Visible Light-Driven Organic Pollutant Removal Using Fe-Based Photocatalysts Supported by Wheat Straw Biochar
Abstract
:1. Introduction
2. Results and Discussion
2.1. Characterization of Catalyst
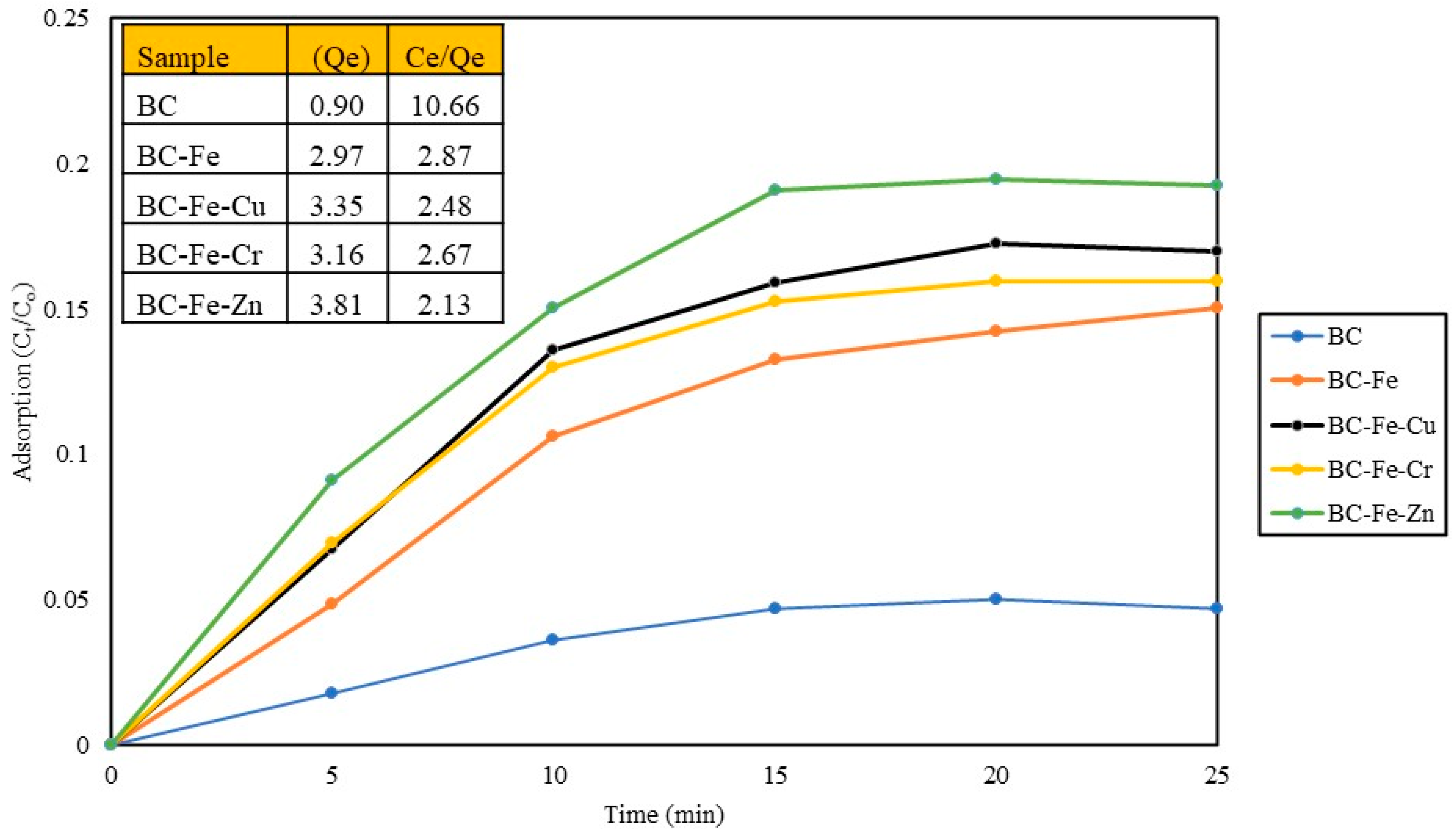
2.2. Adsorption Studies
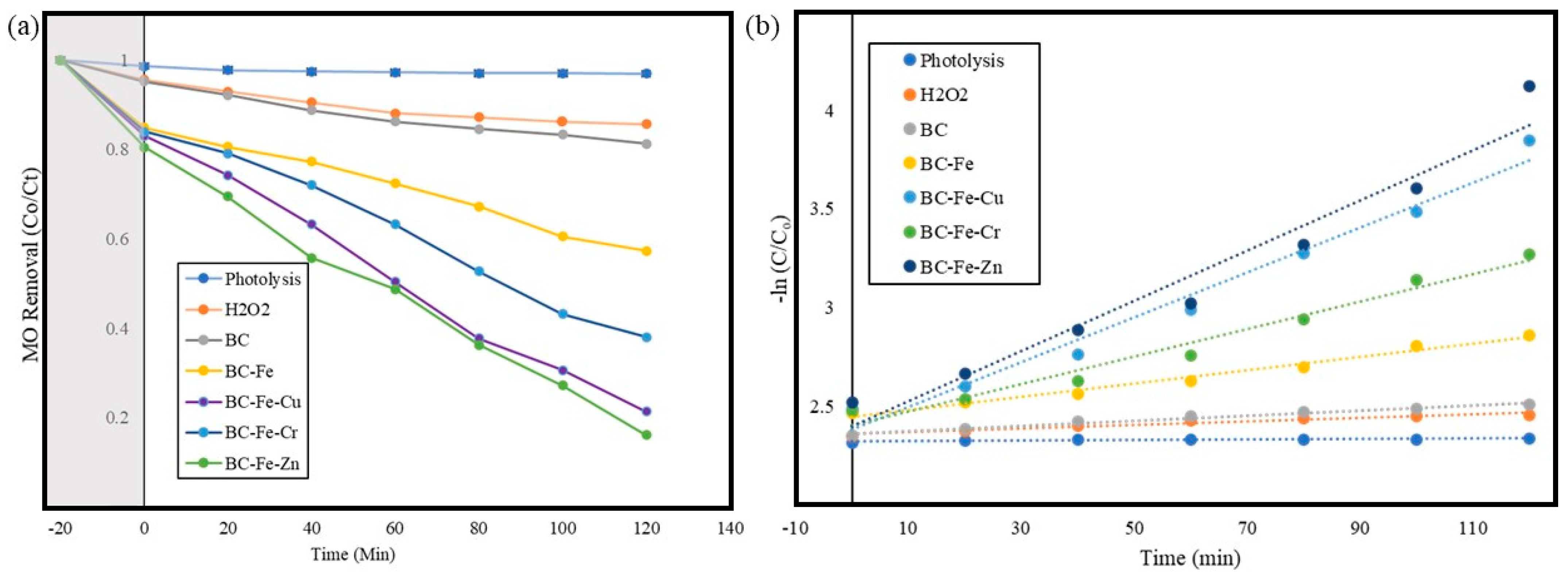
2.3. Photocatalytic Activity of Prepared Catalyst
2.3.1. Effect of Different Metal Dopants on Photoactivity
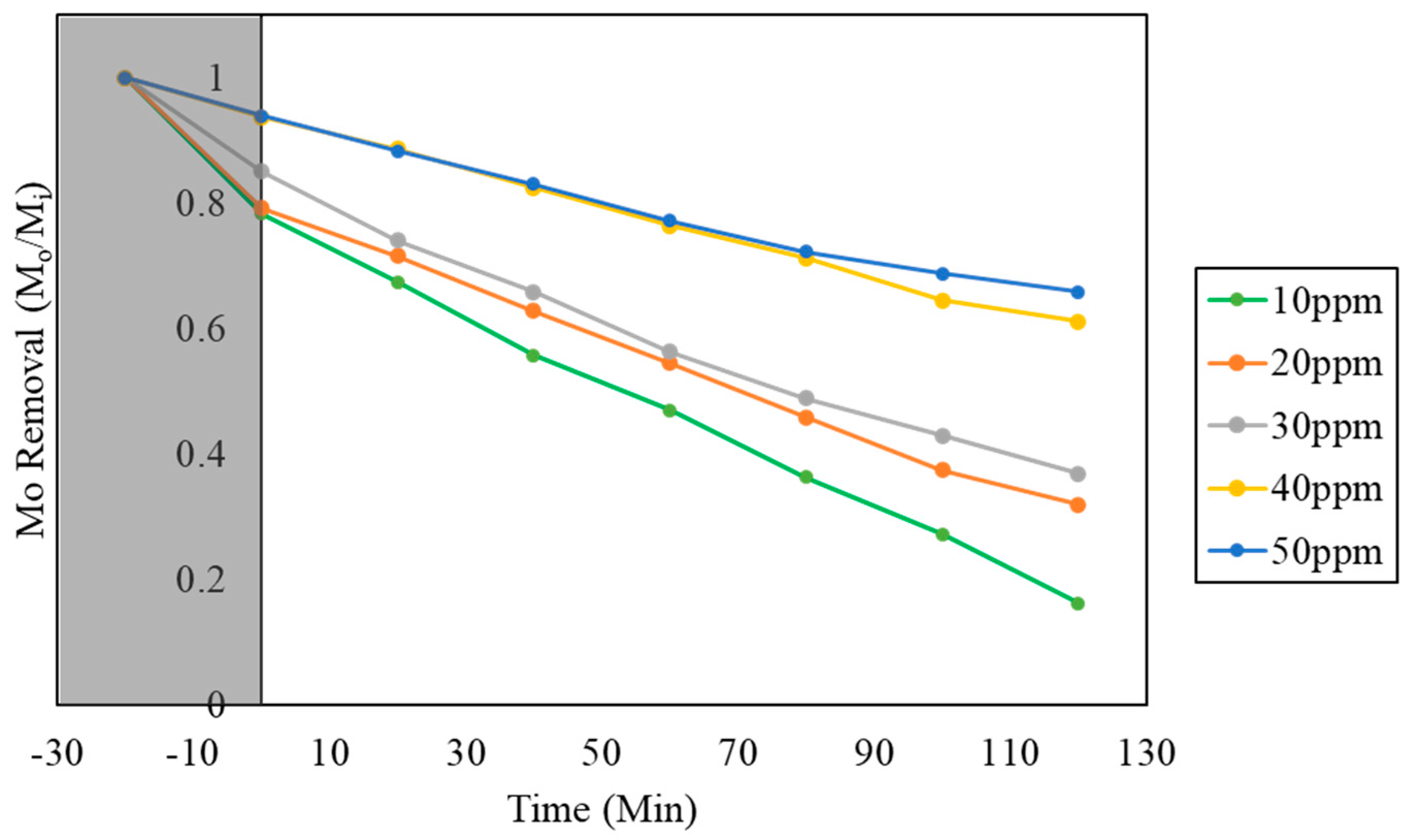
2.3.2. Effect of Different pH Values and Different Initial Concentration
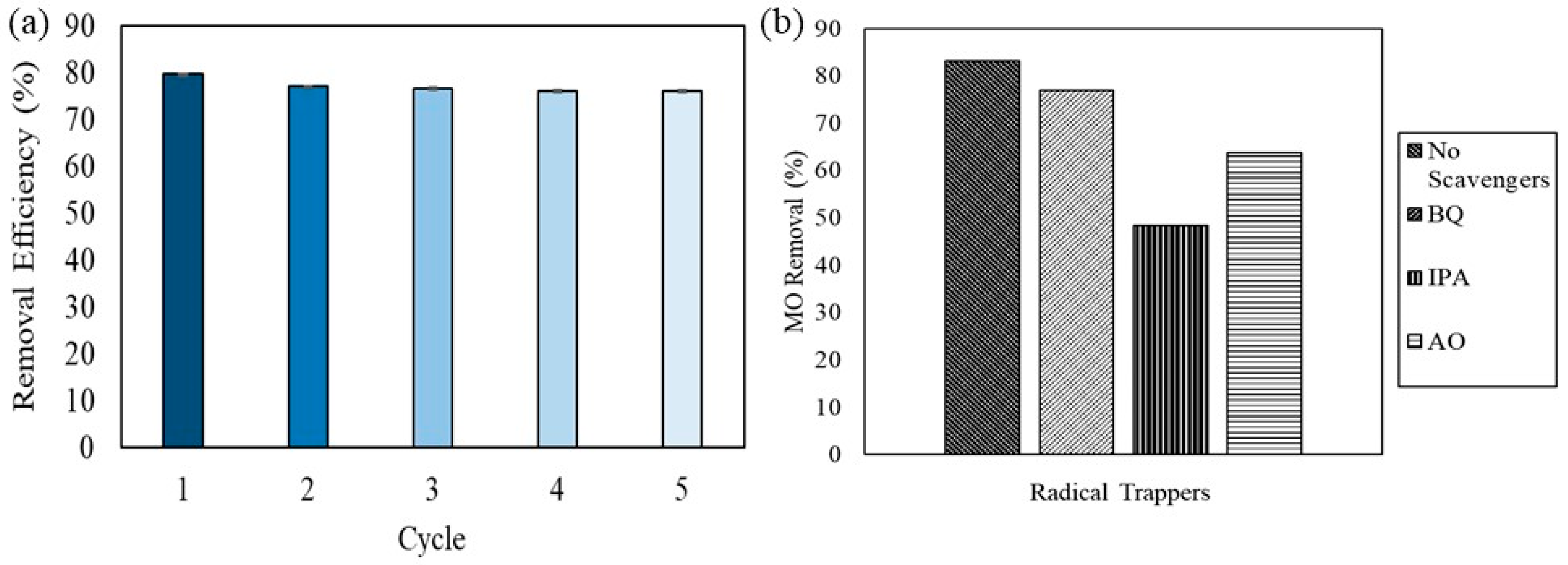
2.4. Recyclability of Photocatalyst and Radical Scavenging
2.5. Benchmarking of Photodegradation of BC–Metal Photocatalyst with Literature
3. Methodology
3.1. Materials
3.2. Synthesis of BC Photocatalyst
3.2.1. Preparation of BC
3.2.2. Synthesis of BC-Fe Photocatalyst with Metallic Dopants
3.3. Characterization of Synthesized Catalyst
3.4. Photocatalytic Tests
3.4.1. Preparation of the Model Pollutant
3.4.2. Adsorption Studies
3.4.3. Evaluation of Photocatalytic Activity
3.4.4. Reactive Oxidative Species Determination
3.4.5. Photocatalyst Recyclability Analysis
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- van Vliet, M.T.H.; Jones, E.R.; Flörke, M.; Franssen, W.H.P.; Hanasaki, N.; Wada, Y.; Yearsley, J.R. Global Water Scarcity Including Surface Water Quality and Expansions of Clean Water Technologies. Environ. Res. Lett. 2021, 16, 024020. [Google Scholar] [CrossRef]
- Ambigadevi, J.; Senthil Kumar, P.; Vo, D.-V.N.; Hari Haran, S.; Srinivasa Raghavan, T.N. Recent Developments in Photocatalytic Remediation of Textile Effluent Using Semiconductor Based Nanostructured Catalyst: A Review. J. Environ. Chem. Eng. 2021, 9, 104881. [Google Scholar] [CrossRef]
- Ighnih, H.; Haounati, R.; Malekshah, R.E.; Ouachtak, H.; Jada, A.; Addi, A.A. Photocatalytic Degradation of RhB Dye Using Hybrid Nanocomposite BiOCl@Kaol under Sunlight Irradiation. J. Water Process Eng. 2023, 54, 103925. [Google Scholar] [CrossRef]
- Al-Tohamy, R.; Ali, S.S.; Li, F.; Okasha, K.M.; Mahmoud, Y.A.G.; Elsamahy, T.; Jiao, H.; Fu, Y.; Sun, J. A Critical Review on the Treatment of Dye-Containing Wastewater: Ecotoxicological and Health Concerns of Textile Dyes and Possible Remediation Approaches for Environmental Safety. Ecotoxicol. Environ. Saf. 2022, 231, 113160. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.; Sun, Y.; Hanif, A.; Shang, J.; Shen, Z.; Hou, D.; Zhou, Y.; Chen, Q.; Ok, Y.S.; Tsang, D.C.W. Design and Fabrication of Exfoliated Mg/Al Layered Double Hydroxides on Biochar Support. J. Clean. Prod. 2021, 289, 125142. [Google Scholar] [CrossRef]
