Overview of Ni-Based Catalysts for Hydrogen Production from Biogas Reforming
Abstract
:1. Introduction
2. Reactions Involved in Biogas Reforming
3. Ni-Based Catalysts for Biogas Dry Reforming
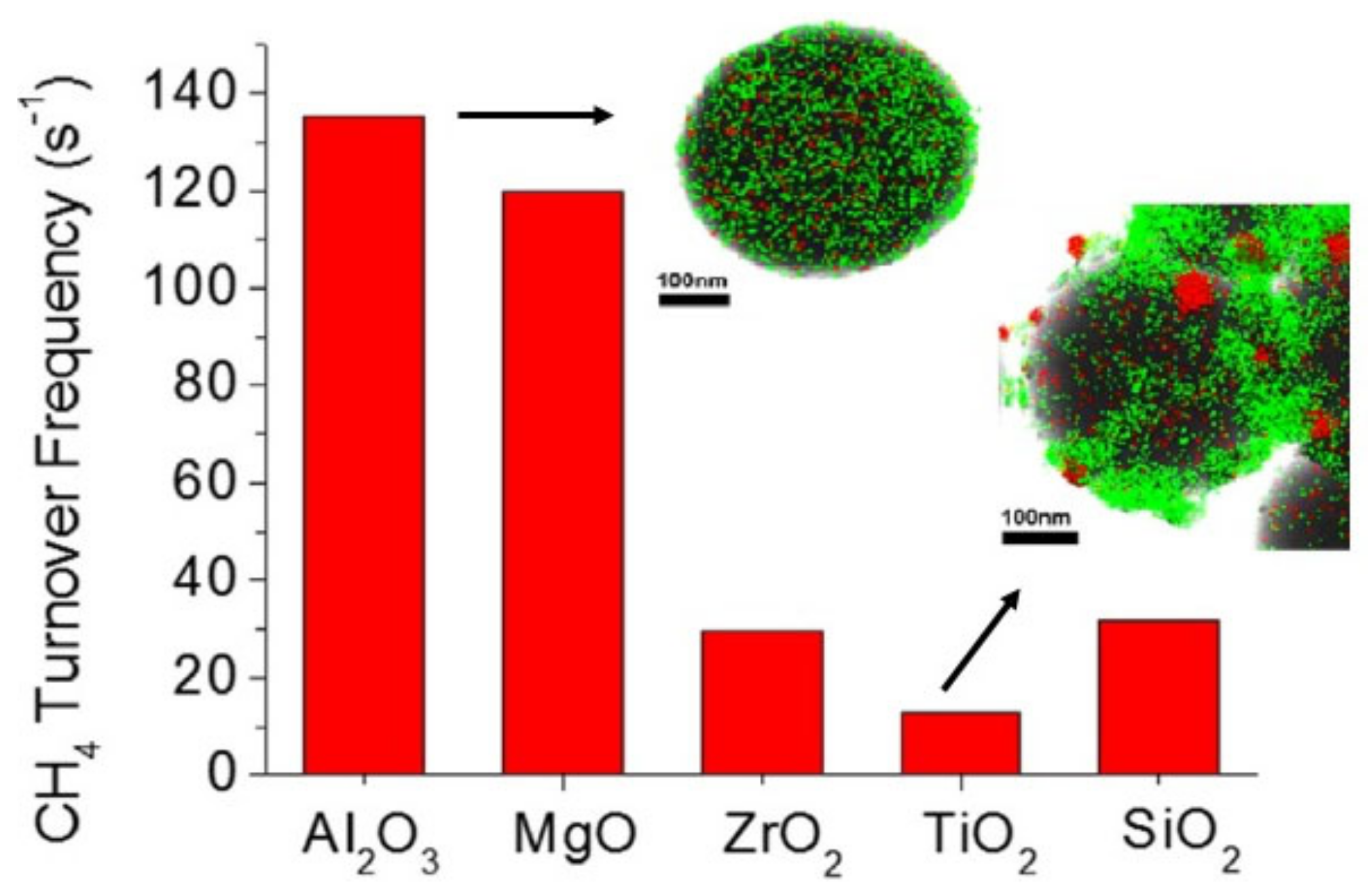
3.1. Effect of the Catalyst Support and Promoters
3.2. Core-Shell Catalysts
3.3. Bimetallic Catalysts
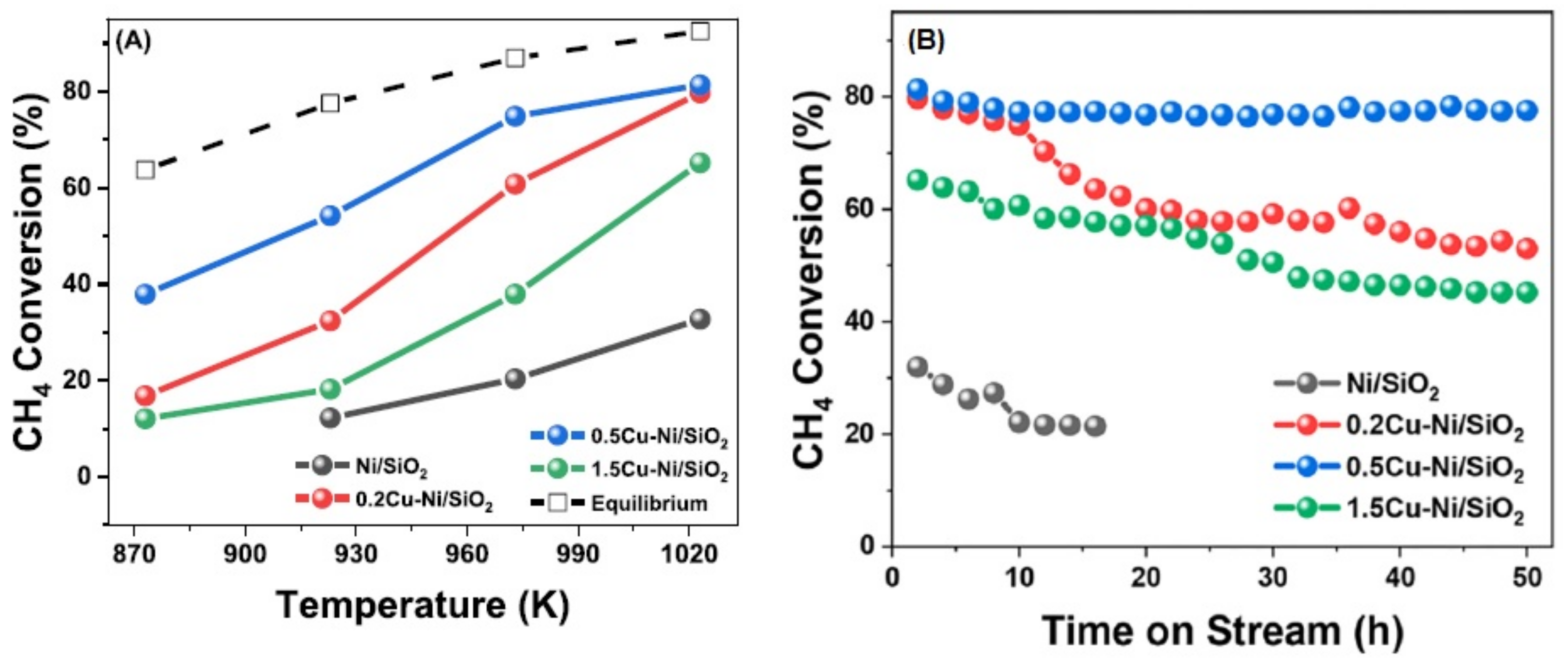
3.3.1. Ni and Transition Metals
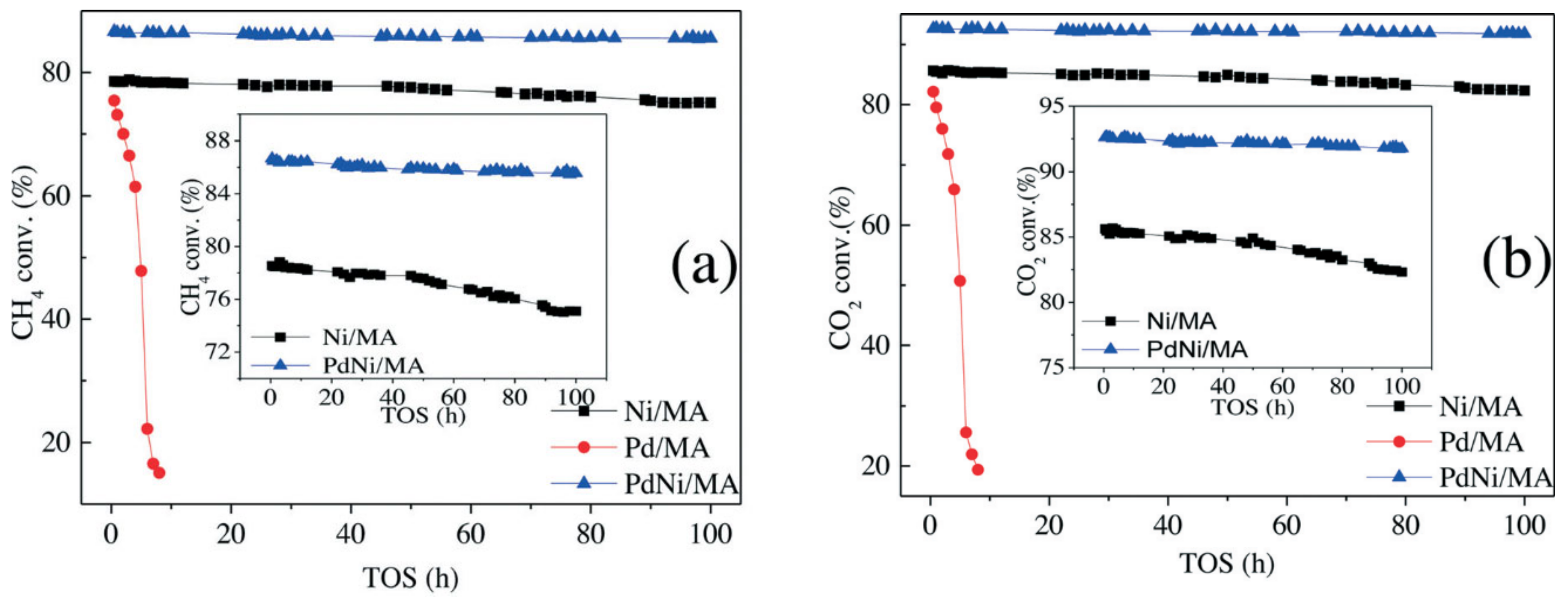
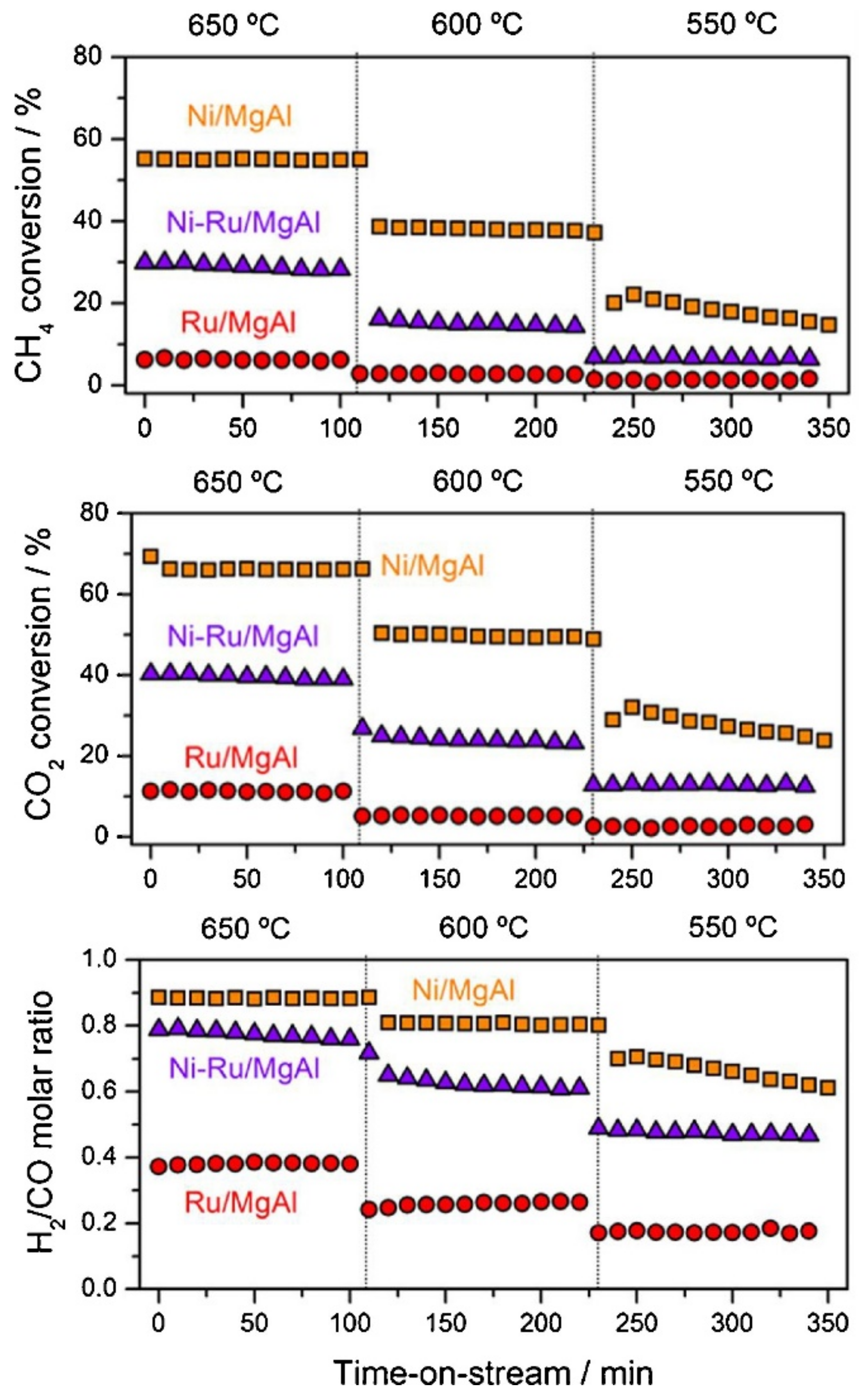
3.3.2. Ni and Noble Metals
4. Conclusions and Prospects
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Gao, Y.; Jiang, J.; Meng, Y.; Yan, F.; Aihemaiti, A. A Review of Recent Developments in Hydrogen Production via Biogas Dry Reforming. Energy Convers. Manag. 2018, 171, 133–155. [Google Scholar] [CrossRef]
- Abanades, S.; Abbaspour, H.; Ahmadi, A.; Das, B.; Ehyaei, M.A.; Esmaeilion, F.; El Haj Assad, M.; Hajilounezhad, T.; Jamali, D.H.; Hmida, A.; et al. A Critical Review of Biogas Production and Usage with Legislations Framework across the Globe. Int. J. Environ. Sci. Technol. 2022, 19, 3377–3400. [Google Scholar] [CrossRef] [PubMed]
- International Energy Agency. Outlook for Biogas and Biomethane: Prospects for Organic Growth. 2020. Available online: https://www.iea.org/reports/outlook-for-biogas-and-biomethane-prospects-for-organic-growth/annex (accessed on 22 February 2023).
- Alves, H.J.; Bley Junior, C.; Niklevicz, R.R.; Frigo, E.P.; Frigo, M.S.; Coimbra-Araújo, C.H. Overview of Hydrogen Production Technologies from Biogas and the Applications in Fuel Cells. Int. J. Hydrogen Energy 2013, 38, 5215–5225. [Google Scholar] [CrossRef]
- Ullah Khan, I.; Hafiz Dzarfan Othman, M.; Hashim, H.; Matsuura, T.; Ismail, A.F.; Rezaei-DashtArzhandi, M.; Wan Azelee, I. Biogas as a Renewable Energy Fuel—A Review of Biogas Upgrading, Utilisation and Storage. Energy Convers. Manag. 2017, 150, 277–294. [Google Scholar] [CrossRef]
- Awe, O.W.; Zhao, Y.; Nzihou, A.; Minh, D.P.; Lyczko, N. A Review of Biogas Utilisation, Purification and Upgrading Technologies. Waste Biomass Valorization 2017, 8, 267–283. [Google Scholar] [CrossRef]
- Sun, Q.; Li, H.; Yan, J.; Liu, L.; Yu, Z.; Yu, X. Selection of appropriate biogas upgrading technology-a review of biogas cleaning, upgrading and utilisation. Renew. Sustain. Energy Rev. 2015, 51, 521–532. [Google Scholar] [CrossRef]
- Jung, S.; Lee, J.; Moon, D.H.; Kim, K.H.; Kwon, E.E. Upgrading biogas into syngas through dry reforming. Renew. Sustain. Energy Rev. 2021, 143, 110949. [Google Scholar] [CrossRef]
- International Energy Agency. The Future of Hydrogen; OECD: Paris, France, 2019. [Google Scholar] [CrossRef]
- International Energy Agency. Global Hydrogen Review 2022; OECD: Paris, France, 2022. [Google Scholar] [CrossRef]
- Prato-Garcia, D.; Robayo-Avendaño, A.; Vasquez-Medrano, R. Hydrogen from Natural Gas and Biogas: Building Bridges for a Sustainable Transition to a Green Economy. Gas Sci. Eng. 2023, 111, 204918. [Google Scholar] [CrossRef]
- Lau, C.S.; Tsolakis, A.; Wyszynski, M.L. Biogas upgrade to syn-gas (H2-CO) via dry and oxidative reforming. Int. J. Hydrogen Energy 2011, 36, 397–404. [Google Scholar] [CrossRef]
- Araki, S.; Hino, N.; Mori, T.; Hikazudani, S. Autothermal reforming of biogas over a monolithic catalyst. J. Nat. Gas Chem. 2010, 19, 477–481. [Google Scholar] [CrossRef]
- Vikrant, K.; Kailasa, S.K.; Tsang, D.C.W.; Lee, S.S.; Kumar, P.; Giri, B.S.; Singh, R.S.; Kim, K.-H. Biofiltration of hydrogen sulfide: Trends and challenges. J. Clean. Prod. 2018, 187, 131–147. [Google Scholar] [CrossRef]
- Kathiraser, Y.; Oemar, U.; Saw, E.T.; Li, Z.; Kawi, S. Kinetic and Mechanistic Aspects for CO2 Reforming of Methane over Ni Based Catalysts. Chem. Eng. J. 2015, 278, 62–78. [Google Scholar] [CrossRef]
- Papadopoulou, C.; Matralis, H.; Verykios, X. Catalysis for Alternative Energy Generation; Guczi, L., Erdôhelyi, A., Eds.; Springer: New York, NY, USA, 2012; ISBN 978-1-4614-0343-2. [Google Scholar]
- Nikoo, M.K.; Amin, N.A.S. Thermodynamic Analysis of Carbon Dioxide Reforming of Methane in View of Solid Carbon Formation. Fuel Process. Technol. 2011, 92, 678–691. [Google Scholar] [CrossRef]
- Aramouni, N.A.K.; Touma, J.G.; Tarboush, B.A.; Zeaiter, J.; Ahmad, M.N. Catalyst Design for Dry Reforming of Methane: Analysis Review. Renew. Sustain. Energy Rev. 2018, 82, 2570–2585. [Google Scholar] [CrossRef]
- Jang, W.; Jeong, D.; Shim, J.; Kim, H.; Roh, H.; Son, I.H.; Lee, S.J. Combined Steam and Carbon Dioxide Reforming of Methane and Side Reactions: Thermodynamic Equilibrium Analysis and Experimental Application. Appl. Energy 2016, 173, 80–91. [Google Scholar] [CrossRef]
- Usman, M.; Wan Daud, W.M.A.; Abbas, H.F. Dry Reforming of Methane: Influence of Process Parameters—A Review. Renew. Sustain. Energy Rev. 2015, 45, 710–744. [Google Scholar] [CrossRef]
- Argyle, M.D.; Bartholomew, C.H. Heterogeneous Catalyst Deactivation and Regeneration: A Review. Catalysts 2015, 5, 145–269. [Google Scholar] [CrossRef]
