Optimization of an Open-Cell Foam-Based Ni-Mg-Al Catalyst for Enhanced CO2 Hydrogenation to Methane
Abstract
:1. Introduction
2. Results
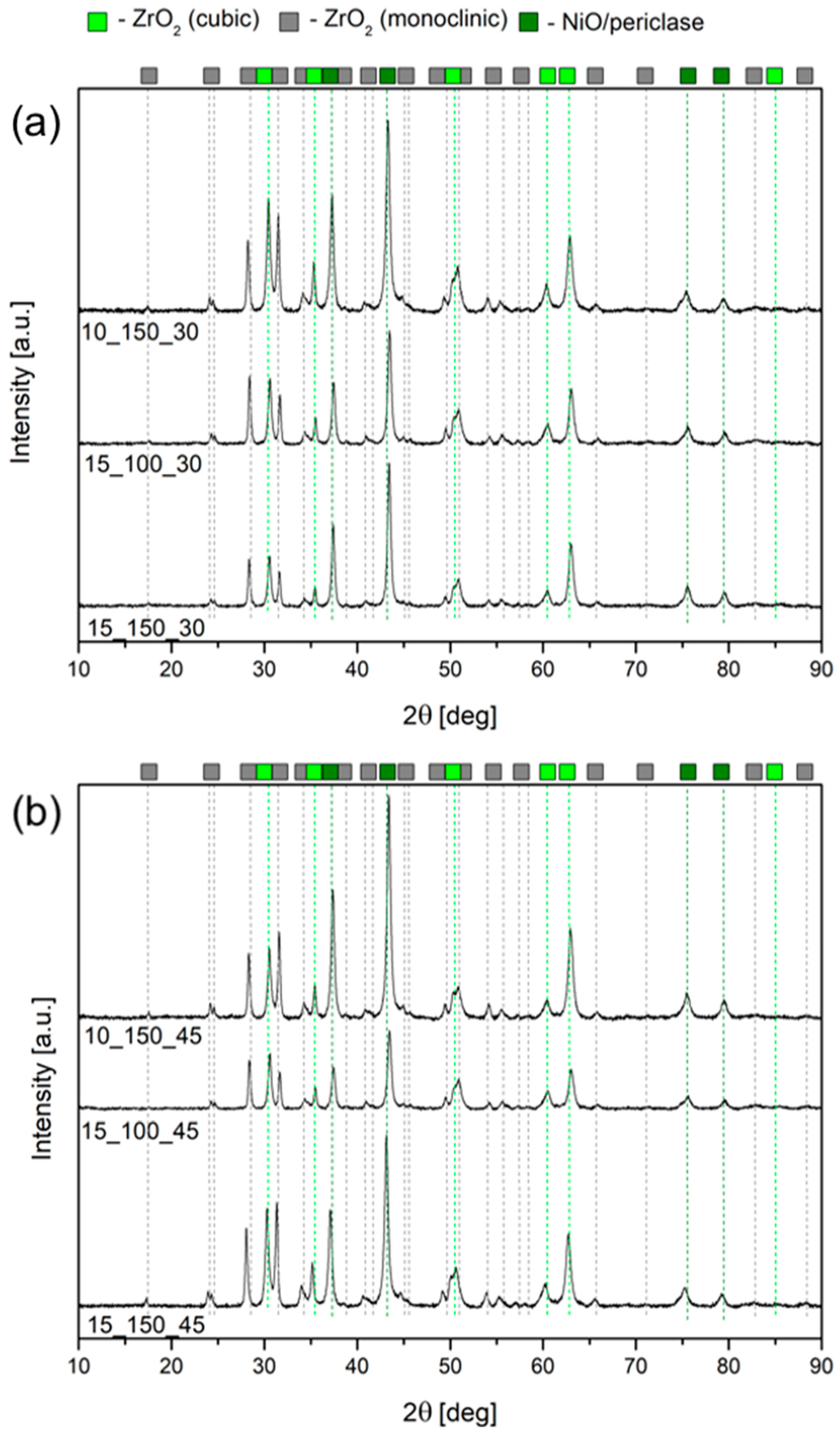
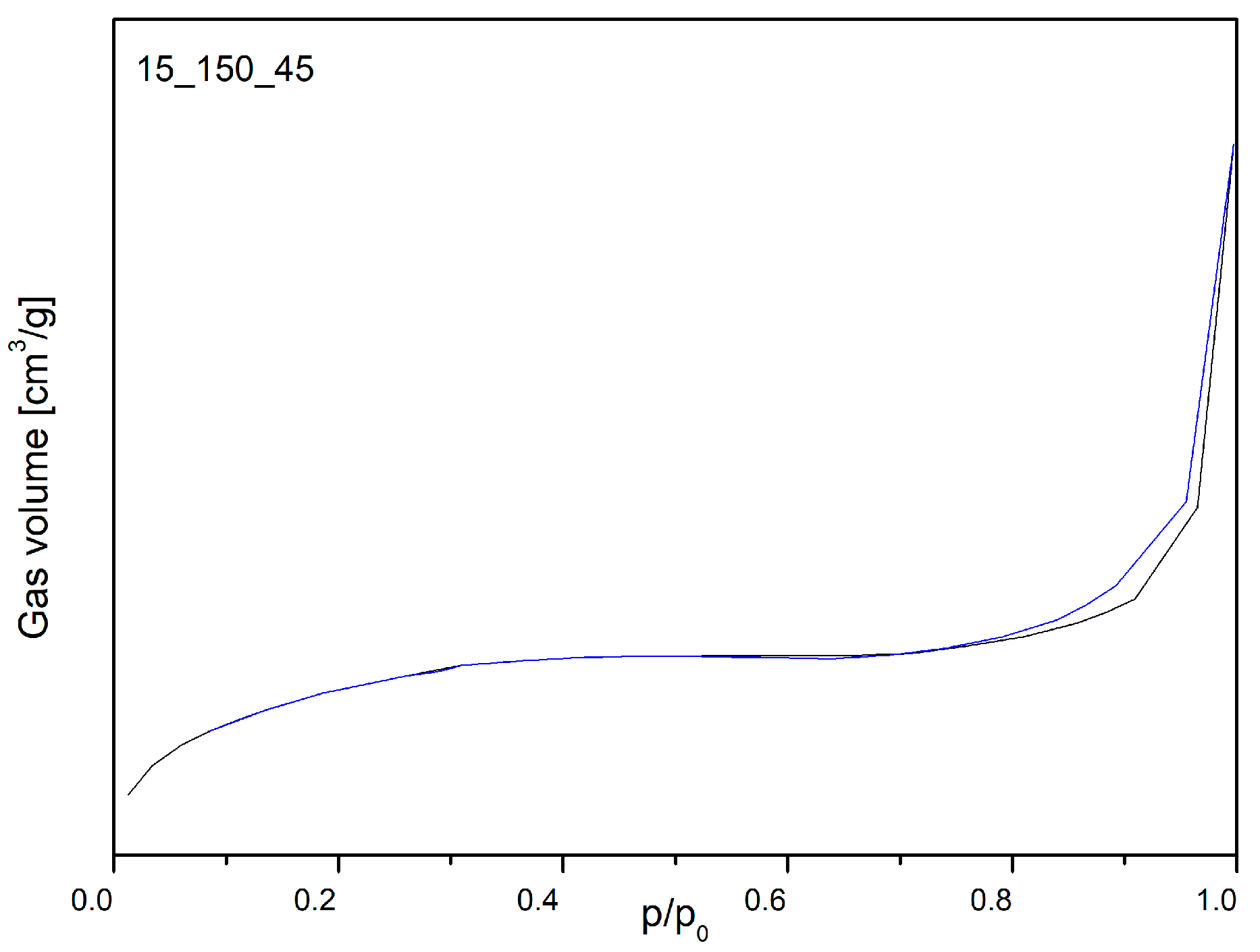
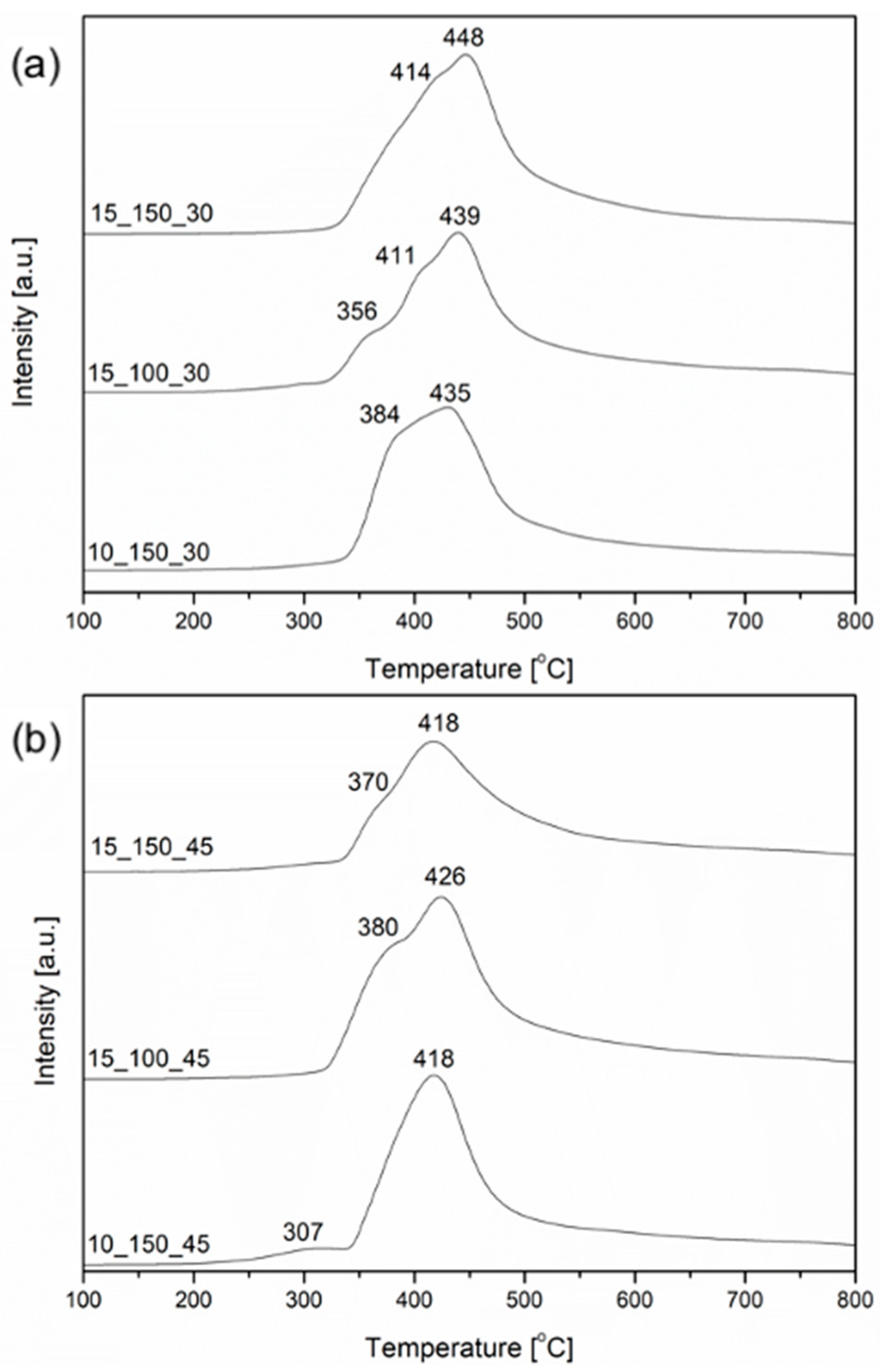
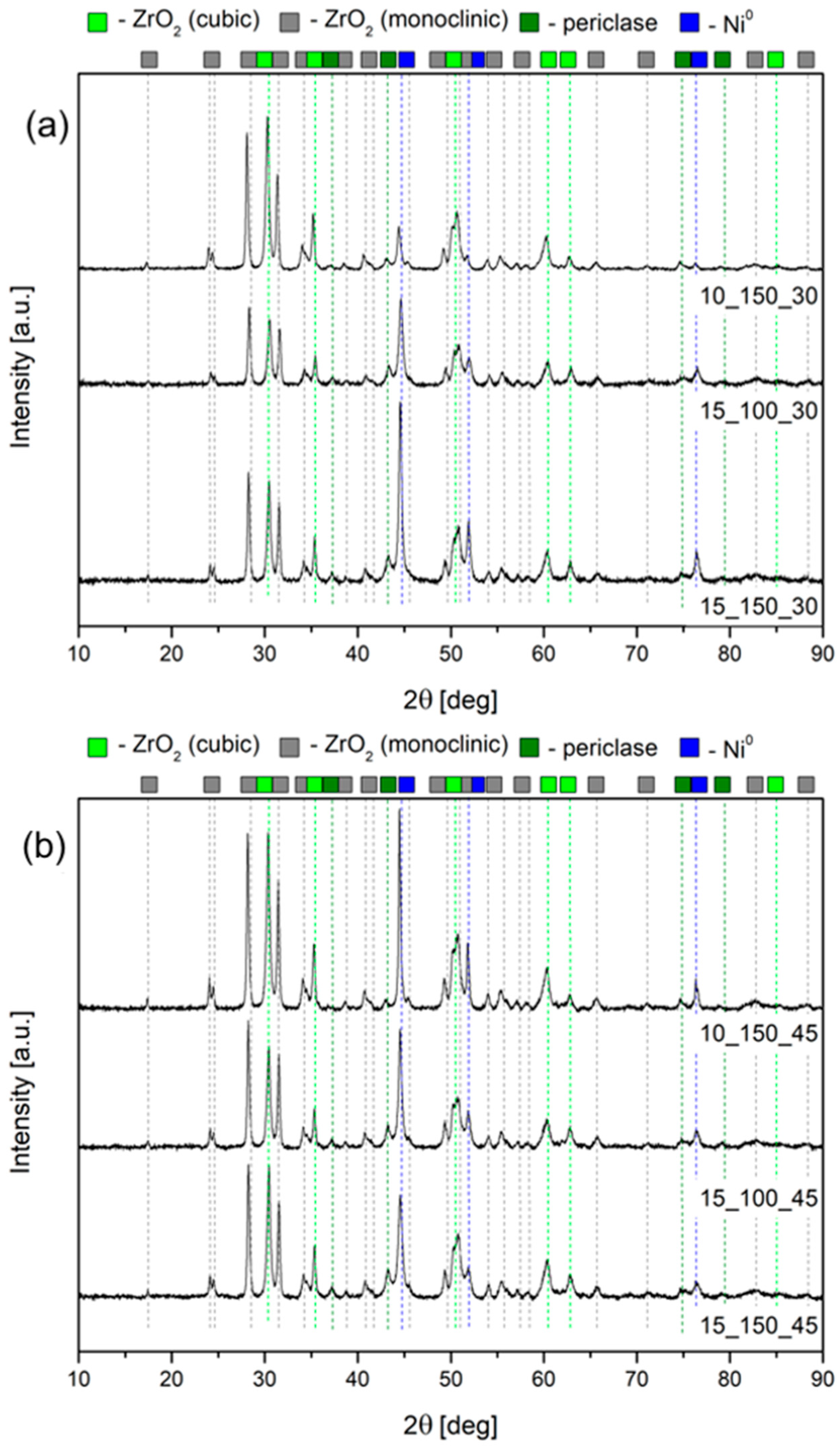
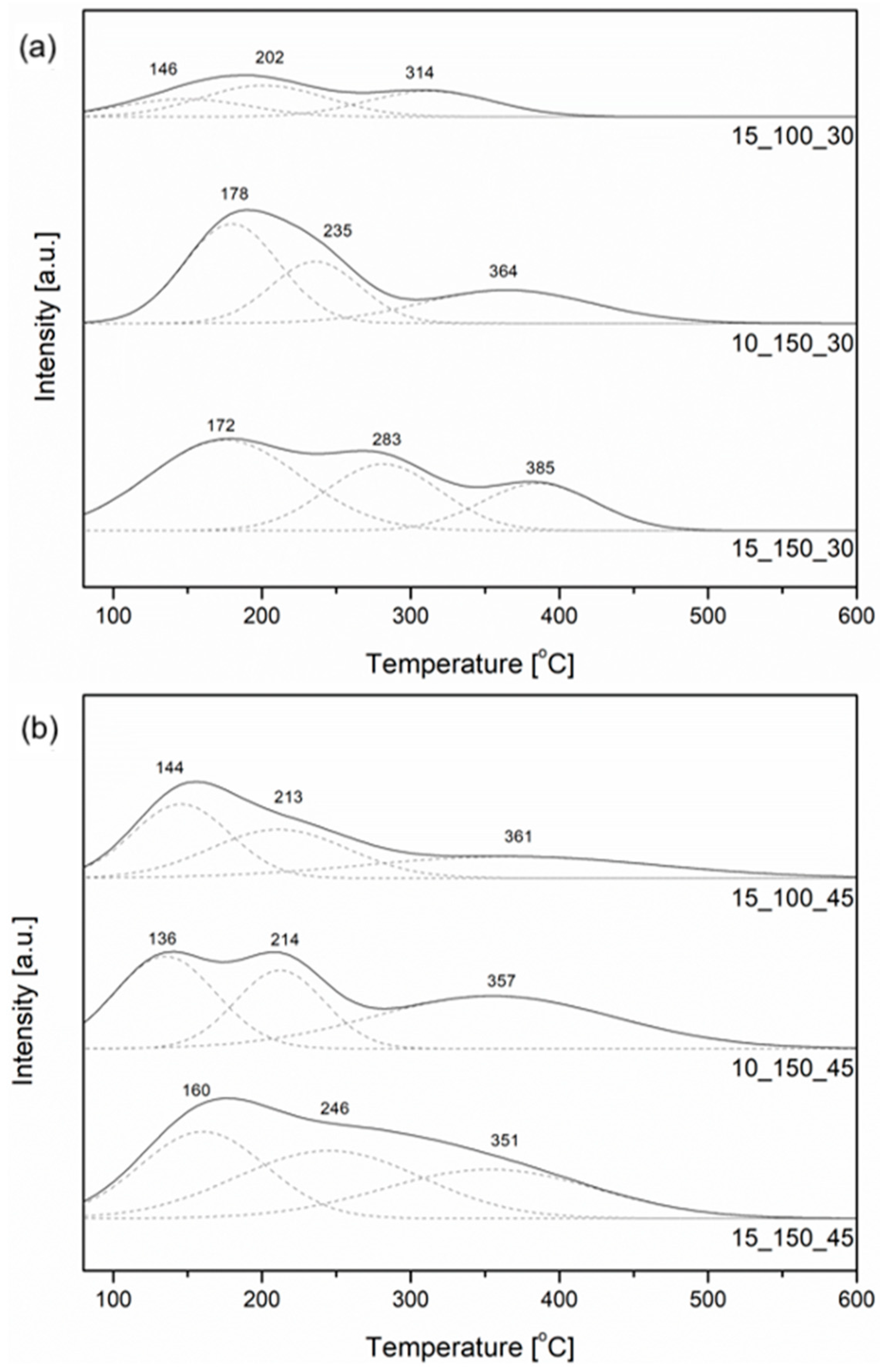
2.1. Characterization of the Open-Cell Foam-Based Catalysts
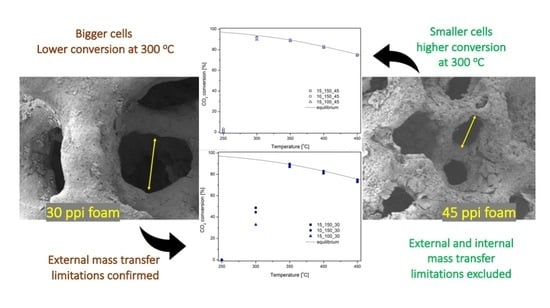
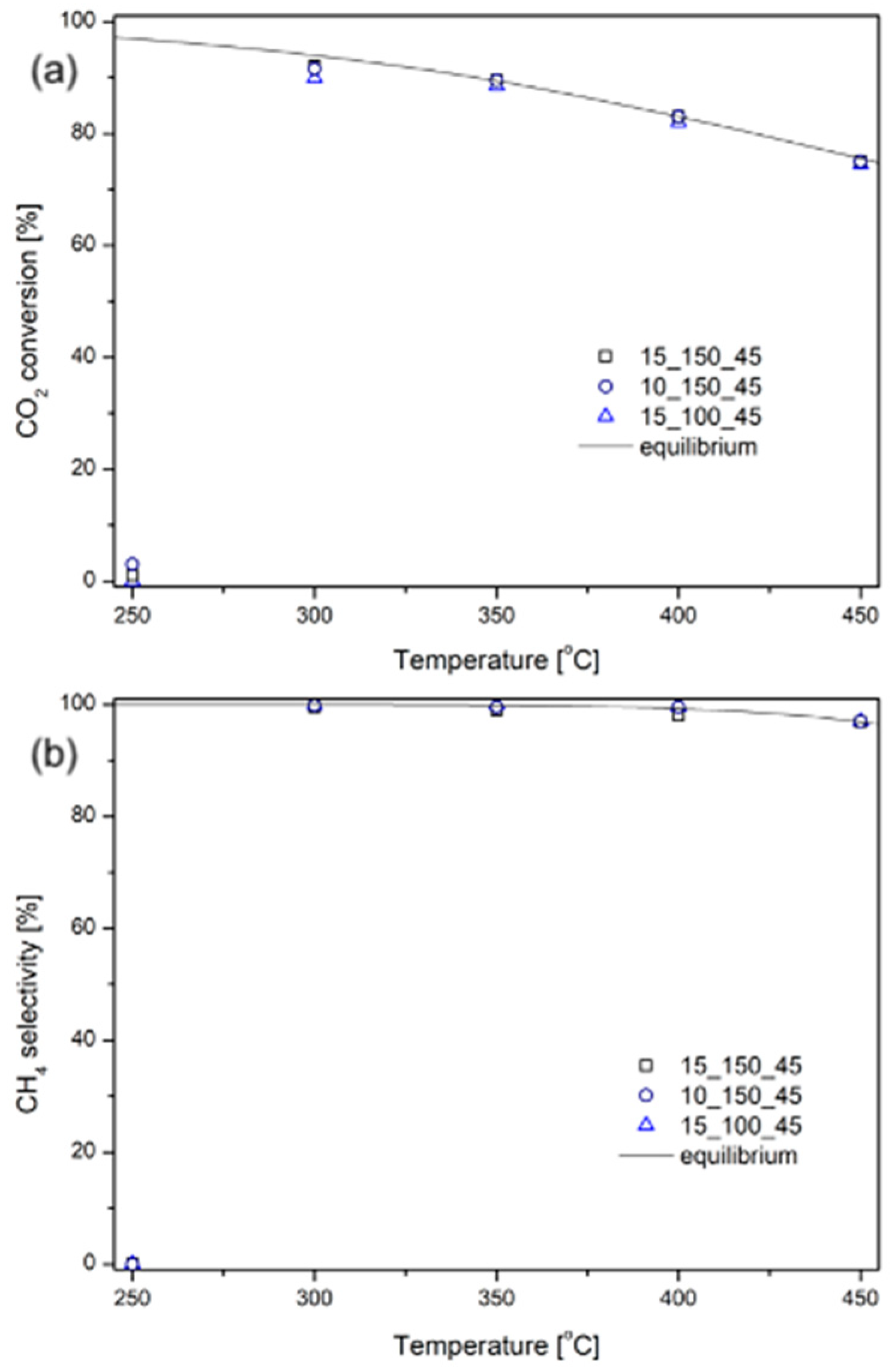
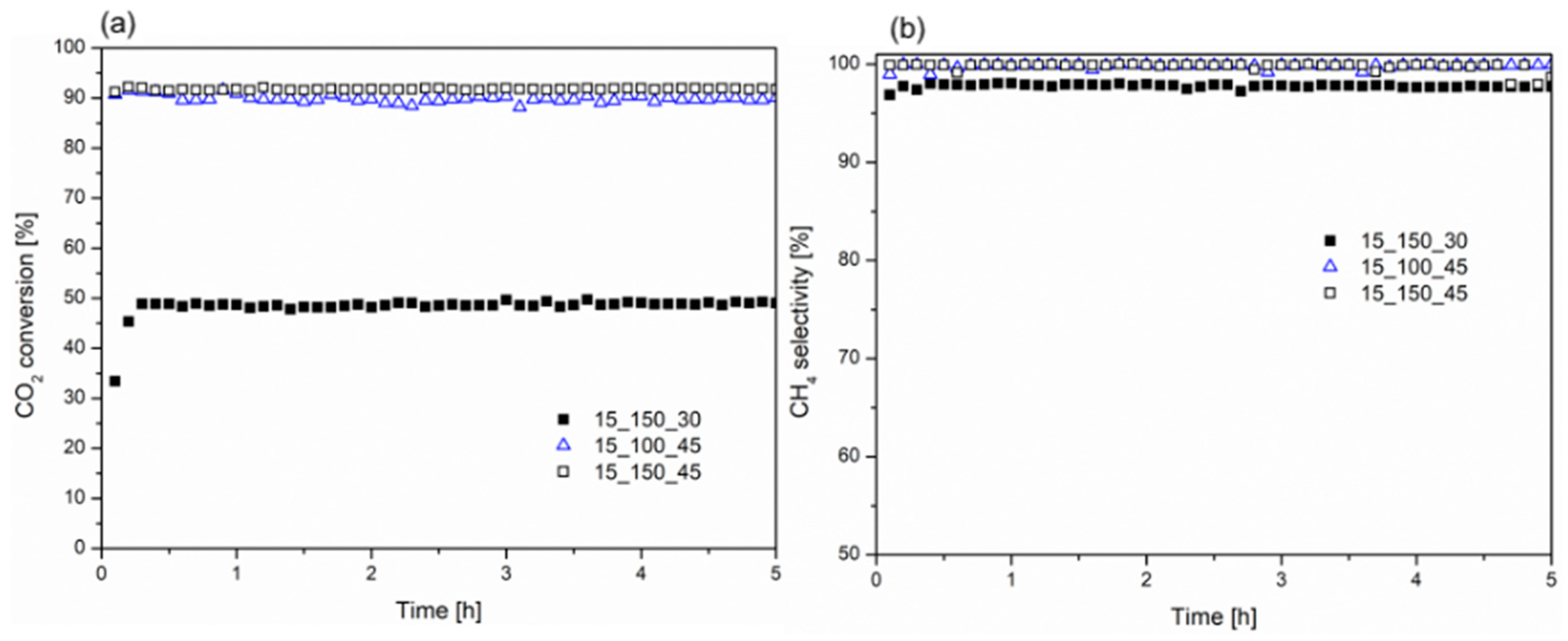
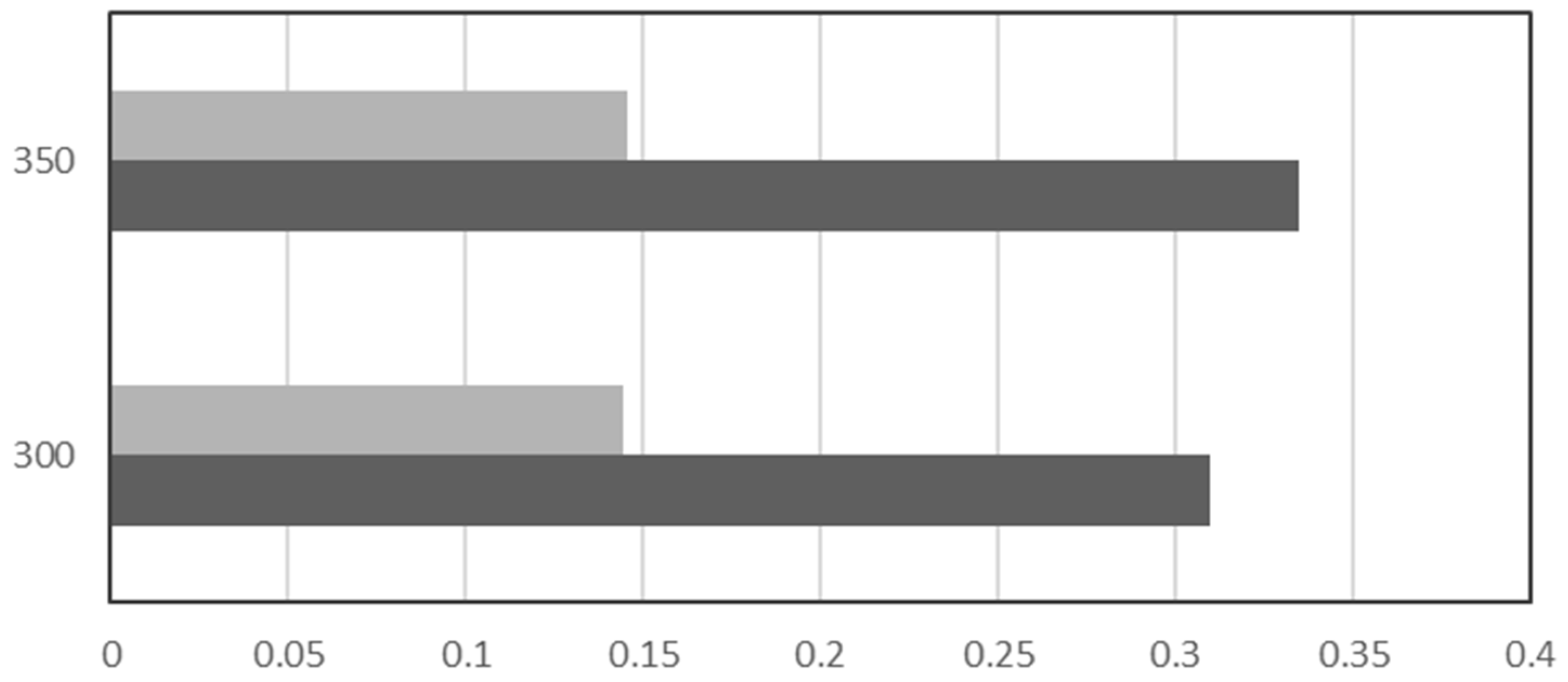
2.2. Catalytic Tests
2.3. Post-Run Characterization
3. Discussion
4. Materials and Methods
4.1. Synthesis of the Catalyst
4.2. Physicochemical Characterization
4.3. Catalytic Tests