- Ou, S.-F.; Yang, D.-S.; Liao, J.-W.; Chen, S.-T. Treating High COD Dyeing Wastewater via a Regenerative Sorption-Oxidation Process Using a Nano-Pored Activated Carbon. Int. J. Mol. Sci. 2022, 23, 4752. [Google Scholar] [CrossRef]
- Karam, A.; Bakhoum, E.S.; Zaher, K. Coagulation/Flocculation Process for Textile Mill Effluent Treatment: Experimental and Numerical Perspectives. Int. J. Sustain. Eng. 2021, 14, 983–995. [Google Scholar] [CrossRef]
- Katiyar, J.; Saharan, V.K. Enhanced Photocatalytic Degradation of Reactive Blue 21 Dye and Textile Dyeing Effluent by Synthesized SmFeO3-RGO Photocatalyst in Combination with Ultrasonication: Characterization and Performance Evaluation. J. Water Process Eng. 2023, 56, 104314. [Google Scholar] [CrossRef]
- Acharya, S.M.; Chakraborty, R.; Tringe, S.G. Emerging Trends in Biological Treatment of Wastewater From Unconventional Oil and Gas Extraction. Front. Microbiol. 2020, 11, 569019. [Google Scholar] [CrossRef]
- Pipil, H.; Yadav, S.; Chawla, H.; Taneja, S.; Verma, M.; Singla, N.; Haritash, A.K. Comparison of TiO2 Catalysis and Fenton’s Treatment for Rapid Degradation of Remazol Red Dye in Textile Industry Effluent. Rend. Lincei. Sci. Fis. Nat. 2022, 33, 105–114. [Google Scholar] [CrossRef]
- Khlyustova, A.; Sirotkin, N.; Kusova, T.; Kraev, A.; Titov, V.; Agafonov, A. Doped TiO2: The Effect of Doping Elements on Photocatalytic Activity. Mater. Adv. 2020, 1, 1193–1201. [Google Scholar] [CrossRef]
- Wang, Y.; Liang, L.; Dai, H.; Li, N.; Song, Y.; Yan, B.; Chen, G.; Hou, L. Activation of Peroxymonosulfate by Food Waste Digestate Derived Biochar for Sulfamethoxazole Degradation: Performance and Mechanism. Sep. Purif. Technol. 2023, 327, 124935. [Google Scholar] [CrossRef]
- Yaashikaa, P.R.; Kumar, P.S.; Varjani, S.; Saravanan, A. A Critical Review on the Biochar Production Techniques, Characterization, Stability and Applications for Circular Bioeconomy. Biotechnol. Rep. 2020, 28, e00570. [Google Scholar] [CrossRef] [PubMed]
- Janu, R.; Mrlik, V.; Ribitsch, D.; Hofman, J.; Sedláček, P.; Bielská, L.; Soja, G. Biochar Surface Functional Groups as Affected by Biomass Feedstock, Biochar Composition and Pyrolysis Temperature. Carbon Resour. Convers. 2021, 4, 36–46. [Google Scholar] [CrossRef]
- Bharathi, D.; Lee, J.; Karthiga, P.; Sandhanasamy, R.M. Kiwi Fruit Peel Biowaste Mediated Green Synthesis of Silver Nanoparticles for Enhanced Dye Degradation and Antibacterial Activity. Waste Biomass Valoriz. 2023, 2023, 1–10. [Google Scholar] [CrossRef]
