Abstract
In line with the development of industrial society, wastewater has caused multiple environmental problems. Contaminants of emerging concern (CECs) in water and wastewater are persistent, and for this reason they can cause serious problems for human health, animal health, and the whole environment. Therefore, it is absolutely necessary to apply efficient methods for the treatment of wastewater that has a high concentration of organic compounds. Over recent years, the prescribed and non-prescribed consumption of antibiotics has increased significantly worldwide. Large quantities of antibiotics are discharged into wastewater because of their incomplete absorption by living organisms. However, even small concentrations present in aquatic environments represent a major risk to human health and environment protection. This paper presents the main advantages and disadvantages of advanced oxidation processes, and the current state and new perspectives in the field of environment protection. This study summarizes data from the most recent specialized scientific literature that focuses on the topic of advanced oxidation processes, thus bringing all these aspects to the attention of researchers in a single work that adds comments and interpretations related to the presented processes. Advanced oxidation processes (AOPs) are often used in the treatment of different types of wastewater. AOPs are based on physicochemical processes that create significant structural changes in chemical species. The majority of antibiotics may be eliminated using physicochemical processes, such as photo-Fenton oxidation, photolysis, ozonation, electrooxidation, heterogeneous catalysis, and other bioprocesses. In comparison to conventional chemical processes, AOPs provide superior oxidation efficiency, ideal operating costs, and zero secondary pollutants.
1. Introduction
The most important natural resource found on Earth is water. It is essential for human life and contributes to the good health of the environment. As is well known, water is unequally allocated in different parts of the world, and the quality is different on all the continents. Conversely, the planet is home to an abundance of aquatic resources; however, the majority of these resources are either inaccessible to humans in their current state or are isolated from them. Some examples of these resources include the salty water found in oceans and seas, as well as glaciers [1,2]. However, continuous access to water and energy is essential to ensure the prosperity and development of the global population. Good management of the two resources is also essential to sustain and improve the health of the environment, and of the human population, respectively [3].
Recently, the availability of fresh water, especially for drinking, has become one of the biggest problems in the world. One of the major causes of this problem, especially in the case of developing countries, is the pollution of surface water by effluent discharges into the surface water from various sectors of activity, such as the chemical and petrochemical industry, the pharmaceutical and cosmetics industry, and the electrical and electronic components manufacturing industry [4,5,6]. Among the many pollutants, persistent organic compounds have a strong negative impact on aquatic flora and fauna [7,8]. Thus, a wide range of technologies have been developed and perfected to remove these organic compounds from effluents before they are discharged into surface water [8]. Among these, the most widely used are advanced oxidation processes (AOPs) such as Fenton oxidation, photo-Fenton oxidation, photocatalysis, and electrochemical and sonochemical advanced oxidation.
AOPs have the potential to be used in the treatment of hazardous effluents, such as those found in hospitals and slaughterhouses, in addition to industrial effluents, which include wastewater from agrochemical and distillery operations, oilfields, textile and pharmaceutical production, and metal plating [1,7]. It is supposed that different contaminants of water, from hazardous contaminants of pesticides, herbicides, detergents, and cosmetics products, to pathogenic agents, can be efficiently removed by photocatalytic processes [1,8].
2. Approaches for Removal of Antibiotics from Wastewater
Pharmaceutical compounds have been a growing concern in recent years due to their negative effects on the environment. In recent years, compounds such as analgesics, antibiotics, and steroids have been detected in public water systems worldwide. A large number of pharmaceutical compounds are used in human and veterinary medicine, and these products are finally discharged into the environment through metabolic processes. These compounds have been introduced in the category of emerging pollutants [8,9].
Antibiotics are produced by the pharmaceutical industry to treat infectious (microbial) diseases and are used extensively in both human and veterinary medicine. The growing demand for antibiotics has led to an increase in their production and, implicitly, in the effluents generated from them, especially those in wastewaters, which have a high content of these compounds. In many cases, due to their poor treatment, these wastewaters end up in surface water in high concentrations [10,11]. Many antibiotics are not totally metabolized by humans and animals, leading to their release in sewage treatment plants and, finally, the environment [12,13].
In the past two years, it has been observed that the pandemic period has contributed to a sudden increase in global antibiotics consumption [14]. Because bacterial co-infections are a viral disease, antibiotics, especially azithromycin, have been used for treatment. Recent reviews have identified that more than 50% of COVID-19 patients receive antibiotics therapy, although less than 10% have a bacterial infection [15]. The fast development pace during the pandemic period represented a direct threat to patients’ safety and public health via an over-prescription of antibiotics [15].
Antibiotics could be considered to be persistent compounds due to the fact that their rate of elimination from the environment is much lower than their rate of their entry into it [16,17]. In addition, the presence of antibiotic residues in the environment is correlated with the pharmacokinetic properties of the compounds. Pharmacies provide different types of antibiotics with oral administration and intravenous administration, and those addressed to different groups (humans, animals) [16].
Several types of antibiotics have been found in industrial, household, pharmaceutical, and hospital wastewaters in different countries worldwide. Depending on the geographical region, seasonal changes, and demographic data, it was found that the concentration value of antibiotics in wastewater may fluctuate from ng/L to μg/L [14]. As a result, numerous natural environments, including groundwater, surface water, soil, and sediments, have been shown to contain antibiotics. The presence of antibiotics in water bodies for extended periods of time may pose a risk to the integrity of ecological systems since it can lead to the evolution of bacteria that are resistant to antibiotics (known as ARBs), as well as genes that are resistant to antibiotics (ARGs) [18,19]. So, in this respect, negative effects of antibiotic resistance and higher antibiotics concentrations in wastewater are reported in some legislation documents (e.g., annual reports from World Health Organization).
One recent analysis estimated that, in the past twenty years, the antibiotics consumption expressed in definite daily doses (DDD) has increased rapidly, by more than 65%. It is estimated that, in the coming years, there will be an increase of up to 200% if major changes are not taken soon. Numerous factors, such as the lack of information, poor health knowledge, the pandemic situation, and fear of disease, drive an excessive use of antibiotics. Setting the quantity of antibiotic residues is very important in establishing a relation between their presence in the environment and their biological effects and ecotoxicological evaluation [16].
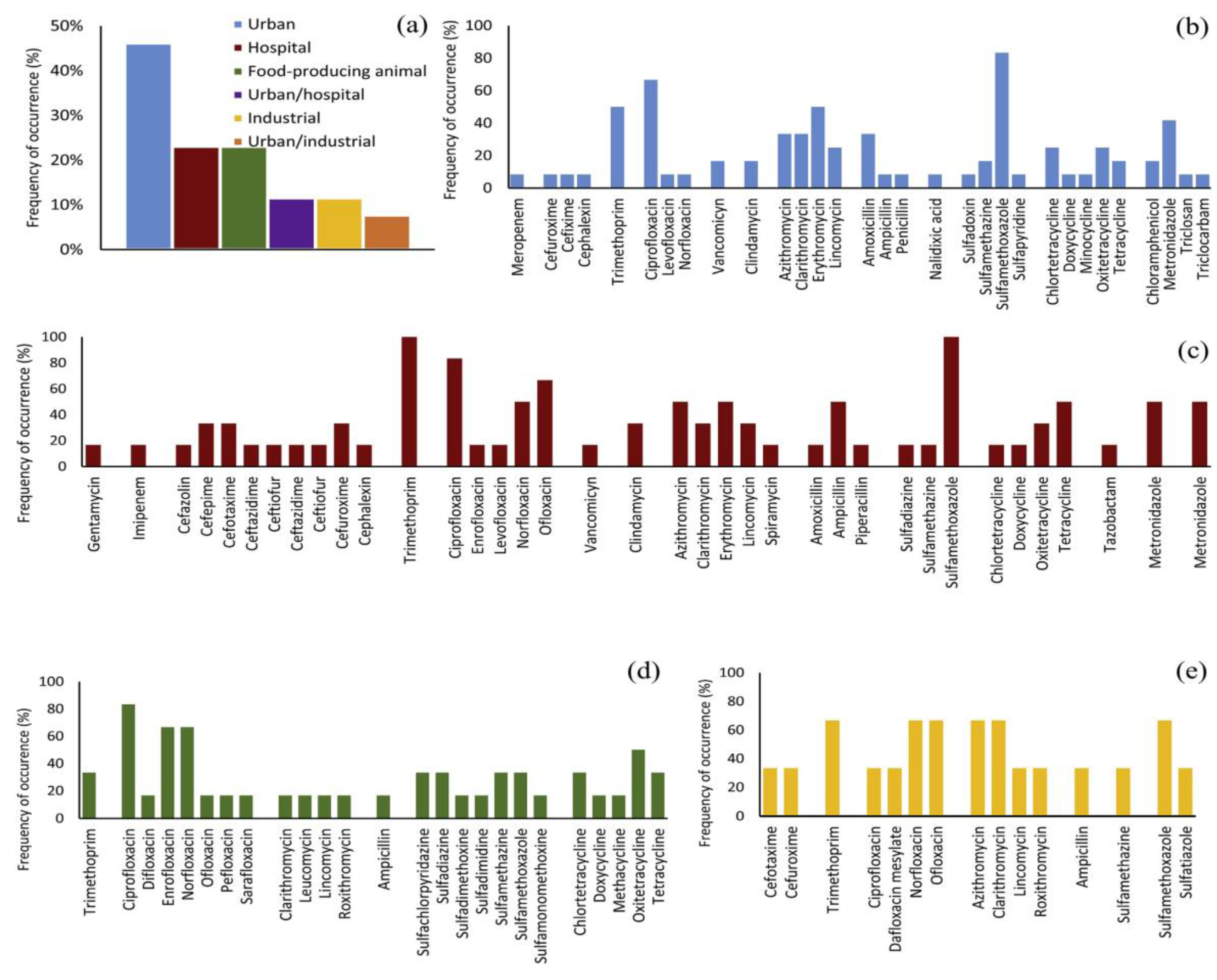
Antibiotics can be classified into two basic categories based on the type of action, namely bacteriostatic (which inhibit the growth and reproduction of bacteria) and bactericidal (which cause the death of bacteria cells) [18]. The occurrence of antibiotics in different wastewaters is shown in Figure 1. Sulfamethoxazole, ciprofloxacin, and trimethoprim are antibiotics that are used to treat a large number of infections of the urinary tract, respiratory system, and gastrointestinal tract. They were detected in hospital, urban, and pharmaceutical wastewaters, often in high concentrations. Antibiotic degradation is a process of breaking down antibiotics into smaller, less harmful substances. This is important because antibiotics that are designed to kill bacteria can have unintended effects on the environment and human health. When antibiotics are not completely degraded, they can persist in the environment, leading to the development of antibiotic-resistant bacteria and other negative consequences.
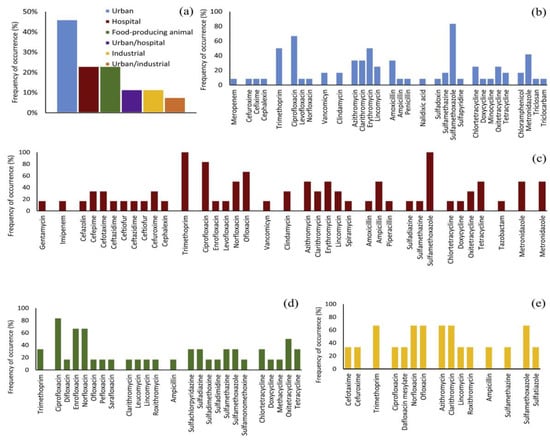
Figure 1.
Occurrence of some antibiotics in different types of wastewater (adapted from [14]): (a) wastewater sources of antibiotics reported in recent studies; antibiotic types and their detection frequency in: (b) urban wastewater, (c) hospital wastewater, (d) wastewater from the production of food, (e) industrial wastewater.
There are several different mechanisms of antibiotic degradation, including chemical, physical, and biological processes. Chemical degradation of antibiotics occurs through reactions with other substances, or water, as well with sunlight, which can break down the chemical structure of the antibiotics. Physical degradation of antibiotics occurs when they are broken down into smaller particles through processes such as grinding or crushing.
Biological degradation of antibiotics, on the other hand, is the process by which microorganisms such as bacteria and fungi break down the antibiotics into smaller, less harmful substances. This is a crucial process because it helps reduce the quantity of antibiotics that persist in the environment. Additionally, by breaking down antibiotics, these microorganisms can help reduce the risk of antibiotic resistance and the spread of antibiotic-resistant bacteria.
The degradation of antibiotics in the environment is a complex process that is influenced by many factors. Some of the most important factors that contribute to the degradation of antibiotics include the type of antibiotic, the presence of other substances in the environment, and the conditions of the environment, such as temperature, pH, and moisture. The effectiveness of antibiotic degradation is also influenced by the presence of other pollutants in the environment, as well as the presence of other microorganisms that may compete for the same resources.
Despite the importance of antibiotic degradation, there is still much that is unknown about this process. To better understand the degradation of antibiotics, researchers have developed a few methods for measuring and tracking the degradation of these compounds in the environment. Some of these methods include the use of bioreactors, which allow researchers to study the degradation of antibiotics in controlled environments, and the use of analytical techniques such as mass spectrometry and chromatography, which allow researchers to identify and quantify the products of degradation.
To help to reduce the negative impact of antibiotics on the environment and human health, it is important to develop new methods for managing the degradation of antibiotics. This may involve developing new methods for removing antibiotics from the environment, such as through the use of bioreactors or other treatment technologies, as well as improving our understanding of the factors that contribute to antibiotic degradation. Additionally, it may be important to develop new antibiotics that are more biodegradable and less likely to persist in the environment, as well as to encourage the responsible use of antibiotics by healthcare providers and patients.
