Reaction Kinetics and Mechanism for the Synthesis of Glycerol Carbonate from Glycerol and Urea Using ZnSO4 as a Catalyst
Abstract
:1. Introduction
2. Results and Discussion
2.1. Activity of Different Zn Salt Catalysts
2.2. Effects of Reaction Parameters
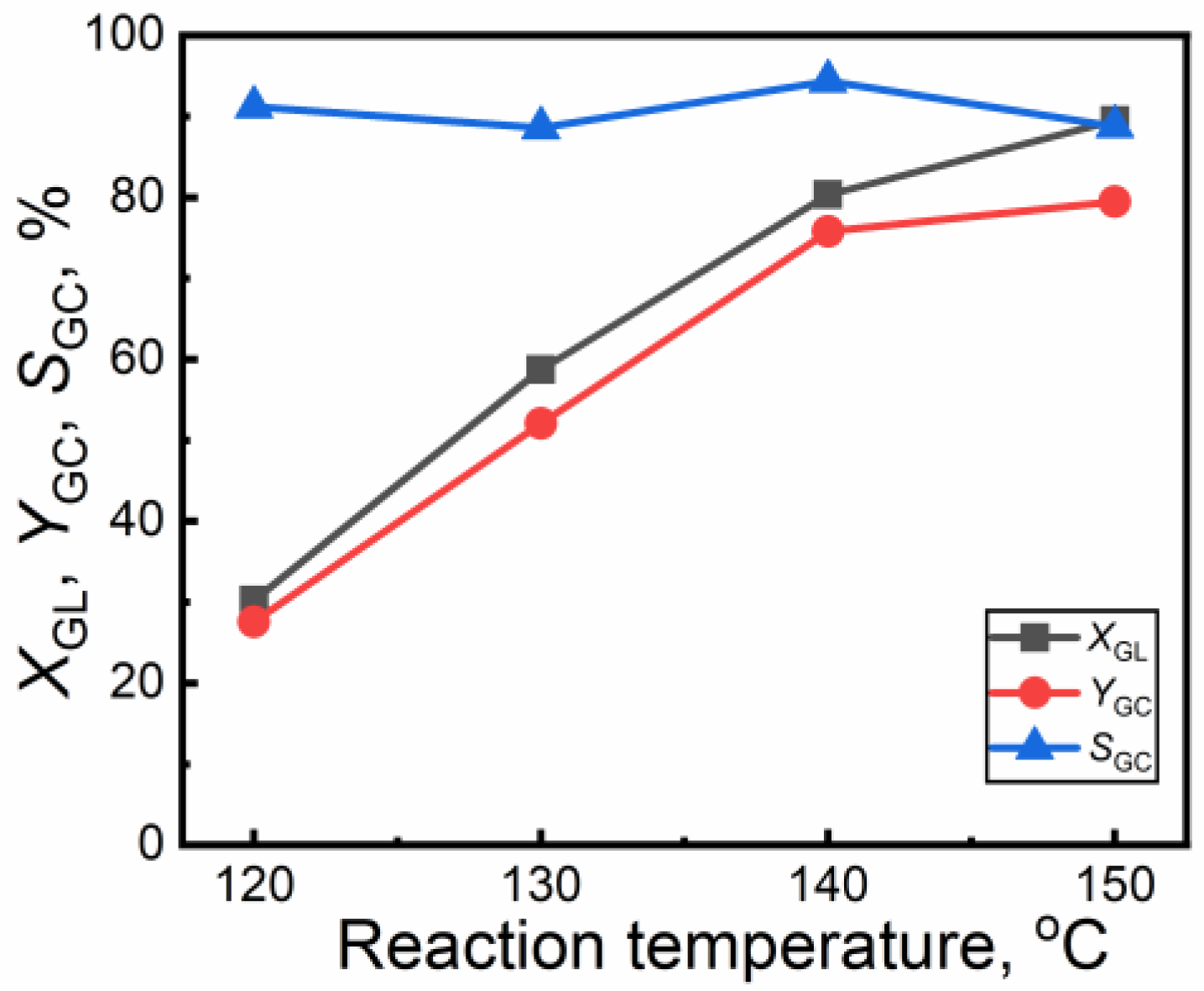
2.2.1. Effect of Reaction Temperature
2.2.2. Effect of Urea/GL Molar Ratio
2.2.3. Effect of Catalyst Amount
2.2.4. Effect of Reaction Time
2.3. Reaction Mechanism
2.4. Reaction Kinetics
2.4.1. Reaction Rate
2.4.2. Solution of Kinetics Model
3. Materials and Methods
3.1. Materials
3.2. Reaction Procedure
3.3. Characterization
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Nomenclature
| Ci | molar concentration of component i (mol/L) |
| Eaj | activation energy of reaction j (kJ/mol) |
| F | F-test function |
| FT | tabulated value of F distribution |
| k1 | reaction rate constant of reaction R1 (L·min−1·mol−1) |
| k2 | reaction rate constant of reaction R2 (min−1) |
| kj* | pre-exponential factor (L·min−1·mol−1 or min−1) |
| Mi | molar mass of component i (kg/mol) |
| mi | mass of component i (kg) |
| ni | molar number of component i (mol) |
| N | number of experimental runs |
| p | number of parameters |
| R | gas constant, 8.314 J/(mol·K) |
| R2 | correlation coefficient |
| rj | reaction rate of reaction j (mol/(L·min)) |
| SGC | selectivity of glycerol carbonate (-) |
| T | temperature (K) |
| t | reaction time (min) |
| V | volume of reaction mixture (L) |
| molar volume of component i (L/mol) | |
| XGL | conversion of glycerol (-) |
| YGC | yield of glycerol carbonate (-) |
| GC | glycerol carbonate |
| GCM | glyceryl carbamate |
| GL | glycerol |
| Greek letters | |
| ψ | objective function |
| ρi | density of component i (kg/L) |
Appendix A
References
- Alibas, I.; Yilmaz, A.; Alibas, K.; Arslan, M.; Koc, C. The effect of different drying methods and microalgae species on the quality parameters of biodiesel obtained by transesterification technique. Biomass Bioenerg. 2023, 168, 106688. [Google Scholar] [CrossRef]
- Javed, F.; Zimmerman, W.B.; Fazal, T.; Hafeez, A.; Mustafa, M.; Rashid, N.; Rehman, F. Green synthesis of biodiesel from microalgae cultivated in industrial wastewater via microbubble induced esterification using bio-MOF based heterogeneous catalyst. Chem. Eng. Res. Des. 2023, 189, 707–720. [Google Scholar] [CrossRef]
- Chen, C.; Mira, D.; Xing, Z.; Jiang, X. Thermophysical property prediction of biodiesel mixtures at extreme conditions using molecular dynamics simulation. J. Mol. Liq. 2022, 367, 120423. [Google Scholar] [CrossRef]
