Ru-Loaded Biphasic TiO2 Nanosheet-Tubes Enriched with Ti3+ Defects and Directionally Deficient Electrons as Highly Efficient Catalysts in Benzene Selective Hydrogenation
Abstract
:1. Introduction
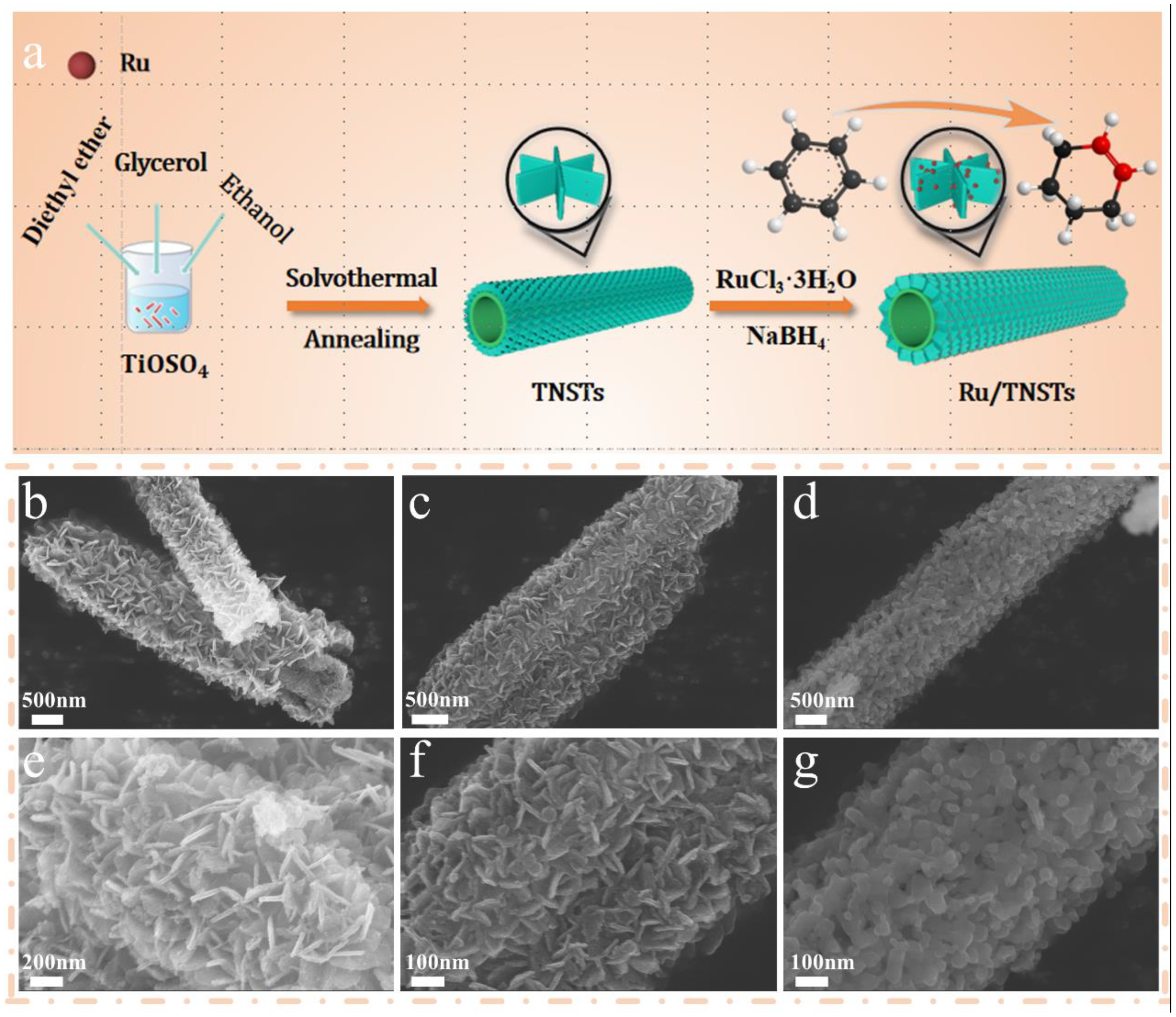
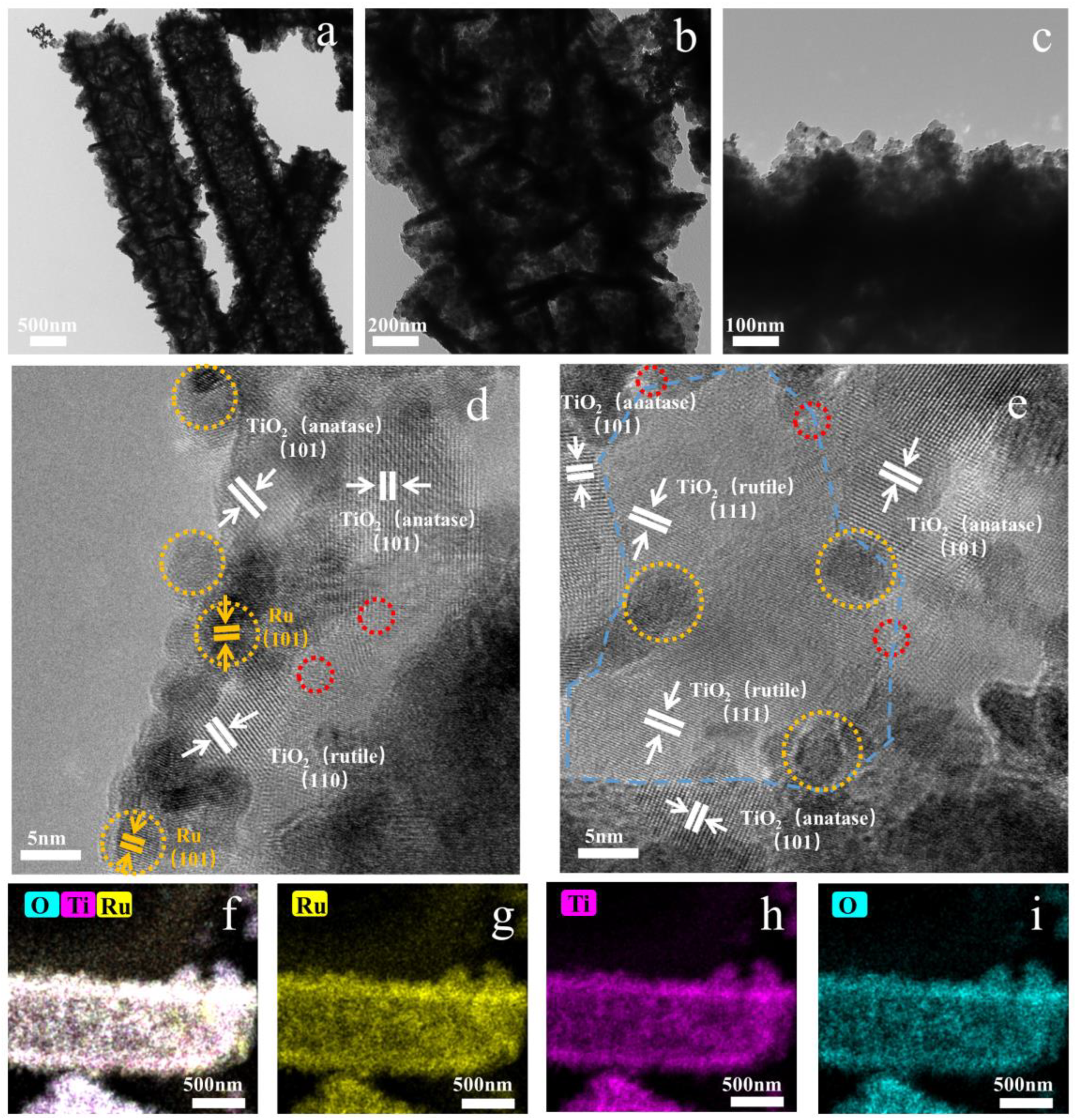
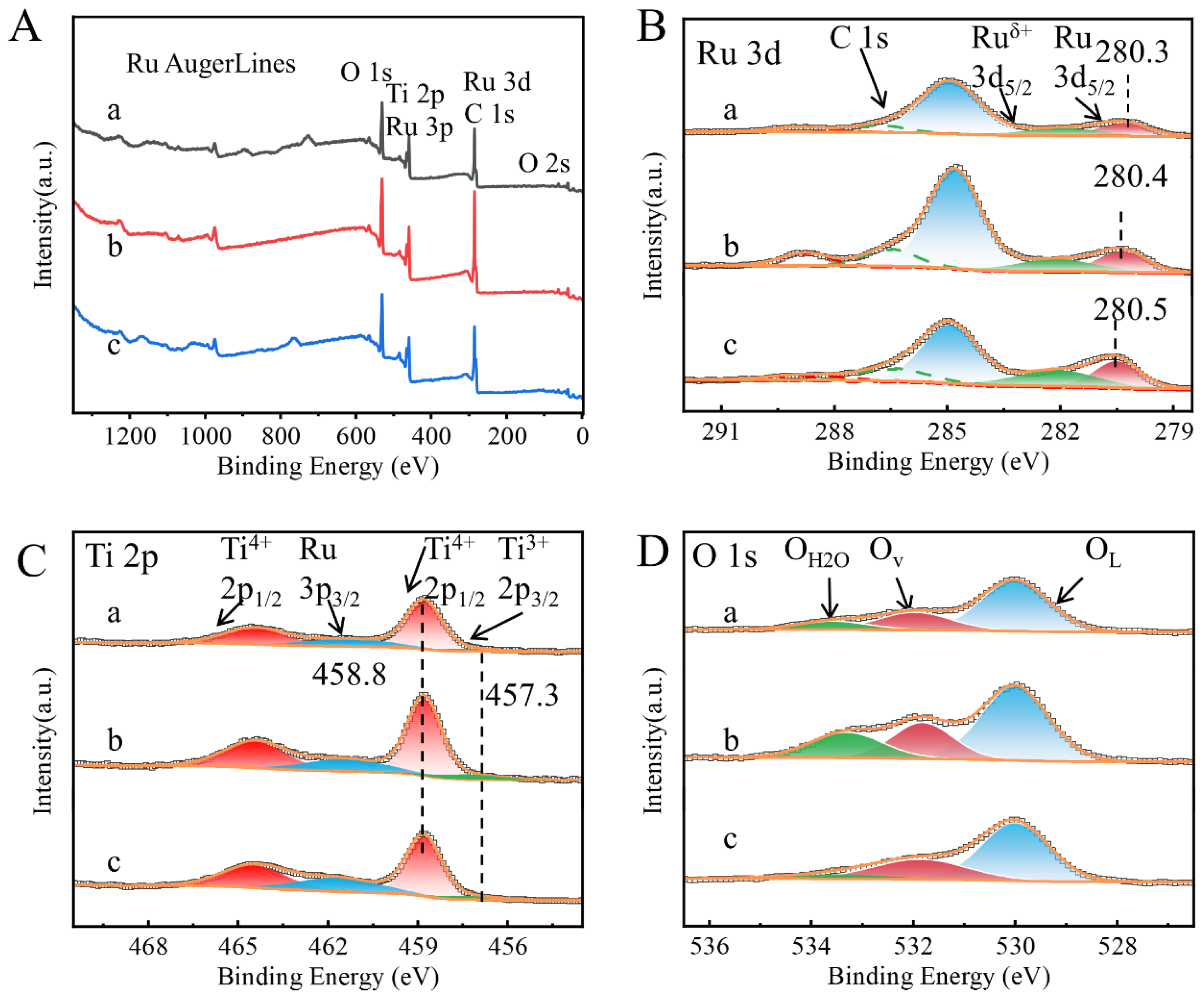
2. Results and Discussion
3. Experimental Section
3.1. Synthesis of TiO2 Nanosheet-Assembled Nanotubes (TNSTs)
3.2. Preparation of TNSTs-Supported Ru Catalysts
3.3. Catalytic Testing
3.4. Instrumentation and Characterization
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Pu, J.-C.; Doka Dari, M.; Tang, X.-Q.; Yuan, P.-Q. Diffusion of benzene through water film confined in silica mesopores: Effect of competitive adsorption of solvent. Chem. Eng. Sci. 2020, 224, 115793. [Google Scholar] [CrossRef]
- Spod, H.; Lucas, M.; Claus, P. Selective Hydrogenation of Benzene to Cyclohexene over 2Ru/La2O3-ZnO Catalyst without Additional Modifiers. ChemCatChem 2016, 8, 2659–2666. [Google Scholar] [CrossRef]
- Melgo, M.S.; Lindner, A.; Schuchardt, U. Wacker oxidation of cyclohexene in the presence of Pd(NO3)2/CuSO4/H3PMo12O40. Appl. Catal. A Gen. 2004, 273, 217–221. [Google Scholar] [CrossRef]
- Chen, Z.; Sun, H.; Peng, Z.; Gao, J.; Li, B.; Liu, Z.; Liu, S. Selective Hydrogenation of Benzene: Progress of Understanding for the Ru-Based Catalytic System Design. Ind. Eng. Chem. Res. 2019, 58, 13794–13803. [Google Scholar] [CrossRef]
- Foppa, L.; Dupont, J. Benzene partial hydrogenation: Advances and perspectives. Chem. Soc. Rev. 2015, 44, 1886–1897. [Google Scholar] [CrossRef]
- He, H.; Meyer, R.J.; Rioux, R.M.; Janik, M.J. Catalyst Design for Selective Hydrogenation of Benzene to Cyclohexene through Density Functional Theory and Microkinetic Modeling. ACS Catal. 2021, 11, 11831–11842. [Google Scholar] [CrossRef]
- Nagahara, H.; Ono, M.; Konishi, M.; Fukuoka, Y. Partial hydrogenation of benzene to cyclohexene. Appl. Surf. Sci. 1997, 121–122, 448–451. [Google Scholar] [CrossRef]
