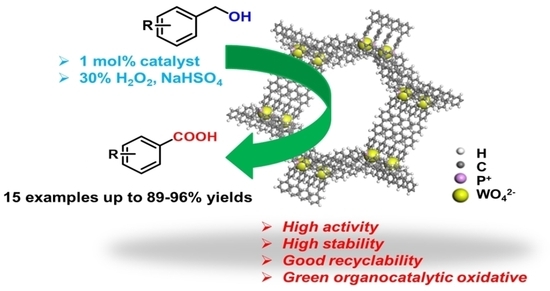
Highly Efficient and Selective Oxidation of Benzyl Alcohol by WO42− Catalyst Immobilized by a Phosphonium-Containing Porous Aromatic Framework
Abstract
:1. Introduction
2. Results
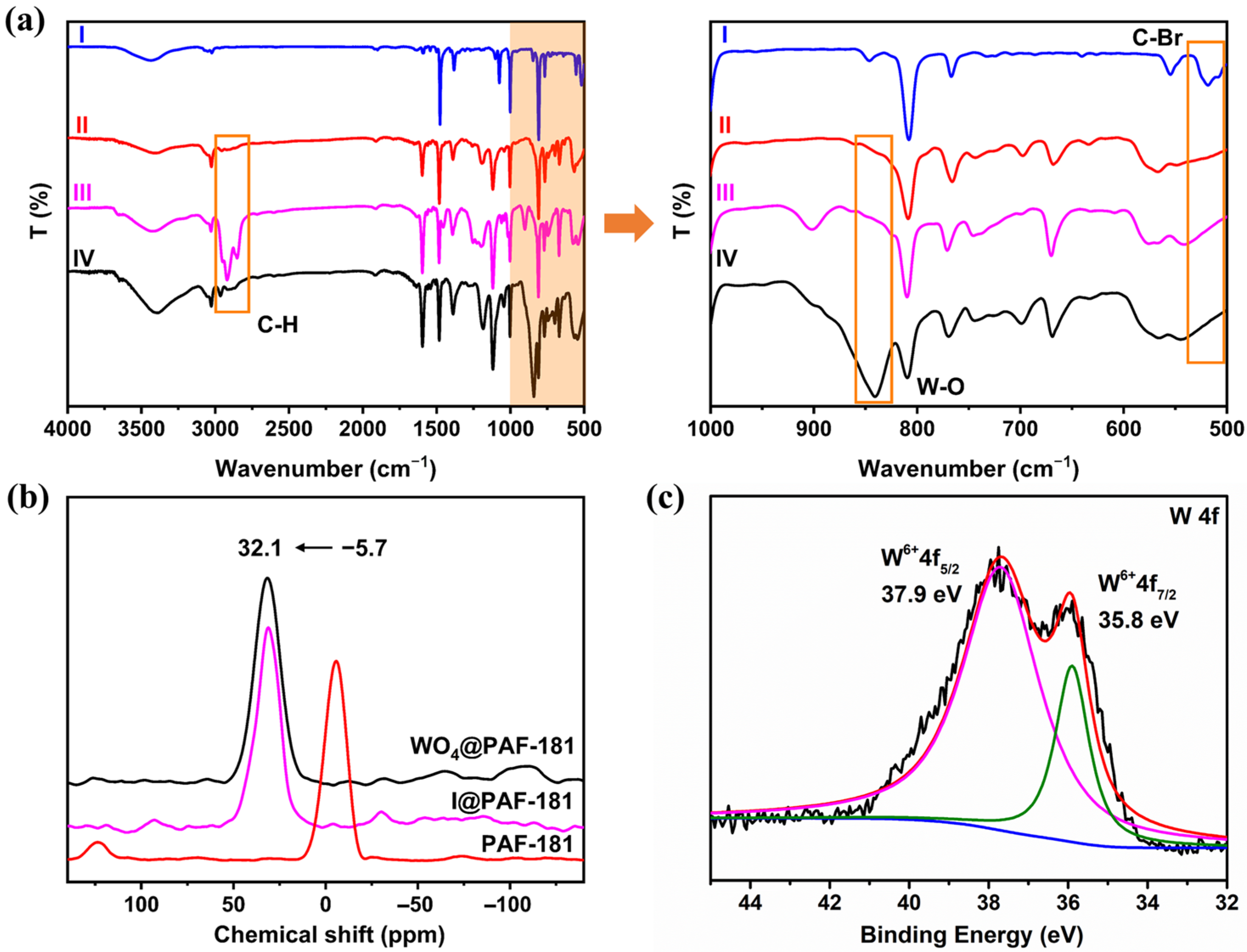
2.1. Structural Characterization
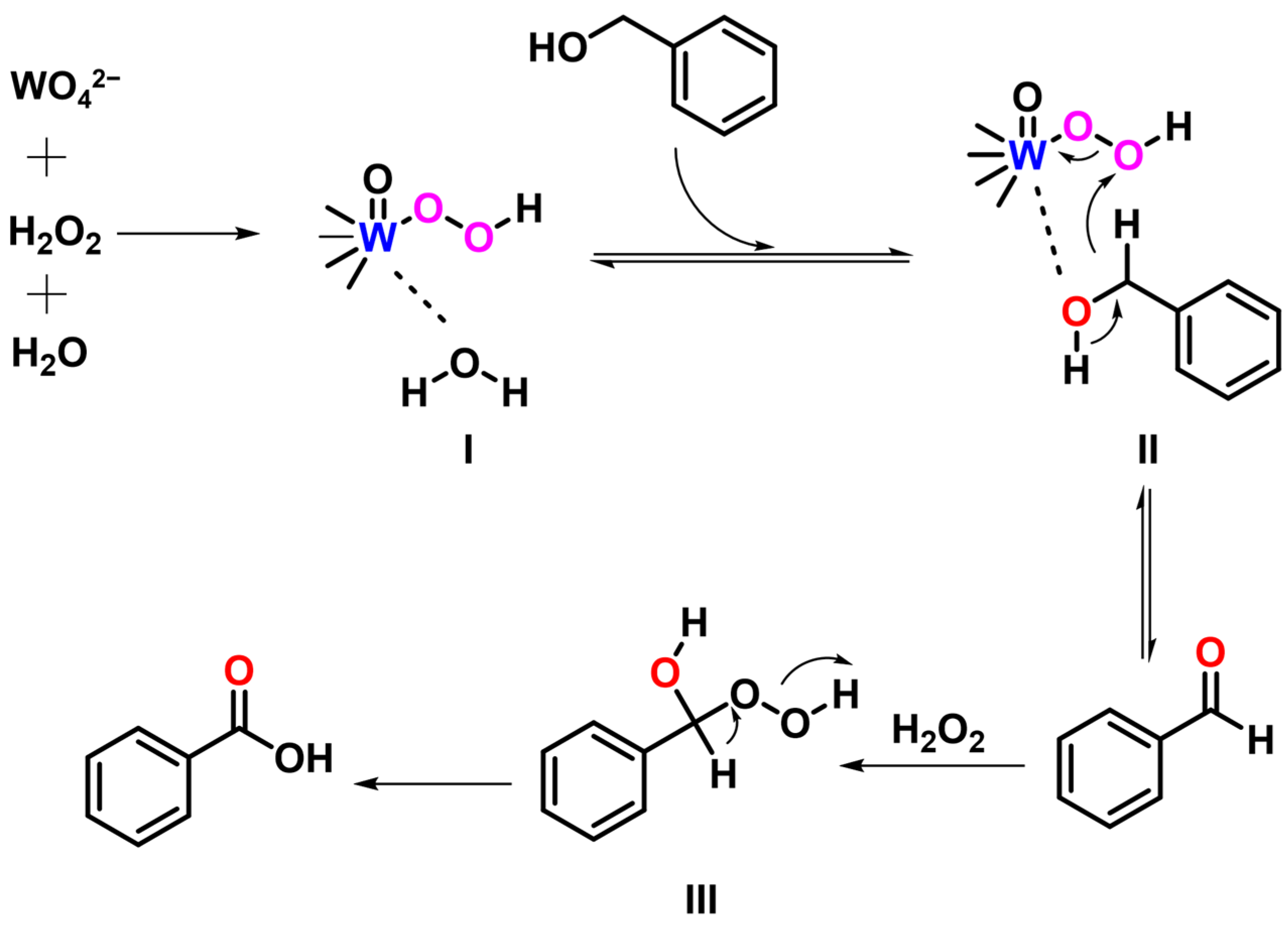
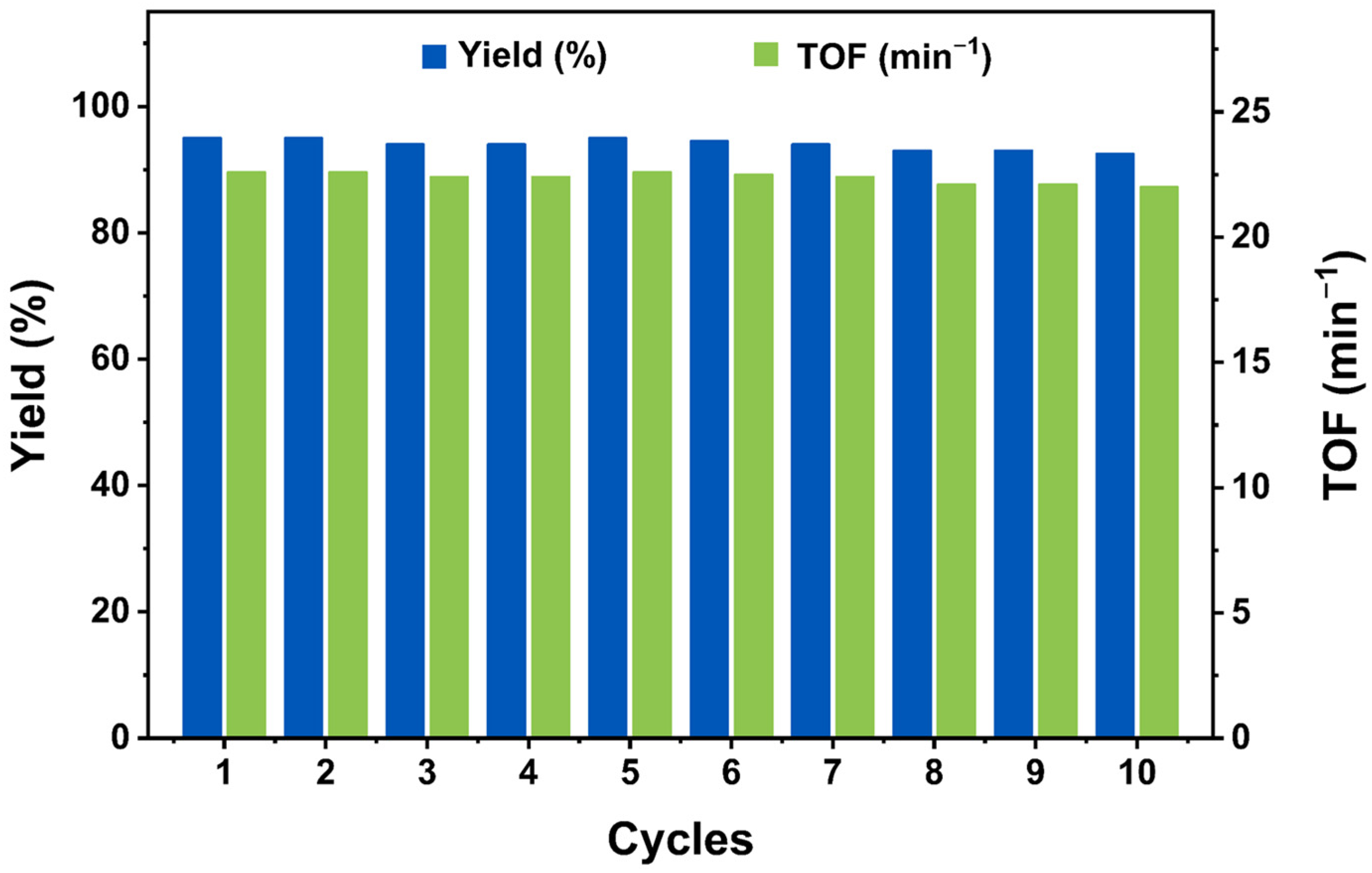
2.2. Catalytic Performance
3. Materials and Methods
3.1. Synthesis of tris(4-bromobiphenyl)phosphine
3.2. Synthesis of PAF-181
3.3. Synthesis of WO4@PAF-181
3.4. Catalytic Reaction
3.5. Leaching Experiment
3.6. Loop Test
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, H.; Shi, Y.; Haruta, M.; Huang, J. Aerobic oxidation of benzyl alcohol in water catalyzed by gold nanoparticles supported on imidazole containing crosslinked polymer. Appl. Catal. A Gen. 2017, 536, 27–34. [Google Scholar] [CrossRef]
- Mao, X.; Yang, Q.; Chen, D.; Yu, B.; He, J. Benzoic acid used as food and feed additives can regulate gut functions. BioMed Res. Int. 2019, 2019, 5721585. [Google Scholar] [CrossRef] [PubMed]
- Oktay, Y.; Leyla, G.; Ecem, A.; Saban, M.; Emel, A.; Ozer, K. Benzoic acid formation and its relationship with microbial properties in traditional Turkish cheese varieties. Food Biosci. 2021, 41, 101040. [Google Scholar] [CrossRef]
- Xu, M.; Geng, J.; Xu, H.; Zhang, S.; Zhang, H. In situ construction of NiCo2O4 nanosheets on nickel foam for efficient electrocatalytic oxidation of benzyl alcohol. Inorg. Chem. Front. 2023, 10, 2053–2059. [Google Scholar] [CrossRef]
- Zhang, S.; Wang, Y.; Ma, Y.; Li, Z.; Du, J.; Han, Z. Three-dimensional silver-containing polyoxotungstate frameworks for photocatalytic aerobic oxidation of benzyl alcohol. Inorg. Chem. 2022, 61, 20596–20607. [Google Scholar] [CrossRef] [PubMed]
- Branko, J. Surfactant assisted permanganate oxidation of aromatic compounds. Can. J. Chem. 1989, 67, 1381–1383. [Google Scholar] [CrossRef]
- Zhao, M.Z.; Li, J.; Song, Z.G.; Desmond, R.; Tschaen, D.M.; Grabowski, E.J.J.; Reider, R.J. A novel chromium trioxide catalyzed oxidation of primary alcohols to the carboxylic acids. Tetrahedron Lett. 1998, 39, 5323–5326. [Google Scholar] [CrossRef]