- Arora, S.; Prasad, R. An overview on dry reforming of methane: Strategies to reduce carbonaceous deactivation of catalysts. RSC Adv. 2016, 6, 108668–108688. [Google Scholar] [CrossRef]
- Djinovic, P.; Crnivec, I.G.O.; Erjavec, B.; Pintar, A. Influence of active metal loading and oxygen mobility on coke-free dry reforming of Ni–Co bimetallic catalysts. Appl. Catal. B Environ. 2012, 125, 259–270. [Google Scholar] [CrossRef]
- Wang, N.; Xu, Z.; Deng, J.; Shen, K.; Yu, X.; Qian, W.; Chu, W.; Wei, F. One-pot Synthesis of Ordered Mesoporous NiCeAl Oxide Catalysts and a Study of Their Performance in Methane Dry Reforming. ChemCatChem 2014, 6, 1470–1480. [Google Scholar] [CrossRef]
- Rostrup-Nielsen, J.R.; Hansen, J.H.B. CO2-Reforming of Methane over Transition Metals. J. Catal. 1993, 144, 38–49. [Google Scholar] [CrossRef]
- Ferreira-Aparicio, P.; Guerrero-Ruiz, A.; Rodríguez-Ramos, I. Comparative Study at Low and Medium Reaction Temperatures of Syngas Production by Methane Reforming with Carbon Dioxide over Silica and Alumina Supported Catalysts. Appl. Catal. A Gen. 1998, 170, 177–187. [Google Scholar] [CrossRef]
- Hou, Z.; Chen, P.; Fang, H.; Zheng, X.; Yashima, T. Production of Synthesis Gas via Methane Reforming with CO2 on Noble Metals and Small Amount of Noble-(Rh-) Promoted Ni Catalysts. Int. J. Hydrogen Energy 2006, 31, 555–561. [Google Scholar] [CrossRef]
- Pakhare, D.; Spivey, J. A Review of Dry (CO2) Reforming of Methane over Noble Metal Catalysts. Chem. Soc. Rev. 2014, 43, 7813–7837. [Google Scholar] [CrossRef]
- Ferreira-Aparicio, P.; Rodríguez-Ramos, I.; Anderson, J.; Guerrero-Ruiz, A. Mechanistic Aspects of the Dry Reforming of Methane over Ruthenium Catalysts. Appl. Catal. A Gen. 2000, 202, 183–196. [Google Scholar] [CrossRef]
- Abdulrasheed, A.; Jalil, A.A.; Gambo, Y.; Ibrahim, M.; Hambali, H.U.; Shahul Hamid, M.Y. A Review on Catalyst Development for Dry Reforming of Methane to Syngas: Recent Advances. Renew. Sustain. Energy Rev. 2019, 108, 175–193. [Google Scholar] [CrossRef]
- Das, S.; Sengupta, M.; Patel, J.; Bordoloi, A. A Study of the Synergy between Support Surface Properties and Catalyst Deactivation for CO2 Reforming over Supported Ni Nanoparticles. Appl. Catal. A Gen. 2017, 545, 113–126. [Google Scholar] [CrossRef]
- Sahli, N.; Petit, C.; Roger, A.C.; Kiennemann, A.; Libs, S.; Bettahar, M.M. Ni Catalysts from NiAl2O4 Spinel for CO2 Reforming of Methane. Catal. Today 2006, 113, 187–193. [Google Scholar] [CrossRef]
- Ribeiro, N.F.P.; Neto, R.C.R.; Moya, S.F.; Souza, M.M.V.M.; Schmal, M. Synthesis of NiAl2O4 with High Surface Area as Precursor of Ni Nanoparticles for Hydrogen Production. Int. J. Hydrogen Energy 2010, 35, 11725–11732. [Google Scholar] [CrossRef]
- Zhou, L.; Li, L.; Wei, N.; Li, J.; Basset, J. Effect of NiAl2O4 Formation on Ni/Al2O3 Stability during Dry Reforming of Methane. ChemCatChem 2015, 7, 2508–2516. [Google Scholar] [CrossRef]
- Bao, Z.; Lu, Y.; Han, J.; Li, Y.; Yu, F. Highly Active and Stable Ni-Based Bimodal Pore Catalyst for Dry Reforming of Methane. Appl. Catal. A Gen. 2015, 491, 116–126. [Google Scholar] [CrossRef]
- Goula, M.A.; Charisiou, N.D.; Papageridis, K.N.; Delimitis, A.; Pachatouridou, E.; Iliopoulou, E.F. Nickel on Alumina Catalysts for the Production of Hydrogen Rich Mixtures via the Biogas Dry Reforming Reaction: Influence of the Synthesis Method. Int. J. Hydrogen Energy 2015, 40, 9183–9200. [Google Scholar] [CrossRef]
- Liu, L.; Wang, S.; Guo, Y.; Wang, B.; Rukundo, P.; Wen, S.; Wang, Z. Synthesis of a Highly Dispersed Ni/Al2O3 Catalyst with Enhanced Catalytic Performance for CO2 Reforming of Methane by an Electrospinning Method. Int. J. Hydrogen Energy 2016, 41, 17361–17369. [Google Scholar] [CrossRef]
- Shah, M.; Das, S.; Nayak, A.K.; Mondal, P.; Bordoloi, A. Smart Designing of Metal-Support Interface for Imperishable Dry Reforming Catalyst. Appl. Catal. A Gen. 2018, 556, 137–154. [Google Scholar] [CrossRef]
- Zhang, R.; Xia, G.; Li, M.; Wu, Y.; Nie, H.; Li, D. Effect of Support on the Performance of Ni-Based Catalyst in Methane Dry Reforming. J. Fuel Chem. Technol. 2015, 43, 1359–1365. [Google Scholar] [CrossRef]
- Gao, Y.; Aihemaiti, A.; Jiang, J.; Meng, Y.; Ju, T.; Han, S.; Chen, X.; Liu, J. Inspection over Carbon Deposition Features of Various Nickel Catalysts during Simulated Biogas Dry Reforming. J. Clean. Prod. 2020, 260, 120944. [Google Scholar] [CrossRef]
- Min, J.; Lee, Y.; Park, H.; Zhang, C.; Jun, K. Carbon Dioxide Reforming of Methane on Ni–MgO–Al2O3 Catalysts Prepared by Sol–Gel Method: Effects of Mg/Al Ratios. J. Ind. Eng. Chem. 2015, 26, 375–383. [Google Scholar] [CrossRef]
- Zhu, Y.; Zhang, S.; Chen, B.; Zhang, Z.; Shi, C. Effect of Mg/Al Ratio of NiMgAl Mixed Oxide Catalyst Derived from Hydrotalcite for Carbon Dioxide Reforming of Methane. Catal. Today 2016, 264, 163–170. [Google Scholar] [CrossRef]
- Zhan, Y.; Han, J.; Bao, Z.; Cao, B.; Li, Y.; Street, J.; Yu, F. Biogas Reforming of Carbon Dioxide to Syngas Production over Ni-Mg-Al Catalysts. Mol. Catal. 2017, 436, 248–258. [Google Scholar] [CrossRef]
