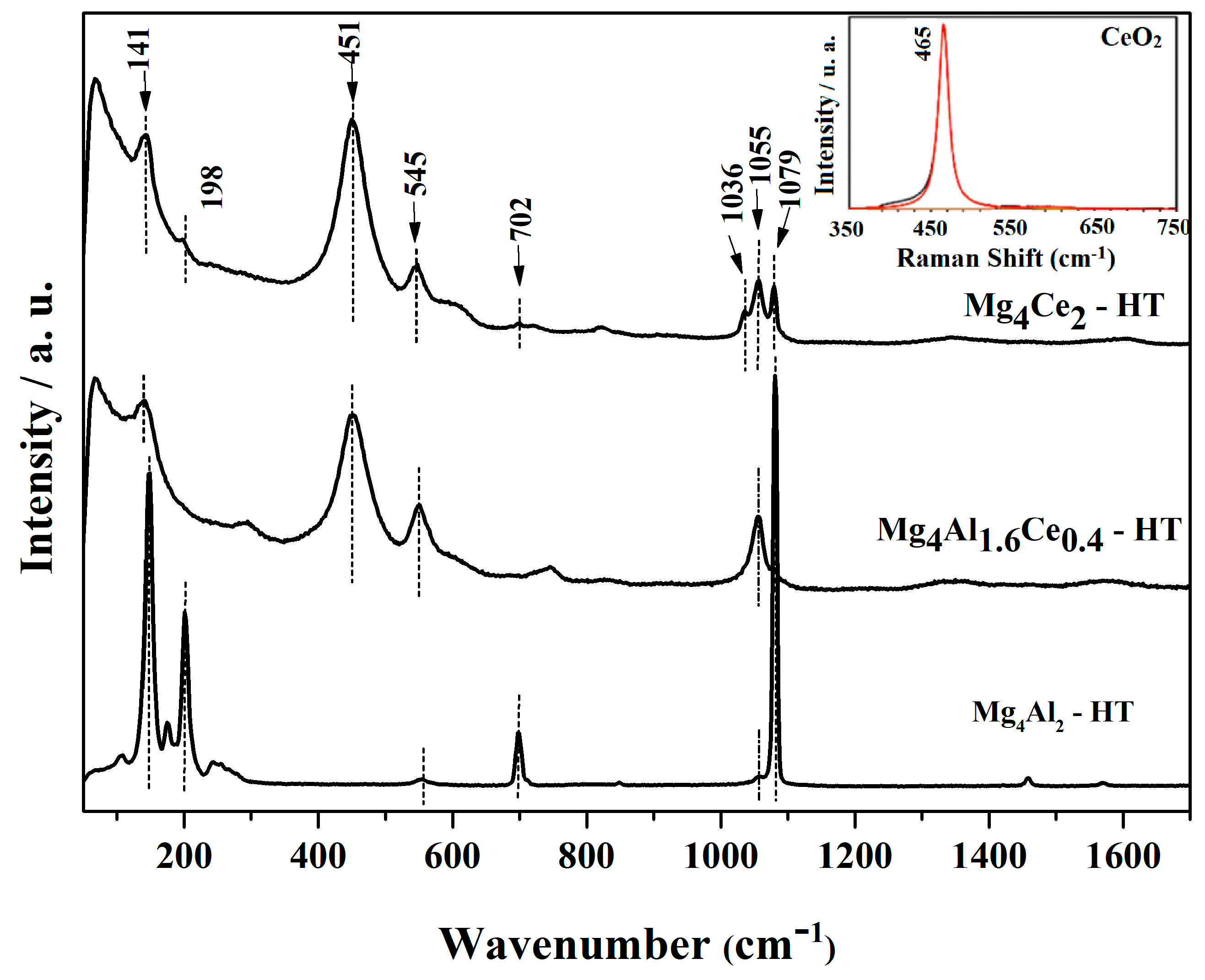
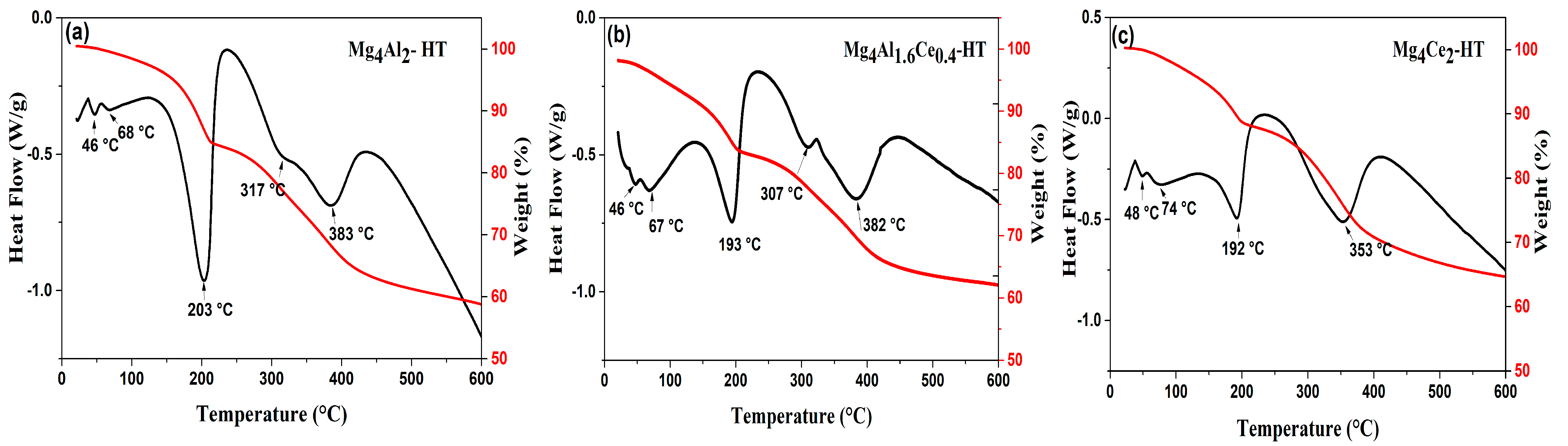
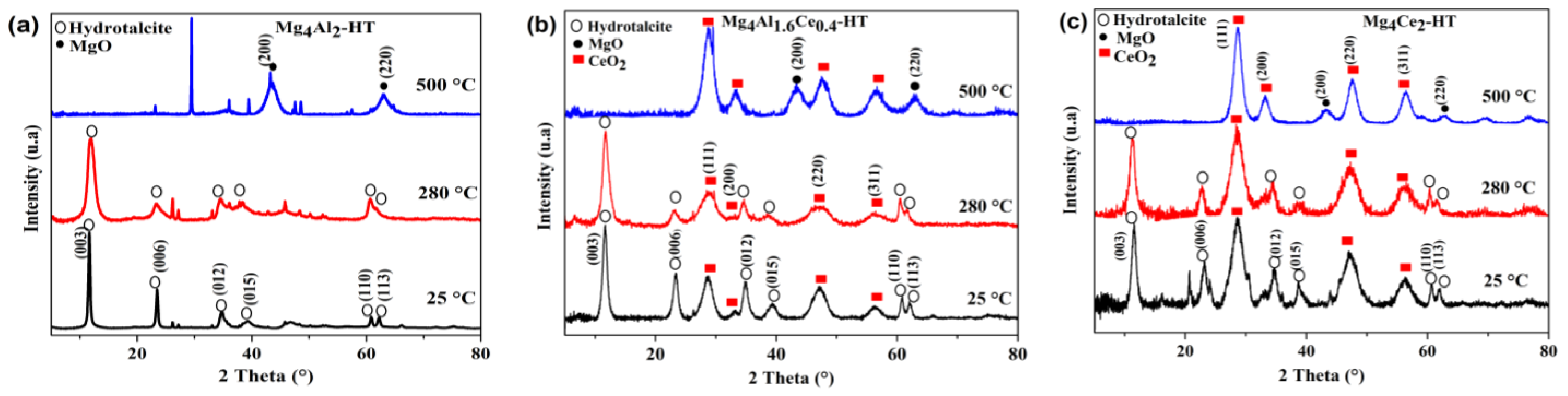



| Sample | Lattice Parameters | |
|---|---|---|
| a (Å) | c (Å) | |
| Mg4Al2-HT | 3.03 | 22,73 |
| Mg4Al1.6Ce0.4-HT | 3.04 | 22.79 |
| Mg4Al1.2Ce0.8-HT | 3.05 | 23.00 |
| Mg4Ce2-HT | 3.05 | 23.00 |
| Sample | Hydrotalcite | CeO2 | MgO | |||
|---|---|---|---|---|---|---|
| L003 (Å) a | L003 (Å) b | L111 (Å) a | L111 (Å) b | L111 (Å) c | L200 (Å) c | |
| Mg4Al2-HT | 183 | 54.6 | - | - | - | 29 |
| Mg4Al1.6Ce0.4-HT | 93.6 | 67.6 | 26.8 | 26.1 | 37 | 34.9 |
| Mg4Ce2-HT | 72.7 | 75.6 | 30.1 | 32.1 | 49.5 | 39.5 |
| Catalyst | SBET (m2/g) | Pore Volume (cm3/g) |
|---|---|---|
| Mg4Al2-HT280 | 58.2 | 0.47 |
| Mg4Al1.6Ce0.4-HT280 | 112.5 | 0.46 |