- Tohma, H.; Takizawa, S.; Maegawa, T.; Prof, Y.K. Facile and clean oxidation of alcohols in water using hypervalent iodine (III) reagents. Angew. Chem. Int. Ed. 2000, 39, 1306–1308. [Google Scholar] [CrossRef]
- Könning, D.; Olbrisch, T.; Sypaseuth, F.D.; Tzschuckea, C.C.; Christmann, M. Oxidation of allylic and benzylic alcohols to aldehydes and carboxylic acids. Chem. Commun. 2014, 50, 5014–5016. [Google Scholar] [CrossRef]
- Gao, W.; Li, S.; Pal, M.; Liu, Y.; Wan, X.; Li, W.; Wang, S.; Wang, C.; Zheng, G.; Zhao, D. Capping agent-free highly dispersed noble metal nanoparticles supported in ordered mesoporous carbon with short channels and their catalytic applications. RSC Adv. 2016, 6, 61064–61072. [Google Scholar] [CrossRef]
- Pai, Z.P.; Kochubey, D.I.; Berdnikova, P.V.; Kanazhevskiy, V.V.; Prikhodʹko, I.Y.; Chesalov, Y.A. Structure and properties of tungsten peroxopolyoxo complexes-promising catalysts for organics oxidation. I. structure of peroxocomplexes studied during the stepwise synthesis of tetra(diperoxotungsten)phosphate-tetra-n-butyl ammonium. J. Mol. Catal. A Chem. 2010, 332, 122–127. [Google Scholar] [CrossRef]
- Sloboda-Rozner, D.; Witte, P.; Alsters, P.L.; Neumann, R. Aqueous biphasic oxidation: A water-soluble polyoxometalate catalyst for selective oxidation of various functional groups with hydrogen peroxide. Adv. Synth. Catal. 2004, 346, 339–345. [Google Scholar] [CrossRef]
- Saisaha, P.; Buettner, L.; Meer, M.V.; Hage, R.; Feringa, B.L.; Browne, W.R.; Boer, J.W. Selective catalytic oxidation of alcohols, aldehydes, alkanes and alkenes employing manganese catalysts and hydrogen peroxide. Adv. Synth. Catal. 2013, 355, 2591–2603. [Google Scholar] [CrossRef]
- Seth, S.; Jhulki, S.; Moorthy, J.N. Catalytic and chemoselective oxidation of activated alcohols and direct conversion of diols to lactones with in situ-generated bis-IBX catalyst. Eur. J. Org. Chem. 2013, 2013, 2445–2452. [Google Scholar] [CrossRef]
- Mirza, M.; Tavana, M.M.; Boukherroub, R. Oxone/iron (II) sulfate/graphite oxide as a highly effective system for oxidation of alcohols under ultrasonic irradiation. Tetrahedron Lett. 2014, 55, 342–345. [Google Scholar] [CrossRef]
