Assessment of Reaction Kinetics for the Dehydrogenation of Perhydro-Dibenzyltoluene Using Mg- and Zn-Modified Pt/Al2O3 Catalysts
Abstract
:1. Introduction
2. Results and Discussion
2.1. Evaluation of Catalytic Performance for Dehydrogenation of H18-DBT Using a Batch Reactor
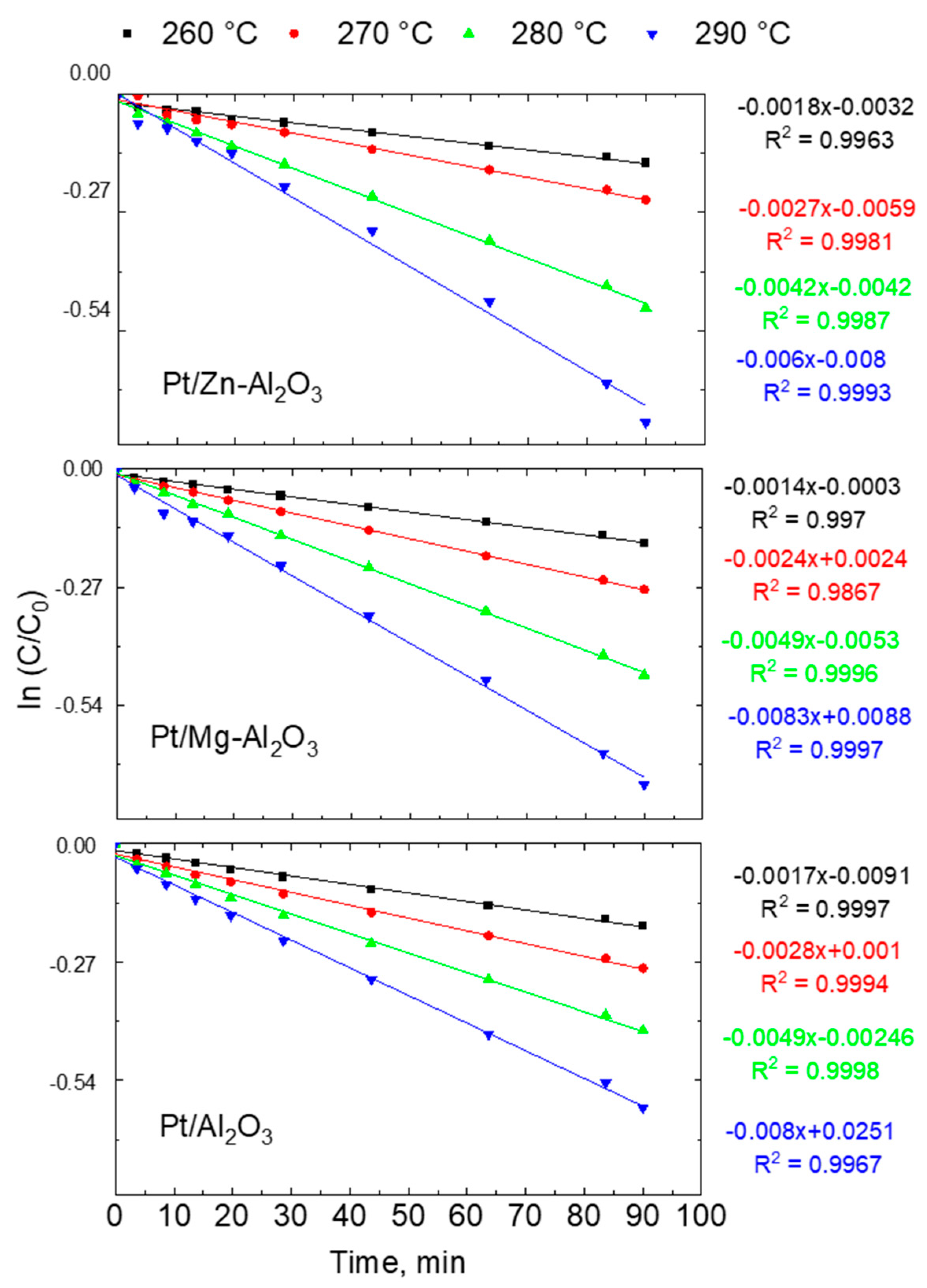
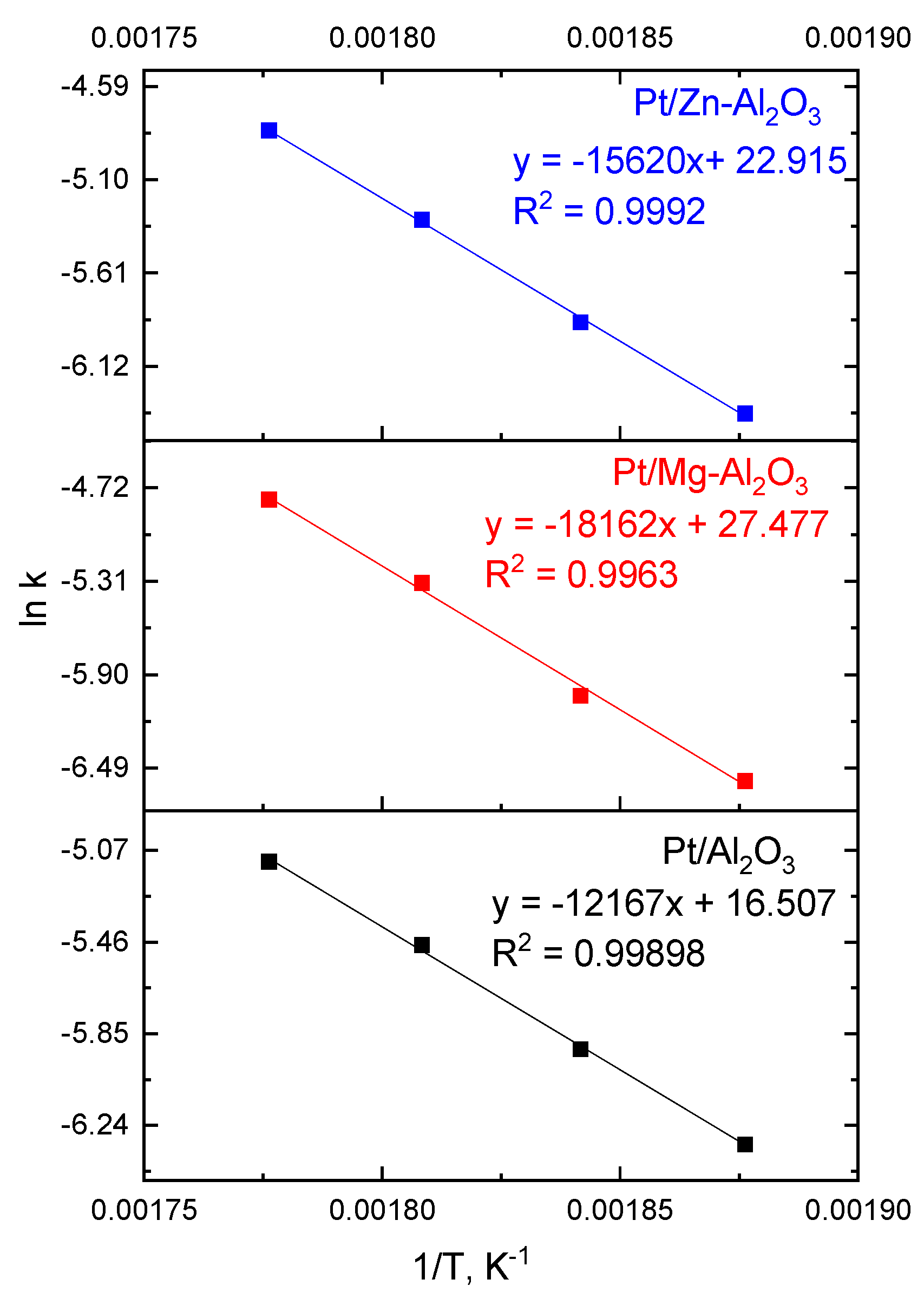
2.2. Reaction Kinetics for Dehydrogenation of H18-DBT
| Reference | Reactor Type | Catalyst | Conditions | Kinetic Values | ||
|---|---|---|---|---|---|---|
| n | k0 | Ea | ||||
| This study | Batch | 0.5 wt % Pt/Al2O3 | 260–290 °C; 0.2 mol % | 1 | 1.77 × 107 | 102 |
| 0.5 wt % Pt/Mg-Al2O3 | 1 | 2.30 × 1011 | 151 | |||
| 0.5 wt % Pt/Zn-Al2O3 | 1 | 1.15 × 1010 | 131 | |||
| [12] | Batch | 1 wt % Pt/Al2O3 | 260–290 °C; 0.4 mol % | 1 | 2.21 × 1016 | 205 |
| 1 wt % Pd/Al2O3 | 1 | 2.26 × 104 | 84 | |||
| 1 wt % Pt-Pd/Al2O3 | 1 | 2.35 × 102 | 66 | |||
| [18] | Batch | 1 wt % Pt/Al2O3 | 300–355 °C; 0.2 mol % | 1 | 1.76 × 1016 | 201 |
| [19] | Fixed bed | 0.3 wt % Pt/Al2O3 | 280–300 °C, 1 bar (2.5 bar) | 2 | 6.49 × 105 (2.66 × 108) | 117(149) |
| [15] | Batch | 0.3 wt % Pt/TiO2 | 270–310 °C; 0.1 mol % | 1.66 | 6.16 × 109 | 145 |
| 0.3 wt % Pt (0.1 wt % S)/TiO2 | 270–310 °C; 0.1 mol % | 1.51 | 3.23 × 1012 | 174 | ||
| [22] | Fixed bed | Pt/Al2O3 | 260–310 °C | 1 | 125 s−1 | 120 |
| [20] | Pd/Ag membrane | Pt/Al2O3 | 300–350 °C | 1 | 2.637 × 10−6 m3/kgcat.s | 156 ± 28.5 |
| [32] | Batch | 0.3 wt % Pt/Al2O3 | 280–310 °C; 0.05 mol %; di 11 nm | - | 1.60 × 1010 | 151 |
| 0.3 wt % Pt/Al2O3 | 280–310 °C; 0.05 mol %; di 14 nm | - | 1.50 × 1011 | 161 | ||
| 0.3 wt %Pt/Al2O3 | 280–310 °C; 0.05 mol %; di 18 nm | - | 1.30 × 1012 | 169 | ||
