Synthesis, Magnetic, and Photocatalytic Activity of Polypyrrole-Based TiO2–Fe Catalyst for Wastewater Treatment
Abstract
:1. Introduction
2. Results and Discussion
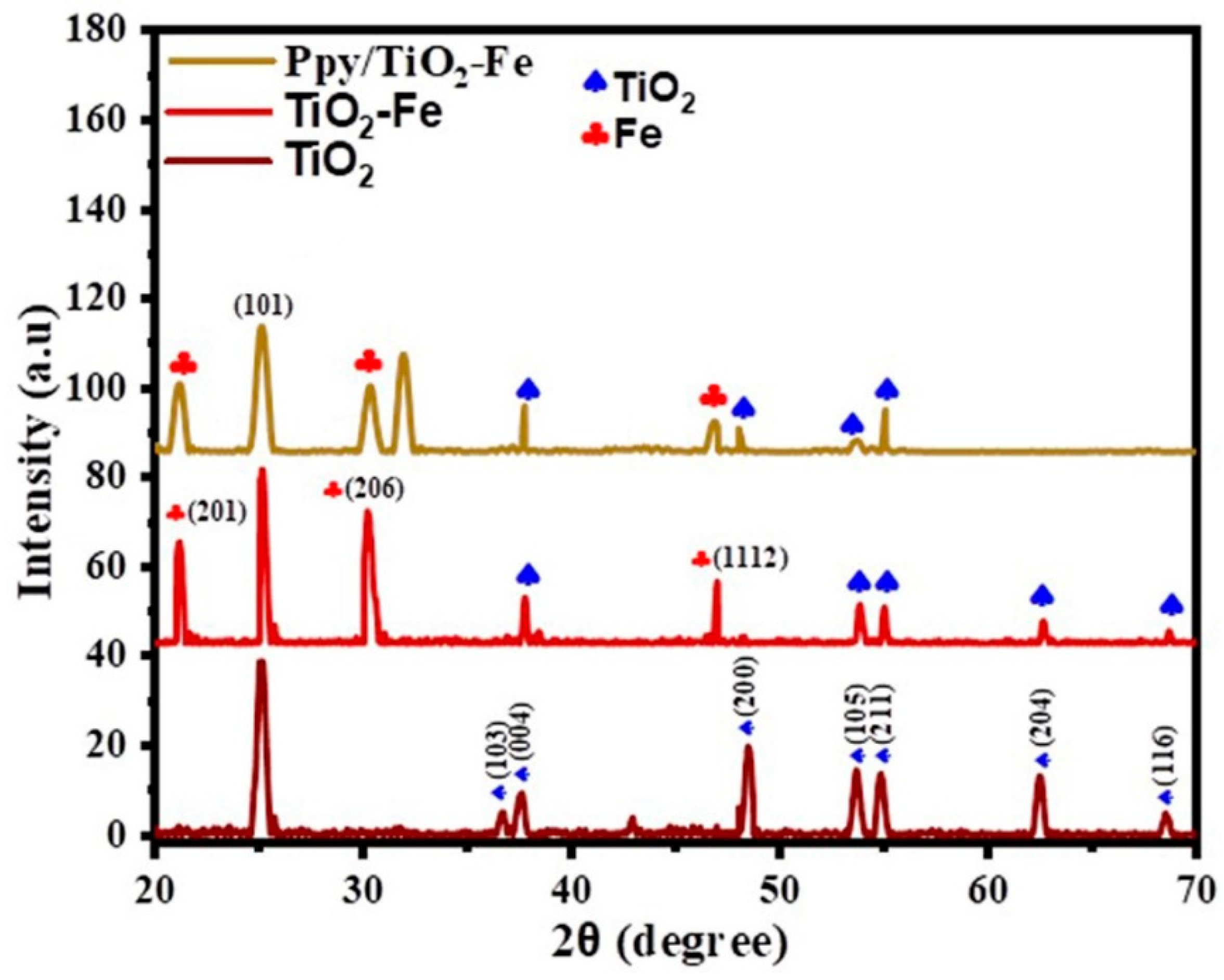
2.1. XRD Analysis
2.2. Fourier Transform Infrared Spectroscopy (FTIR) Analysis
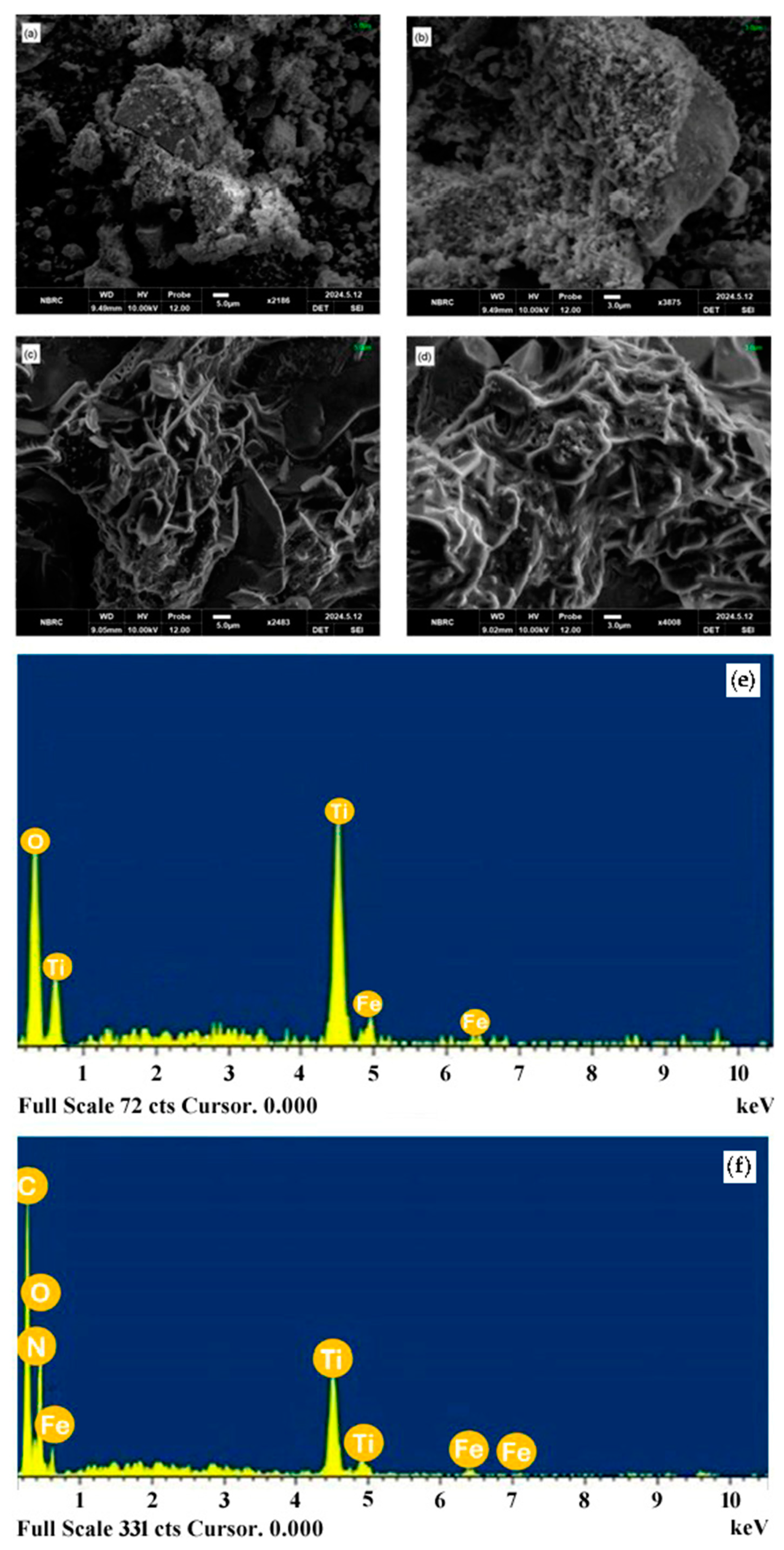
2.3. SEM and EDS Analysis
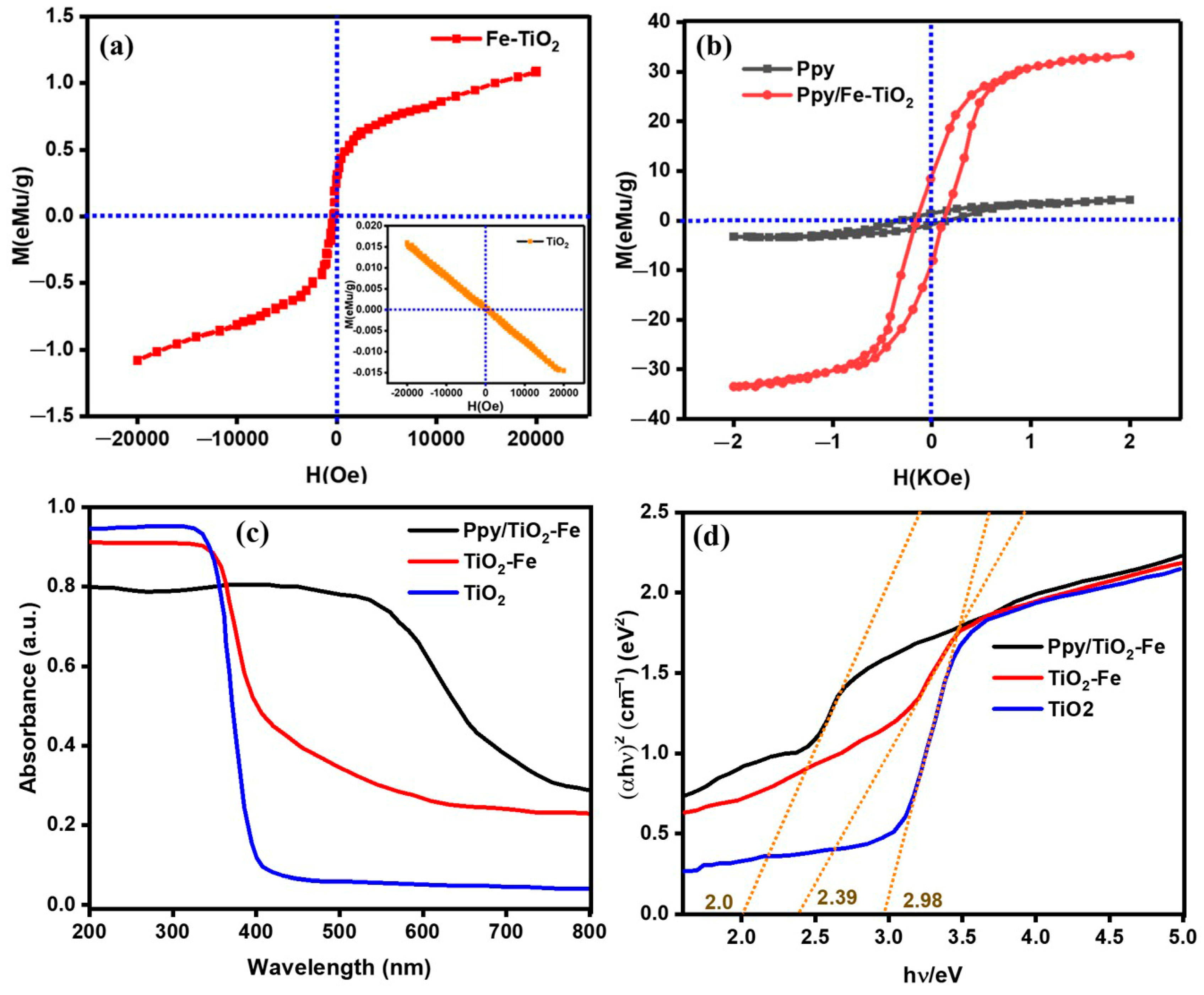
2.4. VSM and DRS Analysis
2.5. N2 Adsorption-Desorption Analysis
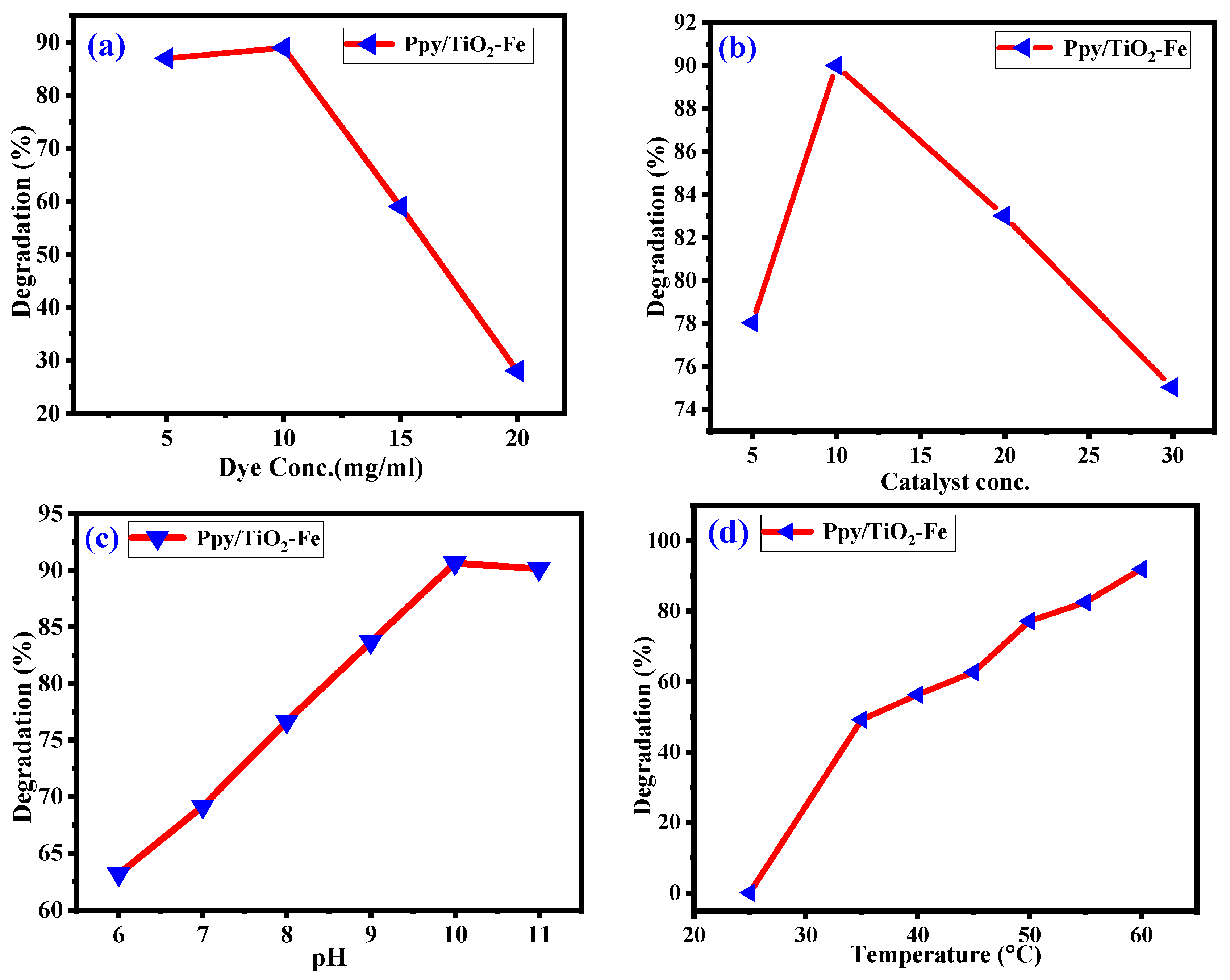
2.6. Parameters Factors Influencing Dye Degradation Rate
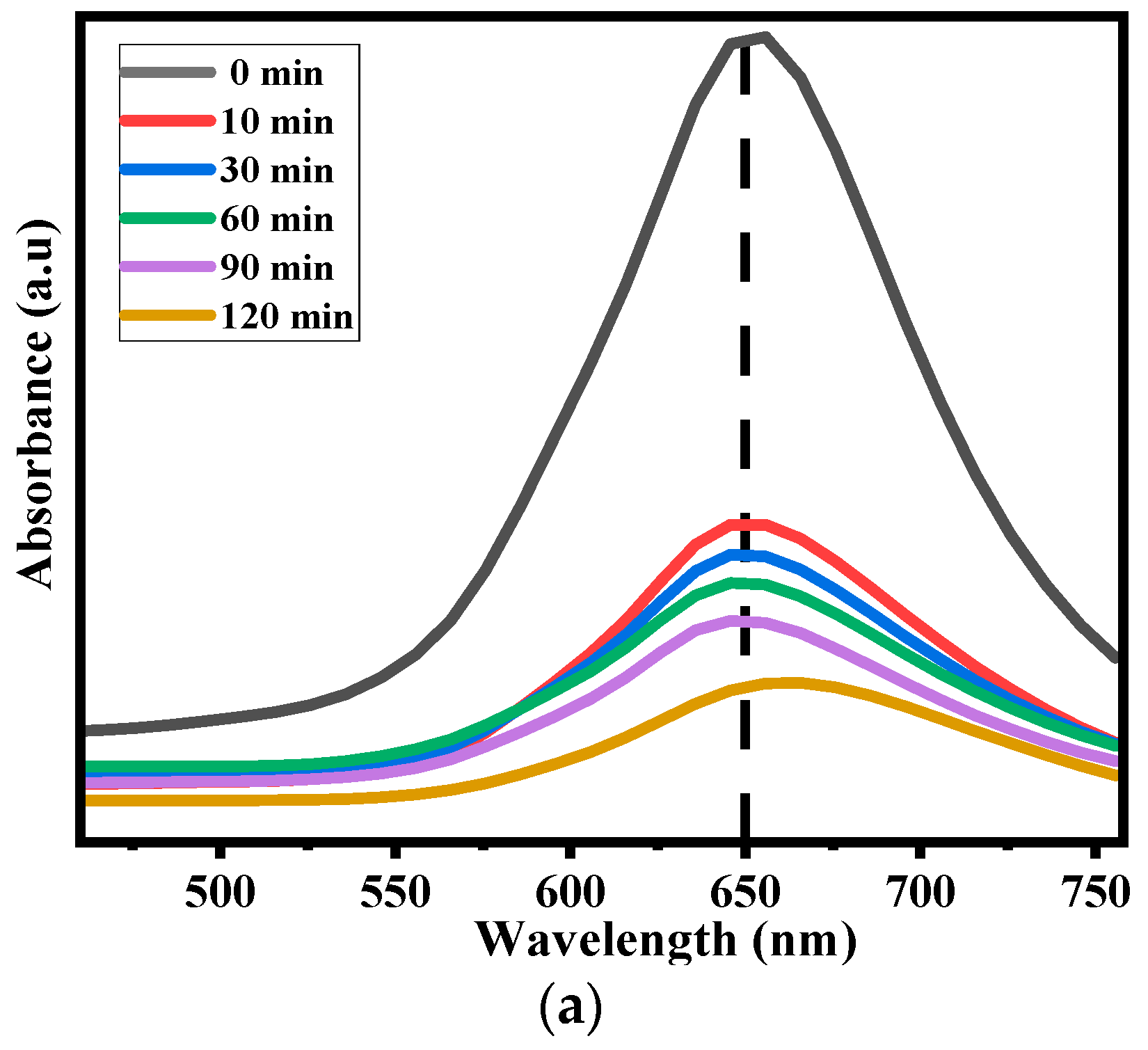
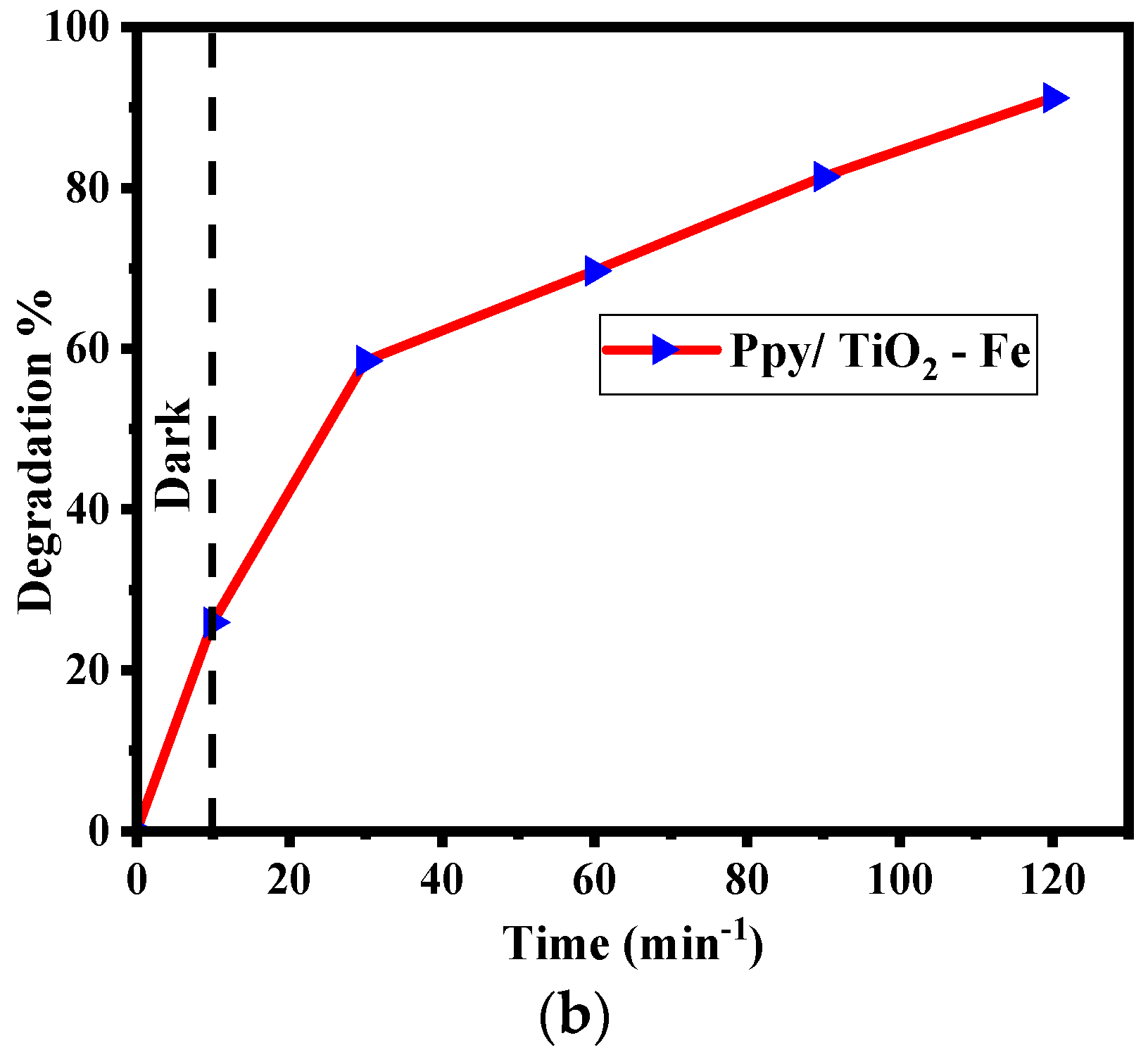
2.6.1. Effect of Contact Time
2.6.2. Photocatalytic Efficiency of Ppy/Fe-TiO2
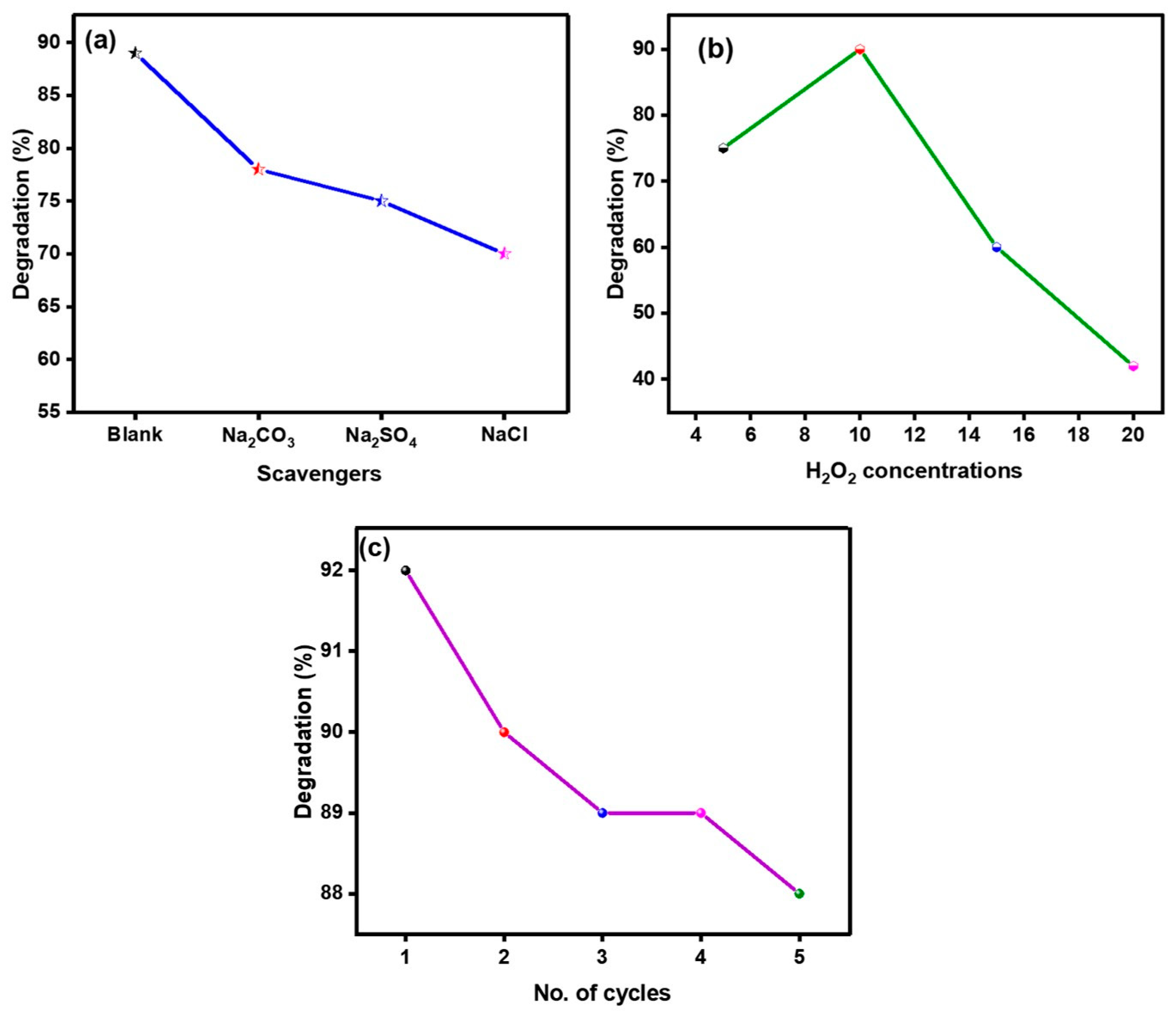
2.6.3. Scavengers Effect
2.6.4. Impact of H2O2 Concentration
2.6.5. Recyclability of Ppy/Fe–TiO2 Photocatalyst
2.7. Degradation Kinetics
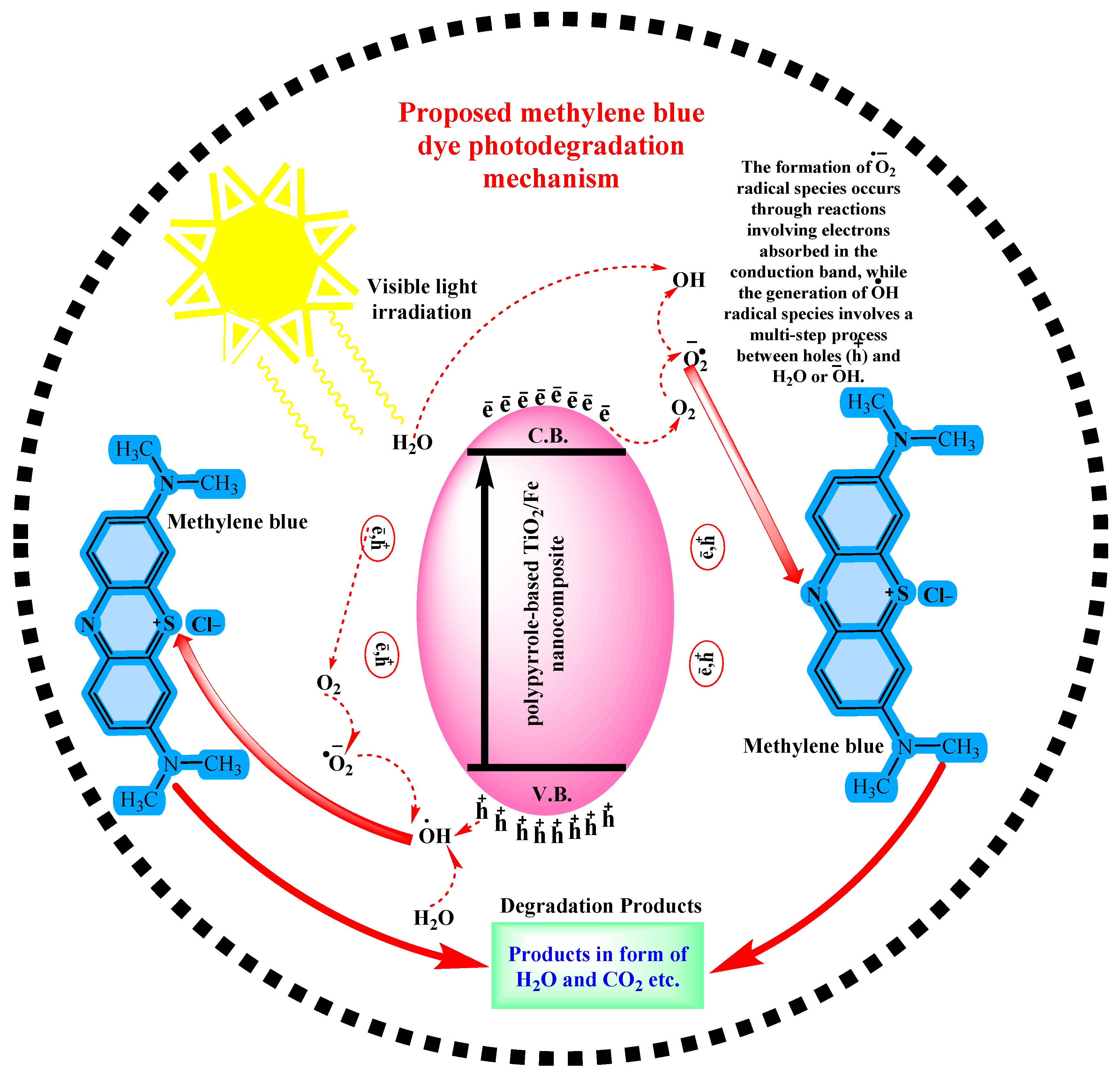
2.8. Mechanism of Photocatalytic Activity
3. Experimental Section
3.1. Materials
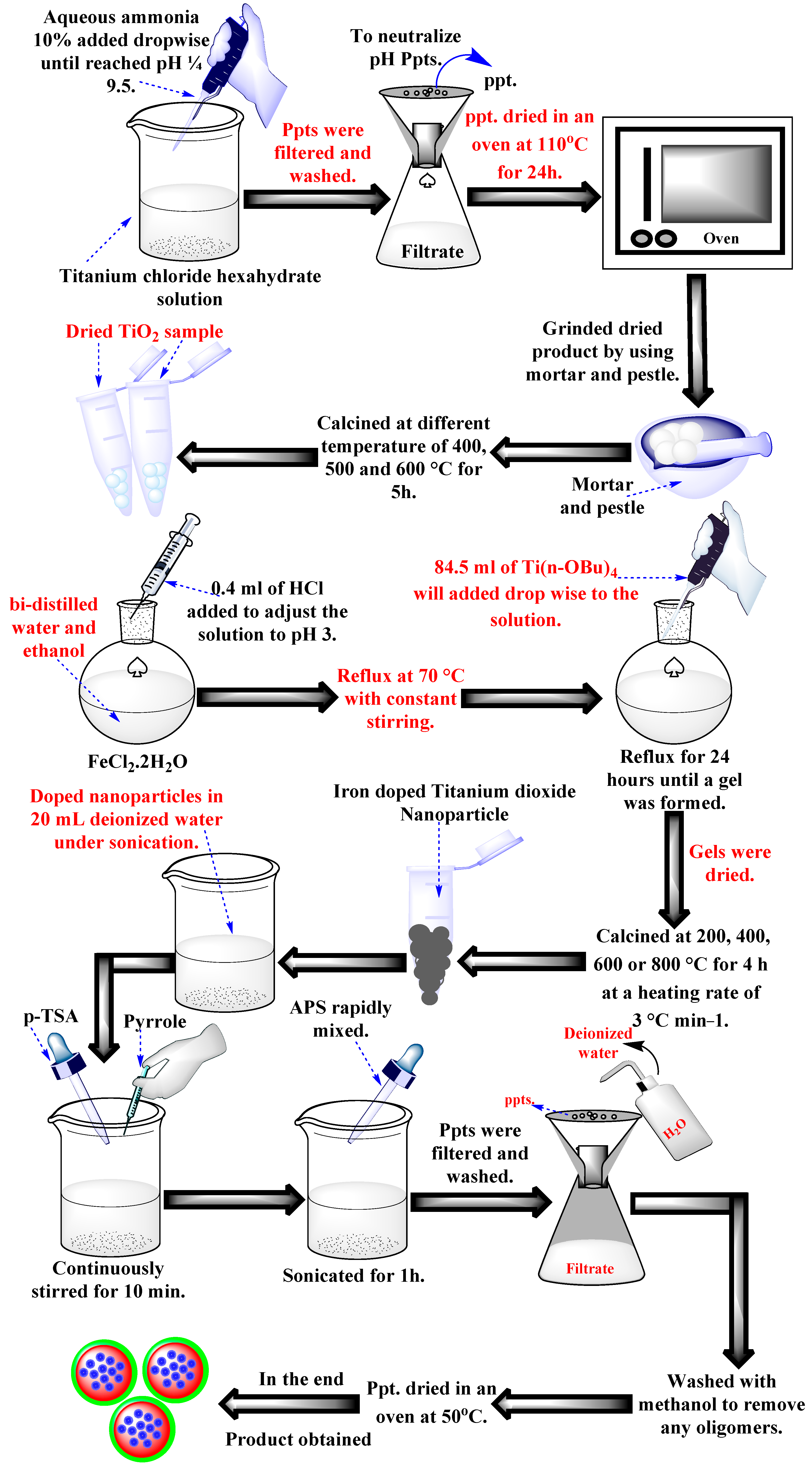
3.2. Synthesis of Titanium Dioxide Nanoparticles
3.3. Synthesis of Iron-Doped Titanium Dioxide Nanoparticles
3.4. Synthesis of Polypyrrole-Based Iron-Doped Titanium Dioxide Photocatalyst
3.5. Structural Characterization
3.6. Photocatalytic Degradation of MB Dye
3.7. Parameters Affecting the Rate of Dye Degradation
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Das, A.; Dey, A. P-Nitrophenol-Bioremediation using potent Pseudomonas strain from the textile dye industry effluent. J. Environ. Chem. Eng. 2020, 8, 103830. [Google Scholar] [CrossRef]
- Lakshmi, S.; Suvedha, K.; Sruthi, R.; Lavanya, J.; Varjani, S.; Nakkeeran, E. Hexavalent chromium sequestration from electronic waste by biomass of Aspergillus carbonarius. Bioengineered 2020, 11, 708–717. [Google Scholar] [CrossRef] [PubMed]
- Shindhal, T.; Rakholiya, P.; Varjani, S.; Pandey, A.; Ngo, H.H.; Guo, W.; Ng, H.Y.; Taherzadeh, M.J. A critical review on advances in the practices and perspectives for the treatment of dye industry wastewater. Bioengineered 2021, 12, 70–87. [Google Scholar] [CrossRef] [PubMed]