- Gowda, R.R.; Chakraborty, D. Ceric ammonium nitrate catalyzed oxidation of aldehydes and alcohols. Chin. J. Chem. 2011, 29, 2379–2384. [Google Scholar] [CrossRef]
- Das, R.; Chakraborty, D. Cu (II) bromide catalyzed oxidation of aldehydes and alcohols. Appl. Organometal. Chem. 2011, 25, 437–442. [Google Scholar] [CrossRef]
- da Silva, M.J.; da Silva, G.R.N.; Sampaio, V.F.C.; Vilanculo, C.B.; Fernandes, S.A.; Teixeira, M.G. One-pot synthesis of benzaldehyde derivatives in PdCl2-catalyzed reactions with H2O2 in alcoholic solutions. Chem. Pap. 2021, 75, 1545–1554. [Google Scholar] [CrossRef]
- Masuda, S.; Takano, S.; Yamazoe, S.; Tsukuda, T. Synthesis of active, robust and cationic Au25 cluster catalysts on double metal hydroxide by long-term oxidative aging of Au25(SR)18. Nanoscale 2022, 14, 3031–3039. [Google Scholar] [CrossRef]
- Tiburcio, E.; Greco, R.; Mon, M.; Ballesteros-Soberanas, J.; Ferrando-Soria, J.; López-Haro, M.; Hernández-Garrido, J.C.; Oliver-Meseguer, J.; Marini, C.; Boronat, M.; et al. Soluble/MOF-supported palladium single atoms catalyze the ligand-, additive-, and solvent-free aerobic oxidation of benzyl alcohols to benzoic acids. J. Am. Chem. Soc. 2021, 143, 2581–2592. [Google Scholar] [CrossRef]
- Zhao, Y.; Yu, C.; Wu, S.; Zhang, W.; Xue, W.; Zeng, Z. Synthesis of benzaldehyde and benzoic acid by selective oxidation of benzyl alcohol with iron (III) tosylate and hydrogen peroxide: A solvent-controlled reaction. Catal. Lett. 2018, 148, 3082–3092. [Google Scholar] [CrossRef]
- Sun, Q.; Dai, Z.; Meng, X.; Xiao, F. Porous polymer catalysts with hierarchical structures. Chem. Soc. Rev. 2015, 44, 6018–6034. [Google Scholar] [CrossRef] [PubMed]
- Sun, Q.; Xiao, F. Porous polymeric catalysts constructed from vinylated functionalities. Acc. Mater. Res. 2022, 3, 772–781. [Google Scholar] [CrossRef]
- Ferreira, G.A.; Loh, W. Planet–satellite nanostructures based on block copolymer-surfactant nanoparticles surface-decorated with gold and silver: A new strategy for interfacial catalysis. Adv. Mater. Interfaces 2019, 6, 1900348. [Google Scholar] [CrossRef]
- Tao, R.; Ma, X.; Wei, X.; Jin, Y.; Qiu, L.; Zhang, W. Porous organic polymer material supported palladium nanoparticles. J. Mater. Chem. A 2020, 8, 17360–17391. [Google Scholar] [CrossRef]
