Nanodiamond Supported Ultra-Small Palladium Nanoparticles as an Efficient Catalyst for Suzuki Cross-Coupling Reactions
Abstract
:1. Introduction
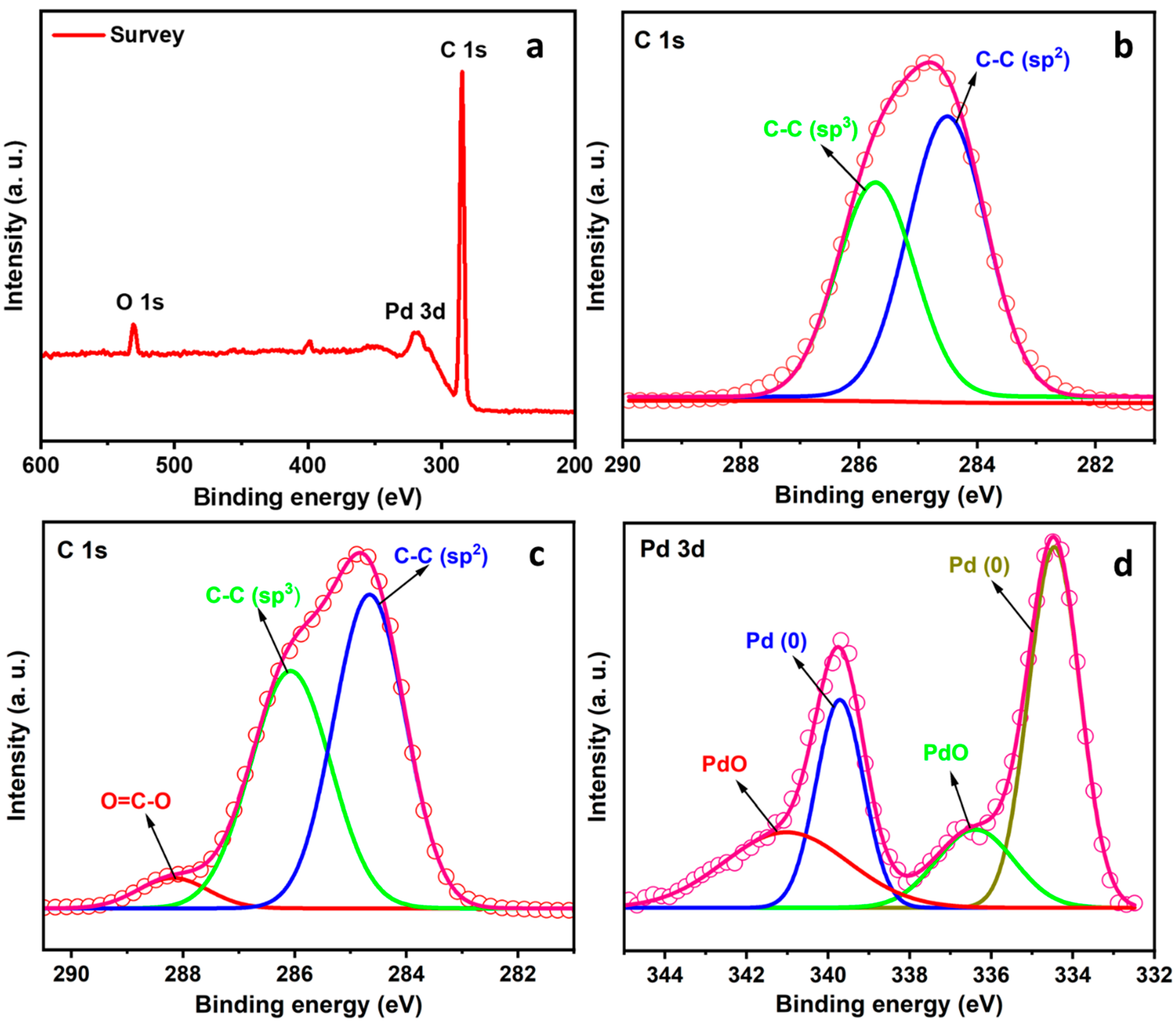
2. Results and Discussion
3. Materials and Methods
3.1. Materials
3.2. General Procedure for the Preparation of Oxidized NDs
3.3. Synthesis of rNDs/Palladium Composite
3.4. Preparation of Palladium Nanoparticles
3.5. General Procedure for Suzuki–Miyaura Reaction of Aryl Halide and Boronic Acid
4. Catalytic Activity
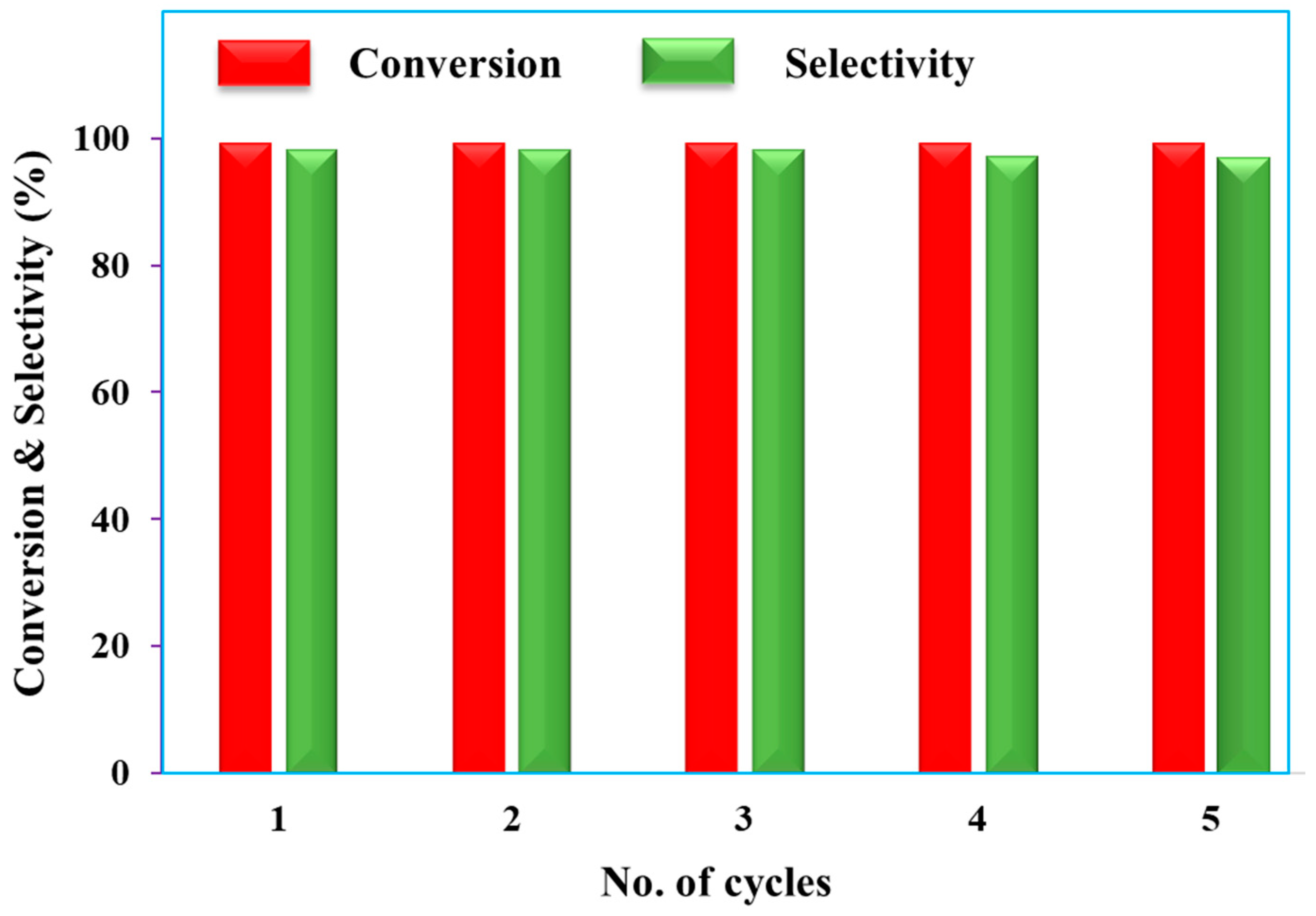
4.1. Recyclability Studies
4.2. Reusability of the Pd/rNDs Catalyst
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Barnard, B.C. Palladium-catalysed C-C coupling: Then and now. Platin. Met. Rev. 2008, 52, 38–45. [Google Scholar] [CrossRef]
- Pagliaro, M.; Pandarus, V.; Ciriminna, R.; Béland, F.; Demma Carà, P. Heterogeneous versus homogeneous palladium catalysts for cross-coupling reactions. ChemCatChem 2012, 4, 432–445. [Google Scholar] [CrossRef]
- Wu, X.-F.; Anbarasan, P.; Neumann, H.; Beller, M. From noble metal to nobel prize: Palladium-catalyzed coupling reactions as key methods in organic synthesis. Angew. Chem. Int. Ed. 2010, 49, 9047–9050. [Google Scholar] [CrossRef] [PubMed]
- Rouhi Maureen, A. Suzuki-coupling chemistry takes hold in commercial practice, from small-scale synthesis of screening compounds to industrial production of active ingredients. Chem. Eng. News 2004, 82, 49–58. [Google Scholar] [CrossRef]
- Garrett, C.E.; Prasad, K. The art of meeting palladium specifications in active pharmaceutical ingredients produced by Pd-catalyzed reactions. Adv. Synth. Catal. 2004, 346, 889–900. [Google Scholar] [CrossRef]
- Nicolaou, K.C.; Bulger, P.G.; Sarlah, D. Palladium-catalyzed cross-coupling reactions in total synthesis. Angew. Chem. Int. Ed. 2005, 44, 4442–4489. [Google Scholar] [CrossRef]
- So, C.M.; Kwong, F.Y. Palladium-catalyzed cross-coupling reactions of aryl mesylates. Chem. Soc. Rev. 2011, 40, 4963–4972. [Google Scholar] [CrossRef]
- Corbet, J.-P.; Mignani, G. Selected patented cross-coupling reaction technologies. Chem. Rev. 2006, 106, 2651–2710. [Google Scholar] [CrossRef]
- Fan, G.; Liu, Q.; Tang, D.; Li, X.; Bi, J.; Gao, D. Nanodiamond supported Ru nanoparticles as an effective catalyst for hydrogen evolution from hydrolysis of ammonia borane. Int. J. Hydrogen Energy 2016, 41, 1542–1549. [Google Scholar] [CrossRef]
- Zhang, L.; Liu, H.; Huang, X.; Sun, X.; Jiang, Z.; Schlögl, R.; Su, D. Stabilization of palladium nanoparticles on nanodiamond-graphene core-shell supports for CO oxidation. Angew. Chem. Int. Ed. 2015, 54, 15823–15826. [Google Scholar] [CrossRef]
- Ba, H.; Truong-Phuoc, L.; Liu, Y.; Duong-Viet, C.; Nhut, J.-M.; Nguyen-Dinh, L.; Granger, P.; Pham-Huu, C. Hierarchical carbon nanofibers/graphene composite containing nanodiamonds for direct dehydrogenation of ethylbenzene. Carbon 2016, 96, 1060–1069. [Google Scholar] [CrossRef]