- Masera, K.; Hossain, A.K. Biofuels and thermal barrier: A review on compression ignition engine performance, combustion and exhaust gas emission. J. Energy Inst. 2019, 92, 783–801. [Google Scholar] [CrossRef]
- Julio, A.A.V.; Milessi, T.S.; Batlle, E.A.O.; Lora, E.E.S.; Maya, D.M.Y.; Palacio, J.C.E. Techno-economic and environmental potential of renewable diesel as complementation for diesel and biodiesel in Brazil: A comprehensive review and perspectives. J. Clean. Prod. 2022, 371, 133431. [Google Scholar] [CrossRef]
- Meng, L.; Su, H.; Du, S.; Wang, Y.; Li, M. Brief discussion on the production technology and development trend of biodiesel in our country. Chem. Eng. Equip. 2019, 7, 274–276. (In Chinese) [Google Scholar]
- Song, S.S.; Tian, B.C.; Chen, H.; Chi, Z.; Liu, G.L.; Chi, Z.M. Transformation of corncob-derived xylose into intracellular lipid by engineered strain of Aureobasidium melanogenum P10 for biodiesel production. Renew. Energy 2022, 200, 1211–1222. [Google Scholar] [CrossRef]
- Akram, F.; Haq, I.; Raja, S.I.; Mir, A.S.; Qureshi, S.S.; Aqeel, A.; Shah, F.I. Current trends in biodiesel production technologies and future progressions: A possible displacement of the petro-diesel. J. Clean. Prod. 2022, 370, 133479. [Google Scholar] [CrossRef]
- Ashine, F.; Kiflie, Z.; Prabhu, S.V.; Tizazu, B.Z.; Varadharajan, V.; Rajasimman, M.; Joo, S.W.; Vasseghian, Y.; Jayakumar, M. Biodiesel production from Argemone mexicana oil using chicken eggshell derived CaO catalyst. Fuel 2013, 332, 126166. [Google Scholar] [CrossRef]
- Caro, P.; Bandres, M.; Urrutigoïty, M.; Cecutti, C.; Thiebaud-Roux, S. Recent progress in synthesis of glycerol carbonate and evaluation of its plasticizing properties. Front. Chem. 2019, 7, 308. [Google Scholar] [CrossRef]
- Pagliaro, M.; Ciriminna, R.; Kimura, H.; Rossi, M.; Pina, C.D. From glycerol to value-added products. Angew. Chem. Int. Ed. 2007, 46, 4434–4440. [Google Scholar] [CrossRef] [PubMed]
- Atadashi, I.M.; Aroua, M.K.; Abdul Aziz, A. High quality biodiesel and its diesel engine application: A review. Renew. Sust. Energy Rev. 2010, 14, 1999–2008. [Google Scholar] [CrossRef]
- Demirbas, A. Biodiesel production from vegetable oils via catalytic and non-catalytic supercritical methanol transesterification methods. Prog. Energy Combust. Sci. 2005, 31, 466–487. [Google Scholar] [CrossRef]
- Sonnati, M.O.; Amigoni, S.; Taffin de Givenchy, E.P.; Darmanin, T.; Choulet, O.; Guittard, F. Glycerol carbonate as a versatile building block for tomorrow: Synthesis, reactivity, properties and applications. Green Chem. 2013, 15, 283–306. [Google Scholar] [CrossRef]