- Ha, Q.; Armbruster, U.; Atia, H.; Schneider, M.; Lund, H.; Agostini, G.; Radnik, J.; Vuong, H.; Martin, A. Development of Active and Stable Low Nickel Content Catalysts for Dry Reforming of Methane. Catalysts 2017, 7, 157. [Google Scholar] [CrossRef]
- Nguyen-Phu, H.; Kim, T.; Kim, Y.; Kang, K.H.; Cho, H.; Kim, J.; Ro, I. Role of Phase in NiMgAl Mixed Oxide Catalysts for CO2 Dry Methane Reforming (DRM). Catal. Today 2023, 411–412, 113894. [Google Scholar] [CrossRef]
- Akbari, E.; Alavi, S.M.; Rezaei, M. Synthesis Gas Production over Highly Active and Stable Nanostructured Ni-MgO-Al2O3 Catalysts in Dry Reforming of Methane: Effects of Ni Contents. Fuel 2017, 194, 171–179. [Google Scholar] [CrossRef]
- Alipour, Z.; Rezaei, M.; Meshkani, F. Effect of Alkaline Earth Promoters (MgO, CaO, and BaO) on the Activity and Coke Formation of Ni Catalysts Supported on Nanocrystalline Al2O3 in Dry Reforming of Methane. J. Ind. Eng. Chem. 2014, 20, 2858–2863. [Google Scholar] [CrossRef]
- Sengupta, S.; Deo, G. Modifying Alumina with CaO or MgO in Supported Ni and Ni–Co Catalysts and Its Effect on Dry Reforming of CH4. J. CO2 Util. 2015, 10, 67–77. [Google Scholar] [CrossRef]
- Charisiou, N.D.; Baklavaridis, A.; Papadakis, V.G.; Goula, M.A. Synthesis Gas Production via the Biogas Reforming Reaction Over Ni/MgO–Al2O3 and Ni/CaO–Al2O3 Catalysts. Waste Biomass Valorization 2016, 7, 725–736. [Google Scholar] [CrossRef]
- Teh, L.P.; Setiabudi, H.D.; Timmiati, S.N.; Aziz, M.A.A.; Annuar, N.H.R.; Ruslan, N.N. Recent Progress in Ceria-Based Catalysts for the Dry Reforming of Methane: A Review. Chem. Eng. Sci. 2021, 242, 116606. [Google Scholar] [CrossRef]
- Damyanova, S.; Pawelec, B.; Palcheva, R.; Karakirova, Y.; Sanchez, M.C.C.; Tyuliev, G.; Gaigneaux, E.; Fierro, J.L.G. Structure and Surface Properties of Ceria-Modified Ni-Based Catalysts for Hydrogen Production. Appl. Catal. B Environ. 2018, 225, 340–353. [Google Scholar] [CrossRef]
- Li, M.; van Veen, A.C. Tuning the Catalytic Performance of Ni-Catalysed Dry Reforming of Methane and Carbon Deposition via Ni-CeO2- Interaction. Appl. Catal. B Environ. 2018, 237, 641–648. [Google Scholar] [CrossRef]
- Lino, A.V.P.; Assaf, E.M.; Assaf, J.M. Hydrotalcites Derived Catalysts for Syngas Production from Biogas Reforming: Effect of Nickel and Cerium Load. Catal. Today 2017, 289, 78–88. [Google Scholar] [CrossRef]
- Rosha, P.; Mohapatra, S.K.; Mahla, S.K.; Dhir, A. Biogas Reforming for Hydrogen Enrichment by Ceria Decorated over Nickel Catalyst Supported on Titania and Alumina. Int. J. Hydrogen Energy 2018, 43, 21246–21255. [Google Scholar] [CrossRef]
- Luisetto, I.; Tuti, S.; Battocchio, C.; Lo Mastro, S.; Sodo, A. Ni/CeO2–Al2O3 Catalysts for the Dry Reforming of Methane: The Effect of CeAlO3 Content and Nickel Crystallite Size on Catalytic Activity and Coke Resistance. Appl. Catal. A Gen. 2015, 500, 12–22. [Google Scholar] [CrossRef]
- Aghamohammadi, S.; Haghighi, M.; Maleki, M.; Rahemi, N. Sequential Impregnation vs. Sol-Gel Synthesized Ni/Al2O3-CeO2 Nanocatalyst for Dry Reforming of Methane: Effect of Synthesis Method and Support Promotion. Mol. Catal. 2017, 431, 39–48. [Google Scholar] [CrossRef]
- Marinho, A.L.A.; Toniolo, F.S.; Noronha, F.B.; Epron, F.; Duprez, D.; Bion, N. Highly Active and Stable Ni Dispersed on Mesoporous CeO2-Al2O3 Catalysts for Production of Syngas by Dry Reforming of Methane. Appl. Catal. B Environ. 2021, 281, 119459. [Google Scholar] [CrossRef]
- Yang, R.; Xing, C.; Lv, C.; Shi, L.; Tsubaki, N. Promotional Effect of La2O3 and CeO2 on Ni/γ-Al2O3 Catalysts for CO2 Reforming of CH4. Appl. Catal. A Gen. 2010, 385, 92–100. [Google Scholar] [CrossRef]
- Charisiou, N.D.; Siakavelas, G.; Papageridis, K.N.; Baklavaridis, A.; Tzounis, L.; Avraam, D.G.; Goula, M.A. Syngas Production via the Biogas Dry Reforming Reaction over Nickel Supported on Modified with CeO2 and/or La2O3 Alumina Catalysts. J. Nat. Gas Sci. Eng. 2016, 31, 164–183. [Google Scholar] [CrossRef]
- Farooqi, A.S.; Al-Swai, B.M.; Ruslan, F.H.; Mohd Zabidi, N.A.; Saidur, R.; Syed Muhammad, S.A.F.; Abdullah, B. Catalytic Conversion of Greenhouse Gases (CO2 and CH4) to Syngas over Ni-Based Catalyst: Effects of Ce-La Promoters. Arab. J. Chem. 2020, 13, 5740–5749. [Google Scholar] [CrossRef]
- Taherian, Z.; Shahed Gharahshiran, V.; Khataee, A.; Meshkani, F.; Orooji, Y. Comparative Study of Modified Ni Catalysts over Mesoporous CaO-Al2O3 Support for CO2/Methane Reforming. Catal. Commun. 2020, 145, 106100. [Google Scholar] [CrossRef]
- Shamskar, F.R.; Meshkani, F.; Rezaei, M. Preparation and Characterization of Ultrasound-Assisted Co-Precipitated Nanocrystalline La-, Ce-, Zr -Promoted Ni-Al2O3 Catalysts for Dry Reforming Reaction. J. CO2 Util. 2017, 22, 124–134. [Google Scholar] [CrossRef]
- Rezaei, M.; Alavi, S.M. Dry Reforming over Mesoporous Nanocrystalline 5% Ni/M-MgAl2O4 (M: CeO2, ZrO2, La2O3) Catalysts. Int. J. Hydrogen Energy 2019, 44, 16516–16525. [Google Scholar] [CrossRef]
