Sterical Self-Consistency of Carbonaceous Nanopolyhedra Triggered by Introduced CNTs to Optimize ORR Performance
Abstract
:1. Introduction
2. Results and Discussion
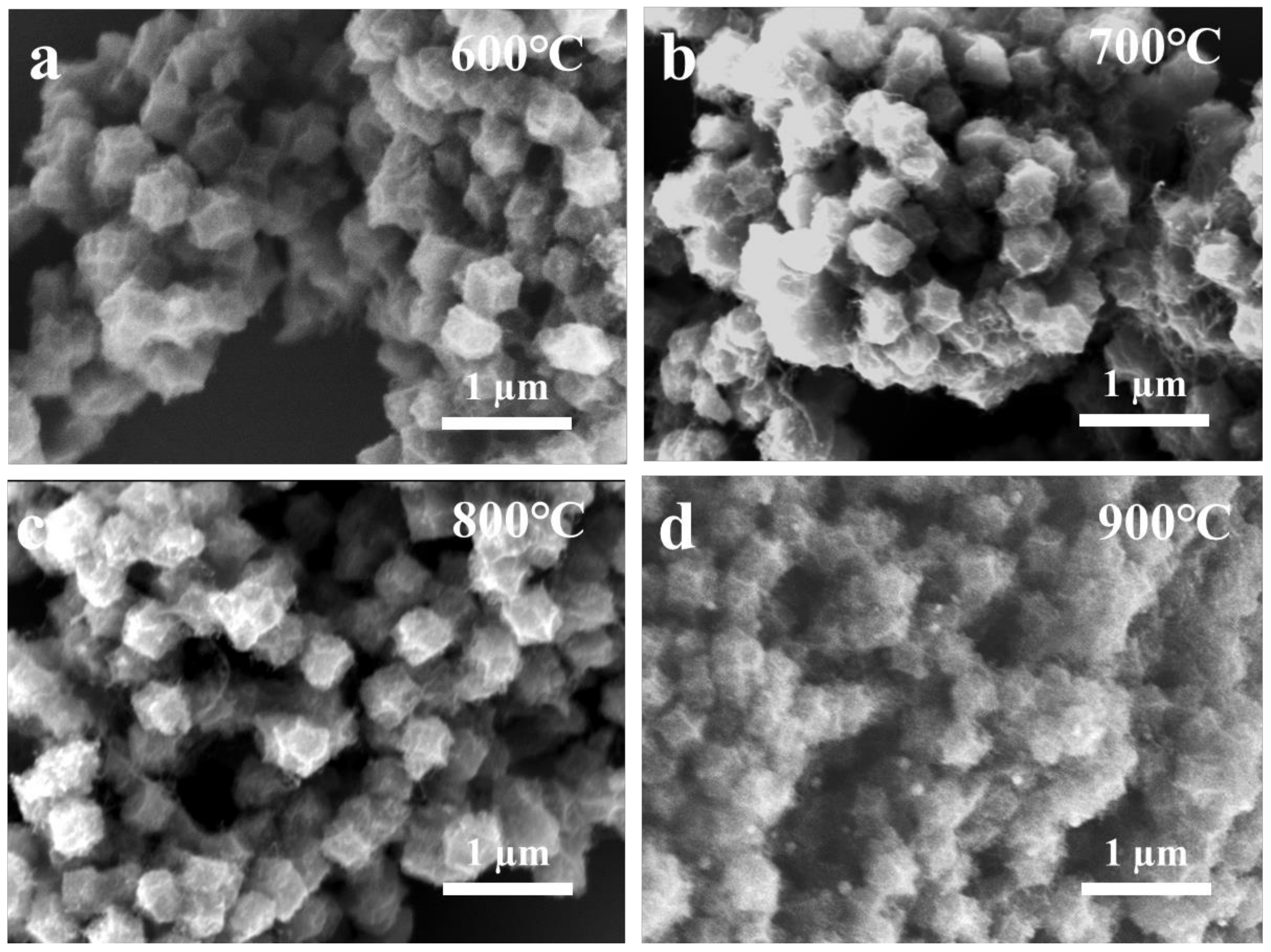
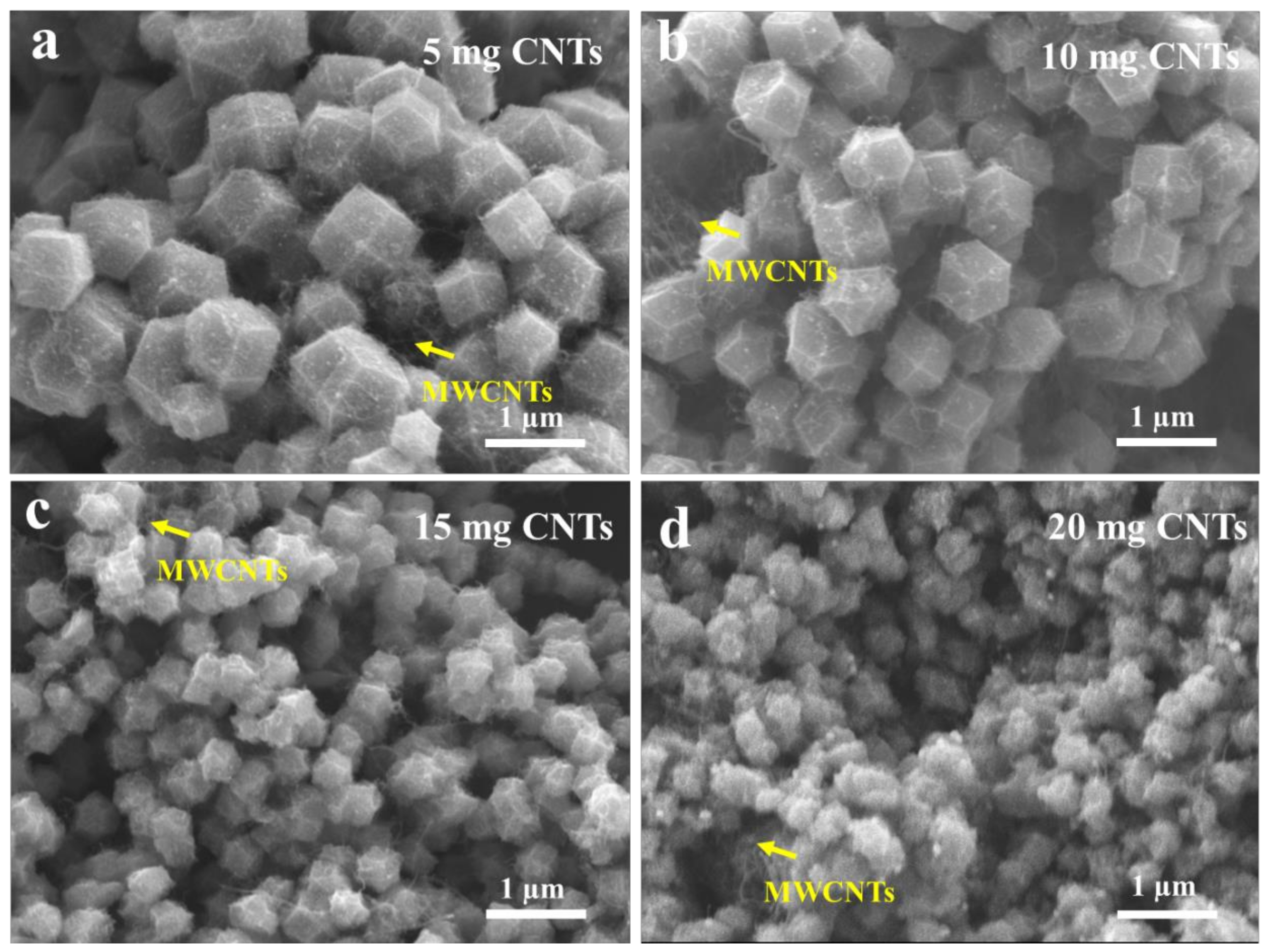
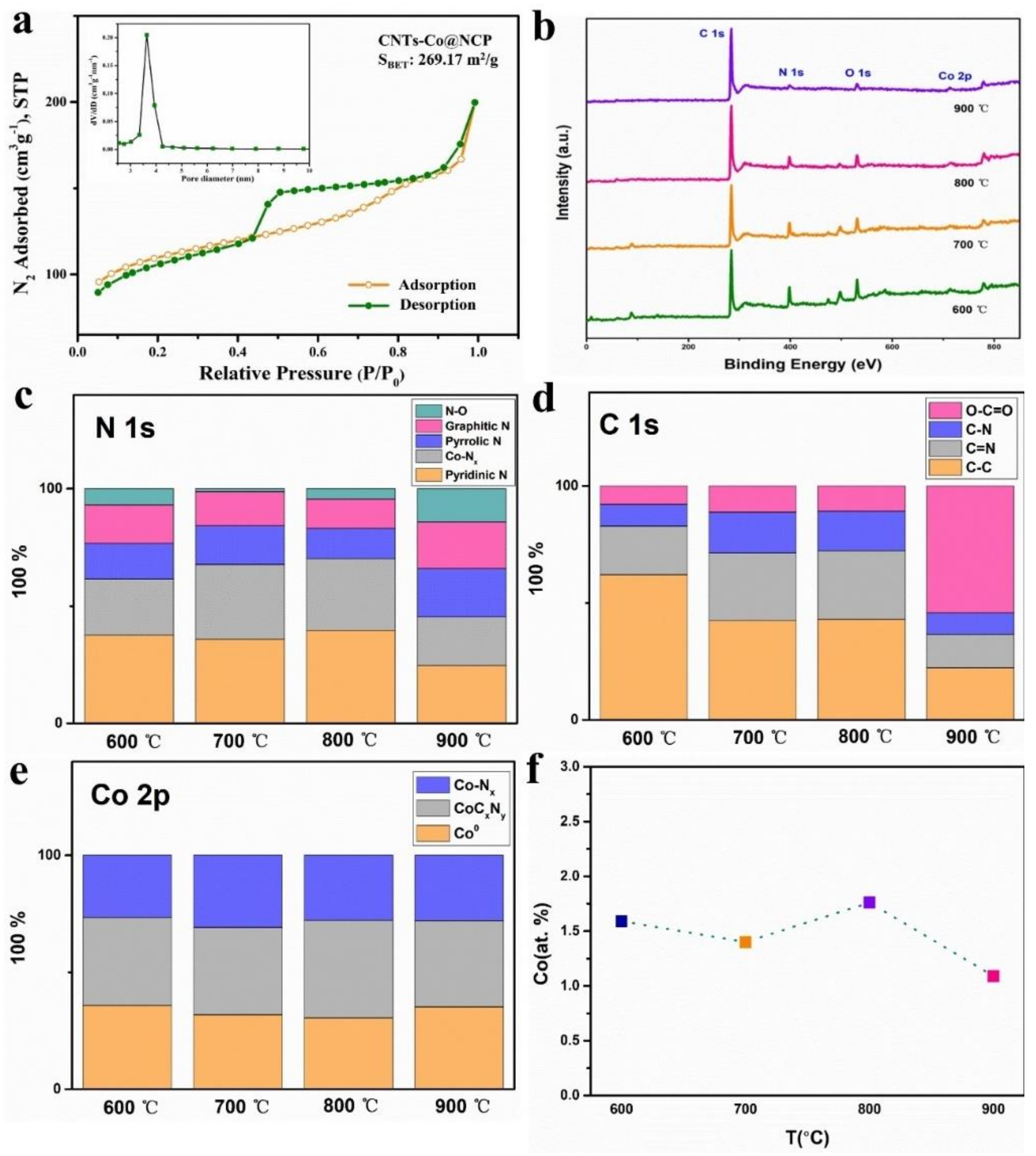
2.1. Preparation and Characterization
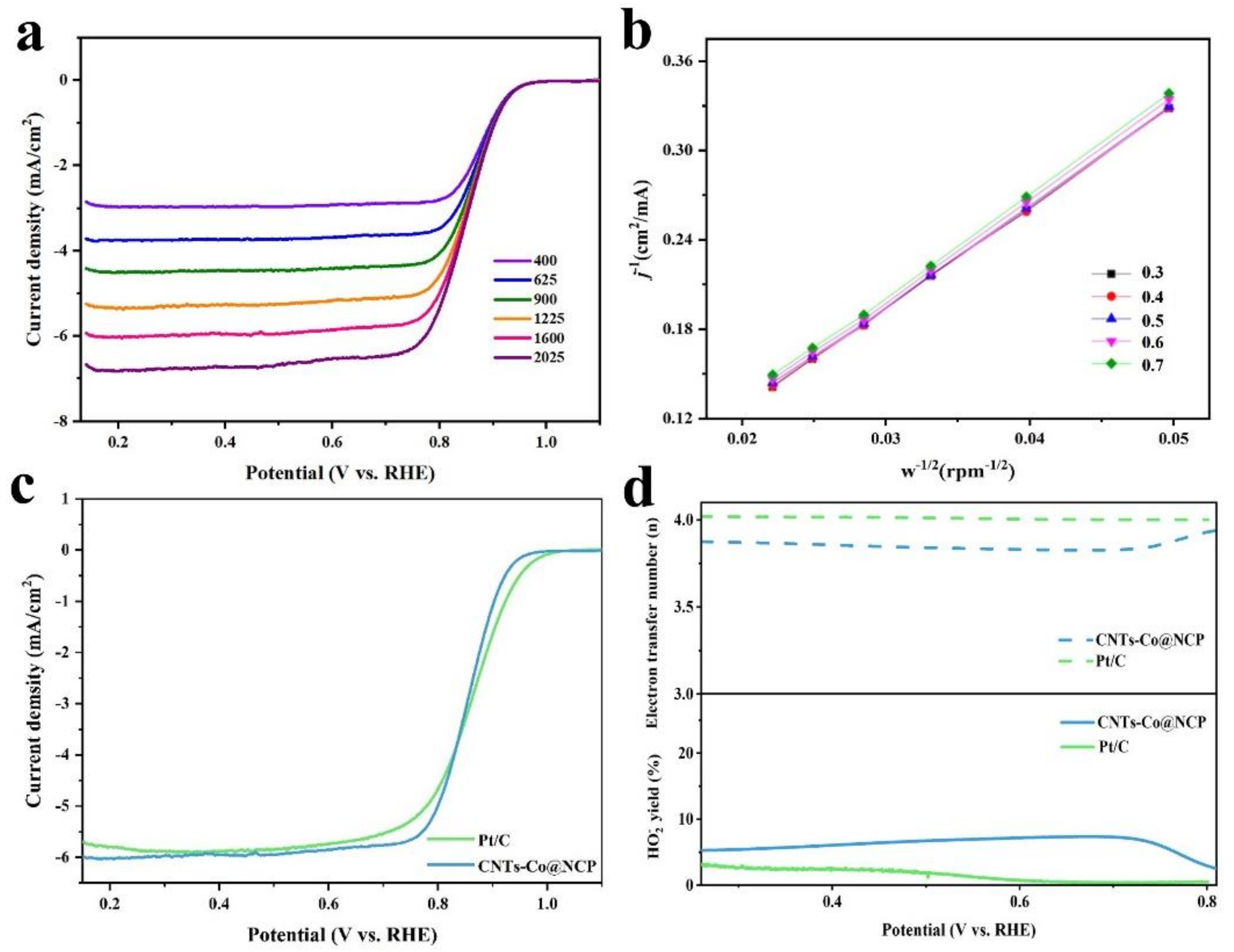
2.2. Electrocatalytic Activity
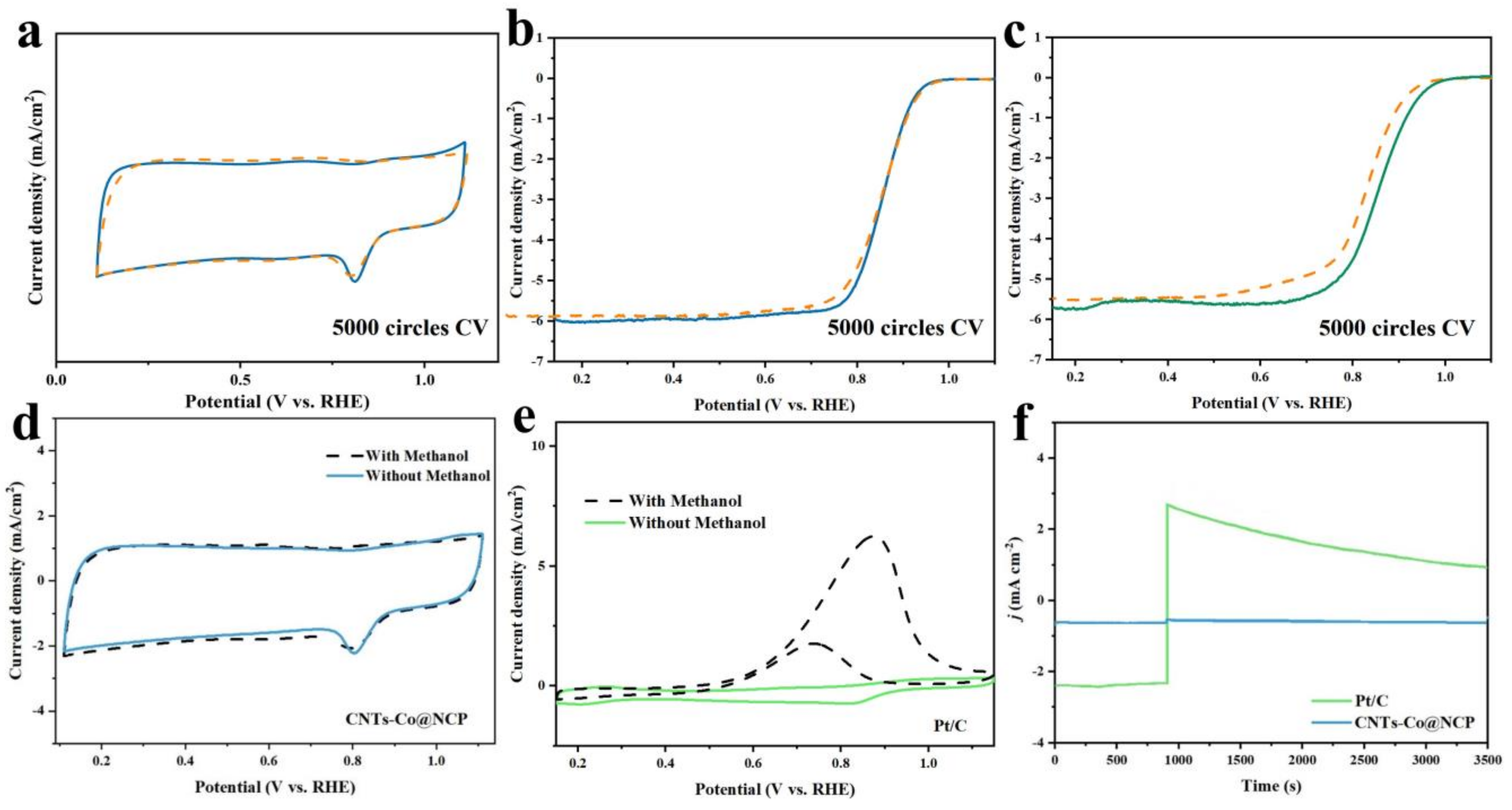
2.3. Stability and Methanol Tolerance
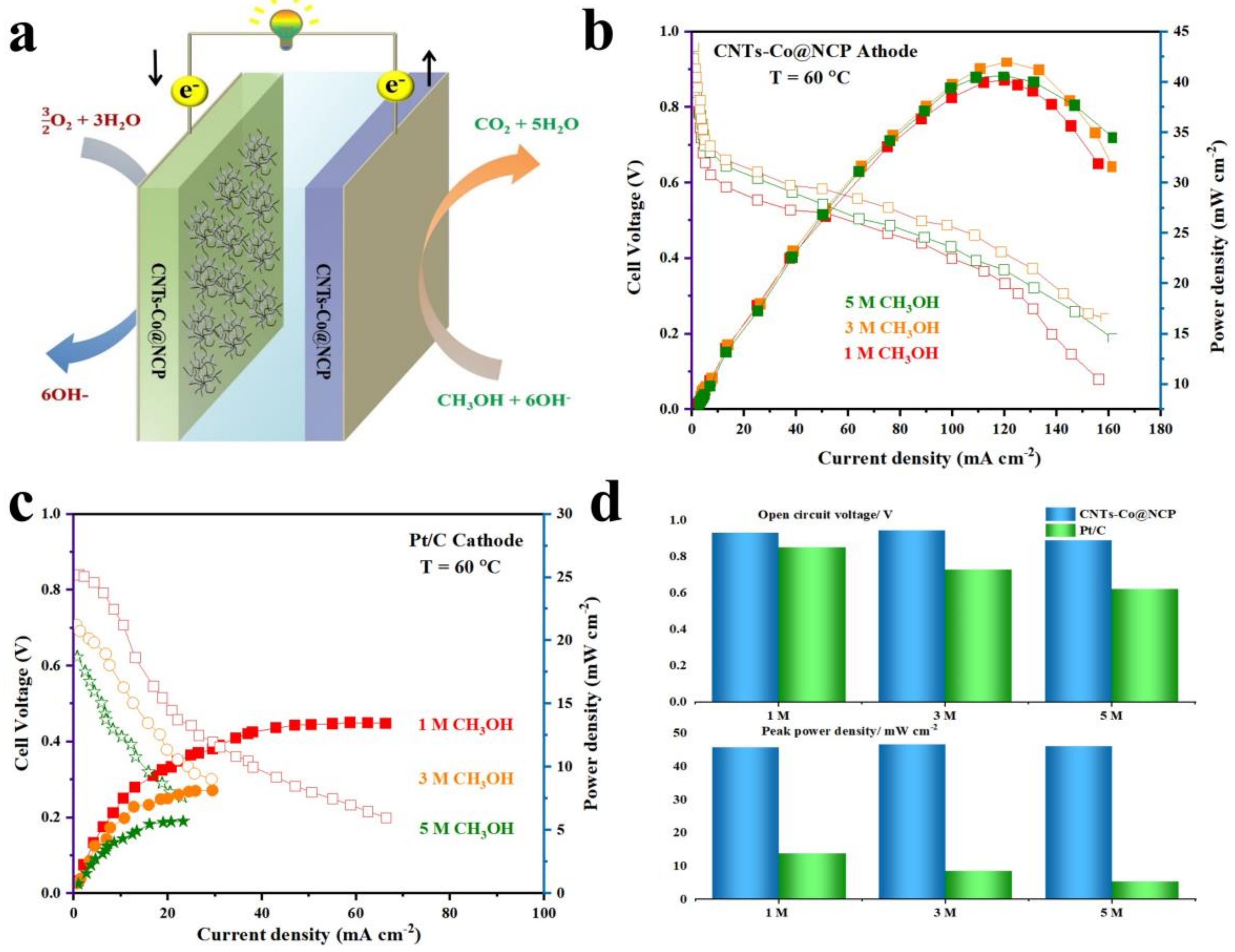
2.4. Fuel Cells Tests
3. Experimental Section
3.1. Chemicals and Materials
3.2. Synthesis of CNTs-Co@NCP
3.3. Electrochemical Measurements
3.4. Characterizations
3.5. Evaluation of the Electron-Transfer rate, Mass and Specific Activities
3.6. Rotating Ring-Disk Electrode (RRDE)
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Shi, H.; Peng, J.; Cheng, X.; Yang, X.; Jin, J.; Hu, J. The CO2 desorption analysis of tri-solvent MEA + BEA + DEEA with several commercial solid acid catalysts. Int. J. Greenh. Gas Control. 2022, 116, 103647. [Google Scholar] [CrossRef]
- Wang, Z.; Huang, J.; Wang, L.; Liu, Y.; Liu, W.; Zhao, S.; Liu, Z. Cation-Tuning Induced d-Band Center Modulation on Co-Based Spinel Oxide for Oxygen Reduction/Evolution Reaction. Angew. Chem. Int. Ed. 2022, 61, e202114696. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Ha, Y.; Mao, X.; Xu, W.; Du, A.; Wu, R.; Chou, S.; Zhang, H. Co Nanoparticles Encapsulated in N-Doped Carbon Nanotubes Grafted CNTs as Electrocatalysts for Enhanced Oxygen Reduction Reaction. Adv. Mater. Interfaces 2022, 9, 2101877. [Google Scholar] [CrossRef]