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- IPCC Climate Change 2021 The Physical Science Basis Summary for Policymakers Working Group I Contribution to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change. 2021. Available online: https://reliefweb.int/report/world/climate-change-2021-physical-science-basis?gad_source=1&gclid=CjwKCAiAvoqsBhB9EiwA9XTWGfIF-QJJ7vhezD_d52Bf-mjsMzz7B7SuS1-ZSvqWt73kdPaMuJvqXxoC11sQAvD_BwE (accessed on 2 June 2021).
- European Commission European Commission—Press Release EU Agrees to COP27 Compromise to Keep Paris Agreement Alive and Protect Those Most Vulnerable to Climate Change. 2023. Available online: https://reliefweb.int/report/world/eu-agrees-cop27-compromise-keep-paris-agreement-alive-and-protect-those-most-vulnerable-climate-change (accessed on 2 June 2023).
- Ashok, J.; Pati, S.; Hongmanorom, P.; Tianxi, Z.; Junmei, C.; Kawi, S. A review of recent catalyst advances in CO2 methanation processes. Catal. Today 2020, 356, 471–489. [Google Scholar] [CrossRef]
- Wierzbicki, D.; Motak, M.; Grzybek, T.; Gálvez, M.E.; Da Costa, P. The influence of lanthanum incorporation method on the performance of nickel-containing hydrotalcite-derived catalysts in CO2 methanation reaction. Catal. Today 2018, 307, 205–211. [Google Scholar] [CrossRef]
- Fan, W.K.; Tahir, M. Recent trends in developments of active metals and heterogenous materials for catalytic CO2 hydrogenation to renewable methane: A review. J. Environ. Chem. Eng. 2021, 9, 105460. [Google Scholar] [CrossRef]
- Younas, M.; Loong Kong, L.; Bashir, M.J.K.; Nadeem, H.; Shehzad, A.; Sethupathi, S. Recent Advancements, Fundamental Challenges, and Opportunities in Catalytic Methanation of CO2. Energy Fuels 2016, 30, 8815–8831. [Google Scholar] [CrossRef]
- Chwoła, T.; Spietz, T.; Więcław-Solny, L.; Tatarczuk, A.; Krótki, A.; Dobras, S.; Wilk, A.; Tchórz, J.; Stec, M.; Zdeb, J. Pilot plant initial results for the methanation process using CO2 from amine scrubbing at the Łaziska power plant in Poland. Fuel 2020, 263, 116804. [Google Scholar] [CrossRef]
- Schirrmeister, S.; Morstein, O.V.; Föcker, H. Innovative Large-Scale Energy Storage Technologies and Power-to-Gas Concepts after Optimisation. D2.3. Demonstration Plant Falkenhagen Commissioned/Commissioning Report. 2017. Available online: https://ec.europa.eu/research/participants/documents/downloadPublic?documentIds=080166e5bd9d423f&appId=PPGMS (accessed on 2 June 2017).