| Mg4Ce2-HT280 | 101.8 | 0.26 |
| Mg4Al2-HT500 | 117.6 | 0.92 |
| Mg4Al1.6Ce0.4-HT500 | 84.2 | 0.31 |
| Mg4Ce2-HT500 | 90.2 | 0.41 |
| Sample | Ce/(Ce +Mg)Bulk | Ce/(Ce + Mg)XPS | O1s (BE)/eV | % u‴ | |
|---|---|---|---|---|---|
| OI | OII | ||||
| Mg4Al1.6Ce0.4-HT280 | 0.09 | 0.021 | 529.7 | 531.9 | 13.3 |
| Mg4Ce2-HT280 | 0.33 | 0.020 | 529.5 | 531.7 | 14.7 |
| Mg4Al1.6Ce0.4-HT250 | 0.09 | 0.027 | 529.8 | 532.0 | 13.9 |
| Mg4Ce2-HT250 | 0.33 | 0.049 | 529.4 | 532.2 | 12 |
| Oxidized Compound | Compound in Mixture | Mg4Ce2-HT280 | |
|---|---|---|---|
| T50 (°C) | T99 (°C) | ||
| n-Butanol | 205 | 250 | |
| Ethyl acetate | 235 | 280 | |
| n-Butanol | Ethyl acetate | 260 | >280 |
| Ethyl acetate | n-Butanol | 240 | >280 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sahraoui, F.; Haddad, N.; Lamonier, J.-F.; Rabia, C. Catalytic Oxidation of Volatile Organic Compounds Alone or in Mixture over Mg4Al2−xCex Mixed Oxides. Catalysts 2023, 13, 1269. https://doi.org/10.3390/catal13091269
Sahraoui F, Haddad N, Lamonier J-F, Rabia C. Catalytic Oxidation of Volatile Organic Compounds Alone or in Mixture over Mg4Al2−xCex Mixed Oxides. Catalysts. 2023; 13(9):1269. https://doi.org/10.3390/catal13091269
Chicago/Turabian StyleSahraoui, Faiza, Naima Haddad, Jean-François Lamonier, and Chérifa Rabia. 2023. "Catalytic Oxidation of Volatile Organic Compounds Alone or in Mixture over Mg4Al2−xCex Mixed Oxides" Catalysts 13, no. 9: 1269. https://doi.org/10.3390/catal13091269