- Yao, X.; Wang, X.; Sun, L.; Li, L.; Kan, E.; Ouyang, B.; Zhang, W. Popcorn-like Co3O4 nanoparticles confined in a three-dimensional hierarchical N-doped carbon nanotube network as a highly-efficient trifunctional electrocatalyst for zinc–air batteries and water splitting devices. Inorg. Chem. Front. 2022, 9, 2517–2529. [Google Scholar] [CrossRef]
- Wang, Z.; Lu, Y.; Yan, Y.; Larissa, T.Y.P.; Zhang, X.; Wuu, D.; Zhang, H.; Yang, Y.; Wang, X. Core-shell carbon materials derived from metal-organic frameworks as an efficient oxygen bifunctional electrocatalyst. Nano Energy 2016, 30, 368–378. [Google Scholar] [CrossRef]
- Hu, Z.; Zhang, Z.; Li, Z.; Dou, M.; Wang, F. One-Step Conversion from Core-Shell Metal-Organic Framework Materials to Cobalt and Nitrogen Codoped Carbon Nanopolyhedra with Hierarchically Porous Structure for Highly Efficient Oxygen Reduction. ACS Appl. Mater. Interfaces 2017, 9, 16109–16116. [Google Scholar] [CrossRef]
- Wei, J.; Hu, Y.; Liang, Y.; Kong, B.; Zheng, Z.; Zhang, J.; Jiang, S.P.; Zhao, Y.; Wang, H. Graphene oxide/core-shell structured metal-organic framework nano-sandwiches and their derived cobalt/N-doped carbon nanosheets for oxygen reduction reactions. J. Mater. Chem. A 2017, 5, 10182–10189. [Google Scholar] [CrossRef]
- Jose, V.; Jayakumar, A.; Lee, J. Bimetal/Metal Oxide Encapsulated in Graphitic Nitrogen Doped Mesoporous Carbon Networks for Enhanced Oxygen Electrocatalysis. ChemElectroChem 2018, 6, 1485–1491. [Google Scholar] [CrossRef]