- Calgaro, C.O.; Lima, D.d.S.; Tonietto, R.; Perez-Lopez, O.W. Biogas Dry Reforming Over Ni–Mg–La–Al Catalysts: Influence of La/Mg Ratio. Catal. Lett. 2021, 151, 267–280. [Google Scholar] [CrossRef]
- Al-Mubaddel, F.S.; Kumar, R.; Sofiu, M.L.; Frusteri, F.; Ibrahim, A.A.; Srivastava, V.K.; Kasim, S.O.; Fakeeha, A.H.; Abasaeed, A.E.; Osman, A.I.; et al. Optimizing Acido-Basic Profile of Support in Ni Supported La2O3-Al2O3 Catalyst for Dry Reforming of Methane. Int. J. Hydrogen Energy 2021, 46, 14225–14235. [Google Scholar] [CrossRef]
- Charisiou, N.D.; Tzounis, L.; Sebastian, V.; Hinder, S.J.; Baker, M.A.; Polychronopoulou, K.; Goula, M.A. Investigating the Correlation between Deactivation and the Carbon Deposited on the Surface of Ni/Al2O3 and Ni/La2O3-Al2O3 Catalysts during the Biogas Reforming Reaction. Appl. Surf. Sci. 2019, 474, 42–56. [Google Scholar] [CrossRef]
- Al-Fatesh, A.S.; Naeem, M.A.; Fakeeha, A.H.; Abasaeed, A.E. Role of La2O3 as Promoter and Support in Ni/γ-Al2O3 Catalysts for Dry Reforming of Methane. Chin. J. Chem. Eng. 2014, 22, 28–37. [Google Scholar] [CrossRef]
- Tsoukalou, A.; Imtiaz, Q.; Kim, S.M.; Abdala, P.M.; Yoon, S.; Müller, C.R. Dry-Reforming of Methane over Bimetallic Ni–M/La2O3 (M = Co, Fe): The Effect of the Rate of La2O2CO3 Formation and Phase Stability on the Catalytic Activity and Stability. J. Catal. 2016, 343, 208–214. [Google Scholar] [CrossRef]
- Li, K.; Chang, X.; Pei, C.; Li, X.; Chen, S.; Zhang, X.; Assabumrungrat, S.; Zhao, Z.J.; Zeng, L.; Gong, J. Ordered Mesoporous Ni/La2O3 Catalysts with Interfacial Synergism towards CO2 Activation in Dry Reforming of Methane. Appl. Catal. B Environ. 2019, 259, 118092. [Google Scholar] [CrossRef]
- Li, K.; Pei, C.; Li, X.; Chen, S.; Zhang, X.; Liu, R.; Gong, J. Dry Reforming of Methane over La2O2CO3-Modified Ni/Al2O3 Catalysts with Moderate Metal Support Interaction. Appl. Catal. B Environ. 2020, 264, 118448. [Google Scholar] [CrossRef]
- Therdthianwong, S.; Siangchin, C.; Therdthianwong, A. Improvement of Coke Resistance of Ni/Al2O3 Catalyst in CH4/CO2 Reforming by ZrO2 Addition. Fuel Process. Technol. 2008, 89, 160–168. [Google Scholar] [CrossRef]
- Morales Anzures, F.; Salinas Hernández, P.; Mondragón Galicia, G.; Gutiérrez Martínez, A.; Tzompantzi Morales, F.; Romero Romo, M.A.; Pérez Hernández, R. Synthetic Gas Production by Dry Reforming of Methane over Ni/Al2O3–ZrO2 Catalysts: High H2/CO Ratio. Int. J. Hydrogen Energy 2021, 46, 26224–26233. [Google Scholar] [CrossRef]
- Shin, S.A.; Noh, Y.S.; Hong, G.H.; Park, J.I.; Song, H.T.; Lee, K.Y.; Moon, D.J. Dry Reforming of Methane over Ni/ZrO2-Al2O3 Catalysts: Effect of Preparation Methods. J. Taiwan Inst. Chem. Eng. 2018, 90, 25–32. [Google Scholar] [CrossRef]
- Iglesias, I.; Baronetti, G.; Alemany, L.; Mariño, F. Insight into Ni/Ce1−xZrxO2−δ Support Interplay for Enhanced Methane Steam Reforming. Int. J. Hydrogen Energy 2019, 44, 3668–3680. [Google Scholar] [CrossRef]
- Kumar, P.; Sun, Y.; Idem, R.O. Nickel-Based Ceria, Zirconia, and Ceria–Zirconia Catalytic Systems for Low-Temperature Carbon Dioxide Reforming of Methane. Energy Fuels 2007, 21, 3113–3123. [Google Scholar] [CrossRef]
- Kambolis, A.; Matralis, H.; Trovarelli, A.; Papadopoulou, C. Ni/CeO2-ZrO2 Catalysts for the Dry Reforming of Methane. Appl. Catal. A Gen. 2010, 377, 16–26. [Google Scholar] [CrossRef]
- Wolfbeisser, A.; Sophiphun, O.; Bernardi, J.; Wittayakun, J.; Föttinger, K.; Rupprechter, G. Methane Dry Reforming over Ceria-Zirconia Supported Ni Catalysts. Catal. Today 2016, 277, 234–245. [Google Scholar] [CrossRef]
- Luisetto, I.; Tuti, S.; Romano, C.; Boaro, M.; Di Bartolomeo, E.; Kesavan, J.K.; Kumar, S.S.; Selvakumar, K. Dry Reforming of Methane over Ni Supported on Doped CeO2: New Insight on the Role of Dopants for CO2 Activation. J. CO2 Util. 2019, 30, 63–78. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, Q.; Tsubaki, N.; Tan, Y.; Han, Y. Influence of Zirconia Phase on the Performance of Ni/ZrO2 for Carbon Dioxide Reforming of Methane. In Advances in CO2 Capture, Sequestration, and Conversion; Jin, F., He, L., Hu, Y., Eds.; ACS Symposium Series; American Chemical Society: Washington, DC, USA, 2015; Volume 1194, pp. 135–153. [Google Scholar]
- Zhang, X.; Zhang, Q.; Tsubaki, N.; Tan, Y.; Han, Y. Carbon Dioxide Reforming of Methane over Ni Nanoparticles Incorporated into Mesoporous Amorphous ZrO2 Matrix. Fuel 2015, 147, 243–252. [Google Scholar] [CrossRef]
- Zhang, M.; Zhang, J.; Wu, Y.; Pan, J.; Zhang, Q.; Tan, Y.; Han, Y. Insight into the Effects of the Oxygen Species over Ni/ZrO2 Catalyst Surface on Methane Reforming with Carbon Dioxide. Appl. Catal. B Environ. 2019, 244, 427–437. [Google Scholar] [CrossRef]
- Lou, Y.; Steib, M.; Zhang, Q.; Tiefenbacher, K.; Horváth, A.; Jentys, A.; Liu, Y.; Lercher, J.A. Design of Stable Ni/ZrO2 Catalysts for Dry Reforming of Methane. J. Catal. 2017, 356, 147–156. [Google Scholar] [CrossRef]
- Goula, M.A.; Charisiou, N.D.; Siakavelas, G.; Tzounis, L.; Tsiaoussis, I.; Panagiotopoulou, P.; Goula, G.; Yentekakis, I.V. Syngas Production via the Biogas Dry Reforming Reaction over Ni Supported on Zirconia Modified with CeO2 or La2O3 Catalysts. Int. J. Hydrogen Energy 2017, 42, 13724–13740. [Google Scholar] [CrossRef]
