Modular Photocatalytic Reactor for the Removal of Estrogens from Aqueous Solutions
Abstract
1. Introduction
2. Results and Discussion
2.1. Characterization of the Synthesized Photocatalysts
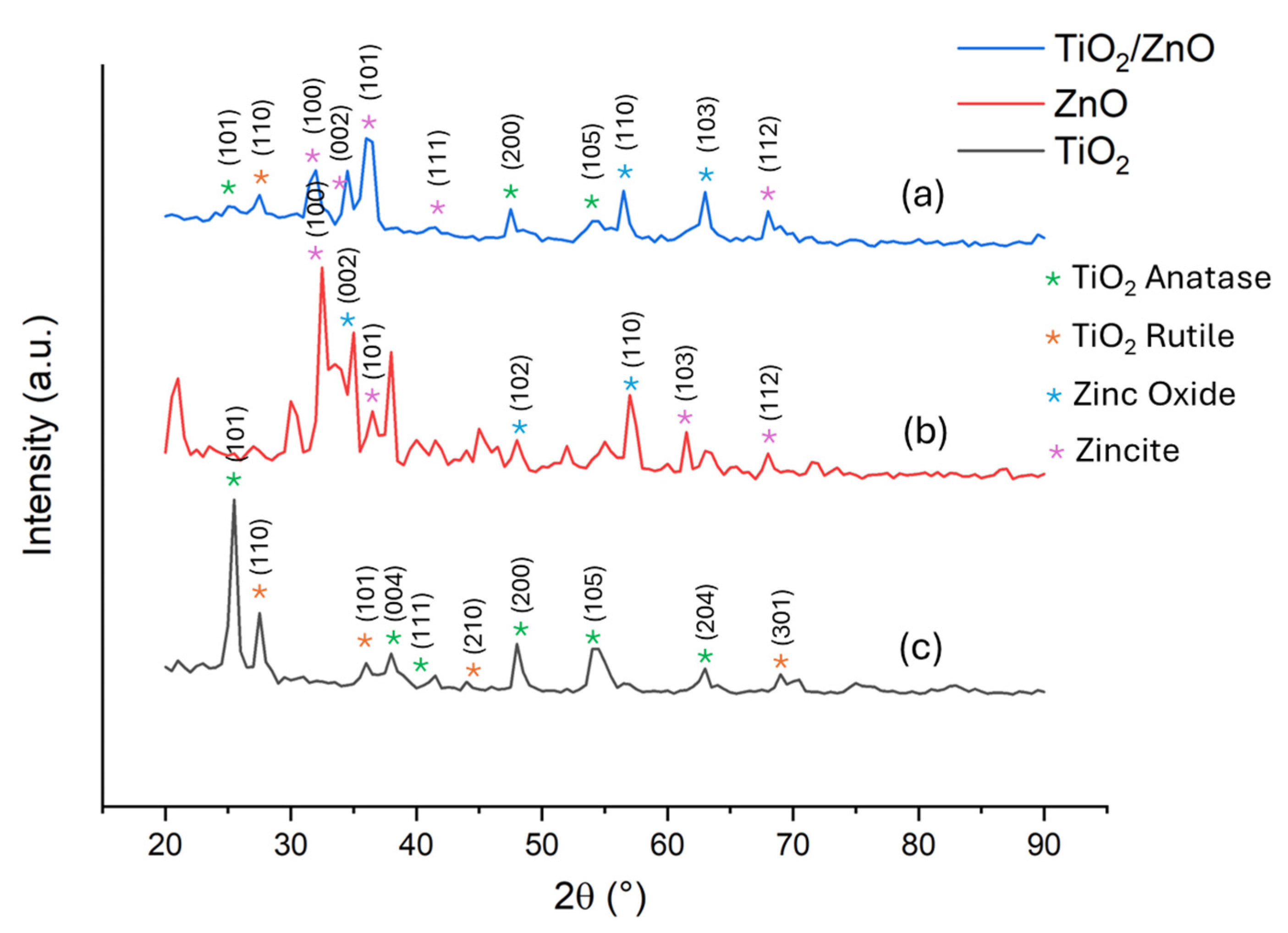
2.1.1. X-ray Diffractometry
2.1.2. X-ray Photoelectron Spectroscopy