3. Advanced Oxidation Processes—Principles, Mechanisms, and Their Applications
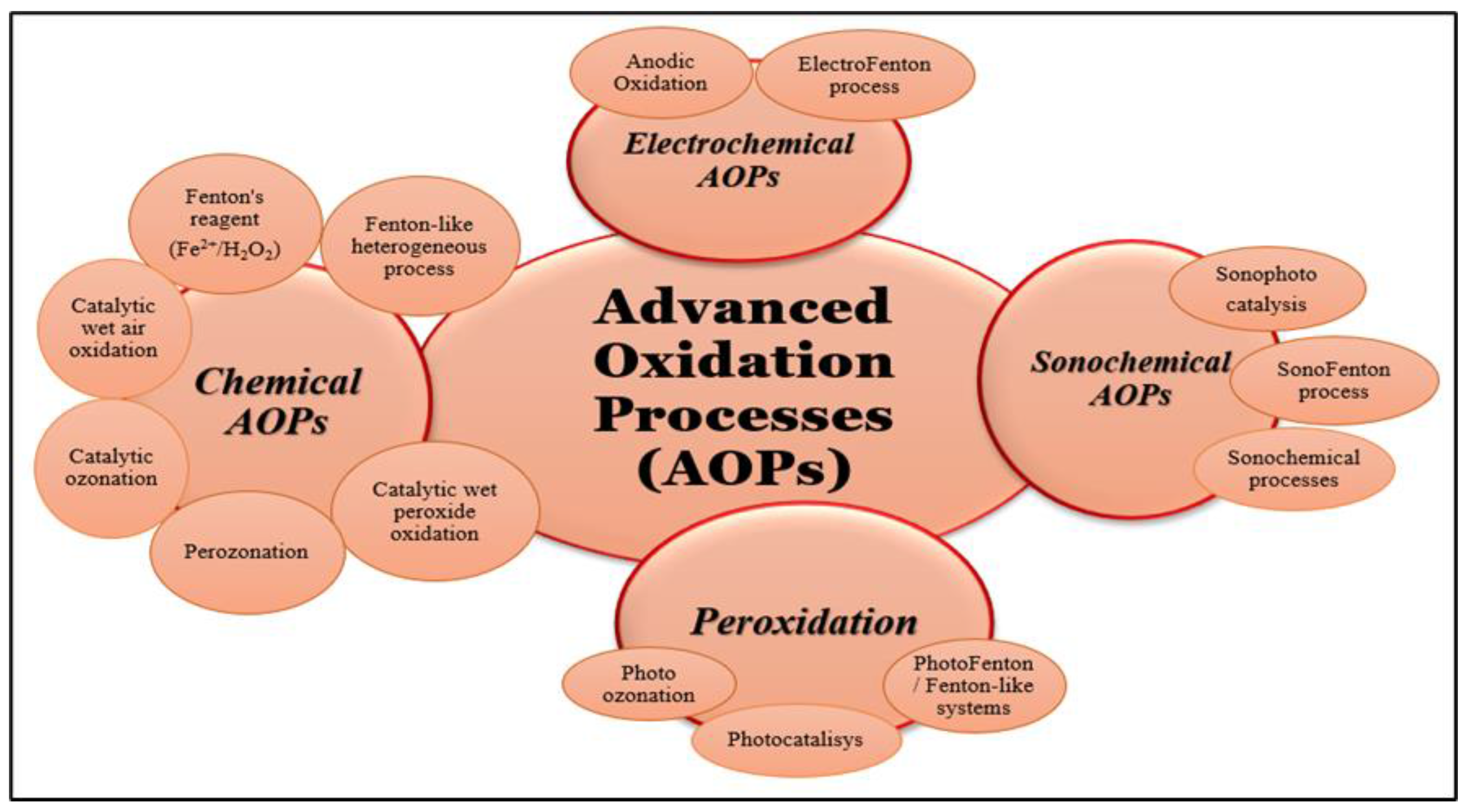
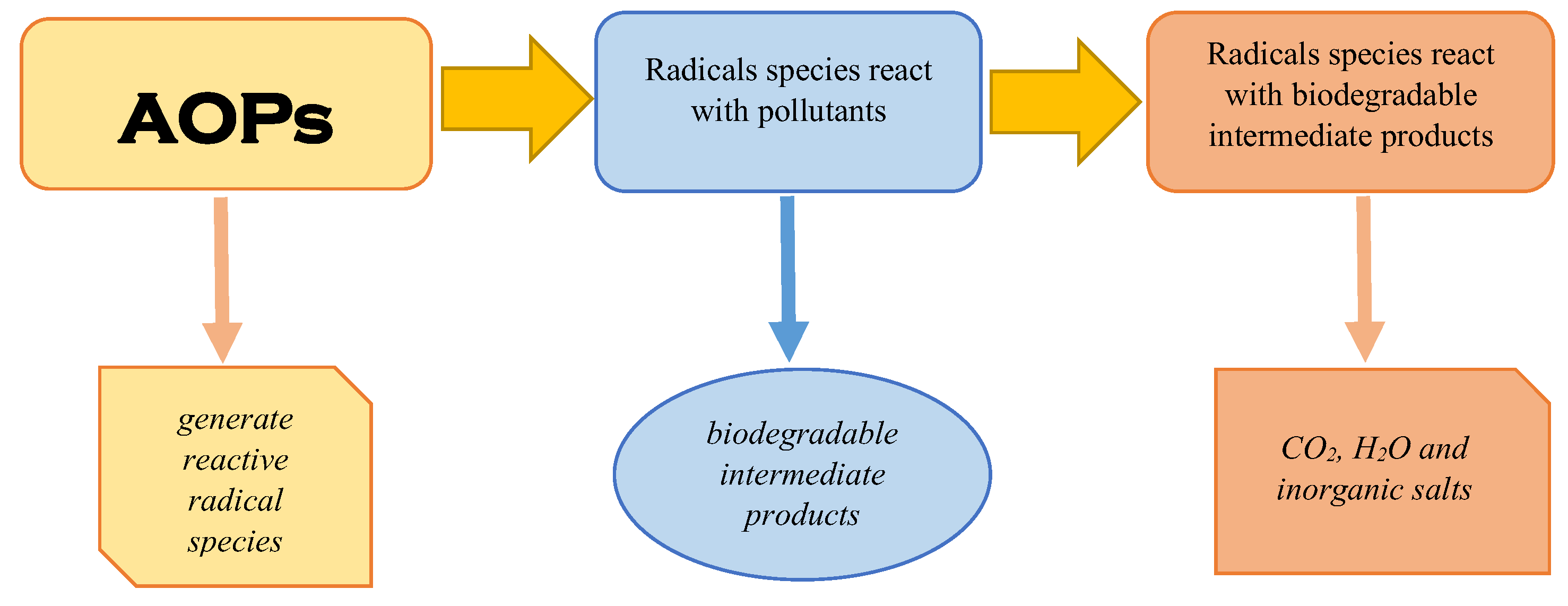
Advanced oxidation processes, often known as AOPs, are methods that generate extremely reactive radicals in order to destroy organic molecules, particularly resistant chemical compounds that are found in aqueous environments. Degradation can be partial, with the formation of organic intermediates, or complete when the mineralization of organic compounds takes place (Figure 2) [18,19]. So, by partial degradation, some compounds are created that are more hydrophilic and biodegradable, have fewer electrons, and are smaller in terms of molecular mass compared with the basic pollutants [20]. These compounds are more easily handled in the next steps of treatment technology [21]. The advanced oxidation processes can be classified depending on the method of generation of reactive radicals, such as chemical, electrochemical, sonochemical, and photochemical, or depending on the reactive phase (homogeneous and heterogeneous) [8,22]. Recently, significant efforts have been made to use solar energy instead of ultraviolet or visible lamps to reduce reactors’ operating costs. However, the results obtained were not always good, thus highlighting the fact that important steps still need to be taken to achieve high efficiencies. In most cases, in order to achieve high efficiencies, advanced oxidation processes using solar energy are placed in ultrasonic or microwave fields, which increases the operating costs of the reactors [8,23]. In addition, regarding the efficiency of the advanced oxidation processes, it must be taken into account that they are based on the strong reactivity of the radicals generated in the system. However, these radicals are non-selective and will act both on the target compounds in the system, which are usually in low concentrations, and on the natural organic material in this system. Therefore, the degradation efficiency of the target compounds may be much lower compared to the overall degradation efficiency of the organic material present in the system.
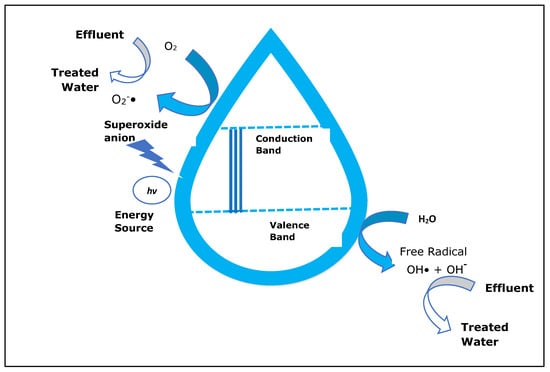
Figure 2.
Advanced oxidation process—schematic of the mechanism.
The advanced oxidation of inhibitory and refractory organic pollutants having low biodegradability and high chemical stability involves the generation of reactive oxygen species (ROS), including hydroxyl radicals (•OH), superoxide radicals (•O2−), and sulphates radicals (SO4•−). All these species are able to be derived from water via the use of hydrogen peroxide (H2O2), ozone (O3), and peroxy-sulphates (PSs), with or without the application of a suitable catalyst or the utilization of solar energy, electrical energy, or sound energy [1,18]. Generally, there are differences in the degradation efficiency of different AOPs for organic pollutants. The combination of different AOPs, including UV/H2O2, ultrasonic/photocatalytic oxidation, UV/O3 and UV/Fe2+/H2O2, and photo/sono/electro-assisted Fenton reaction processes, has been developed to address the limitations of a single AOP in terms of efficient ROS (reactive oxygen species) generation and operating parameters (Figure 3). Due to the synergistic effect of different substances, the combination of different AOPs can significantly improve the oxidation efficiency of contaminants compared with individual treatment technology [6]. The redox potentials of a selection of typical oxidants are shown in Table 1 as a summary in relation to the normal hydrogen electrode (NHE) [1].
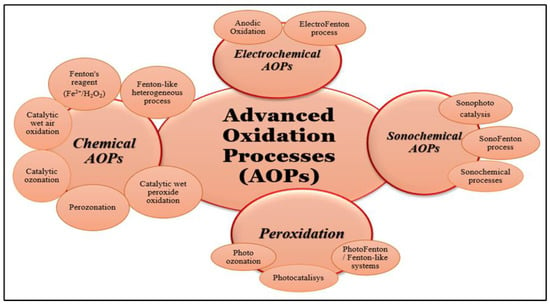
Figure 3.
Advanced oxidation processes that generate reactive oxygen species (adapted from [1,24]).

Table 1.
Redox potentials of common oxidants in reference to a normal hydrogen electrode [1].
When hydrogen peroxide comes into contact with active surfaces, such as catalysts, which typically have a large specific surface area, it is more likely to undergo a chain reaction that results in the release of oxygen in the form of a number of different free radicals. Some of these radicals include •OH and HO2•. Therefore, when combined with an effective catalyst, hydrogen peroxide demonstrates good reactivity and application efficiency, therefore lowering the high operating costs [1]. Regarding the toxicity of H2O2, if ingested, solutions of hydrogen peroxide up to concentrations of 9% are generally nontoxic; however, even a 3% solution is mildly irritating to mucosal tissue and may cause vomiting. Ingestion of industrial-strength solutions (10%) causes systemic toxicity and has been associated with fatalities. In general, the most important mechanisms involved in advanced oxidation are broken down and discussed in Figure 4.
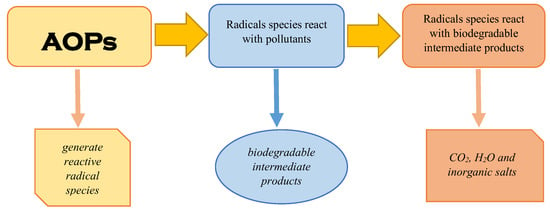
Figure 4.
The processes involved in advanced oxidation processes.
Briefly, the main characteristics of the AOPs are presented as follows:
- -
- The possibility of controlling the oxidation of inorganic chemicals and ions such as chlorides and nitrates, as well as the mineralization of organic contaminants of CO2 (carbon dioxide) and H2O2 (water).
- -
- Non-selective reactivity with practically all organic substances is necessary to avoid the existence of potentially hazardous intermediates produced from primary pollutants. This non-selective reactivity may be achieved using a variety of methods that do not entail the entire oxidation of the pollutant.
- -
- One of the main disadvantages of AOPs is the high cost of employing expensive reagents (H2O2) and the increased energy usage (generation of O3 or UV radiation) [21].
4. Advantages and Disadvantages of AOPs
The AOP processes are relevant for the removal of antibiotics from wastewater, but they have a series of advantages and disadvantages that depend on the type of processes applied and working conditions; thus, all these aspects are reflected in the efficiency of the treatment of wastewater. Next, we synthesize these advantages and disadvantages. The advantages include the following: organic compounds are transformed into stable inorganic compounds such as carbon dioxide, water, and salts; they have high reaction rates [3]; they have the potential to reduce toxicity and completely mineralize organic contaminants; there is no need to concentrate waste for further treatment through methods using membranes or activated carbon adsorption [25]; different organics can be treated at the same time; the cost is relatively low compared with other technologies; during oxidation processes, heavy metals could precipitate as hydroxides and can be removed in a subsequent stage [8,23]; hydroxyl radicals could help in the disinfection process during the wastewater treatment simultaneously with the degradation of organic compounds [8]; and no new organic compounds with higher toxicity are formed [2,8].
In addition to these advantages, the advanced oxidation processes have some disadvantages, including the following: a large consumption of acid and base is determined by the AOP (Fenton oxidation), which is usually conducted in acid conditions [4]; the use of H2O2 can be dangerous for humans; the efficiency of the process depends on the dosage, so it is important to use the right amount in order to form an appropriate amount of hydroxyl radicals [4]; the cost of AOPs can be high because of the need for chemicals and the high energy consumption, as well as the possibility of forming unknown, persistent by-products; and AOPs are used for the elimination of radicals by non-target substances, but they are not effective for toxic compounds that resist hydroxyl radical involvement [4,8].