- Charisiou, N.D.; Siakavelas, G.; Tzounis, L.; Sebastian, V.; Monzon, A.; Baker, M.A.; Hinder, S.J.; Polychronopoulou, K.; Yentekakis, I.V.; Goula, M.A. An in Depth Investigation of Deactivation through Carbon Formation during the Biogas Dry Reforming Reaction for Ni Supported on Modified with CeO2 and La2O3 Zirconia Catalysts. Int. J. Hydrogen Energy 2018, 43, 18955–18976. [Google Scholar] [CrossRef]
- Fakeeha, A.H.; Khan, W.U.; Al-Fatesh, A.S.; Abasaeed, A.E. Stabilities of Zeolite-Supported Ni Catalysts for Dry Reforming of Methane. Chin. J. Catal. 2013, 34, 764–768. [Google Scholar] [CrossRef]
- Moradi, G.; Khezeli, F.; Hemmati, H. Syngas Production with Dry Reforming of Methane over Ni/ZSM-5 Catalysts. J. Nat. Gas Sci. Eng. 2016, 33, 657–665. [Google Scholar] [CrossRef]
- Singh, S.; Kumar, R.; Setiabudi, H.D.; Nanda, S.; Vo, D.N. Advanced Synthesis Strategies of Mesoporous SBA-15 Supported Catalysts for Catalytic Reforming Applications: A State-of-the-Art Review. Appl. Catal. A Gen. 2018, 559, 57–74. [Google Scholar] [CrossRef]
- Gálvez, M.E.; Albarazi, A.; Da Costa, P. Enhanced Catalytic Stability through Non-Conventional Synthesis of Ni/SBA-15 for Methane Dry Reforming at Low Temperatures. Appl. Catal. A Gen. 2015, 504, 143–150. [Google Scholar] [CrossRef]
- Karam, L.; Casale, S.; El Zakhem, H.; El Hassan, N. Tuning the Properties of Nickel Nanoparticles inside SBA-15 Mesopores for Enhanced Stability in Methane Reforming. J. CO2 Util. 2017, 17, 119–124. [Google Scholar] [CrossRef]
- Omoregbe, O.; Danh, H.T.; Nguyen-Huy, C.; Setiabudi, H.D.; Abidin, S.Z.; Truong, Q.D.; Vo, D.N. Syngas Production from Methane Dry Reforming over Ni/SBA-15 Catalyst: Effect of Operating Parameters. Int. J. Hydrogen Energy 2017, 42, 11283–11294. [Google Scholar] [CrossRef]
- Kiani, P.; Meshksar, M.; Rahimpour, M.R. Biogas Reforming over La-Promoted Ni/SBA-16 Catalyst for Syngas Production: Catalytic Structure and Process Activity Investigation. Int. J. Hydrogen Energy 2023, 48, 6262–6274. [Google Scholar] [CrossRef]
- Lovell, E.; Jiang, Y.; Scott, J.; Wang, F.; Suhardja, Y.; Chen, M.; Huang, J.; Amal, R. CO2 Reforming of Methane over MCM-41-Supported Nickel Catalysts: Altering Support Acidity by One-Pot Synthesis at Room Temperature. Appl. Catal. A Gen. 2014, 473, 51–58. [Google Scholar] [CrossRef]
- Aguiar, M.; Cazula, B.B.; Saragiotto Colpini, L.M.; Borba, C.E.; Alves da Silva, F.; Noronha, F.B.; Alves, H.J. Si-MCM-41 Obtained from Different Sources of Silica and Its Application as Support for Nickel Catalysts Used in Dry Reforming of Methane. Int. J. Hydrogen Energy 2019, 44, 32003–32018. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, G.; Liu, J.; Li, T.; Wang, Y.; Zhao, Y.; Li, G.; Zhang, Y. Dry Reforming of Methane over Ni/SiO2 Catalysts: Role of Support Structure Properties. Fuel 2023, 340, 127490. [Google Scholar] [CrossRef]
- Zhang, J.; Li, F. Coke-Resistant Ni@SiO2 Catalyst for Dry Reforming of Methane. Appl. Catal. B Environ. 2015, 176–177, 513–521. [Google Scholar] [CrossRef]
- Wang, F.; Han, B.; Zhang, L.; Xu, L.; Yu, H.; Shi, W. CO2 Reforming with Methane over Small-Sized Ni@SiO2 Catalysts with Unique Features of Sintering-Free and Low Carbon. Appl. Catal. B Environ. 2018, 235, 26–35. [Google Scholar] [CrossRef]
- Han, J.W.; Kim, C.; Park, J.S.; Lee, H. Highly Coke-Resistant Ni Nanoparticle Catalysts with Minimal Sintering in Dry Reforming of Methane. ChemSusChem 2014, 7, 451–456. [Google Scholar] [CrossRef] [PubMed]
- Han, J.W.; Park, J.S.; Choi, M.S.; Lee, H. Uncoupling the Size and Support Effects of Ni Catalysts for Dry Reforming of Methane. Appl. Catal. B Environ. 2017, 203, 625–632. [Google Scholar] [CrossRef]
- Das, S.; Ashok, J.; Bian, Z.; Dewangan, N.; Wai, M.H.; Du, Y.; Borgna, A.; Hidajat, K.; Kawi, S. Silica–Ceria Sandwiched Ni Core–Shell Catalyst for Low Temperature Dry Reforming of Biogas: Coke Resistance and Mechanistic Insights. Appl. Catal. B Environ. 2018, 230, 220–236. [Google Scholar] [CrossRef]
- Han, K.; Xu, S.; Wang, Y.; Wang, S.; Zhao, L.; Kambonde, J.; Yu, H.; Shi, W.; Wang, F. Confining Ni and Ceria in Silica Shell as Synergistic Multifunctional Catalyst for Methane Dry Reforming Reaction. J. Power Sources 2021, 506, 230232. [Google Scholar] [CrossRef]
- Lin, S.; Wang, J.; Mi, Y.; Yang, S.; Wang, Z.; Liu, W.; Wu, D.; Peng, H. Trifunctional Strategy for the Design and Synthesis of a Ni-CeO2@SiO2 Catalyst with Remarkable Low-Temperature Sintering and Coking Resistance for Methane Dry Reforming. Chin. J. Catal. 2021, 42, 1808–1820. [Google Scholar] [CrossRef]
- Liu, W.; Li, L.; Zhang, X.; Wang, Z.; Wang, X.; Peng, H. Design of Ni-ZrO2@SiO2 Catalyst with Ultra-High Sintering and Coking Resistance for Dry Reforming of Methane to Prepare Syngas. J. CO2 Util. 2018, 27, 297–307. [Google Scholar] [CrossRef]