| [21] | Batch | 0.5 wt % Pt/Al2O3 | 270–320 °C; 0.05 mol % | 1.84 | 8.30 × 10 | 97 |
| 0.3 wt % Pt/Al2O3 | 270–320 °C; 0.05 mol % | 2.21 | 2.27 × 104 | 131 | ||
| 0.3 wt % Pt (0.14 wt % S)/Al2O3 | 270–320 °C; 0.05 mol % | 1.62 | 6.76 × 106 | 143 | ||
| [23] | Fixed bed | 5 wt % Pt/Al2O3 | 250–320 °C; WHSV > 2 h−1 | 2.35–2.44 | 5.40 × 1011 | 171 |
| [8] | Microchannel reactor | 2 wt % Pt/Al2O3 | 260–320 °C; 0.01 mL/min | 1 | 3.2725 s−1 | 13.79 |
3. Characterization
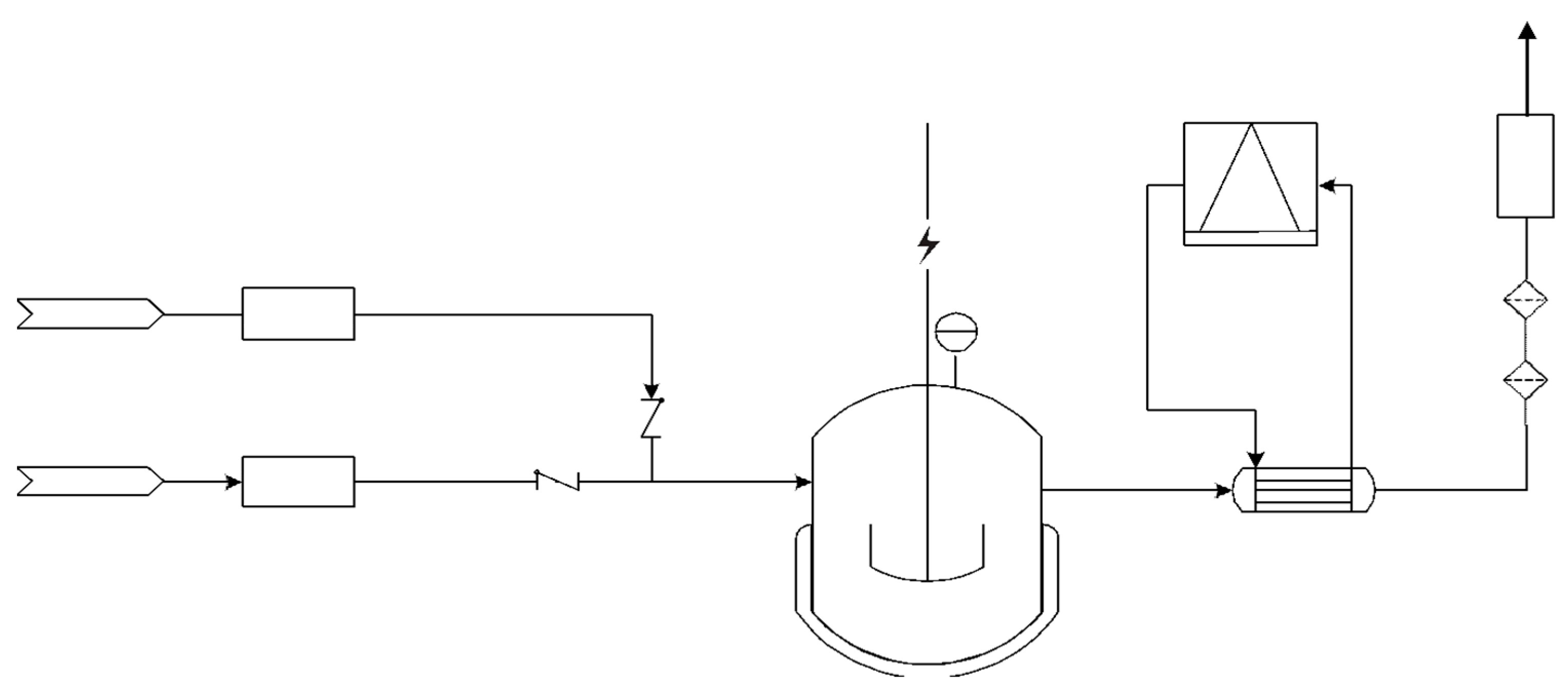
4. Experimental
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Preuster, P.; Fang, Q.; Peters, R.; Deja, R.; Nguyen, V.N.; Blum, L.; Stolten, D.; Wasserscheid, P. Solid Oxide Fuel Cell Operating on Liquid Organic Hydrogen Carrier-Based Hydrogen—Making Full Use of Heat Integration Potentials. Int. J. Hydrogen Energy 2018, 43, 1758–1768. [Google Scholar] [CrossRef]
- Fitchner, M.; Idrisova, F. Fundamental Properties of Hydrogen. In The Hydrogen Economy: Opportunities and Challenges; Cambridge University Press: New York, NY, USA, 2018; pp. 271–276. [Google Scholar]
- Aakko-Saksa, P.T.; Cook, C.; Kiviaho, J.; Repo, T. Liquid Organic Hydrogen Carriers for Transportation and Storing of Renewable Energy—Review and Discussion. J. Power Sources 2018, 396, 803–823. [Google Scholar] [CrossRef]
- Modisha, P.M.; Ouma, C.N.M.; Garidzirai, R.; Wasserscheid, P.; Bessarabov, D. The Prospect of Hydrogen Storage Using Liquid Organic Hydrogen Carriers. Energy Fuels 2019, 33, 2778–2796. [Google Scholar] [CrossRef]