- Jiang, L.; Zhou, G. Promoting the performances of Ru on hierarchical TiO2 nanospheres exposed {0 0 1} facets in benzene semi-hydrogenation by manipulating the metal-support interfaces. J. Catal. 2020, 382, 97–108. [Google Scholar] [CrossRef]
- Sun, H.; Fan, Y.; Sun, X.; Chen, Z.; Li, H.; Peng, Z.; Liu, Z. Effect of ZnSO4, MnSO4 and FeSO4 on the Partial Hydrogenation of Benzene over Nano Ru-Based Catalysts. Int. J. Mol. Sci. 2021, 22, 7756. [Google Scholar] [CrossRef]
- Peng, Z.; Liu, X.; Lin, H.; Wang, Z.; Li, Z.; Li, B.; Liu, Z.; Liu, S. Surface engineering on a nanocatalyst: Basic zinc salt nanoclusters improve catalytic performances of Ru nanoparticles. J. Mater. Chem. A 2016, 4, 17694–17703. [Google Scholar] [CrossRef]
- Zhong, Z.; Luo, B.; Lin, C.; Yin, T.; Tian, Z.; Wang, C.; Chen, Y.; Wu, Y.; Shu, R. Ultrafast microfluidic preparation of highly dispersed Ru/TiO2 catalyst for the hydrodeoxygenation of lignin-derived phenolic compounds. Fuel 2023, 340, 127567. [Google Scholar] [CrossRef]
- Zhang, X.; Luo, L.; Yun, R.; Pu, M.; Zhang, B.; Xiang, X. Increasing the Activity and Selectivity of TiO2-Supported Au Catalysts for Renewable Hydrogen Generation from Ethanol Photoreforming by Engineering Ti3+ Defects. ACS Sustain. Chem. Eng. 2019, 7, 13856–13864. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, X.; Yan, Z.; Xu, C.; Zhang, W.; Ban, H.; Li, C. Activation reconstructing CuZnO/SiO2 catalyst for CO2 hydrogenation. J. Catal. 2022, 412, 10–20. [Google Scholar] [CrossRef]
- Wang, S.; Feng, K.; Zhang, D.; Yang, D.; Xiao, M.; Zhang, C.; He, L.; Yan, B.; Ozin, G.A.; Sun, W. Stable Cu Catalysts Supported by Two-dimensional SiO(2) with Strong Metal-Support Interaction. Adv. Sci. 2022, 9, e2104972. [Google Scholar] [CrossRef] [PubMed]
- Diao, Y.; Wang, H.; Chen, B.; Zhang, X.; Shi, C. Modulating morphology and textural properties of Al2O3 for supported Ni catalysts toward plasma-assisted dry reforming of methane. Appl. Catal. B Environ. 2023, 330, 122573. [Google Scholar] [CrossRef]