- Das, S.; Jangam, A.; Jayaprakash, S.; Xi, S.; Hidajat, K.; Tomishige, K.; Kawi, S. Role of Lattice Oxygen in Methane Activation on Ni-Phyllosilicate@Ce1-XZrxO2 Core-Shell Catalyst for Methane Dry Reforming: Zr Doping Effect, Mechanism, and Kinetic Study. Appl. Catal. B Environ. 2021, 290, 119998. [Google Scholar] [CrossRef]
- Marinho, A.L.A.; Rabelo-Neto, R.C.; Epron, F.; Bion, N.; Toniolo, F.S.; Noronha, F.B. Embedded Ni Nanoparticles in CeZrO2 as Stable Catalyst for Dry Reforming of Methane. Appl. Catal. B Environ. 2020, 268, 118387. [Google Scholar] [CrossRef]
- Marinho, A.L.A.; Rabelo-Neto, R.C.; Epron, F.; Toniolo, F.S.; Noronha, F.B.; Bion, N. Effect of Metal Dopant on the Performance of Ni@CeMeO2 Embedded Catalysts (Me = Gd, Sm and Zr) for Dry Reforming of Methane. Methane 2022, 1, 300–319. [Google Scholar] [CrossRef]
- Meloni, E.; Martino, M.; Palma, V. A Short Review on Ni Based Catalysts and Related Engineering Issues for Methane Steam Reforming. Catalysts 2020, 10, 352. [Google Scholar] [CrossRef]
- Angeli, S.D.; Monteleone, G.; Giaconia, A.; Lemonidou, A.A. State-of-the-Art Catalysts for CH4 Steam Reforming at Low Temperature. Int. J. Hydrogen Energy 2014, 39, 1979–1997. [Google Scholar] [CrossRef]
- Gao, X.; Ge, Z.; Zhu, G.; Wang, Z.; Ashok, J.; Kawi, S. Anti-Coking and Anti-Sintering Ni/Al2O3 Catalysts in the Dry Reforming of Methane: Recent Progress and Prospects. Catalysts 2021, 11, 1003. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, H.; Dalai, A.K. Development of Stable Bimetallic Catalysts for Carbon Dioxide Reforming of Methane. J. Catal. 2007, 249, 300–310. [Google Scholar] [CrossRef]
- Kim, D.H.; Youn, J.R.; Seo, J.C.; Kim, S.B.; Kim, M.J.; Lee, K. One-Pot Synthesis of NiCo/MgAl2O4 Catalyst for High Coke-Resistance in Steam Methane Reforming: Optimization of Ni/Co Ratio. Catal. Today 2023, 411–412, 113910. [Google Scholar] [CrossRef]
- Braga, A.H.; De Oliveira, D.C.; Taschin, A.R.; Santos, J.B.O.; Gallo, J.M.R.; Buen, J.M.C. Steam Reforming of Ethanol Using Ni-Co Catalysts Supported on MgAl2O4: Structural Study and Catalytic Properties at Different Temperatures. ACS Catal. 2021, 11, 2047–2061. [Google Scholar] [CrossRef]
- Koh, A.C.W.; Chen, L.; Kee Leong, W.; Johnson, B.F.G.; Khimyak, T.; Lin, J. Hydrogen or Synthesis Gas Production via the Partial Oxidation of Methane over Supported Nickel-Cobalt Catalysts. Int. J. Hydrogen Energy 2007, 32, 725–730. [Google Scholar] [CrossRef]
- Sheng, K.; Luan, D.; Jiang, H.; Zeng, F.; Wei, B.; Pang, F.; Ge, J. NixCoy Nanocatalyst Supported by ZrO2 Hollow Sphere for Dry Reforming of Methane: Synergetic Catalysis by Ni and Co in Alloy. ACS Appl. Mater. Interfaces 2019, 11, 24078–24087. [Google Scholar] [CrossRef]
- Bahari, M.B.; Setiabudi, H.D.; Ainirazali, N.; Vo, D.V.N. A Short Review on Bimetallic Co-Based Catalysts for Carbon Dioxide Reforming of Methane. Mater. Today Proc. 2019, 42, 94–100. [Google Scholar] [CrossRef]
- You, X.; Wang, X.; Ma, Y.; Liu, J.; Liu, W.; Xu, X.; Peng, H.; Li, C.; Zhou, W.; Yuan, P.; et al. Ni-Co/Al2O3 Bimetallic Catalysts for CH4 Steam Reforming: Elucidating the Role of Co for Improving Coke Resistance. ChemCatChem 2014, 6, 3377–3386. [Google Scholar] [CrossRef]
- Horlyck, J.; Lawrey, C.; Lovell, E.C.; Amal, R.; Scott, J. Elucidating the Impact of Ni and Co Loading on the Selectivity of Bimetallic NiCo Catalysts for Dry Reforming of Methane. Chem. Eng. J. 2018, 352, 572–580. [Google Scholar] [CrossRef]
- Lyu, L.; Shengene, M.; Ma, Q.; Sun, J.; Gao, X.; Fan, H.; Zhang, J.; Zhao, T.S. Synergy of Macro-Meso Bimodal Pore and Ni-Co Alloy for Enhanced Stability in Dry Reforming of Methane. Fuel 2022, 310, 122375. [Google Scholar] [CrossRef]
- Zolghadri, S.; Honarvar, B.; Rahimpour, M.R. Synthesis, Application, and Characteristics of Mesoporous Alumina as a Support of Promoted Ni-Co Bimetallic Catalysts in Steam Reforming of Methane. Fuel 2023, 335, 127005. [Google Scholar] [CrossRef]
- Sharifi, M.; Haghighi, M.; Rahmani, F.; Karimipour, S. Syngas Production via Dry Reforming of CH4 over Co- and Cu-Promoted Ni/Al2O3-ZrO2 Nanocatalysts Synthesized via Sequential Impregnation and Sol-Gel Methods. J. Nat. Gas Sci. Eng. 2014, 21, 993–1004. [Google Scholar] [CrossRef]
- Bernardo, C.A.; Alstrup, I.; Rostrup-Nielsen, J.R. Carbon Deposition and Methane Steam Reforming on Silica-Supported NiCu Catalysts. J. Catal. 1985, 96, 517–534. [Google Scholar] [CrossRef]
- Moradi, G.R.; Khosravian, F.; Rahmanzadeh, M. Effects of Partial Substitution of Ni by Cu in LaNiO3 Perovskite Catalyst for Dry Methane Reforming. Chin. J. Catal. 2012, 33, 797–801. [Google Scholar] [CrossRef]
- Quirino, P.P.S.; Amaral, A.F.; Manenti, F.; Pontes, K.V. Mapping and Optimization of an Industrial Steam Methane Reformer by the Design of Experiments (DOE). Chem. Eng. Res. Des. 2022, 184, 349–365. [Google Scholar] [CrossRef]
- Chatla, A.; Ghouri, M.M.; El Hassan, O.W.; Mohamed, N.; Prakash, A.V.; Elbashir, N.O. An Experimental and First Principles DFT Investigation on the Effect of Cu Addition to Ni/Al2O3 Catalyst for the Dry Reforming of Methane. Appl. Catal. A Gen. 2020, 602, 117699. [Google Scholar] [CrossRef]