- Ghumro, S.S.; Lal, B.; Pirzada, T. Visible-Light-Driven Carbon-Doped TiO2-Based Nanocatalysts for Enhanced Activity toward Microbes and Removal of Dye. ACS Omega 2022, 7, 4333–4341. [Google Scholar] [CrossRef] [PubMed]
- Salunkhe, T.T.; Gurugubelli, T.R.; Babu, B.; Yoo, K. Recent Innovative Progress of Metal Oxide Quantum-Dot-Integrated g-C3N4 (0D-2D) Synergistic Nanocomposites for Photocatalytic Applications. Catalysts 2023, 13, 1414. [Google Scholar] [CrossRef]
- Albero, J.; Mateo, D.; García, H. Graphene-Based Materials as Efficient Photocatalysts for Water Splitting. Molecules 2019, 24, 906. [Google Scholar] [CrossRef]
- Lu, K.; Li, Y.; Tang, Z.; Xu, Y. Roles of Graphene Oxide in Heterogeneous Photocatalysis. ACS Mater. 2021, 1, 37–54. [Google Scholar] [CrossRef]
- Saini, D.; Garg, A.K.; Dalal, C.; Anand, S.R.; Sonkar, S.K.; Sonker, A.K.; Westman, G. Visible-Light-Promoted Photocatalytic Applications of Carbon Dots: A Review. ACS Appl. Nano Mater. 2022, 5, 3087–3109. [Google Scholar] [CrossRef]
- Yao, Y.; Zhang, H.; Hu, K.; Nie, G.; Yang, Y.; Wang, Y.; Duan, X.; Wang, S. Carbon Dots Based Photocatalysis for Environmental Applications. J. Environ. Chem. Eng. 2022, 10, 107336. [Google Scholar] [CrossRef]
- Phin, H.-Y.; Ong, Y.-T.; Sin, J.-C. Effect of Carbon Nanotubes Loading on the Photocatalytic Activity of Zinc Oxide/Carbon Nanotubes Photocatalyst Synthesized via a Modified Sol-Gel Method. J. Environ. Chem. Eng. 2020, 8, 103222. [Google Scholar] [CrossRef]
- Zhao, F.; Yang, Y.; Ji, S.; Yu, R.; Li, X.; Zhou, Z. Photocatalysis-Fenton Mechanism of RGO-Enhanced Fe-Doped Carbon Nitride with Boosted Degradation Performance towards Rhodamine B. J. Water Process Eng. 2023, 55, 104080. [Google Scholar] [CrossRef]
- Mondal, A.; Prabhakaran, A.; Gupta, S.; Subramanian, V.R. Boosting Photocatalytic Activity Using Reduced Graphene Oxide (RGO)/Semiconductor Nanocomposites: Issues and Future Scope. ACS Omega 2021, 6, 8734–8743. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Xu, W.; Liu, H.; Bao, Y. Titanium Dioxide–Reduced Graphene Oxide Composites for Photocatalytic Degradation of Dyes in Water. Catalysts 2022, 12, 1340. [Google Scholar] [CrossRef]
- Pinna, M.; Binda, G.; Altomare, M.; Marelli, M.; Dossi, C.; Monticelli, D.; Spanu, D.; Recchia, S. Biochar Nanoparticles over TiO2 Nanotube Arrays: A Green Co-Catalyst to Boost the Photocatalytic Degradation of Organic Pollutants. Catalysts 2021, 11, 1048. [Google Scholar] [CrossRef]