5. Fenton and Photo-Fenton Oxidation Processes
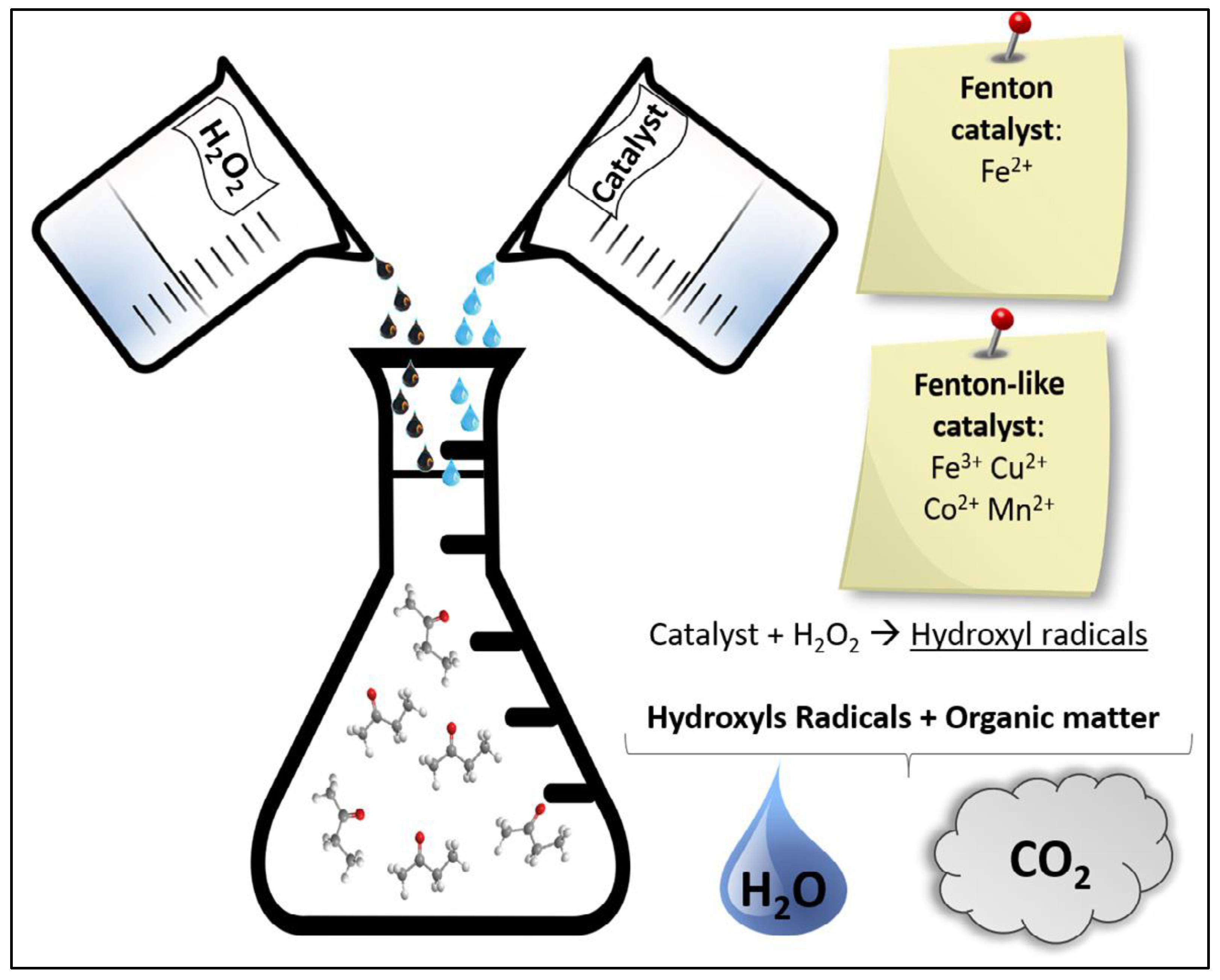
The first considered AOP is the Fenton process. Using Fenton oxidation, it is possible to treat complex wastewater with the increase in the biodegradability of the effluent’s recalcitrant organic compounds, such as antibiotics, decreasing the toxicity and removing the remnant COD and the color [26,27]. In the Fenton treatment process, a mixture of H2O2 and ferrous iron salts (Fe2+), which form the Fenton reagent generates reactive (•OH) radicals, causing the organic removal of wastewater by involving a complex reaction sequence [23]. The generation of •OH radicals is caused by the decomposition of H2O2 in acidic conditions initiated and catalyzed by the Fe2+. The mechanism of Fenton oxidation is presented in the following reaction:
Fe2+ + H2O2 → Fe3+ + HO− + HO•
According to Equation (2), ferric ions can be reduced by the hydrogen peroxide reaction to reconstruct ferrous ions, but also to produce additional radicals. This type of reaction is called a Fenton-like reaction.
Fe3+ + H2O2 → Fe2+ + H+ + HO2•
While iron is considered a catalyst, the consumption of hydrogen peroxide is continuous as it serves both as a generator of hydroxyl radicals and as a radical scavenger, as demonstrated in Equation (3):
HO• + H2O2 → H2O + HO2•
Figure 5 shows a diagram of the Fenton process. The primary advantages of this method are its ability to be performed under normal conditions (room temperature and atmospheric pressure) and the availability of readily accessible and easy to store and handle chemicals. Another advantage is that mass transfer is minimal due to its uniform structure; therefore, the reactor design is easy. However, this mechanism has two drawbacks: self-decomposition and oxidant loss due to the radical scavenging action of H2O2, as shown in Equation (4) [23].
2 H2O2 → 2 H2O + O2
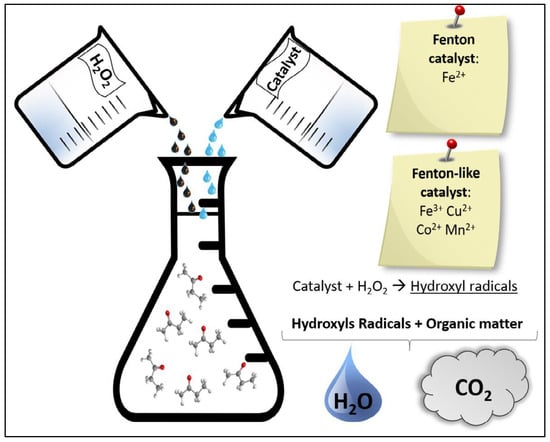
Figure 5.
Diagram of the Fenton process (adapted from [23]).
The second downside refers to the pH conditions. The pH value has to be around the value of 3 because the value of the pH is dependent on the Fenton reaction. In most cases, the pH must be decreased before the treatment because most wastewaters do not have a pH value around 3. In the next phase, it was observed that, in order to precipitate the excess iron, the pH has to be increased with the further solid sludge formation [10].
The concentration of the hydrogen peroxide has a significative role in this process. It was highlighted that a higher concentration of H2O2 leads to greater removal of organic compounds. However, care must be taken with the toxicity induced by high concentrations of hydrogen peroxide in aqueous systems. In this respect, for proper control of the concentration, a continuous dosage of the hydrogen peroxide during the oxidative treatment is preferred [2,23]. It was also highlighted that when the temperature is increased, the reaction rate could also be increased. On the other hand, for low organic concentrations, the temperature could be increased by a few degrees because the reaction of H2O2 with the catalyst is an exothermal one. When organic contaminants are present in large quantities in the wastewater, hydrogen peroxide decomposition (Equation (22)) might be expedited, resulting in increased consumption and higher operational costs [2,23]. It was observed that three other categories of processes derived from Fenton technology were used, namely:
- Fenton-like;
- Heterogeneous Fenton;
- Zero-valent iron (ZVI).
Influencing Factors of the Fenton and Photo-Fenton Oxidation Processes
- Fenton catalysts
Even if the Fenton oxidation process can efficiently degrade organic pollutants, in practical applications some problems may occur. Thus, if the usage rate of H2O2 is low, it could cause a low decomposition rate of pollutants. Furthermore, as stated in the preceding sections, the Fenton process requires a pH of 3, which is lower than the pH used in actual wastewater. As a conclusion, adjusting the pH value could increase the operational costs. Finally, the heterogeneous Fenton or Fenton-like processes can be performed over a broad pH range and the catalyst can be reused repeatedly. These characteristics may help to reduce the development of iron sludge [2,10].
Heterogeneous Fenton catalysts can contain:
- Iron minerals, such as ferrite and magnetite.
- Zero-valent iron.
- Metals and metal oxides such as MnO2.
- Materials containing iron and iron oxide; typically, the supports used include activated carbon, alumina, silica, and zeolite.
- Metal-organic frameworks are crystalline functional materials made of a combination of transition metal ions and organic ligands [10].
- Catalyst dosage
The amount of catalyst used, which plays a major role in breaking down organic pollutants, is a crucial factor in the Fenton and Fenton-like oxidation processes. Overuse of the catalyst may reduce the formation of hydroxyl radicals (•OH) and hinder the degradation of contaminants. As a result, overdosing on catalyst might raise operational expenses [26].
- Concentration of H2O2
H2O2 is the primary generator of hydroxyl radicals (•OH), and it plays a crucial role in the Fenton oxidation process. The reduction in the degradation efficiency is possible if an insufficient H2O2 dosage leads to an insufficient amount of hydroxyl radicals (•OH). The actual added H2O2 concentration is frequently more than the estimated amount based on the chemical equations, which can be evaluated by preparatory tests [2,23].
- pH value
As shown above, in Fenton and Fenton processes, the pH value has a significant role and is an important parameter for efficient treatment of wastewater. The ideal pH value for the homogeneous Fenton process is 3, and the appropriate pH value in the Fenton process is contingent on the reaction system, especially when the reaction mechanisms depend on the catalyst’s efficacy Table 2 [28].

Table 2.
Removal of antibiotics by Fenton and Fenton-like oxidation.
There are several strategies for optimizing the Fenton technology. To avoid sludge production, the operation conditions have to be near a neutral pH value to bypass the neutralization acidification and to operate with a low iron concentration. On the other hand, this technology might be placed in microwave or ultrasound fields. Regarding operation conditions, several strategies are described below:
- One of the strategies is to use some chelating agents such as oxalate, citrate, EDDS (ethylenediamine-N,N’-disuccinic acid), or EDTA (ethylenediaminetetraacetic acid) in order to build an iron ligand complex that can continue to be at a neutral pH in the solution.
- Copper, manganese, and cobalt are other metals that can be a good alternative to ferrous iron. These are derived in the Fenton-like processes.
- Another strategy is to immobilize the ferrous iron on the mesoporous materials, which can be used to conduct a heterogeneous Fenton process [23].
The Fenton process contributes to the generation of reactive species by having Fe(II) and H2O2 in solution, which also leads to the elimination of organic pollutants through the photolysis of H2O2 to produce hydroxyl radicals. It has been observed that the photo-Fenton technology takes place when these three elements are applied [23]. Furthermore, compared with the classical Fenton process, the main improvement is the continuous generation of the catalyst as described in Equation (5). The key results are that a lower amount of iron salt is needed, and a smaller amount of iron residue is produced. Both factors lead to cost reductions and improved process efficiency [23].
Fe3+ + H2O + hv → Fe2+ + HO• + H+
Solar energy, on the other hand, is appealing from a sustainability viewpoint. Moreover, it was observed that when the Fe(OH)2+ is present in the solution, the pH is around the value of 3.0 because of the photoactivity of the Fe(OH)2+, and the process is very efficient [23]. However, the pH adjustment cannot be avoided due to the implications of higher reagent costs, and in these circumstances pH limits remain a concern. It is also suggested that various catalysts should be developed that are more efficient at pH levels near to neutrality. An alternative to using humic acids is chelating agents such as EDTA (ethylenediaminetetraacetic acid), citrate, and oxalate. Furthermore, if sunlight is employed as a source of radiation, the wavelength might shift closer to the visible range depending on the chelating agent. However, the costs will be increased if a chelating agent is used, but the value of TOC (total organic carbon) will be increased [23,37,38].
Lower efficiency is obtained when an excess of H2O2 concentration is used, causing a scavenging effect. Moreover, some undesirable results can be obtained in the improbable case of using a catalyst that can decrease the efficiency. As a conclusion, all these variables have to be studied for every single case [23]. Under appropriate irradiation, solid iron oxides such as hematite might behave as semiconductors, creating electron–hole pairs. Additionally, their zero-point charge might vary, leading to substantial differences in their effectiveness at various pH levels.
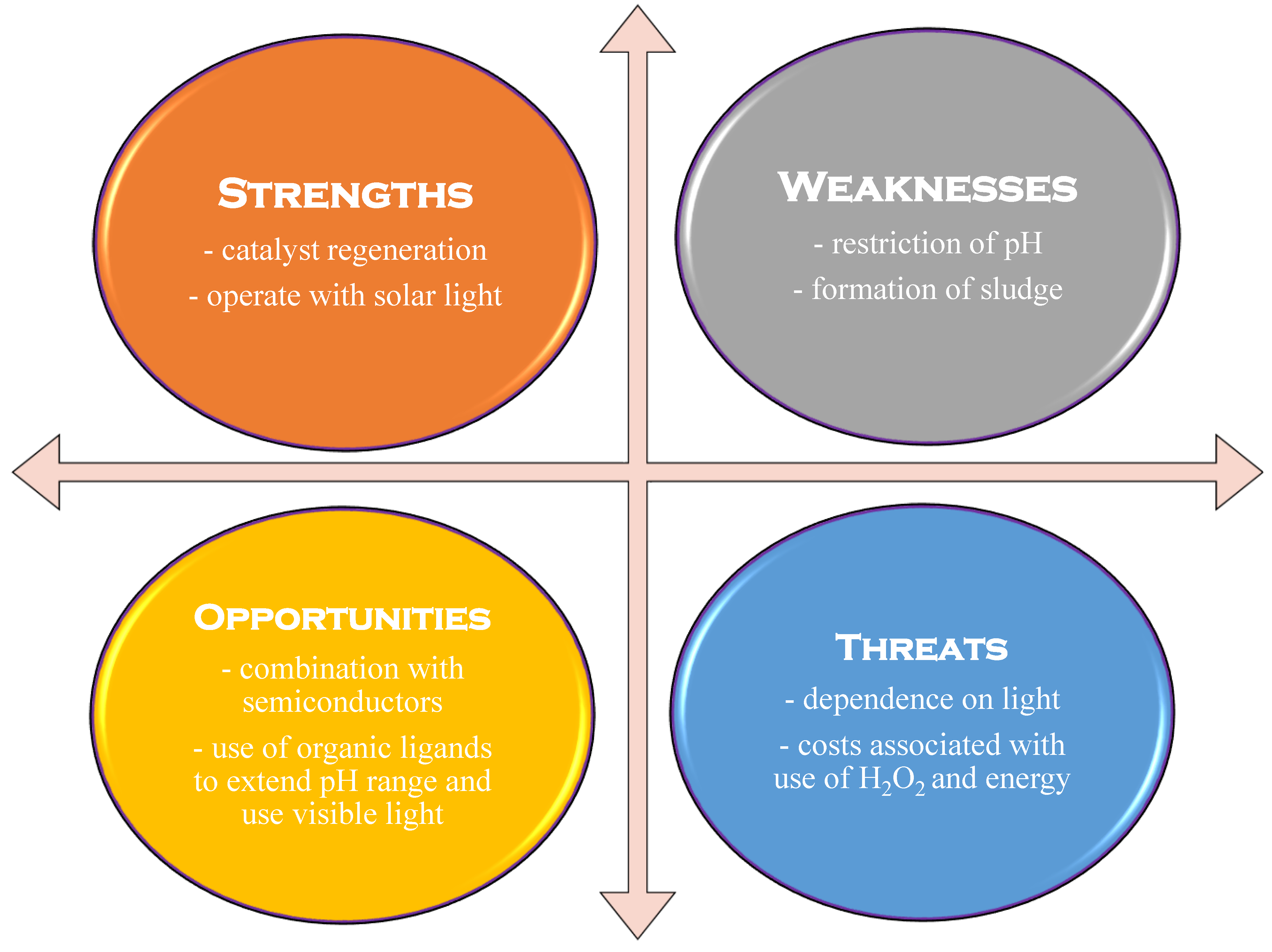
Adding ultrasound to the system leads to a further increase in hydroxyl radical production, resulting in the sono-photo-Fenton process. In this case, the intermediate species produced with Fe3+ can be converted to Fe2+ not only through photolysis, but also through sonolysis. However, the energy needed for ultrasonic generation increases the process’s energy consumption when using this technique [2,23]. The entire energy consumption can be lowered when solar light is used. However, if the treatment time is too long or if there are geographical limitations, research might consider alternative parameters, such as the electrical energy per order (EEO) [23,26]. Figure 6 shows a typical SWOT analysis for a photo-Fenton process.