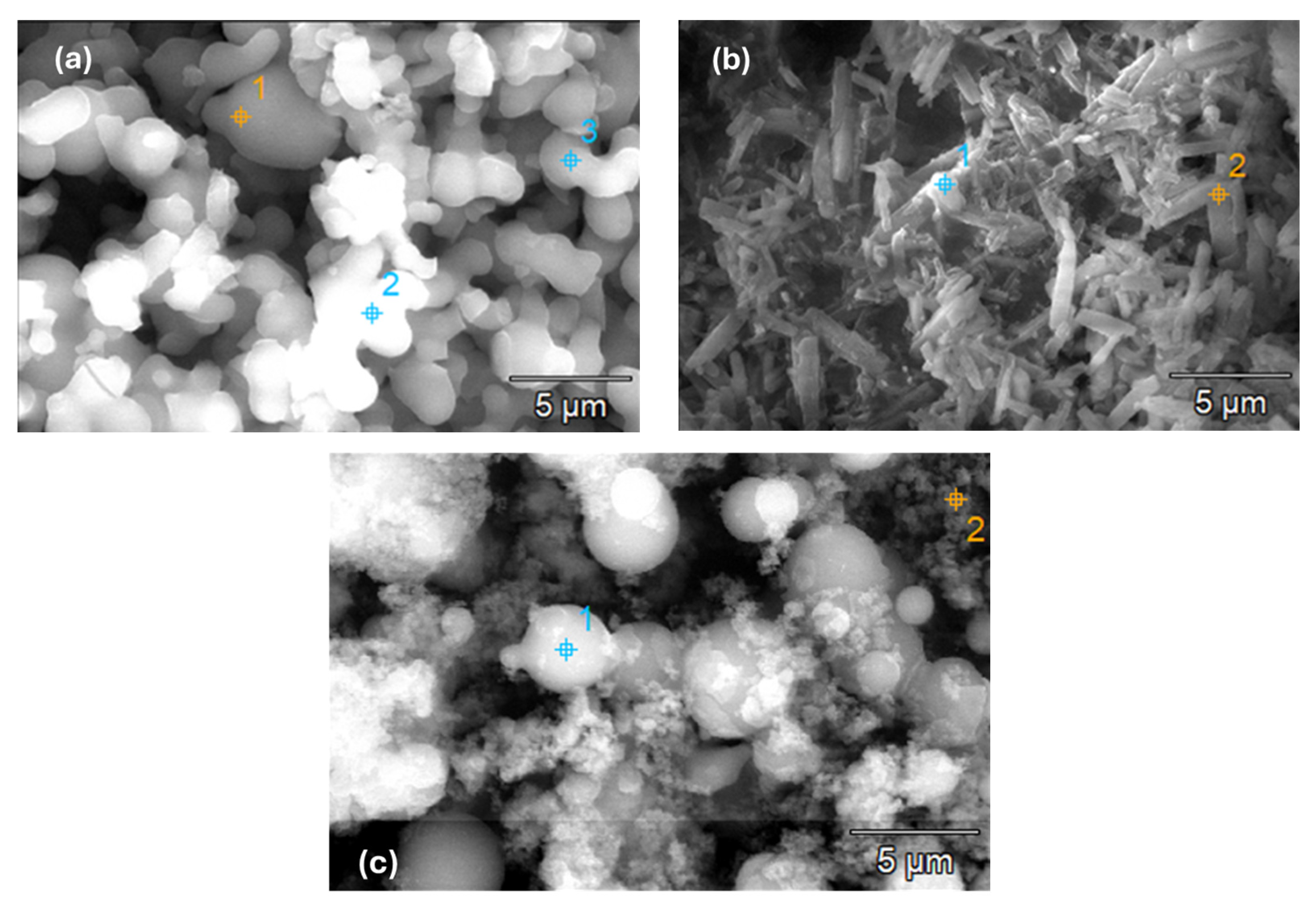
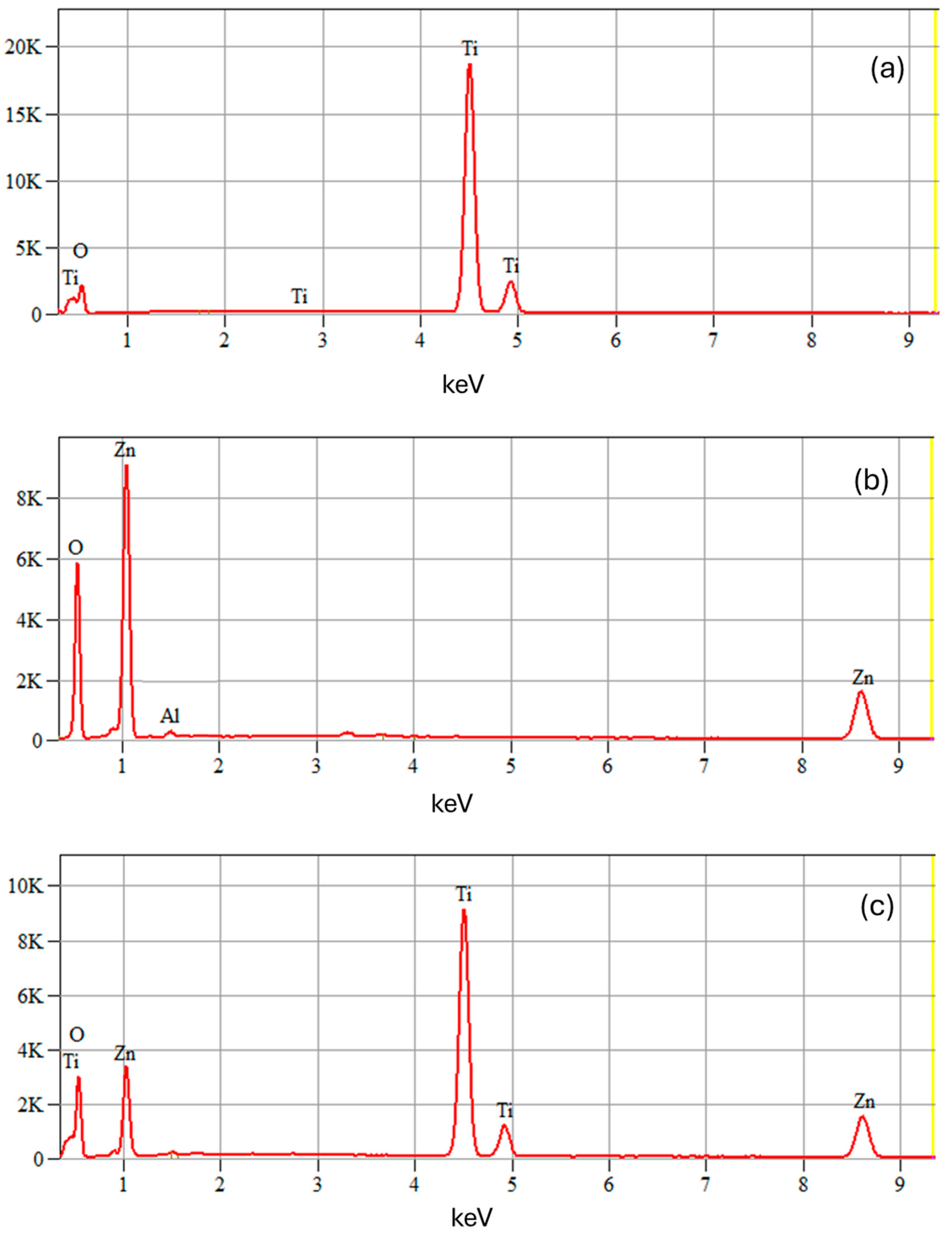
2.1.3. Scanning Electron Microscopy/Energy-Dispersive X-ray Spectroscopy
2.2. Photocatalytic Degradation of Estrogenic Mixture
3. Materials and Methods
3.1. Materials
3.2. Synthesis of Photocatalysts and Their Deposition on the Inert Support