- Lv, C.; Xu, L.; Chen, M.; Cui, Y.; Wen, X.; Li, Y.; Wu, C.E.; Yang, B.; Miao, Z.; Hu, X.; et al. Recent Progresses in Constructing the Highly Efficient Ni Based Catalysts With Advanced Low-Temperature Activity Toward CO2 Methanation. Front. Chem. 2020, 8, 269. [Google Scholar] [CrossRef]
- Wierzbicki, D.; Baran, R.; Dębek, R.; Motak, M.; Grzybek, T.; Gálvez, M.E.; Da Costa, P. The influence of nickel content on the performance of hydrotalcite-derived catalysts in CO2 methanation reaction. Int. J. Hydrogen Energy 2017, 42, 23548–23555. [Google Scholar] [CrossRef]
- Summa, P.; Gajewska, M.; Li, L.; Hu, C.; Samojeden, B.; Motak, M.; Da Costa, P. Solution combustion synthesis as an alternative synthesis route for novel Ni-Mg-Al mixed-oxide catalyst for CO2 methanation. J. CO2 Util. 2022, 60, 101983. [Google Scholar] [CrossRef]
- Summa, P.; Montero, D.; Samojeden, B.; Motak, M.; Da Costa, P. Nickel–magnesium-modified cenospheres for CO2 methanation. Int. J. Hydrogen Energy 2022, 47, 27944–27960. [Google Scholar] [CrossRef]
- Summa, P.; Świrk, K.; Wang, Y.; Samojeden, B.; Rønning, M.; Hu, C.; Motak, M.; Da Costa, P. Effect of cobalt promotion on hydrotalcite-derived nickel catalyst for CO2 methanation. Appl. Mater. Today 2021, 25, 101211. [Google Scholar] [CrossRef]
- Wierzbicki, D.; Debek, R.; Motak, M.; Grzybek, T.; Gálvez, M.E.; Da Costa, P. Novel Ni-La-hydrotalcite derived catalysts for CO2 methanation. Catal. Commun. 2016, 83, 5–8. [Google Scholar] [CrossRef]
- Świrk, K.; Summa, P.; Wierzbicki, D.; Motak, M.; Da Costa, P. Vanadium promoted Ni(Mg,Al)O hydrotalcite-derived catalysts for CO2 methanation. Int. J. Hydrogen Energy 2021, 46, 17776–17783. [Google Scholar] [CrossRef]
- Turek, V.; Bébar, L.; Jegla, Z. Simplified Pressure Drop and Flow Distribution Modelling in Radial Catalytic Converters. Chem. Endineering Trans. 2014, 39, 853–858. [Google Scholar] [CrossRef]
- Ahmadi, S.; Sefidvash, F. Study of Pressure Drop in Fixed Bed Reactor Using a Computational Fluid Dynamics (CFD) Code. ChemEngineering 2018, 2, 14. [Google Scholar] [CrossRef]
- Chaparro-Garnica, C.Y.; Bailón-García, E.; Davó-Quiñonero, A.; Da Costa, P.; Lozano-Castelló, D.; Bueno-López, A. High Performance Tunable Catalysts Prepared by Using 3D Printing. Materials 2021, 14, 5017. [Google Scholar] [CrossRef] [PubMed]
- Maiorano, L.P.; Chaparro-Garnica, C.Y.; Bailón García, E.; Lozano-Castelló, D.; Bueno-López, A.; Molina-Jordá, J.M. Guefoams (guest-containing foams) as novel heterogeneous catalysts: Preparation, characterization and proof-of-concept testing for CO2 methanation. Mater. Des. 2022, 217, 110619. [Google Scholar] [CrossRef]
- Navarro, J.C.; Centeno, M.A.; Laguna, O.H.; Odriozola, J.A. Ru–Ni/MgAl2O4 structured catalyst for CO2 methanation. Renew. Energy 2020, 161, 120–132. [Google Scholar] [CrossRef]
- Ratchahat, S.; Sudoh, M.; Suzuki, Y.; Kawasaki, W.; Watanabe, R.; Fukuhara, C. Development of a powerful CO2 methanation process using a structured Ni/CeO2 catalyst. J. CO2 Util. 2018, 24, 210–219. [Google Scholar] [CrossRef]
- Vita, A.; Italiano, C.; Pino, L.; Frontera, P.; Ferraro, M.; Antonucci, V. Activity and stability of powder and monolith-coated Ni/GDC catalysts for CO2 methanation. Appl. Catal. B Environ. 2018, 226, 384–395. [Google Scholar] [CrossRef]
- Huynh, H.L.; Yu, Z. CO2 Methanation on Hydrotalcite-Derived Catalysts and Structured Reactors: A Review. Energy Technol. 2020, 8, 1901475. [Google Scholar] [CrossRef]
- Danaci, S.; Protasova, L.; Lefevere, J.; Bedel, L.; Guilet, R.; Marty, P. Efficient CO2 methanation over Ni/Al2O3 coated structured catalysts. Catal. Today 2016, 273, 234–243. [Google Scholar] [CrossRef]
- Italiano, C.; Drago Ferrante, G.; Pino, L.; Laganà, M.; Ferraro, M.; Antonucci, V.; Vita, A. Silicon carbide and alumina open-cell foams activated by Ni/CeO2-ZrO2 catalyst for CO2 methanation in a heat-exchanger reactor. Chem. Eng. J. 2022, 434, 134685. [Google Scholar] [CrossRef]
- Sinn, C.; Wentrup, J.; Pesch, G.R.; Thöming, J. Heat Transport in Open-Cell Foams: CFD Analysis of Artificial Heat Sources vs Fully Resolved Exothermal Reactions. Ind. Eng. Chem. Res. 2021, 60, 4542–4551. [Google Scholar] [CrossRef]
- Sinn, C.; Wentrup, J.; Pesch, G.R.; Thöming, J.; Kiewidt, L. Structure-heat transport analysis of periodic open-cell foams to be used as catalyst carriers. Chem. Eng. Res. Des. 2021, 166, 209–219. [Google Scholar] [CrossRef]
- Fukuhara, C.; Hayakawa, K.; Suzuki, Y.; Kawasaki, W.; Watanabe, R. A novel nickel-based structured catalyst for CO2 methanation: A honeycomb-type Ni/CeO2 catalyst to transform greenhouse gas into useful resources. Appl. Catal. A Gen. 2017, 532, 12–18. [Google Scholar] [CrossRef]
- Ahn, J.Y.; Chang, S.W.; Lee, S.M.; Kim, S.S.; Chung, W.J.; Lee, J.C.; Cho, Y.J.; Shin, K.S.; Moon, D.H.; Nguyen, D.D. Developing Ni-based honeycomb-type catalysts using different binary oxide-supported species for synergistically enhanced CO2 methanation activity. Fuel 2019, 250, 277–284. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, Q.; Chai, R.; Zhao, G.; Liu, Y.; Lu, Y.; Cao, F. Ni-Al2O3/Ni-foam catalyst with enhanced heat transfer for hydrogenation of CO2 to methane. AIChE J. 2015, 61, 4323–4331. [Google Scholar] [CrossRef]
- Cimino, S.; Cepollaro, E.M.; Lisi, L.; Fasolin, S.; Musiani, M.; Vázquez-Gómez, L. Ru/Ce/Ni Metal Foams as Structured Catalysts for the Methanation of CO2. Catalysts 2021, 11, 13. [Google Scholar] [CrossRef]
- Ho, P.H.; Luna, G.S.D.; Ospitali, F.; Fornasari, G.; Vaccari, A.; Benito, P. Open-cell foams coated by Ni/X/Al hydrotalcite-type derived catalysts (X = Ce, La, Y) for CO2 methanation. J. CO2 Util. 2020, 42, 101327. [Google Scholar] [CrossRef]
- Balzarotti, R.; Drago Ferrante, G.; Italiano, C.; Laganà, M.; Francis, L.F.; Vita, A.; Cristiani, C.; Pino, L. RhNi/CeO2 catalytic activation of alumina open cell foams by dip-spin coating for the CO2 methanation of biogas. Surf. Coat. Technol. 2022, 441, 128563. [Google Scholar] [CrossRef]
- Frey, M.; Romero, T.; Roger, A.-C.; Edouard, D. Open cell foam catalysts for CO2 methanation: Presentation of coating procedures and in situ exothermicity reaction study by infrared thermography. Catal. Today 2016, 273, 83–90. [Google Scholar] [CrossRef]
- Frey, M.; Bengaouer, A.; Geffraye, G.; Edouard, D.; Roger, A.-C. Aluminum Open Cell Foams as Efficient Supports for Carbon Dioxide Methanation Catalysts: Pilot-Scale Reaction Results. Energy Technol. 2017, 5, 2078–2085. [Google Scholar] [CrossRef]
- Italiano, C.; Pino, L.; Laganà, M.; Vita, A. Ceramic monolith- and foam-structured catalysts via in-situ combustion deposition for energetic applications. Ann. Chim. Sci. Des Mater. 2018, 42, 405–418. [Google Scholar] [CrossRef]
- Palma, V.; Pisano, D.; Martino, M. Comparative study between aluminum monolith and foam as carriers for the intensification of the CO water gas shift process. Catalysts 2018, 8, 489. [Google Scholar] [CrossRef]
- Lanik Lanik. Available online: https://www.lanik.eu/ (accessed on 1 May 2023).
