Photocatalytic Degradation of Vehicle Exhaust by Nano-TiO2 Cement Slurry: Experimental Factors and Field Application
Abstract
:1. Introduction
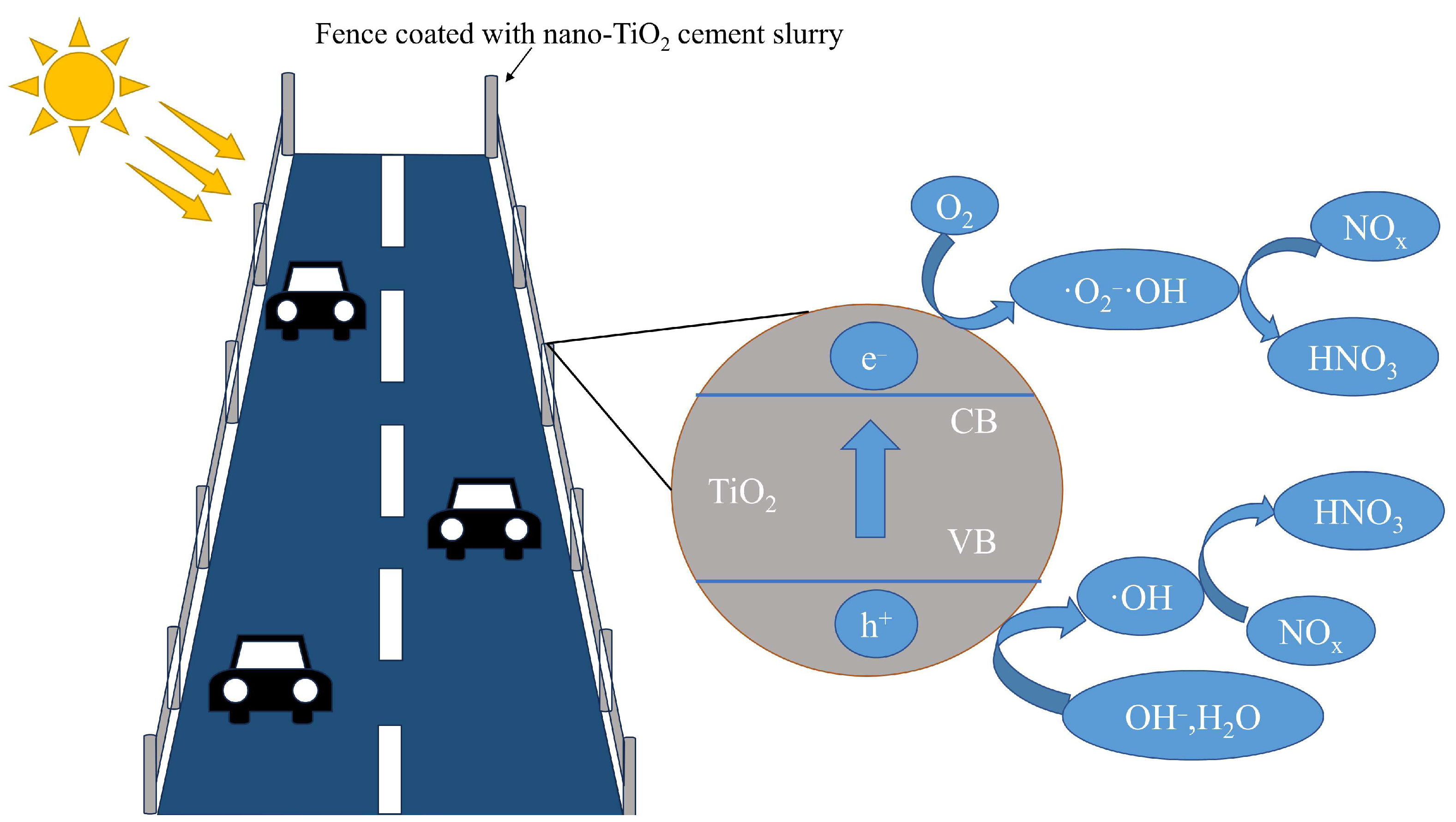
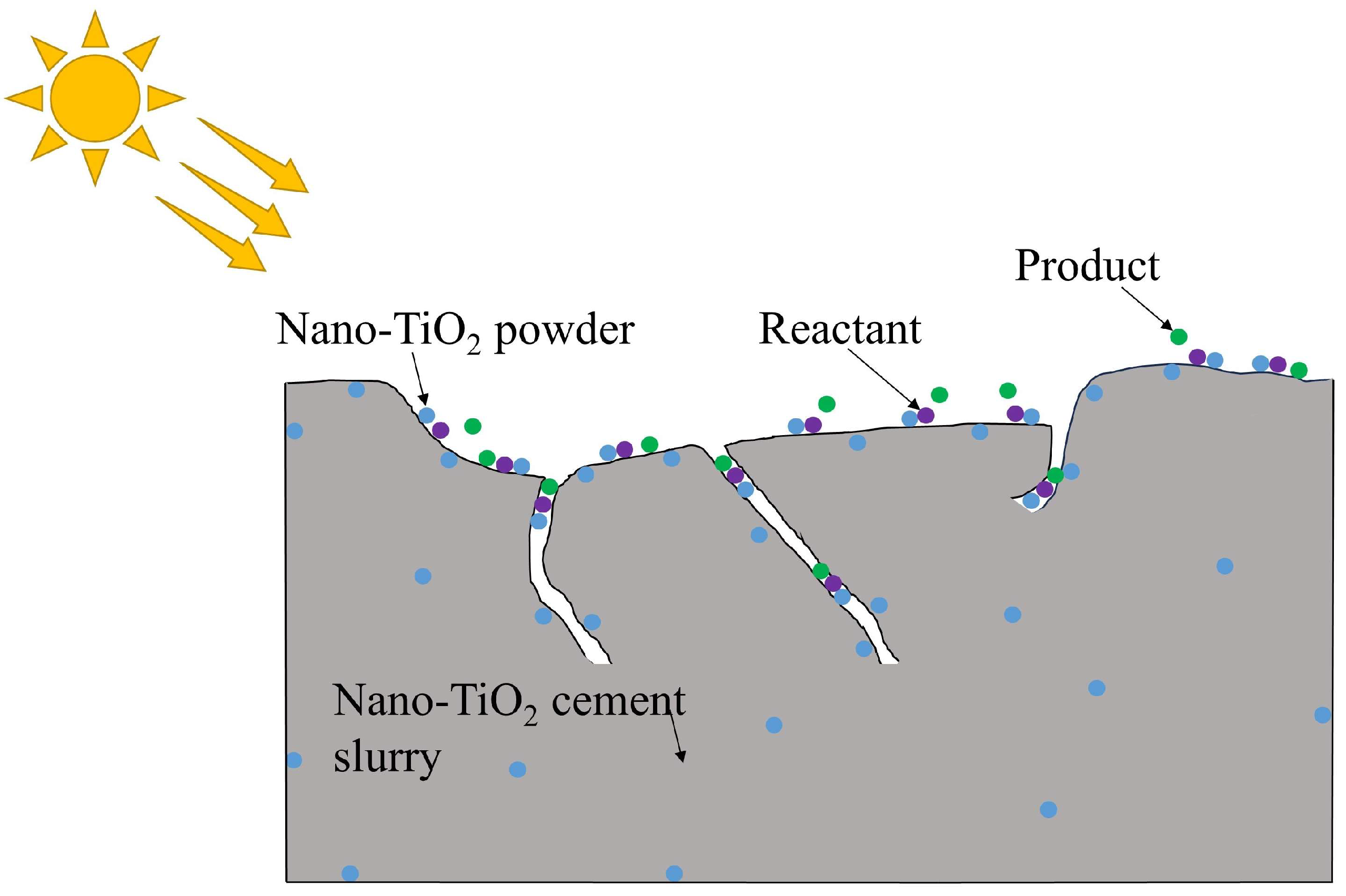
2. Mechanism of Nano-TiO2 Cement Slurry Photocatalytic Degradation of Vehicle Exhaust
3. Results and Discussions