3.2.1. TiO2 Photocatalyst
3.2.2. ZnO Photocatalyst
3.2.3. TiO2/ZnO Photocatalyst
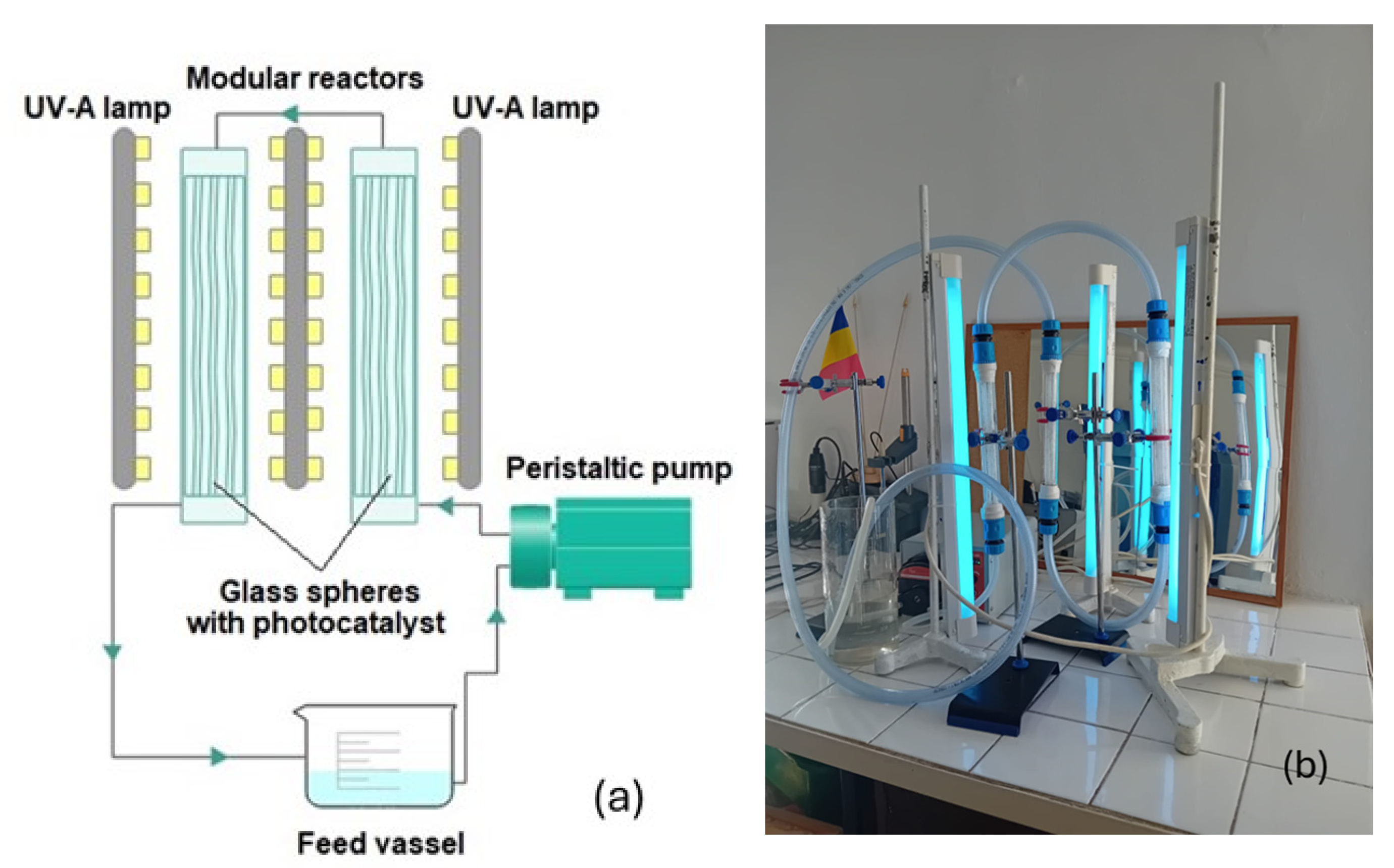
3.3. Photocatalytic Experiments
3.4. Photocatalysts Characterization
4. Conclusions
- ○
- The photocatalytic degradation efficiency of the organic substrate, initially formed from the estrogenic estradiol valerate/norgestrel mixture, largely depends on the dose of photocatalyst used regardless of its type. The dose can be easily modified by adding or removing photocatalytic modules that contain approximately the same amount of photocatalysts.