- Wang, W.; Zhang, J.; Chen, T.; Sun, J.; Ma, X.; Wang, Y.; Wang, J.; Zhonglei, X. Preparation of TiO2 -Modified Biochar and Its Characteristics of Photo-Catalysis Degradation for Enrofloxacin. Sci. Rep. 2020, 10, 6588. [Google Scholar] [CrossRef] [PubMed]
- Jamdagni, P.; Rana, J.S.; Khatri, P. Comparative Study of Antifungal Effect of Green and Chemically Synthesised Silver Nanoparticles in Combination with Carbendazim, Mancozeb, and Thiram. IET Nanobiotechnol. 2018, 12, 1102–1107. [Google Scholar] [CrossRef]
- Fatimah, I.; Purwiandono, G.; Sahroni, I.; Wijayana, A.; Faraswati, M.; Dwi Putri, A.; Oh, W.-C.; Doong, R. Magnetically-Separable Photocatalyst of Magnetic Biochar from Snake Fruit Peel for Rhodamine B Photooxidation. Environ. Nanotechnol. Monit. Manag. 2022, 17, 100669. [Google Scholar] [CrossRef]
- Adepu, A.K.; Goskula, S.; Chirra, S.; Siliveri, S.; Gujjula, S.R.; Narayanan, V. Synthesis of a High-Surface Area V2O5/TiO2-SiO2 Catalyst and Its Application in the Visible Light Photocatalytic Degradation of Methylene Blue. RSC Adv. 2019, 9, 24368–24376. [Google Scholar] [CrossRef]
- Zhao, X.; Han, R. Characterization of Bio-Char from Pyrolysis of Wheat Straw and Its Evaluation on Methylene Blue Adsorption. Desalinat. Water Treat. 2012, 46, 115–123. [Google Scholar] [CrossRef]
- Armynah, B.; Djafar, Z.; Piarah, W.H. Analysis of Chemical and Physical Properties of Biochar from Rice Husk Biomass Analysis of Chemical and Physical Properties of Biochar from Rice Husk Biomass. J. Phys. Conf. Ser. Pap. 2018, 979, 012038. [Google Scholar] [CrossRef]
- Phul, R.; Kaur, C.; Farooq, U.; Ahmad, T. Ascorbic Acid Assisted Synthesis, Characterization and Catalytic Application of Copper Nanoparticles. Mater. Sci. Eng. Int. J. 2018, 2, 90–94. [Google Scholar]
- Nomura, K. Self-Dual Leonard Pairs Green Synthesis of Copper Oxide Nanoparticles Extract Assessment and Biological Properties. De Gruyter 2019, 8, 557–567. [Google Scholar]
- Bansal, J.; Tabassum, R.; Swami, S.K.; Bishnoi, S.; Vashishtha, P.; Gupta, G.; Sharma, S.N.; Hafiz, A.K. Performance Analysis of Anomalous Photocatalytic Activity of Cr-Doped TiO2 Nanoparticles [Cr(x)TiO2(1−x)]. Appl. Phys. A Mater. Sci. Process. 2020, 126, 363. [Google Scholar] [CrossRef]
- Muhammad, W.; Ullah, N.; Haroon, M.; Abbasi, B.H. Optical, Morphological and Biological Analysis of Zinc Oxide Nanoparticles ( ZnO NPs ) Using Papaver. RSC Adv. 2019, 9, 29541–29548. [Google Scholar] [CrossRef] [PubMed]
- Tomczyk, A. Biochar Physicochemical Properties: Pyrolysis Temperature and Feedstock Kind Effects. Rev. Environ. Sci. Biol. Technol. 2020, 19, 191–215. [Google Scholar] [CrossRef]