- Brückner, N.; Obesser, K.; Bösmann, A.; Teichmann, D.; Arlt, W.; Dungs, J.; Wasserscheid, P. Evaluation of Industrially Applied Heat-Transfer Fluids as Liquid Organic Hydrogen Carrier Systems. ChemSusChem 2014, 7, 229–235. [Google Scholar] [CrossRef] [PubMed]
- Shi, L.; Zhou, Y.; Qi, S.; Smith, K.J.; Tan, X.; Yan, J.; Yi, C. Pt Catalysts Supported on H2 and O2 Plasma-Treated Al2O3 for Hydrogenation and Dehydrogenation of the Liquid Organic Hydrogen Carrier Pair Dibenzyltoluene and Perhydrodibenzyltoluene. ACS Catal. 2020, 10, 10661–10671. [Google Scholar] [CrossRef]
- Shi, L.; Qi, S.; Qu, J.; Che, T.; Yi, C.; Yang, B. Integration of Hydrogenation and Dehydrogenation Based on Dibenzyltoluene as Liquid Organic Hydrogen Energy Carrier. Int. J. Hydrogen Energy 2019, 44, 5345–5354. [Google Scholar] [CrossRef]
- Ali, A.; Rohini, A.K.; Lee, H.J. Dehydrogenation of Perhydro-Dibenzyltoluene for Hydrogen Production in a Microchannel Reactor. Int. J. Hydrogen Energy 2022, 47, 20905–20914. [Google Scholar] [CrossRef]
- Kwak, Y.; Kirk, J.; Moon, S.; Ohm, T.; Lee, Y.J.; Jang, M.; Park, L.H.; Il Ahn, C.; Jeong, H.; Sohn, H.; et al. Hydrogen Production from Homocyclic Liquid Organic Hydrogen Carriers (LOHCs): Benchmarking Studies and Energy-Economic Analyses. Energy Convers. Manag. 2021, 239, 114124. [Google Scholar] [CrossRef]
- Lee, S.; Han, G.; Kim, T.; Yoo, Y.S.; Jeon, S.Y.; Bae, J. Connected Evaluation of Polymer Electrolyte Membrane Fuel Cell with Dehydrogenation Reactor of Liquid Organic Hydrogen Carrier. Int. J. Hydrogen Energy 2020, 45, 13398–13405. [Google Scholar] [CrossRef]
- Lee, S.; Lee, J.; Kim, T.; Han, G.; Lee, J.; Lee, K.; Bae, J. Pt/CeO2 Catalyst Synthesized by Combustion Method for Dehydrogenation of Perhydro-Dibenzyltoluene as Liquid Organic Hydrogen Carrier: Effect of Pore Size and Metal Dispersion. Int. J. Hydrogen Energy 2021, 46, 5520–5529. [Google Scholar] [CrossRef]