- Javaid, R.; Qazi, U.Y. Catalytic oxidation process for the degradation of synthetic dyes: An overview. Int. J. Environ. Res. Public Health 2019, 16, 2066. [Google Scholar] [CrossRef] [PubMed]
- Bilal, M.; Iqbal, M.; Hu, H.; Zhang, X. Mutagenicity and cytotoxicity assessment of biodegraded textile effluent by Ca-alginate encapsulated manganese peroxidase. Biochem. Eng. J. 2016, 109, 153–161. [Google Scholar] [CrossRef]
- Varjani, S.; Rakholiya, P.; Ng, H.Y.; You, S.; Teixeira, J.A. Microbial degradation of dyes: An overview. Bioresour. Technol. 2020, 314, 123728. [Google Scholar] [CrossRef]
- Adeyemo, A.A.; Adeoye, I.O.; Bello, O.S. Adsorption of dyes using different types of clay: A review. Appl. Water Sci. 2017, 7, 543–568. [Google Scholar] [CrossRef]
- Tichapondwa, S.M.; Newman, J.; Kubheka, O. Effect of TiO2 phase on the photocatalytic degradation of methylene blue dye. Phys. Chem. Earth Parts A/B/C 2020, 118, 102900. [Google Scholar] [CrossRef]
- Rajoriya, S.; Bargole, S.; Saharan, V.K. Degradation of reactive blue 13 using hydrodynamic cavitation: Effect of geometrical parameters and different oxidizing additives. Ultrason. Sonochem. 2017, 37, 192–202. [Google Scholar] [CrossRef]
- Chen, C.; Ma, W.; Zhao, J. Semiconductor-mediated photodegradation of pollutants under visible-light irradiation. Chem. Soc. Rev. 2010, 39, 4206–4219. [Google Scholar] [CrossRef]
- Chopra, L. Photocatalytic activity of zinc oxide for dye and drug degradation: A review. Mater. Today Proc. 2022, 52, 1653–1656. [Google Scholar]
- Ahmed, M.; El-Katori, E.E.; Gharni, Z.H. Photocatalytic degradation of methylene blue dye using Fe2O3/TiO2 nanoparticles prepared by sol–gel method. J. Alloys Compd. 2013, 553, 19–29. [Google Scholar] [CrossRef]
- Kazemi, M.; Mohammadizadeh, M. Simultaneous improvement of photocatalytic and superhydrophilicity properties of nano TiO2 thin films. Chem. Eng. Res. Des. 2012, 90, 1473–1479. [Google Scholar] [CrossRef]