- Starodubtseva, E.V.; Vinogradov, M.G.; Turova, O.V.; Bumagin, N.A.; Rakov, E.G.; Sokolov, V.I. Palladium(0) supported on carbon nanotubes as an efficient catalyst of the C≡C bond hydrogenation. Catal. Commun. 2009, 10, 1441–1442. [Google Scholar] [CrossRef]
- Golubina, E.V.; Lokteva, E.S.; Erokhin, A.V.; Veligzhanin, A.A.; Zubavichus, Y.V.; Likholobov, V.A.; Lunin, V.V. The role of metal-support interaction in catalytic activity of nanodiamond-supported nickel in selective phenylacetylene hydrogenation. J. Catal. 2016, 344, 90–99. [Google Scholar] [CrossRef]
- Siamaki, A.R.; Lin, Y.; Woodberry, K.; Connell, J.W.; Gupton, B.F. Palladium nanoparticles supported on carbon nanotubes from solventless preparations: Versatile catalysts for ligand-free Suzuki cross coupling reactions. J. Mater. Chem. A 2013, 1, 12909–12918. [Google Scholar] [CrossRef]
- Martin, R.; Buchwald, S.L. Palladium-catalyzed Suzuki-Miyaura cross-coupling reactions employing dialkylbiaryl phosphine ligands. Acc. Chem. Res. 2008, 41, 1461–1473. [Google Scholar] [CrossRef] [PubMed]
- Rathi, A.K.; Gawande, M.B.; Pechousek, J.; Tucek, J.; Aparicio, C.; Petr, M.; Tomanec, O.; Krikavova, R.; Travnicek, Z.; Varma, R.S.; et al. Maghemite decorated with ultra-small palladium nanoparticles (γ-Fe2O3-Pd): Applications in the heck-mizoroki olefination, Suzuki reaction and allylic oxidation of alkenes. Green Chem. 2016, 18, 2363–2373. [Google Scholar] [CrossRef]
- Sharma, R.K.; Dutta, S.; Sharma, S.; Zboril, R.; Varma, R.S.; Gawande, M.B. Fe3O4 (iron oxide)-supported nanocatalysts: Synthesis, characterization and applications in coupling reactions. Green Chem. 2016, 18, 3184–3209. [Google Scholar] [CrossRef]
- Sharma, R.K.; Yadav, M.; Gaur, R.; Gupta, R.; Adholeya, A.; Gawande, M.B. Synthesis of iron oxide palladium nanoparticles and their catalytic applications for direct coupling of acyl chlorides with alkynes. ChemPlusChem 2016, 81, 1312–1319. [Google Scholar] [CrossRef]
- Sharma, R.K.; Yadav, S.; Dutta, S.; Kale, H.B.; Warkad, I.R.; Zbořil, R.; Varma, R.S.; Gawande, M.B. Silver nanomaterials: Synthesis and (electro/photo) catalytic applications. Chem. Soc. Rev. 2021, 50, 11293–11380. [Google Scholar] [CrossRef]
- Heck, R.F. Palladium-catalyzed reactions of organic halides with olefins. Acc. Chem. Res. 1979, 12, 146–151. [Google Scholar] [CrossRef]
- Miyaura, N.; Suzuki, A. Palladium-catalyzed cross-coupling reactions of organoboron compounds. Chem. Rev. 1995, 95, 2457–2483. [Google Scholar] [CrossRef]
- Butters, M.; Catterick, D.; Craig, A.; Curzons, A.; Dale, D.; Gillmore, A.; Green, S.P.; Marziano, I.; Sherlock, J.-P.; White, W. Critical assessment of pharmaceutical processes-a rationale for changing the synthetic route. Chem. Rev. 2006, 106, 3002–3027. [Google Scholar] [CrossRef] [PubMed]
- Welch, C.J.; Albaneze-Walker, J.; Leonard, W.R.; Biba, M.; DaSilva, J.; Henderson, D.; Laing, B.; Mathre, D.J.; Spencer, S.; Bu, X.; et al. Adsorbent screening for metal impurity removal in pharmaceutical process research. Org. Process Res. Dev. 2005, 9, 198–205. [Google Scholar] [CrossRef]