- Qi, H.; Feng, Y.; Chi, Z.; Cui, Y.; Wang, M.; Liu, J.; Guo, Z.; Wang, L.; Feng, S. In situ encapsulation of Co-based nanoparticles into nitrogen-doped carbon nanotubes-modified reduced graphene oxide as an air cathode for high-performance Zn–air batteries. Nanoscale 2019, 11, 21943–21952. [Google Scholar] [CrossRef]
- Fang, F.; Wu, Z.; Zheng, D.; Guo, M.; Li, X.; Li, Z.; Wei, Y.; Liu, X.; Tong, Y.; Dong, X.; et al. ZnO@zeolitic imidazolate frameworks derived porous hybrid hollow carbon shell as an efficient electrocatalyst for oxygen reduction. J. Mater. Sci. 2021, 56, 14989–15003. [Google Scholar] [CrossRef]
- Lee, J.S.; Rajan, H.; Christy, M.; Yi, S.C. Optimization of active sites by sulfurization of the core–shell ZIF 67@ZIF 8 for rapid oxygen reduction kinetics in acidic media. Int. J. Hydrogen Energy 2021, 46, 10739–10748. [Google Scholar] [CrossRef]
- Li, G.; Deng, W.; He, L.; Wu, J.; Liu, J.; Wu, T.; Wang, Y.; Wang, X. Zn, Co, and Fe Tridoped N-C Core-Shell Nanocages as the High-Efficiency Oxygen Reduction Reaction Electrocatalyst in Zinc-Air Batteries. ACS Appl. Mater. Interfaces 2021, 13, 28324–28333. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Zhang, Y.; Wang, P.; Long, X.; Zheng, L.; Liu, G.; He, X.; Qiu, J. Core-Shell ZIF-67@ZIF-8-derived multi-dimensional cobalt-nitrogen doped hierarchical carbon nanomaterial for efficient oxygen reduction reaction. J. Alloy. Compd. 2022, 903, 163701. [Google Scholar] [CrossRef]
- Zhang, Y.; Yang, M.; Wang, P.; Li, K.; Li, S.; Zhang, Z.; He, X.; Duan, Y. Co/N-codoped carbon nanotube hollow polyhedron hybrid derived from salt-encapsulated core-shell ZIF-8@ZIF-67 for efficient oxygen reduction reaction. J. Alloy. Compd. 2022, 904, 164083. [Google Scholar] [CrossRef]