- Modisha, P.; Gqogqa, P.; Garidzirai, R.; Ouma, C.N.M.; Bessarabov, D. Evaluation of Catalyst Activity for Release of Hydrogen from Liquid Organic Hydrogen Carriers. Int. J. Hydrogen Energy 2019, 44, 21926–21935. [Google Scholar] [CrossRef]
- Garidzirai, R.; Modisha, P.; Shuro, I.; Visagie, J.; van Helden, P.; Bessarabov, D. The Effect of Mg and Zn Dopants on Pt/Al2O3 for the Dehydrogenation of Perhydrodibenzyltoluene. Catalysts 2021, 11, 490. [Google Scholar] [CrossRef]
- Auer, F.; Blaumeiser, D.; Bauer, T.; Bösmann, A.; Szesni, N.; Libuda, J.; Wasserscheid, P. Boosting the Activity of Hydrogen Release from Liquid Organic Hydrogen Carrier Systems by Sulfur-Additives to Pt on Alumina Catalysts. Catal. Sci. Technol. 2019, 9, 3537–3547. [Google Scholar] [CrossRef]
- Chen, X.; Gierlich, C.H.; Schötz, S.; Blaumeiser, D.; Bauer, T.; Libuda, J.; Palkovits, R. Hydrogen Production Based on Liquid Organic Hydrogen Carriers through Sulfur Doped Platinum Catalysts Supported on TiO2. ACS Sustain. Chem. Eng. 2021, 9, 6561–6573. [Google Scholar] [CrossRef]
- Modisha, P.; Garidzirai, R.; Günes, H.; Bozbag, S.E.; Rommel, S.; Uzunlar, E.; Aindow, M.; Erkey, C.; Bessarabov, D. A Promising Catalyst for the Dehydrogenation of Perhydro-Dibenzyltoluene: Pt/Al2O3 Prepared by Supercritical CO2 Deposition. Catalysts 2022, 12, 489. [Google Scholar] [CrossRef]
- Catalyst Development for Improved Economic Viability of LOHC Technology. Available online: https://cordis.europa.eu/programme/id/H2020_FCH-02-1-2020 (accessed on 10 October 2023).