- Yamashina, M.; Sei, Y.; Akita, M.; Yoshizawa, M. Safe storage of radical initiators within a polyaromatic nanocapsule. Nat. Commun. 2014, 5, 4662. [Google Scholar] [CrossRef]
- Boonpangrak, S.; Whitcombe, M.J.; Prachayasittikul, V.; Mosbach, K.; Ye, L. Preparation of molecularly imprinted polymers using nitroxide-mediated living radical polymerization. Biosens. Bioelectron. 2006, 22, 349–354. [Google Scholar] [CrossRef]
- Huang, Z.; Liu, K.; Liu, M.; Yun, J.; Dong, J.; Chen, Z.; Xie, Z.; Pan, X. Photocatalytic metal-free radical hydrosilylation for polymer functionalization. Chin. J. Chem. 2023, 41, 2275–2281. [Google Scholar] [CrossRef]
- Ben, T.; Ren, H.; Ma, S.Q.; Cao, D.; Lan, J.; Jing, X.; Wang, W.; Xu, J.; Deng, F.; Simmons, J.M.; et al. Targeted synthesis of a porous aromatic framework with high stability and exceptionally high surface area. Angew. Chem. Int. Ed. 2009, 48, 9457–9460. [Google Scholar] [CrossRef]
- Tian, Y.; Zhu, G. Porous aromatic frameworks (PAFs). Chem. Rev. 2020, 120, 8934–8986. [Google Scholar] [CrossRef]
- Wang, Q.; Chen, Q.; Zhao, R.; Wang, H.; Diao, W.; Cui, F.; Li, S.; Wang, H.; Zhu, G. Salen-based porous aromatic frameworks with multi-active sites as anode materials for lithium-ion batteries. J. Colloid Interface Sci. 2023, 648, 616–622. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.; Das, A.; Maji, B. Phosphorus containing porous organic polymers: Synthetic techniques and applications in organic synthesis and catalysis. Org. Biomol. Chem. 2021, 19, 4174–4192. [Google Scholar] [CrossRef]
- Bayne, J.M.; Fasano, V.; Szkop, K.M.; Ingleson, M.J.; Stephan, D.W. Phosphorous(V) Lewis acids: Water/base tolerant P3-trimethylated trications. Chem. Commun. 2018, 54, 12467–12470. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Ding, M.; Ding, J.; Kang, J.; Yan, Z.; Zhao, P.; Zhou, X.; Jin, Y.; Chen, S.; Xia, C. Targeted synthesis of a high-stability cationic porous aromatic framework for highly efficient remediation of 99TcO4−. Chem. Eng. J. 2022, 435, 134785. [Google Scholar] [CrossRef]
- Tian, Y.; Song, J.; Zhu, Y.; Zhao, H.; Muhammad, F.; Ma, T.; Chen, M.; Zhu, G. Understanding the desulphurization process in an ionic porous aromatic framework. Chem. Sci. 2019, 10, 606–613. [Google Scholar] [CrossRef] [PubMed]