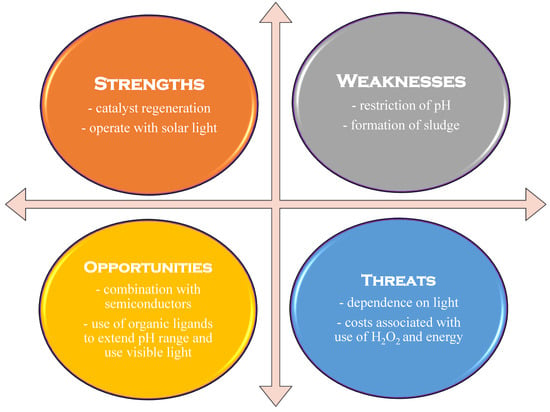
Figure 6.
SWOT analysis for the photo-Fenton process (adapted from [25]).
6. Photochemical Advanced Oxidation Processes
In photochemical accelerated oxidation processes, light energy is the principal source of transient species production. Compared to previous AOPs, this procedure is clean, reasonably inexpensive, straightforward, and much more efficient. In photochemical AOPs, UV/Visible light radiations are linked with potent oxidants such as O3 and H2O2 and catalysts such as titanium dioxide (TiO2). These photochemical reactions may destroy pollutants via three distinct mechanisms: photodecomposition in the presence of O3 under UV irradiation, photooxidation in the presence of H2O2, and oxidation by photocatalysis [1].
6.1. Photo-Peroxidation (H2O2/UV)
H2O2 can be photolyzed by UV radiations at different wavelengths ranging from 200 to 300 nm. Homolytic fission of the O–O bond of the H2O2 molecule is possible and leads to the formation of •OH radicals through a series of successive reactions, as shown in the following equations:
H2O2 + hv → 2•OH
•OH + H2O2 → H2O + HO2•
HO2• + H2O2 → •OH + H2O + O2
•OH + HO2− → HO2• + OH−
2HO2• → H2O2 + O2
•OH + HO2• → H2O + O2
2•OH → H2O2
The reaction rate is faster in an alkaline medium at a value of pH >10, as the UV radiations can produce the free radicals HO2• and •OH. However, the molar absorption coefficient of H2O2 in the UV area is quite low; therefore, a higher concentration of hydrogen peroxide is required for the safe destruction of target contaminants [1,2].
6.2. Photo-Ozonation (O3/UV)
Ozone dissolved in water effectively absorbs UV radiation in the range of 200–360 nm, with the highest absorption occurring at 253.7 nm. This absorption is measured by the molar absorption coefficient, εmax, which has a value of 3600 L mol−1cm−1. Due to this high εmax value, the process of ozone photolysis in water is more efficient than the photolysis of hydrogen peroxide. The photolysis of ozone in water leads to the formation of •OH radicals, which are highly reactive and effective oxidizing agents. These radicals can participate in a series of chemical reactions, as shown in the following equations [23]:
O3 + H2O + hv → 2•OH + O2
O3 + •OH → HO2• + O2
O3 + HO2• → •OH + 2 O2
•OH + HO2• → H2O + O2
The use of nanoparticles made from ZnO and TiO2 as catalysts in the photo-ozonation process has yielded significant results. These materials have proven to be effective in producing oxidizing agents when they are exposed to light [1].
6.3. Heterogeneous Photocatalysis
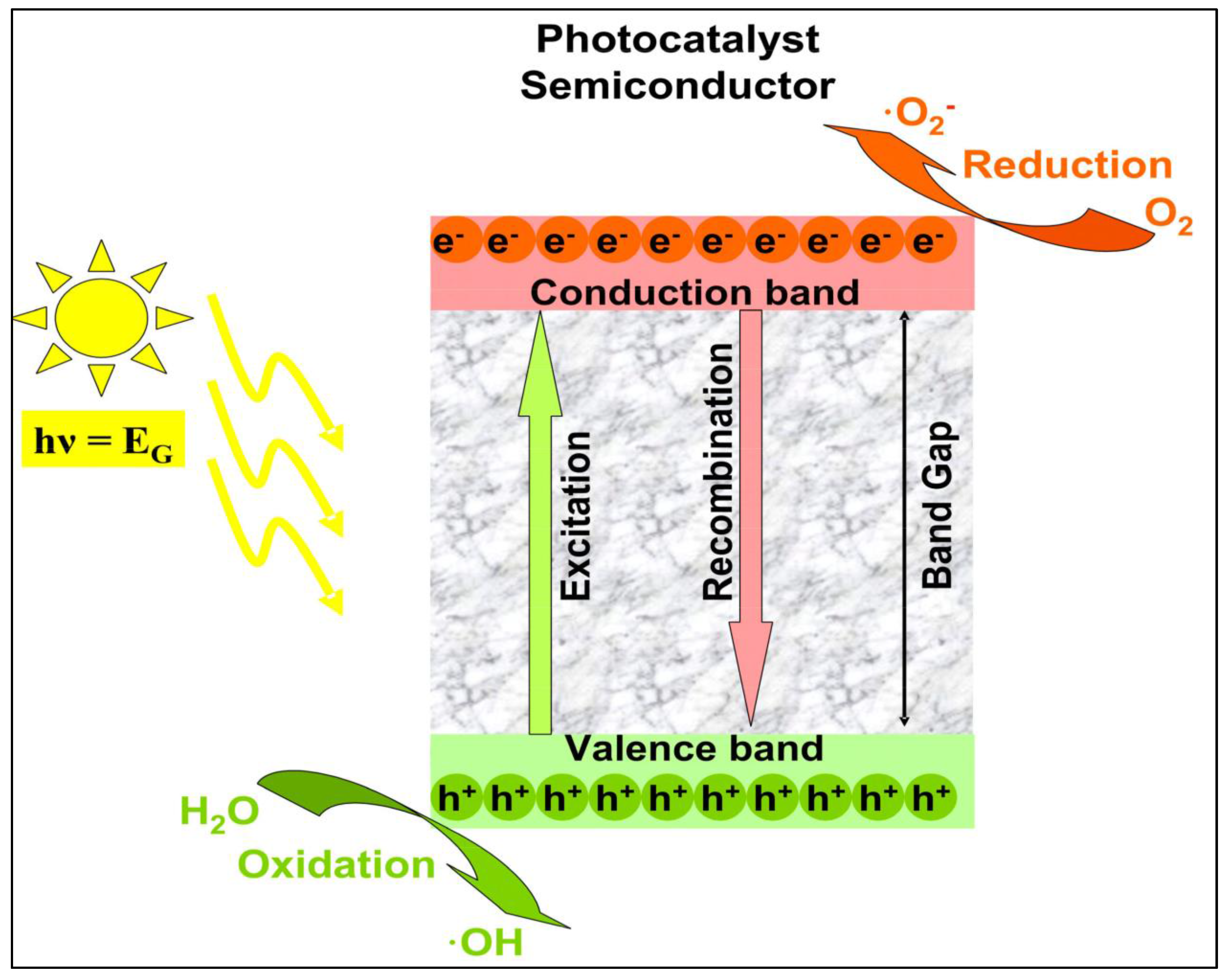
Photocatalysis is a process in which a chemical reaction is accelerated by a catalyst that is activated by the absorption of photons with energy above its bandgap. The term heterogeneous refers to the fact that pollutants are present in a fluid phase while the catalyst is in a solid phase [39]. Various methods of this technique have been attempted, resulting in the development of a new advanced oxidation process (AOP) technology for environmental and energy applications based on semiconductor photocatalysis [1].
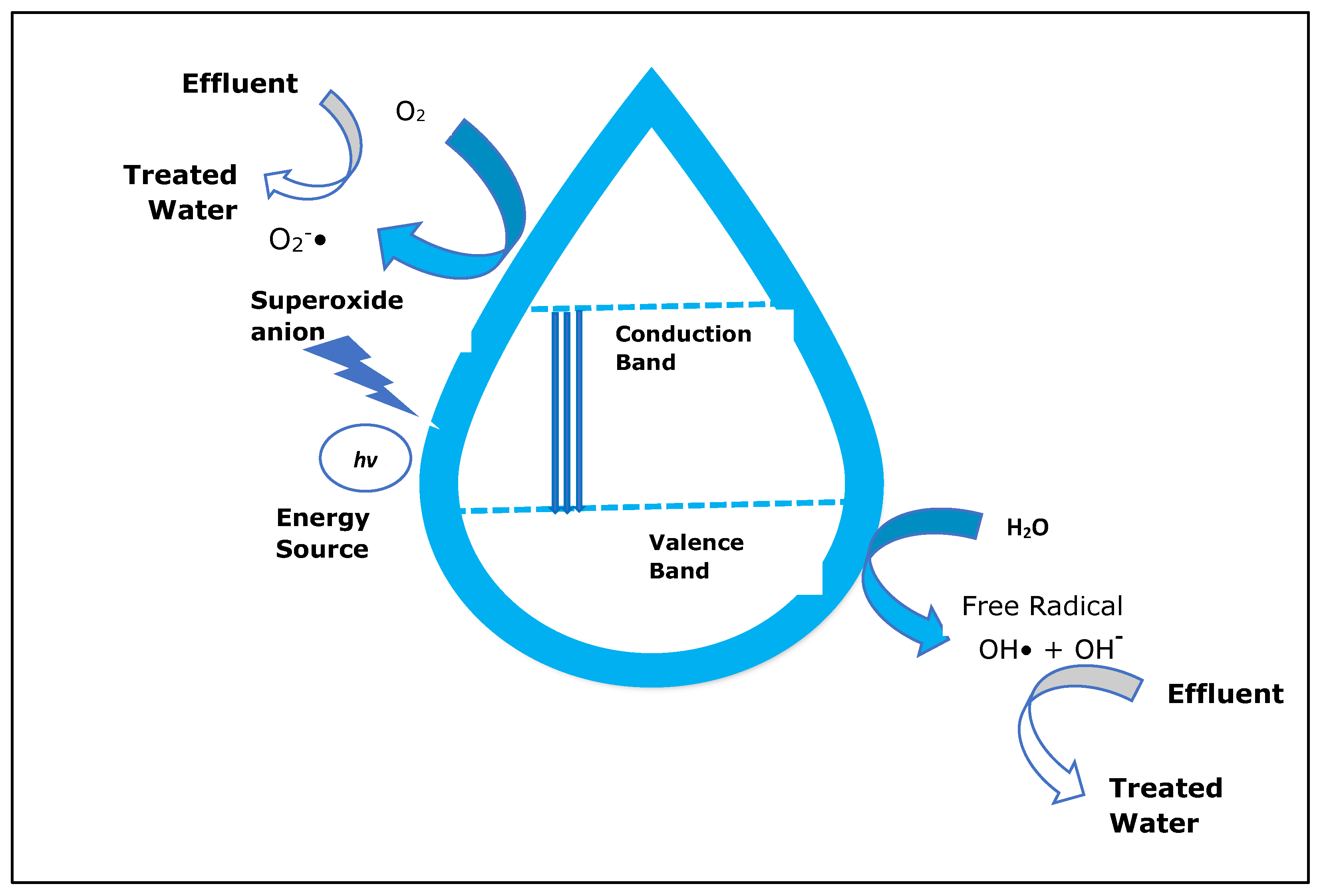
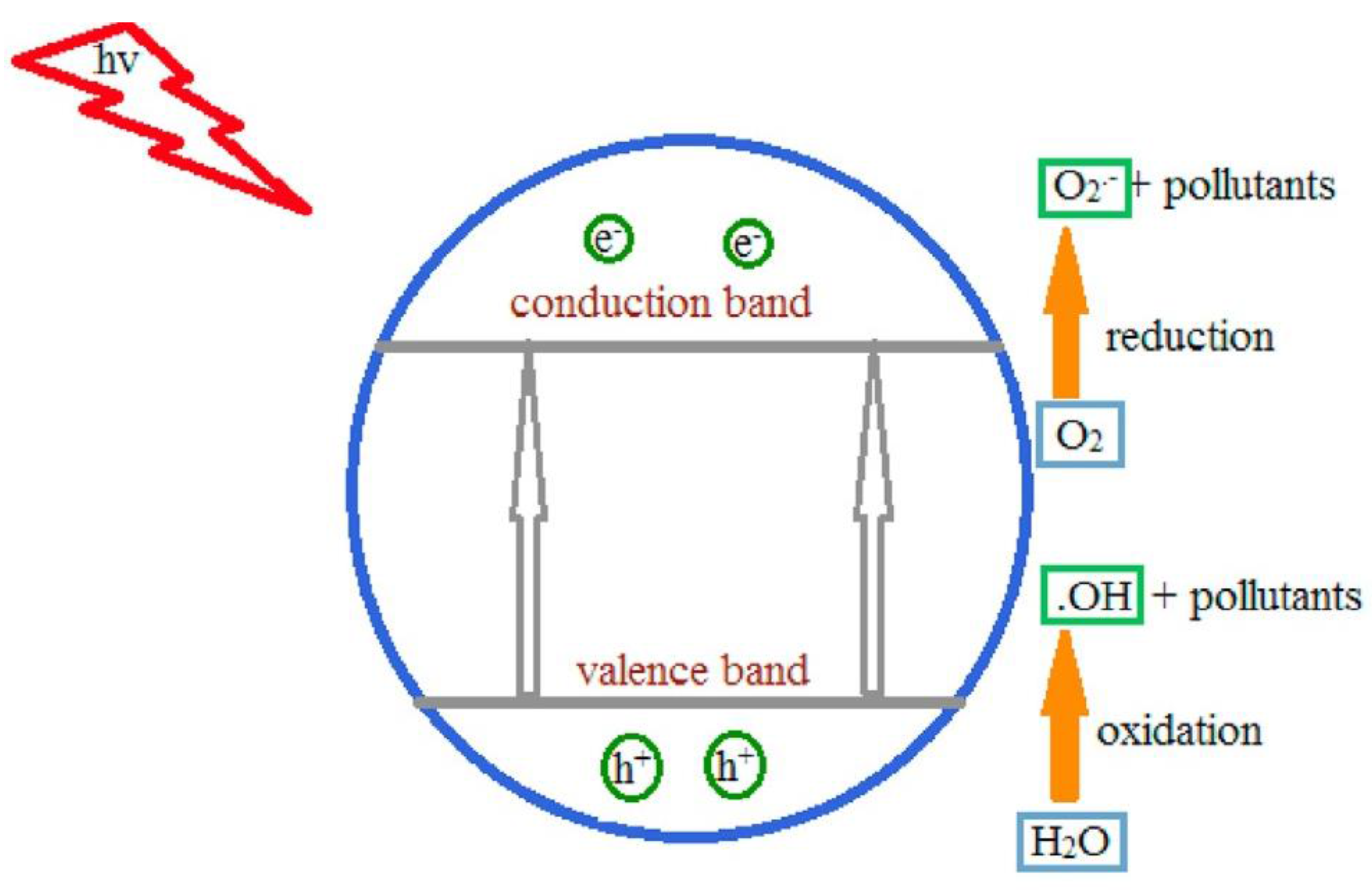
When a semiconductor is exposed to light with ultra-bandgap energy, a valence band electron is excited to the conduction band, leaving a photogenerated hole (h+) in the valence band [39]. The e−/h+ pairs can then migrate to the surface of the semiconductor and participate in redox reactions that generate hydroxyl radicals (•OH), h+, and superoxide ion radicals (•O2−) in the photocatalytic degradation of pollutants in wet conditions, as shown in Figure 7 [40]. The photogenerated h+ is considered an oxidant that directly degrades organic contaminants, and its effectiveness is determined by the catalyst used and the oxidation states [1].