- Modisha, P.; Bessarabov, D. Stress Tolerance Assessment of Dibenzyltoluene-Based Liquid Organic Hydrogen Carriers. Sustain. Energy Fuels 2020, 4, 4662–4670. [Google Scholar] [CrossRef]
- Bulgarin, A.; Jorschick, H.; Preuster, P.; Bösmann, A.; Wasserscheid, P. Purity of Hydrogen Released from the Liquid Organic Hydrogen Carrier Compound Perhydro Dibenzyltoluene by Catalytic Dehydrogenation. Int. J. Hydrogen Energy 2020, 45, 712–720. [Google Scholar] [CrossRef]
- Wunsch, A.; Mohr, M.; Pfeifer, P. Intensified LOHC-Dehydrogenation Using Multi-Stage Microstructures and Pd-Based Membranes. Membranes 2018, 8, 112. [Google Scholar] [CrossRef]
- Seidel, A. Entwicklung Eines Technischen Platin-Trägerkatalysators zur Dehydrierung von Perhydro-Dibenzyltoluol. Ph.D. Thesis, Friedrich Alexander Universität Erlangen, Nürnberg, Germany, 2019; 177p. [Google Scholar]
- Peters, R.; Deja, R.; Fang, Q.; Nguyen, V.N.; Preuster, P.; Blum, L.; Wasserscheid, P.; Stolten, D. A Solid Oxide Fuel Cell Operating on Liquid Organic Hydrogen Carrier-Based Hydrogen—A Kinetic Model of the Hydrogen Release Unit and System Performance. Int. J. Hydrogen Energy 2019, 44, 13794–13806. [Google Scholar] [CrossRef]
- Park, S.; Naseem, M.; Lee, S. Experimental Assessment of Perhydro-Dibenzyltoluene Dehydrogenation Reaction Kinetics in a Continuous Flow System for Stable Hydrogen Supply. Materials 2021, 14, 7613. [Google Scholar] [CrossRef]
- Ballarini, A.; Basile, F.; Benito, P.; Bersani, I.; Fornasari, G.; De Miguel, S.; Maina, S.C.P.; Vilella, J.; Vaccari, A.; Scelza, O.A. Platinum Supported on Alkaline and Alkaline Earth Metal-Doped Alumina as Catalysts for Dry Reforming and Partial Oxidation of Methane. Appl. Catal. A Gen. 2012, 433–434, 1–11. [Google Scholar] [CrossRef]