3.1. Effect of Temperature
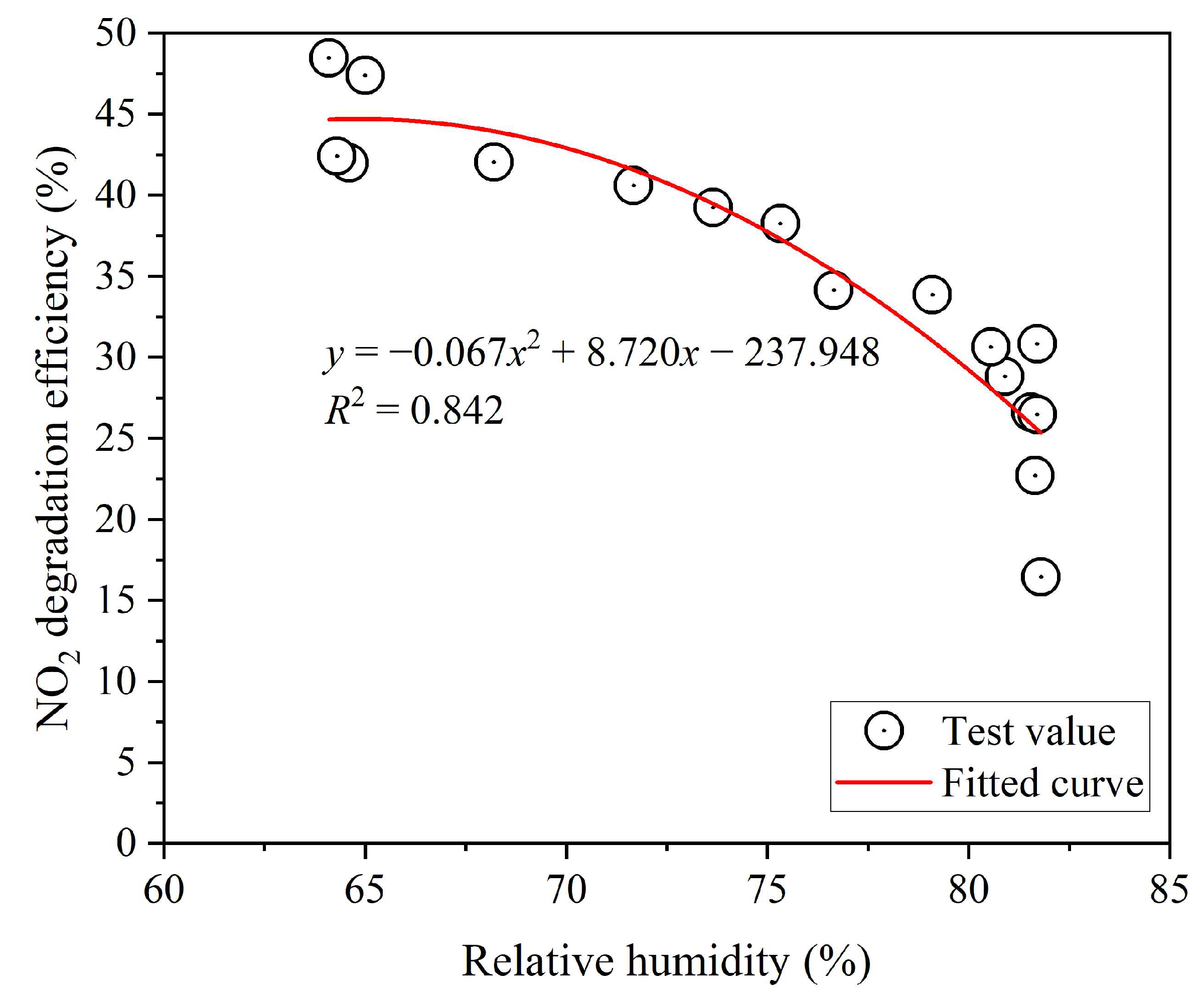
3.2. Effect of Relative Humidity
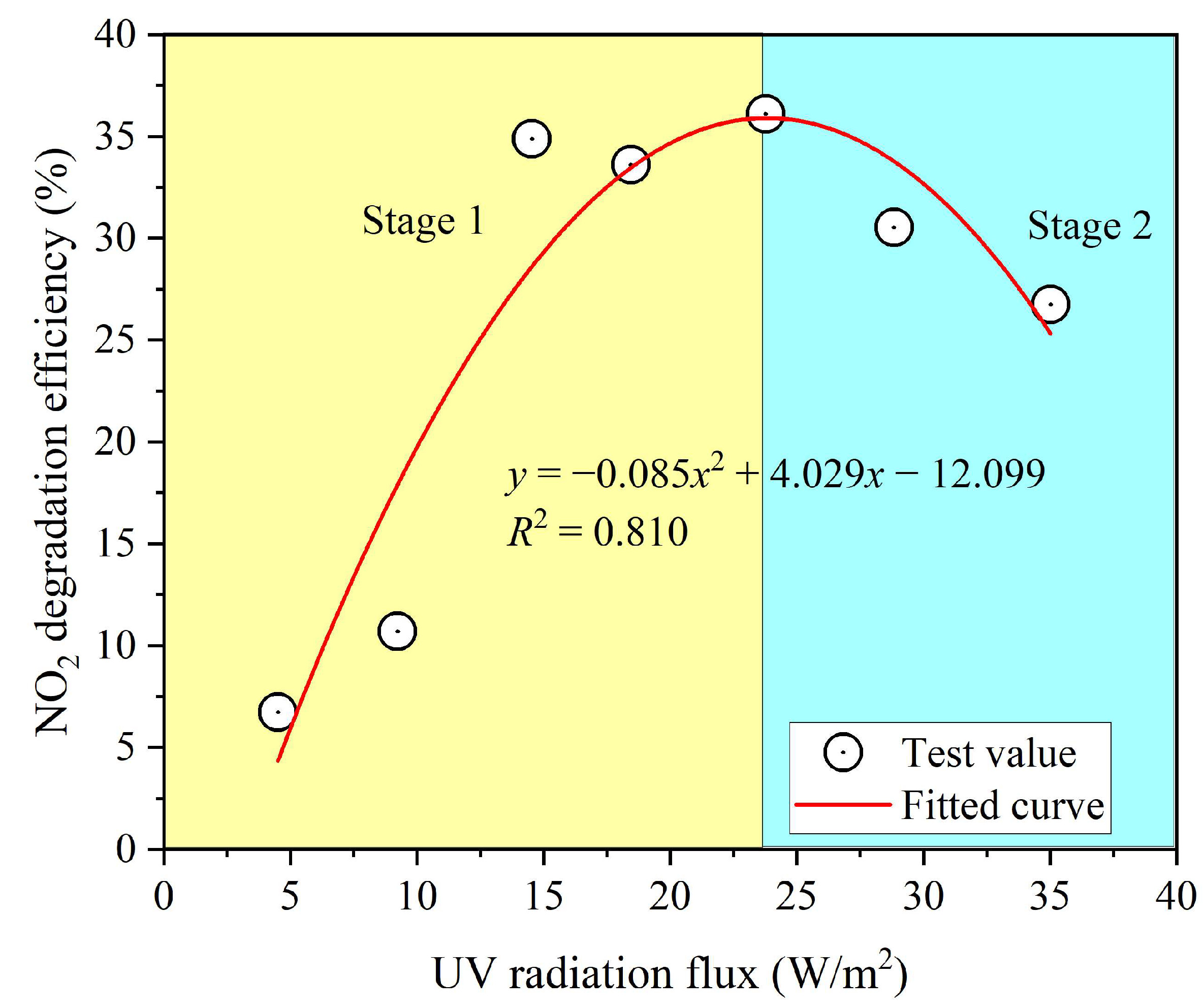
3.3. Effect of UV Radiation Flux
3.4. Effect of Thickness of Cement Slurry
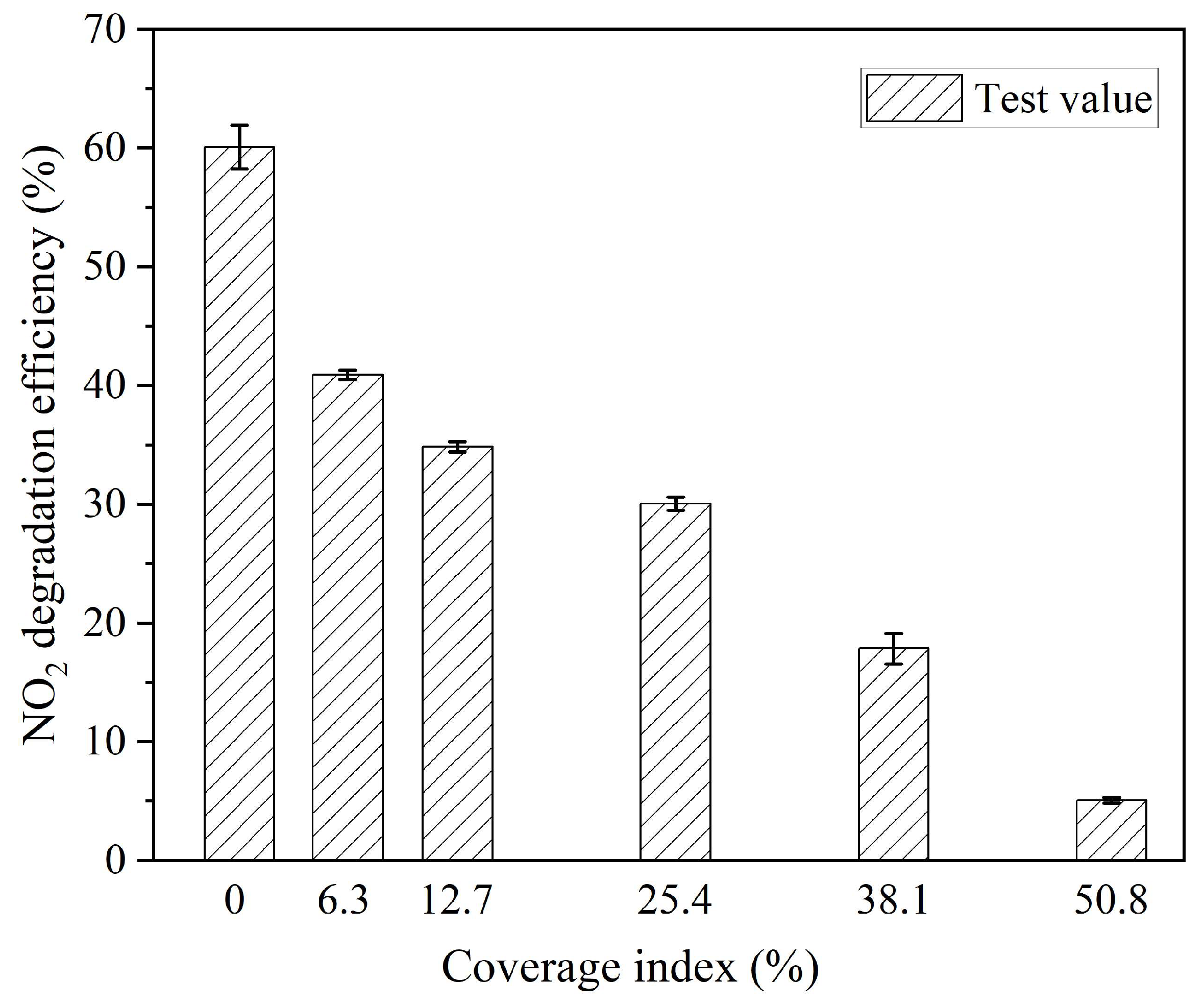
3.5. Effect of Amount of Dust Adhering to the Surface
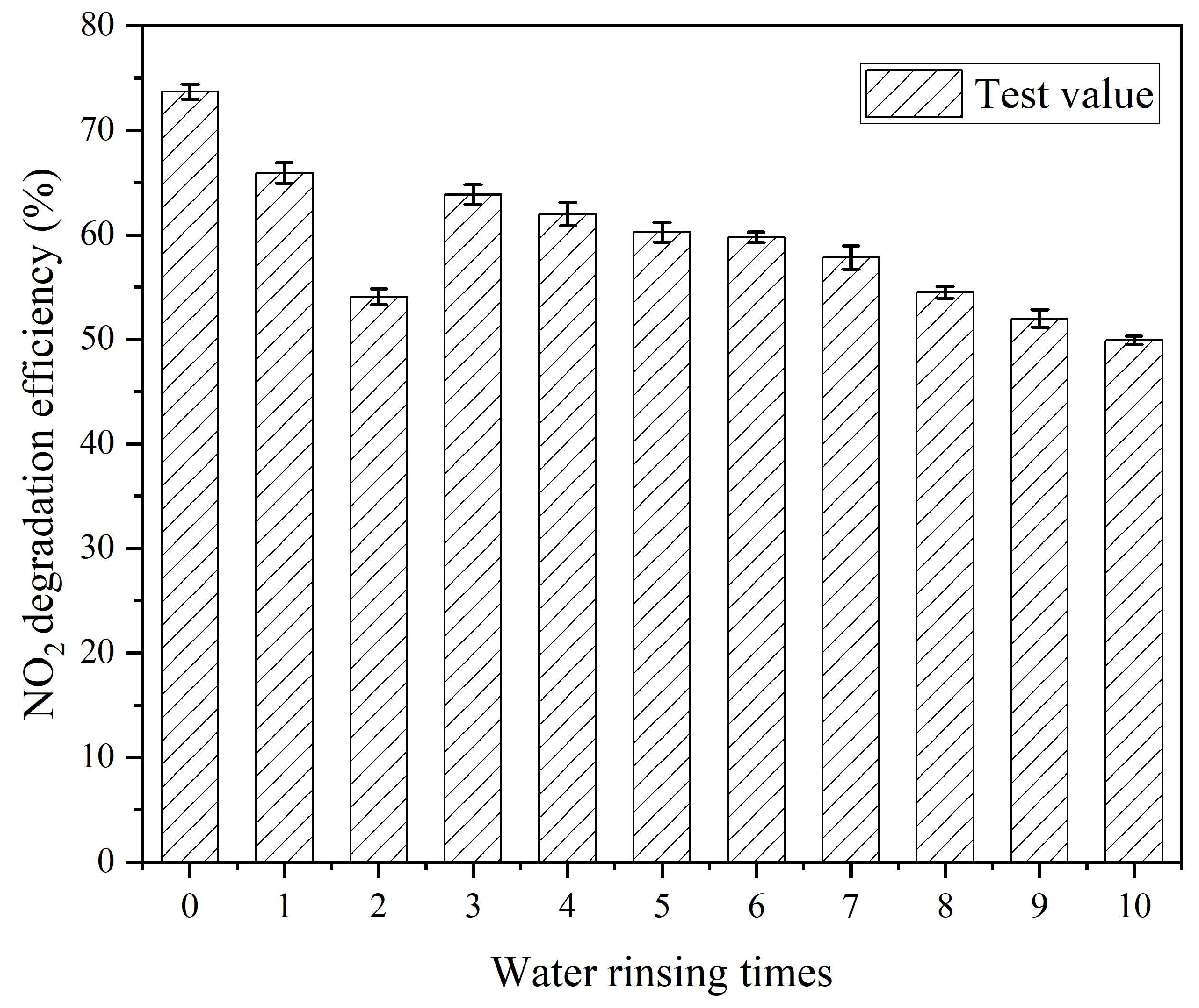