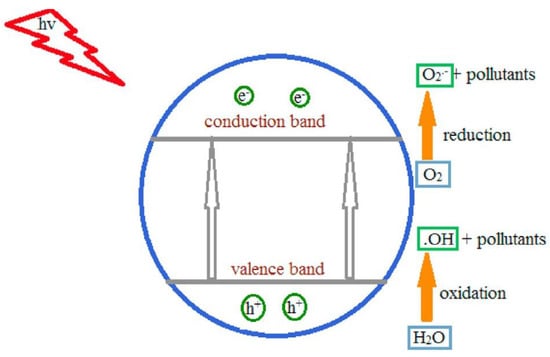
Figure 7.
Mechanism of photocatalysis. Superoxide anions and hydroxyl radicals produced by electron–hole pairs completely degrade pollutants (adapted from [1]).
In this process, when the catalyst surfaces are exposed to photoirradiation, valence band holes and conduction band electrons are generated. When the valence band holes react with water, •OH radicals are generated, whereas when they react with molecular oxygen absorbed on the catalyst surface, superoxide radical anions are generated.
Different classes of therapeutic drugs, such as analgesics, antibiotics, anticonvulsants, and psychiatric drugs, are oxidized by the UV/TiO2 photocatalyst. Different operational parameters, such as initial concentration of the substrate, catalyst loading, type of TiO2 photocatalyst, wavelength/light intensity, pH of the solution, and the water matrix, influence the degradation kinetics for pharmaceutical pollutants.
Heterogeneous photocatalysis requires several items to be fulfilled, such as the following:
- (a)
- A photon emitted at the suitable wavelength.
- (b)
- A semiconductor material as a catalyst.
- (c)
- A strong oxidizing agent.
The main challenge is connected to the growth of new types of catalysts with improved quality. There are some restrictions that need to be detailed, as follows:
- Bandgap regulation to obtain a type of catalyst that has activity in the visible range.
- Morphology improvement, which can optimize shape and size.
- Delay or avoidance of recombination of electron holes [23].
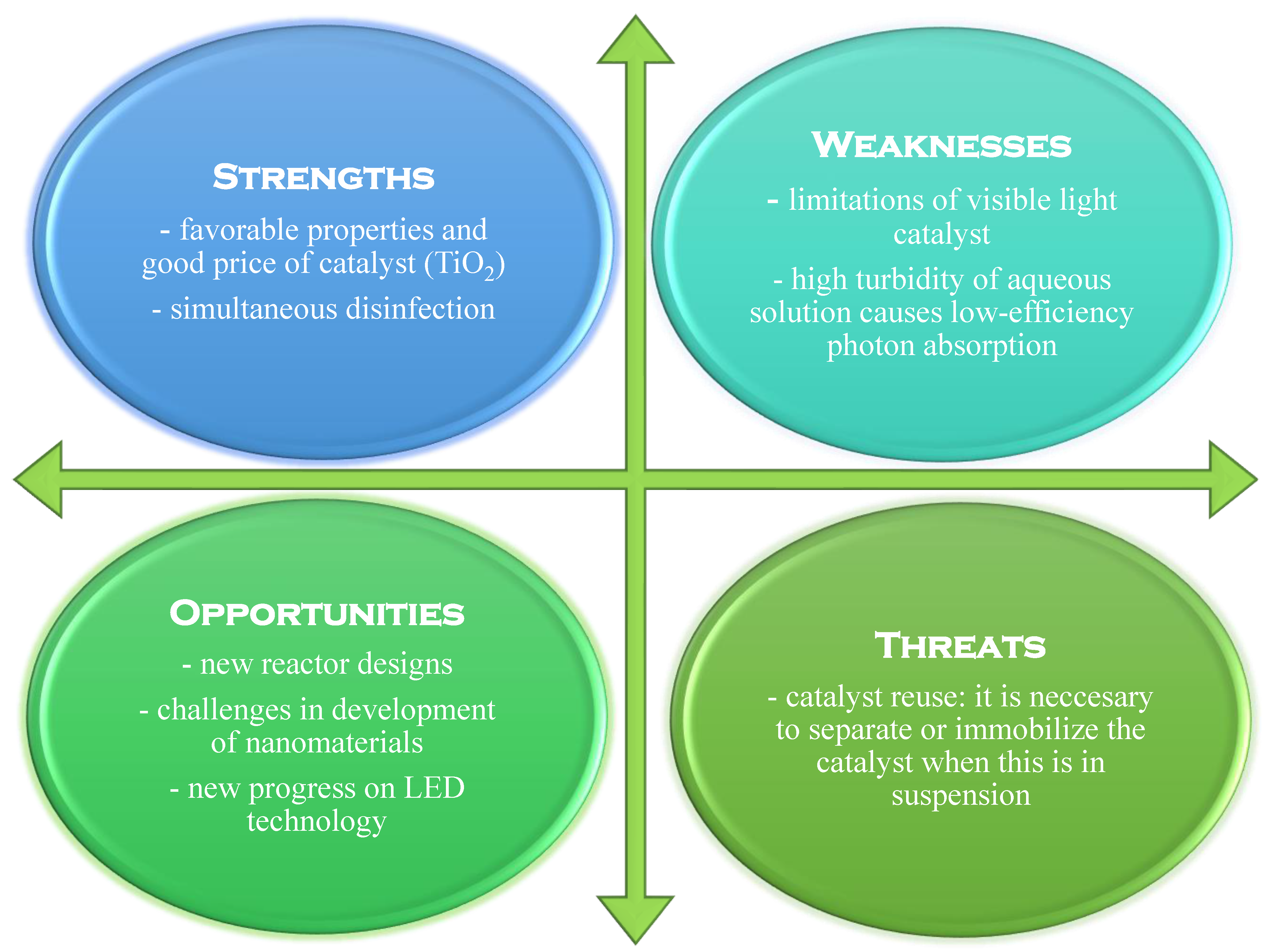
Figure 8 shows a typical SWOT analysis for the heterogeneous photocatalysis process.
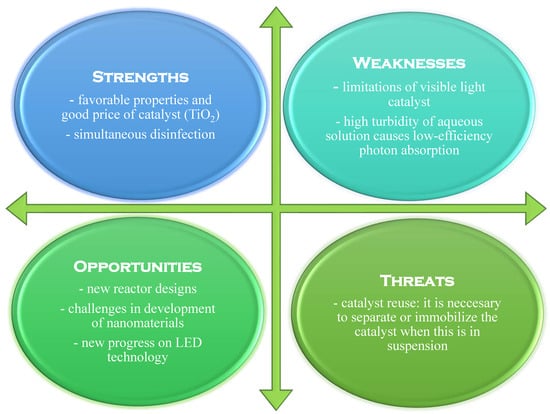
Figure 8.
SWOT analysis for the heterogeneous photocatalysis process (adapted from [25]).
The current trend is to develop new, mixed advanced oxidation processes that combine microwaves or ultrasounds. Some preliminary results show that the reaction rate can be increased to an acceptable level and a successful rate of removing organic substrate can be reached. Solar energy could be used, in association with some photovoltaic panels, to reduce the operation costs regarding the implementation of the AOPs on a large scale. This system has some major advantages, such as the reduced costs of operation and maintenance; it is considered a clean energy; the process of energy generation is noiseless; and it can be close to the final consumer. It was observed that this system still has significant problems, such as the limited ability of the system, the high-rate initial cost, the large area of land required for the system to be installed, and the inability to install the system in a geographical area that has low rates of solar radiation [23].
6.4. TiO2/UV System
The process of photocatalytic oxidation is a widely recognized technique for eliminating a variety of organic compounds. This process involves the combination of UV light, a photocatalyst (typically TiO2), and air or oxygen. However, it is not widely implemented due to the need to separate the photocatalyst, despite its relatively simple application. TiO2 is highly effective when used in powder form, with particle sizes in the range of tens of nanometers. Moreover, the light that reaches the photocatalyst surface must have low energy for the promotion of electrons from the semiconductor’s valence band to the conduction band, thus enabling electron–hole pairs to be created [2].
In this case, TiO2 radiation in the near-ultraviolet spectrum is needed. Because of its relatively broad bandgap, TiO2 may absorb up to 5% of the solar spectrum (UV radiation with a wavelength of 380 nm) [41]. Consequently, researchers have paid more attention to the catalytic activity of TiO2 within the visible zone of the solar spectrum in recent years [42].
In this respect, efforts were made to improve the photocatalytic properties of TiO2 under visible irradiation, such as surface modification with organic molecules or nanoparticles, or doping with metal and non-metal ions [43]. Table 3 summarizes the results, and it can be stated that the UV-TiO2 system is effective at removing antibiotics from wastewater. However, when significant catalyst dosages are used, the process efficiency drops. Nonetheless, the most significant impediment to the adoption of this technology is probably the difficulty in separating and reusing a costly photocatalyst, such as TiO2 [2].

Table 3.
Removal efficiency of antibiotics from wastewaters by TiO2/UV.
7. Sustainable Photocatalytic Wastewater Treatment
To increase the biodegradability of dangerous and non-biodegradable contaminants, such as persistent organic pollutants and antibiotics, photocatalytic degradation can be used as an efficient and advantageous treatment technology for wastewater. This technology allows the transformation of chemical pollutants into less toxic compounds with structural features that are much more readily biodegradable. The photocatalytic treatment process consists of the combination of oxidizing agents with an appropriate catalyst and/or light [10,44]. For the treatment of effluents containing toxic compounds for which biological process are not efficient, the photocatalytic oxidation process may be used. Photoexcitation of a semiconductor that is solid, sometimes due to the absorption of electromagnetic radiation but not always in the near-UV spectrum, is the basic stage of photocatalysis [26,48].
Over the years, several photocatalytic oxidation processes that could be effective for the degradation of various types of organic contaminants have been investigated. As an example, some semi-conductor materials, including TiO2, ZnS, WO3, and SnO2, are used as photocatalysts. A photocatalyst must absorb energy in order to produce electrons (e−) with strong reducing capacity, according to numerous studies. Moreover, the excited electrons (e−) could be used to reduce the quantity of O2 to form superoxide radicals (•O2−), whereas holes (h+) go to the photocatalyst surface where they oxidize water to generate hydroxyl radicals (•OH), which will begin to break down the organic contaminants. After that, either the superoxide radicals (•O2−) or hydroxyl radicals (•OH) are generated. It was found that titanium dioxide, due to its high catalytic efficiency and stability, is the most significant catalyst [10,49].
The mechanisms of photocatalytic oxidation using TiO2 as a catalyst are presented in Figure 9 and are described by the following equations:
TiO2 + hv → TiO2 + h+ + e−
h+ + OH−→ •OH
h+ + H2O +O2 → •OH + H+ + •O2−
O2 + e− → O2−
O2− + H+→ HO2−
2HO2− → O2 + H2O2
H2O2 + •O2− → •OH + OH− + O2
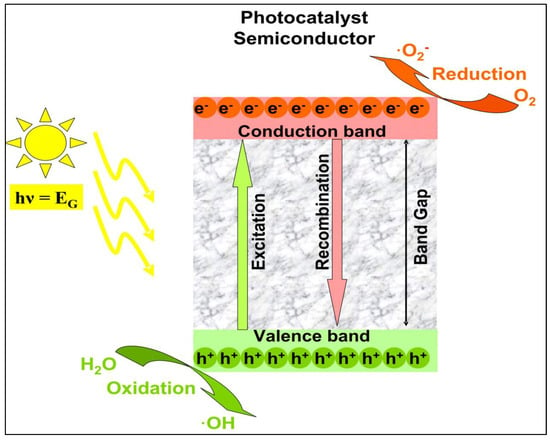
Figure 9.
Mechanism of oxidative radicals’ development [50].
7.1. Photocatalytic Materials
A photocatalytic reaction is a redox process and a photochemical reaction that takes place between a photocatalyst and the substrates on its surface, such as hydrogen peroxide, oxygen, and target pollutants, when light is applied. Photocatalysts are relevant components in photocatalytic processes. The photocatalysts can be classified into two main categories:
- Oxide photocatalysts: TiO2-based photocatalysts, Bi2O3-based photocatalysts, and other oxide photocatalysts such as ZnO, WO3, or Fe2O3.
- Non-oxide photocatalyst: CdS series photocatalysts, CuS series photocatalysts, ZnS series photocatalysts, and nitride series photocatalysts.