- Belskaya, O.B.; Stepanova, L.N.; Gulyaeva, T.I.; Erenburg, S.B.; Trubina, S.V.; Kvashnina, K.; Nizovskii, A.I.; Kalinkin, A.V.; Zaikovskii, V.I.; Bukhtiyarov, V.I.; et al. Zinc Influence on the Formation and Properties of Pt/Mg(Zn)AlOx Catalysts Synthesized from Layered Hydroxides. J. Catal. 2016, 341, 13–23. [Google Scholar] [CrossRef]
- Jorschick, H.; Dürr, S.; Preuster, P.; Bösmann, A.; Wasserscheid, P. Operational Stability of a LOHC-Based Hot Pressure Swing Reactor for Hydrogen Storage. Energy Technol. 2019, 7, 146–152. [Google Scholar] [CrossRef]
- Modisha, P.M.; Jordaan, J.H.L.; Bösmann, A.; Wasserscheid, P.; Bessarabov, D. Analysis of Reaction Mixtures of Perhydro-Dibenzyltoluene Using Two-Dimensional Gas Chromatography and Single Quadrupole Gas Chromatography. Int. J. Hydrogen Energy 2018, 43, 5620–5636. [Google Scholar] [CrossRef]
- Sotoodeh, F. Hydrogenation and Dehydrogenation Kinetics and Catalysts for New Hydrogen Storage Liquids. Ph.D Thesis, University of British Columbia, Vancouver, BC, Canada, 2011; 223p. [Google Scholar]
- Triyono, T. Correlation between Preexponential Factor and Activation Energy of Isoamylalcohol Hydrogenolysis on Platinum Catalysts. Indones. J. Chem. 2010, 4, 1–5. [Google Scholar] [CrossRef]
- Jorschick, H.; Geißelbrecht, M.; Eßl, M.; Preuster, P.; Bösmann, A.; Wasserscheid, P. Benzyltoluene/Dibenzyltoluene-Based Mixtures as Suitable Liquid Organic Hydrogen Carrier Systems for Low Temperature Applications. Int. J. Hydrogen Energy 2020, 45, 14897–14906. [Google Scholar] [CrossRef]