- Chen, Z.; Vorobyeva, E.; Mitchell, S.; Fako, E.; Ortuño, M.A.; López, N.; Collins, S.M.; Midgley, P.A.; Richard, S.; Vilé, G.; et al. A heterogeneous single-atom palladium catalyst surpassing homogeneous systems for Suzuki coupling. Nat. Nanotechnol. 2018, 13, 702–707. [Google Scholar] [CrossRef]
- Saptal, V.B.; Saptal, M.V.; Mane, R.S.; Sasaki, T.; Bhanage, B.M. Amine-functionalized graphene oxide-stabilized Pd nanoparticles (Pd@APGO): A novel and efficient catalyst for the Suzuki and carbonylative Suzuki-Miyaura coupling reactions. ACS Omega 2019, 4, 643–649. [Google Scholar] [CrossRef] [PubMed]
- Duan, X.; Ao, Z.; Li, D.; Sun, H.; Zhou, L.; Suvorova, A.; Saunders, M.; Wang, G.; Wang, S. Surface-tailored nanodiamonds as excellent metal-free catalysts for organic oxidation. Carbon 2016, 103, 404–411. [Google Scholar] [CrossRef]
- Kuuloja, N.; Kylmälä, T.; Xu, Y.; Franzén, R. Synthesis of Xenbucin using Suzuki reaction catalyzed by Pd/C in water. Open Chem. 2008, 6, 390–392. [Google Scholar] [CrossRef]
- King, A.K.; Brar, A.; Li, G.; Findlater, M. Homogeneous and recyclable palladium catalysts: Application in Suzuki–Miyaura cross-coupling reactions. Organometallics 2023, 42, 2353–2358. [Google Scholar] [CrossRef]
- Miyaura, N.; Yamada, K.; Suzuki, A. A new stereospecific cross-coupling by the palladium-catalyzed reaction of 1-alkenylboranes with 1-alkenyl or 1-alkynyl halides. Tetrahedron Lett. 1979, 20, 3437–3440. [Google Scholar] [CrossRef]
- Hooshmand, S.E.; Heidari, B.; Sedghi, R.; Varma, R.S. Recent advances in the Suzuki–Miyaura cross-coupling reaction using efficient catalysts in eco-friendly media. Green Chem. 2019, 21, 381–405. [Google Scholar] [CrossRef]
- Suzuki, A. Organoborates in new synthetic reactions. Acc. Chem. Res. 1982, 15, 178–184. [Google Scholar] [CrossRef]
- Miyaura, N.; Suzuki, A. Stereoselective synthesis of arylated (E)-alkenes by the reaction of alk-1-enylboranes with aryl halides in the presence of palladium catalyst. J. Chem. Soc. Chem. Commun. 1979, 19, 866–867. [Google Scholar] [CrossRef]
- Genkin, A.D.; Evstigneeva, T.L. Associations of platinum-group minerals of the Noril’sk copper-nickel sulfide ores. Econ. Geol. 1986, 81, 1203–1212. [Google Scholar] [CrossRef]
- Sanderson, K. Chemistry: It’s not easy being green. Nature 2011, 469, 18–20. [Google Scholar] [CrossRef] [PubMed]
- Bernardis, F.L.; Grant, R.A.; Sherrington, D.C. A review of methods of separation of the platinum-group metals through their chloro-complexes. React. Funct. Polym. 2005, 65, 205–217. [Google Scholar] [CrossRef]
- Molnár, Á. Efficient, selective, and recyclable palladium catalysts in carbon-carbon coupling reactions. Chem. Rev. 2011, 111, 2251–2320. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Wang, Z. Diatomite-supported Pd nanoparticles: An efficient catalyst for Heck and Suzuki reactions. J. Org. Chem. 2006, 71, 7485–7487. [Google Scholar] [CrossRef]
- Kann, N. Recent applications of polymer supported organometallic catalysts in organic synthesis. Molecules 2010, 15, 6306–6331. [Google Scholar] [CrossRef]