To enhance the efficiency of the photodegradation process, some photocatalysts, such as metal oxides (i.e., titanium dioxide, zinc oxide), metal sulfides (i.e., CdS), precious metal semiconductors (i.e., BiOBr, BiOCl, BiVO4, Ag3O4, SmVO4, GdVO4), and non-metallic semiconductors (i.e., g-C3N4) have been tested in the photochemical oxidation process [10,51].
TiO2 photocatalysts were used in the living world and wastewater treatment for the elimination of hazardous organic pollutants, notably antibiotics, due to higher photocatalytic activity, non-toxicity, and high photo-stability [51]. Another important photocatalyst, zinc oxide (ZnO), has been studied in the literature for the degradation of a series of antibiotics (such as ciprofloxacin, norfloxacin, sulfamethoxazole, cefixime trihydrate, and tetracycline hydrochloride), due to its reduced cost, non-toxicity, raised redox potential, and environmentally friendly properties [10].
Photocatalyst Preparation Methods
All types of photocatalyst are obtained by different techniques. Over time, a large number of methods to prepare photocatalysts have been developed. For example, the electrospinning, solid phase, gas phase, liquid phase, sol–gel, precipitation, liquid deposition, and hydrothermal methods are the most commonly used methods for obtaining photocatalysts [52].
- ▪
- Electrospinning method
The electrospinning method is used to directly produce polymer nanofibers. This technique has been used to make a variety of nanofibers, including organic and inorganic composites. The electrospinning process requires several components: a nozzle, a liquid supply device, a fiber-receiving device, and a high-voltage power source. The high-voltage power supply, which uses a direct current, produces thousands of volts and generates an electromagnetic field to polarize and charge the liquid. The fiber-receiving device, which may take the form of a rotating metallic roller, is located on the opposite side of the nozzle, ground with a thread, and connected to the negative electrode of the high-voltage power supply. The most important factors that contribute to a highly efficient electrospinning process include the voltage, surface tension, solution viscosity, conductivity, and solvent evaporation rate [52,53].
- ▪
- Solid Phase method
The solid phase method is a process where the reactant ingredients are ground and blended together in a specified ratio, and then calcined at a specific temperature to yield the desired product [52]. This method has many benefits, such as simple setup, low cost, ease of use, uniform particle size, and controlled reaction conditions [54]. The absence of a solvent also minimizes the formation of hard agglomerates and reduces environmental pollution. However, this method also has some drawbacks, such as a tendency for particle aggregation, a coarser powder, and a higher likelihood of ion oxidation, as well as easy impurity contamination [52,54].
- ▪
- Gas Phase method
The gas phase method involves directly using gas or converting a substance into gas to allow it to react either physically or chemically in the gas phase. During the cooling process, the nanoparticles can then aggregate and form [52]. This method results in high-purity nanoparticles with desirable properties, but it requires advanced techniques and equipment. It encompasses various vapor phase methods, including physical vapor deposition, chemical vapor deposition, and molecular beam epitaxy.
The chemical vapor deposition (CVD) process involves the use of chemical reactions between vapor phase components to produce thin films on a substrate surface. This technique can be used to deposit various materials, such as metals, nitrides, and oxides. CVD can be further divided into four categories, namely atmospheric pressure CVD, low pressure CVD, plasma pressure CVD, and laser pressure CVD, depending on the conditions under which it is performed. However, there are certain limitations to the CVD process, such as inconsistent surface roughness and particle size (ranging from 50 to 150 nm) [52,55].
The physical vapor deposition (PVD) technique involves physically vaporizing a paint to create a film on the surface of a substrate. This method has advantages over conventional vacuum deposition techniques, such as ion beam deposition, ion plating, and ion-beam-assisted deposition. Additionally, the PVD process operates at low deposition temperatures and does not result in substrate deformation or cracking. This process has been used to deposit ceramic compounds, polymer films, and metal and alloy films [52,56].
The molecular beam epitaxy (MBE) method is a cutting-edge film production process that allows for the creation of single crystal films. In this process, a hot beam of atoms or molecules is directed onto the surface of a heated substrate under vacuum conditions. The MBE technique offers several benefits, such as the ability to precisely control beam intensity and shape the surface of the epitaxial material, which can be transformed into a multi-layer structure with various compositions [52].
- ▪
- Liquid Phase method
The liquid phase method, also referred to as the wet chemical method, is a process for producing material. To start, a soluble metal salt must be selected based on the desired composition of the material. The metal ions in the solution must then be precipitated or crystallized using appropriate agents. Finally, the desired raw material powder is produced through thermal decomposition of the precipitate [57].
- ▪
- Sol–Gel method
The sol–gel method is a common technique for preparing photocatalysts. It involves dissolving a precursor in a solvent to form a sol via hydrolysis. The sol–gel process is influenced by various parameters such as the pH of the solution, concentration, reaction time, and temperature. The sol–gel process has numerous benefits, including high purity and homogeneity, low synthesis temperature, and easy control of reaction parameters. However, it also has some drawbacks, such as the high cost of titanium alkoxide raw material, the use of a large amount of organic solvent, high cost of film production due to high-temperature heat treatment, low film adhesion, and weak transparency [52].
- ▪
- Precipitation method
The precipitation method is a commonly used technique for synthesizing nanomaterials. In this method, a precipitant is added to form a precursor precipitate which is then mixed with other substances having various chemical components. However, some heavy metals introduced during the precipitation process may not react in solution and could potentially introduce impurities into the solution, affecting the usage of this approach. The reaction temperature, duration, pH value, and titration rate all have an effect on the material manufacture throughout this method’s operation. Some advantages of the precipitation method are the low cost of operation and its simple and safe process [58,59].
- ▪
- Liquid deposition method
In this method, a uniform film is put into the substrate, before which the substrate is placed in a reaction solution. For this method, a high temperature during film formation or expensive equipment are not needed. This approach may also be used to create composite oxide films, multicomponent oxide films, metal fine particle dispersion oxide films, and laminated oxide films [52,60]. The most significant advantages are that environmental pollution is minimal and the benefit-to-cost ratio is high. It has these advantages because all of the problems have been solved; as a result, the liquid deposition method has a broad application area [60].
- ▪
- Hydrothermal method
In this method, an enhanced inorganic material is created by combining an inorganic or organic component with water under high pressure at temperatures ranging from 100 °C to 300 °C. The resulting inorganic compound is filtered, washed, and dried to produce ultrafine particles of high purity. Some advantages of the hydrothermal method are mild conditions, stable system, easy process, low cost, and low environmental pollution. Other advantages are that films obtained using this method have good uniformity and are not limited by the shape and size of the substrate [52]. On the other hand, by adjusting the amount of the product using the hydrothermal approach, homogenous nanocomposites may be obtained.
7.2. Factors That Influence the Photocatalytic Oxidation Process
- pH value
An important parameter of the photocatalytic oxidation processes is the pH of the aqueous solutions. It was discovered that the charge on the surface of the catalyst can vary depending on whether the pH is above or below the potential of zero charge. Likewise, if the pH is higher or lower than the pKa (acid dissociation constant), the substrates may exhibit a different charge form [61]. In certain studies, a UV-A/TiO2 photocatalyst was used to degrade amoxicillin, and it was discovered that the degradation was unaffected by the rise in pH from 5.0 to 7.5. This rise may be ascribed to the ionization states of both the catalyst and the pollutant. The mineralization of amoxicillin was reduced from 95% to 75% when the pH was increased from 5 to 7. Moreover, the photocatalytic degradation of cefotaxime in various aqueous solutions was studied by employing TiO2 and ZnO as a photocatalyst. It was discovered that, when the pH of cefotaxime was elevated from 4 to 6.2 using TiO2 as a catalyst, the removal rate rose, but it decreased when the pH was increased from 6.2 to 7.6. Other results were noted in scientific papers in the case of cefotaxime degradation using ZnO as a catalyst [10,62].
- Catalyst dosage
Another significant parameter in the photocatalytic oxidation process is the photocatalyst dosage. It has been discovered that having a higher amount of photocatalyst in the system can result in more active sites and an improved ability to remove pollutants through oxidation and mineralization. However, having too much catalyst can also obstruct light energy from reaching the system and reduce the effectiveness of light energy due to reflection, scattering, and light blockage caused by solid particles [2,10].
- Mineralization of antibiotics
It has been frequently remarked that the degradation efficiency due to the formation of short-term organic intermediates that are formed during the photocatalytic process is higher than the mineralization efficiency [63]. In this respect, it was found that for oxytetracycline degradation under UV irradiation for 10 h, the total organic carbon (TOC) removal was only 9.5%. It was also found that in the case of tetracycline under UV radiation for 5 h with the addition of 0.2 g/L (multi-walled carbon nanotube) MWCNT/TiO2, TOC removal reached 83%. Flumequine’s mineralization ratio reached 74% after 15 min of UV irradiation, and it remained unchanged after 60 min, or even extended the irradiation times. It was observed that some aromatic organic intermediates remained intact after they were exposed several times to irradiation, for a longer time [10,64] Table 4.

Table 4.
Removal of several antibiotics by photocatalytic oxidation.
7.3. Photocatalysis—Challenges and Opportunities
In many applications areas, such as environmental remediation, energy production, chemical engineering, medicine/biochemistry, and agriculture, the heterogeneous photocatalysis has shown great efficiency [88]. The applicability of the photocatalytic technology for wastewater treatment is constrained by some important technical issues that need more research in the near future, such as the following:
- The first issue is if the photocatalytic process is a pre-treatment or a separate system.
- The photocatalytic process can be used as a pre-treatment step to improve the biodegradation of recalcitrant organic pollutants before biological wastewater treatment due to the non-selective reactivity of the non-biodegradable wastewater soluble pollutants [89].
In order to try to obtain significant results regarding the efficiency of this process, it is necessary to consider other technical aspects, from catalyst development to reactor design and process optimization, such as the following:
- Catalyst improvement for high photo-efficiency.
- Catalyst immobilization to ensure a cost-effective solid–liquid separation.
- Improvement in the photocatalytic operation for a wider pH range and for a minimization of the addition of oxidant additives.
- A new, integrated or coupling system for improved photo-mineralization or photo-disinfection kinetics.
- An efficient design of the reactor photocatalytic system or the use of solar energy to reduce the electricity costs [89].
Therefore, a photocatalytic wastewater treatment process with large-scale applicability, high efficiency, and low costs of operation, using solar energy, can be realized in order to perform a rapid evaluation of different possible issues and ambiguities that may appear.
Nevertheless, the applicability of heterogeneous photocatalysis based on ZnO and TiO2 is still doubtful due to some limitations:
- The quantum efficiency is reduced under sunlight because of the use of large bandgap energy.
- An e−/h+ pair is generated by high energy consumption.
- The photocatalytic activities are decreased due to reactive oxidative species reduction of a rapid e−/h+ pair recombination.
- There is limited ability to extend the approach due to the complex modification process needed to increase the photocatalysis.
- The kinetic reaction rate decreases due to a fast active surface site and the activation by interaction with intermediate secondary products.
- The low photocatalyst stability reduces the reuse of its lifetime services.
- The solid photocatalyst is eliminated as a secondary solid waste.
- A low photocatalytic efficiency against high concentrations of pollutants at high air and water flow rates [88].
As a remark, water can also be considered as a solvent or a chemical component because the water properties are changed due to the rapid increases in the temperature or the applied pressure. The operation and synthesis costs depend on many factors involved in the usage of catalyst, such as energy consumption, the regeneration process, the long-term stability, and the individual component costs [90].
8. Opportunities and Future Perspectives
In recent years, scientists have been involved in looking for a strategy to allow for total removal of the low concentration of contaminants found in wastewater that was previously treated with conventional removal techniques. Furthermore, it has been observed that using AOPs is a proper option in which the pollutants are adsorbed in a porous material that is removed following in situ AOP reactions; these reactions could take place on the surface of the exhausted adsorbent [24,91,92].
Advanced oxidation processes could be considered suitable technologies that can used to treat wastewaters when they include recalcitrant or toxic organic compounds. In some cases, an excellent option could be when the full mineralization can be obtained with suitable costs and time. These processes are efficient and some of them could be seen as “green” technologies. The combination of two or three methods can increase the mineralization efficiencies in eliminating pesticide pollutants. This approach not only transforms organic compounds into non-toxic by-products, but also proves to be cost effective as it requires minimal space and chemicals, and generates little to no sludge.
It has been observed that most of the heterogeneous photocatalysis research was conducted using bench setups in unrealistic conditions that are too different from the real conditions. Only some limited efforts have been made to test the real performance of the heterogeneous photocatalysis using water and air feed streams. On the other hand, it has been discovered that there are many inconsistent data in the existing research regarding heterogeneous photocatalysis application for the environment [89].
Moreover, the scalability of the heterogeneous photocatalysis technology has barely been implemented for industrial purposes such as wastewater treatment or air purification. The most recent studies suggest that the photocatalytic splitting of water into hydrogen, which could be used as a renewable energy, is impractical, because it requires expensive sacrificial agents or electron donors, for example, methanol, glucose, or isopropanol.
9. Conclusions
Antibiotics are widespread contaminants found in a wide range of surface waters, wastewaters, WWTPs (wastewater treatment plants), and hospital effluents. Furthermore, the presence of antibiotics in wastewater may have negative consequences for human health and may enhance the resistance of bacteria to antibiotics. Advanced oxidation processes have frequently proven their great efficacy in the removal of a wide range of contaminants, including antibiotics.
The high efficiency and non-selectiveness of AOPs have made them a highly sought-after method for removing organic pollutants in various matrices, as evidenced by the high removal rates, effective remediation of water, and low energy consumption. Therefore, it is important to consider harnessing the full potential of AOPs in future developments by implementing them on an industrial scale for removing organic pollutants. These techniques show great promise as effective tools for wastewater treatment and further research should focus on improving the stability of solid catalysts, as well as on evaluating the cost effectiveness and long-term efficacy of large-scale AOP applications. From the point of view of the photochemical properties, some AOPs such as UV/H2O2 can be considered to be undisguised, and their development will be consistent with new UV light sources such as LEDs. In some places with high insolation, solar-powered photo-Fenton and heterogeneous photocatalysis may be advantageous for the future implementation of the technology. From these perspectives, it is clear that a significant effort is taking place in order to develop new catalysts for heterogenous photocatalysis, to thereby increase the efficiency of this technology and reach the commercial stage.