- Zheng, Y.; Chen, X.; Shen, Y. Commodity chemicals derived from glycerol, an important biorefinery feedstock. Chem. Rev. 2008, 108, 5253–5277. [Google Scholar] [CrossRef] [PubMed]
- An, S.; Sun, Y.; Song, D.; Zhang, Q.; Guo, Y.; Shang, Q. Arenesulfonic acid-functionalized alkyl-bridged organosilica hollow nanospheres for selective esterification of glycerol with lauric acid to glycerol mono- and dilaurate. J. Catal. 2016, 342, 40–54. [Google Scholar] [CrossRef]
- Binhayeeding, N.; Klomklao, S.; Sangkharak, K. Utilization of waste glycerol from biodiesel process as a substrate for mono-, di-, and triacylglycerol production. Energy Procedia 2017, 138, 895–900. [Google Scholar] [CrossRef]
- Islam, Z.; Klein, M.; Aßkamp, M.R.; Ødum, A.S.R.; Nevoigt, E. A modular metabolic engineering approach for the production of 1,2- propanediol from glycerol by Saccharomyces cerevisiae. Metab. Eng. 2017, 44, 223–235. [Google Scholar] [CrossRef]
- Zhou, S.; Lama, S.; Sankaranarayanan, M.; Park, S. Metabolic engineering of Pseudomonas denitrificans for the 1,3-propanediol production from glycerol. Bioresour. Technol. 2019, 292, 121933. [Google Scholar] [CrossRef]
- Maina, S.; Kachrimanidou, V.; Ladakis, D.; Papanikolaou, S.; Castro, A.M.; Koutinas, A. Evaluation of 1,3-propanediol production by two Citrobacter freundii strains using crude glycerol and soybean cake hydrolysate. Environ. Sci. Pollut. Res. 2019, 26, 35523–35532. [Google Scholar] [CrossRef]
- Guedes, P.H.P.S.; Luz, R.F.; Cavalcante, R.M.; Young, A.F. Process simulation for technical and economic evaluation of acrolein and glycerol carbonate production from glycerol. Biomass Bioenerg. 2023, 168, 106659. [Google Scholar] [CrossRef]
- Rittiron, P.; Niamnuy, C.; Donphai, W.; Chareonpanich, M.; Seubsai, A. Production of glycerol carbonate from glycerol over templated-sodium-aluminate catalysts prepared using a spray-drying method. ACS Omega 2019, 4, 9001–9009. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Chen, D.; Wang, Z.; Wu, Q.; Yin, Z.; Wei, Z. Synthesis of glycerol carbonate from glycerol and dimethyl carbonate over CaO-SBA-15 catalyst. Chem. Eng. Sci. 2022, 258, 117760. [Google Scholar] [CrossRef]
- Pradhan, G.; Jaiswal, S.; Sharma, Y.C. Exploring the promotional effect of transition metals (Cr and V) on the catalytic activity of MgO for glycerol carbonate synthesis. Mol. Catal. 2022, 526, 112332. [Google Scholar] [CrossRef]