- Saha, S.; Wang, J.; Pal, A. Nano silver impregnation on commercial TiO2 and a comparative photocatalytic account to degrade malachite green. Sep. Purif. Technol. 2012, 89, 147–159. [Google Scholar] [CrossRef]
- Zhao, W.; Bai, Z.; Ren, A.; Guo, B.; Wu, C. Sunlight photocatalytic activity of CdS modified TiO2 loaded on activated carbon fibers. Appl. Surf. Sci. 2010, 256, 3493–3498. [Google Scholar] [CrossRef]
- Puma, G.L.; Bono, A.; Krishnaiah, D.; Collin, J.G. Preparation of titanium dioxide photocatalyst loaded onto activated carbon support using chemical vapor deposition: A review paper. J. Hazard. Mater. 2008, 157, 209–219. [Google Scholar] [CrossRef]
- Wang, T.; Zhu, Y.-C.; Sun, Z.-X.; Wu, L.-G. Preparation of weak light driven TiO2 multi composite photocatalysts via adsorption phase synthesis. Huan Jing Ke Xue = Huanjing Kexue 2015, 36, 559–567. [Google Scholar]
- Chen, M.-N.; Mo, L.-P.; Cui, Z.-S.; Zhang, Z.-H. Magnetic nanocatalysts: Synthesis and application in multicomponent reactions. Curr. Opin. Green Sustain. Chem. 2019, 15, 27–37. [Google Scholar] [CrossRef]
- Suliman, Z.A.; Mecha, A.C.; Mwasiagi, J.I. Effect of TiO2/Fe2O3 nanopowder synthesis method on visible light photocatalytic degradation of reactive blue dye. Heliyon 2024, 10, e29648. [Google Scholar] [CrossRef]
- Deng, Q.; Shen, Y.; Zhu, H.; Tu, T. A magnetic nanoparticle-supported N-heterocyclic carbene-palladacycle: An efficient and recyclable solid molecular catalyst for Suzuki–Miyaura cross-coupling of 9-chloroacridine. Chem. Commun. 2017, 53, 13063–13066. [Google Scholar] [CrossRef]
- Xu, L.; Zhang, S.Z.; Li, W.; Zhang, Z.H. Visible-Light-Mediated Oxidative Amidation of Aldehydes by Using Magnetic CdS Quantum Dots as a Photocatalyst. Chem.—Eur. J. 2021, 27, 5483–5491. [Google Scholar] [CrossRef] [PubMed]
- Rakibuddin, M.; Ananthakrishnan, R. Novel nano coordination polymer based synthesis of porous ZnO hexagonal nanodisk for higher gas sorption and photocatalytic activities. Appl. Surf. Sci. 2016, 362, 265–273. [Google Scholar] [CrossRef]