- ○
- The photocatalytic degradation of the organic substrate takes place in two stages with different speeds but following the same type of kinetics, namely pseudo-first-order kinetics. The number of photocatalytic modules required for the complete mineralization of a certain type of organic substrate can be determined based on the results obtained in the second stage of photocatalytic degradation in terms of degradation efficiency.
- ○
- The best results in terms of photocatalytic degradation efficiency were obtained for the photocatalytic system with a TiO2/ZnO-type photocatalyst. The organic substrate was almost completely mineralized in 120 min of irradiation with only two photocatalytic modules.
- ○
- The morphological, mineralogical and spectroscopic analyses showed that the synthesized photocatalysts are of high purity, and that the high performance of the TiO2/ZnO photocatalyst is due to its superior characteristics such as small crystallites, compact structure and highly reactive specific surface. The manifestation of specific interactions between the elements that form the oxide mixture leads to the appearance of surface defects beneficial to charge transfer, and thus, to photocatalytic activity.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Yang, X.; He, X.; Lin, H.; Lin, X.; Mo, J.; Chen, C.; Dai, X.; Liao, D.; Gao, C.; Li, Y. Occurrence and distribution of natural and synthetic progestins, androgens, and estrogens in soils from agricultural production areas in China. Sci. Total Environ. 2021, 751, 141766. [Google Scholar] [CrossRef]
- Aubead, N.M. Role of Sex Hormones in Human Body. In Reproductive Hormones; Marsh, C., Ed.; IntechOpen: Kansas City, MO, USA, 2021. [Google Scholar] [CrossRef]
- Amenyogbe, E.; Chen, G.; Wang, Z.; Lu, X.; Lin, M.; Lin, A.I. A Review on Sex Steroid Hormone Estrogen Receptors in Mammals and Fish. Int. J. Endocrinol. 2020, 2020, 5386193. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Grimes, K.L.; Colosi, L.M.; Lung, W.-S. Attenuation, transport, and management of estrogens: A review. Chemosphere 2019, 230, 462–478. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Li, J.; Zhang, Q.; Gao, S.; Quan, X.; Liu, P.; Xu, C. Effects of endocrine disrupting chemicals in host health: Three-way interactions between environmental exposure, host phenotypic responses, and gut microbiota. Environ. Pollut. 2021, 271, 116387. [Google Scholar] [CrossRef]
- Directive 2013/39/EU of the European Parliament and of the Council of 12 August 2013 Amending Directives 2000/60/EC and 2008/105/EC as Regards Priority Substances in the Field of Water Policy Text with EEA Relevance. OJ L 226. 2013, pp. 1–17. Available online: http://data.europa.eu/eli/dir/2013/39/oj (accessed on 9 July 2024).
- Commission Implementing Decision (EU) 2018/840 of 5 June 2018 Establishing a Watch List of Substances for Union-Wide Monitoring in the Field of Water Policy Pursuant to Directive 2008/105/EC of the European Parliament and of the Council and Repealing Commission Implementing Decision (EU) 2015/495 (Notified under Document C(2018) 3362). OJ L 141. 2018, pp. 9–12. Available online: http://data.europa.eu/eli/dec_impl/2018/840/oj (accessed on 9 July 2024).
- Commission Implementing Decision (EU) 2022/679 of 19 January 2022 Establishing a Watch List of Substances and Compounds of Concern for Water Intended for Human Consumption as Provided for in Directive (EU) 2020/2184 of the European Parliament and of the Council (Notified under Document C(2022) 142). OJ L 124. 2022, pp. 41–43. Available online: http://data.europa.eu/eli/dec_impl/2022/679/oj (accessed on 9 July 2024).