- Dhepe, P.L.; Fukuoka, A. Cellulose conversion under heterogeneous catalysis. ChemSusChem 2008, 1, 969–975. [Google Scholar] [CrossRef]
- Lam, E.; Hrapovic, S.; Majid, E.; Chong, J.H.; Luong, J.H.T. Catalysis using gold nanoparticles decorated on nanocrystalline cellulose. Nanoscale 2012, 4, 997–1002. [Google Scholar] [CrossRef]
- Djakovitch, L.; Koehler, K. Heck reaction catalyzed by Pd-modified zeolites. J. Am. Chem. Soc. 2001, 123, 5990–5999. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.; Johnson, M.; Veinot, J.G.C. Iron/iron oxide nanoparticles: A versatile support for catalytic metals and their application in Suzuki-Miyaura cross-coupling reactions. ChemComm 2010, 46, 2411–2413. [Google Scholar]
- Baig, R.B.N.; Nadagouda, M.N.; Varma, R.S. Ruthenium on chitosan: A recyclable heterogeneous catalyst for aqueous hydration of nitriles to amides. Green Chem. 2014, 16, 2122–2127. [Google Scholar] [CrossRef]
- El Hankari, S.; El Kadib, A.; Finiels, A.; Bouhaouss, A.; Moreau, J.J.; Crudden, C.M.; Brunel, D.; Hesemann, P. SBA-15-type organosilica with 4-mercapto-N,N-bis-(3-Si-propyl)butanamide for palladium scavenging and cross-coupling catalysis. Chemistry 2011, 17, 8984–8994. [Google Scholar] [CrossRef] [PubMed]
- Lim, Y.; Kim, S.K.; Lee, S.-C.; Choi, J.; Nahm, K.S.; Yoo, S.J.; Kim, P. One-step synthesis of carbon-supported Pd@Pt/C core-shell nanoparticles as oxygen reduction electrocatalysts and their enhanced activity and stability. Nanoscale 2014, 6, 4038–4042. [Google Scholar] [CrossRef]
- 46 Köhler, K.; Heidenreich, R.G.; Soomro, S.S.; Pröckl, S.S. Supported palladium catalysts for Suzuki reactions: Structure-property relationships, optimized reaction protocol and control of palladium leaching. Adv. Synth. Catal. 2008, 350, 2930–2936. [Google Scholar] [CrossRef]
- Moussa, S.; Siamaki, A.R.; Gupton, B.F.; El-Shall, M.S. Pd-partially reduced graphene oxide catalysts (Pd/PRGO): Laser synthesis of Pd nanoparticles supported on PRGO nanosheets for carbon-carbon cross coupling reactions. ACS Catal. 2012, 2, 145–154. [Google Scholar] [CrossRef]
- Maleki, A.; Hajizadeh, Z.; Abbasi, H. Surface modification of graphene oxide by citric acid and its application as a heterogeneous nanocatalyst in organic condensation reaction. Carbon Lett. 2018, 27, 42–49. [Google Scholar]
- Goswami, A.; Kadam, R.G.; Tuček, J.; Sofer, Z.; Bouša, D.; Varma, R.S.; Gawande, M.B.; Zbořil, R. Fe(0)-Embedded thermally reduced graphene oxide as efficient nanocatalyst for reduction of nitro compounds to amines. J. Chem. Eng. 2020, 382, 122469. [Google Scholar] [CrossRef]
- Sullivan, J.A.; Flanagan, K.A.; Hain, H. Suzuki coupling activity of an aqueous phase Pd nanoparticle dispersion and a carbon nanotube/Pd nanoparticle composite. Catal. Today 2009, 145, 108–113. [Google Scholar] [CrossRef]
- Gómez-Gualdrón, D.A.; McKenzie, G.D.; Alvarado, J.F.J.; Balbuena, P.B. Dynamic evolution of supported metal nanocatalyst/carbon structure during single-walled carbon nanotube growth. ACS Nano 2012, 6, 720–735. [Google Scholar] [CrossRef]