- Kumar, R.; El-Shishtawy, R.M.; Barakat, M.A. Synthesis and characterization of Ag-Ag2O/TiO2@ polypyrrole heterojunction for enhanced photocatalytic degradation of methylene blue. Catalysts 2016, 6, 76. [Google Scholar] [CrossRef]
- Lin, C.-C.; Ho, J.-M. Structural analysis and catalytic activity of Fe3O4 nanoparticles prepared by a facile co-precipitation method in a rotating packed bed. Ceram. Int. 2014, 40, 10275–10282. [Google Scholar] [CrossRef]
- Khan, S.; Noor, A.; Khan, I.; Muhammad, M.; Sadiq, M.; Muhammad, N. Photocatalytic degradation of organic dyes contaminated aqueous solution using binary CdTiO2 and ternary NiCdTiO2 nanocomposites. Catalysts 2022, 13, 44. [Google Scholar] [CrossRef]
- Moalej, N.S.; Ahadi, S.; Sheibani, S. Photocatalytic degradation of methylene blue by 2 wt.% Fe doped TiO2 nanopowder under visible light irradiation. J. Ultrafine Grained Nanostruct. Mater. 2019, 52, 133–141. [Google Scholar]
- Li, R.; Jia, Y.; Bu, N.; Wu, J.; Zhen, Q. Photocatalytic degradation of methyl blue using Fe2O3/TiO2 composite ceramics. J. Alloys Compd. 2015, 643, 88–93. [Google Scholar] [CrossRef]
- Feng, J.; Sun, N.; Wu, D.; Yang, H.; Xu, H.; Yan, W. Preparation of Fe3O4/TiO2/polypyrrole ternary magnetic composite and using as adsorbent for the removal of acid red G. J. Polym. Environ. 2017, 25, 781–791. [Google Scholar] [CrossRef]
- Ali, H.; Ismail, A. Fabrication of magnetic Fe3O4/Polypyrrole/carbon black nanocomposite for effective uptake of congo red and methylene blue dye: Adsorption investigation and mechanism. J. Polym. Environ. 2023, 31, 976–998. [Google Scholar] [CrossRef]
- Sood, S.; Umar, A.; Mehta, S.K.; Kansal, S.K. Highly effective Fe-doped TiO2 nanoparticles photocatalysts for visible-light driven photocatalytic degradation of toxic organic compounds. J. Colloid Interface Sci. 2015, 450, 213–223. [Google Scholar] [CrossRef]
- Kumar, M.A.; Abebe, B.; Nagaswarupa, H.; Murthy, H.A.; Ravikumar, C.; Sabir, F.K. Enhanced photocatalytic and electrochemical performance of TiO2-Fe2O3 nanocomposite: Its applications in dye decolorization and as supercapacitors. Sci. Rep. 2020, 10, 1249. [Google Scholar] [CrossRef] [PubMed]