3.6. Effect of Number of Water Rinsing Cycles
4. Experimental Details
4.1. Materials and Instruments
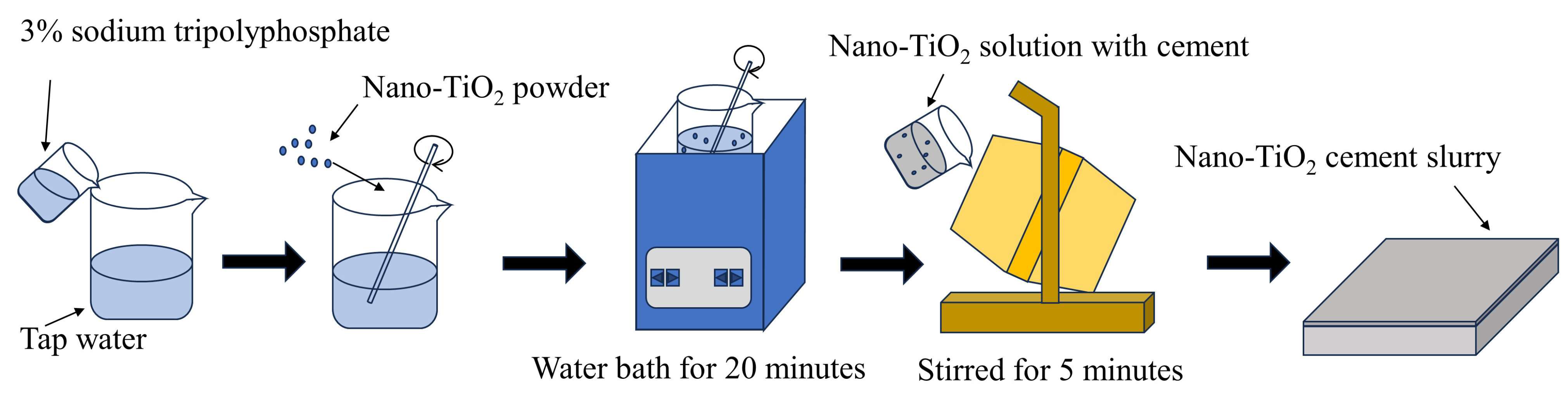
4.2. Fabrication of Nano-TiO2 Cement Slurry
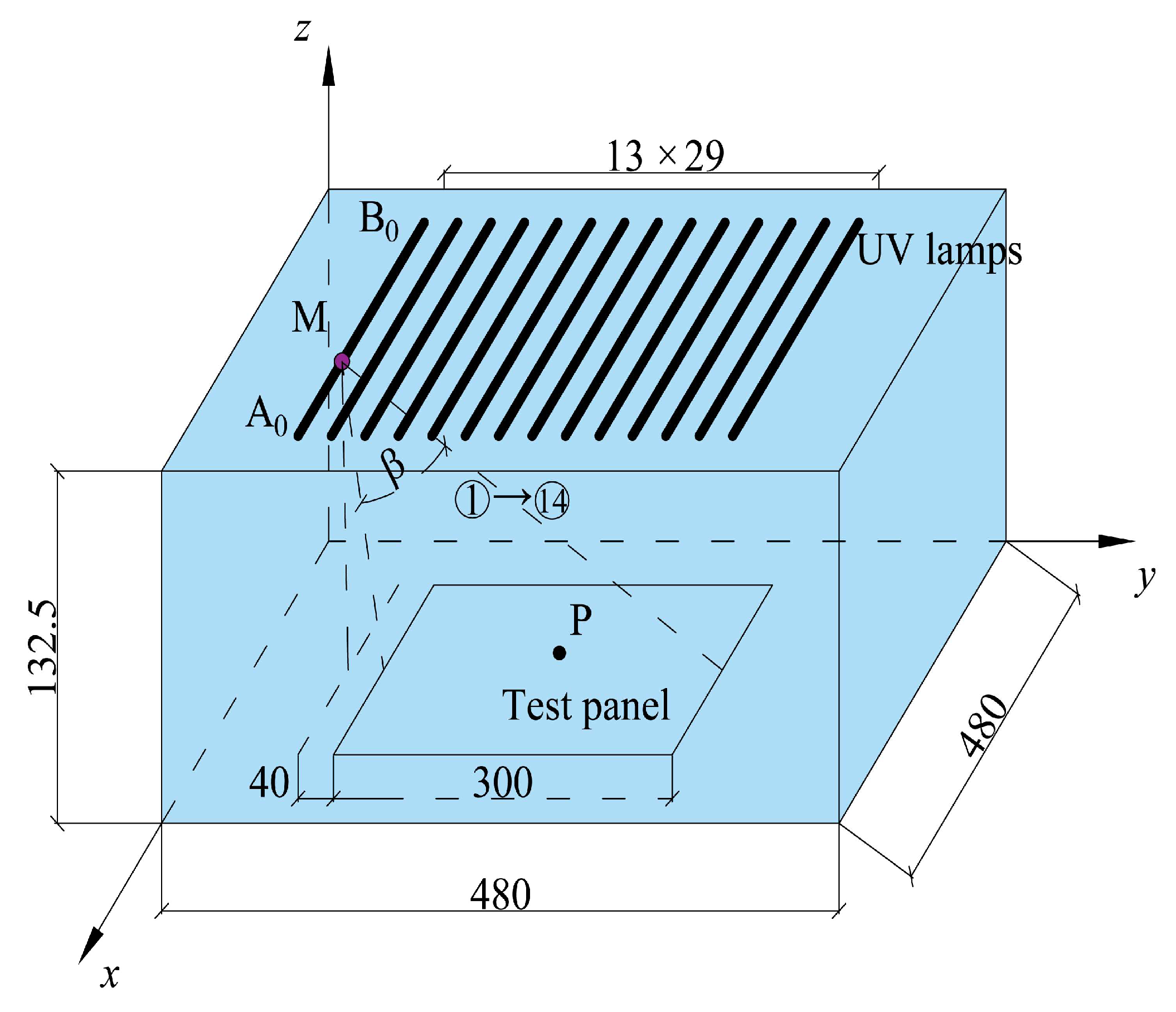
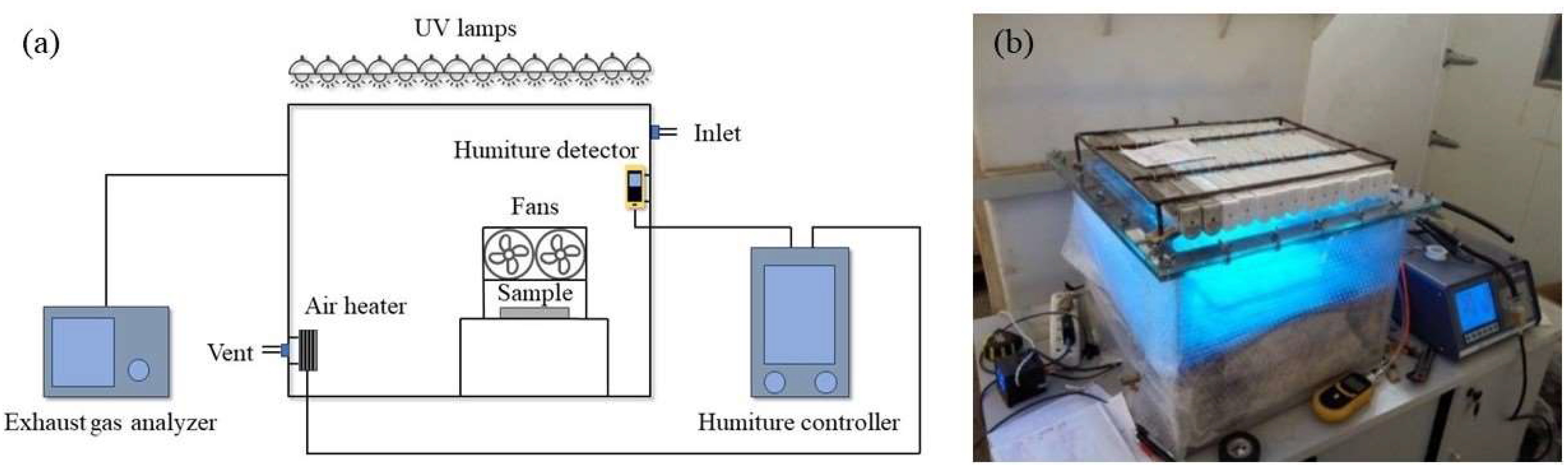
4.3. Degradation of Vehicle Exhaust Test