- Krueger, A. Beyond the shine: Recent progress in applications of nanodiamond. J. Mater. Chem. 2011, 21, 12571–12578. [Google Scholar] [CrossRef]
- Zhou, Q.; Zhao, Z. Sulfate surfactant assisted approach to fabricate sulphur-doped supported nanodiamond catalyst on carbon nanotube with unprecedented catalysis for ethylbenzene dehydrogenation. ChemCatChem 2020, 12, 342–349. [Google Scholar] [CrossRef]
- Huang, F.; Deng, Y.; Chen, Y.; Cai, X.; Peng, M.; Jia, Z.; Xie, J.; Xiao, D.; Wen, X.; Wang, N.; et al. Anchoring Cu1 species over nanodiamond-graphene for semi-hydrogenation of acetylene. Nat. Commun. 2019, 10, 4431. [Google Scholar] [CrossRef] [PubMed]
- Gupta, N.; Ding, Y.; Feng, Z.; Su, D. Palladium supported on nanodiamonds as an efficient catalyst for the hydrogenating deamination of benzonitrile and related compounds. ChemCatChem 2016, 8, 922–928. [Google Scholar] [CrossRef]
- Mochalin, V.N.; Shenderova, O.; Ho, D.; Gogotsi, Y. The properties and applications of nanodiamonds. Nat. Nanotechnol. 2012, 7, 11–23. [Google Scholar] [CrossRef] [PubMed]
- Krueger, A. Diamond nanoparticles: Jewels for chemistry and physics. Adv. Mater. 2008, 20, 2445–2449. [Google Scholar] [CrossRef]
- Bondon, N.; Raehm, L.; Charnay, C.; Boukherroub, R.; Durand, J.-O. Nanodiamonds for bioapplications, recent developments. J. Mater. Chem. B 2020, 8, 10878–10896. [Google Scholar] [CrossRef]
- Reina, G.; Zhao, L.; Bianco, A.; Komatsu, N. Chemical functionalization of nanodiamonds: Opportunities and challenges ahead. Angew. Chem. Int. Ed. 2019, 58, 17918–17929. [Google Scholar] [CrossRef]
- Basso, L.; Cazzanelli, M.; Orlandi, M.; Miotello, A. Nanodiamonds: Synthesis and application in sensing, catalysis, and the possible connection with some processes occurring in space. Appl. Sci. 2020, 10, 4094. [Google Scholar] [CrossRef]
- Gupta, N.; Wang, Q.; Wen, G.; Su, D. Nanodiamonds for catalytic reactions. In Nanodiamonds; Chapter 18; Arnault, J.-C., Ed.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 439–463. [Google Scholar]
- Fan, J.; Chu, P.K. Group IV nanoparticles: Synthesis, properties, and biological applications. Small 2010, 6, 2080–2098. [Google Scholar] [CrossRef]
- Basiuk, E.V.; Basiuk, V.A. Green chemistry of carbon nanomaterials. J. Nanosci. Nanotechnol. 2014, 14, 644–672. [Google Scholar] [CrossRef]
- Chen, D.; Holmen, A.; Sui, Z.; Zhou, X. Carbon mediated catalysis: A review on oxidative dehydrogenation. Chin. J. Catal. 2014, 35, 824–841. [Google Scholar] [CrossRef]
- Zhang, J.; Su, D.S.; Blume, R.; Schlögl, R.; Wang, R.; Yang, X.; Gajović, A. Surface chemistry and catalytic reactivity of a nanodiamond in the steam-free dehydrogenation of ethylbenzene. Angew. Chem. Int. Ed. 2010, 49, 8640–8644. [Google Scholar] [CrossRef] [PubMed]
- Obraztsova, I.I.; Eremenko, N.K.; Velyakina, Y.N. Reaction kinetics of nitrobenzene hydrogenation on a palladium catalyst supported on nanodiamonds. Kinet. Catal. 2008, 49, 401–406. [Google Scholar] [CrossRef]