- Muravev, V.; Simons, J.F.M.; Parastaev, A.; Verheijen, M.A.; Struijs, J.J.C.; Kosinov, N.; Hensen, E.J.M. Operando Spectroscopy Unveils the Catalytic Role of Different Palladium Oxidation States in CO Oxidation on Pd/CeO(2) Catalysts. Angew. Chem. Int. Ed. Engl. 2022, 61, e202200434. [Google Scholar] [CrossRef] [PubMed]
- Tsiotsias, A.I.; Hafeez, S.; Charisiou, N.D.; Al-Salem, S.M.; Manos, G.; Constantinou, A.; AlKhoori, S.; Sebastian, V.; Hinder, S.J.; Baker, M.A.; et al. Selective catalytic deoxygenation of palm oil to produce green diesel over Ni catalysts supported on ZrO2 and CeO2–ZrO2: Experimental and process simulation modelling studies. Renew. Energy 2023, 206, 582–596. [Google Scholar] [CrossRef]
- Hu, X.; Fan, Q.; Tan, M.; Luo, Y.; Wu, X.; Manuputty, M.Y.; Ding, J.; Choksi, T.S.; Kraft, M.; Xu, R.; et al. Investigating the impact of dynamic structural changes of Au/rutile catalysts on the catalytic activity of CO oxidation. Carbon. Energy 2023, e412. [Google Scholar] [CrossRef]
- Wang, K.; He, S.; Lin, Y.; Chen, X.; Dai, W.; Fu, X. Photo-enhanced thermal catalytic CO2 methanation activity and stability over oxygen-deficient Ru/TiO2 with exposed TiO2 {001} facets: Adjusting photogenerated electron behaviors by metal-support interactions. Chin. J. Catal. 2022, 43, 391–402. [Google Scholar] [CrossRef]
- Wang, F.; Kishimoto, H.; Develos-Bagarinao, K.; Yamaji, K.; Horita, T.; Yokokawa, H. Encroachment of titanium oxide on Ni surface for Ni/TiO2 under reducing atmosphere. Solid. State Ion. 2016, 288, 130–134. [Google Scholar] [CrossRef]
- Shi, R.; Wang, X.; Zhou, G. Electronic metal—Support interaction directed electron-deficient nanoparticulate Ru on Ti3C2 MXene-derived TiO2 nanoflowers for robust benzene semi-hydrogenation. Appl. Surf. Sci. 2023, 624, 157159. [Google Scholar] [CrossRef]