4.4. Degradation Efficiency Evaluation Index
5. Field Application of Nano-TiO2 Cement Slurry
5.1. Field Application of Nano-TiO2 Cement Slurry
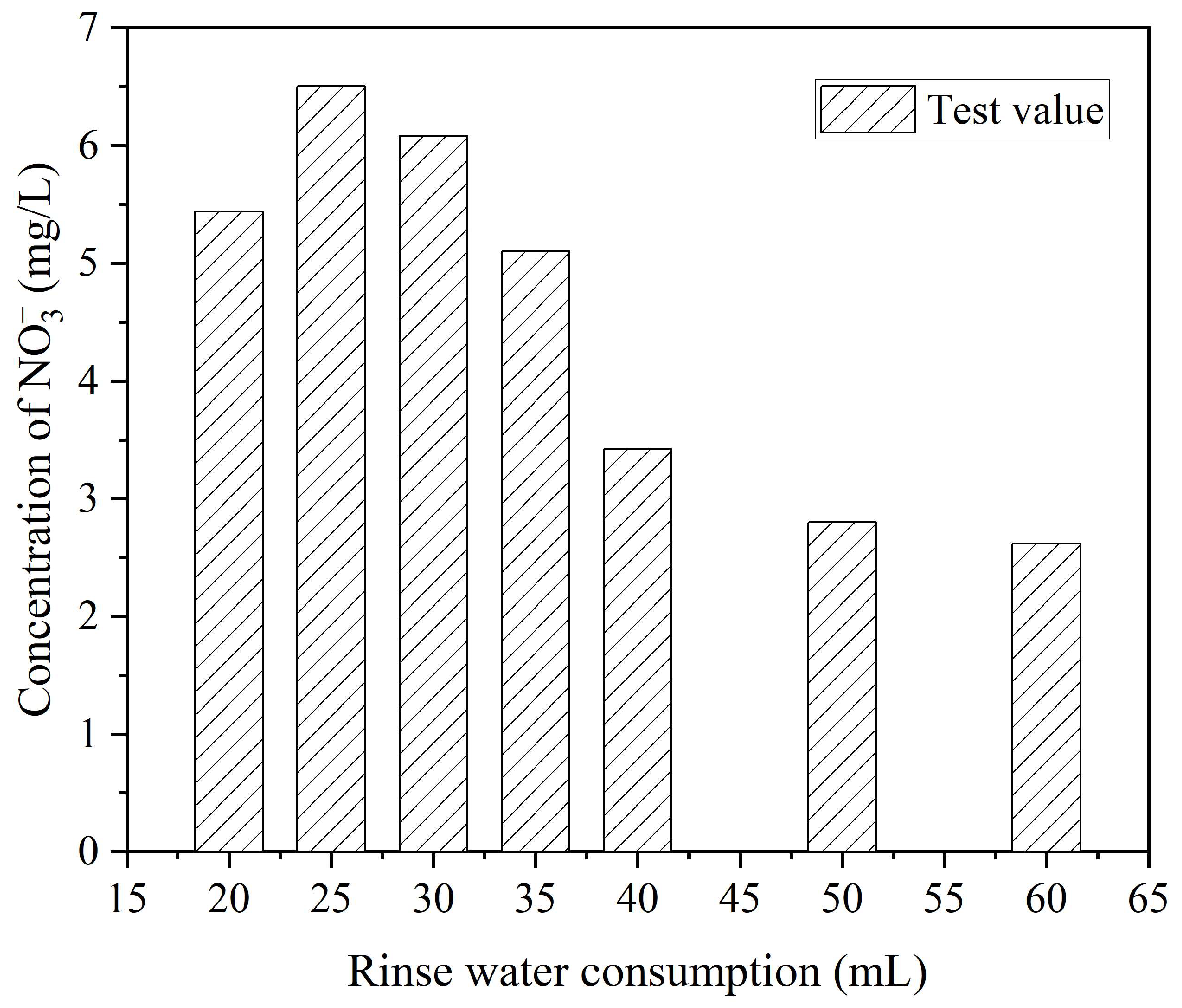
5.2. Determination of Optimal Rinsing Water Consumption
5.3. Evaluation of the Performance of Nano-TiO2 Cement Slurry for Field Application
6. Conclusions
- (1)
- The efficiency of NO2 photocatalytic degradation by nano-TiO2 cement slurry was significantly affected by temperature and UV illumination. There was an optimal temperature (28.8 °C in this test) at which the degradation efficiency was maximized. As the temperature increased, the degradation efficiency initially increased and then declined.
