Aspects of Reaction Engineering for Biodiesel Production
Abstract
:1. Introduction
2. Thermodynamics of Transesterification and Esterification
2.1. Reaction
2.2. Separation
3. Kinetics of Heterogeneously Catalysed Transesterification
- excess methanol drives the transesterification equilibrium forward, and hydrolysis is negligible;
- the methanol concentration is constant, and hence, transesterification is pseudo-first-order;
- glycerol does not react with methanol;
- reactions are free from mass-transport limitations, and the reaction mixture is homogeneous;
- methanol adsorption is rate-limiting;
- quasi-steady-state kinetics can be assumed;
- reaction intermediates are rapidly converted into glycerol.
- bimolecular surface reaction is the limiting step
- methanol adsorption is the limiting step
- the intermediate steps are assumed to be rapid
4. Heterogeneously Catalysed Transesterification
5. Heterogeneous Solid-Acid Catalysis
5.1. Zeolites
5.2. Metal Oxides
5.3. Carbon-Based Materials
5.4. Heteropolyacids
6. Solid Base Catalysts
6.1. Solid Earth Metal Oxides
6.2. Alkali-Doped Metal Oxides
6.3. Hydrotalcites
6.4. Basic Zeolites
6.5. Natural Waste Sources
7. Reactor Engineering
7.1. Microreactors
7.2. Ultrasonic Reactors
7.3. Rotating Fields
7.4. Microwave Reactors
7.5. Static Mixers
7.6. Oscillatory Baffled Reactors (OBRs)
8. Chemical and Physical Properties of Biodiesel
8.1. Viscosity
8.2. Density
8.3. Flash Point
8.4. Cold Flow Properties
8.5. Acid Number
8.6. Cetane Number
8.7. Stability
8.8. Compliance with Standards
9. Conclusions and Future Research Focus
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Pérez-Almada, D.; Galán-Martín, Á.; Contreras, M.d.M.; Castro, E. Integrated techno-economic and environmental assessment of biorefineries: Review and future research directions. Sustain. Energy Fuels 2023, 7, 4031–4050. [Google Scholar] [CrossRef]
- Jeswani, H.K.; Chilvers, A.; Azapagic, A. Environmental sustainability of biofuels: A review. Proc. R. Soc. A 2020, 476, 20200351. [Google Scholar] [CrossRef] [PubMed]
- Ubando, A.T.; Felix, C.B.; Chen, W.-H. Biorefineries in circular bioeconomy: A comprehensive review. Bioresour. Technol. 2020, 299, 122585. [Google Scholar] [CrossRef]
- Wilson, K.; Lee, A.F. Catalyst design for biorefining. Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 2016, 374, 20150081. [Google Scholar] [CrossRef]
- Abbas, A.; Cross, M.; Duan, X.; Jeschke, S.; Konarova, M.; Huber, G.W.; Lee, A.F.; Lovell, E.C.; Lim, J.Y.C.; Polyzos, A.; et al. Catalysis at the intersection of sustainable chemistry and a circular economy. One Earth 2024, 7, 738–741. [Google Scholar] [CrossRef]
- Atabani, A.E.; Silitonga, A.S.; Badruddin, I.A.; Mahlia, T.M.I.; Masjuki, H.H.; Mekhilef, S. A comprehensive review on biodiesel as an alternative energy resource and its characteristics. Renew. Sustain. Energy Rev. 2012, 16, 2070–2093. [Google Scholar] [CrossRef]
- Bastan, F.; Kazemeini, M.; Larimi, A.S. Aqueous-phase reforming of glycerol for production of alkanes over Ni/CexZr1−xO2 nano-catalyst: Effects of the support’s composition. Renew. Energy 2017, 108, 417–424. [Google Scholar] [CrossRef]
- Larimi, A.; Khorasheh, F. Renewable hydrogen production by ethylene glycol steam reforming over Al2O3 supported Ni-Pt bimetallic nano-catalysts. Renew. Energy 2018, 128, 188–199. [Google Scholar] [CrossRef]
- Atadashi, I.M.; Aroua, M.K.; Aziz, A.A. Biodiesel separation and purification: A review. Renew. Energy 2011, 36, 437–443. [Google Scholar] [CrossRef]
- Fayyazi, E.; Ghobadian, B.; Van De Bovenkamp, H.H.; Najafi, G.; Hosseinzadehsamani, B.; Heeres, H.J.; Yue, J. Optimization of Biodiesel Production over Chicken Eggshell-Derived CaO Catalyst in a Continuous Centrifugal Contactor Separator. Ind. Eng. Chem. Res. 2018, 57, 12742–12755. [Google Scholar] [CrossRef]
- Maleki, H.; Kazemeini, M.; Larimi, A.S.; Khorasheh, F. Transesterification of canola oil and methanol by lithium impregnated CaO–La2O3 mixed oxide for biodiesel synthesis. J. Ind. Eng. Chem. 2017, 47, 399–404. [Google Scholar] [CrossRef]