- Liu, C.; Wang, W.; Wu, R.; Liu, Y.; Lin, X.; Kan, H.; Zheng, Y. Preparation of Acid- and Alkali-Modified Biochar for Removal of Methylene Blue Pigment. ACS Omega 2020, 5, 30906–30922. [Google Scholar] [CrossRef]
- Wei, X.; Yu, F.; Ji, J.; Cai, Y.; Zou, W.; Zheng, Y.; Huang, J.; Zhang, Y.; Yang, Y.; Naushad, M.; et al. Porous Biochar Supported Ag3PO4 Photocatalyst for “Two-in-One” Synergistic Adsorptive-Photocatalytic Removal of Methylene Blue under Visible Light Irradiation. J. Environ. Chem. Eng. 2021, 9, 106753. [Google Scholar] [CrossRef]
- Zehtab-Lotfi, E.; Amani-Ghadim, A.R.; Soltani, B. Visible Light-Driven Photocatalytic Activity of Wide Band Gap ATiO3 (A = Sr, Zn and Cd) Perovskites by Lanthanide Doping and the Formation of a Mesoporous Heterostructure with ZnS QDs. Dalt. Trans. 2022, 51, 12198–12212. [Google Scholar] [CrossRef]
- Lai, M.T.L.; Lee, K.M.; Yang, T.C.K.; Pan, G.T.; Lai, C.W.; Chen, C.-Y.; Johan, M.R.; Juan, J.C. The Improved Photocatalytic Activity of Highly Expanded MoS(2) under Visible Light Emitting Diodes. Nanoscale Adv. 2021, 3, 1106–1120. [Google Scholar] [CrossRef]
- Lu, Y.; Cai, Y.; Zhang, S.; Zhuang, L.; Hu, B.; Wang, S.; Chen, J.; Wang, X. Application of Biochar-Based Photocatalysts for Adsorption-(Photo)Degradation/Reduction of Environmental Contaminants: Mechanism, Challenges and Perspective. Biochar 2022, 4, 45. [Google Scholar] [CrossRef]
- Talukdar, K.; Jun, B.-M.; Yoon, Y.; Kim, Y.; Fayyaz, A.; Park, C.M. Novel Z-Scheme Ag3PO4/Fe3O4-Activated Biochar Photocatalyst with Enhanced Visible-Light Catalytic Performance toward Degradation of Bisphenol A. J. Hazard. Mater. 2020, 398, 123025. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, V.H.; Nguyen, T.D.; Bach, L.G.; Hoang, T.; Bui, Q.T.P.; Tran, L.D.; Nguyen, C.V.; Vo, D.V.N.; Do, S.T. Effective Photocatalytic Activity of Mixed Ni/Fe-Base Metal-Organic Framework under a Compact Fluorescent Daylight Lamp. Catalysts 2018, 8, 487. [Google Scholar] [CrossRef]
- Wang, Y.; Qiao, L.; Zhang, X.; Liu, Z.; Li, T.; Wang, H. Green Synthesis of FeCu@biochar Nanocomposites through a Mechanochemical Method for Enhanced Tetracycline Degradation via Peroxymonosulfate Activation. Sep. Purif. Technol. 2024, 328, 125077. [Google Scholar] [CrossRef]
- Monteiro, F.C.; Guimaraes, I.D.L.; de Almeida Rodrigues, P.; da Anunciação de Pinho, J.V.; Conte-Junior, C.A. Degradation of PAHs Using TiO2 as a Semiconductor in the Heterogeneous Photocatalysis Process: A Systematic Review. J. Photochem. Photobiol. A Chem. 2023, 437, 114497. [Google Scholar] [CrossRef]