- Moghaddam, M.; Abbassi, A.; Ghazanfarian, J.; Jalilian, S. Investigation of microstructure effects on performance of hierarchically structured porous catalyst using a novel pore network model. Chem. Eng. J. 2020, 388, 124261. [Google Scholar] [CrossRef]
- Sun, Y.; Zhang, C.; Zhu, X.; Dong, L.; Sun, X. Mass and heat transfer of pressure swing adsorption oxygen production process with small adsorbent particles. Processes 2023, 11, 2485. [Google Scholar] [CrossRef]
- Hyodo, M.; Iwano, H.; Kasakado, T.; Fukuyama, T.; Ryu, I. Using high-power UV-LED to accelerate a decatungstate-anion-catalyzed reaction: A model study for the quick oxidation of benzyl alcohol to benzoic acid using molecular oxygen. Micromachines 2021, 12, 1307. [Google Scholar] [CrossRef]
- Bamoharram, F.F.; Roshani, M.; Heravi, M.M.; Safaie, S. The catalytic performance of preyssler’s anion, [NaP5W30O110]14−, in the oxidation of benzyl alcohols. Phosphorus Sulfur Silicon Relat. Elem. 2006, 181, 2833–2841. [Google Scholar] [CrossRef]
- Neves, P.; Lysenko, A.B.; Gomes, A.C.; Pillinger, M.; Gonçalves, I.S.; Valente, A.A. Behavior of triazolylmolybdenum (VI) oxide hybrids as oxidation catalysts with hydrogen peroxide. Catal. Lett. 2017, 147, 1133–1143. [Google Scholar] [CrossRef]
- Su, H.; Yang, C. Selective oxidation of benzyl alcohol catalyzed by (TEAH)nH3-nPW12O40 and its reaction mechanism. Chin. J. Catal. 2014, 35, 1224–1234. [Google Scholar] [CrossRef]
- Aguinaco, A.; Pocostales, J.P.; García-Araya, J.F.; Beltran, F.J. Decomposition of hydrogen peroxide in the presence of activated carbons with different characteristics. J. Chem. Technol. Biotechnol. 2011, 86, 595–600. [Google Scholar] [CrossRef]
- Mao, D.; Jia, M.; Qiu, J.; Zhang, X.F.; Yao, J. N-doped porous carbon supported Au nanoparticles for benzyl alcohol oxidation. Catal. Lett. 2020, 150, 74–81. [Google Scholar] [CrossRef]
- Jacobson, S.E.; Muccigrosso, D.A.; Mares, F. Oxidation of alcohols by molybdenum and tungsten peroxo complexes. J. Org. Chem. 1979, 44, 921–924. [Google Scholar] [CrossRef]
- Carlo, V.; Marco, R. Oxidative cleavage of 1,2-diols to carboylic acids by hydrogen peroxide. J. Org. Chem. 1986, 51, 1599–1602. [Google Scholar] [CrossRef]