- Zhang, C.-L.; Lu, B.-R.; Cao, F.-H.; Wu, Z.-Y.; Zhang, W.; Cong, H.-P.; Yu, S.-H. Electrospun metal-organic framework nanoparticle fibers and their derived electrocatalysts for oxygen reduction reaction. Nano Energy 2018, 55, 226–233. [Google Scholar] [CrossRef]
- Agarwal, S.; Yu, X.; Manthiram, A. A pair of metal organic framework (MOF)-derived oxygen reduction reaction (ORR) and oxygen evolution reaction (OER) catalysts for zinc-air batteries. Mater. Today Energy 2020, 16, 100405. [Google Scholar] [CrossRef]
- Lee, Y.-R.; Yoo, H.; Choi, J.; Ahn, W.-S. Electrocatalytic oxygen reduction over Co@Co3O4/N-doped porous carbon derived from pyrolysis of ZIF-8/67 on cellulose nanofibers. Cellulose. 2020, 27, 2723–2735. [Google Scholar] [CrossRef]
- Li, C.; Li, Y.; Yao, Z.; Wang, J.; Zhong, Q. A mild approach to bimetallic ZIF-derived porous carbons as highly efficient oxygen reduction electrocatalysts. Int. J. Hydrogen Energy 2021, 46, 6188–6196. [Google Scholar] [CrossRef]
- Li, J.; Meng, Y.; Zhang, L.; Li, G.; Shi, Z.; Hou, P.; Liu, C.; Cheng, H.; Shao, M. Dual-Phasic Carbon with Co Single Atoms and Nanoparticles as a Bifunctional Oxygen Electrocatalyst for Rechargeable Zn-Air Batteries. Adv. Funct. Mater. 2021, 31, 2103360. [Google Scholar] [CrossRef]
- Chen, D.; Huang, Q.; Ding, J.; Li, T.-T.; Yu, D.; Nie, H.; Qian, J.; Yang, Z. Heteroepitaxial metal-organic frameworks derived cobalt and nitrogen codoped carbon nanosheets to boost oxygen reduction. J. Colloid Interface Sci. 2022, 623, 1210–1219. [Google Scholar] [CrossRef]