- (2)
- Excessive water molecules and dust on the cement surface will compete with NO2 for adsorption on nano-TiO2, thereby occupying specific active sites of the catalyst. Consequently, an increase in relative humidity (falling within the range of 64.1% to 81.8%) results in a reduced degradation efficiency of nano-TiO2 cement slurry for NO2.
- (3)
- The thickness of the nano-TiO2 cement slurry had only a minor impact on the efficiency of NO2 photocatalytic degradation by nano-TiO2 cement slurry. The photocatalytic properties of the samples were significantly diminished by surface dust and rain erosion.
- (4)
- The evaluation method based on photocatalytic products for assessing the photocatalytic performance of nano-TiO2 cement slurry in degrading vehicle exhaust is less affected by natural environmental factors. Field application has shown that the nano-TiO2 cement paste exhibits good performance in the photocatalytic degradation of vehicle exhaust. The efficiency of NO2 photocatalytic degradation by nano-TiO2 cement slurry was approximately 0.28 mg/m2/day.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zhang, T.; Jin, T.; Qi, J.; Liu, S.; Hu, J.; Wang, Z.; Li, Z.; Mao, H.; Xu, X. Influence of test cycle and fuel property on fuel consumption and exhaust emissions of a heavy-duty diesel engine. Energy 2022, 244, 122705. [Google Scholar] [CrossRef]
- Marques, B.; Kostenidou, E.; Valiente, A.M.; Vansevenant, B.; Sarica, T.; Fine, L.; Temime-Roussel, B.; Tassel, P.; Perret, P.; Liu, Y.; et al. Detailed Speciation of Non-Methane Volatile Organic Compounds in Exhaust Emissions from Diesel and Gasoline Euro 5 Vehicles Using Online and Offline Measurements. Toxics 2022, 10, 184. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Liu, K.; Chong, D.; Niu, D.; Lin, P.; Liu, X.; Niu, Y.; Jing, R. Evaluation of photocatalytic micro-surfacing mixture: Road performance, vehicle exhaust gas degradation capacity and environmental impacts. Constr. Build. Mater. 2022, 345, 128367. [Google Scholar] [CrossRef]
- Zhu, Y.; Pan, Z.; Jing, D.; Liang, H.; Cheng, J.; Li, D.; Zhou, X.; Lin, F.; Liu, H.; Pan, P.; et al. Association of air pollution, genetic risk, and lifestyle with incident adult-onset asthma: A prospective cohort study. Ecotoxicol. Environ. Saf. 2023, 257, 114922. [Google Scholar] [CrossRef] [PubMed]
- Bronte, O.; García-García, F.; Lee, D.-J.; Urrutia, I.; Uranga, A.; Nieves, M.; Martínez-Minaya, J.; Quintana, J.M.; Arostegui, I.; Zalacain, R.; et al. Impact of outdoor air pollution on severity and mortality in COVID-19 pneumonia. Sci. Total Environ. 2023, 894, 164877. [Google Scholar] [CrossRef] [PubMed]
- Tyukavkina, V.V.; Shchelokova, E.A.; Tsyryatyeva, A.V.; Kasikov, A.G. TiO2–SiO2 nanocomposites from technological wastes for self-cleaning cement composition. J. Build. Eng. 2021, 44, 102648. [Google Scholar] [CrossRef]
- Bej, D.; Chattaraj, N. Air pollution from vehicle-tailpipe emissions and diagnostic approaches through cyber–physical platform—A review. Microprocess. Microsyst. 2023, 98, 104805. [Google Scholar] [CrossRef]
- Pérez-Nicolás, M.; Navarro-Blasco, I.; Fernández, J.M.; Alvarez, J.I. Atmospheric NOx removal: Study of cement mortars with iron- and vanadium-doped TiO2 as visible light–sensitive photocatalysts. Constr. Build. Mater. 2017, 149, 257–271. [Google Scholar] [CrossRef]
- Huang, M.; Huang, Y.; Cao, J.; Tao, W. Study on mitigation of automobile exhaust pollution in an urban street canyon: Emission reduction and air cleaning street lamps. Build. Environ. 2021, 193, 107651. [Google Scholar] [CrossRef]
- Paul, L.A.; Burnett, R.T.; Kwong, J.C.; Hystad, P.; Donkelaar, A.; Bai, L.; Goldberg, M.S.; Lavigne, E.; Copes, R.; Martin, R.V.; et al. The impact of air pollution on the incidence of diabetes and survival among prevalent diabetes cases. Environ. Int. 2020, 134, 105333. [Google Scholar] [CrossRef]
- Tamayo, T.; Rathmann, W.; Stahl-Pehe, A.; Landwehr, S.; Sugiri, D.; Krämer, U.; Hermann, J.; Holl, R.; Rosenbauer, J. No adverse effect of outdoor air pollution on HbA1c in children and young adults with type 1 diabetes. Int. J. Hyg. Environ. Health 2016, 219, 349–355. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zhang, L.; Li, L.; Hu, Y.; Liu, J.; Xu, Y.; Luo, C.; Zheng, C. NO removal from flue gas using conventional imidazolium-based ionic liquids at high pressures. Energy Fuels 2018, 32, 6039–6048. [Google Scholar] [CrossRef]
- Huang, R.-J.; Zhang, Y.; Bozzetti, C.; Ho, K.-F.; Cao, J.-J.; Han, Y.; Daellenbach, K.R.; Slowik, J.G.; Platt, S.M.; Canonaco, F.; et al. High secondary aerosol contribution to particulate pollution during haze events in China. Nature 2014, 514, 218–222. [Google Scholar] [CrossRef] [PubMed]
- Mochida, I.; Korai, Y.; Shirahama, M.; Kawano, S.; Hada, T.; Seo, Y.; Yoshikawa, M.; Yasutake, A. Removal of SOx and NOx over activated carbon fibers. Carbon 2000, 38, 227–239. [Google Scholar] [CrossRef]
- Hooftman, N.; Messagie, M.; Joint, F.; Segard, J.-B.; Coosemans, T. In-life range modularity for electric vehicles: The environmental impact of a range-extender trailer system. Appl. Sci. 2018, 8, 1016. [Google Scholar] [CrossRef]
- Venu, H.; Subramani, L.; Dhana, R.V. Emission reduction in a DI diesel engine using exhaust gas recirculation (EGR) of palm biodiesel blended with TiO2 nano additives. Renew. Energy 2019, 140, 245–263. [Google Scholar] [CrossRef]