- Yaşar, F. Comparision of fuel properties of biodiesel fuels produced from different oils to determine the most suitable feedstock type. Fuel 2020, 264, 116817. [Google Scholar] [CrossRef]
- Atadashi, I.M.; Aroua, M.K.; Aziz, A.A. High quality biodiesel and its diesel engine application: A review. Renew. Sustain. Energy Rev. 2010, 14, 1999–2008. [Google Scholar] [CrossRef]
- Salvi, B.L.; Panwar, N.L. Biodiesel resources and production technologies—A review. Renew. Sustain. Energy Rev. 2012, 16, 3680–3689. [Google Scholar] [CrossRef]
- Zhang, Y.; Dubé, M.A.; McLean, D.D.; Kates, M. Biodiesel production from waste cooking oil: 1. Process design and technological assessment. Bioresour. Technol. 2003, 89, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Basso, R.C.; Meirelles, A.J.D.A.; Batista, E.A.C. Experimental data, thermodynamic modeling and sensitivity analyses for the purification steps of ethyl biodiesel from fodder radish oil production. Braz. J. Chem. Eng. 2017, 34, 341–353. [Google Scholar] [CrossRef]
- Rostami, M.; Raeissi, S.; Mahmoodi, M.; Nowroozi, M. Liquid–Liquid Equilibria in Biodiesel Production. J. Am. Oil Chem. Soc. 2013, 90, 147–154. [Google Scholar] [CrossRef]
- Narasimharao, K.; Lee, A.; Wilson, K. Catalysts in Production of Biodiesel: A Review. J. Biobased Mater. Bioenergy 2007, 1, 19–30. [Google Scholar] [CrossRef]
- Salam, K.A.; Velasquez-Orta, S.B.; Harvey, A.P. A sustainable integrated in situ transesterification of microalgae for biodiesel production and associated co-product-a review. Renew. Sustain. Energy Rev. 2016, 65, 1179–1198. [Google Scholar] [CrossRef]
- Tomasevic, A.V.; Siler-Marinkovic, S.S. Methanolysis of used frying oil. Fuel Process. Technol. 2003, 81, 1–6. [Google Scholar] [CrossRef]
- Wang, Y.; Ou, S.; Liu, P.; Zhang, Z. Preparation of biodiesel from waste cooking oil via two-step catalyzed process. Energy Convers. Manag. 2007, 48, 184–188. [Google Scholar] [CrossRef]
- Zhang, Y.; Dubé, M.A.; McLean, D.D.; Kates, M. Biodiesel production from waste cooking oil: 2. Economic assessment and sensitivity analysis. Bioresour. Technol. 2003, 90, 229–240. [Google Scholar] [CrossRef] [PubMed]
- Chisti, Y. Biodiesel from microalgae. Biotechnol. Adv. 2007, 25, 294–306. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, M.G.; Dalai, A.K.; Bakhshi, N.N. Transesterification of canola oil in mixed methanol/ethanol system and use of esters as lubricity additive. Bioresour. Technol. 2007, 98, 2027–2033. [Google Scholar] [CrossRef] [PubMed]
- Meher, L.C.; Dharmagadda, V.S.S.; Naik, S.N. Optimization of alkali-catalyzed transesterification of Pongamia pinnata oil for production of biodiesel. Bioresour. Technol. 2006, 97, 1392–1397. [Google Scholar] [CrossRef]
- Kumar Tiwari, A.; Kumar, A.; Raheman, H. Biodiesel production from jatropha oil (Jatropha curcas) with high free fatty acids: An optimized process. Biomass Bioenergy 2007, 31, 569–575. [Google Scholar] [CrossRef]
- Bošnjaković, M.; Sinaga, N. The Perspective of Large-Scale Production of Algae Biodiesel. Appl. Sci. 2020, 10, 8181. [Google Scholar] [CrossRef]
- USDA National Agricultural Statistics Service. General Economic Reports. Available online: https://www.nass.usda.gov/Statistics_by_Subject/index.php?sector=CROPS (accessed on 12 August 2024).
- USDA Foreign Agricultural Service. Oilseeds: World Markets and Trade. Available online: https://www.fas.usda.gov/data/oilseeds-world-markets-and-trade (accessed on 12 August 2024).
- USDA Economic Research Service. Oil Crops Yearbook. Available online: https://www.ers.usda.gov/data-products/oil-crops-yearbook/ (accessed on 14 August 2024).
- U.S. Department of Energy Alternative Fuels Data Center. Alternative Fuel Price. Available online: https://afdc.energy.gov/fuels/prices.html (accessed on 11 August 2024).
- USDA Foreign Agricultural Service. Available online: https://www.fas.usda.gov/data (accessed on 12 August 2024).