- Coello-Garcia, T.; Curtis, T.P.; Mrozik, W.; Davenpor, R.J. Enhanced estrogen removal in activated sludge processes through the optimization of the hydraulic flow pattern. Water Res. 2019, 164, 114905. [Google Scholar] [CrossRef] [PubMed]
- Bilal, M.; Rizwan, K.; Adeel, M.; Barceló, D.; Awad, Y.A.; Iqbal, H.M.N. Robust strategies to eliminate endocrine disruptive estrogens inwater resources. Environ. Pollut. 2022, 306, 119373. [Google Scholar] [CrossRef]
- Al-Nuaim, M.A.; Alwasiti, A.A.; Shnain, Z.Y. The photocatalytic process in the treatment of polluted water. Chem. Pap. 2023, 77, 677–701. [Google Scholar] [CrossRef]
- Joseph, A.; Vijayanandan, A. Review on support materials used for immobilization of nano-photocatalysts for water treatment applications. Inorganica Chim. Acta 2023, 545, 121284. [Google Scholar] [CrossRef]
- Huang, H.J.; Wu, J.C.-S.; Chiang, H.-P.; Chau, Y.-F.C.; Lin, Y.-S.; Wang, Y.H.; Chen, P.-J. Review of Experimental Setups for Plasmonic Photocatalytic Reactions. Catalysts 2020, 10, 46. [Google Scholar] [CrossRef]
- Visan, A.; van Ommen, J.R.; Kreutzer, M.T.; Lammertink, R.G.H. Photocatalytic Reactor Design: Guidelines for Kinetic Investigation. Ind. Eng. Chem. Res. 2019, 58, 5349–5357. [Google Scholar] [CrossRef]
- Erdem, B.; Hunsicker, R.A.; Simmons, G.W.; Sudol, E.D.; Dimonie, V.L.; El-Aasser, M.S. XPS and FTIR Surface Characterization of TiO2 Particles Used in Polymer Encapsulation. Langmuir 2001, 17, 2664–2669. [Google Scholar] [CrossRef]
- Kubiak, A.; Żółtowska, S.; Bartkowiak, A.; Gabała, E.; Sacharczuk, N.; Zalas, M.; Siwińska-Ciesielczyk, K.; Jesionowski, T. The TiO2-ZnO Systems with Multifunctional Applications in Photoactive Processes—Efficient Photocatalyst under UV-LED Light and Electrode Materials in DSSCs. Materials 2021, 14, 6063. [Google Scholar] [CrossRef] [PubMed]
- Saad, S.K.M.; Umar, A.A.; Nguyen, H.Q.; Dee, C.F.; Salleh, M.M.; Oyama, M. Porous (001)-faceted Zn-doped anatase TiO2 nanowalls and their heterogeneous photocatalytic characterization. RSC Adv. 2014, 4, 57054. [Google Scholar] [CrossRef]
