Synthesis, Characterization, and Attrition Resistance of Kaolin and Boehmite Alumina-Reinforced La0.7Sr0.3FeO3 Perovskite Catalysts for Chemical Looping Partial Oxidation of Methane
Abstract
1. Introduction
2. Results and Discussion
2.1. Characterization of the Materials
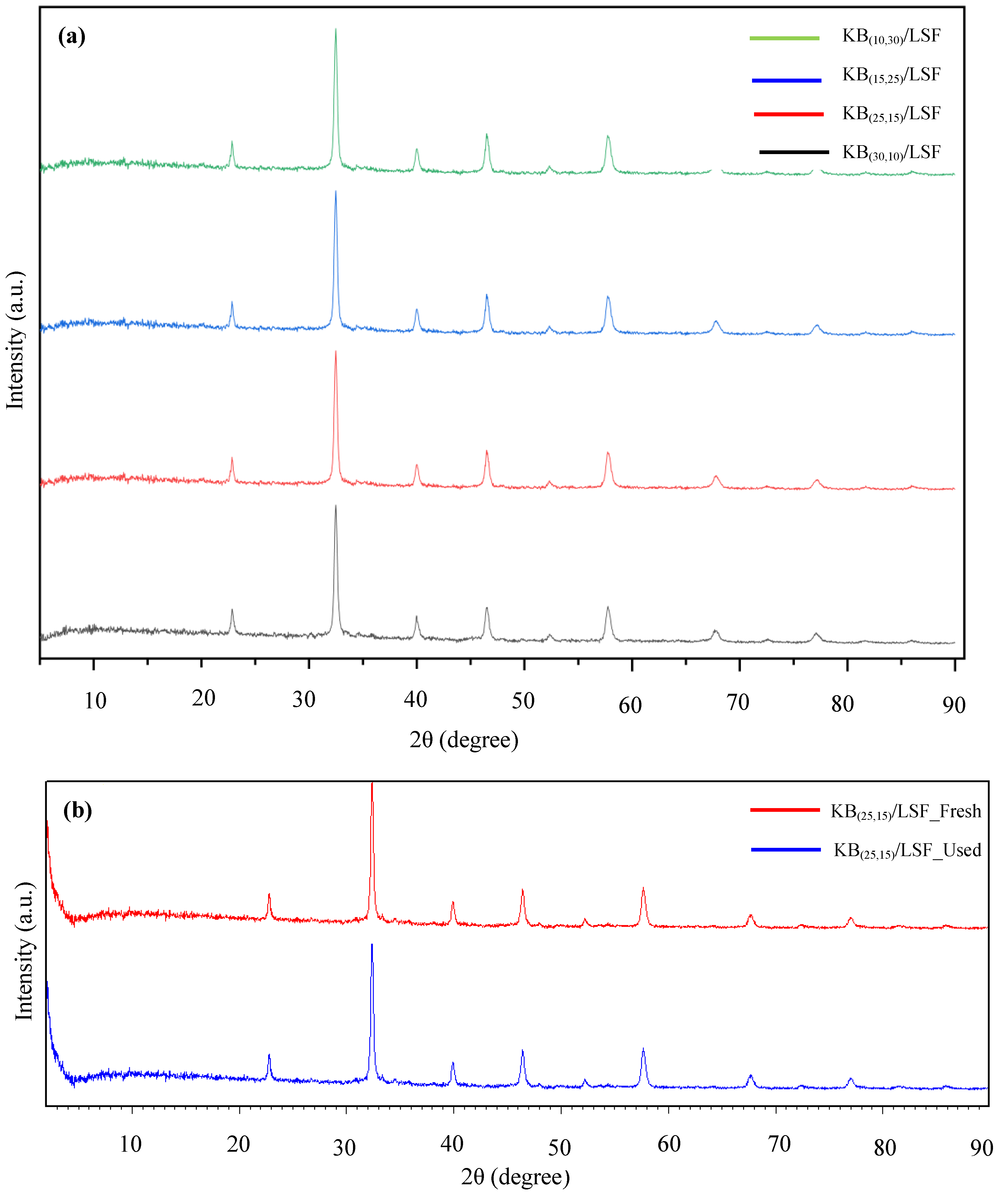
2.1.1. XRD Analysis
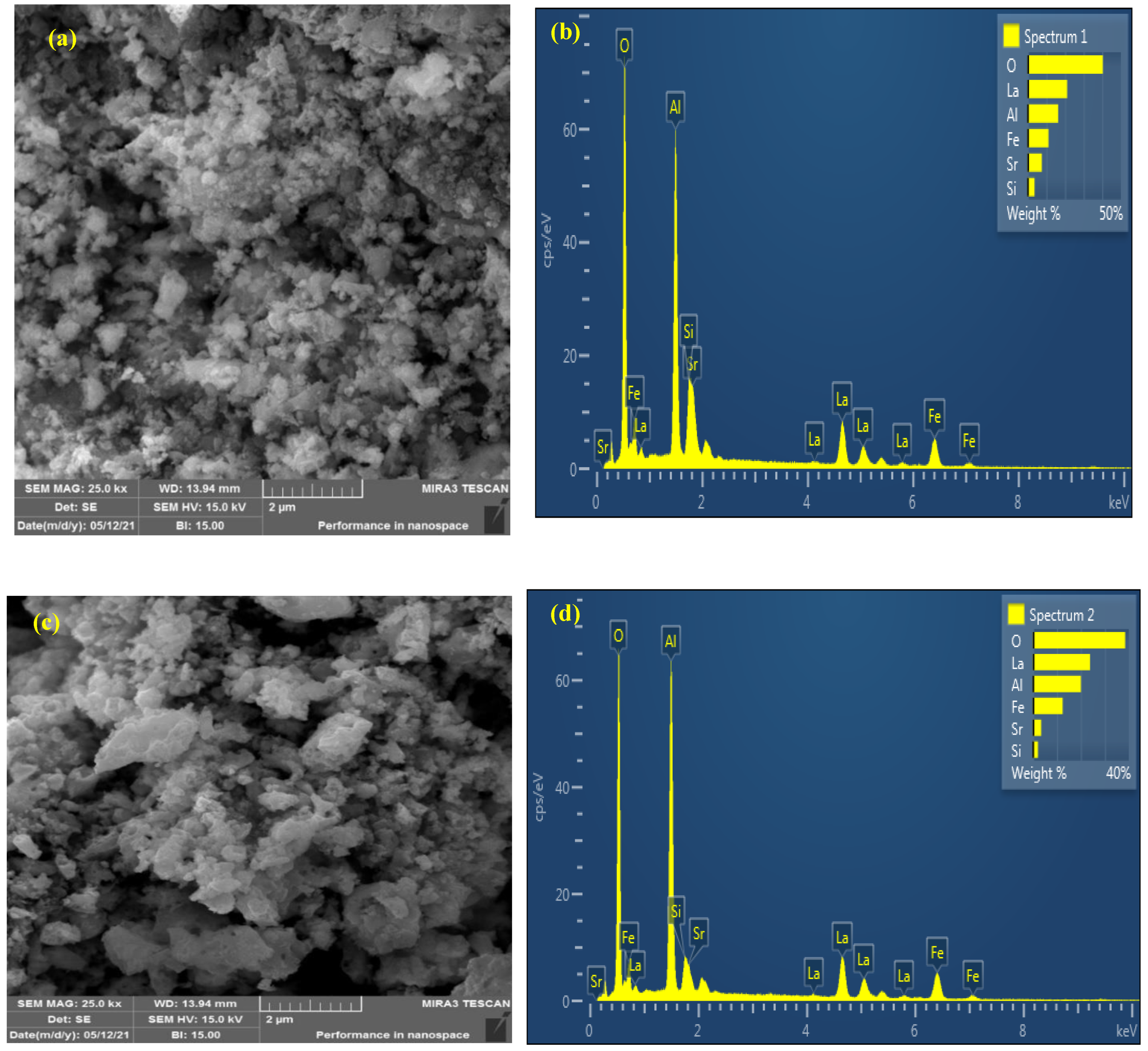