- Choe, E.; Min, D.B. Chemistry of Deep-Fat Frying Oils. J. Food Sci. 2007, 72, R77–R86. [Google Scholar] [CrossRef]
- Kalogianni, E.P.; Karapantsios, T.D.; Miller, R. Effect of repeated frying on the viscosity, density and dynamic interfacial tension of palm and olive oil. J. Food Eng. 2011, 105, 169–179. [Google Scholar] [CrossRef]
- Ruiz-Méndez, M.V.; Marmesat, S.; Liotta, A.; Dobarganes, M.C. Analysis of used frying fats for biodiesel production. Grasas Aceites 2008, 59, 45–50. [Google Scholar] [CrossRef]
- Leung, D.Y.C.; Wu, X.; Leung, M.K.H. A review on biodiesel production using catalyzed transesterification. Appl. Energy 2010, 87, 1083–1095. [Google Scholar] [CrossRef]
- Sendzikiene, E.; Makareviciene, V.; Janulis, P.; Kitrys, S. Kinetics of free fatty acids esterification with methanol in the production of biodiesel fuel. Eur. J. Lipid Sci. Technol. 2004, 106, 831–836. [Google Scholar] [CrossRef]
- Carmo, A.C.; De Souza, L.K.C.; Da Costa, C.E.F.; Longo, E.; Zamian, J.R.; Da Rocha Filho, G.N. Production of biodiesel by esterification of palmitic acid over mesoporous aluminosilicate Al-MCM-41. Fuel 2009, 88, 461–468. [Google Scholar] [CrossRef]
- Srilatha, K.; Lingaiah, N.; Devi, B.L.A.P.; Prasad, R.B.N.; Venkateswar, S.; Prasad, P.S.S. Esterification of free fatty acids for biodiesel production over heteropoly tungstate supported on niobia catalysts. Appl. Catal. A Gen. 2009, 365, 28–33. [Google Scholar] [CrossRef]
- Do Nascimento, L.A.S.; Angélica, R.S.; Da Costa, C.E.F.; Zamian, J.R.; Da Rocha Filho, G.N. Comparative study between catalysts for esterification prepared from kaolins. Appl. Clay Sci. 2011, 51, 267–273. [Google Scholar] [CrossRef]
- Wongjaikham, W.; Wongsawaeng, D.; Ratnitsai, V.; Kamjam, M.; Ngaosuwan, K.; Kiatkittipong, W.; Hosemann, P.; Assabumrungrat, S. Low-cost alternative biodiesel production apparatus based on household food blender for continuous biodiesel production for small communities. Sci. Rep. 2021, 11, 13827. [Google Scholar] [CrossRef] [PubMed]
- Abdulkareem-Alsultan, G.; Asikin-Mijan, N.; Mansir, N.; Lee, H.V.; Zainal, Z.; Islam, A.; Taufiq-Yap, Y.H. Pyro-lytic de-oxygenation of waste cooking oil for green diesel production over Ag2O3-La2O3/AC nano-catalyst. J. Anal. Appl. Pyrolysis 2019, 137, 171–184. [Google Scholar] [CrossRef]
- Sarve, A.N.; Varma, M.N.; Sonawane, S.S. Ultrasound assisted two-stage biodiesel synthesis from non-edible Schleichera triguga oil using heterogeneous catalyst: Kinetics and thermodynamic analysis. Ultrason. Sonochem. 2016, 29, 288–298. [Google Scholar] [CrossRef]
- Bayat, A.; Baghdadi, M.; Bidhendi, G.N. Tailored magnetic nano-alumina as an efficient catalyst for transesterification of waste cooking oil: Optimization of biodiesel production using response surface methodology. Energy Convers. Manag. 2018, 177, 395–405. [Google Scholar] [CrossRef]
- Roy, T.; Sahani, S.; Chandra Sharma, Y. Study on kinetics-thermodynamics and environmental parameter of biodiesel production from waste cooking oil and castor oil using potassium modified ceria oxide catalyst. J. Clean. Prod. 2020, 247, 119166. [Google Scholar] [CrossRef]
- Nautiyal, P.; Subramanian, K.A.; Dastidar, M.G. Kinetic and thermodynamic studies on biodiesel production from Spirulina platensis algae biomass using single stage extraction–transesterification process. Fuel 2014, 135, 228–234. [Google Scholar] [CrossRef]
- Wu, L.; Wei, T.; Lin, Z.; Zou, Y.; Tong, Z.; Sun, J. Bentonite-enhanced biodiesel production by NaOH-catalyzed transesterification: Process optimization and kinetics and thermodynamic analysis. Fuel 2016, 182, 920–927. [Google Scholar] [CrossRef]
- Encinar, J.M.; Pardal, A.; Sánchez, N. An improvement to the transesterification process by the use of co-solvents to produce biodiesel. Fuel 2016, 166, 51–58. [Google Scholar] [CrossRef]
- Deshmane, V.G.; Adewuyi, Y.G. Synthesis and kinetics of biodiesel formation via calcium methoxide base catalyzed transesterification reaction in the absence and presence of ultrasound. Fuel 2013, 107, 474–482. [Google Scholar] [CrossRef]
- Sun, C.; Hu, Y.; Sun, F.; Sun, Y.; Song, G.; Chang, H.; Lunprom, S. Comparison of biodiesel production using a novel porous Zn/Al/Co complex oxide prepared from different methods: Physicochemical properties, reaction kinetic and thermodynamic studies. Renew. Energy 2022, 181, 1419–1430. [Google Scholar] [CrossRef]
- Franca, B.; Villardi, H.; Esteves, T.; Uller, A.M.C.; Pessoa, F.L. Phase equilibrium and emulsion stability on ethyl biodiesel production. Chem. Eng. Trans. 2011, 24, 745–750. [Google Scholar]
- EN 14214; Automotive Fuels—Fatty Acid Methyl Esters (FAME) for Diesel Engines—Requirements and Test Methods. European Committee for Standardization: Brussels, Belgium, 2008.