- Yuan, X.; Kobylanski, M.P.; Cui, Z.; Li, J.; Beaunier, P.; Dragoe, D.; Colbeau-Justin, C.; Zaleska-Medynska, A.; Remita, H. Highly active composite TiO2-polypyrrole nanostructures for water and air depollution under visible light irradiation. J. Environ. Chem. Eng. 2020, 8, 104178. [Google Scholar] [CrossRef]
- Wei, J.; Zhao, L.; Peng, S.; Shi, J.; Liu, Z.; Wen, W. Wettability of urea-doped TiO2 nanoparticles and their high electrorheological effects. J. Sol-Gel Sci. Technol. 2008, 47, 311–315. [Google Scholar] [CrossRef]
- Wang, D.; Wang, Y.; Li, X.; Luo, Q.; An, J.; Yue, J. Sunlight photocatalytic activity of polypyrrole–TiO2 nanocomposites prepared by ‘in situ’method. Catal. Commun. 2008, 9, 1162–1166. [Google Scholar] [CrossRef]
- Deng, F.; Li, Y.; Luo, X.; Yang, L.; Tu, X. Preparation of conductive polypyrrole/TiO2 nanocomposite via surface molecular imprinting technique and its photocatalytic activity under simulated solar light irradiation. Colloids Surf. A Physicochem. Eng. Asp. 2012, 395, 183–189. [Google Scholar] [CrossRef]
- Guo, G.-S.; He, C.-N.; Wang, Z.-H.; Gu, F.-B.; Han, D.-M. Synthesis of titania and titanate nanomaterials and their application in environmental analytical chemistry. Talanta 2007, 72, 1687–1692. [Google Scholar] [CrossRef]
- Arora, K.; Chaubey, A.; Singhal, R.; Singh, R.P.; Pandey, M.; Samanta, S.; Malhotra, B.; Chand, S. Application of electrochemically prepared polypyrrole–polyvinyl sulphonate films to DNA biosensor. Biosens. Bioelectron. 2006, 21, 1777–1783. [Google Scholar] [CrossRef]
- Roy, S.; Mishra, S.; Yogi, P.; Saxena, S.K.; Sagdeo, P.R.; Kumar, R. Synthesis of conducting polypyrrole-titanium oxide nanocomposite: Study of structural, optical and electrical properties. J. Inorg. Organomet. Polym. Mater. 2017, 27, 257–263. [Google Scholar] [CrossRef]
- Li, Q.; Zhang, C.; Li, J. Photocatalytic and microwave absorbing properties of polypyrrole/Fe-doped TiO2 composite by in situ polymerization method. J. Alloys Compd. 2011, 509, 1953–1957. [Google Scholar] [CrossRef]