- Tavares, M.T.; Alstrup, I.; Bernardo, C.A.A. Coking and Decoking during Methanation and Methane Decomposition on Ni-Cu Supported Catalysts. Mater. Corros.—Werkst. Und Korros. 1999, 50, 681–685. [Google Scholar] [CrossRef]
- La Rosa, D.; Lo Faro, M.; Monforte, G.; Antonucci, V.; Aricò, A.S.; Sin, A. Recent Advances on the Development of NiCu Alloy Catalysts for IT-SOFCs. ECS Trans. 2007, 7, 1685–1693. [Google Scholar] [CrossRef]
- Kitla, A.; Safonova, O.V.; Föttinger, K. Infrared Studies on Bimetallic Copper/Nickel Catalysts Supported on Zirconia and Ceria/Zirconia. Catal. Lett. 2013, 143, 517–530. [Google Scholar] [CrossRef] [PubMed]
- Naghash, A.R.; Etsell, T.H.; Xu, S. XRD and XPS Study of Cu-Ni Interactions on Reduced Copper-Nickel-Aluminum Oxide Solid Solution Catalysts. Chem. Mater. 2006, 18, 2480–2488. [Google Scholar] [CrossRef]
- Song, K.; Lu, M.; Xu, S.; Chen, C.; Zhan, Y.; Li, D.; Au, C.; Jiang, L.; Tomishige, K. Effect of Alloy Composition on Catalytic Performance and Coke-Resistance Property of Ni-Cu/Mg(Al)O Catalysts for Dry Reforming of Methane. Appl. Catal. B Environ. 2018, 239, 324–333. [Google Scholar] [CrossRef]
- Nataj, S.M.M.; Alavi, S.M.; Mazloom, G. Modeling and Optimization of Methane Dry Reforming over Ni–Cu/Al2O3 Catalyst Using Box–Behnken Design. J. Energy Chem. 2018, 27, 1475–1488. [Google Scholar] [CrossRef]
- Lee, J.H.; Lee, E.G.; Joo, O.S.; Jung, K.D. Stabilization of Ni/Al2O3 Catalyst by Cu Addition for CO2 Reforming of Methane. Appl. Catal. A Gen. 2004, 269, 1–6. [Google Scholar] [CrossRef]
- Han, K.; Wang, S.; Liu, Q.; Wang, F. Optimizing the Ni/Cu Ratio in Ni-Cu Nanoparticle Catalysts for Methane Dry Reforming. ACS Appl. Nano Mater. 2021, 4, 5340–5348. [Google Scholar] [CrossRef]
- Braga, A.; Armengol-Profitós, M.; Pascua-Solé, L.; Vendrell, X.; Soler, L.; Serrano, I.; Villar-Garcia, I.J.; Pérez-Dieste, V.; Divins, N.J.; Llorca, J. Bimetallic NiFe Nanoparticles Supported on CeO2 as Catalysts for Methane Steam Reforming. ACS Appl. Nano Mater. 2023, 6, 7173–7185. [Google Scholar] [CrossRef]
- Kim, S.M.; Abdala, P.M.; Margossian, T.; Hosseini, D.; Foppa, L.; Armutlulu, A.; Van Beek, W.; Comas-Vives, A.; Copéret, C.; Müller, C. Cooperativity and Dynamics Increase the Performance of NiFe Dry Reforming Catalysts. J. Am. Chem. Soc. 2017, 139, 1937–1949. [Google Scholar] [CrossRef]
- Tomishige, K.; Li, D.; Tamura, M.; Nakagawa, Y. Nickel-Iron Alloy Catalysts for Reforming of Hydrocarbons: Preparation, Structure, and Catalytic Properties. Catal. Sci. Technol. 2017, 7, 3952–3979. [Google Scholar] [CrossRef]
- Li, Y.; Wang, Q.; Cao, M.; Li, S.; Song, Z.; Qiu, L.; Yu, F.; Li, R.; Yan, X. Structural Evolution of Robust Ni3Fe1 Alloy on Al2O3 in Dry Reforming of Methane: Effect of Iron-Surplus Strategy from Ni1Fe1 to Ni3Fe1. Appl. Catal. B Environ. 2023, 331, 122669. [Google Scholar] [CrossRef]
- Song, Z.; Wang, Q.; Guo, C.; Li, S.; Yan, W.; Jiao, W.; Qiu, L.; Yan, X.; Li, R. Improved Effect of Fe on the Stable NiFe/Al2O3 Catalyst in Low-Temperature Dry Reforming of Methane. Ind. Eng. Chem. Res. 2020, 59, 17250–17258. [Google Scholar] [CrossRef]
- Thalinger, R.; Gocyla, M.; Heggen, M.; Dunin-Borkowski, R.; Grünbacher, M.; Stöger-Pollach, M.; Schmidmair, D.; Klötzer, B.; Penner, S. Ni-Perovskite Interaction and Its Structural and Catalytic Consequences in Methane Steam Reforming and Methanation Reactions. J. Catal. 2016, 337, 26–35. [Google Scholar] [CrossRef]
- Kim, W.Y.; Jang, J.S.; Ra, E.C.; Kim, K.Y.; Kim, E.H.; Lee, J.S. Reduced Perovskite LaNiO3 Catalysts Modified with Co and Mn for Low Coke Formation in Dry Reforming of Methane. Appl. Catal. A Gen. 2019, 575, 198–203. [Google Scholar] [CrossRef]
- García-Diéguez, M.; Pieta, I.S.; Herrera, M.C.; Larrubia, M.A.; Alemany, L.J. Improved Pt-Ni Nanocatalysts for Dry Reforming of Methane. Appl. Catal. A Gen. 2010, 377, 191–199. [Google Scholar] [CrossRef]
- Rostrup-Nielsen, J.R. Promotion by Poisoning. Stud. Surf. Sci. Catal. 1991, 68, 85–101. [Google Scholar]
- Pawelec, B.; Damyanova, S.; Arishtirova, K.; Fierro, J.L.G.; Petrov, L. Structural and Surface Features of PtNi Catalysts for Reforming of Methane with CO2. Appl. Catal. A Gen. 2007, 323, 188–201. [Google Scholar] [CrossRef]
- Dai, C.; Zhang, S.; Zhang, A.; Song, C.; Shi, C.; Guo, X. Hollow Zeolite Encapsulated Ni-Pt Bimetals for Sintering and Coking Resistant Dry Reforming of Methane. J. Mater. Chem. A 2015, 3, 16461–16468. [Google Scholar] [CrossRef]
- García-Diéguez, M.; Finocchio, E.; Larrubia, M.Á.; Alemany, L.J.; Busca, G. Characterization of Alumina-Supported Pt, Ni and PtNi Alloy Catalysts for the Dry Reforming of Methane. J. Catal. 2010, 274, 11–20. [Google Scholar] [CrossRef]
- García-Diéguez, M.; Pieta, I.S.; Herrera, M.C.; Larrubia, M.A.; Alemany, L.J. Nanostructured Pt- and Ni-Based Catalysts for CO2-Reforming of Methane. J. Catal. 2010, 270, 136–145. [Google Scholar] [CrossRef]
- Egelske, B.T.; Keels, J.M.; Monnier, J.R.; Regalbuto, J.R. An Analysis of Electroless Deposition Derived Ni-Pt Catalysts for the Dry Reforming of Methane. J. Catal. 2020, 381, 374–384. [Google Scholar] [CrossRef]