- Gonçalves, J.D.; Aznar, M.; Santos, G.R. Liquid–liquid equilibrium data for systems containing Brazil nut biodiesel+methanol+glycerin at 303.15 K and 323.15 K. Fuel 2014, 133, 292–298. [Google Scholar] [CrossRef]
- Oliveira, M.B.; Varanda, F.R.; Marrucho, I.M.; Queimada, A.J.; Coutinho, J.A.P. Prediction of Water Solubility in Biodiesel with the CPA Equation of State. Ind. Eng. Chem. Res. 2008, 47, 4278–4285. [Google Scholar] [CrossRef]
- Michelsen, M.L.; Hendriks, E.M. Physical properties from association models. Fluid Phase Equilib. 2001, 180, 165–174. [Google Scholar] [CrossRef]
- Negi, D.S.; Sobotka, F.; Kimmel, T.; Wozny, G.; Schomäcker, R. Liquid–Liquid Phase Equilibrium in Glycerol–Methanol–Methyl Oleate and Glycerol–Monoolein–Methyl Oleate Ternary Systems. Ind. Eng. Chem. Res. 2006, 45, 3693–3696. [Google Scholar] [CrossRef]
- Ardila, Y.C.; Pinto, G.M.F.; Machado, A.B.; Wolf Maciel, M.R. Experimental Determination of Binodal Curves and Study of the Temperature in Systems Involved in the Production of Biodiesel with Ethanol. J. Chem. Eng. Data 2010, 55, 4592–4596. [Google Scholar] [CrossRef]
- Bell, J.C.; Messerly, R.A.; Gee, R.; Harrison, A.; Rowley, R.L.; Wilding, W.V. Ternary Liquid–Liquid Equilibrium of Biodiesel Compounds for Systems Consisting of a Methyl Ester + Glycerin + Water. J. Chem. Eng. Data 2013, 58, 1001–1004. [Google Scholar] [CrossRef]
- Mazutti, M.A.; Voll, F.A.P.; Cardozo-Filho, L.; Corazza, M.L.; Lanza, M.; Priamo, W.L.; Oliveira, J.V. Thermophysical properties of biodiesel and related systems: (Liquid + liquid) equilibrium data for soybean biodiesel. J. Chem. Thermodyn. 2013, 58, 83–94. [Google Scholar] [CrossRef]
- Santos, T.; Gomes, J.F.; Puna, J. Liquid-liquid equilibrium for ternary system containing biodiesel, methanol and water. J. Environ. Chem. Eng. 2018, 6, 984–990. [Google Scholar] [CrossRef]
- Filho, J.C.G.; Bispo, F.B.; Corazza, M.L.; Arce, P.F.; Voll, F.A.P.; de Gusmão Coêlho, D.; Ferreira-Pinto, L.; de Carvalho, S.H.V.; Soletti, J.I. Liquid–Liquid Equilibrium Measurement and Thermodynamic Modeling of the {Sterculia striata Biodiesel + Glycerol + Ethanol} System. J. Chem. Eng. Data 2021, 66, 3293–3299. [Google Scholar] [CrossRef]
- Lee, M.-J.; Lo, Y.-C.; Lin, H.-M. Liquid–liquid equilibria for mixtures containing water, methanol, fatty acid methyl esters, and glycerol. Fluid Phase Equilib. 2010, 299, 180–190. [Google Scholar] [CrossRef]
- Andreatta, A.E.; Casás, L.M.; Hegel, P.; Bottini, S.B.; Brignole, E.A. Phase Equilibria in Ternary Mixtures of Methyl Oleate, Glycerol, and Methanol. Ind. Eng. Chem. Res. 2008, 47, 5157–5164. [Google Scholar] [CrossRef]
- Do Carmo, F.R.; Evangelista, N.S.; De Santiago-Aguiar, R.S.; Fernandes, F.A.N.; De Sant’Ana, H.B. Evaluation of optimal activity coefficient models for modeling and simulation of liquid–liquid equilibrium of biodiesel+glycerol+alcohol systems. Fuel 2014, 125, 57–65. [Google Scholar] [CrossRef]
- Machado, A.B.; Ardila, Y.C.; de Oliveira, L.H.; Aznar, M.; Wolf Maciel, M.R. Liquid–Liquid Equilibria in Ternary and Quaternary Systems Present in Biodiesel Production from Soybean Oil at (298.2 and 333.2) K. J. Chem. Eng. Data 2012, 57, 1417–1422. [Google Scholar] [CrossRef]
- Silva, W.L.G.D.; Souza, P.T.D.; Shimamoto, G.G.; Tubino, M. Separation of the Glycerol-Biodiesel Phases in an Ethyl Transesterification Synthetic Route Using Water. J. Braz. Chem. Soc. 2015, 26, 1745–1750. [Google Scholar] [CrossRef]
- Kapil, A.; Wilson, K.; Lee, A.F.; Sadhukhan, J. Kinetic Modeling Studies of Heterogeneously Catalyzed Biodiesel Synthesis Reactions. Ind. Eng. Chem. Res. 2011, 50, 4818–4830. [Google Scholar] [CrossRef]