- Rouhi, M.; Babamoradi, M.; Hajizadeh, Z.; Maleki, A.; Maleki, S.T. Design and performance of polypyrrole/halloysite nanotubes/Fe3O4/Ag/Co nanocomposite for photocatalytic degradation of methylene blue under visible light irradiation. Optik 2020, 212, 164721. [Google Scholar] [CrossRef]
- Choudhury, B.; Verma, R.; Choudhury, A. Oxygen defect assisted paramagnetic to ferromagnetic conversion in Fe doped TiO2 nanoparticles. RSC Adv. 2014, 4, 29314–29323. [Google Scholar] [CrossRef]
- Han, K.-H.; Kim, Y.-H.; Mun, M.-H.; Yu, J.-H.; Han, R.-H. Synthesis of polypyrrole-modified Fe3O4/SiO2/TiO2 nanocomposite microspheres and their photocatalytic activity. Mater. Res. Express 2022, 9, 025007. [Google Scholar] [CrossRef]
- Li, K.; Liu, S.; Xue, Y.; Zhang, L.; Han, Y. A superparamagnetic Fe3O4–TiO2 composite coating on titanium by micro-arc oxidation for percutaneous implants. J. Mater. Chem. B 2019, 7, 5265–5276. [Google Scholar] [CrossRef]
- Wuang, S.C.; Neoh, K.G.; Kang, E.-T.; Pack, D.W.; Leckband, D.E. Synthesis and functionalization of polypyrrole-Fe3O4 nanoparticles for applications in biomedicine. J. Mater. Chem. 2007, 17, 3354–3362. [Google Scholar] [CrossRef]
- Park, D.E.; Chae, H.S.; Choi, H.J.; Maity, A. Magnetite–polypyrrole core–shell structured microspheres and their dual stimuli-response under electric and magnetic fields. J. Mater. Chem. C 2015, 3, 3150–3158. [Google Scholar] [CrossRef]
- Hassani, A.; Khataee, A.; Karaca, S. Photocatalytic degradation of ciprofloxacin by synthesized TiO2 nanoparticles on montmorillonite: Effect of operation parameters and artificial neural network modeling. J. Mol. Catal. A Chem. 2015, 409, 149–161. [Google Scholar] [CrossRef]
- Feng, Y.; Liu, H.; Lu, Q.; Liu, Y.; Li, J.; He, X.; Liu, X.; Mikhailova, D. Designing hierarchical MnO/polypyrrole heterostructures to couple polysulfides adsorption and electrocatalysis in lithium-sulfur batteries. J. Power Sources 2022, 520, 230885. [Google Scholar] [CrossRef]