- Nomanbhay, S.; Ong, M.Y.; Chew, K.W.; Show, P.L.; Lam, M.K.; Chen, W.H. Organic carbonate production utilizing crude glycerol derived as by-product of biodiesel production: A review. Energies 2020, 13, 1483–1506. [Google Scholar] [CrossRef]
- Deshmukh, G.P.; Yadav, G.D. Tuneable transesterification of glycerol with dimethyl carbonate for synthesis of glycerol carbonate and glycidol on MnO2 nanorods and efficacy of different polymorphs. Mol. Catal. 2021, 515, 111934. [Google Scholar] [CrossRef]
- Arora, S.; Gosu, V.; Arun Kumar, U.K.; Subbaramaiah, V. Valorization of glycerol into glycerol carbonate using the stable heterogeneous catalyst of Li/MCM-41. J. Clean. Prod. 2021, 295, 126437. [Google Scholar] [CrossRef]
- Jaiswal, S.; Sahani, S.; Shar, Y.C. Enviro-benign synthesis of glycerol carbonate utilizing bio-waste glycerol over Na-Ti based heterogeneous catalyst: Kinetics and E- metrics studies. J. Environ. Chem. Eng. 2022, 10, 107485. [Google Scholar] [CrossRef]
- Rousseau, J.; Rousseau, C.; Lynikaite, B.; Šačkus, A.; Leon, C.; Rollin, P.; Tatibouet, A. Tosylated glycerol carbonate, a versatile bis-electrophile to access new functionalized glycidol derivatives. Tetrahedron 2009, 65, 8571–8581. [Google Scholar] [CrossRef]
- Hu, J.; Li, J.; Gu, Y.; Guan, Z.; Mo, W.; Ni, Y.; Li, T.; Li, G. Oxidative carbonylation of glycerol to glycerol carbonate catalyzed by PdCl2(phen)/KI. Appl. Catal. A Gen. 2010, 386, 188–193. [Google Scholar] [CrossRef]
- Li, J.; Wang, T. Chemical equilibrium of glycerol carbonate synthesis from glycerol. J. Chem. Thermodyn. 2011, 43, 731–736. [Google Scholar] [CrossRef]
- Aresta, M.; Dibenedetto, A.; Nocito, F.; Pastore, C. A study on the carboxylation of glycerol to glycerol carbonate with carbon dioxide: The role of the catalyst, solvent and reaction conditions. J. Mol. Catal. A Chem. 2006, 257, 149–153. [Google Scholar] [CrossRef]
- Patel, Y.; George, J.; Pillai, S.M.; Munshi, P. Effect of liophilicity of catalyst in cyclic carbonate formation by transesterification of polyhydric alcohols. Green Chem. 2009, 11, 1056–1060. [Google Scholar] [CrossRef]
- Kondawar, S.E.; Mane, R.B.; Vasishta, A.; More, S.B.; Dhengale, S.D.; Rode, C.V. Carbonylation of glycerol with urea to glycerol carbonate over supported Zn catalysts. Appl Petrochem. Res. 2017, 7, 41–53. [Google Scholar] [CrossRef]
- Fujita, S.; Yamanishi, Y.; Arai, M. Synthesis of glycerol carbonate from glycerol and urea using zinc-containing solid catalysts: A homogeneous reaction. J. Catal. 2013, 297, 137–141. [Google Scholar] [CrossRef]