- Andreo-Martínez, P.; García-Martínez, N.; Durán-del-Amor, M.D.M.; Quesada-Medina, J. Advances on kinetics and thermodynamics of non-catalytic supercritical methanol transesterification of some vegetable oils to biodiesel. Energy Convers. Manag. 2018, 173, 187–196. [Google Scholar] [CrossRef]
- Ahmad Farid, M.A.; Hassan, M.A.; Taufiq-Yap, Y.H.; Ibrahim, M.L.; Hasan, M.Y.; Ali, A.A.M.; Othman, M.R.; Shirai, Y. Kinetic and thermodynamic of heterogeneously K3PO4/AC-catalysed transesterification via pseudo-first order mechanism and Eyring-Polanyi equation. Fuel 2018, 232, 653–658. [Google Scholar] [CrossRef]
- Moradi, G.R.; Mohadesi, M.; Ghanbari, M.; Moradi, M.J.; Hosseini, S.; Davoodbeygi, Y. Kinetic comparison of two basic heterogenous catalysts obtained from sustainable resources for transesterification of waste cooking oil. Biofuel Res. J. 2015, 2, 236–241. [Google Scholar] [CrossRef]
- Kumar, D.; Ali, A. Transesterification of Low-Quality Triglycerides over a Zn/CaO Heterogeneous Catalyst: Kinetics and Reusability Studies. Energy Fuels 2013, 27, 3758–3768. [Google Scholar] [CrossRef]
- Kaur, N.; Ali, A. Biodiesel production via ethanolysis of jatropha oil using molybdenum impregnated calcium oxide as solid catalyst. RSC Adv. 2015, 5, 13285–13295. [Google Scholar] [CrossRef]
- Kaur, N.; Ali, A. Lithium zirconate as solid catalyst for simultaneous esterification and transesterification of low quality triglycerides. Appl. Catal. A Gen. 2015, 489, 193–202. [Google Scholar] [CrossRef]
- Devaraj Naik, B.; Udayakumar, M. Kinetics and thermodynamic analysis of transesterification of waste cooking sunflower oil using bentonite-supported sodium methoxide catalyst. Biomass Convers. Biorefin. 2023, 13, 9701–9714. [Google Scholar] [CrossRef]
- Feyzi, M.; Shahbazi, Z. Preparation, kinetic and thermodynamic studies of Al–Sr nanocatalysts for biodiesel production. J. Taiwan Inst. Chem. Eng. 2017, 71, 145–155. [Google Scholar] [CrossRef]
- Sahani, S.; Roy, T.; Chandra Sharma, Y. Clean and efficient production of biodiesel using barium cerate as a heterogeneous catalyst for the biodiesel production; kinetics and thermodynamic study. J. Clean. Prod. 2019, 237, 117699. [Google Scholar] [CrossRef]
- Al-Sakkari, E.G.; El-Sheltawy, S.T.; Attia, N.K.; Mostafa, S.R. Kinetic study of soybean oil methanolysis using cement kiln dust as a heterogeneous catalyst for biodiesel production. Appl. Catal. B Environ. 2017, 206, 146–157. [Google Scholar] [CrossRef]
- Xiao, Y.; Gao, L.; Xiao, G.; Lv, J. Kinetics of the Transesterification Reaction Catalyzed by Solid Base in a Fixed-Bed Reactor. Energy Fuels 2010, 24, 5829–5833. [Google Scholar] [CrossRef]
- Hattori, H.; Shima, M.; Kabashima, H. Alcoholysis of ester and epoxide catalyzed by solid bases. In Studies in Surface Science and Catalysis; Elsevier: Amsterdam, The Netherlands, 2000; Volume 130, pp. 3507–3512. [Google Scholar]
- Dossin, T.F.; Reyniers, M.-F.; Berger, R.J.; Marin, G.B. Simulation of heterogeneously MgO-catalyzed transesterification for fine-chemical and biodiesel industrial production. Appl. Catal. B Environ. 2006, 67, 136–148. [Google Scholar] [CrossRef]
- Malani, R.S.; Patil, S.; Roy, K.; Chakma, S.; Goyal, A.; Moholkar, V.S. Mechanistic analysis of ultrasound-assisted biodiesel synthesis with Cu2O catalyst and mixed oil feedstock using continuous (packed bed) and batch (slurry) reactors. Chem. Eng. Sci. 2017, 170, 743–755. [Google Scholar] [CrossRef]