- Han, Y.; Duan, H.; Zhou, C.; Meng, H.; Jiang, Q.; Wang, B.; Yan, W.; Zhang, R. Stabilizing Cobalt Single Atoms via Flexible Carbon Membranes as Bifunctional Electrocatalysts for Binder-Free Zinc-Air Batteries. Nano Lett. 2022, 22, 2497–2505. [Google Scholar] [CrossRef]
- Lian, J.; Bai, Q.; Zhao, J.; Wang, X. Highly dispersed Co-N-RGO electrocatalyst based on an interconnected hierarchical pore framework for proton exchange membrane fuel cells. Catal. Sci. Technol. 2022, 12, 6285–6293. [Google Scholar] [CrossRef]
- Liu, Z.; Wang, D.; Kou, X.; Dong, X.; Chi, X.; Ma, H.; Wang, G. High-performance oxygen reduction electrocatalysts derived from bimetal-organic framework and sulfur-doped precursors for use in microbial fuel cells. J. Power Sources 2021, 521, 230944. [Google Scholar] [CrossRef]
- Peng, Y.; Zhang, F.; Zhang, Y.; Luo, X.; Chen, L.; Shi, Y. N,S-Doped hollow carbon nanosheet-encapsulated Co9S8 nanoparticles as a highly efficient bifunctional electrocatalyst for rechargeable zinc–air batteries. Dalton Trans. 2022, 51, 12630–12640. [Google Scholar] [CrossRef] [PubMed]
- Qin, T.; Li, F.; Liu, X.; Yuan, J.; Jiang, R.; Sun, Y.; Zheng, H.; O’Mullane, A.P. Template-Assisted Synthesis of High-Efficiency Bifunctional Catalysts with Roller-Comb-Like Nanostructure for Rechargeable Zinc-Air Batteries. Chem. Eng. J. 2022, 429, 132199. [Google Scholar] [CrossRef]