- Sethy, N.K.; Arif, Z.; Mishra, P.K.; Kumar, P. Green synthesis of TiO2 nanoparticles from Syzygium cumini extract for photo-catalytic removal of lead (Pb) in explosive industrial wastewater. Green Process. Synth. 2020, 9, 171–181. [Google Scholar] [CrossRef]
- Kupolati, W.; Sadiku, E.R.; Eze, A.; Ibrahim, I.; Agboola, O. Life cycle assessment, recycling and re-use of the bionanocomposites. In Polymer Based Bio-Nanocomposites: Properties, Durability and Applications; Springer: Singapore, 2022; pp. 205–216. [Google Scholar]
- Fatima, S.; Javaid, S.; Ahmad, H.; Almasoudi, A.; Baamer, D.F.; Ali, O.M.; Carabineiro, S.A.; Taj, M.B. Facile Synthesis of Sodium Alginate (SA)-Based Quaternary Bio-Nanocomposite (SA@ Co-Zn-Ce) for Antioxidant Activity and Photocatalytic Degradation of Reactive Red 24. Catalysts 2024, 14, 471. [Google Scholar] [CrossRef]
- Maseeh, I.; Anwar, F.; Aroob, S.; Javed, T.; Bibi, I.; Almasoudi, A.; Raheel, A.; Javid, M.A.; Carabineiro, S.A.; Taj, M.B. Multifunctional MgAl LDH/Zn-MOF S-scheme heterojunction: Efficient hydrogen production, methyl red removal, and CO2 adsorption. Mater. Adv. 2024, 5, 5080–5095. [Google Scholar]
- Usman, M.; Taj, M.B.; Almasoudi, A.; Baamer, D.F.; Ali, O.M.; Khan, M.I.; Bibi, I.; Rehman, M.U.; Rasheed, R.; Raheel, A. Novel Starch-Modified NiCrMn-LDH-Based Composite for Photocatalytic Degradation of Reactive Orange 13. Catalysts 2024, 14, 449. [Google Scholar] [CrossRef]
- Shaban, M.; Ahmed, A.M.; Shehata, N.; Betiha, M.A.; Rabie, A.M. Ni-doped and Ni/Cr co-doped TiO2 nanotubes for enhancement of photocatalytic degradation of methylene blue. J. Colloid Interface Sci. 2019, 555, 31–41. [Google Scholar] [CrossRef] [PubMed]
- Rabie, A.M.; Abukhadra, M.R.; Rady, A.M.; Ahmed, S.A.; Labena, A.; Mohamed, H.S.; Betiha, M.A.; Shim, J.-J. Instantaneous photocatalytic degradation of malachite green dye under visible light using novel green Co–ZnO/algae composites. Res. Chem. Intermed. 2020, 46, 1955–1973. [Google Scholar] [CrossRef]




| Photocatalysts | Dye | Synthesis Method | Degradation% | Time | Ref. |
|---|---|---|---|---|---|