- Noyori, R.; Aokib, M.; Sato, K. Green oxidation with aqueous hydrogen peroxide. Chem. Commun. 2003, 34, 1977–1986. [Google Scholar] [CrossRef]
- Correia, L.M.M.; Soliman, M.M.A.; Granadeiro, C.M.; Balula, S.S.; Martins, L.M.D.R.S.; Pombeiro, A.J.L.; Alegria, E.C.B.A. Vanadium C-scorpionate supported on mesoporous aptes-functionalized SBA-15 as catalyst for the peroxidative oxidation of benzyl alcohol. Microporous Mesoporous Mater. 2021, 320, 111111. [Google Scholar] [CrossRef]
- Shul’pin, G.B.; Kozlov, Y.N.; Nizova, G.V.; Süss-Fink, G.; Stanislas, S.; Kitaygorodskiyc, A.; Kulikovad, V.S. Oxidations by the reagent “O2–H2O2–vanadium derivative–pyrazine-2-carboxylic acid”. Part 12.1 Main features, kinetics and mechanism of alkane hydroperoxidation. J. Chem. Soc. Perkin Trans. 2001, 2, 1351–1371. [Google Scholar] [CrossRef]
- Wu, H.; Zhang, X.; Chen, X.; Chen, Y.; Zheng, X. Preparation, characterization and catalytic properties of MCM-48 supported tungstophosphoric acid mesoporous materials for green synthesis of benzoic acid. J. Solid State Chem. 2014, 211, 51–57. [Google Scholar] [CrossRef]
- Huang, X.; Wang, X.; Tan, M.; Zou, X.; Ding, W.; Lu, X. Selective oxidation of alcohols on P123-stabilized Au-Ag alloy nanoparticles in aqueous solution with molecular oxygen. Appl. Catal. A Gen. 2013, 467, 407–413. [Google Scholar] [CrossRef]
- Rautiainena, S.; Simakova, O.; Guo, H.; Leinoc, A.; Kordás, K.; Murzin, D.; Leskelä, M.; Repo, T. Solvent controlled catalysis: Synthesis of aldehyde, acid or ester by selective oxidation of benzyl alcohol with gold nanoparticles on alumina. Appl. Catal. A Gen. 2014, 485, 202–206. [Google Scholar] [CrossRef]
- Ghadamgahi, S.; Williamson, B.; Golovko, V. Activity of catalysts derived from Au101 immobilized on activated carbon. Catal. Lett. 2016, 146, 1027–1032. [Google Scholar] [CrossRef]
- Wang, C.; Jiao, L.; Ji, P. Interfacial synthesis of Co-based ultrathin nanosheets with NaF crystal as template and inducer: Fluorine doped Co(OH)2, porous fluorine doped Co3O4, and porous Co3O4. Appl. Surf. Sci. 2022, 589, 152923. [Google Scholar] [CrossRef]