- Zheng, W.-W.; Hsieh, Y.-H.; Chiu, Y.-C.; Cai, S.-J.; Cheng, C.-L.; Chen, C. Organic functionalization of ultradispersed nanodiamond: Synthesis and applications. J. Mater. Chem. 2009, 19, 8432–8441. [Google Scholar] [CrossRef]
- Lin, Y.; Su, D. Fabrication of nitrogen-modified annealed nanodiamond with improved catalytic activity. ACS Nano 2014, 8, 7823–7833. [Google Scholar] [CrossRef]
- Kalmykov, P.A.; Magdalinova, N.A.; Gruzdev, M.S.; Lysenok, A.A.; Belkina, E.G.; Klyuev, M.V. Tetrachloromethane hydrodechlorination over palladium-containing nanodiamonds. Pet. Chem. 2020, 60, 1148–1153. [Google Scholar] [CrossRef]
- Tveritinova, E.A.; Zhitnev, Y.N.; Kulakova, I.I.; Cherkasov, N.; Maslakov, K.I.; Nesterova, E.A.; Ivanov, A.S.; Savilov, S.V.; Lunin, V.V. The role of structure and surface chemistry of carbon nanomaterials in catalytic conversion of 1,2-dichloroethane. Appl. Surf. Sci. 2015, 355, 74–81. [Google Scholar] [CrossRef]
- Turova, O.V.; Starodubtseva, E.V.; Vinogradov, M.G.; Sokolov, V.I.; Abramova, N.V.; Vul, A.Y.; Alexenskiy, A.E. Palladium supported on detonation nanodiamond as a highly effective catalyst of the C=C and C≡C bond hydrogenation. Catal. Commun. 2011, 12, 577–579. [Google Scholar] [CrossRef]
- Magdalinova, N.A.; Klyuev, M.V.; Vershinin, N.N.; Efimov, O.N. Pt- and Pd-containing nanodiamonds in hydrogenation and hydroamination reactions. Kinet. Catal. 2012, 53, 482–485. [Google Scholar] [CrossRef]
- Volkov, D.S.; Proskurnin, M.A.; Korobov, M.V. Elemental analysis of nanodiamonds by inductively-coupled plasma atomic emission spectroscopy. Carbon 2014, 74, 1–13. [Google Scholar] [CrossRef]
- Paci, J.T.; Man, H.B.; Saha, B.; Ho, D.; Schatz, G.C. Understanding the surfaces of nanodiamonds. J. Phys. Chem. C 2013, 117, 17256–17267. [Google Scholar] [CrossRef]
- Alekseev, O.S.; Nosova, L.V.; Ryndin, Y.A. Formation and properties of dispersed Pd particles over graphite and diamond. In Studies in Surface Science and Catalysis; Guczi, L., Solymosi, F., Tétényi, P., Eds.; Elsevier: Amsterdam, The Netherlands, 1993; Volume 75, pp. 837–847. [Google Scholar]
- Piña-Salazar, E.-Z.; Kukobat, R.; Futamura, R.; Hayashi, T.; Toshio, S.; Ōsawa, E.; Kaneko, K. Water-selective adsorption sites on detonation nanodiamonds. Carbon 2018, 139, 853–860. [Google Scholar] [CrossRef]
- Piña-Salazar, E.-Z.; Sakai, T.; Ōsawa, E.; Futamura, R.; Kaneko, K. Unusual hygroscopic nature of nanodiamonds in comparison with well-known porous materials. J. Colloid Interface Sci. 2019, 549, 133–139. [Google Scholar] [CrossRef] [PubMed]
- Baylet, A.; Marécot, P.; Duprez, D.; Castellazzi, P.; Groppi, G.; Forzatti, P. In situ Raman and in situ XRD analysis of PdO reduction and Pd° oxidation supported on γ-Al2O3 catalyst under different atmospheres. Phys. Chem. Chem. Phys. 2011, 13, 4607–4613. [Google Scholar] [CrossRef]
- Brun, M.; Berthet, A.; Bertolini, J.C. XPS, AES and auger parameter of Pd and PdO. J. Electron Spectrosc. Relat. Phenom. 1999, 104, 55–60. [Google Scholar] [CrossRef]