- Wang, J.; Mao, Y.; Zhang, L.Z.; Li, Y.; Liu, W.; Ma, Q.; Wu, D.; Peng, H. Remarkable Basic-Metal Oxides Promoted Confinement Catalysts for CO2 Reforming. Fuel 2022, 315, 123167. [Google Scholar] [CrossRef]
- Mao, Y.; Zhang, L.; Zheng, X.; Liu, W.; Cao, Z.; Peng, H. Coke-Resistance over Rh–Ni Bimetallic Catalyst for Low Temperature Dry Reforming of Methane. Int. J. Hydrogen Energy 2023, 48, 13890–13901. [Google Scholar] [CrossRef]
- Jóźwiak, W.K.; Nowosielska, M.; Rynkowski, J. Reforming of Methane with Carbon Dioxide over Supported Bimetallic Catalysts Containing Ni and Noble Metal I. Characterization and Activity of SiO2 Supported Ni-Rh Catalysts. Appl. Catal. A Gen. 2005, 280, 233–244. [Google Scholar] [CrossRef]
- Romano, P.N.; de Carvalho Filho, J.F.S.; de Almeida, J.M.A.R.; Sousa-Aguiar, E.F. Screening of Mono and Bimetallic Catalysts for the Dry Reforming of Methane. Catal. Today 2022, 394–396, 348–356. [Google Scholar] [CrossRef]
- Steinhauer, B.; Kasireddy, M.R.; Radnik, J.; Martin, A. Development of Ni-Pd Bimetallic Catalysts for the Utilization of Carbon Dioxide and Methane by Dry Reforming. Appl. Catal. A Gen. 2009, 366, 333–341. [Google Scholar] [CrossRef]
- Ma, Q.; Sun, J.; Gao, X.; Zhang, J.; Zhao, T.; Yoneyama, Y.; Tsubaki, N. Ordered Mesoporous Alumina-Supported Bimetallic Pd-Ni Catalysts for Methane Dry Reforming Reaction. Catal. Sci. Technol. 2016, 6, 6542–6550. [Google Scholar] [CrossRef]
- Singha, R.K.; Shukla, A.; Sandupatla, A.; Deo, G.; Bal, R. Synthesis and Catalytic Activity of a Pd Doped Ni-MgO Catalyst for Dry Reforming of Methane. J. Mater. Chem. A 2017, 5, 15688–15699. [Google Scholar] [CrossRef]
- Li, D.; Nakagawa, Y.; Tomishige, K. Methane Reforming to Synthesis Gas over Ni Catalysts Modified with Noble Metals. Appl. Catal. A Gen. 2011, 408, 1–24. [Google Scholar] [CrossRef]
- Ashcroft, A.T.; Cheetham, A.K.; Green, M.L.H.; Vernon, P.D.F. Partial Oxidation of Methane to Synthesis Gas Using Carbon Dioxide. Nature 1991, 352, 225–226. [Google Scholar] [CrossRef]
- Luisetto, I.; Sarno, C.; De Felicis, D.; Basoli, F.; Battocchio, C.; Tuti, S.; Licoccia, S.; Di Bartolomeo, E. Ni Supported on γ-Al2O3 promoted by Ru for the Dry Reforming of Methane in Packed and Monolithic Reactors. Fuel Process. Technol. 2017, 158, 130–140. [Google Scholar] [CrossRef]
- Zhou, H.; Zhang, T.; Sui, Z.; Zhu, Y.A.; Han, C.; Zhu, K.; Zhou, X. A Single Source Method to Generate Ru-Ni-MgO Catalysts for Methane Dry Reforming and the Kinetic Effect of Ru on Carbon Deposition and Gasification. Appl. Catal. B Environ. 2018, 233, 143–159. [Google Scholar] [CrossRef]
- Wysocka, I.; Hupka, J.; Rogala, A. Catalytic Activity of Nickel and Ruthenium–Nickel Catalysts Supported on SiO2, ZrO2, Al2O3, and MgAl2O4 in a Dry Reforming Process. Catalysts 2019, 9, 540. [Google Scholar] [CrossRef]
- Álvarez, A.M.; Bobadilla, L.F.; Garcilaso, V.; Centeno, M.A.; Odriozola, J.A. CO2 Reforming of Methane over Ni-Ru Supported Catalysts: On the Nature of Active Sites by Operando DRIFTS Study. J. CO2 Util. 2018, 24, 509–515. [Google Scholar]

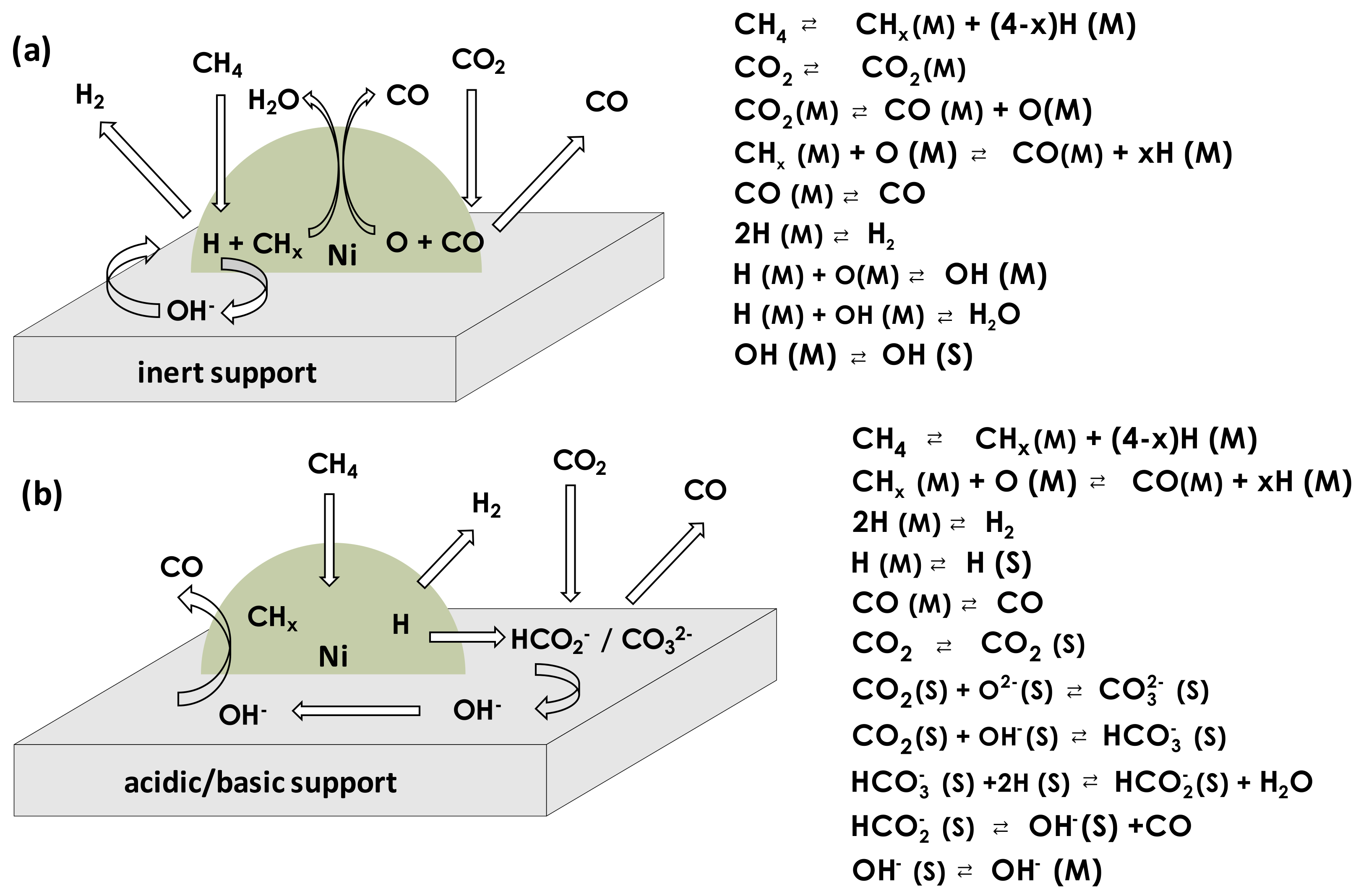

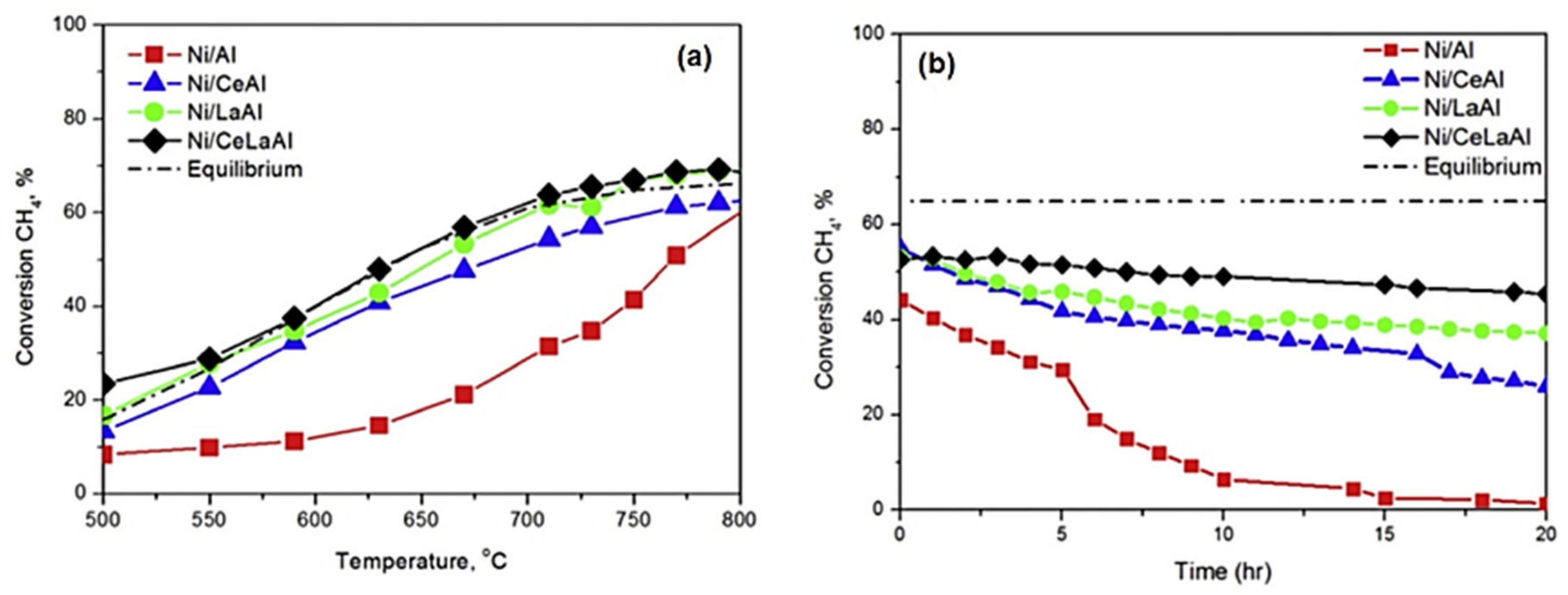

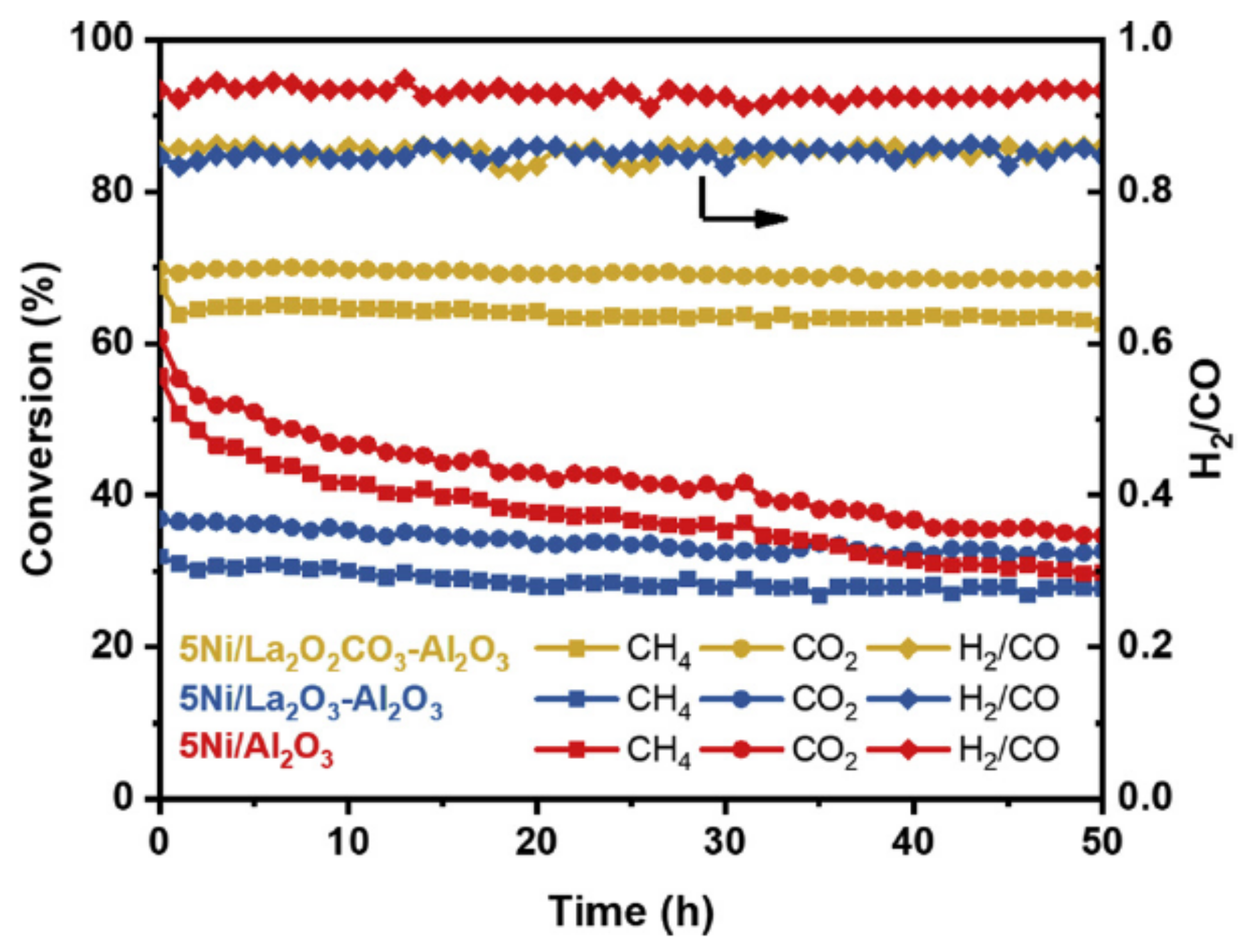
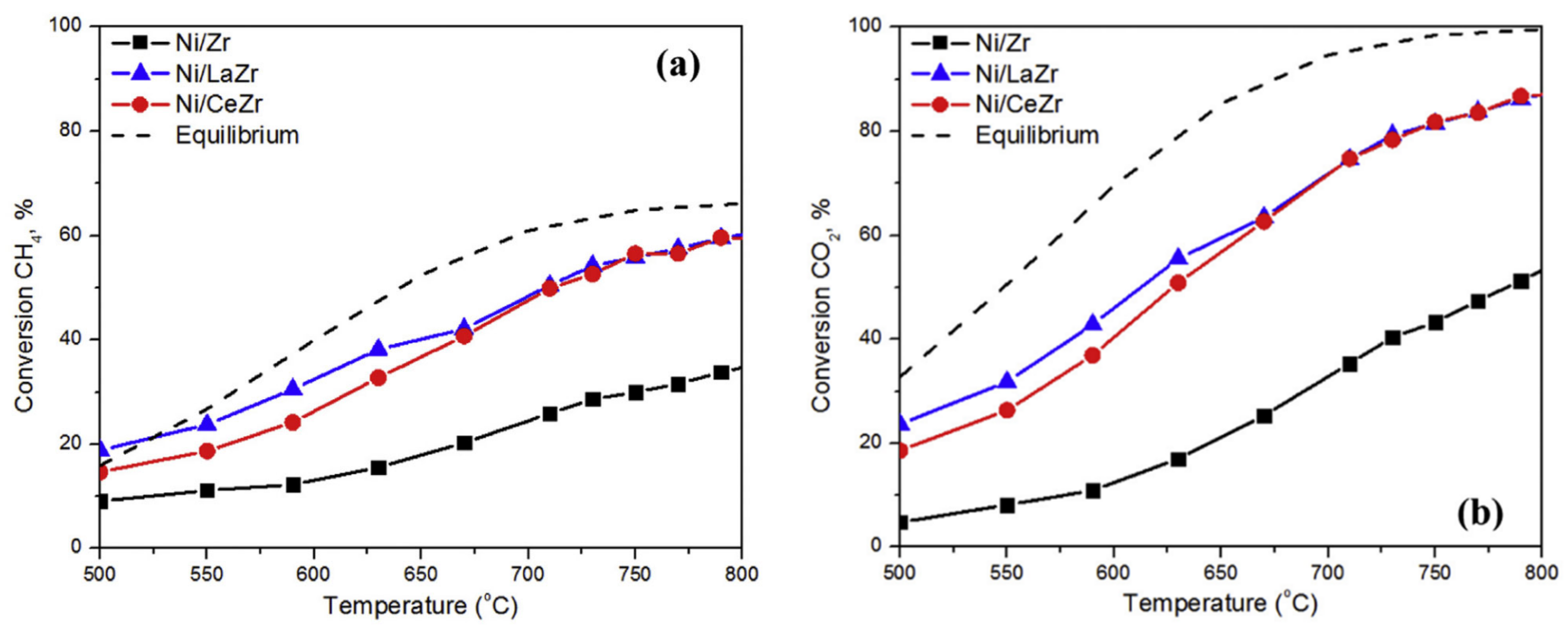



| Promoter | |||
|---|---|---|---|
| Support | Al2O3 | Basic oxides | Reducible oxides |
| Ex: MgO, CaO Reduce support acidity Increase Ni dispersion, promote CO2 adsorption, and inhibit carbon formation | Ex: CeO2, La2O3 Redox properties with active surface oxygen species Improve Ni dispersion and prevent carbon deposition | ||
| Inert oxides | Ex: SiO2 Weak interaction between Ni and support favors sintering | ||
| Basic oxides | Ex: MgO Strong basicity decreases Ni reducibility and favors deactivation by metal oxidation | ||
| Reducible oxides | Ex: CeO2, La2O3, Ce1−xZrxO2 High oxygen storage capacity inhibits carbon deposition Metal decorating effect due to strong metal-support interaction decreases catalytic activity | ||
| Mesoporous | Ex: SBA-15, MCM-41 High surface area increases Ni dispersion and confinement of Ni particles into pore channels prevents sintering | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Manfro, R.L.; Souza, M.M.V.M. Overview of Ni-Based Catalysts for Hydrogen Production from Biogas Reforming. Catalysts 2023, 13, 1296. https://doi.org/10.3390/catal13091296
Manfro RL, Souza MMVM. Overview of Ni-Based Catalysts for Hydrogen Production from Biogas Reforming. Catalysts. 2023; 13(9):1296. https://doi.org/10.3390/catal13091296
Chicago/Turabian StyleManfro, Robinson L., and Mariana M. V. M. Souza. 2023. "Overview of Ni-Based Catalysts for Hydrogen Production from Biogas Reforming" Catalysts 13, no. 9: 1296. https://doi.org/10.3390/catal13091296
APA StyleManfro, R. L., & Souza, M. M. V. M. (2023). Overview of Ni-Based Catalysts for Hydrogen Production from Biogas Reforming. Catalysts, 13(9), 1296. https://doi.org/10.3390/catal13091296