2.1.2. FE-SEM/EDS Analyses
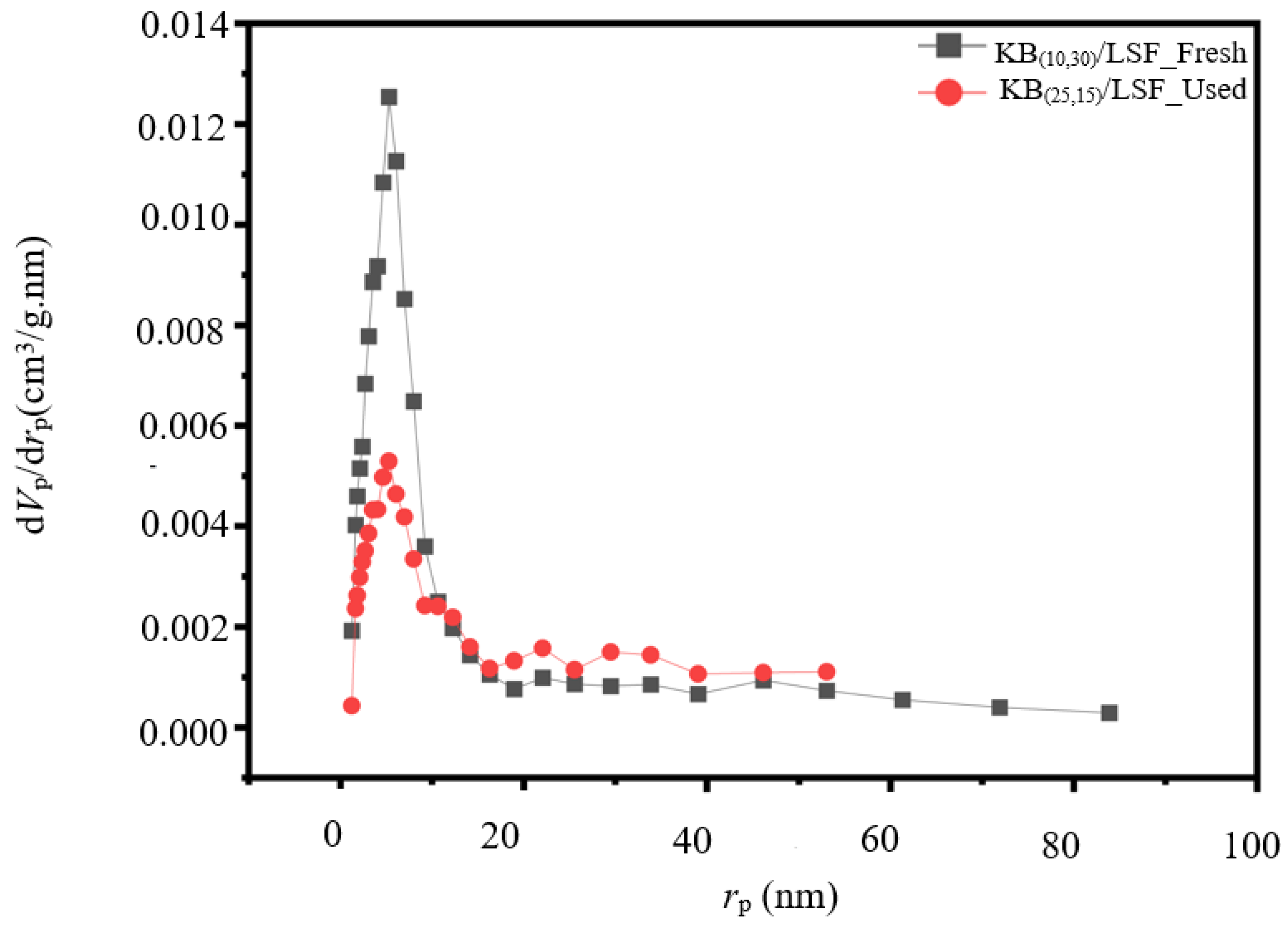
2.1.3. BET/BJH Analysis
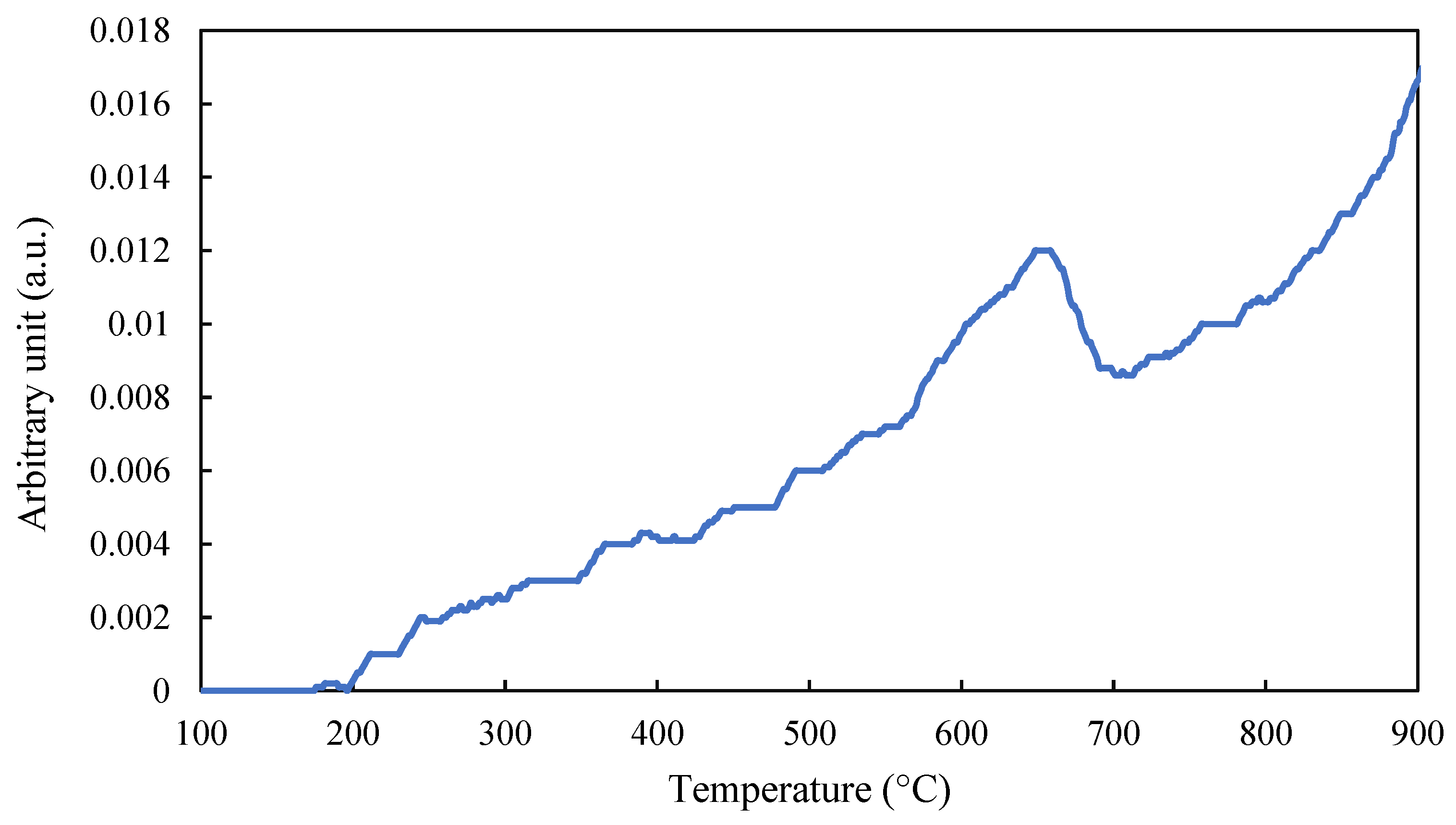
2.1.4. TPD-O2 and TPD-NH3 Tests
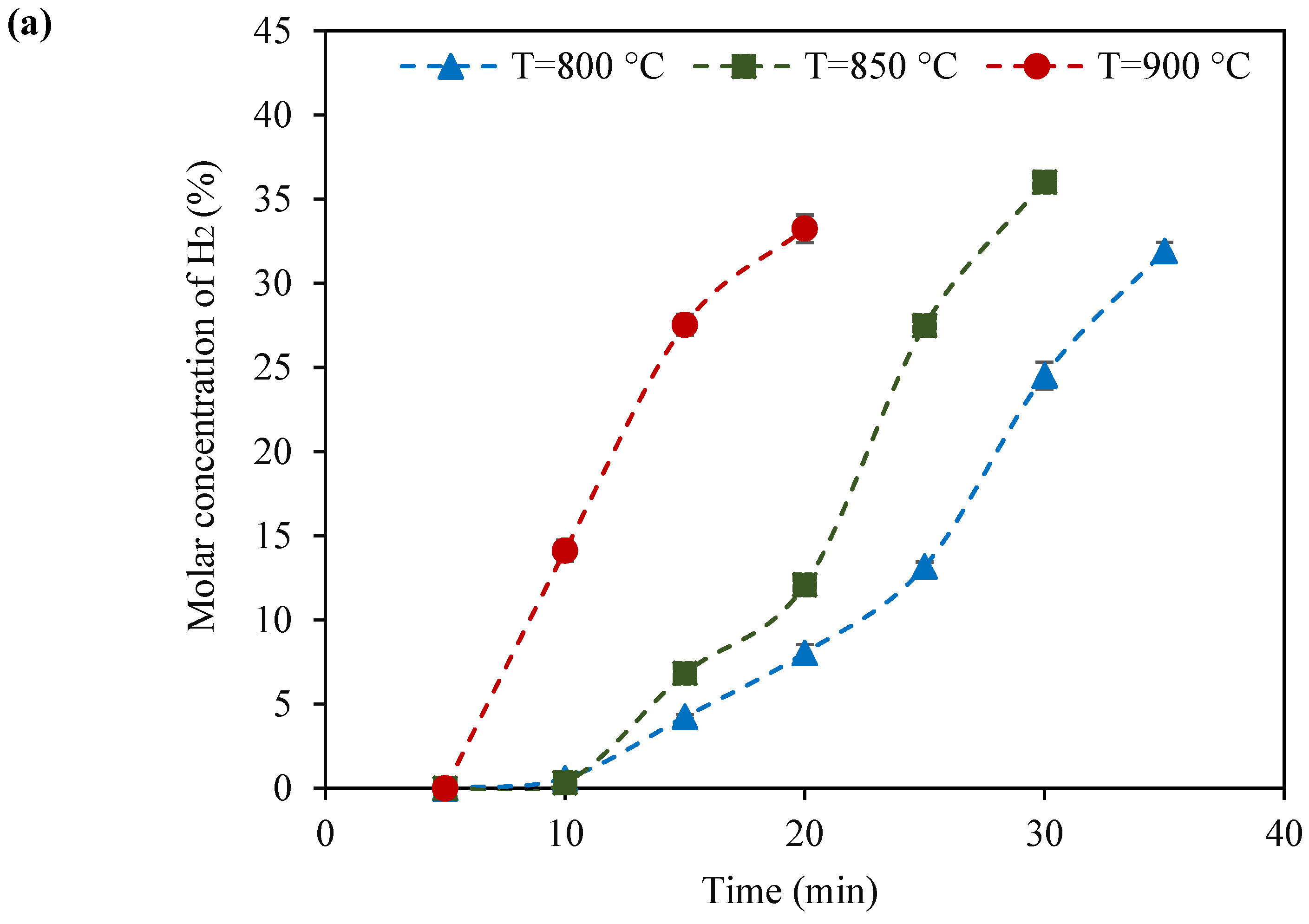
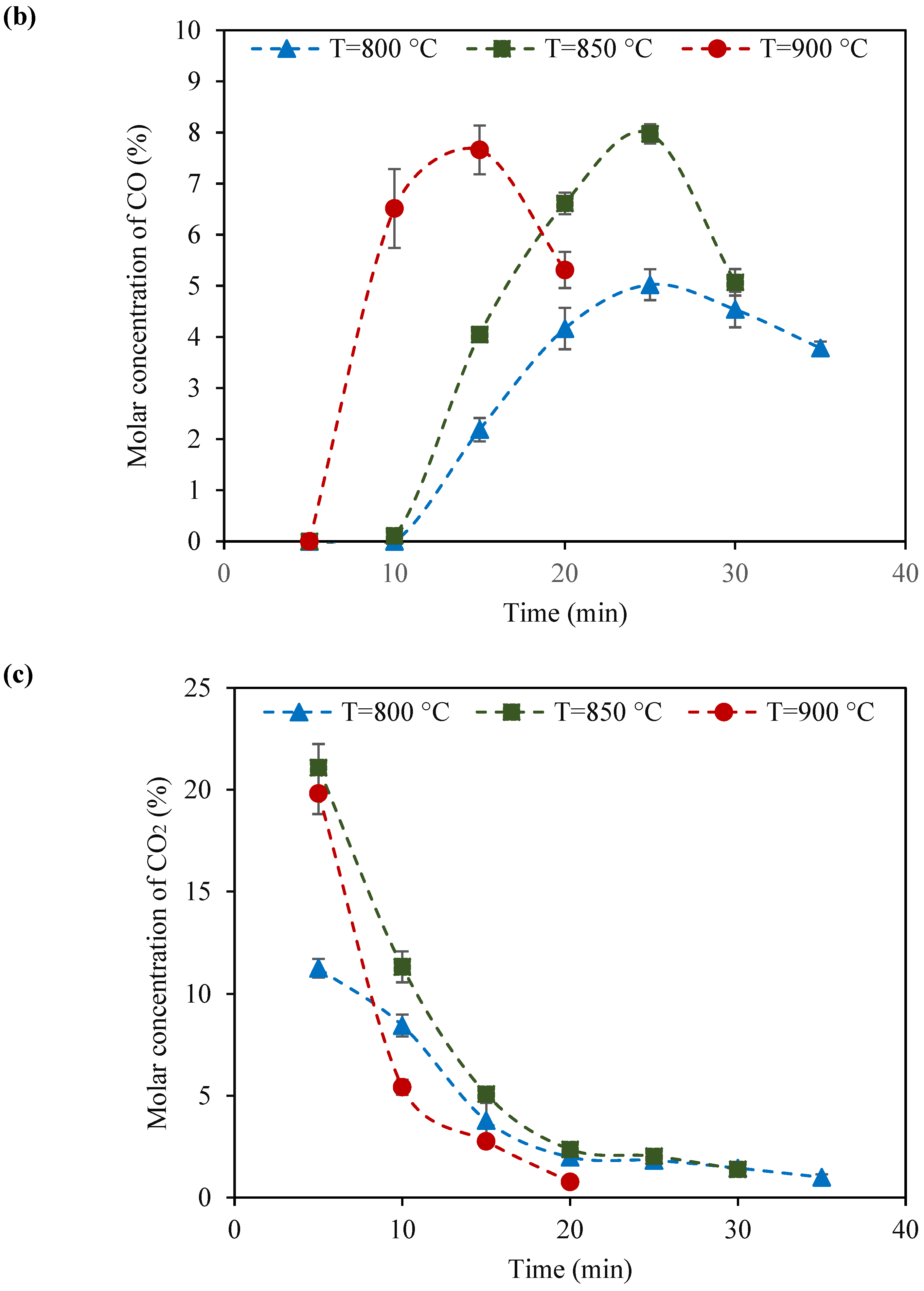
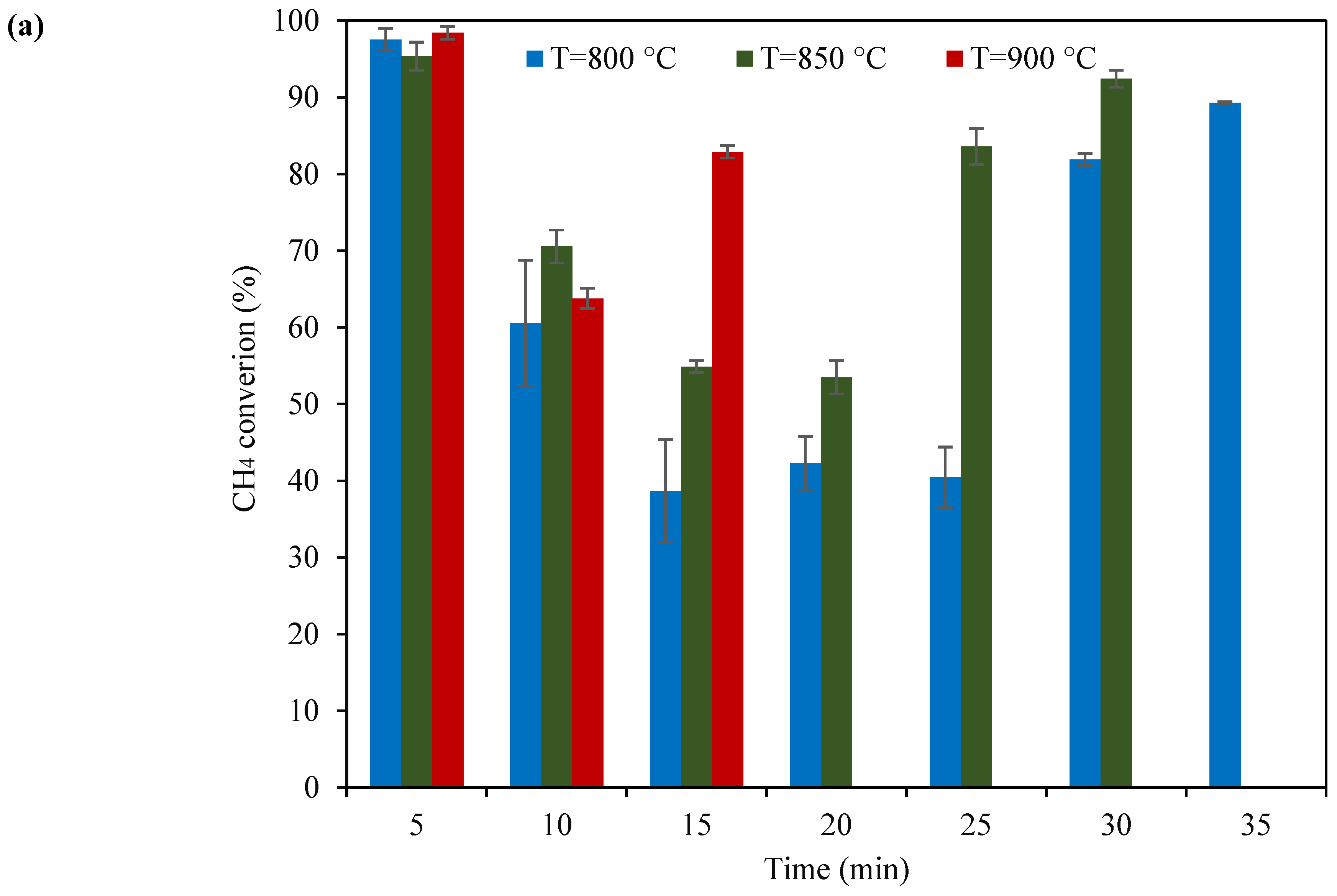
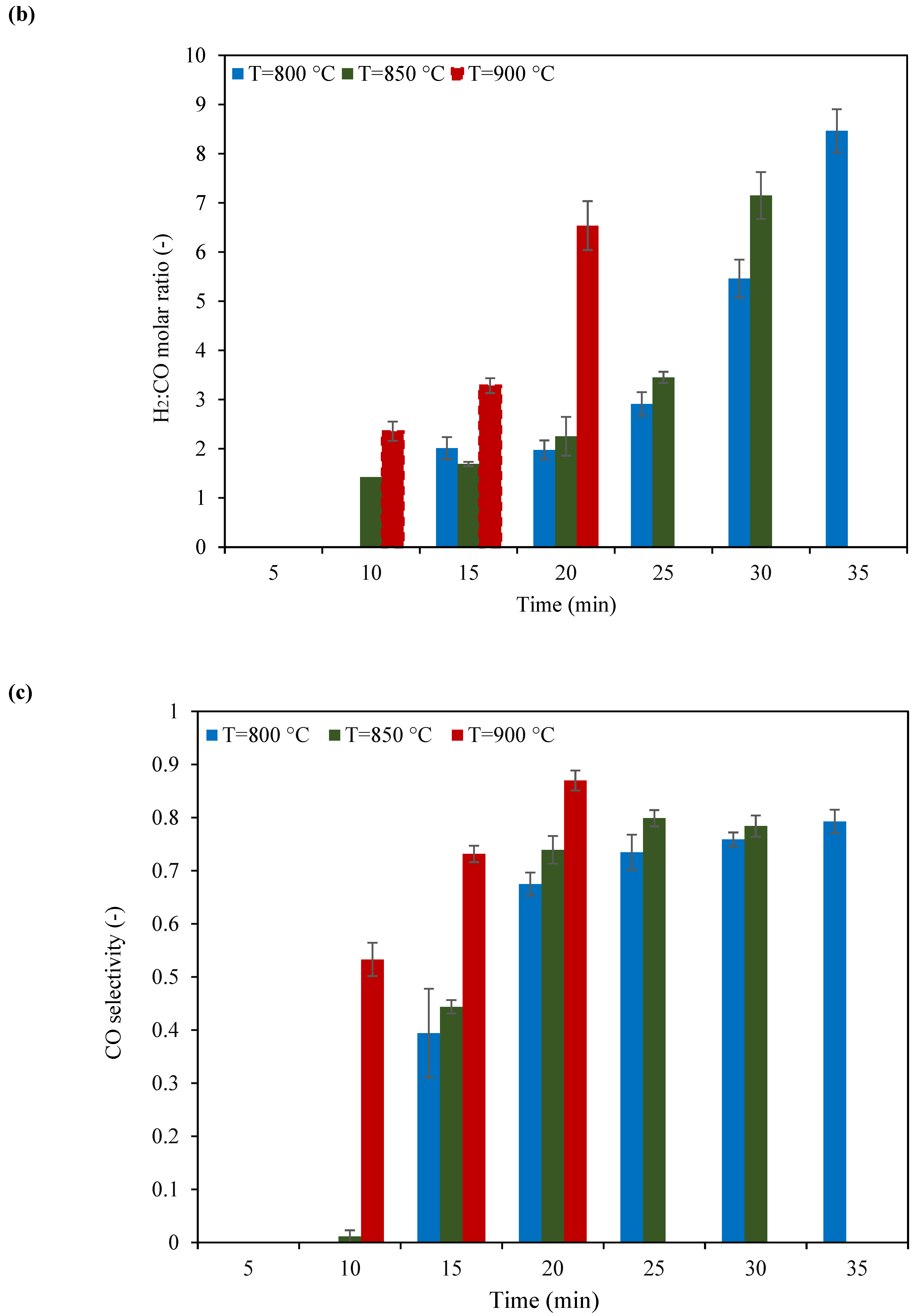
2.2. Catalyst Testing of KB(25,15)/LSF