- Zhou, G.; Wang, F.; Shi, R. Nanoparticulate Ru on morphology-manipulated and Ti3+ defect-riched TiO2 nanosheets for benzene semi-hydrogenation. J. Catal. 2021, 398, 148–160. [Google Scholar] [CrossRef]
- Zhan, Y.; Zhou, C.; Jin, F.; Chen, C.; Jiang, L. Ru/TiO2 catalyst for selective hydrogenation of benzene: Effect of surface hydroxyl groups and spillover hydrogen. Appl. Surf. Sci. 2020, 525, 146627. [Google Scholar] [CrossRef]
- Song, Y.; Sun, Z.; Fan, G.; Yang, L.; Li, F. Regulating Surface-Interface Structures of Zn-Incorporated LiAl-LDH Supported Ru Catalysts for Efficient Benzene Hydrogenation to Produce Cyclohexene. ChemCatChem 2022, 14, e202200125. [Google Scholar] [CrossRef]
- Zhou, G.; Jiang, L.; He, D. Ru nanoparticles on TiO2 with various anatase-to-rutile ratios tuned by selective chemical dissolution: Effect of support polymorph composition on selective benzene hydrogenation. Appl. Catal. A Gen. 2019, 575, 65–73. [Google Scholar] [CrossRef]
- Zhou, G.; Dou, R.; Bi, H.; Xie, S.; Pei, Y.; Fan, K.; Qiao, M.; Sun, B.; Zong, B. Ru nanoparticles on rutile/anatase junction of P25 TiO2: Controlled deposition and synergy in partial hydrogenation of benzene to cyclohexene. J. Catal. 2015, 332, 119–126. [Google Scholar] [CrossRef]
- Jiang, L.; Zhou, G. Distinguishing the roles of TiO2 {1 0 1}, {0 0 1}, and {0 1 0} facets in benzene semi-hydrogenation over Ru/TiO2 catalysts. Appl. Surf. Sci. 2021, 535, 147709. [Google Scholar] [CrossRef]
- Lin, X.; Sun, M.; Gao, B.; Ding, W.; Zhang, Z.; Anandan, S.; Umar, A. Hydrothermally regulating phase composition of TiO2 nanocrystals toward high photocatalytic activity. J. Alloys Compd. 2021, 850, 156653. [Google Scholar] [CrossRef]
- Elsellami, L.; Dappozze, F.; Fessi, N.; Houas, A.; Guillard, C. Highly photocatalytic activity of nanocrystalline TiO2 (anatase, rutile) powders prepared from TiCl4 by sol–gel method in aqueous solutions. Process Saf. Environ. Prot. 2018, 113, 109–121. [Google Scholar] [CrossRef]