- Gunawan, M.A.; Moncea, O.; Poinsot, D.; Keskes, M.; Domenichini, B.; Heintz, O.; Chassagnon, R.; Herbst, F.; Carlson, R.M.K.; Dahl, J.E.P.; et al. Nanodiamond-palladium core-shell organohybrid synthesis: A mild vapor-phase procedure enabling nanolayering metal onto functionalized sp3-carbon. Adv. Funct. Mater. 2018, 28, 1705786. [Google Scholar] [CrossRef]
- Burueva, D.B.; Sviyazov, S.V.; Huang, F.; Prosvirin, I.P.; Bukhtiyarov, A.V.; Bukhtiyarov, V.I.; Liu, H.; Koptyug, I.V. Pd on nanodiamond/graphene in hydrogenation of propyne with parahydrogen. J. Phys. Chem. C 2021, 125, 27221–27229. [Google Scholar] [CrossRef]
- Kim, K.S.; Gossmann, A.F.; Winograd, N. X-ray photoelectron spectroscopic studies of palladium oxides and the palladium-oxygen electrode. Anal. Chem. 1974, 46, 197–200. [Google Scholar] [CrossRef]
- Csákai, Z.; Skoda-Földes, R.; Kollár, L. NMR investigation of Pd(II)–Pd(0) reduction in the presence of mono- and ditertiary phosphines. Inorg. Chim. Acta 1999, 286, 93–97. [Google Scholar] [CrossRef]
- Ibrahim, A.A.; Lin, A.; Adly, M.S.; El-Shall, M.S. Enhancement of the catalytic activity of Pd nanoparticles in Suzuki coupling by partial functionalization of the reduced graphene oxide support with p-phenylenediamine and benzidine. J. Catal. 2020, 385, 194–203. [Google Scholar] [CrossRef]
- Rana, S.; Maddila, S.; Yalagala, K.; Jonnalagadda, S.B. Organo functionalized graphene with Pd nanoparticles and its excellent catalytic activity for Suzuki coupling reaction. Appl. Catal. A Gen. 2015, 505, 539–547. [Google Scholar] [CrossRef]
- Chen, X.; Hou, Y.; Wang, H.; Cao, Y.; He, J. Facile deposition of Pd nanoparticles on carbon nanotube microparticles and their catalytic activity for Suzuki coupling reactions. J. Phys. Chem. C 2008, 112, 8172–8176. [Google Scholar] [CrossRef]
- Zhong, L.; Chokkalingam, A.; Cha, W.S.; Lakhi, K.S.; Su, X.; Lawrence, G.; Vinu, A. Pd nanoparticles embedded in mesoporous carbon: A highly efficient catalyst for Suzuki-Miyaura reaction. Catal. Today 2015, 243, 195–198. [Google Scholar] [CrossRef]
- Phukan, S.; Mahanta, A.; Kakati, D.; Rashid, M.H. Green chemical synthesis of Pd nanoparticles for use as efficient catalyst in Suzuki-Miyaura cross-coupling reaction. Appl. Organomet. Chem. 2019, 33, e4758. [Google Scholar] [CrossRef]
- Sahu, D.; Das, P. Phosphine-stabilized Pd nanoparticles supported on silica as a highly active catalyst for the Suzuki–Miyaura cross-coupling reaction. RSC Adv. 2015, 5, 3512–3520. [Google Scholar] [CrossRef]
- Lichtenegger, G.J.; Maier, M.; Hackl, M.; Khinast, J.G.; Gössler, W.; Griesser, T.; Kumar, V.S.P.; Gruber-Woelfler, H.; Deshpande, P.A. Suzuki-Miyaura coupling reactions using novel metal oxide supported ionic palladium catalysts. J. Mol. Catal. A Chem. 2017, 426, 39–51. [Google Scholar] [CrossRef]
- Han, Y.; Di, J.-Q.; Zhao, A.-D.; Zhang, Z.-H. Synthesis, characterization and catalytic performance of palladium supported on pyridine-based covalent organic polymer for Suzuki-Miyaura reaction. Appl. Organomet. Chem. 2019, 33, e5172. [Google Scholar] [CrossRef]