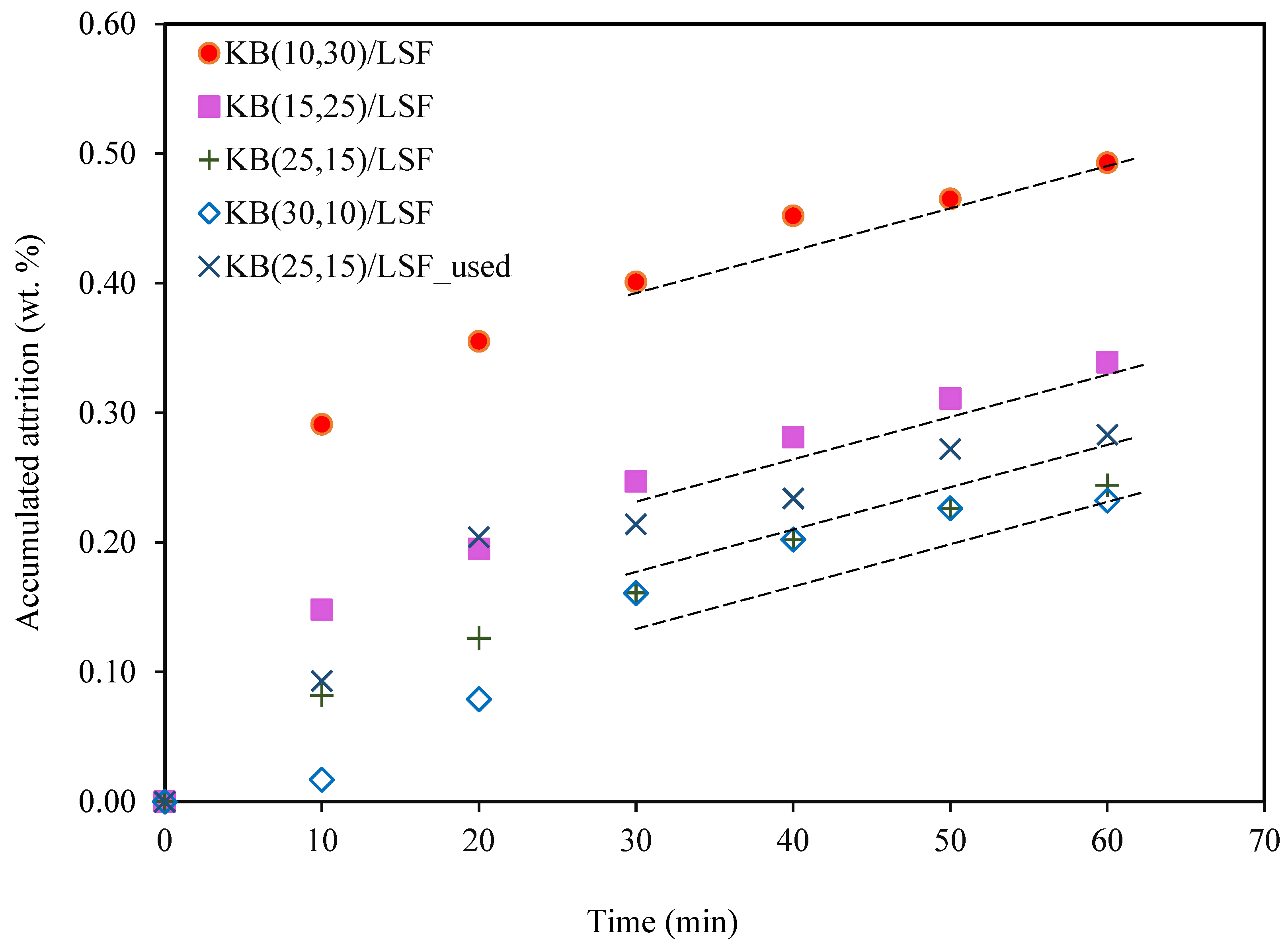
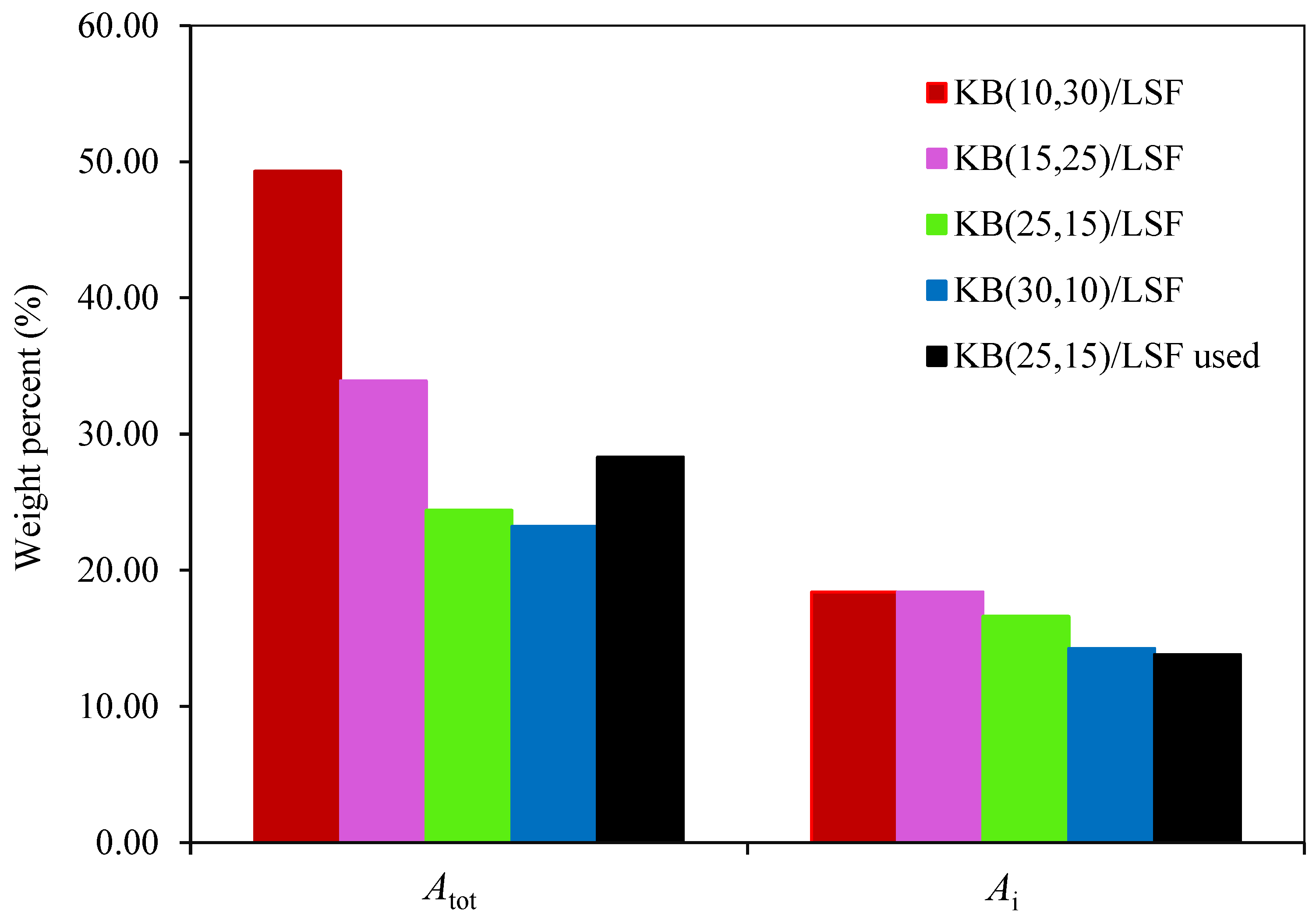
2.3. Analysis of the Attrition Tests
3. Materials and Methods
3.1. Materials
3.2. Preparation of Oxygen Carrier Materials
3.2.1. Synthesis of LSF
3.2.2. Synthesis of KB(x,y)/LSF
3.3. Characterizations
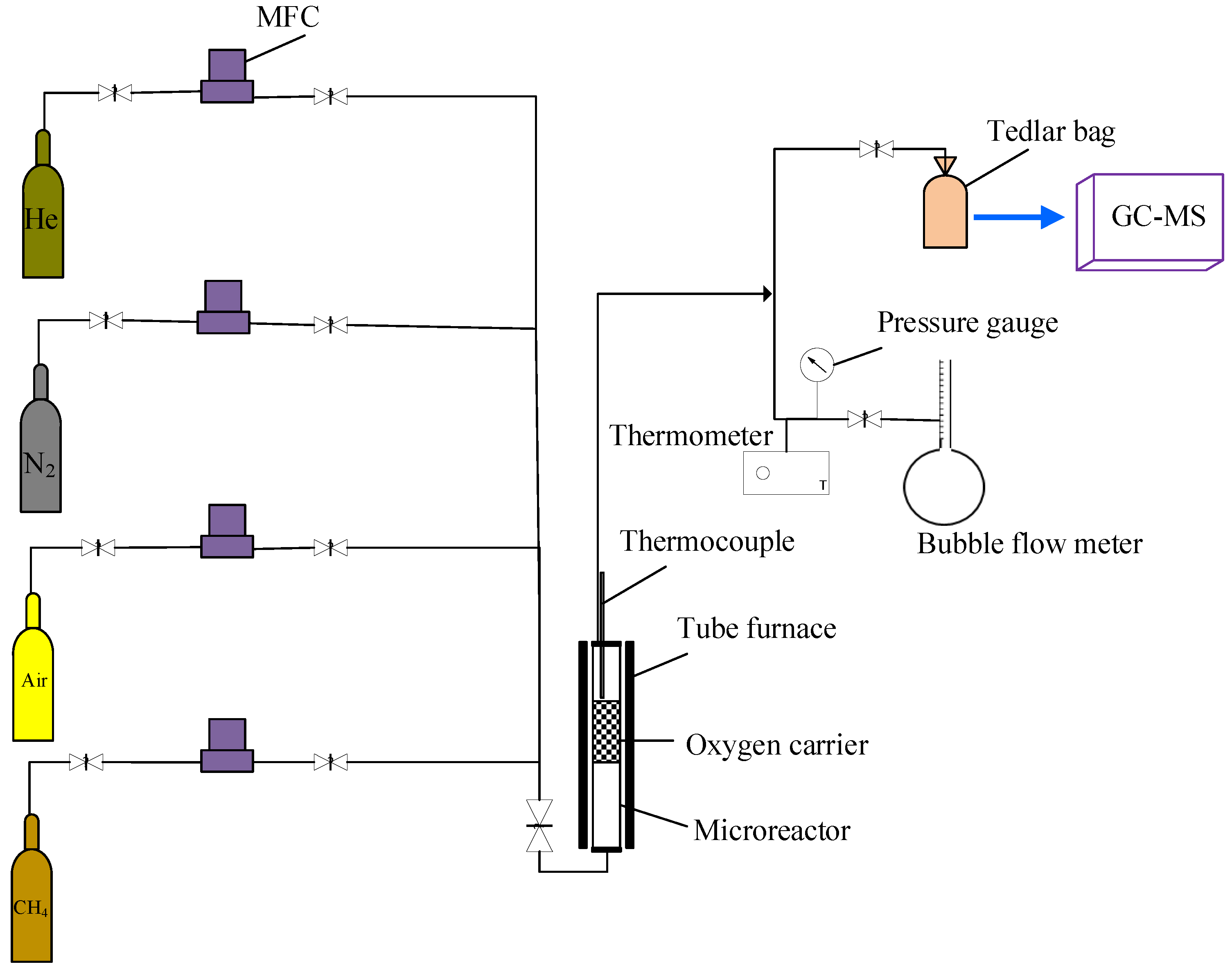
3.4. Reactivity Tests
3.5. Attrition Tests
4. Conclusions
- The characterization results confirmed the pure perovskite crystal structure of KB(x,y)/LSF catalysts. This structure demonstrated an adequate oxygen adsorption capacity, effective coke mitigation capability, robust thermal stability, and resilience to agglomeration during repetitive redox cycles;
- Among the tested catalysts, KB(25,15)/LSF emerged as the superior sample. Consistently, this catalyst produced syngas with a suitable H2:CO molar ratio (ranging from 2 to 3) within ten redox cycles, at 900 °C. The CH4 conversion and CO selectivity values reached up to 64% and 87%, respectively, showcasing its superb performance;
- The synthesized catalysts exhibited a logarithmic attrition pattern in the jet cup tests conducted at room temperature. These tests revealed high attrition resistance after the erosion of particle shape irregularities or weakly bound particles, indicating their durability;
- The KB(25,15)/LSF catalyst, when used at 900 °C, demonstrated great resistance in the attrition test. This finding suggests its potential endurance in the harsh conditions of fluidized bed reactors employed for the CLPO process.