- Endah, Y.K.; Kim, M.S.; Choi, J.; Jae, J.; Lee, S.D.; Lee, H. Consecutive carbonylation and decarboxylation of glycerol with urea for the synthesis of glycidol via glycerol carbonate. Catal. Today 2017, 293–294, 136–141. [Google Scholar] [CrossRef]
- Zhang, P.; Liu, L.; Fan, M.; Dong, Y.; Jiang, P. The value-added utilization of glycerol for the synthesis of glycerol carbonate catalyzed with a novel porous ZnO catalyst. RSC Adv. 2016, 6, 76223–76230. [Google Scholar] [CrossRef]
- Fernandes, G.P.; Yadav, G.D. Selective glycerolysis of urea to glycerol carbonate using combustion synthesized magnesium oxide as catalyst. Catal. Today 2018, 309, 153–160. [Google Scholar] [CrossRef]
- Zhao, W.; Peng, W.; Wang, D.; Zhao, N.; Li, J.; Xiao, F.; Wei, W.; Sun, Y. Zinc oxide as the precursor of homogenous catalyst for synthesis of dialkyl carbonate from urea and alcohols. Catal. Commun. 2009, 10, 655–658. [Google Scholar] [CrossRef]
- Wang, L.; Ma, Y.; Wang, Y.; Liu, S.; Deng, Y. Efficient synthesis of glycerol carbonate from glycerol and urea with lanthanum oxide as a solid base catalyst. Catal. Commun. 2011, 12, 1458–1462. [Google Scholar] [CrossRef]
- Baek, J.; Ryu, Y.B.; Kim, M.H.; Moon, M.J.; Lee, M.S. Synthesis of glycerol carbonate from biomass glycerol: Focus on the calcination temperature. Mater. Sci. Forum. 2012, 724, 374–377. [Google Scholar] [CrossRef]
- Kumar, C.R.; Jagadeeswaraiah, K.; Sai Prasad, P.S.; Lingaiah, N. Samarium-exchanged heteropoly tungstate: An efficient solid acid catalyst for synthesis of glycerol carbonate from glycerol and benzylation of anisole. ChemCatChem 2012, 4, 1360–1367. [Google Scholar] [CrossRef]
- Kim, D.W.; Park, K.A.; Kim, M.J.; Kang, D.H.; Yang, J.G.; Park, D.W. Synthesis of glycerol carbonate from urea and glycerol using polymer-supported metal containing ionic liquid catalysts. Appl. Catal. A Gen. 2014, 473, 31–40. [Google Scholar] [CrossRef]
- Rahim, M.H.A.; He, Q.; Lopez-Sanchez, J.A.; Hammond, C.; Dimitratos, N.; Sankar, M.; Carley, A.F.; Kiely, C.J.; Knight, D.W.; Hutchings, G.J. Gold, palladium and gold–palladium supported nanoparticles for the synthesis of glycerol carbonate from glycerol and urea. Catal. Sci. Technol. 2012, 2, 1914–1924. [Google Scholar] [CrossRef]
- Sun, Y.; Tong, X.; Wu, Z.; Liu, J.; Yan, Y.; Xue, S. A sustainable preparation of glycerol carbonate from glycerol and urea catalyzed by hydrotalcite-like solid catalysts. Energy Technol. 2014, 2, 263–268. [Google Scholar] [CrossRef]