- Wang, F.; Xiao, Z.; Liu, X.; Ren, J.; Xing, T.; Li, Z.; Li, X.; Chen, Y. Strategic design of cellulose nanofibers@zeolitic imidazolate frameworks derived mesoporous carbon-supported nanoscale CoFe2O4/CoFe hybrid composition as trifunctional electrocatalyst for Zn-air battery and self-powered overall water-splitting. J. Power Sources 2022, 521, 230925. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhang, L.; Qi, P.; Luo, H.; Lin, C.; Liu, W.; Zhang, D. Space-confined pyrolysis strategy to self-catalyze the growth of carbon nanotube-wrapped Co3O4 electrocatalyst for lithium-O2 batteries. J. Alloy. Compd. 2022, 905, 164203. [Google Scholar] [CrossRef]
- Etesami, M.; Nguyen, M.T.; Yonezawa, T.; Tuantranont, A.; Somwangthanaroj, A.; Kheawhom, S. 3D carbon nanotubes-graphene hybrids for energy conversion and storage applications. Chem. Eng. J. 2022, 446. [Google Scholar] [CrossRef]
- Sun, J.; Zuo, Y.; Wang, H.; Shi, H.; Lu, S. Global-Local CNTs Conductive Network Couple with Co-Based Polyhedral Promotes the Electrocatalytic Reduction of Oxygen. Catalysts 2022, 12, 1508. [Google Scholar] [CrossRef]
- Tang, J.; Salunkhe, R.R.; Liu, J.; Torad, N.L.; Imura, M.; Furukawa, S.; Yamauchi, Y. Thermal Conversion of Core–Shell Metal–Organic Frameworks: A New Method for Selectively Functionalized Nanoporous Hybrid Carbon. J. Am. Chem. Soc. 2015, 137, 1572–1580. [Google Scholar] [CrossRef]






| Catalysts | SSA (m2/g) | Pore Volume (cm3/g) | Average Pore Size (nm) |
|---|---|---|---|
| C-Z8/C | 212.90 | 0.28 | 4.60 |
| C-Z67/C | 203.31 | 0.21 | 4.01 |
| C-Z8/67/C | 275.09 | 0.39 | 5.22 |
| C-Z8/67 | 280.72 | 0.30 | 4.08 |
| C-Z8/67/C-600 | 189.73 | 0.18 | 3.89 |
| C-Z8/67/C-700 | 242.34 | 0.23 | 3.76 |