- Modrogan, C.; Cǎprǎrescu, S.; Purcar, V.; Radit, V. Modified Composite Based on Magnetite and Polyvinyl Alcohol: Synthesis, Characterization, and Degradation Studies of the Methyl Orange Dye from Synthetic Wastewater. Polymers 2021, 13, 3911. [Google Scholar] [CrossRef]
- Li, M.; Dong, B.; Chang, Z.; Dang, H.; Ma, S.; Li, W. Synthesis of TiO2/g-C3N4 Photocatalyst with Recovered TiO2 from Spent SCR Catalyst for Photodegrading Rhodamine B. Waste Biomass Valoriz. 2023, 14, 687–701. [Google Scholar] [CrossRef]
- Wang, X.; Yang, Z.; Jiang, Y.; Zhao, P.; Meng, X. Adsorption and Catalytic Degradation of Phenol in Water by a Mn, N Co-Doped Biochar via a Non-Radical Oxidation Process. Sep. Purif. Technol. 2024, 330, 125267. [Google Scholar] [CrossRef]
- Saeed, M.; Muneer, M.; Mumtaz, N.; Siddique, M.; Akram, N.; Hamayun, M. Ag-Co3O4: Synthesis, Characterization and Evaluation of Its Photo-Catalytic Activity towards Degradation of Rhodamine B Dye in Aqueous Medium. Chinese J. Chem. Eng. 2018, 26, 1264–1269. [Google Scholar] [CrossRef]
- Zhang, C.; Ma, Y.; Li, C.; Qin, F.; Hu, C.; Hu, Q.; Duo, S. Spatially Confined Growth of Bi2O4 into Hierarchical TiO2 Spheres for Improved Visible Light Photocatalytic Activity. J. Mater. Sci. 2020, 55, 3181–3194. [Google Scholar] [CrossRef]
- Vorontsov, A.V.; Valdés, H.; Smirniotis, P.G.; Paz, Y. Recent Advancements in the Understanding of the Surface Chemistry in TiO2 Photocatalysis. Surfaces 2020, 3, 72–92. [Google Scholar] [CrossRef]
- Youssef, N.A.; Shaban, S.A.; Ibrahim, F.A.; Mahmoud, A.S. Degradation of Methyl Orange Using Fenton Catalytic Reaction. Egypt. J. Pet. 2016, 25, 317–321. [Google Scholar] [CrossRef]
- Cui, X.; Wang, Y.; Jiang, G.; Zhao, Z.; Xu, C.; Duan, A.; Liu, J.; Wei, Y.; Bai, W. The Encapsulation of CdS in Carbon Nanotubes for Stable and Efficient Photocatalysis. J. Mater. Chem. A 2014, 2, 20939–20946. [Google Scholar] [CrossRef]
- Song, C.; Chen, K.; Chen, M.; Jin, X.; Liu, G.; Du, X.; Chen, D.; Huang, Q. Sequential Combined Adsorption and Solid-Phase Photocatalysis to Remove Aqueous Organic Pollutants by H3PO4-Modified TiO2 Nanoparticles Anchored on Biochar. J. Water Process Eng. 2022, 45, 102467. [Google Scholar] [CrossRef]
- Asgharzadeh, F.; Rezaei, R.; Gholami, M.; Jafari, A.J.; Kermani, M. TiO2—Decorated Magnetic Biochar Mediated Heterogeneous Photocatalytic Degradation of Tetracycline and Evaluation of Antibacterial Activity. Biomass Convers. Biorefinery 2021, 2021, 1–11. [Google Scholar] [CrossRef]