- Kim, T.W.; Kim, M.; Kim, S.K.; Choi, Y.N.; Jung, M.; Oh, H.; Suh, Y.W. Remarkably Fast Low-Temperature Hydrogen Storage into Aromatic Benzyltoluenes over MgO-Supported Ru Nanoparticles with Homolytic and Heterolytic H2 Adsorption. Appl. Catal. B Environ. 2021, 286, 119889. [Google Scholar] [CrossRef]
- Auer, F. Katalysatorentwicklung für Die Dehydrierung von Perhydro-Dibenzyltoluol. Ph.D. Thesis, Friedrich Alexander Universität Erlangen, Nürnberg, Germany, 2020. [Google Scholar]


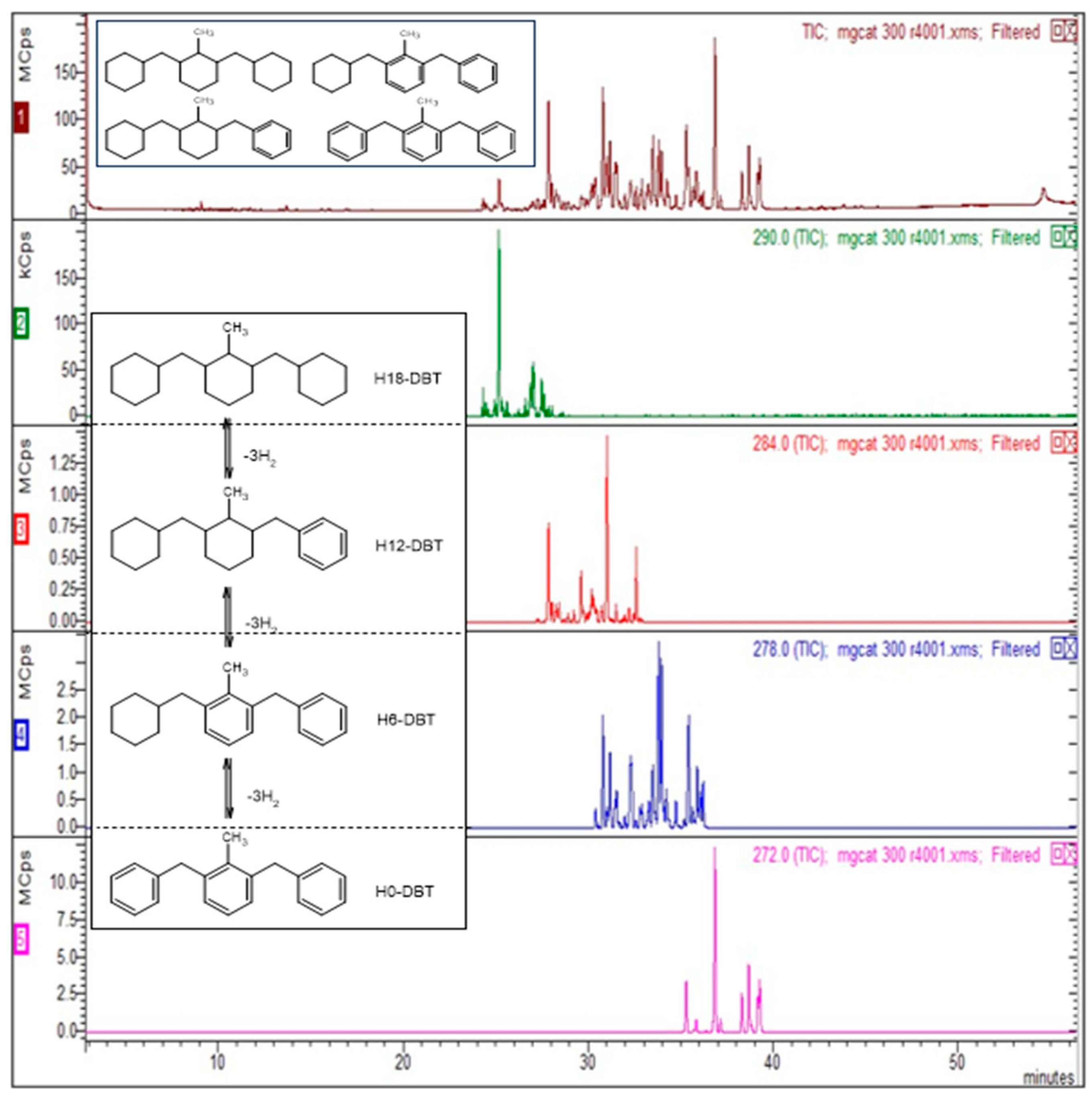

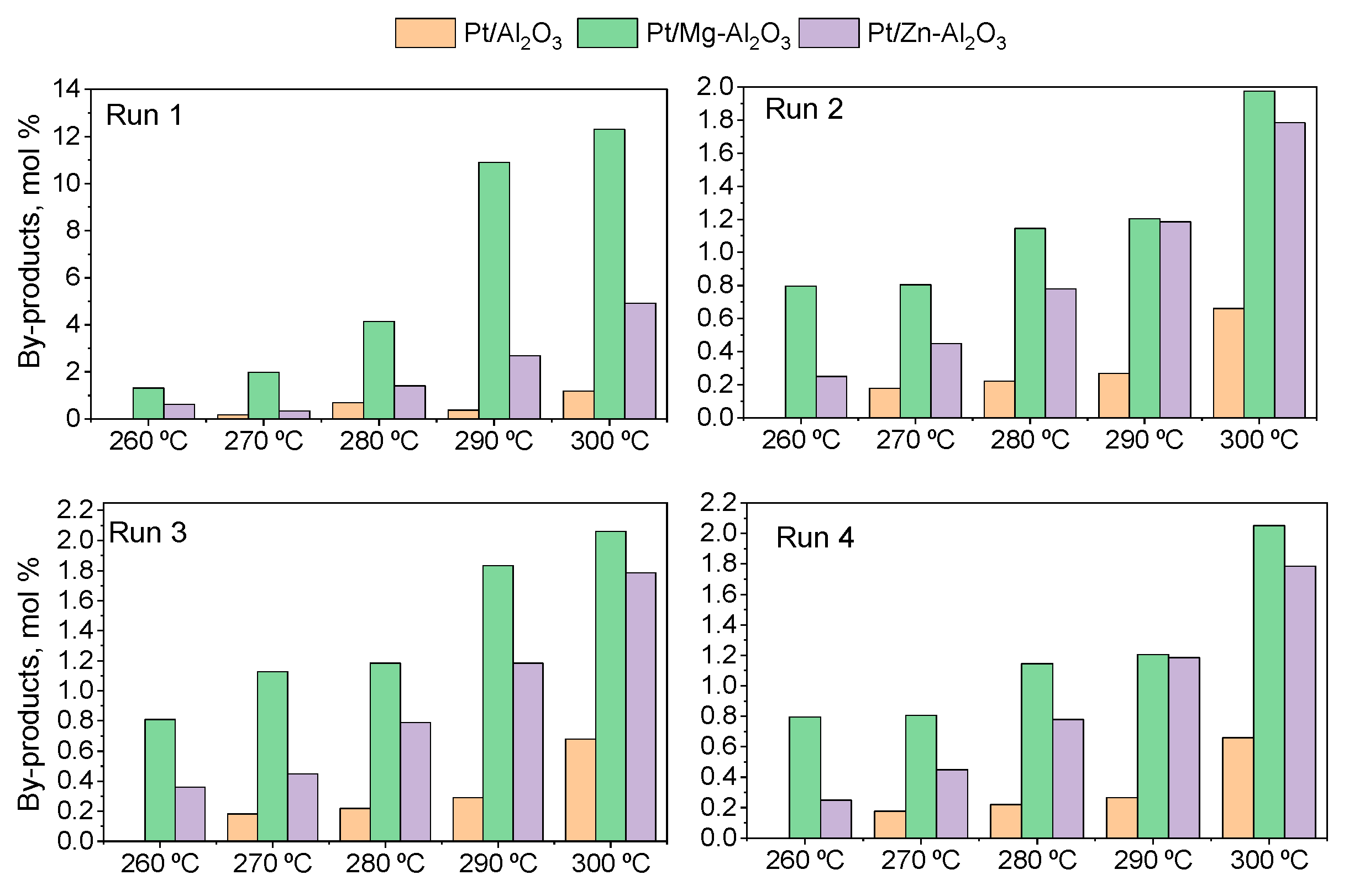


| By-Product Quantities in Mol. % per Catalyst Used | ||||
|---|---|---|---|---|
| By-Product Compounds | Category | Pt/Al2O3 | Pt/Mg-Al2O3 | Pt/Zn-Al2O3 |
| Benzyltoluene (m/z =182) | Low boilers | 0.2 | 2.5 | 1.3 |
| Toluene (m/z = 92) | Low boilers | 0.01 | 0.3 | 0.2 |
| H6-BT (m/z = 188) | Low boilers | 0.2 | 1.2 | 0.6 |
| Benzylmethylfluorene (m/z = 270) | High boilers | 0.8 | 8.0 | 2.7 |
| Total quantity (mol. %) | 1.2 | 12 | 5 | |
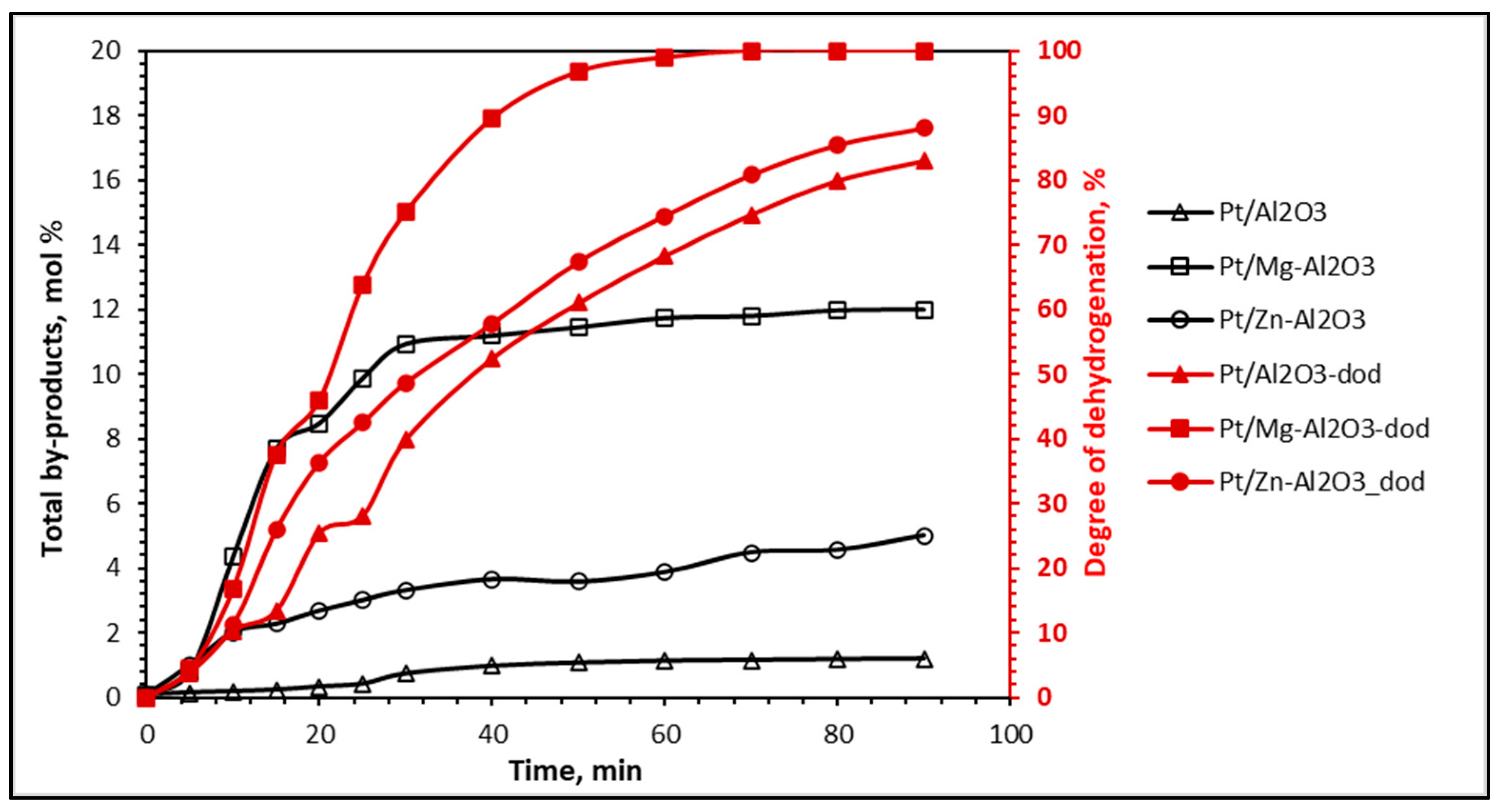
| Catalyst | Dodi | Dodrun 4 | Pi Prun 4 (gH2/gPt/min) | Si | Srun 4 | Xi | Xrun 4 | ΔX | |
|---|---|---|---|---|---|---|---|---|---|
| Pt/Al2O3 | 82 | 50 | 1.62 | 0.53 | 79 | 35 | 99.3 | 92 | 7.4 |
| Pt/Mg-Al2O3 | 100 | 73 | 1.84 | 0.58 | 87 | 62 | 99.9 | 98 | 1.9 |
| Pt/Zn-Al2O3 | 88 | 60 | 1.68 | 0.55 | 82 | 49 | 99.9 | 95 | 4.9 |
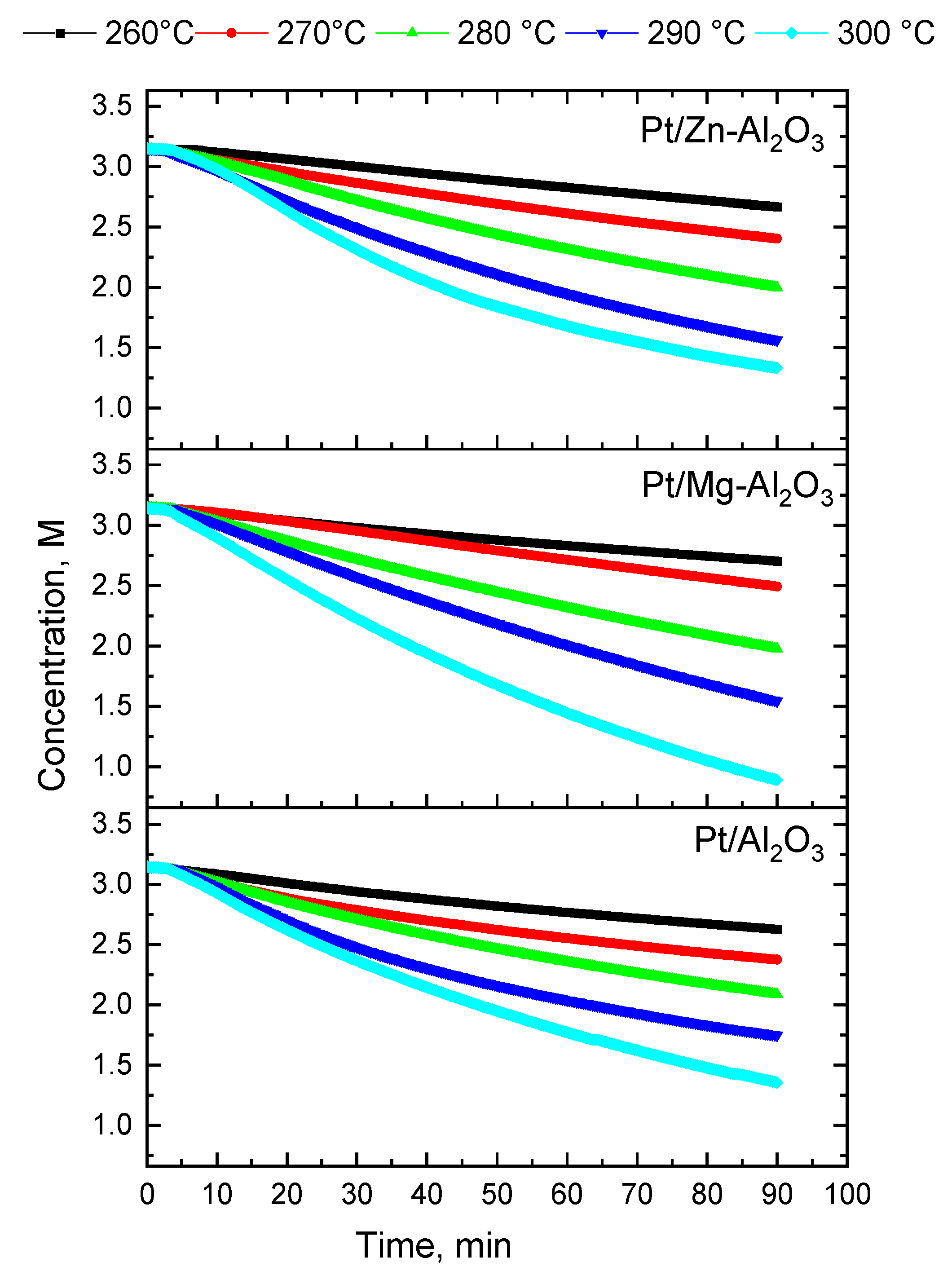
| Catalyst | Temp, °C | k, (min−1) | Initial Rate of Reaction (M min−1 gcat−1) | TOF (min−1) |
|---|---|---|---|---|
| Pt/Al2O3 | 260 | 0.00180 | 0.02352 | 47 |
| 270 | 0.00270 | 0.03696 | 70 | |
| 280 | 0.00420 | 0.05376 | 109 | |