- Wang, D.; Zhang, X.; Cong, X.; Liu, S.; Zhou, D. Influence of Zr on the performance of Mg-Al catalysts via hydrotalcite-like precursors for the synthesis of glycerol carbonate from urea and glycerol. Appl. Catal. A Gen. 2018, 555, 36–46. [Google Scholar] [CrossRef]
- Park, J.H.; Choi, J.S.; Woo, S.K.; Lee, S.D.; Cheong, M.; Kim, H.S.; Lee, H. Isolation and characterization of intermediate catalytic species in the Zn-catalyzed glycerolysis of urea. Appl. Catal. A Gen. 2012, 433–434, 35–40. [Google Scholar] [CrossRef]
- Aresta, M.; Dibenedetto, A.; Nocito, F.; Ferragina, C. Valorization of bio-glycerol: New catalytic materials for the synthesis of glycerol carbonate via glycerolysis of urea. J. Catal. 2009, 268, 106–114. [Google Scholar] [CrossRef]
- Rubio-Marcos, F.; Calvino-Casilda, V.; Bañares, M.A.; Fernandez, J.F. Novel hierarchical Co3O4/ZnO mixtures by dry nanodispersion and their catalytic application in the carbonylation of glycerol. J. Catal. 2010, 275, 288–293. [Google Scholar] [CrossRef]
- Schaber, P.M.; Colson, J.; Higgins, S.; Thielen, D.; Anspach, B.; Brauer, J. Thermal decomposition (pyrolysis) of urea in an open reaction vessel. Thermochim. Acta 2004, 424, 131–142. [Google Scholar] [CrossRef]
- Climent, M.J.; Corma, A.; De Frutos, P.; Iborra, S.; Noy, M.; Velty, A.; Concepción, P. Chemicals from biomass: Synthesis of glycerol carbonate by transesterification and carbonylation with urea with hydrotalcite catalysts. The role of acid–base pairs. J. Catal. 2010, 269, 140–149. [Google Scholar] [CrossRef]
- Luo, W.; Sun, L.; Yang, Y.; Chen, Y.; Zhou, Z.; Liu, J.; Wang, F. Cu–Mn composite oxides: Highly efficient and reusable acid–base catalysts for the carbonylation reaction of glycerol with urea. Catal. Sci. Technol. 2018, 8, 6468–6477. [Google Scholar] [CrossRef]
- Wang, D.; Zhang, X.; Liu, C.; Cheng, T. Synthesis of glycerol carbonate from glycerol and urea over lanthanum compounds. React. Kinet. Mech. Catal. 2015, 115, 597–609. [Google Scholar] [CrossRef]
- Turney, T.W.; Patti, A.; Gates, W.; Shaheen, U.; Kulasegaram, S. Formation of glycerol carbonate from glycerol and urea catalysed by metal monoglycerolatest. Green Chem. 2013, 15, 1925–1931. [Google Scholar] [CrossRef]
- Wang, H.; Li, G. Kinetic study on the synthesis of ethyl nitrite by the reaction of C2H5OH, O2, and NO in a trickle bed reactor. Chem. Eng. J. 2010, 163, 422–428. [Google Scholar] [CrossRef]
- Lertlukkanasuk, N.; Phiyanalinmat, S.; Kiatkittipong, W.; Arpornwichanop, A.; Aiouache, F.; Assabumrungrat, S. Reactive distillation for synthesis of glycerol carbonate via glycerolysis of urea. Chem. Eng. Process. 2013, 70, 103–109. [Google Scholar] [CrossRef]