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sokolovskii, V.D.; Coville, N.J.; Parmaliana, A.; Eskendirov, I.; Makoa, M. Methane Partial Oxidation. Challenge and Perspective. Catal. Today 1998, 42, 191–195. [Google Scholar] [CrossRef]
- Fotovat, F.; Rahimpour, M. Comparison and Reduction of the Chemical Kinetic Mechanisms Proposed for Thermal Partial Oxidation of Methane (TPOX) in Porous Media. Int. J. Hydrogen Energy 2021, 46, 19312–19322. [Google Scholar] [CrossRef]
- Sengodan, S.; Lan, R.; Humphreys, J.; Du, D.; Xu, W.; Wang, H.; Tao, S. Advances in Reforming and Partial Oxidation of Hydrocarbons for Hydrogen Production and Fuel Cell Applications. Renew. Sustain. Energy Rev. 2018, 82, 761–780. [Google Scholar] [CrossRef]
- Lv, Y.; Cheng, B.; Yang, H.; Cui, X.; Zhao, M. Chemical Looping Partial Oxidation (CLPO) of Toluene on LaFeO3 Perovskites for Tunable Syngas Production. Chem. Eng. J. 2023, 451, 138968. [Google Scholar] [CrossRef]
- Tang, M.; Xu, L.; Fan, M. Progress in Oxygen Carrier Development of Methane-Based Chemical-Looping Reforming: A Review. Appl. Energy 2015, 151, 143–156. [Google Scholar] [CrossRef]
- Johansson, M.; Mattisson, T.; Lyngfelt, A.; Abad, A. Using Continuous and Pulse Experiments to Compare Two Promising Nickel-Based Oxygen Carriers for Use in Chemical-Looping Technologies. Fuel 2008, 87, 988–1001. [Google Scholar] [CrossRef]
- Qin, L.; Chen, Y.; Guo, M.; Liu, Y.; Fan, J.A.; Fan, L. Driving towards Highly Selective and Coking-resistant Natural Gas Reforming through a Hybrid Oxygen Carrier Design. ChemCatChem 2021, 13, 617–626. [Google Scholar] [CrossRef]
- Mi, J.; Chen, J.; Chen, X.; Liu, X.; Li, J. Recent Status and Developments of Vacancies Modulation in the ABO3 Perovskites for Catalytic Applications. Chem. Eur. J. 2023, 29, e202202713. [Google Scholar] [CrossRef]
- O’Connell, M.; Norman, A.K.; Hüttermann, C.F.; Morris, M.A. Catalytic Oxidation over Lanthanum-Transition Metal Perovskite Materials. Catal. Today 1999, 47, 123–132. [Google Scholar] [CrossRef]
- Li, R.-J.; Yu, C.-C.; Ji, W.-J.; Shen, S.-K. Methane Oxidation to Synthesis Gas Using Lattice Oxygen in La1–XSrxFeO3 Perovskite Oxides Instead of Molecular Oxygen. In Studies in Surface Science and Catalysis; Elsevier: Amsterdam, The Netherlands, 2004; Volume 147, pp. 199–204. ISBN 978-0-444-50480-7. [Google Scholar]
- Zhao, K.; He, F.; Huang, Z.; Wei, G.; Zheng, A.; Li, H.; Zhao, Z. Perovskite-Type Oxides LaFe1 − XCoxO3 for Chemical Looping Steam Methane Reforming to Syngas and Hydrogen Co-Production. Appl. Energy 2016, 168, 193–203. [Google Scholar] [CrossRef]
- Dawa, T.; Sajjadi, B. Exploring the Potential of Perovskite Structures for Chemical Looping Technology: A State-of-the-Art Review. Fuel Process. Technol. 2024, 253, 108022. [Google Scholar] [CrossRef]
- De Vos, Y.; Jacobs, M.; Van Der Voort, P.; Van Driessche, I.; Snijkers, F.; Verberckmoes, A. Development of Stable Oxygen Carrier Materials for Chemical Looping Processes—A Review. Catalysts 2020, 10, 926. [Google Scholar] [CrossRef]
- Chang, W.; Hu, Y.; Xu, W.; Huang, C.; Chen, H.; He, J.; Han, Y.; Zhu, Y.; Ma, X.; Wang, X. Recent Advances of Oxygen Carriers for Hydrogen Production via Chemical Looping Water-Splitting. Catalysts 2023, 13, 279. [Google Scholar] [CrossRef]
- Manfro, R.L.; Souza, M.M.V.M. Overview of Ni-Based Catalysts for Hydrogen Production from Biogas Reforming. Catalysts 2023, 13, 1296. [Google Scholar] [CrossRef]
- Di Giuliano, A.; Capone, S.; Anatone, M.; Gallucci, K. Chemical Looping Combustion and Gasification: A Review and a Focus on European Research Projects. Ind. Eng. Chem. Res. 2022, 61, 14403–14432. [Google Scholar] [CrossRef]
- Fotovat, F.; Gill, K.; Grace, J.R.; Bi, X.T. Impact of Column Material on Electrostatics and Entrainment of Particles from Gas-Solid Fluidized Beds. Chem. Eng. Sci. 2017, 167, 120–134. [Google Scholar] [CrossRef]
- Fotovat, F. Entrainment from Bubbling and Turbulent Beds. In Essentials of Fluidization Technology; Grace, J.R., Bi, X.T., Ellis, N., Eds.; Wiley-VCH Verlag GmbH & Co. KGaA: Hoboken, NJ, USA, 2020; pp. 181–202. [Google Scholar]
- Fotovat, F.; Grace, J.R.; Bi, X.T. Particle Entrainment from Gas-Solid Fluidized Beds: Conductive vs. Dielectric Fines. AIChE J. 2016, 63, 1194–1202. [Google Scholar] [CrossRef]
- Nurherdiana, S.D.; Gunawan, T.; Widiastuti, N.; Fansuri, H. Grand Challenges of Perovskite and Metal Oxide-Based Membrane: A Form of Dual-Layer Hollow Fibre. J. Appl. Membr. Sci. Technol. 2021, 25, 17–28. [Google Scholar] [CrossRef]
- Murugan, A. Iron-Containing Perovskite Materials for Stable Hydrogen Production by Chemical Looping Water Splitting. Ph.D. Thesis, Newcastle University, Newcastle upon Tyne, UK, 2012; 224p. [Google Scholar]
- Zhang, R.; Cao, Y.; Li, H.; Zhao, Z.; Zhao, K.; Jiang, L. The Role of CuO Modified La0· 7Sr0· 3FeO3 Perovskite on Intermediate-Temperature Partial Oxidation of Methane via Chemical Looping Scheme. Int. J. Hydrogen Energy 2020, 45, 4073–4083. [Google Scholar] [CrossRef]
- McNeary, W.; Dameron, A.; Watson, M. Enhanced Catalyst Durability for the Oxidative Production of Biobased Chemicals (Cooperative Research and Development Final Report); National Renewable Energy Laboratory (NREL): Golden, CO, USA, 2022.
- Hwang, J.; Rao, R.R.; Giordano, L.; Katayama, Y.; Yu, Y.; Shao-Horn, Y. Perovskites in Catalysis and Electrocatalysis. Science 2017, 358, 751–756. [Google Scholar] [CrossRef]
- Yuan, C.; Li, Z.; Zhou, L.; Ju, G. Synthesis of Si-Modified Pseudo-Boehmite@ Kaolin Composite and Its Application as a Novel Matrix Material for FCC Catalyst. Materials 2022, 15, 2169. [Google Scholar] [CrossRef] [PubMed]
- Aimdate, K.; Srifa, A.; Koo-Amornpattana, W.; Sakdaronnarong, C.; Klysubun, W.; Kiatphuengporn, S.; Assabumrungrat, S.; Wongsakulphasatch, S.; Kaveevivitchai, W.; Sudoh, M. Natural Kaolin-Based Ni Catalysts for CO2 Methanation: On the Effect of Ce Enhancement and Microwave-Assisted Hydrothermal Synthesis. ACS Omega 2021, 6, 13779–13794. [Google Scholar] [CrossRef] [PubMed]
- Scherrer, P. Bestimmung Der Grosse Und Inneren Struktur von Kolloidteilchen Mittels Rontgenstrahlen. Nach. Ges. Wiss. Gottingen. 1918, 2, 8–100. [Google Scholar]
- He, F.; Li, X.; Zhao, K.; Huang, Z.; Wei, G.; Li, H. The Use of La1−xSrxFeO3 Perovskite-Type Oxides as Oxygen Carriers in Chemical-Looping Reforming of Methane. Fuel 2013, 108, 465–473. [Google Scholar] [CrossRef]
- Karouia, F.; Boualleg, M.; Digne, M.; Alphonse, P. The Impact of Nanocrystallite Size and Shape on Phase Transformation: Application to the Boehmite/Alumina Transformation. Adv. Powder Technol. 2016, 27, 1814–1820. [Google Scholar] [CrossRef]