- Asgharzadeh, F.; Gholami, M.; Jonidi, A.; Kermani, M. Heterogeneous Photocatalytic Degradation of Metronidazole from Aqueous Solutions Using Fe3O4/TiO2 Supported on Biochar. Desalinat. Water Treat. 2020, 175, 24789. [Google Scholar] [CrossRef]
- Sadati, H.; Ayati, B. Using a Promising Biomass-Based Biochar in Photocatalytic Degradation: Highly Impressive Performance of RHB/SnO2/Fe3O4 for Elimination of AO7. Photochem. Photobiol. Sci. 2023, 22, 1445–1462. [Google Scholar] [CrossRef]
- Silvestri, S.; Stefanello, N.; Sulkovski, A.A.; Foletto, E.L. Preparation of TiO2 Supported on MDF Biochar for Simultaneous Removal of Methylene Blue by Adsorption and Photocatalysis. J. Chem. Technol. Biotechnol. 2020, 95, 2723–2729. [Google Scholar] [CrossRef]
- Syed Abd Halim, S.N.Q.; Nazri, N.A.M.; Nordin, N.A.H.M. Photocatalytic Degradation of Anthracene by Biochar-Based Graphitic Carbon Nitride. IOP Conf. Ser. Mater. Sci. Eng. 2021, 1195, 012053. [Google Scholar] [CrossRef]
- Rego, F.; Xiang, H.; Yang, Y.; Ordovás, J.L.; Chong, K.; Wang, J.; Bridgwater, A. Investigation of the Role of Feedstock Properties and Process Conditions on the Slow Pyrolysis of Biomass in a Continuous Auger Reactor. J. Anal. Appl. Pyrolysis 2022, 161, 105378. [Google Scholar] [CrossRef]
- Leichtweis, J.; Silvestri, S.; Welter, N.; Vieira, Y.; Zaragoza-sánchez, P.I.; Chávez-mejía, A.C.; Carissimi, E. Wastewater Containing Emerging Contaminants Treated by Residues from the Brewing Industry Based on Biochar as a New CuFe2O4/Biochar Photocatalyst. Process Saf. Environ. Prot. 2021, 150, 497–509. [Google Scholar] [CrossRef]
- Nguyen, M.B.; Le, G.H.; Nguyen, T.D.; Nguyen, Q.K.; Pham, T.T.T.; Lee, T.; Vu, T.A. Bimetallic Ag-Zn-BTC/GO Composite as Highly Efficient Photocatalyst in the Photocatalytic Degradation of Reactive Yellow 145 Dye in Water. J. Hazard. Mater. 2021, 420, 126560. [Google Scholar] [CrossRef]
- Revellame, E.D.; Fortela, D.L.; Sharp, W.; Hernandez, R.; Zappi, M.E. Adsorption Kinetic Modeling Using Pseudo-First Order and Pseudo-Second Order Rate Laws: A Review. Clean. Eng. Technol. 2020, 1, 100032. [Google Scholar] [CrossRef]
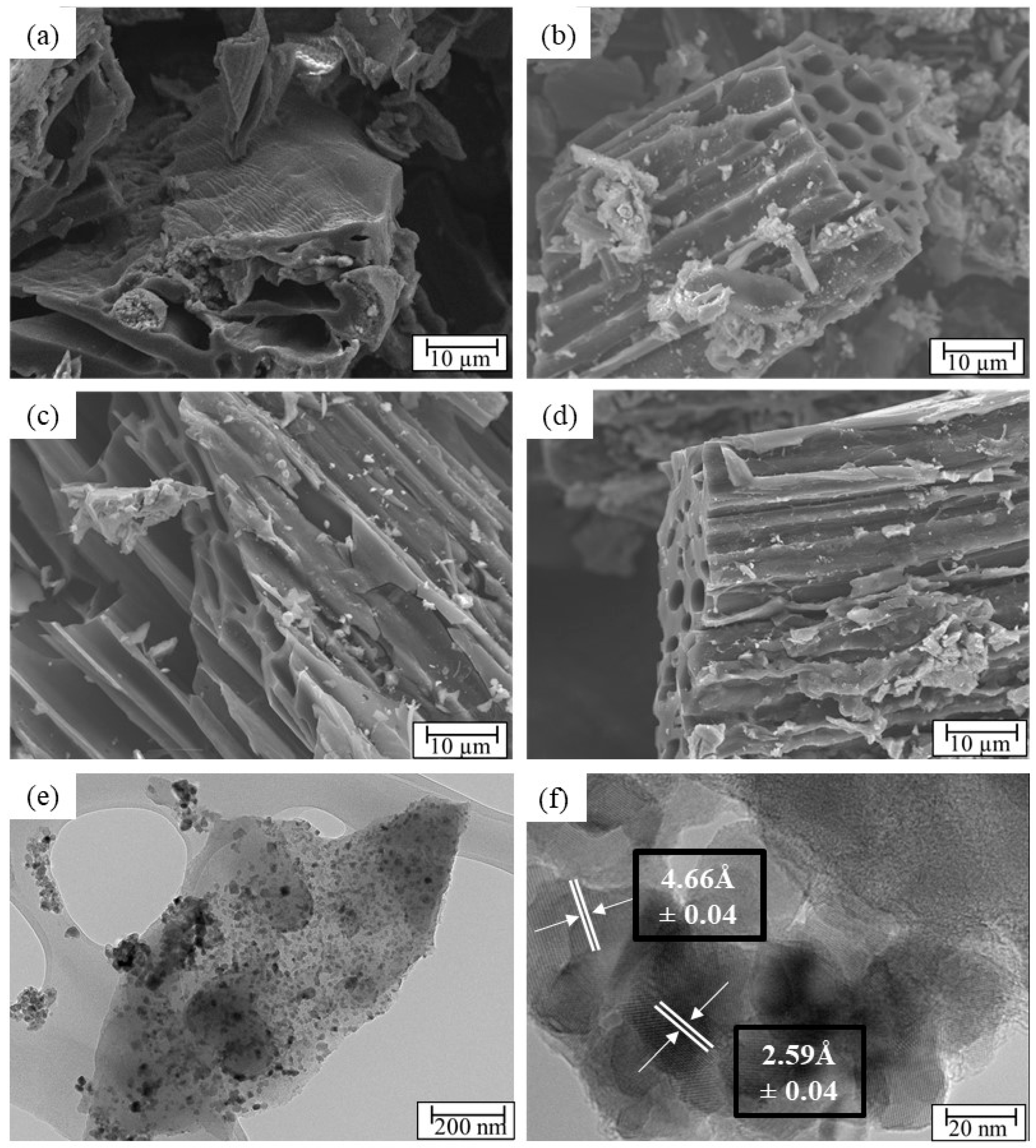

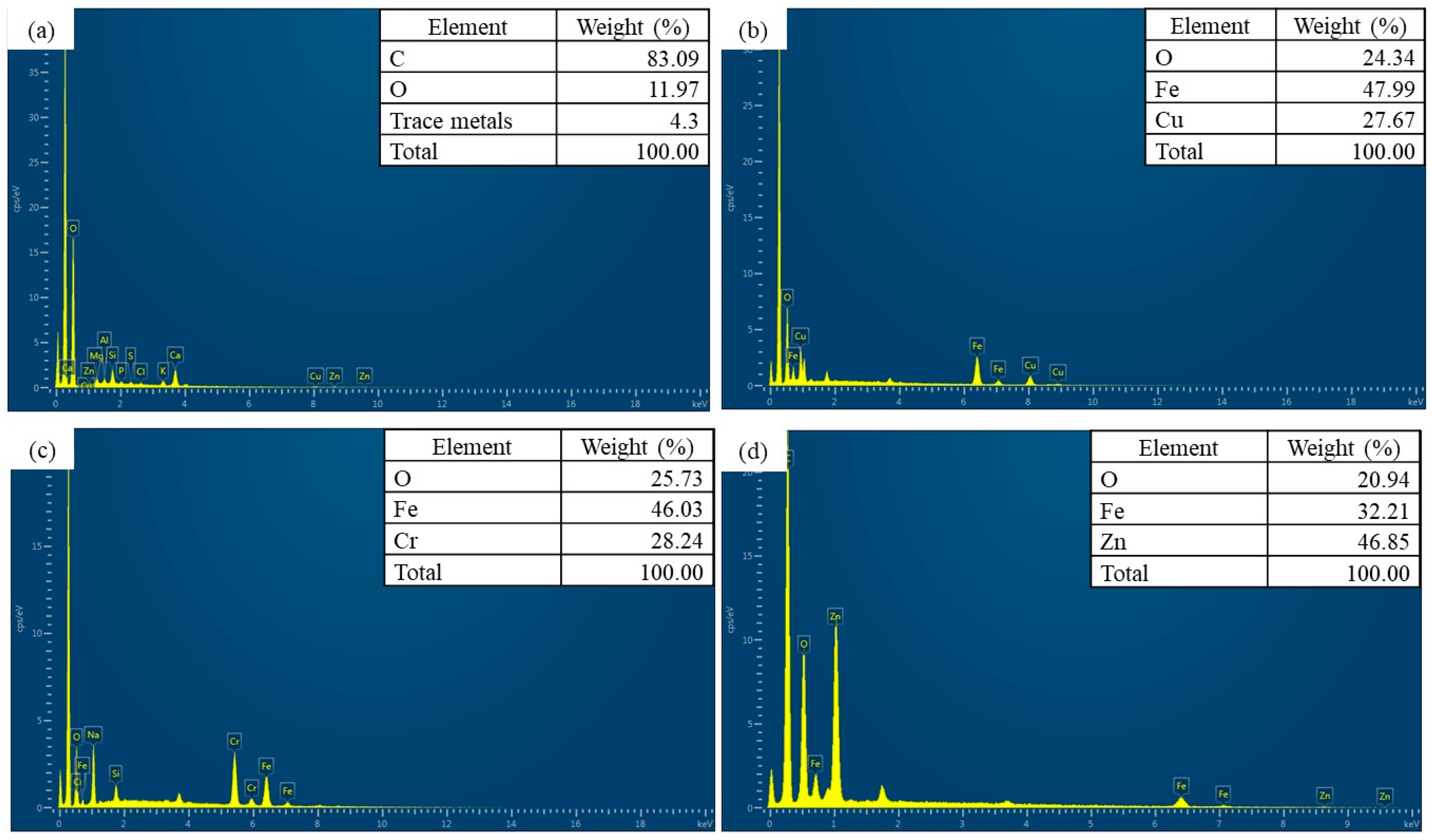
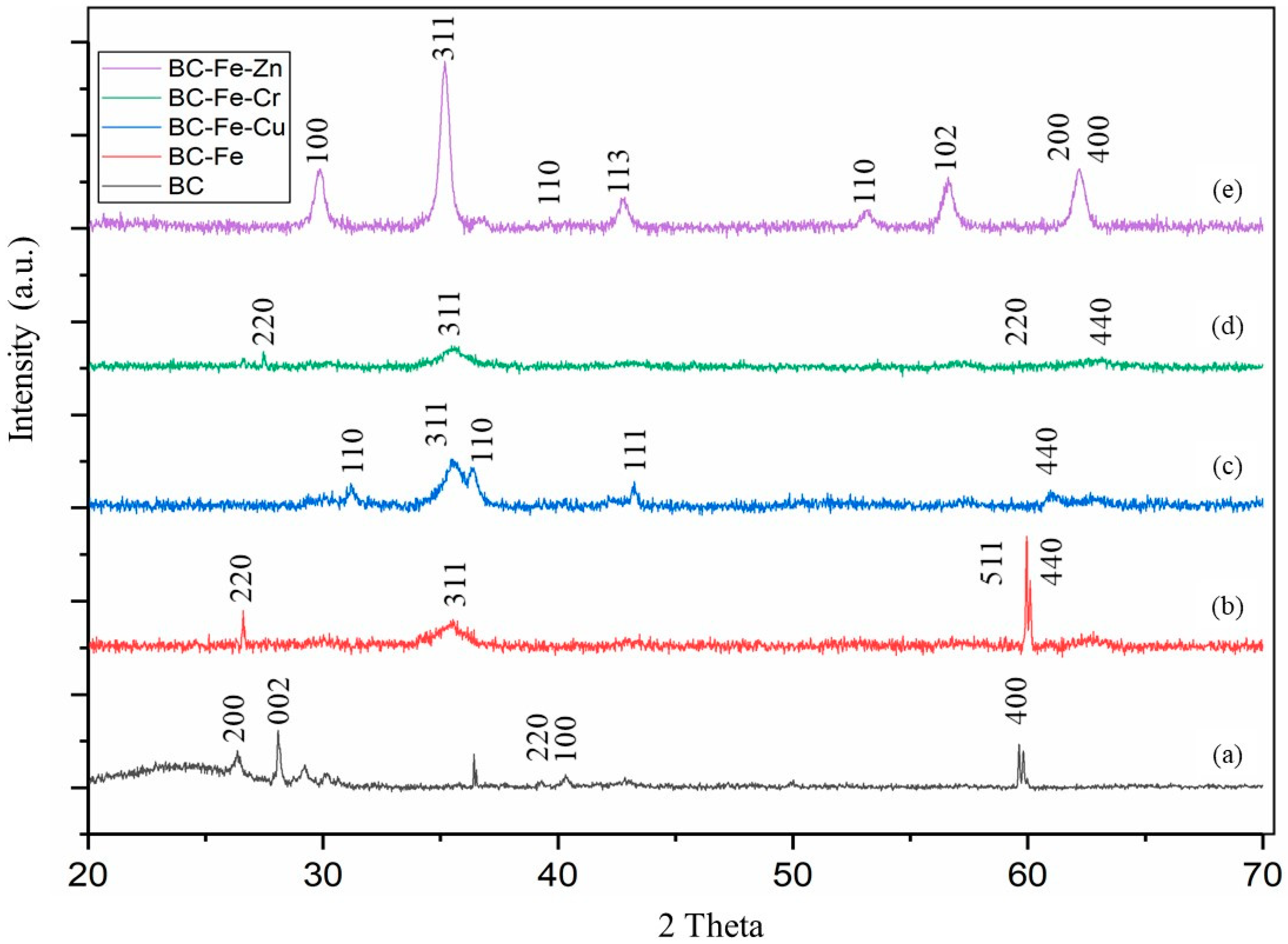
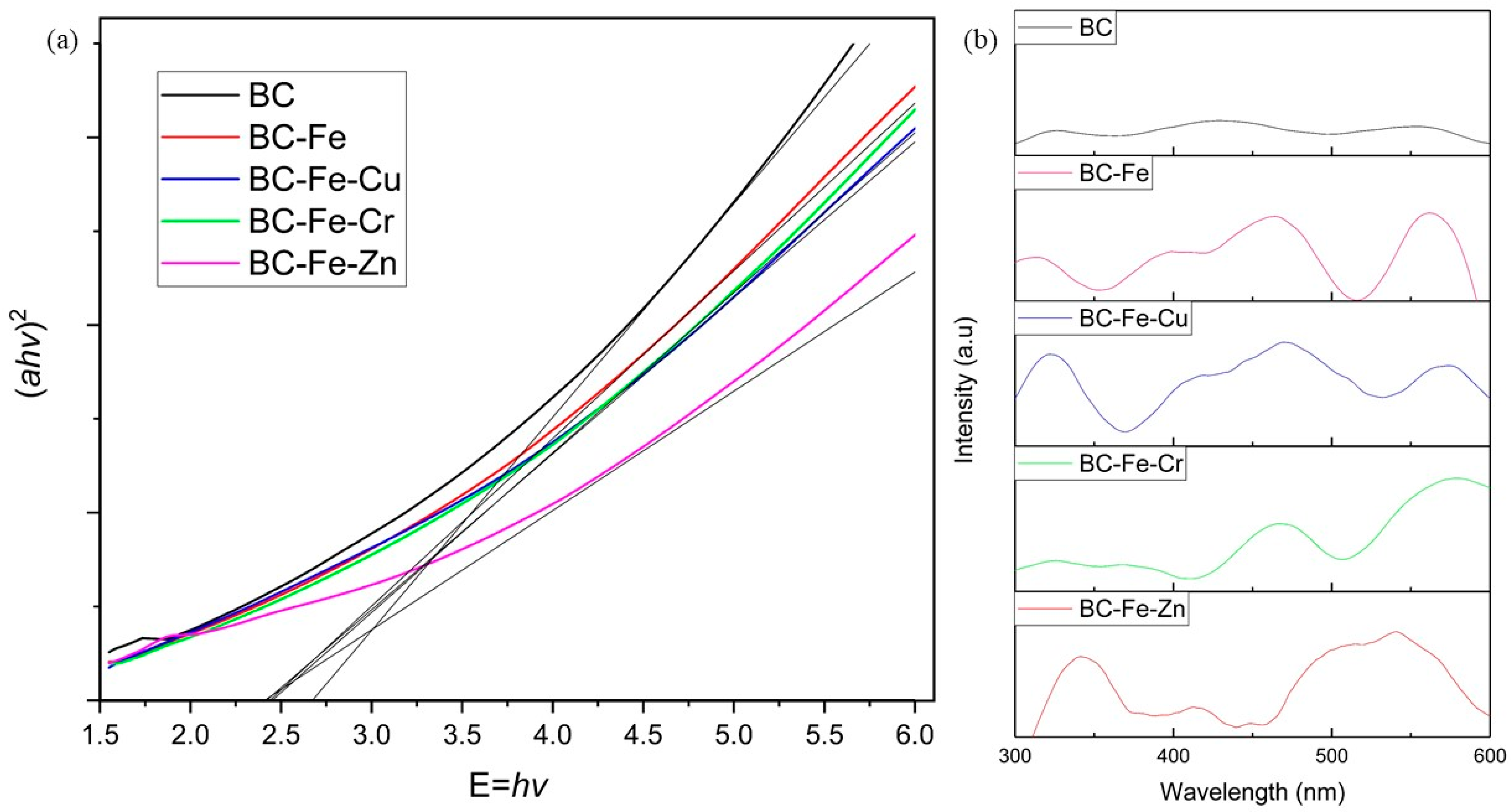


| Sample | 2θ (Corresponding Planes) |
|---|---|
| BC | 26.3° (200), 28.2° (002), 39.3° (220), 40.3° (100), 59.4° (400) |
| BC-Fe | 26.6° (220), 35.7° (311), 59.9° (511), 62.9° (440) |