- Galván Muciño, G.E.; Romero, R.; Ramírez, A.; Ramos, M.J.; Baeza-Jiménez, R.; Natividad, R. Kinetics of Transesterification of Safflower Oil to Obtain Biodiesel Using Heterogeneous Catalysis. Int. J. Chem. React. Eng. 2016, 14, 929–938. [Google Scholar] [CrossRef]
- Thoai, D.N.; Tongurai, C.; Prasertsit, K.; Kumar, A. A novel two-step transesterification process catalyzed by homogeneous base catalyst in the first step and heterogeneous acid catalyst in the second step. Fuel Process. Technol. 2017, 168, 97–104. [Google Scholar] [CrossRef]
- Klaewkla, R.; Arend, M.; Hoelderich, W.F. A Review of Mass Transfer Controlling the Reaction Rate in Heterogeneous Catalytic Systems. In Mass Transfer—Advanced Aspects; Nakajima, H., Ed.; InTech: London, UK, 2011. [Google Scholar]
- Ma, F.; Hanna, M.A. Biodiesel production: A review. Bioresour. Technol. 1999, 70, 1–15. [Google Scholar] [CrossRef]
- Zakaria, R.; Harvey, A.P. Direct production of biodiesel from rapeseed by reactive extraction/in situ transesterification. Fuel Process. Technol. 2012, 102, 53–60. [Google Scholar] [CrossRef]
- Porwal, J.; Bangwal, D.; Garg, M.; Kaul, S. Reactive-extraction of pongamia seeds for biodiesel production. J. Sci. Ind. Res. 2012, 71, 822–828. [Google Scholar]
- Amalia Kartika, I.; Yani, M.; Ariono, D.; Evon, P.; Rigal, L. Biodiesel production from jatropha seeds: Solvent extraction and in situ transesterification in a single step. Fuel 2013, 106, 111–117. [Google Scholar] [CrossRef]
- Silitonga, A.S.; Ong, H.C.; Mahlia, T.M.I.; Masjuki, H.H.; Chong, W.T. Biodiesel Conversion from High FFA Crude Jatropha curcas, Calophyllum inophyllum and Ceiba pentandra Oil. Energy Procedia 2014, 61, 480–483. [Google Scholar] [CrossRef]
- Dawodu, F.A.; Ayodele, O.O.; Bolanle-Ojo, T. Biodiesel production from Sesamum indicum L. seed oil: An optimization study. Egypt. J. Pet. 2014, 23, 191–199. [Google Scholar] [CrossRef]
- Chen, K.-T.; Wang, J.-X.; Dai, Y.-M.; Wang, P.-H.; Liou, C.-Y.; Nien, C.-W.; Wu, J.-S.; Chen, C.-C. Rice husk ash as a catalyst precursor for biodiesel production. J. Taiwan Inst. Chem. Eng. 2013, 44, 622–629. [Google Scholar] [CrossRef]
- Savaliya, M.L.; Dhorajiya, B.D.; Dholakiya, B.Z. Current Trends in Separation and Purification of Fatty Acid Methyl Ester. Sep. Purif. Rev. 2015, 44, 28–40. [Google Scholar] [CrossRef]
- Stojković, I.J.; Stamenković, O.S.; Povrenović, D.S.; Veljković, V.B. Purification technologies for crude biodiesel obtained by alkali-catalyzed transesterification. Renew. Sustain. Energy Rev. 2014, 32, 1–15. [Google Scholar] [CrossRef]
- Lotero, E.; Liu, Y.; Lopez, D.E.; Suwannakarn, K.; Bruce, D.A.; Goodwin, J.G. Synthesis of Biodiesel via Acid Catalysis. Ind. Eng. Chem. Res. 2005, 44, 5353–5363. [Google Scholar] [CrossRef]
- Javidialesaadi, A.; Raeissi, S. Biodiesel Production from High Free Fatty Acid-Content Oils: Experimental Investigation of the Pretreatment Step. APCBEE Procedia 2013, 5, 474–478. [Google Scholar] [CrossRef]
- Brucato, A.; Busciglio, A.; Stefano, F.D.; Grisafi, F.; Micale, G.; Scargiali, F. High temperature solid-catalized transesterification for biodiesel production. Chem. Eng. Trans. 2010, 19, 31–36. [Google Scholar]
- Muthu, H.; SathyaSelvabala, V.; Varathachary, T.K.; Kirupha Selvaraj, D.; Nandagopal, J.; Subramanian, S. Synthesis of biodiesel from Neem oil using sulfated zirconia via tranesterification. Braz. J. Chem. Eng. 2010, 27, 601–608. [Google Scholar] [CrossRef]