- Ma, Y.-J.; Lu, F.; Xiang, B.-X.; Zhao, J.-L.; Ruan, S.-C. Fabrication of TiO2 thin films with both anatase and rutile structures together using the ion-implantation method. Opt. Mater. Express 2018, 8, 532–540. [Google Scholar] [CrossRef]
- Zhang, X.; Hu, W.; Zhang, K.; Wang, J.; Sun, B.; Li, H.; Qiao, P.; Wang, L.; Zhou, W. Ti3+ Self-Doped Black TiO2 Nanotubes with Mesoporous Nanosheet Architecture as Efficient Solar-Driven Hydrogen Evolution Photocatalysts. ACS Sustain. Chem. Eng. 2017, 5, 6894–6901. [Google Scholar] [CrossRef]
- Zhu, F.; Wen, J.; Guo, H.; An, J.; Wang, G.; Ren, G.; Ma, X. Low-temperature catalytic performance improvement of Ru/TiO2{001} for o-dichlorobenzene oxidation. Chem. Eng. J. 2023, 473, 145186. [Google Scholar] [CrossRef]
- Lin, W.; Chen, Y.; Zhang, Y.; Zhang, Y.; Wang, J.; Wang, L.; Xu, C.C.; Nie, R. Surface Synergetic Effects of Ni–ReOx for Promoting the Mild Hydrogenation of Furfural to Tetrahydrofurfuryl Alcohol. ACS Catal. 2023, 13, 11256–11267. [Google Scholar] [CrossRef]
- Wang, X.; Wang, Z.; Zhou, L.; Liu, Y.; Yang, Y.; Zhang, L.; Shang, Z.; Li, H.; Xiao, T.; Zhang, C.; et al. Efficient hydrodeoxygenation of guaiacol to phenol over Ru/Ti–SiO2 catalysts: The significance of defect-rich TiOx species. Green. Chem. 2022, 24, 5822–5834. [Google Scholar] [CrossRef]
- Wu, T.; Zhao, H.; Zhu, X.; Xing, Z.; Liu, Q.; Liu, T.; Gao, S.; Lu, S.; Chen, G.; Asiri, A.M.; et al. Identifying the Origin of Ti3+ Activity toward Enhanced Electrocatalytic N2 Reduction over TiO2 Nanoparticles Modulated by Mixed-Valent Copper. Adv. Mater. 2020, 32, e2000299. [Google Scholar] [CrossRef]
- Hu, J.; Zhang, S.; Cao, Y.; Wang, H.; Yu, H.; Peng, F. Novel Highly Active Anatase/Rutile TiO2 Photocatalyst with Hydrogenated Heterophase Interface Structures for Photoelectrochemical Water Splitting into Hydrogen. ACS Sustain. Chem. Eng. 2018, 6, 10823–10832. [Google Scholar] [CrossRef]