- Moncada, C.; Ercolino, G.; Specchia, S. Combined silicon carbide and zirconia open cell foams for the process intensification of catalytic methane combustion in lean conditions: Impact on heat and mass transfer. Chem. Eng. J. 2021, 429, 132448. [Google Scholar] [CrossRef]
- Moncada, C.; Ercolino, G.; Specchia, S. Effect of the Co3O4 load on the performance of PdO/Co3O4/ZrO2 open cell foam catalysts for the lean combustion of methane: Kinetic and mass transfer regimes catalysts for the lean combustion of methane: Kinetic and mass transfer regimes. Catal. Today 2021, 383, 247–258. [Google Scholar] [CrossRef]
- Moncada, C.; Ercolino, G.; Poozhikunnath, A.; Maric, R. Analysis of heat and mass transfer limitations for the combustion of methane emissions on PdO/Co3O4 coated on ceramic open cell foams. Chem. Eng. J. 2020, 405, 126970. [Google Scholar] [CrossRef]
- Italiano, C.; Ashraf, M.A.; Pino, L.; Quintero, C.W.M.; Specchia, S.; Vita, A. Rh/CeO2 thin catalytic layer deposition on alumina foams: Catalytic performance and controlling regimes in biogas reforming processes. Catalysts 2018, 8, 448. [Google Scholar] [CrossRef]
- Italiano, C.; Balzarotti, R.; Vita, A.; Latorrata, S.; Fabiano, C.; Pino, L.; Cristiani, C. Preparation of structured catalysts with Ni and Ni–Rh/CeO2 catalytic layers for syngas production by biogas reforming processes. Catal. Today 2016, 273, 3–11. [Google Scholar] [CrossRef]
- Cristiani, C.; Finocchio, E.; Latorrata, S.; Giorgio, C.; Bianchi, E.; Tronconi, E.; Groppi, G.; Pollesel, P. Activation of metallic open-cell foams via washcoat deposition of Ni/MgAl2O4 catalysts for steam reforming reaction. Catal. Today 2012, 197, 256–264. [Google Scholar] [CrossRef]
- Balzarotti, R.; Ambrosetti, M.; Beretta, A.; Groppi, G.; Tronconi, E. Investigation of packed conductive foams as a novel reactor configuration for methane steam reforming. Chem. Eng. J. 2020, 391, 123494. [Google Scholar] [CrossRef]
- Tarifa, P.; Schiaroli, N.; Ho, P.H.; Cañaza, F.; Ospitali, F.; Sanghez de Luna, G.; Lucarelli, C.; Fornasari, G.; Vaccari, A.; Monzon, A.; et al. Steam reforming of clean biogas over Rh and Ru open-cell metallic foam structured catalysts. Catal. Today 2022, 383, 74–83. [Google Scholar] [CrossRef]
- Balzarotti, R.; Beretta, A.; Groppi, G.; Tronconi, E. A comparison between washcoated and packed copper foams for the intensification of methane steam reforming. React. Chem. Eng. 2019, 4, 1387–1392. [Google Scholar] [CrossRef]
- Zheng, L.; Ambrosetti, M.; Marangoni, D.; Beretta, A.; Groppi, G.; Tronconi, E. Electrified methane steam reforming on a washcoated SiSiC foam for low-carbon hydrogen production. AIChE J. 2023, 69, e17620. [Google Scholar] [CrossRef]
- Egaña, A.; Sanz, O.; Merino, D.; Moriones, X.; Montes, M. Fischer–Tropsch Synthesis Intensification in Foam Structures. Ind. Eng. Chem. Res. 2018, 57, 10187–10197. [Google Scholar] [CrossRef]