- Koberg, M.; Gedanken, A. Using Microwave Radiation and SrO as a Catalyst for the Complete Conversion of Oils, Cooked Oils, and Microalgae to Biodiesel. In New and Future Developments in Catalysis; Elsevier: Amsterdam, The Netherlands, 2013; pp. 209–227. [Google Scholar]
- Musa, I.A. The effects of alcohol to oil molar ratios and the type of alcohol on biodiesel production using transesterification process. Egypt. J. Pet. 2016, 25, 21–31. [Google Scholar] [CrossRef]
- Mandari, V.; Devarai, S.K. Biodiesel Production Using Homogeneous, Heterogeneous, and Enzyme Catalysts via Transesterification and Esterification Reactions: A Critical Review. Bioenergy Res. 2022, 15, 935–961. [Google Scholar] [CrossRef] [PubMed]
- Atadashi, I.M.; Aroua, M.K.; Abdul Aziz, A.R.; Sulaiman, N.M.N. The effects of catalysts in biodiesel production: A review. J. Ind. Eng. Chem. 2013, 19, 14–26. [Google Scholar] [CrossRef]
- Lam, M.K.; Lee, K.T.; Mohamed, A.R. Homogeneous, heterogeneous and enzymatic catalysis for transesterification of high free fatty acid oil (waste cooking oil) to biodiesel: A review. Biotechnol. Adv. 2010, 28, 500–518. [Google Scholar] [CrossRef] [PubMed]
- Sagiroglu, A.; Selen, I.; Ozcan, M.; Paluzar, H.; Toprakkiran, N. Comparison of biodiesel productivities of different vegetable oils by acidic catalysis. Chem. Ind. Chem. Eng. Q. 2011, 17, 53–58. [Google Scholar] [CrossRef]
- Limpanuparb, T.; Punyain, K.; Tantirungrotechai, Y. A DFT investigation of methanolysis and hydrolysis of triacetin. J. Mol. Struct. Theochem 2010, 955, 23–32. [Google Scholar] [CrossRef]
- Almeida, E.L.; Andrade, C.M.G.; Andreo dos Santos, O. Production of Biodiesel Via Catalytic Processes: A Brief Review. Int. J. Chem. React. Eng. 2018, 16, 20170130. [Google Scholar] [CrossRef]
- Tan, Y.H.; Abdullah, M.O.; Nolasco-Hipolito, C.; Taufiq-Yap, Y.H. Waste ostrich- and chicken-eggshells as heterogeneous base catalyst for biodiesel production from used cooking oil: Catalyst characterization and biodiesel yield performance. Appl. Energy 2015, 160, 58–70. [Google Scholar] [CrossRef]
- Vicente, G.; Martínez, M.; Aracil, J. Integrated biodiesel production: A comparison of different homogeneous catalysts systems. Bioresour. Technol. 2004, 92, 297–305. [Google Scholar] [CrossRef]
- Zhang, M.; Sun, A.; Meng, Y.; Wang, L.; Jiang, H.; Li, G. Catalytic Performance of Biomass Carbon-Based Solid Acid Catalyst for Esterification of Free Fatty Acids in Waste Cooking Oil. Catal. Surv. Asia 2015, 19, 61–67. [Google Scholar] [CrossRef]
- Lin, L.; Cunshan, Z.; Vittayapadung, S.; Xiangqian, S.; Mingdong, D. Opportunities and challenges for biodiesel fuel. Appl. Energy 2011, 88, 1020–1031. [Google Scholar] [CrossRef]
- Vasudevan, P.T.; Briggs, M. Biodiesel production—Current state of the art and challenges. J. Ind. Microbiol. Biotechnol. 2008, 35, 421. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.-W.; Park, Y.-M.; Lee, K.-Y. Heterogeneous Base Catalysts for Transesterification in Biodiesel Synthesis. Catal. Surv. Asia 2009, 13, 63–77. [Google Scholar] [CrossRef]
- Md Radzi, M.R.; Manogaran, M.D.; Yusoff, M.H.M.; Zulqarnain; Anuar, M.R.; Shoparwe, N.F.; Rahman, M.F.A. Production of Propanediols through In Situ Glycerol Hydrogenolysis via Aqueous Phase Reforming: A Review. Catalysts 2022, 12, 945. [Google Scholar] [CrossRef]
- Rattanaphra, D.; Harvey, A.; Srinophakun, P. Simultaneous Conversion of Triglyceride/Free Fatty Acid Mixtures into Biodiesel Using Sulfated Zirconia. Top. Catal. 2010, 53, 773–782. [Google Scholar] [CrossRef]
- Canakci, M.; Gerpen, J.V. Biodiesel production from oils and fats with high free fatty acids. Trans. ASAE 2001, 44, 1429–1436. [Google Scholar] [CrossRef]