| 290 | 0.00600 | 0.08064 | 155 | |
| 300 | 0.00780 | 0.09702 | 202 | |
| Pt/Mg-Al2O3 | 260 | 0.00140 | 0.01806 | 61 |
| 270 | 0.00240 | 0.03150 | 103 | |
| 280 | 0.00490 | 0.06258 | 209 | |
| 290 | 0.00830 | 0.09996 | 355 | |
| 300 | 0.01370 | 0.17640 | 586 | |
| Pt/Zn-Al2O3 | 260 | 0.00170 | 0.02016 | 49 |
| 270 | 0.00280 | 0.03318 | 81 | |
| 280 | 0.00490 | 0.05999 | 142 | |
| 290 | 0.00800 | 0.09576 | 231 | |
| 300 | 0.00930 | 0.10836 | 269 |
| Catalyst Properties | Pt/Al2O3 | Pt/Mg-Al2O3 | Pt/Zn-Al2O3 |
|---|---|---|---|
| a Pt dispersion, % | 38 | 34 | 23 |
| a Metallic surface area, m2/gsample | 0.42 | 0.42 | 0.28 |
| b H2 consumption, µmol/g | 34 | 64 | 55 |
| c Total Acidity, NH3/gcat | 0.48 | 0.41 | 0.49 |
| d Particle size, nm | 1.72 | 1.70 | 1.54 |
| e Pt loading, wt% | 0.48 | 0.49 | 0.48 |
| e Dopant loading, wt % | 3.89 | 3.44 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Garidzirai, R.; Modisha, P.; Bessarabov, D. Assessment of Reaction Kinetics for the Dehydrogenation of Perhydro-Dibenzyltoluene Using Mg- and Zn-Modified Pt/Al2O3 Catalysts. Catalysts 2024, 14, 32. https://doi.org/10.3390/catal14010032
Garidzirai R, Modisha P, Bessarabov D. Assessment of Reaction Kinetics for the Dehydrogenation of Perhydro-Dibenzyltoluene Using Mg- and Zn-Modified Pt/Al2O3 Catalysts. Catalysts. 2024; 14(1):32. https://doi.org/10.3390/catal14010032
Chicago/Turabian StyleGaridzirai, Rudaviro, Phillimon Modisha, and Dmitri Bessarabov. 2024. "Assessment of Reaction Kinetics for the Dehydrogenation of Perhydro-Dibenzyltoluene Using Mg- and Zn-Modified Pt/Al2O3 Catalysts" Catalysts 14, no. 1: 32. https://doi.org/10.3390/catal14010032