- Lan, K.; Wang, R.; Wei, Q.; Wang, Y.; Hong, A.; Feng, P.; Zhao, D. Stable Ti3+ Defects in Oriented Mesoporous Titania Frameworks for Efficient Photocatalysis. Angew. Chem. Int. Ed. 2020, 59, 17676–17683. [Google Scholar] [CrossRef]
- Wu, P.; Lyu, S.; Tian, Y.; Zhao, D.; Ye, J.; She, M.; Song, S.; Ding, T.; Li, X. Identification of active sites for preferential oxidation of CO over Ru/TiO2 catalysts via tuning metal–support interaction. Chem. Eng. J. 2023, 475, 146051. [Google Scholar] [CrossRef]
- Zhao, Z.; Jiang, Q.; Wang, Q.; Wang, M.; Zuo, J.; Chen, H.; Kuang, Q.; Xie, Z. Effect of Rutile Content on the Catalytic Performance of Ru/TiO2 Catalyst for Low-Temperature CO2 Methanation. ACS Sustain. Chem. Eng. 2021, 9, 14288–14296. [Google Scholar] [CrossRef]
- Qiu, J.-Y.; Feng, H.-Z.; Chen, Z.-H.; Ruan, S.-H.; Chen, Y.-P.; Xu, T.-T.; Su, J.-Y.; Ha, E.-N.; Wang, L.-Y. Selective introduction of surface defects in anatase TiO2 nanosheets for highly efficient photocatalytic hydrogen generation. Rare Met. 2022, 41, 2074–2083. [Google Scholar] [CrossRef]
- Chen, L.-N.; Wang, S.-H.; Zhang, P.-Y.; Chen, Z.-X.; Lin, X.; Yang, H.-J.; Sheng, T.; Lin, W.-F.; Tian, N.; Sun, S.-G.; et al. Ru nanoparticles supported on partially reduced TiO2 as highly efficient catalyst for hydrogen evolution. Nano Energy 2021, 88, 106211. [Google Scholar] [CrossRef]
- Tang, M.; Tong, Q.; Li, Y.; Jiang, R.; Shi, L.; Shen, F.; Wei, Y.; Liu, Z.; Liu, S.; Zhang, J.; et al. Effective and selective electrocatalytic nitrate reduction to ammonia on urchin-like and defect-enriched titanium oxide microparticles. Chin. Chem. Lett. 2023, 34, 108410. [Google Scholar] [CrossRef]
- Zhou, G.; Dong, Y.; He, D. Bimetallic Ru–M/TiO2 (M = Fe, Ni, Cu, Co) nanocomposite catalysts facribated by galvanic replacement: Structural elucidation and catalytic behavior in benzene selective hydrogenation. Appl. Surf. Sci. 2018, 456, 1004–1013. [Google Scholar] [CrossRef]
- Hao, F.; Zheng, J.; Ouyang, D.; Xiong, W.; Liu, P.; Luo, H. Selective hydrogenation of benzene over Ru supported on surface modified TiO2. Korean J. Chem. Eng. 2021, 38, 736–746. [Google Scholar] [CrossRef]