- Ganapathy, D.; Sekar, G. Palladium nanoparticles stabilized by metal-carbon covalent bond: An efficient and reusable nanocatalyst in cross-coupling reactions. Catal. Commun. 2013, 39, 50–54. [Google Scholar] [CrossRef]
- Dutta, P.; Sarkar, A. Palladium nanoparticles immobilized on chemically modified silica gel: Efficient heterogeneous catalyst for Suzuki, Stille and Sonogashira cross-coupling reactions. Adv. Synth. Catal. 2011, 353, 2814–2822. [Google Scholar] [CrossRef]



| Entry | Catalysts | Conversion (%) |
|---|---|---|
| 1 | - | 0 |
| 2 | ND | 0 |
| 3 | oxND | 0 |
| 4 | PdCl2 (0.02 mmol) | 72 |
| 5 | 0.02 mmol Pd NPs | 80 |
| 6 | Pd/rND (0.01 mmol Pd) | 65 |
| 7 | Pd/rND (0.025 mmol Pd) | ≥99 |
| 8 | Pd/rND (0.05 mmol Pd) | ≥99 |
| Entry | Aryl Iodide | Boronic Acid | Products | Time (h) | Yield (%) a |
|---|---|---|---|---|---|
| 1 | 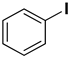 |  |  | 1 | 93 |
| 2 |  | 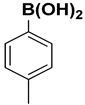 |  | 1 | 98 |
| 3 |  |  | 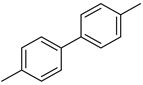 | 1 | 98 |
| 4 |  |  | 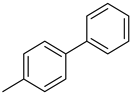 | 1 | 90 |
| 5 | 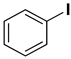 | 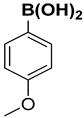 | 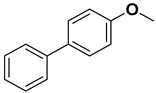 | 1 | 90 |
| 6 |  | 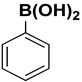 | 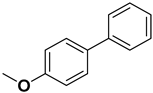 | 1.5 | 95 |
| 7 |  |  | 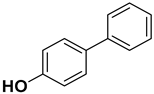 | 1.5 | 86 |
| 8 |  | 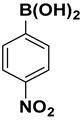 |  | 2 | 80 |
| 9 |  | 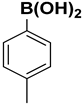 | 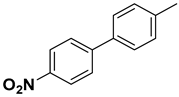 | 2 | 82 |
| 10 | 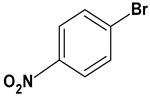 | 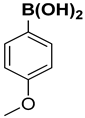 | 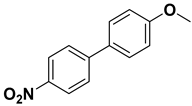 | 2 | 90 |
| 11 | 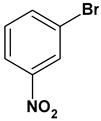 |  | 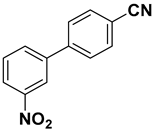 | 2 | 84 |
| 12 | 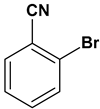 | 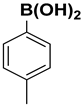 | 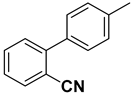 | 2 | 75 |
| 13 | 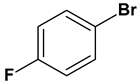 | 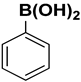 |  | 2.5 | 85 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pocklanová, R.; Warkad, I.R.; Prucek, R.; Balzerová, A.; Panáček, A.; Kadam, R.G.; Kvítek, L.; Gawande, M.B. Nanodiamond Supported Ultra-Small Palladium Nanoparticles as an Efficient Catalyst for Suzuki Cross-Coupling Reactions. Catalysts 2024, 14, 53. https://doi.org/10.3390/catal14010053
Pocklanová R, Warkad IR, Prucek R, Balzerová A, Panáček A, Kadam RG, Kvítek L, Gawande MB. Nanodiamond Supported Ultra-Small Palladium Nanoparticles as an Efficient Catalyst for Suzuki Cross-Coupling Reactions. Catalysts. 2024; 14(1):53. https://doi.org/10.3390/catal14010053
Chicago/Turabian StylePocklanová, Radka, Indrajeet R. Warkad, Robert Prucek, Anna Balzerová, Aleš Panáček, Ravishankar G. Kadam, Libor Kvítek, and Manoj B. Gawande. 2024. "Nanodiamond Supported Ultra-Small Palladium Nanoparticles as an Efficient Catalyst for Suzuki Cross-Coupling Reactions" Catalysts 14, no. 1: 53. https://doi.org/10.3390/catal14010053