- Aguirre, A.; Scholman, E.; van der Shaaf, J.; Neira d’Angelo, M.F. Controlling the selectivity in the Fischer-Tropsch synthesis using foam catalysts: An integrated experimental and modeling approach. Chem. Eng. J. 2021, 409, 128139. [Google Scholar] [CrossRef]
- Liu, Y.; Edouard, D.; Nguyen, L.D.; Begin, D.; Nguyen, P.; Pham, C.; Pham-Huu, C. High performance structured platelet milli-reactor filled with supported cobalt open cell SiC foam catalyst for the Fischer–Tropsch synthesis. Chem. Eng. J. 2013, 222, 265–273. [Google Scholar] [CrossRef]
- Fratalocchi, L.; Groppi, G.; Visconti, C.G.; Lietti, L.; Tronconi, E. Adoption of 3D printed highly conductive periodic open cellular structures as an effective solution to enhance the heat transfer performances of compact Fischer-Tropsch fixed-bed reactors. Chem. Eng. J. 2020, 386, 123988. [Google Scholar] [CrossRef]
- Fratalocchi, L.; Visconti, C.G.; Groppi, G.; Lietti, L.; Tronconi, E. Intensifying heat transfer in Fischer-Tropsch tubular reactors through the adoption of conductive packed foams. Chem. Eng. J. 2018, 349, 829–837. [Google Scholar] [CrossRef]
- Shimizu, K.; Oka, M.; Kumar, P.; Kotoura, Y.; Yamamuro, T.; Makinouchi, K.; Nakamura, T. Time-dependent changes in the mechanical properties of zirconia ceramic. J. Biomed. Mater. Res. 1993, 27, 729–734. [Google Scholar] [CrossRef]
- Donohue, M.D.; Aranovich, G.L. Classification of Gibbs adsorption isotherms. Adv. Colloid Interface Sci. 1998, 76–77, 137–152. [Google Scholar] [CrossRef]
- Li, C.; Chen, Y.W. Temperature-programmed-reduction studies of nickel oxide/alumina catalysts: Effects of the preparation method. Thermochim. Acta 1995, 256, 457–465. [Google Scholar] [CrossRef]
- Summa, P.; Samojeden, B.; Motak, M.; Wierzbicki, D.; Alxneit, I.; Świerczek, K.; Da Costa, P. Investigation of Cu promotion effect on hydrotalcite-based nickel catalyst for CO2 methanation. Catal. Today 2022, 384–386, 133–145. [Google Scholar] [CrossRef]
- Ashraf, M.A.; Sanz, O.; Italiano, C.; Vita, A.; Montes, M.; Specchia, S. Analysis of Ru/La-Al2O3 catalyst loading on alumina monoliths and controlling regimes in methane steam reforming. Chem. Eng. J. 2018, 334, 1792–1807. [Google Scholar] [CrossRef]
- Khairullin, A.; Haibullina, A.; Sinyavin, A.; Balzamov, D.; Ilyin, V.; Khairullina, L.; Bronskaya, V. Heat Transfer in 3D Laguerre–Voronoi Open-Cell Foams under Pulsating Flow. Energies 2022, 15, 8660. [Google Scholar] [CrossRef]
- Sadeghi, M.; Mirdrikvand, M.; Pesch, G.R.; Dreher, W.; Thöming, J. Full—Field analysis of gas flow within open—Cell foams: Comparison of micro—Computed tomography—Based CFD simulations with experimental magnetic resonance flow mapping data. Exp. Fluids 2020, 61, 124. [Google Scholar] [CrossRef]
- Buciuman, F.C.; Kraushaar-Czarnetzki, B. Ceramic foam monoliths as catalyst carriers. 1. Adjustment and description of the morphology. Ind. Eng. Chem. Res. 2003, 42, 1863–1869. [Google Scholar] [CrossRef]
- Perry, R.H.; Green, D.W. Perry’s Chemical Engineers’ Handbook; McGraw-Hill Professional: New York, NY, USA, 1997. [Google Scholar]