- Zhou, G.; Jiang, L.; Dong, Y.; Li, R.; He, D. Engineering the exposed facets and open-coordinated sites of brookite TiO2 to boost the loaded Ru nanoparticle efficiency in benzene selective hydrogenation. Appl. Surf. Sci. 2019, 486, 187–197. [Google Scholar]
- Jiang, L.; Dong, Y.; Zhou, G.; Li, R.; He, D. Promoting the Performances of TiO2 Submicrosphere-Embedded Ru Nanoparticles in Benzene Selective Hydrogenation by Morphology Manipulation. Ind. Eng. Chem. Res. 2019, 59, 1083–1092. [Google Scholar] [CrossRef]
- Zhou, G.; Jiang, L.; He, D. Nanoparticulate Ru on TiO2 exposed the {100} facets: Support facet effect on selective hydrogenation of benzene to cyclohexene. J. Catal. 2019, 369, 352–362. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, M.; Yang, H. Tuning Biphasic Catalysis Reaction with a Pickering Emulsion Strategy Exemplified by Selective Hydrogenation of Benzene. ChemCatChem 2018, 10, 5224–5230. [Google Scholar] [CrossRef]
- Yu, X.-L.; Li, Y.; Xin, S.-M.; Yuan, P.-Q.; Yuan, W.-K. Partial Hydrogenation of Benzene to Cyclohexene on Ru@XO2 (X = Ti, Zr, or Si). Ind. Eng. Chem. Res. 2018, 57, 1961–1967. [Google Scholar] [CrossRef]
- Xue, X.; Liu, J.; Rao, D.; Xu, S.; Bing, W.; Wang, B.; He, S.; Wei, M. Double-active site synergistic catalysis in Ru–TiO2 toward benzene hydrogenation to cyclohexene with largely enhanced selectivity. Catal. Sci. Technol. 2017, 7, 650–657. [Google Scholar]
- Wu, T.; Zhang, P.; Jiang, T.; Yang, D.; Han, B. Enhancing the selective hydrogenation of benzene to cyclohexene over Ru/TiO2 catalyst in the presence of a very small amount of ZnO. Sci. China Chem. 2014, 58, 93–100. [Google Scholar]
- Liu, S.C.; Guo, Y.Q.; Yang, X.L.; Ji, Y.L.; Luo, G. Kinetic Equations for Liquid-Phase Selective Hydrogenation of Benzene to Cyclohexene. Chin. J. Catal. 2003, 24, 42–461. [Google Scholar]