| C-Z8/67/C-800 | 270.80 | 0.32 | 4.40 |
| C-Z8/67/C-900 | 275.09 | 0.39 | 5.22 |
| C-Z8/67/C-5 | 259.12 | 0.25 | 3.69 |
| C-Z8/67/C-10 | 263.96 | 0.29 | 4.24 |
| C-Z8/67/C-15 | 269.17 | 0.31 | 4.27 |
| C-Z8/67/C-20 | 270.80 | 0.32 | 4.40 |
| Catalysts | E1/2 (V) | JL (mA/cm2) | Tafel Slope (mV/dec) |
|---|---|---|---|
| C-Z8/C | 0.698 | 3.765 | 97 |
| C-Z67/C | 0.804 | 4.965 | 60 |
| C-Z8/67/C | 0.836 | 5.343 | 59 |
| C-Z8/67 | 0.840 | 4.894 | 78 |
| C-Z8/67/C-600 | 0.794 | 4.613 | 68 |
| C-Z8/67/C-700 | 0.812 | 4.502 | 63 |
| C-Z8/67/C-800 | 0.845 | 5.315 | 53 |
| C-Z8/67/C-900 | 0.834 | 4.824 | 60 |
| C-Z8/67/C-5 | 0.850 | 4.991 | 60 |
| C-Z8/67/C-10 | 0.856 | 5.194 | 58 |
| C-Z8/67/C-15 (CNTs-Co@NCP) | 0.860 | 5.940 | 54 |
| C-Z8/67/C-20 | 0.851 | 5.706 | 59 |
| Sample | E1/2 (V) | JL (mA/cm2) | Electrolyte | Ref. |
|---|---|---|---|---|
| CNTs-Co@NCP | 0.86 | 5.94 | 0.1 M KOH | This work |
| NAC@Co3O4/NCNTs/CNF | 0.83 | 5.6 | 0.1 M KOH | [4] |
| NC@CC | 0.89 | ~4.4 | 0.1 M KOH | [5] |
| Core-shell Co,N-HCNP | 0.855 | 5.0 | 0.1 M KOH | [6] |
| GO/ZIF-8@ZIF-67-900 | ~0.82 | 5.1 | 0.1 M KOH | [7] |
| FCNC900 | 0.868 | - | 0.1 M KOH | [8] |
| Co/Co4N@N-CNTs/rGO | ~0.83 | 4.82 | 0.1 M KOH | [9] |
| Zn/Co-NC | 0.856 | ~5.6 | 0.1 M KOH | [10] |
| Co-NSC 200 | 0.74 | 5.53 | 0.1 M HClO4 | [11] |
| ZnCoFe-N-C | 0.878 | ~5.0 | 0.1 M KOH | [12] |
| Co@N-CNT-HC | 0.84 | 4.7 | 0.1 M KOH | [13] |
| CoNHPC-920 | 0.87 | 5.41 | 0.1 M KOH | [14] |
| ES-CNCo-5 | –0.155 vs. Ag/AgCl | 4.82 | 0.1 M KOH | [15] |
| CoFeZn@pCNT | 0.87 | ~5.2 | 0.1 M KOH | [16] |
| Co,N-C/TOCNF | 0.74 V vs. SHE | 4.5 | 0.1 M KOH | [17] |