- Sarkheil, M.; Lashanizadegan, M.; Ghiasi, M. High catalytic activity of magnetic Fe3O4@SiO2-Schiff base-Co (II) nanocatalyst for aerobic oxidation of alkenes and alcohols and DFT study. J. Mol. Struct. 2019, 1179, 278–288. [Google Scholar] [CrossRef]
- Feng, D.; Dong, Y.; Zhang, L.; Ge, X.; Zhang, W.; Dai, S.; Qiao, Z. Holey lamellar high entropy oxide as ultra-highly active heterogeneous catalyst for solvent-free aerobic oxidation of benzyl alcohol. Angew. Chem. Int. Ed. 2020, 59, 19503–19509. [Google Scholar] [CrossRef]
- Gao, W.; Song, Y.; Jiao, W.; Liu, Y. A catalyst-free and highly efficient approach to ozonation of benzyl alcohol to benzoic acid in a rotating packed bed. J. Taiwan Inst. Chem. Eng. 2019, 103, 1–6. [Google Scholar] [CrossRef]
- Sheldon, R.A.; Wallau, M.; Arends, I.W.C.E.; Schuchardt, U. Heterogeneous catalysts for liquid-phase oxidations: Philosophers’ stones or trojan horses? Acc. Chem. Res. 1998, 31, 485–493. [Google Scholar] [CrossRef]


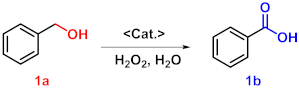 | |||||
|---|---|---|---|---|---|
| Entry | Catalyst | Acid | Temperature (°C) | T (h) | Yield (%) b |
| 1 | WO4@PAF-181 | None | 110 | 24 | 13 |
| 2 | WO4@PAF-181 | Tartaric acid | 110 | 7 | 69 |
| 3 | WO4@PAF-181 | TsOH | 110 | 7 | 73 |
| 4 c | WO4@PAF-181 | NaHSO4 | 110 | 7 | 53 |
| 5 | None | NaHSO4 | 110 | 7 | 26 |
| 6 | PAF-181 | NaHSO4 | 110 | 7 | 10 |
| 7 | I@PAF-181 | NaHSO4 | 110 | 7 | 16 |
| 8 | Na2WO4 | NaHSO4 | 110 | 7 | 77 |
| 9 d | WO4@PAF-181 | NaHSO4 | 110 | 7 | 0 |
| 10 e | WO4@PAF-181 | NaHSO4 | 110 | 7 | 96 |
| 11 | WO4@PAF-181 | NaHSO4 | 110 | 7 | 99 |
| 12 | WO4@PAF-181 | NaHSO4 | 60 | 7 | 81 |
| 13 | WO4@PAF-181 | NaHSO4 | r.t. | 7 | 58 |
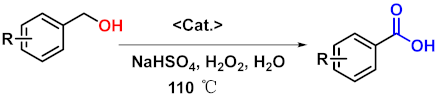 | |||
|---|---|---|---|
| Entry | Substrates | Products | Yield (%) b |
| 1 |  |  | 95 |
| 2 | 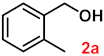 |  | 94 |
| 3 |  |  | 93 |
| 4 | 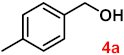 | 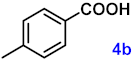 | 95 |
| 5 |  |  | 96 |
| 6 |  | 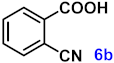 | 92 |
| 7 | 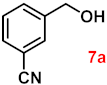 | 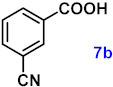 | 91 |
| 8 | 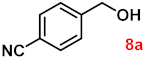 | 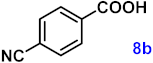 | 91 |