| Fe3O4 nanoparticles | MB | Co-precipitation | 80% | 120 min. | [24] |
| NiCdTiO2 | MB | Co-precipitation | 86% | 100 min. | [25] |
| Fe3O4/SiO2/TiO2 | MB | Co-precipitation | 90% | 60 min. | [26] |
| Fe2O3/TiO2 nanopowders | MB | Co-precipitation | 88% | - | [27] |
| Polypyrrole-based TiO2-Fe | MB | Co-precipitation | 91.92 | 120 min. | Present work |
| Sample Name | Crystallite Size (nm) |
|---|---|
| TiO2 | 24.99 |
| TiO2-Fe | 21.94 |
| Ppy/TiO2-Fe | 21.84 |
| Magnetic Parameters | HC (Oe) | MS (emu/g) | Mr (emu/g) | Squareness (Mr/MS) | Experimental Magnetic Moment η (Ms/Hc) (μB) | Behavior |
|---|---|---|---|---|---|---|
| TiO2-Fe | 341.39 | 1.09 | 0.29 | 0.266 | 0.0032 | Ferromagnetic |
| Ppy/TiO2-Fe | 0.160 | 33.11 | 8.39 | 0.253 | 206.94 | Superparamagnetic |
| Initial Concentration (ppm) | R2 | Rate Constant (1/min) | Straight Line Equation |
|---|---|---|---|
| 5 ppm | 0.83 | 0.002 | 0.12–0.02 |
| 10 ppm | 0.99 | 0.009 | 0.19–0.01 |
| 15 ppm | 0.97 | 0.019 | 0.42–0.02 |
| 20 ppm | 0.96 | 0.021 | 0.52–0.03 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nazir, M.K.; Javaid, S.; Afzal, H.; Taj, M.B.; Baamer, D.F.; Almasoudi, A.; Aldahiri, R.H.; Ali, O.M.; Khan, M.I.; Ahmed, M.M.; et al. Synthesis, Magnetic, and Photocatalytic Activity of Polypyrrole-Based TiO2–Fe Catalyst for Wastewater Treatment. Catalysts 2024, 14, 692. https://doi.org/10.3390/catal14100692
Nazir MK, Javaid S, Afzal H, Taj MB, Baamer DF, Almasoudi A, Aldahiri RH, Ali OM, Khan MI, Ahmed MM, et al. Synthesis, Magnetic, and Photocatalytic Activity of Polypyrrole-Based TiO2–Fe Catalyst for Wastewater Treatment. Catalysts. 2024; 14(10):692. https://doi.org/10.3390/catal14100692
Chicago/Turabian StyleNazir, Muhammad Khalid, Sana Javaid, Hina Afzal, Muhammad Babar Taj, Doaa F. Baamer, Afaf Almasoudi, Reema H. Aldahiri, Omar Makram Ali, Muhammad Imran Khan, Muhammad Mahboob Ahmed, and et al. 2024. "Synthesis, Magnetic, and Photocatalytic Activity of Polypyrrole-Based TiO2–Fe Catalyst for Wastewater Treatment" Catalysts 14, no. 10: 692. https://doi.org/10.3390/catal14100692