| BM2-C6-50T | 0.84 | 5.26 | 0.1 M KOH | [18] |
| CNT@SAC-Co/NCP | 0.870 | ~5.25 | 0.1 M KOH | [19] |
| CoZnNC-2 | 0.85 | 5.41 | 0.1 M KOH | [20] |
| Co SA/NCFs | 0.85 | ~6.0 | 0.1 M KOH | [21] |
| Co-N-RGO | 0.75 | 5.25 | 0.5 M H2SO4 | [22] |
| Co-N/S-C-3.5 | 0.80 | 4.2 | 0.1 M KOH | [23] |
| Co9S8/NSC | 0.82 | ~5.4 | 0.1 M KOH | [24] |
| ZnCo2@NCNTs-800 | 0.85 | ~6.2 | 0.1 M KOH | [25] |
| FeZn4Co@CNFs | 0.84 | ~4.95 | 0.1 M KOH | [26] |
| Co3O4/CNTs | 0.86 | 5.65 | 0.1 M KOH | [27] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zuo, Y.; Tang, Y.; Shi, H.; Lu, S.; Tontiwachwuthikul, P. Sterical Self-Consistency of Carbonaceous Nanopolyhedra Triggered by Introduced CNTs to Optimize ORR Performance. Catalysts 2023, 13, 1307. https://doi.org/10.3390/catal13091307
Zuo Y, Tang Y, Shi H, Lu S, Tontiwachwuthikul P. Sterical Self-Consistency of Carbonaceous Nanopolyhedra Triggered by Introduced CNTs to Optimize ORR Performance. Catalysts. 2023; 13(9):1307. https://doi.org/10.3390/catal13091307
Chicago/Turabian StyleZuo, Yuanhui, Yanlong Tang, Huancong Shi, Shijian Lu, and Paitoon Tontiwachwuthikul. 2023. "Sterical Self-Consistency of Carbonaceous Nanopolyhedra Triggered by Introduced CNTs to Optimize ORR Performance" Catalysts 13, no. 9: 1307. https://doi.org/10.3390/catal13091307
APA StyleZuo, Y., Tang, Y., Shi, H., Lu, S., & Tontiwachwuthikul, P. (2023). Sterical Self-Consistency of Carbonaceous Nanopolyhedra Triggered by Introduced CNTs to Optimize ORR Performance. Catalysts, 13(9), 1307. https://doi.org/10.3390/catal13091307