| 9 |  | 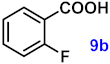 | 90 |
| 10 | 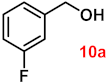 | 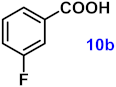 | 91 |
| 11 |  | 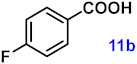 | 89 |
| 12 | 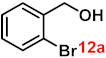 |  | 93 |
| 13 |  | 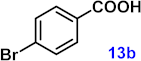 | 92 |
| 14 |  | 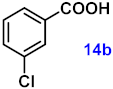 | 89 |
| 15 |  | 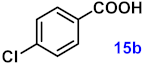 | 91 |
| Entry | Catalysts | Oxidant | Solvent | Yield (%) | Ref. |
|---|---|---|---|---|---|
| 1 | WO4@PAF-181 | H2O2 | H2O | 99 | this work |
| 2 | I@PAF-181 | H2O2 | H2O | 16 | |
| 3 | PAF-181 | H2O2 | H2O | 10 | |
| 4 | Na2WO4 | H2O2 | H2O | 77 | |
| 5 | HPW/MCM-48 | H2O2 | H2O | 66 | [49] |
| 6 | Au/PDVB-VI-n | O2 | H2O | 80 | [1] |
| 7 | Au1–xAgx | O2 | H2O | 67 | [50] |
| 8 | Au/Al2O3 | O2 | EtOH | 81 | [51] |
| 9 | Au101/AC | O2 | EtOH | 76 | [52] |
| 10 | p-Co3O4 | O2 | CH3CN | 30 | [53] |
| 11 | Fe3O4@SiO2-Schiff base-Co(II) | O2 | CH3CN | 91 | [54] |
| 12 | Holey HEO | O2 | - | 16 | [55] |
| 13 | RPB-O3 | O3 | ethyl acetate | 94 | [56] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
You, B.; Cheng, Z.; Tian, Y.; Wang, S.; Wang, B. Highly Efficient and Selective Oxidation of Benzyl Alcohol by WO42− Catalyst Immobilized by a Phosphonium-Containing Porous Aromatic Framework. Catalysts 2023, 13, 1309. https://doi.org/10.3390/catal13091309
You B, Cheng Z, Tian Y, Wang S, Wang B. Highly Efficient and Selective Oxidation of Benzyl Alcohol by WO42− Catalyst Immobilized by a Phosphonium-Containing Porous Aromatic Framework. Catalysts. 2023; 13(9):1309. https://doi.org/10.3390/catal13091309
Chicago/Turabian StyleYou, Bingxin, Zeliang Cheng, Yuyang Tian, Shaolei Wang, and Baolin Wang. 2023. "Highly Efficient and Selective Oxidation of Benzyl Alcohol by WO42− Catalyst Immobilized by a Phosphonium-Containing Porous Aromatic Framework" Catalysts 13, no. 9: 1309. https://doi.org/10.3390/catal13091309
APA StyleYou, B., Cheng, Z., Tian, Y., Wang, S., & Wang, B. (2023). Highly Efficient and Selective Oxidation of Benzyl Alcohol by WO42− Catalyst Immobilized by a Phosphonium-Containing Porous Aromatic Framework. Catalysts, 13(9), 1309. https://doi.org/10.3390/catal13091309