AOPs have the potential to effectively eliminate a wide range of stubborn organic compounds due to their high oxidation capability, which can be further enhanced by pairing with various oxidants and catalysts. One of the promising advanced oxidation process technologies for industrial applications, such as wastewater treatment, is photocatalysis. Selecting an appropriate photocatalyst and optimizing conditions can result in high degradation rates of persistent organic contaminants from wastewater. Therefore, the main motivation for AOPs is the use of renewable energy sources to reduce the costs and perhaps to substitute electricity with them. An area of future research that could prove to be of interest is the use of solar energy on a large scale, which has the potential to significantly reduce the environmental impact, by over 90%.
Future developments will have to occur with the direct participation of engineers, analytical chemists, and electrochemists, to create and conduct research for an efficient application and operation of the advanced oxidation processes. AOPs could represent an efficient, important, and environmentally friendly method to remove or to degrade a series of organic pollutants from wastewaters.
Author Contributions
All authors contributed to the study conception and design. Material preparation and data collection were performed by G.-I.L., C.B., C.O., L.B. and L.F.P. The first draft of the manuscript was written by the first author, G.-I.L. and all authors commented on previous versions of the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This work has been funded by the European Social Fund from the Sectoral Operational Programme Human Capital 2014–2020, through the Financial Agreement with the title “Training of PhD students and postdoctoral researchers in order to acquire applied research skills-SMART”, Contract no. 13530/16.06.2022-SMIS code: 153734.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare that they have no competing interest.
References
- Kurian, M. Advanced oxidation processes and nanomaterials—A review. Clean. Eng. Technol. 2021, 2, 100090. [Google Scholar] [CrossRef]
- Cuerda-Correa, E.M.; Alexandre-Franco, M.F.; Fernández-González, C. Advanced Oxidation Processes for the Removal of Antibiotics from Water. An Overview. Water 2020, 12, 102. [Google Scholar] [CrossRef]
- WWAP. The United Nations World Water Development Report 2014: Water and Energy; UNESCO: Paris, France, 2014. [Google Scholar]
- Khare, P.; Patel, R.K.; Sharan, S.; Shankar, R. Recent trends in advanced oxidation process for treatment of recalcitrant industrial effluents. In Advanced Oxidation Processes for Effluent Treatment Plants; Elsevier: Amsterdam, The Netherlands, 2021. [Google Scholar] [CrossRef]
- Giwa, A.; Yusuf, A.; Balogun, H.A.; Sambudi, N.S.; Bilad, M.R.; Adeyemi, I.; Chakraborty, S.; Curcio, S. Recent advances in advanced oxidation processes for removal of contaminants from water: A comprehensive review. Process. Saf. Environ. Prot. 2020, 146, 220–256. [Google Scholar] [CrossRef]
- Gkika, D.A.; Mitropoulos, A.C.; Lambropoulou, D.A.; Kalavrouziotis, I.K.; Kyzas, G.Z. Cosmetic wastewater treatment technologies: A review. Environ. Sci. Pollut. Res. 2022, 29, 75223–75247. [Google Scholar] [CrossRef]
- Ma, D.; Yi, H.; Lai, C.; Liu, X.; Huo, X.; An, Z.; Li, L.; Fu, Y.; Li, B.; Zhang, M.; et al. Critical review of advanced oxidation processes in organic wastewater treatment. Chemosphere 2021, 275, 130104. [Google Scholar] [CrossRef]
- Stanton, I.C.; Bethel, A.; Leonard, A.F.C.; Gaze, W.H.; Garside, R. Existing evidence on antibiotic resistance exposure and transmission to humans from the environment: A systematic map. Environ. Évid. 2022, 11, 8. [Google Scholar] [CrossRef]
- Samal, K.; Mahapatra, S.; Ali, H. Pharmaceutical wastewater as Emerging Contaminants (EC): Treatment technologies, impact on environment and human health. Energy Nexus 2022, 6, 100076. [Google Scholar] [CrossRef]
- Dodson, L.G.; Vogt, R.A.; Marks, J.; Reichardt, C.; Crespo-Hernández, C.E. Photophysical and photochemical properties of the pharmaceutical compound salbutamol in aqueous solutions. Chemosphere 2011, 83, 1513–1523. [Google Scholar] [CrossRef]
- Wang, J.; Zhuan, R. Degradation of antibiotics by advanced oxidation processes: An overview. Sci. Total Environ. 2020, 701, 135023. [Google Scholar] [CrossRef]
- Manzetti, S.; Ghisi, R. The environmental release and fate of antibiotics. Mar. Pollut. Bull. 2014, 79, 7–15. [Google Scholar] [CrossRef]
- Gao, Y.; Wang, Q.; Ji, G.; Li, A. Degradation of antibiotic pollutants by persulfate activated with various carbon materials. Chem. Eng. J. 2022, 429, 132387. [Google Scholar] [CrossRef]
- Langbehn, R.K.; Michels, C.; Soares, H.M. Antibiotics in wastewater: From its occurrence to the biological removal by environmentally conscious technologies. Environ. Pollut. 2021, 275, 116603. [Google Scholar] [CrossRef] [PubMed]
- Pulia, M.S.; Wolf, I.; Schulz, L.T.; Pop-Vicas, A.; Schwei, R.J.; Lindenauer, P.K. COVID-19: An emerging threat to antibiotic stewardship in the emergency department. West. J. Emerg. Med. 2020, 21, 1283–1286. [Google Scholar] [CrossRef] [PubMed]
- Polianciuc, S.I.; Gurzău, A.E.; Kiss, B.; Ștefan, M.G.; Loghin, F. Antibiotics in the environment: Causes and consequences. Med. Pharm. Rep. 2020, 93, 231–240. [Google Scholar] [CrossRef]
- Ebrahimi, S.M.; Reyhani, R.D.; Asghari-JafarAbadi, M.; Fathifar, Z. Diversity of antibiotics in hospital and municipal wastewaters and receiving water bodies and removal efficiency by treatment processes: A systematic review protocol. Environ. Évid. 2020, 9, 19. [Google Scholar] [CrossRef]
- Mangla, D.; Annu; Sharma, A.; Ikram, S. Critical review on adsorptive removal of antibiotics: Present situation, challenges and future perspective. J. Hazard. Mater. 2022, 425, 127946. [Google Scholar] [CrossRef]
- Stasinakis, A.S. Use of selected advanced oxidation processes (AOPs) for wastewater treatment—A mini review. Glob. NEST J. 2008, 10, 376–385. [Google Scholar]
- Ghime, D.; Ghosh, P. Advanced Oxidation Processes: A Powerful Treatment Option for the Removal of Recalcitrant Organic Compounds. In Advanced Oxidation Processes-Applications, Trends, and Prospects; IntechOpen: London, UK, 2020. [Google Scholar] [CrossRef]
- Coha, M.; Farinelli, G.; Tiraferri, A.; Minella, M.; Vione, D. Advanced oxidation processes in the removal of organic substances from produced water: Potential, configurations, and research needs. Chem. Eng. J. 2021, 414, 128668. [Google Scholar] [CrossRef]
- Seshadri, S.S.; Ramachandran, S.; Thirumavalavan, K.; Kumar, B.P. A review of solar photocatalytic degradation of wastewater using advanced oxidation processes. J. Ind. Pollut. Control 2015, 31, 297–309. [Google Scholar]
- Ortiz, I.; Rivero, M.J.; Margallo, M. Chapter 6—Advanced Oxidative and Catalytic Processes, Sustainable Water and Wastewater Processing; Elsevier: Amsterdam, The Netherlands, 2021; pp. 161–201. [Google Scholar] [CrossRef]
- Pandis, P.K.; Kalogirou, C.; Kanellou, E.; Vaitsis, C.; Savvidou, M.G.; Sourkouni, G.; Zorpas, A.A.; Argirusis, C. Key Points of Advanced Oxidation Processes (AOPs) for Wastewater, Organic Pollutants and Pharmaceutical Waste Treatment: A Mini Review. ChemEngineering 2022, 6, 8. [Google Scholar] [CrossRef]
- Ameta, S.C. Chapter 1—Introduction, Advanced Oxidation Processes for Wastewater Treatment; Academic Press: Cambridge, MA, USA, 2018; pp. 1–12. [Google Scholar] [CrossRef]
- Kumar, V.; Shah, M.P.; Singh, K. Advanced oxidation processes for complex wastewater treatment. In Advanced Oxidation Processes for Effluent Treatment Plants; Elsevier: Amsterdam, The Netherlands, 2021; pp. 1–31. [Google Scholar] [CrossRef]
- Bhoite, G.M.; Vaidya, P.D. Fenton oxidation and adsorption pretreatment for superior biogas recovery from biomethanated spent wash. Chem. Eng. Commun. 2019, 207, 1347–1357. [Google Scholar] [CrossRef]
- Sharma, S.; Ruparelia, J.P.; Patel, M.L. A General Review on Advanced Oxidation Processes for Waste Water Treatment; Institute of Technology, Nirma University: Ahmedabad, India, 2011; Volume 382, pp. 8–10. [Google Scholar]
- Guo, R.; Xie, X.; Chen, J. The degradation of antibiotic amoxicillin in the Fenton-activated sludge combined system. Environ. Technol. 2015, 36, 844–851. [Google Scholar] [CrossRef]
- Mackuľak, T.; Nagyová, K.; Faberová, M.; Grabic, R.; Koba, O.; Gál, M.; Birošová, L. Utilization of Fenton-like reaction for antibiotics and resistant bacteria elimination in different parts of WWTP. Environ. Toxicol. Pharmacol. 2015, 40, 492–497. [Google Scholar] [CrossRef]
- Hassani, A.; Karaca, M.; Karaca, S.; Khataee, A.; Açışlı, Ö.; Yılmaz, B. Preparation of magnetite nanoparticles by high-energy planetary ball mill and its application for ciprofloxacin degradation through heterogeneous Fenton process. J. Environ. Manag. 2018, 211, 53–62. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.; Xu, S.; Wang, Y.; Sun, X.; Gao, Y.; Gao, B. Enhanced degradation of ciprofloxacin by graphitized mesoporous carbon (GMC)-TiO2 nanocomposite: Strong synergy of adsorption-photocatalysis and antibiotics degradation mechanism. J. Colloid Interface Sci. 2018, 527, 202–213. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Chen, J.; Fang, Z.; Tsang, E.P. Ceria accelerated nanoscale zerovalent iron assisted heterogenous Fenton oxidation of tetracycline. Chem. Eng. J. 2019, 369, 588–599. [Google Scholar] [CrossRef]
- Pan, Y.; Zhang, Y.; Zhou, M.; Cai, J.; Tian, Y. Enhanced removal of antibiotics from secondary wastewater effluents by novel UV/pre-magnetized Fe0/H2O2 process. Water Res. 2019, 153, 144–159. [Google Scholar] [CrossRef] [PubMed]
- Nasseh, N.; Taghavi, L.; Barikbin, B.; Nasseri, M.A.; Allahresani, A. FeNi3/SiO2 magnetic nanocomposite as an efficient and recyclable heterogeneous fenton-like catalyst for the oxidation of metronidazole in neutral environments: Adsorption and degradation studies. Compos. Part B Eng. 2019, 166, 328–340. [Google Scholar] [CrossRef]
- Qi, Y.; Mei, Y.; Li, J.; Yao, T.; Yang, Y.; Jia, W.; Tong, X.; Wu, J.; Xin, B. Highly efficient microwave-assisted Fenton degradation of metacycline using pine-needle-like CuCo2O4 nanocatalyst. Chem. Eng. J. 2019, 373, 1158–1167. [Google Scholar] [CrossRef]
- Clarizia, L.; Russo, D.; Di Somma, I.; Marotta, R.; Andreozzi, R. Homogeneous photo-Fenton processes at near neutral pH: A review. Appl. Catal. B Environ. 2017, 209, 358–371. [Google Scholar] [CrossRef]
- Khan, S.H.; Pathak, B. Zinc oxide based photocatalytic degradation of persistent pesticides: A comprehensive review. Environ. Nanotechnol. Monit. Manag. 2020, 13, 100290. [Google Scholar] [CrossRef]
- Wu, Q.; Liu, Y.; Jing, H.; Yu, H.; Lu, Y.; Huo, M.; Huo, H. Peculiar synergetic effect of γ-Fe2O3 nanoparticles and graphene oxide on MIL-53 (Fe) for boosting photocatalysis. Chem. Eng. J. 2020, 390, 124615. [Google Scholar] [CrossRef]
- Rahbar, M.; Behpour, M. Multi-walled carbon nanotubes/TiO2 thin layer for photocatalytic degradation of organic pollutant under visible light irradiation. J. Mater. Sci. Mater. Electron. 2016, 27, 8348–8355. [Google Scholar] [CrossRef]
- Oturan, M.A.; Aaron, J.-J. Advanced Oxidation Processes in Water/Wastewater Treatment: Principles and Applications. A Review. Crit. Rev. Environ. Sci. Technol. 2014, 44, 2577–2641. [Google Scholar] [CrossRef]
- Wang, D.; Wang, S.; Li, B.; Zhang, Z.; Zhang, Q. Tunable band gap of N V co-doped Ca:TiO2B (CaTi5O11) for visible-light photocatalysis. Int. J. Hydrogen Energy 2019, 44, 4716–4723. [Google Scholar] [CrossRef]
- Humayun, M.; Raziq, F.; Khan, A.; Luo, W. Modification strategies of TiO2 for potential applications in photocatalysis: A critical review. Green Chem. Lett. Rev. 2018, 11, 86–102. [Google Scholar] [CrossRef]
- Serna-Galvis, E.A.; Giraldo-Aguirre, A.L.; Silva-Agredo, J.; Flórez-Acosta, O.A.; Torres-Palma, R.A. Removal of antibiotic cloxacillin by means of electrochemical oxidation, TiO2 photocatalysis, and photo-Fenton processes: Analysis of degradation pathways and effect of the water matrix on the elimination of antimicrobial activity. Environ. Sci. Pollut. Res. 2017, 24, 6339–6352. [Google Scholar] [CrossRef]