| BC-Fe-Cu | 30.6° (110), 35.5° (311), 36.3° (110), 43.2° (111), 62.7° (440) |
| BC-Fe-Cr | 27.4° (220), 35.7° (311), 63.1° (220), 62.6° (440) |
| BC-Fe-Zn | 29.8° (100), 35.2° (311), 39.9° (110), 42.7° (113) 53.1° (110), 59.6° (102), 62.2° (200) (440) |
| Material | SSA (m2/g) | Average Pore Size (nm) | Average Pore Volume (cm3/g) | Zeta Potential (mV) |
|---|---|---|---|---|
| BC | 41.07 ± 1.1 | 3.09 ± 0.2 | 3.73 ± 0.5 | −31.13 |
| BC-Fe | 55.26 ± 1.2 | 7.88 ± 0.5 | 10.42 ± 0.6 | −21.23 |
| BC-Fe-Cu | 119.41 ± 0.9 | 2.01 ± 0.4 | 5.62 ± 0.6 | −52.60 |
| BC-Fe-Cr | 102.27 ± 2.1 | 3.15 ± 0.3 | 7.37 ± 0.7 | −30.87 |
| BC-Fe-Zn | 117.99 ± 1.8 | 2.72 ± 0.4 | 7.72 ± 0.8 | −45.10 |
| Samples | k (ppm min−1) | R2 |
|---|---|---|
| Photolysis | 0.0181 | 0.8024 |
| H2O2 | 0.0565 | 0.9413 |
| BC | 0.0585 | 0.9788 |
| BC-Fe | 0.5505 | 0.9833 |
| BC-Fe-Cu | 1.4477 | 0.9732 |
| BC-Fe-Cr | 0.9419 | 0.9768 |
| BC-Fe-Zn | 1.621 | 0.9531 |
| Photocatalyst | Biomass Source | Pollutant | Performance (Time) | Recyclability | Reference |
|---|---|---|---|---|---|
| BC-Fe-Zn | Wheat straw | MO (10 ppm) | 83.33% (120 min) | >90% (5 cycles) | This work |
| BC-Sn-Fe | Rice husk | Acid Orange 7 (OA7, 10 ppm) | 82.22% (120 min) | >70% (5 cycles) | [58] |
| BC-TiO2 | Microalgae (Nannochloropsis sp.) | MB (10 ppm) | 90.00% (180 min) | - | [26] |
| BC-TiO2 | Medium Density Fiberboard (MDF) | MB (10 ppm) | 90.00% (180 min) | >80% (3 cycles) | [59] |
| BC(SB)/g-C3N4 | Waste biomass | Anthracene (2 ppm) | 95.00% (240 min) | >80% (5 cycles) | [60] |
| Sample Name | Metal Dopant | Sample Denomination |
|---|---|---|
| Biochar | Nil | BC |
| Biochar-Fe | Fe | BC-Fe |
| Biochar-Fe-Cu | Fe, Cu | BC-Fe-Cu |
| Biochar-Fe-Cr | Fe, Cr | BC-Fe-Cr |
| Biochar-Fe-Zn | Fe, Zn | BC-Fe-Zn |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Subramaniam, M.N.; Zheng, J.; Wu, Z.; Goh, P.S.; Zhang, G. Visible Light-Driven Organic Pollutant Removal Using Fe-Based Photocatalysts Supported by Wheat Straw Biochar. Catalysts 2024, 14, 43. https://doi.org/10.3390/catal14010043
Subramaniam MN, Zheng J, Wu Z, Goh PS, Zhang G. Visible Light-Driven Organic Pollutant Removal Using Fe-Based Photocatalysts Supported by Wheat Straw Biochar. Catalysts. 2024; 14(1):43. https://doi.org/10.3390/catal14010043
Chicago/Turabian StyleSubramaniam, Mahesan Naidu, Jiaojiao Zheng, Zhentao Wu, Pei Sean Goh, and Guangru Zhang. 2024. "Visible Light-Driven Organic Pollutant Removal Using Fe-Based Photocatalysts Supported by Wheat Straw Biochar" Catalysts 14, no. 1: 43. https://doi.org/10.3390/catal14010043