- Li, R.; Wu, J.; Liu, H.; Gao, Z.; Sun, H.; Ding, R.; Tang, T. Crowded urban traffic: Co-evolution among land development, population, roads and vehicle ownership. Nonlinear Dyn. 2019, 95, 2783–2795. [Google Scholar] [CrossRef]
- Lapidus, A.; Korolev, E.; Topchiy, D.; Kuzmina, T.; Shekhovtsova, S.; Shestakov, N. Self-cleaning cement-based building materials. Buildings 2022, 12, 606. [Google Scholar] [CrossRef]
- Zhang, Z.; Tan, J.; Cheng, L.; Yang, W. Carbon nano-layer coated TiO2 nanoparticles for efficient photocatalytic CO2 reduction into CH4 and CO. Ceram. Int. 2021, 47, 34106–34114. [Google Scholar] [CrossRef]
- Qian, G.; Yu, H.; Gong, X.; Zhao, L. Impact of Nano-TiO2 on the NO2 degradation and rheological performance of asphalt pavement. Constr. Build. Mater. 2019, 218, 53–63. [Google Scholar] [CrossRef]
- Li, R.; Xiao, F.; Amirkhanian, S.; You, Z.; Huang, J. Developments of nano materials and technologies on asphalt materials—A review. Constr. Build. Mater. 2017, 143, 633–648. [Google Scholar] [CrossRef]
- Jia, Z.; Zhao, Y.; Shi, J. Adsorption kinetics of the photocatalytic reaction of nano-TiO2 cement-based materials: A review. Constr. Build. Mater. 2023, 370, 130462. [Google Scholar] [CrossRef]
- Nada, A.A.; El Rouby, W.M.A.; Bekheet, M.F.; Antuch, M.; Weber, M.; Miele, P.; Viter, R.; Roualdes, S.; Millet, P.; Bechelany, M. Highly textured boron/nitrogen co-doped TiO2 with honeycomb structure showing enhanced visible-light photoelectrocatalytic activity. Appl. Surf. Sci. 2020, 505, 144419. [Google Scholar] [CrossRef]
- Guo, M.; Poon, C. Photocatalytic NO removal of concrete surface layers intermixed with TiO2. Build. Environ. 2013, 70, 102–109. [Google Scholar] [CrossRef]
- Dylla, H.; Hassan, M.M.; Osborn, D. Field evaluation of photocatalytic concrete pavements’ ability to remove nitrogen oxides. Trans. Res. Rec. 2012, 2290, 154–160. [Google Scholar] [CrossRef]
- Shen, S.; Burtou, M.; Jobson, B.; Haselbach, L. Pervious concrete with titanium dioxide as a photocatalyst compound for a greener urban road environment. Constr. Build. Mater. 2012, 35, 874–883. [Google Scholar] [CrossRef]
- Xia, H.; Liu, G.; Zhang, R.; Song, L.; Chen, H. The Photocatalytic Degradation of Vehicle Exhausts by an Fe/N/Co–TiO2 Waterborne Coating under Visible Light. Materials 2019, 12, 3378. [Google Scholar] [CrossRef]
- Witkowski, H.; Jarosławski, J.; Tryfon-Bojarska, A. Application of Photocatalytic Concrete Paving Blocks in Poland—Verification of Effectiveness of Nitric Oxides Reduction and Novel Test Method. Materials 2020, 13, 5183. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, X.; Niu, D.; Niu, Y.; Xia, H.; Wang, Y. Effect of Photocatalyst on Rheological Behavior and NO Degradation Capacity of Asphalt Binder. Catalysts 2023, 13, 1083. [Google Scholar] [CrossRef]
- Bhattu, M.; Singh, J. Recent advances in nanomaterials based sustainable approaches for mitigation of emerging organic pollutants. Chemosphere 2023, 321, 138072. [Google Scholar] [CrossRef]
- Das, S.; Mukherjee, A. Chapter 1—Nanotechnology as sustainable strategy for remediation of soil contaminants, air pollutants, and mitigation of food biodeterioration. In Environmental Applications of Microbial Nanotechnology; Elsevier: Amsterdam, The Netherlands, 2023; pp. 3–16. [Google Scholar] [CrossRef]
- Qian, C.; Zhao, L.; Wang, R. Effect of concentration of nitrogen oxidizes on photocatalytic oxidation efficiency of nano-TiO2 photocatalyst immobilized on cement-based material. Mater. Sci. Technol. 2007, 15, 582–585. (In Chinese) [Google Scholar]
- Luo, G.; Liu, H.; Li, W.; Lyu, X. Automobile Exhaust Removal Performance of Pervious Concrete with Nano TiO2 under Photocatalysis. Nanomaterials 2020, 10, 2088. [Google Scholar] [CrossRef] [PubMed]
- Jin, J.; Chen, B.; Liu, L.; Liu, R.; Qian, G.; Wei, H.; Zheng, J. A Study on Modified Bitumen with Metal Doped Nano-TiO2 Pillared Montmorillonite. Materials 2019, 12, 1910. [Google Scholar] [CrossRef] [PubMed]
- Kuang, Y.; Zhang, Z.; Ji, X.; Zhang, X. Study on nano-TiO2 photocatalytic cement paste with of automobile exhaust degradation performance. J. Funct. Mater. 2017, 48, 2241–2246. (In Chinese) [Google Scholar] [CrossRef]
- Jin, J.; Xiao, T.; Tan, Y.; Zheng, J.; Liu, R.; Qian, G.; Wei, H.; Zhang, J. Effects of TiO2 pillared montmorillonite nanocomposites on the properties of asphalt with exhaust catalytic capacity. J. Clean. Prod. 2018, 205, 339–349. [Google Scholar] [CrossRef]
- Saber, N.B.; Mezni, A.; Alrooqi, A.; Altalhi, T. Fabrication of efficient Au@TiO2/rGO heterojunction nanocomposite: Boosted photocatalytic activity under ultraviolet and visible light irradiation. J. Mater. Res. Technol. 2021, 12, 2238–2246. [Google Scholar] [CrossRef]
- Guo, M.; Chen, J.; Xia, M.; Wang, T.; Poon, C. Pathways of conversion of nitrogen oxides by nano TiO2 incorporated in cement-based materials. Build. Environ. 2018, 144, 412–418. [Google Scholar] [CrossRef]
- Liu, S.; Shi, Z. Study on catalytic decomposition of vehicle exhaust pavement materials by TiO2. Energy Rep. 2022, 8, 161–170. [Google Scholar] [CrossRef]
- Chen, C.; Tang, B.; Cao, X.; Gu, F.; Huang, W. Enhanced photocatalytic decomposition of NO on portland cement concrete pavement using nano-TiO2 suspension. Constr. Build. Mater. 2021, 275, 122135. [Google Scholar] [CrossRef]
- Xie, J.; Kuang, Y. Photocatalytic technology of nano-TiO2 and its application in photodegradation of automobile exhaust. Mater. Rep. 2012, 26, 141–145. (In Chinese) [Google Scholar] [CrossRef]