- Garrido, G.I.; Patcas, F.C.; Lang, S. Mass transfer and pressure drop in ceramic foams: A description for different pore sizes and porosities. Chem. Eng. Sci. 2008, 63, 5202–5217. [Google Scholar] [CrossRef]


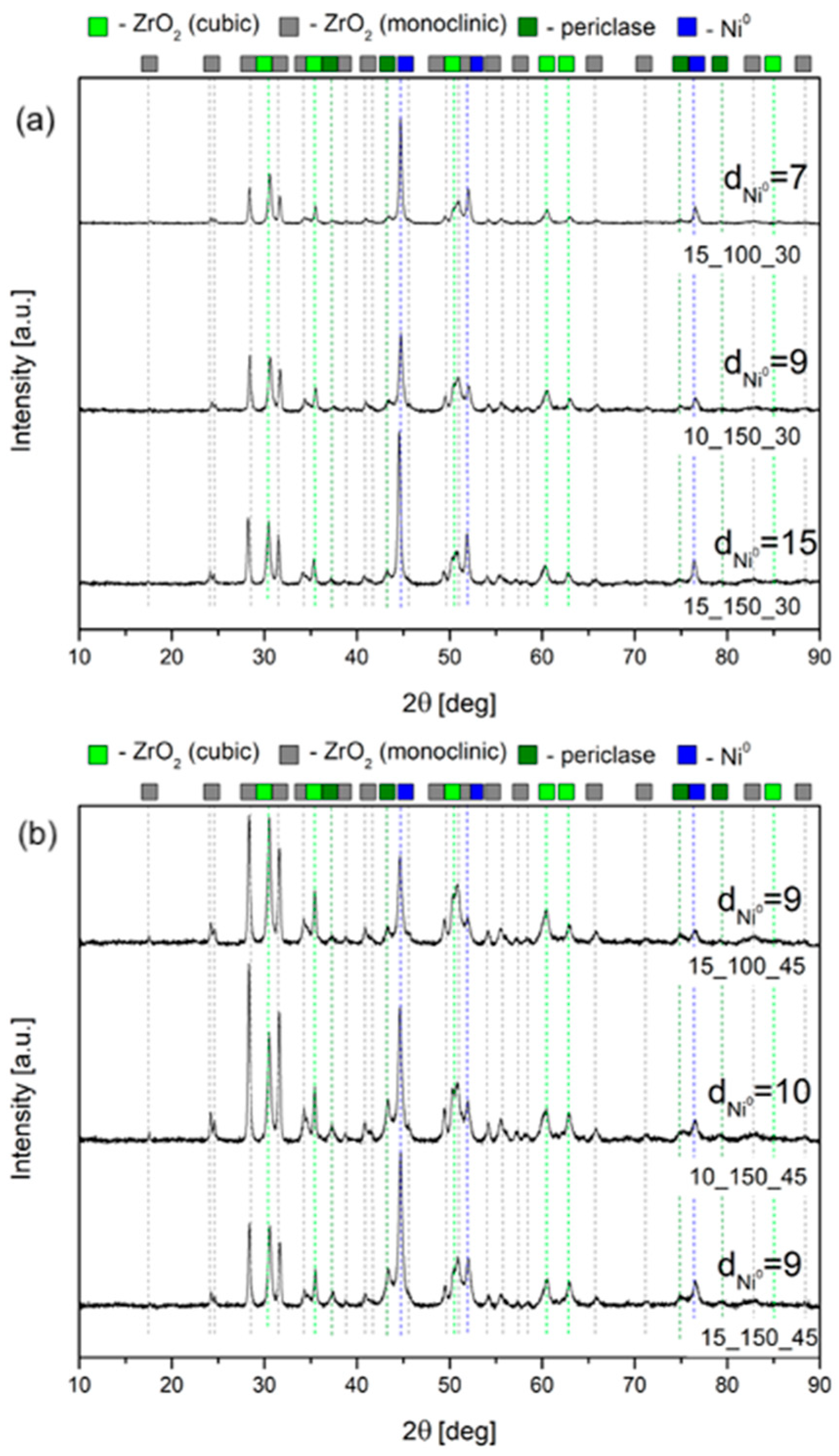
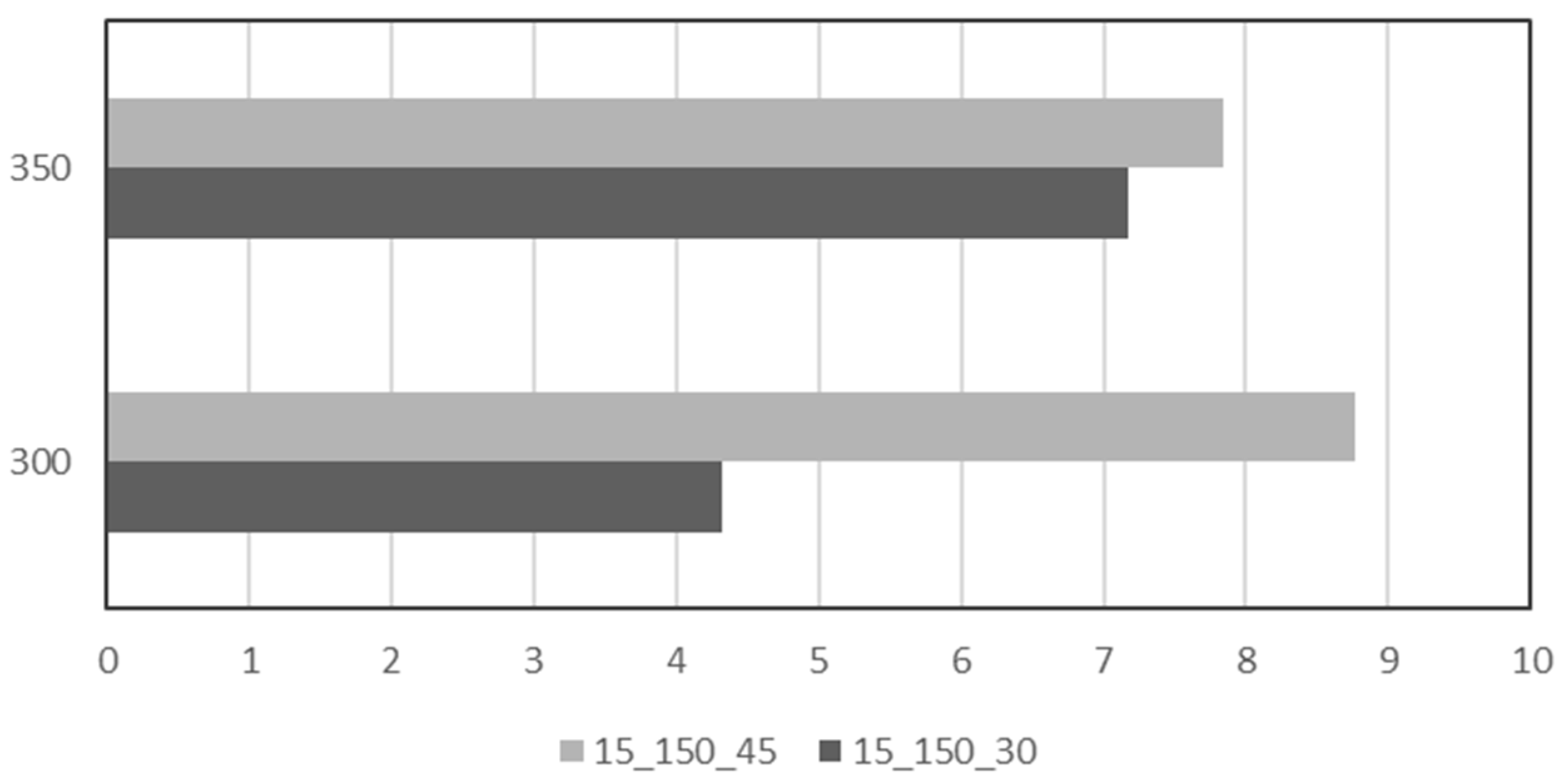
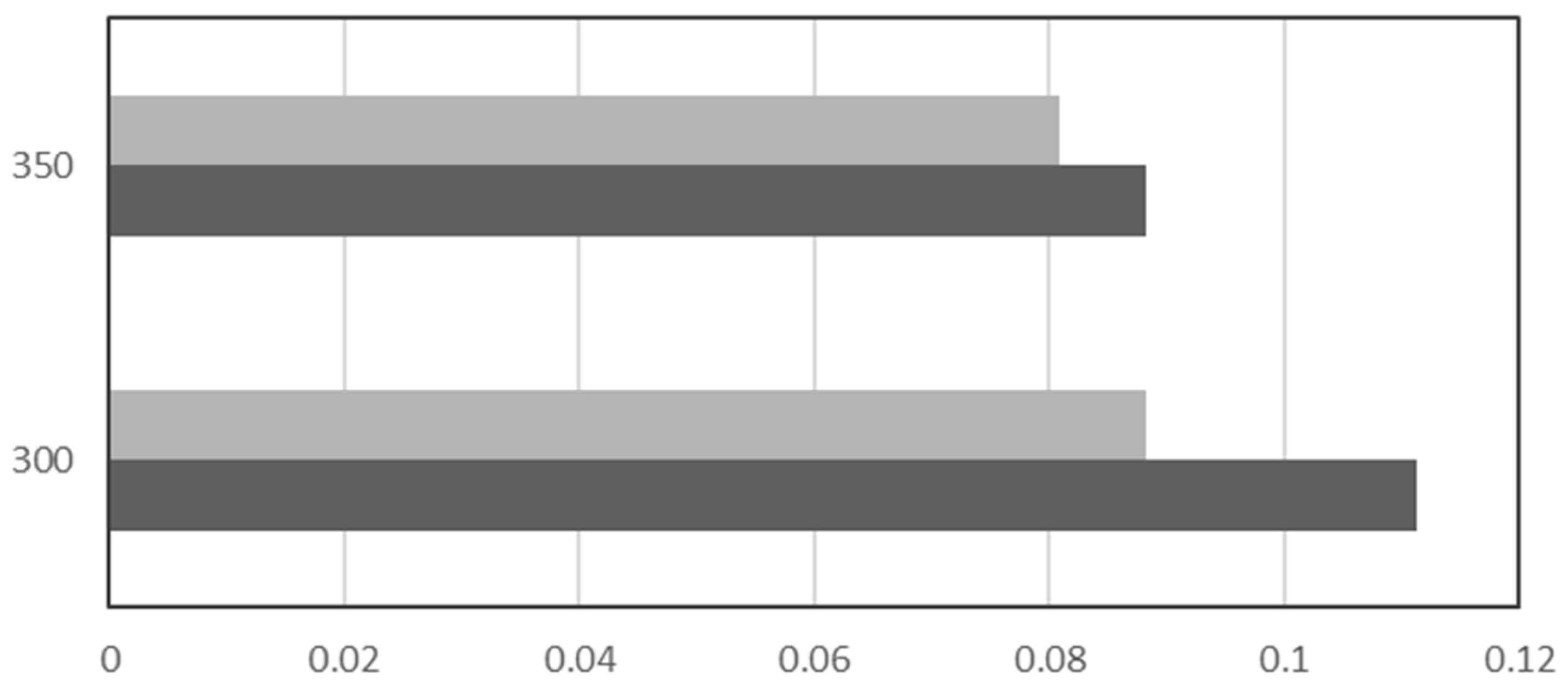

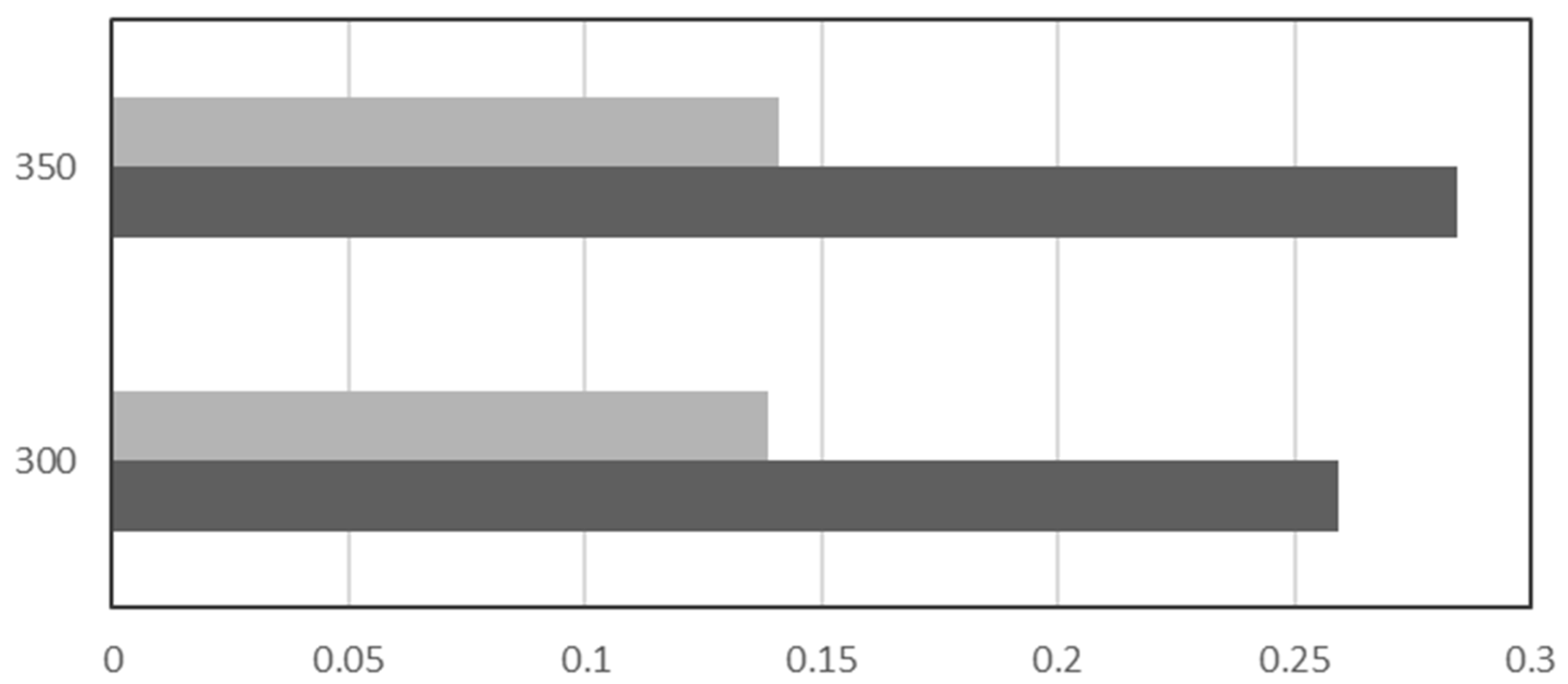
| Name | Location | Partners | Power Input | Status | Year |
|---|---|---|---|---|---|
| PtG test plant Stuttgart | Germany, Stuttgart | ZSW, IWES, Etogas | 250 kW | Pilot plant | 2012 |
| PTG test plant Rapperswil | Switzerland, Rapperswil | Erdgas Obersee AG, Etogas, HSR | 25 kW | Pilot plant | 2014 |
| E-gas/PtG BETA plant | Germany, Werlte | ZSW, Audi, EWE, IWES | 6300 kW | Commercial | 2013 |
| Copenhagen project of PtG | Denmark, Avedore Copenhagen | Electrochaea, BCH | 1000 kW | Commercial | 2016 |
| CO2-SNG | Poland, Łaziska Górne | Tauron, Atmostat, CEA, Rafako | 4930 kW | Pilot plant | 2019 |
| Store and Go | Germany, Falkenhagen | Atmostat, Electrochaea, KIT, Uniper | 580 kW | Pilot plant | 2017 |
| Catalyst | SBET (m2/g) | SBET (m2/gcoating) | Total Pore Volume (cm3/g) | dp (nm) | Vmesopores (cm3/g) |
|---|---|---|---|---|---|
| ZrO2 foam | <1 | - | - | - | |
| 15_150_45 | 14 | 165 | 0.016 | 4.6 | 0.010 |
| 15_150_30 | 9 | 111 | 0.010 | 4.7 | 0.006 |
| Sample | Weak (μmol/g) | Medium (μmol/g) | Strong (μmol/g) | Total (μmol/g) | Ni0 Crystallite Size (nm) |
|---|---|---|---|---|---|
| 15_100_30 | 1 | 6 | 5 | 13 | 8 |
| 10_150_30 | 15 | 9 | 23 | 47 | 11 |
| 15_150_30 | 7 | 7 | 6 | 20 | 9 |
| 15_100_45 | 9 | 10 | 9 | 28 | 10 |
| 10_150_45 | 7 | 7 | 20 | 35 | 8 |
| 15_150_45 | 18 | 21 | 5 | 44 | 7 |
| Catalyst | Nickel Content (%) | NiO (mg) | Mg-Al (mg) | Volumetric Catalyst Loading g/lfoam | Porosity (ppi) |
|---|---|---|---|---|---|
| 15_150_30 | 12.87 | 317 | 157 | 212.9 | 30 |
| 10_150_30 | 8.71 | 230 | 155 | 176.0 | |
| 15_100_30 | 12.61 | 314 | 120 | 192.2 | |
| 15_150_45 | 12.39 | 316 | 170 | 219.3 | 45 |
| 10_150_45 | 9.60 | 239 | 157 | 180.7 | |
| 15_100_45 | 13.26 | 339 | 114 | 199.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Summa, P.; Motak, M.; Da Costa, P. Optimization of an Open-Cell Foam-Based Ni-Mg-Al Catalyst for Enhanced CO2 Hydrogenation to Methane. Catalysts 2024, 14, 11. https://doi.org/10.3390/catal14010011
Summa P, Motak M, Da Costa P. Optimization of an Open-Cell Foam-Based Ni-Mg-Al Catalyst for Enhanced CO2 Hydrogenation to Methane. Catalysts. 2024; 14(1):11. https://doi.org/10.3390/catal14010011
Chicago/Turabian StyleSumma, Paulina, Monika Motak, and Patrick Da Costa. 2024. "Optimization of an Open-Cell Foam-Based Ni-Mg-Al Catalyst for Enhanced CO2 Hydrogenation to Methane" Catalysts 14, no. 1: 11. https://doi.org/10.3390/catal14010011
APA StyleSumma, P., Motak, M., & Da Costa, P. (2024). Optimization of an Open-Cell Foam-Based Ni-Mg-Al Catalyst for Enhanced CO2 Hydrogenation to Methane. Catalysts, 14(1), 11. https://doi.org/10.3390/catal14010011