- Ohno, T.; Tsubota, T.; Toyofukum, M.; Inaba, R. Preparation of S-doped TiO2 Photocatalysts and their Photocatalytic Activities Under Visible Light. Appl. Catal. A Gen. 2004, 265, 255–258. [Google Scholar] [CrossRef]
- Pandey, S.K.; Pandey, S.K.; Mukherjee, C.; Mishra, P.; Gupta, M.; Barman, S.R.; D’Souza, S.W.; Mukherjee, S. Effect of growth temperature on structural, electrical and optical properties of dual ion beam sputtered ZnO thin films. J. Mater. Sci. Mater. Electron. 2013, 24, 2541–2547. [Google Scholar] [CrossRef]
- Fu, R.; Wang, Q.; Gao, S.; Wang, Z.; Huang, B.; Dai, Y.; Lu, J. Effect of different processes and Ti/Zn molar ratios on the structure, morphology, and enhanced photoelectrochemical and photocatalytic performance of Ti3+ self-doped titanium–zinc hybrid oxides. J. Power Sources 2015, 285, 449–459. [Google Scholar] [CrossRef]
- Krylova, G.; Brioude, A.; Ababou-Girard, S.; Mrazeka, J.; Spanhel, L. Natural superhydrophilicity and photocatalytic properties of sol-gel derived ZnTiO3-ilmenite/r-TiO2 films. Chem. Phys. 2010, 12, 15101–15110. [Google Scholar] [CrossRef]
- Kumar, A.; Ahmad, M.I. Role of defects and microstructure on the electrical properties of solution-processed Al-doped ZnO transparent conducting films. Appl. Phys. A 2020, 126, 598. [Google Scholar] [CrossRef]
- Fan, C.; Chen, C.; Wang, J.; Fu, X.; Ren, Z.; Qian, G.; Wang, Z. Black Hydroxylated Titanium Dioxide Prepared Via Ultrasonication with Enhanced Photocatalytic Activity. Sci. Rep. 2015, 5, 11712. [Google Scholar] [CrossRef]
- Claros, M.; Setka, M.; Jimenez, Y.; Vallejos, S. AACVD Synthesis and Characterization of Iron and Copper Oxides Modified ZnO Structured Films. Nanomaterials 2020, 10, 471. [Google Scholar] [CrossRef]
- Idriss, H. On the wrong assignment of the XPS O1s signal at 531–532 eV attributed to oxygen vacancies in photo- and electro-catalysts for water splitting and other materials applications. Surf. Sci. 2021, 712, 121894. [Google Scholar] [CrossRef]