- Li, X.; Wang, F.; You, L.; Wu, S.; Yang, C.; Zhang, L.; Barbieri, D.M. A review on photocatalytic asphalt pavement designed for degradation of vehicle exhausts. Transp. Res. D Transp. Environ. 2023, 115, 103605. [Google Scholar] [CrossRef]
- Fan, W.; Chan, K.; Zhang, C.; Leung, M.K.H. Advanced solar photocatalytic asphalt for removal of vehicular NOx. Energy Procedia 2017, 143, 811–816. [Google Scholar] [CrossRef]
- Zhang, L.; Lu, Q.; Shan, R.; Zhang, F.; Muhammad, Y.; Huang, K. Photocatalytic degradation of vehicular exhaust by nitrogen-doped titanium dioxide modified pavement material. Transp. Res. D Transp. Environ. 2021, 91, 102690. [Google Scholar] [CrossRef]
- Ballari, M.M.; Yu, Q.L.; Brouwers, H.J.H. Experimental study of the NO and NO2 degradation by photocatalytically active concrete. Catal. Today 2011, 161, 175–180. [Google Scholar] [CrossRef]
- Lin, Y.T.; Weng, C.H.; Chen, F.Y. Key operating parameters affecting photocatalytic activity of visible-light-induced C-doped TiO2 catalyst for ethylene oxidation. Chem. Eng. J. 2014, 248, 175–183. [Google Scholar] [CrossRef]
- Xiao, L.; Chen, P.; Yang, W.; Zhao, X.; Dong, F. Photocatalytic reaction mechanisms at the gas–solid interface for environmental and energy applications. Catal. Sci. Technol. 2021, 11, 7807–7839. [Google Scholar] [CrossRef]
- Hager, S.; Bauer, R.; Kudielka, G. Photocatalytic oxidation of gaseous chlorinated organics over titanium dioxide. Chemosphere 2000, 41, 1219–1225. [Google Scholar] [CrossRef]
- Sánchez, B.; Cardona, A.I.; Romero, M.; Avila, P.; Bahamonde, A. Influence of temperature on gas-phase photo-assisted mineralization of TCE using tubular and monolit. Catal. Today 1999, 54, 369–377. [Google Scholar] [CrossRef]
- Qian, C.; Zhao, L.; Fu, D.; Li, L.; Wang, R. Study on the effect of temperature, humidity and light intensity on photocatalytic oxidation of NO2 by Nano-TiO2 immobilized on cement-based materials. Acta Sci. Circumstantiae 2005, 25, 623–630. (In Chinese) [Google Scholar] [CrossRef]
- Pellegrino, F.; Zangirolami, M.; Minero, C.; Maurino, V. Portable photoreactor for on-site measurement of the activity of photocatalytic surfaces. Catal. Today 2020, 340, 363–368. [Google Scholar] [CrossRef]
- Casagrande, C.A.; Repette, W.L.; Hotza, D. Effect of environmental conditions on degradation of NOx gases by photocatalytic nanotitania-based cement mortars after long-term hydration. J. Clean. Prod. 2020, 274, 123067. [Google Scholar] [CrossRef]
- Puddu, V.; Choi, H.; Dionysiou, D.D.; Li Puma, G. TiO2 photocatalyst for indoor air remediation: Influence of crystallinity, crystal phase, and UV radiation intensity on trichloroethylene degradation. Appl. Catal. B 2010, 94, 211–218. [Google Scholar] [CrossRef]
- Wang, K.H.; Tsai, H.H.; Hsieh, Y.H. A study of photocatalytic degradation of trichloroethylene in vapor phase on TiO2 photocatalyst. Chemosphere 1998, 36, 2763–2773. [Google Scholar] [CrossRef]
- Guo, M.Z.; Ling, T.C.; Poon, C.S. Photocatalytic NOx degradation of concrete surface layers intermixed and spray-coated with nano-TiO2: Influence of experimental factors. Cem. Concr. Compos. 2017, 83, 279–289. [Google Scholar] [CrossRef]
- Folli, A.; Strøm, M.; Madsen, T.P.; Henriksen, T.; Lang, J.; Emenius, J.; Klevebrant, T.; Nilsson, A. Field study of air purifying paving elements containing TiO2. Atmos. Environ. 2015, 107, 44–51. [Google Scholar] [CrossRef]
- GB/T 5750.5-2023; Standard Examination Methods for Drinking Water—Part 5: Inorganic Nonmetallic Indices. Standards Press of China: Beijing, China, 2023. (In Chinese)





| Item | Photograph | Object | Range | Precision |
|---|---|---|---|---|
| FGA-4100 auto exhaust gas analyzer |  | NO | 0–5000 ppm | 1 ppm |
| HD-P900 nitrogen dioxide detector |  | NO2 | 0–500 ppm | 0.01 ppm |
| Saipwell HG040-45W air heater |  | temperature | −45–80 °C | 0.1 °C |
| CSTH80 intelligent digital temperature and humidity controller |  | temperature and humidity | −19.9–99.9 °C 0.0–99.9% RH | 0.1 °C 0.1% RH |
| Test Days | Weather | Temperature (°C) | Relative Humidity (%) |
|---|---|---|---|
| Day 1 | sunny to cloudy | 3–15 | 63 |
| Day 2 | cloudy | 6–10 | 70 |
| Day 3 | sunny to cloudy | 5–12 | 64 |
| Day 4 | cloudy | 5–13 | 59 |
| Day 5 | cloudy | 8–12 | 54 |
| Day 6 | sunny | 4–15 | 66 |
| Day 7 | sunny | 6–15 | 52 |
| Day 8 | sunny to cloudy | 6–17 | 47 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kuang, Y.; Ding, F.; Peng, Z.; Fan, F.; Zhang, Z.; Ji, X. Photocatalytic Degradation of Vehicle Exhaust by Nano-TiO2 Cement Slurry: Experimental Factors and Field Application. Catalysts 2024, 14, 21. https://doi.org/10.3390/catal14010021
Kuang Y, Ding F, Peng Z, Fan F, Zhang Z, Ji X. Photocatalytic Degradation of Vehicle Exhaust by Nano-TiO2 Cement Slurry: Experimental Factors and Field Application. Catalysts. 2024; 14(1):21. https://doi.org/10.3390/catal14010021
Chicago/Turabian StyleKuang, Yachuan, Fuzheng Ding, Zhiwei Peng, Fan Fan, Zhaohuan Zhang, and Xiaoyong Ji. 2024. "Photocatalytic Degradation of Vehicle Exhaust by Nano-TiO2 Cement Slurry: Experimental Factors and Field Application" Catalysts 14, no. 1: 21. https://doi.org/10.3390/catal14010021
APA StyleKuang, Y., Ding, F., Peng, Z., Fan, F., Zhang, Z., & Ji, X. (2024). Photocatalytic Degradation of Vehicle Exhaust by Nano-TiO2 Cement Slurry: Experimental Factors and Field Application. Catalysts, 14(1), 21. https://doi.org/10.3390/catal14010021