- Burhan, M.; Shahzad, M.W.; Ng, K.C. Energy Distribution Function Based Universal Adsorption Isotherm Model for All Types of Isotherm. Int. J. Low-Carbon Technol. 2018, 13, 292–297. [Google Scholar] [CrossRef]
- Martins, G.V.A.; Berlier, G.; Bisio, C.; Coluccia, S.; Pastore, H.O.; Marchese, L. Quantification of Brønsted Acid Sites in Microporous Catalysts by a Combined FTIR and NH3-TPD Study. J. Phys. Chem. C 2008, 112, 7193–7200. [Google Scholar] [CrossRef]
- Shen, Y.; Zhao, K.; He, F.; Li, H.; Yang, S.; Kun, Z.; Fang, H.E.; Li, H. Synthesis of Three-Dimensionally Ordered Macroporous LaFe0.7Co0.3O3 Perovskites and Their Performance for Chemical-Looping Steam Reforming of Methane. J. Fuel Chem. Technol. 2016, 44, 1168–1176. [Google Scholar] [CrossRef]
- Padula, S.; Tregambi, C.; Troiano, M.; Di Benedetto, A.; Salatino, P.; Landi, G.; Solimene, R. Chemical Looping Reforming with Perovskite-Based Catalysts for Thermochemical Energy Storage. Energies 2022, 15, 8556. [Google Scholar] [CrossRef]
- Polo-Garzon, F.; Fung, V.; Zhang, J.; Bao, Z.; Meyer, H.M., III; Kidder, M.; Wu, Z. CH4 Activation over Perovskite Catalysts: True Density and Reactivity of Active Sites. ACS Catal. 2022, 12, 11845–11853. [Google Scholar] [CrossRef]
- Bhattar, S.; Abedin, M.A.; Kanitkar, S.; Spivey, J.J. A Review on Dry Reforming of Methane over Perovskite Derived Catalysts. Catal. Today 2021, 365, 2–23. [Google Scholar] [CrossRef]
- Nurherdiana, S.D.; Widiastuti, N.; Gunawan, T.; Wahyu, W. Perovskites Boosted by Metal Oxides as a Catalyst for the Partial Oxidation of Methane. Ceramics–Silikáty 2022, 66, 128–136. [Google Scholar] [CrossRef]
- Bian, Z.; Wang, Z.; Jiang, B.; Hongmanorom, P.; Zhong, W.; Kawi, S. A Review on Perovskite Catalysts for Reforming of Methane to Hydrogen Production. Renew. Sustain. Energy Rev. 2020, 134, 110291. [Google Scholar] [CrossRef]
- Yao, X.; Cheng, Q.; Bai, X.; Davaasuren, B.; Melinte, G.; Morlanes, N.; Cerrillo, J.L.; Velisoju, V.K.; Mohamed, H.O.; Kolubah, P.D. Enlarging the Three-Phase Boundary to Raise CO2/CH4 Conversions on Exsolved Ni–Fe Alloy Perovskite Catalysts by Minimal Rh Doping. ACS Catal. 2024, 14, 5639–5653. [Google Scholar] [CrossRef]
- Rydén, M.; Moldenhauer, P.; Lindqvist, S.; Mattisson, T.; Lyngfelt, A. Measuring Attrition Resistance of Oxygen Carrier Particles for Chemical Looping Combustion with a Customized Jet Cup. Powder Technol. 2014, 256, 75–86. [Google Scholar] [CrossRef]
- Alvarez-Galvan, C.; Melian, M.; Ruiz-Matas, L.; Eslava, J.L.; Navarro, R.M.; Ahmadi, M.; Roldan Cuenya, B.; Fierro, J.L.G. Partial Oxidation of Methane to Syngas over Nickel-Based Catalysts: Influence of Support Type, Addition of Rhodium, and Preparation Method. Front. Chem. 2019, 7, 104. [Google Scholar] [CrossRef]
- Kumar, D.; Singh, A.K.; Yadav, R.S.; Singh, A.K.; Rai, S.B. Synthesis Techniques and Applications of Perovskite Materials. In Perovskite Materials, Devices and Integration; IntechOpen: London, UK, 2020. [Google Scholar] [CrossRef]
- Dai, X.P.; Li, J.; Fan, J.T.; Wei, W.S.; Xu, J. Synthesis Gas Generation by Chemical-Looping Reforming in a Circulating Fluidized Bed Reactor Using Perovskite LaFeO3-Based Oxygen Carriers. Ind. Eng. Chem. Res. 2012, 51, 11072–11082. [Google Scholar] [CrossRef]
- Levenspiel, O. Chemical Reaction Engineering; John Wiley & Sons: Hoboken, NJ, USA, 1998; ISBN 047125424X. [Google Scholar]
- Fogler, H.S. Essentials of Chemical Reaction Engineering: Essenti Chemica Reactio Engi; Pearson Education: London, UK, 2010; ISBN 0132317176. [Google Scholar]
- Mayer, K.; Piesenberger, S.; Penthor, S.; Pröll, T.; Hofbauer, H. Chemical Looping Combustion Using Two Different Perovskite Based Oxygen Carriers: A Pilot Study. Energy Technol. 2018, 6, 2333–2343. [Google Scholar] [CrossRef] [PubMed]
- Chang, H.; Bjørgum, E.; Mihai, O.; Yang, J.; Lein, H.L.; Grande, T.; Raaen, S.; Zhu, Y.A.; Holmen, A.; Chen, D. Effects of Oxygen Mobility in La-Fe-Based Perovskites on the Catalytic Activity and Selectivity of Methane Oxidation. ACS Catal. 2020, 10, 3707–3719. [Google Scholar] [CrossRef]
- Ten Elshof, J.E.; Lankhorst, M.H.R.; Bouwmeester, H.J.M. Oxygen Exchange and Diffusion Coefficients of Strontium-doped Lanthanum Ferrites by Electrical Conductivity Relaxation. J. Electrochem. Soc. 1997, 144, 1060. [Google Scholar] [CrossRef]
- Ovtar, S.; Søgaard, M.; Norrman, K.; Hendriksen, P.V. Oxygen Exchange and Transport in (La0.6Sr0.4)0.98FeO3-d–Ce0.9Gd0.1O1.95 Dual-Phase Composites. J. Electrochem. Soc. 2018, 165, F220. [Google Scholar] [CrossRef]
- Arp, V.D.; McCarty, R.D. Thermophysical Properties of Helium-4 from 0.8 to 1500 K with Pressures to 2000 MPa; U.S. Government Printing Office: Washington, DC, USA, 1998.
- List of Viscosities. Available online: https://en.wikipedia.org/wiki/List_of_viscosities (accessed on 7 August 2024).



| Catalyst | Specific Surface Area (m2/g) | Total Pore Volume (cm3/g) | Average Pore Diameter (nm) |
|---|---|---|---|
| KB(30,10)/LSF | 14.04 | 0.094 | 26.9 |
| KB(25,15)/LSF | 16.95 | 0.100 | 24.9 |
| Used KB(25,15)/LSF | 16.62 | 0.098 | 23.8 |
| KB(10,30)/LSF | 25.82 | 0.124 | 19.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fotovat, F.; Beyzaei, M.; Ebrahimi, H.; Mohebolkhames, E. Synthesis, Characterization, and Attrition Resistance of Kaolin and Boehmite Alumina-Reinforced La0.7Sr0.3FeO3 Perovskite Catalysts for Chemical Looping Partial Oxidation of Methane. Catalysts 2024, 14, 670. https://doi.org/10.3390/catal14100670
Fotovat F, Beyzaei M, Ebrahimi H, Mohebolkhames E. Synthesis, Characterization, and Attrition Resistance of Kaolin and Boehmite Alumina-Reinforced La0.7Sr0.3FeO3 Perovskite Catalysts for Chemical Looping Partial Oxidation of Methane. Catalysts. 2024; 14(10):670. https://doi.org/10.3390/catal14100670
Chicago/Turabian StyleFotovat, Farzam, Mohammad Beyzaei, Hadi Ebrahimi, and Erfan Mohebolkhames. 2024. "Synthesis, Characterization, and Attrition Resistance of Kaolin and Boehmite Alumina-Reinforced La0.7Sr0.3FeO3 Perovskite Catalysts for Chemical Looping Partial Oxidation of Methane" Catalysts 14, no. 10: 670. https://doi.org/10.3390/catal14100670
APA StyleFotovat, F., Beyzaei, M., Ebrahimi, H., & Mohebolkhames, E. (2024). Synthesis, Characterization, and Attrition Resistance of Kaolin and Boehmite Alumina-Reinforced La0.7Sr0.3FeO3 Perovskite Catalysts for Chemical Looping Partial Oxidation of Methane. Catalysts, 14(10), 670. https://doi.org/10.3390/catal14100670