- Frankcombe, T.J.; Liu, Y. Interpretation of Oxygen 1s X-ray Photoelectron Spectroscopy of ZnO. Chem. Mater. 2023, 35, 5468–5474. [Google Scholar] [CrossRef]
- Li, B.; Yuan, D.; Gao, C.; Zhang, H.; Li, Z. Synthesis and characterization of TiO2/ZnO heterostructural composite for ultraviolet photocatalytic degrading DOM in landfill leachate. Environ. Sci. Pollut. Res. 2022, 29, 85510–85524. [Google Scholar] [CrossRef] [PubMed]





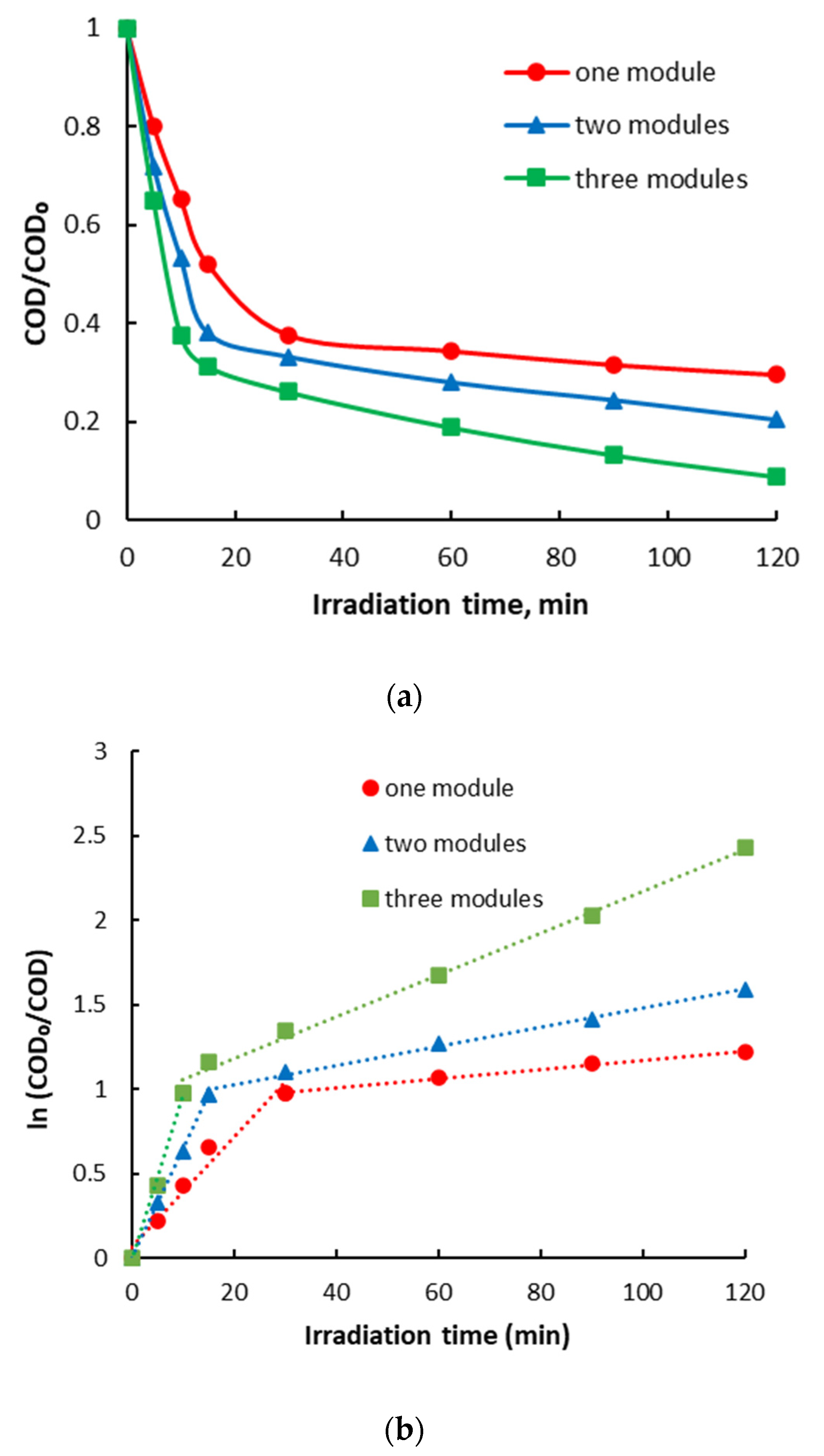


| Photocatalyst | One Module | Two Modules | Three Modules | |
|---|---|---|---|---|
| k, min−1 | ||||
| TiO2 | 1st stage | 0.0207 | 0.0418 | 0.0624 |
| 2nd stage | 0.0018 | 0.0045 | 0.0101 | |
| ZnO | 1st stage | 0.0324 | 0.0641 | 0.0978 |
| 2nd stage | 0.0027 | 0.0057 | 0.0124 | |
| TiO2/ZnO | 1st stage | 0.0838 | 0.1317 | - |
| 2nd stage | 0.0060 | 0.0230 | - | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bobirică, L.; Orbeci, C.; Pîrvu, C.; Constantinescu, A.; Bîru, E.I.; Ionică, G.I.; Matei, E.; Berbecaru, A.C.; Bobirică, C. Modular Photocatalytic Reactor for the Removal of Estrogens from Aqueous Solutions. Catalysts 2024, 14, 661. https://doi.org/10.3390/catal14100661
Bobirică L, Orbeci C, Pîrvu C, Constantinescu A, Bîru EI, Ionică GI, Matei E, Berbecaru AC, Bobirică C. Modular Photocatalytic Reactor for the Removal of Estrogens from Aqueous Solutions. Catalysts. 2024; 14(10):661. https://doi.org/10.3390/catal14100661
Chicago/Turabian StyleBobirică, Liliana, Cristina Orbeci, Cristian Pîrvu, Alexandra Constantinescu, Elena Iuliana Bîru, Giovanina Iuliana Ionică, Ecaterina Matei, Andrei Constantin Berbecaru, and Constantin Bobirică. 2024. "Modular Photocatalytic Reactor for the Removal of Estrogens from Aqueous Solutions" Catalysts 14, no. 10: 661. https://doi.org/10.3390/catal14100661
APA StyleBobirică, L., Orbeci, C., Pîrvu, C., Constantinescu, A., Bîru, E. I., Ionică, G. I., Matei, E., Berbecaru, A. C., & Bobirică, C. (2024). Modular Photocatalytic Reactor for the Removal of Estrogens from Aqueous Solutions. Catalysts, 14(10), 661. https://doi.org/10.3390/catal14100661