- Wang, H.; Liu, T.; Jiang, C.; Wang, Y.; Ma, J. Synthesis of glycidol and glycerol carbonate from glycerol and dimethyl carbonate using deep-eutectic solvent as a catalyst. Chem. Eng. J. 2022, 442, 136196. [Google Scholar] [CrossRef]
- Ayastuy, J.L.; Gutierrez-Ortiz, M.A.; Gonzalez-Marcos, J.A.; Aranzabal, A.; Gonzalez-Velasco, J.R. Kinetics of the low-temperature WGS reaction over a CuO/ZnO/Al2O3 catalyst. Ind. Eng. Chem. Res. 2005, 44, 41–50. [Google Scholar] [CrossRef]















| No. | Catalyst | XGL, % b | YGC, % c | SGC, % d |
|---|---|---|---|---|
| 1 | - | 42.24 | 32.29 | 76.44 |
| 2 | KOH | trace | trace | trace |
| 3 | KNO3 | trace | trace | trace |
| 4 | ZnO | 71.20 | 64.73 | 90.91 |
| 5 | ZnCl2 | 81.75 | 73.83 | 90.31 |
| 6 | ZnBr2 | 78.33 | 69.91 | 89.25 |
| 7 | ZnI2 | 74.41 | 67.95 | 91.32 |
| 8 | Zn(NO3)·6H2O | 68.11 | 61.19 | 89.84 |
| 9 | Zn3(PO4)2 | 67.14 | 62.30 | 92.79 |
| 10 | ZnSO4 | 80.33 | 75.81 | 94.37 |
| No. | Catalyst | XGL, % b | YGC, % c | SGC, % d |
|---|---|---|---|---|
| 11 | Zn(C3H6O3) | 65.78 | 60.84 | 92.49 |
| 12 | (NH4)2SO4 | 47.01 | 38.69 | 82.30 |
| 13 | Zn(C3H6O3) + (NH4)2SO4 | 76.38 | 71.62 | 93.77 |
| ki a | Inch | Ea, kJ/mol b | R2 c |
|---|---|---|---|
| L·mol−1·min−1 | 143.39 | 0.9995 | |
| min−1 | 87.29 | 0.9995 |
| No. | Cat. | Temperature, °C | Pressure, kPa | Ea, kJ/mol a | Ref. |
|---|---|---|---|---|---|
| 1 | MgO | 135~150 | 101.3 b | 117.85 | [38] |
| 2 | Co3O4/ZnO | 100~160 | 101.3 | 31.89 | [56] |
| Catalyst | F | 10 × FT |
|---|---|---|
| ZnSO4 | 216.37 | 23.6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, H.; Ma, J. Reaction Kinetics and Mechanism for the Synthesis of Glycerol Carbonate from Glycerol and Urea Using ZnSO4 as a Catalyst. Catalysts 2024, 14, 41. https://doi.org/10.3390/catal14010041
Wang H, Ma J. Reaction Kinetics and Mechanism for the Synthesis of Glycerol Carbonate from Glycerol and Urea Using ZnSO4 as a Catalyst. Catalysts. 2024; 14(1):41. https://doi.org/10.3390/catal14010041
Chicago/Turabian StyleWang, Huajun, and Jingjing Ma. 2024. "Reaction Kinetics and Mechanism for the Synthesis of Glycerol Carbonate from Glycerol and Urea Using ZnSO4 as a Catalyst" Catalysts 14, no. 1: 41. https://doi.org/10.3390/catal14010041
APA StyleWang, H., & Ma, J. (2024). Reaction Kinetics and Mechanism for the Synthesis of Glycerol Carbonate from Glycerol and Urea Using ZnSO4 as a Catalyst. Catalysts, 14(1), 41. https://doi.org/10.3390/catal14010041