- Fan, C.; Zhu, Y.-A.; Zhou, X.-G.; Liu, Z.-P. Catalytic hydrogenation of benzene to cyclohexene on Ru(0001) from density functional theory investigations☆. Catal. Today 2011, 160, 234–241. [Google Scholar] [CrossRef]
- Zhang, P.; Wu, T.; Jiang, T.; Wang, W.; Liu, H.; Fan, H.; Zhang, Z.; Han, B. Ru–Zn supported on hydroxyapatite as an effective catalyst for partial hydrogenation of benzene. Green. Chem. 2013, 15, 152–159. [Google Scholar] [CrossRef]
- Fan, G.-Y.; Jiang, W.-D.; Wang, J.-B.; Li, R.-X.; Chen, H.; Li, X.-J. Selective hydrogenation of benzene to cyclohexene over RuCoB/γ-Al2O3 without additive. Catal. Commun. 2008, 10, 98–102. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, S.; Chen, X.; Xiong, S.; Zhang, X.; Hou, L.; Zhang, Q.; Wang, Y.; Gao, F. Ru-Loaded Biphasic TiO2 Nanosheet-Tubes Enriched with Ti3+ Defects and Directionally Deficient Electrons as Highly Efficient Catalysts in Benzene Selective Hydrogenation. Catalysts 2024, 14, 31. https://doi.org/10.3390/catal14010031
Wang S, Chen X, Xiong S, Zhang X, Hou L, Zhang Q, Wang Y, Gao F. Ru-Loaded Biphasic TiO2 Nanosheet-Tubes Enriched with Ti3+ Defects and Directionally Deficient Electrons as Highly Efficient Catalysts in Benzene Selective Hydrogenation. Catalysts. 2024; 14(1):31. https://doi.org/10.3390/catal14010031
Chicago/Turabian StyleWang, Shuo, Xianrui Chen, Shuangsheng Xiong, Xiaoting Zhang, Li Hou, Qian Zhang, Yatao Wang, and Faming Gao. 2024. "Ru-Loaded Biphasic TiO2 Nanosheet-Tubes Enriched with Ti3+ Defects and Directionally Deficient Electrons as Highly Efficient Catalysts in Benzene Selective Hydrogenation" Catalysts 14, no. 1: 31. https://doi.org/10.3390/catal14010031
APA StyleWang, S., Chen, X., Xiong, S., Zhang, X., Hou, L., Zhang, Q., Wang, Y., & Gao, F. (2024). Ru-Loaded Biphasic TiO2 Nanosheet-Tubes Enriched with Ti3+ Defects and Directionally Deficient Electrons as Highly Efficient Catalysts in Benzene Selective Hydrogenation. Catalysts, 14(1), 31. https://doi.org/10.3390/catal14010031