- Tran, M.L.; Fu, C.-C.; Juang, R.-S. Effects of water matrix components on degradation efficiency and pathways of antibiotic metronidazole by UV/TiO2 photocatalysis. J. Mol. Liq. 2019, 276, 32–38. [Google Scholar] [CrossRef]
- Shankaraiah, G.; Poodari, S.; Bhagawan, D.; Himabindu, V.; Vidyavathi, S. Degradation of antibiotic norfloxacin in aqueous solution using advanced oxidation processes (AOPs)—A comparative study. Desalination Water Treat. 2016, 57, 27804–27815. [Google Scholar] [CrossRef]
- Giraldo-Aguirre, A.L.; Serna-Galvis, E.A.; Erazo-Erazo, E.D.; Silva-Agredo, J.; Giraldo-Ospina, H.; Flórez-Acosta, O.A.; Torres-Palma, R.A. Removal of β-lactam antibiotics from pharmaceutical wastewaters using photo-Fenton process at near-neutral pH. Environ. Sci. Pollut. Res. 2018, 25, 20293–20303. [Google Scholar] [CrossRef]
- Serna-Galvis, E.A.; Silva-Agredo, J.; Giraldo, A.L.; Flórez-Acosta, O.A.; Torres-Palma, R.A. Comparative study of the effect of pharmaceutical additives on the elimination of antibiotic activity during the treatment of oxacillin in water by the photo-Fenton, TiO2-photocatalysis and electrochemical processes. Sci. Total Environ. 2016, 541, 1431–1438. [Google Scholar] [CrossRef] [PubMed]
- Leong, S.; Razmjou, A.; Wang, K.; Hapgood, K.; Zhang, X.; Wang, H. TiO2 based photocatalytic membranes: A review. J. Membr. Sci. 2014, 472, 167–184. [Google Scholar] [CrossRef]
- Xia, X.; Zhu, F.; Li, J.; Yang, H.; Wei, L.; Li, Q.; Jiang, J.; Zhang, G.; Zhao, Q. A Review Study on Sulfate-Radical-Based Advanced Oxidation Processes for Domestic/Industrial Wastewater Treatment: Degradation, Efficiency, and Mechanism. Front. Chem. 2020, 8, 592056. [Google Scholar] [CrossRef]
- Li, M.-F.; Liu, Y.-G.; Zeng, G.-M.; Liu, N.; Liu, S.-B. Graphene and graphene-based nanocomposites used for antibiotics removal in water treatment: A review. Chemosphere 2019, 226, 360–380. [Google Scholar] [CrossRef]
- Uluseker, C.; Kaster, K.M.; Thorsen, K.; Basiry, D.; Shobana, S.; Jain, M.; Kumar, G.; Kommedal, R.; Pala-Ozkok, I. A Review on Occurrence and Spread of Antibiotic Resistance in Wastewaters and in Wastewater Treatment Plants: Mechanisms and Perspectives. Front. Microbiol. 2021, 12, 717809. [Google Scholar] [CrossRef] [PubMed]
- Rizzo, L.; Manaia, C.; Merlin, C.; Schwartz, T.; Dagot, C.; Ploy, M.C.; Michael, I.; Fatta-Kassinos, D. Urban wastewater treatment plants as hotspots for antibiotic resistant bacteria and genes spread into the environment: A review. Sci. Total Environ. 2013, 447, 345–360. [Google Scholar] [CrossRef]
- Yang, S.; Höti, N.; Yang, W.; Liu, Y.; Chen, L.; Li, S.; Zhang, H. Simultaneous analyses of N-linked and O-linked glycans of ovarian cancer cells using solid-phase chemoenzymatic method. Clin. Proteom. 2017, 14, 3. [Google Scholar] [CrossRef]
- Duan, X.; Chen, G.; Guo, L.; Zhu, Y.; Ye, H.; Wu, Y. A template-free CVD route to synthesize hierarchical porous ZnO films. Superlattices Microstruct. 2015, 88, 501–507. [Google Scholar] [CrossRef]
- Richter, G.; Hillerich, K.; Gianola, D.S.; Mönig, R.; Kraft, O.; Volkert, C.A. Ultrahigh strength single crystalline nanowhiskers grown by physical vapor deposition. Nano Lett. 2009, 9, 3048–3052. [Google Scholar] [CrossRef]
- Sambaza, S.S.; Naicker, N. Contribution of wastewater to antimicrobial resistance: A review article. J. Glob. Antimicrob. Resist. 2023, 34, 23–29. [Google Scholar] [CrossRef]
- Jiang, X.; Zhao, X.; Duan, L.; Shen, H.; Liu, H.; Hou, T.; Wang, F. Enhanced photoluminescence and photocatalytic activity of ZnO-ZnWO4 nanocomposites synthesized by a precipitation method. Ceram. Int. 2016, 42, 15160–15165. [Google Scholar] [CrossRef]
- Meng, Q.; Wang, J. Synthesis of Fe-doped ZnO by parallel flow precipitation method and its photocatalytic denitrification performance. CIESC J. 2017, 68, 437–443. [Google Scholar] [CrossRef]
- Wenderich, K.; Mul, G. Methods, mechanism, and applications of photodeposition in photocatalysis: A review. Chem. Rev. 2016, 116, 14587–14619. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, M.; Kaur, N.; Bansal, P.; Kumar, R.; Srinivasan, S.; Chaudhary, G.R. Proficient Photocatalytic and Sonocatalytic Degradation of Organic Pollutants Using CuO Nanoparticles. J. Nanomater. 2020, 2020, 6123178. [Google Scholar] [CrossRef]
- León, D.E.; Zúñiga-Benítez, H.; Peñuela, G.A.; Mansilla, H.D. Photocatalytic Removal of the Antibiotic Cefotaxime on TiO2 and ZnO Suspensions Under Simulated Sunlight Radiation. Water Air Soil Pollut. 2017, 228, 361. [Google Scholar] [CrossRef]
- Orbeci, C.; Modrogan, C.; Dancila, A.M. Degradation of Pharmaceutical Effluents by Photo-assisted Techniques. Reactions 2016, 12, 13. [Google Scholar]
- Song, C.; Liu, H.-Y.; Guo, S.; Wang, S.-G. Photolysis mechanisms of tetracycline under UV irradiation in simulated aquatic environment surrounding limestone. Chemosphere 2019, 244, 125582. [Google Scholar] [CrossRef]
- Dimitrakopoulou, D.; Rethemiotaki, I.; Frontistis, Z.; Xekoukoulotakis, N.P.; Venieri, D.; Mantzavinos, D. Degradation, mineralization and antibiotic inactivation of amoxicillin by UV-A/TiO2 photocatalysis. J. Environ. Manag. 2012, 98, 168–174. [Google Scholar] [CrossRef]
- Melchionna, M.; Fornasiero, P. Updates on the Roadmap for Photocatalysis. ACS Catal. 2020, 10, 5493–5501. [Google Scholar] [CrossRef]
- Sheydaei, M.; Shiadeh, H.R.K.; Ayoubi-Feiz, B.; Ezzati, R. Preparation of nano N-TiO2/graphene oxide/titan grid sheets for visible light assisted photocatalytic ozonation of cefixime. Chem. Eng. J. 2018, 353, 138–146. [Google Scholar] [CrossRef]
- Shooshtari, N.M.; Ghazi, M.M. An investigation of the photocatalytic activity of nano α-Fe2O3/ZnO on the photodegradation of cefixime trihydrate. Chem. Eng. J. 2017, 315, 527–536. [Google Scholar] [CrossRef]
- Dong, H.; Fu, Y.; Wang, P.; Jiang, W.; Gao, G.; Zhang, X. Degradation of chloramphenicol by Ti/PbO2-La anodes and alteration in bacterial community and antibiotics resistance genes. Environ. Pollut. 2022, 301, 119031. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Shao, Y.; Gao, N.; Gao, Y.; Chu, W.; Li, S.; Wang, Y.; Xu, S. Kinetics and by-products formation of chloramphenicol (CAP) using chlorination and photocatalytic oxidation. Chem. Eng. J. 2018, 333, 85–91. [Google Scholar] [CrossRef]
- Zeng, Y.; Chen, D.; Chen, T.; Cai, M.; Zhang, Q.; Xie, Z.; Li, R.; Xiao, Z.; Liu, G.; Lv, W. Study on heterogeneous photocatalytic ozonation degradation of ciprofloxacin by TiO2/carbon dots: Kinetic, mechanism and pathway investigation. Chemosphere 2019, 227, 198–206. [Google Scholar] [CrossRef]
- Yan, Y.; Sun, S.; Song, Y.; Yan, X.; Guan, W.; Liu, X.; Shi, W. Microwave-assisted in situ synthesis of reduced graphene oxide-BiVO4 composite photocatalysts and their enhanced photocatalytic performance for the degradation of ciprofloxacin. J. Hazard. Mater. 2013, 250–251, 106–114. [Google Scholar] [CrossRef] [PubMed]
- Sarkhosh, M.; Sadani, M.; Abtahi, M.; Mohseni, S.M.; Sheikhmohammadi, A.; Azarpira, H.; Najafpoor, A.A.; Atafar, Z.; Rezaei, S.; Alli, R.; et al. Enhancing photo-degradation of ciprofloxacin using simultaneous usage of eaq− and OH over UV/ZnO/I- process: Efficiency, kinetics, pathways, and mechanisms. J. Hazard. Mater. 2019, 377, 418–426. [Google Scholar] [CrossRef]
- Wu, G.; Xiao, L.; Gu, W.; Shi, W.; Jiang, D.; Liu, C. Fabrication and excellent visible-light-driven photodegradation activity for antibiotics of SrTiO3 nanocube coated CdS microsphere heterojunctions. RSC Adv. 2016, 6, 19878–19886. [Google Scholar] [CrossRef]
- Zhu, Z.; Huo, P.; Lu, Z.; Yan, Y.; Liu, Z.; Shi, W.; Li, C.; Dong, H. Fabrication of magnetically recoverable photocatalysts using g-C3N4 for effective separation of charge carriers through like-Z-scheme mechanism with Fe3O4 mediator. Chem. Eng. J. 2018, 331, 615–625. [Google Scholar] [CrossRef]
- Du, H.; Pu, W.; Wang, Y.; Yan, K.; Feng, J.; Zhang, J.; Yang, C.; Gong, J. Synthesis of BiVO4/WO3 composite film for highly efficient visible light induced photoelectrocatalytic oxidation of norfloxacin. J. Alloys Compd. 2019, 787, 284–294. [Google Scholar] [CrossRef]
- Mamba, G.; Kiwi, J.; Pulgarin, C.; Sanjines, R.; Giannakis, S.; Rtimi, S. Evidence for the degradation of an emerging pollutant by a mechanism involving iso-energetic charge transfer under visible light. Appl. Catal. B Environ. 2018, 233, 175–183. [Google Scholar] [CrossRef]
- Espíndola, J.C.; Cristóvão, R.O.; Santos, S.G.; Boaventura, R.A.; Dias, M.M.; Lopes, J.C.B.; Vilar, V.J. Intensification of heterogeneous TiO2 photocatalysis using the NETmix mili-photoreactor under microscale illumination for oxytetracycline oxidation. Sci. Total Environ. 2019, 681, 467–474. [Google Scholar] [CrossRef] [PubMed]
- Ye, S.; Zhou, X.; Xu, Y.; Lai, W.; Yan, K.; Huang, L.; Ling, J.; Zheng, L. Photocatalytic performance of multi-walled carbon nanotube/BiVO4 synthesized by electro-spinning process and its degradation mechanisms on oxytetracycline. Chem. Eng. J. 2019, 373, 880–890. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, M.; He, X.; Du, T.; Wang, Y.; Li, Y.; Hao, T. Facile prepared ball-like TiO2 at GO composites for oxytetracycline removal under solar and visible lights. Water Res. 2019, 160, 197–205. [Google Scholar] [CrossRef]
- Yuan, S.; Fan, Y.; Zhang, Y.; Tong, M.; Liao, P. Pd-catalytic in situ generation of H2O2 from H2 and O2 produced by water electrolysis for the efficient electro-Fenton degradation of Rhodamine B. Environ. Sci. Technol. 2011, 45, 8514–8520. [Google Scholar] [CrossRef] [PubMed]
- Orbeci, C.; Stefan, M.; Dancila, A.M.; Tudosie, M.S. Removal of Antibiotics from Wastewater Using Photocatalytic Membranes. Rev. Chim. 2016, 67, 1666–1668. [Google Scholar]
- Liu, X.; Guo, Z.; Zhou, L.; Yang, J.; Cao, H.; Xiong, M.; Xie, Y.; Jia, G. Hierarchical biomimetic BiVO4 for the treatment of pharmaceutical wastewater in visible-light photocatalytic ozonation. Chemosphere 2019, 222, 38–45. [Google Scholar] [CrossRef]
- Liu, X.; Ji, H.; Li, S.; Liu, W. Graphene modified anatase/titanate nanosheets with enhanced photocatalytic activity for efficient degradation of sulfamethazine under simulated solar light. Chemosphere 2019, 233, 198–206. [Google Scholar] [CrossRef] [PubMed]
- Mirzaei, A.; Yerushalmi, L.; Chen, Z.; Haghighat, F.; Guo, J. Enhanced photocatalytic degradation of sulfamethoxazole by zinc oxide photocatalyst in the presence of fluoride ions: Optimization of parameters and toxicological evaluation. Water Res. 2018, 132, 241–251. [Google Scholar] [CrossRef] [PubMed]
- Sandikly, N.; Kassir, M.; El Jamal, M.; Takache, H.; Arnoux, P.; Mokh, S.; Al-Iskandarani, M.; Roques-Carmes, T. Comparison of the toxicity of waters containing initially sulfaquinoxaline after photocatalytic treatment by TiO2 and polyaniline/TiO2. Environ. Technol. 2021, 42, 419–428. [Google Scholar] [CrossRef] [PubMed]
- Lofrano, G.; Carotenuto, M.; Uyguner-Demirel, C.S.; Vitagliano, A.; Siciliano, A.; Guida, M. An integrated chemical and ecotoxicological assessment for the photocatalytic degradation of vancomycin. Environ. Technol. 2014, 35, 1234–1242. [Google Scholar] [CrossRef][Green Version]
- Mishra, N.S.; Reddy, R.; Kuila, A.; Rani, A.; Nawaz, A.; Pichiah, S. A Review on Advanced Oxidation Processes for Effective Water Treatment. Curr. World Environ. J. 2017, 12, 469–489. [Google Scholar] [CrossRef]
- Chong, M.N.; Jin, B.; Chow, C.W.K.; Saint, C. Recent developments in photocatalytic water treatment technology: A review. Water Res. 2010, 44, 2997–3027. [Google Scholar] [CrossRef] [PubMed]
- Djurišić, A.B.; He, Y.; Ng, A.M.C. Visible-light photocatalysts: Prospects and challenges. APL Mater. 2020, 8, 030903. [Google Scholar] [CrossRef]
- Zhang, T. Heterogeneous Catalytic Process for Wastewater Treatment, Advanced Oxidation Processes; Intechopen: London, UK, 2020. [Google Scholar] [CrossRef]
- Milan, E.G.; Mahvi, A.H.; Nabizadeh, R.; Alimohammadi, M. What is the effect on antibiotic resistant genes of chlorine disinfection in drinking water supply systems? A systematic review protocol. Environ. Évid. 2022, 11, 11. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).