- Hayyan, A.; Alam, M.Z.; Mirghani, M.E.S.; Kabbashi, N.A.; Hakimi, N.I.N.M.; Siran, Y.M.; Tahiruddin, S. Sludge palm oil as a renewable raw material for biodiesel production by two-step processes. Bioresour. Technol. 2010, 101, 7804–7811. [Google Scholar] [CrossRef]
- Charoenchaitrakool, M.; Thienmethangkoon, J. Statistical optimization for biodiesel production from waste frying oil through two-step catalyzed process. Fuel Process. Technol. 2011, 92, 112–118. [Google Scholar] [CrossRef]
- Patil, P.; Deng, S.; Isaac Rhodes, J.; Lammers, P.J. Conversion of waste cooking oil to biodiesel using ferric sulfate and supercritical methanol processes. Fuel 2010, 89, 360–364. [Google Scholar] [CrossRef]
- Boro, J.; Konwar, L.J.; Deka, D. Transesterification of non edible feedstock with lithium incorporated egg shell derived CaO for biodiesel production. Fuel Process. Technol. 2014, 122, 72–78. [Google Scholar] [CrossRef]
- Munyentwali, A.; Li, H.; Yang, Q. Review of advances in bifunctional solid acid/base catalysts for sustainable biodiesel production. Appl. Catal. A Gen. 2022, 633, 118525. [Google Scholar] [CrossRef]
- Al-Saadi, A.; Mathan, B.; He, Y. Biodiesel production via simultaneous transesterification and esterification reactions over SrO–ZnO/Al2O3 as a bifunctional catalyst using high acidic waste cooking oil. Chem. Eng. Res. Des. 2020, 162, 238–248. [Google Scholar] [CrossRef]
- Isaacs, M.A.; Parlett, C.M.A.; Robinson, N.; Durndell, L.J.; Manayil, J.C.; Beaumont, S.K.; Jiang, S.; Hondow, N.S.; Lamb, A.C.; Jampaiah, D.; et al. A spatially orthogonal hierarchically porous acid–base catalyst for cascade and antagonistic reactions. Nat. Catal. 2020, 3, 921–931. [Google Scholar] [CrossRef]
- Ding, S.; Fernandez Ainaga, D.L.; Hu, M.; Qiu, B.; Khalid, U.; D’Agostino, C.; Ou, X.; Spencer, B.; Zhong, X.; Peng, Y.; et al. Spatial segregation of catalytic sites within Pd doped H-ZSM-5 for fatty acid hydrodeoxygenation to alkanes. Nat. Commun. 2024, 15, 7718. [Google Scholar] [CrossRef] [PubMed]
- Canakci, M.; Gerpen, J.V. Biodiesel production via acid catalysis. Trans. ASAE 1999, 42, 1203–1210. [Google Scholar] [CrossRef]
- Enweremadu, C.C.; Mbarawa, M.M. Technical aspects of production and analysis of biodiesel from used cooking oil—A review. Renew. Sustain. Energy Rev. 2009, 13, 2205–2224. [Google Scholar] [CrossRef]
- Romano, S. Vegetable Oils: A New Alternative. In Proceedings of the International Conference on Plant and Vegetable Oils as Fuels, Fargo, ND, USA, 2 August 1982. [Google Scholar]
- Iglesias, J.; Melero, J.A.; Bautista, L.F.; Morales, G.; Sánchez-Vázquez, R.; Andreola, M.T.; Lizarraga-Fernández, A. Zr-SBA-15 as an efficient acid catalyst for FAME production from crude palm oil. Catal. Today 2011, 167, 46–55. [Google Scholar] [CrossRef]
- Parangi, T.; Mishra, M.K. Solid Acid Catalysts for Biodiesel Production. Comments Inorg. Chem. 2020, 40, 176–216. [Google Scholar] [CrossRef]
- Puna, J.F.; Gomes, J.F.; Correia, M.J.N.; Soares Dias, A.P.; Bordado, J.C. Advances on the development of novel heterogeneous catalysts for transesterification of triglycerides in biodiesel. Fuel 2010, 89, 3602–3606. [Google Scholar] [CrossRef]
- Chouhan, A.P.S.; Sarma, A.K. Modern heterogeneous catalysts for biodiesel production: A comprehensive review. Renew. Sustain. Energy Rev. 2011, 15, 4378–4399. [Google Scholar] [CrossRef]
- Diamantopoulos, N. Comprehensive Review on the Biodiesel Production using Solid Acid Heterogeneous Catalysts. J. Therm. Catal. 2015, 6, 1–8. [Google Scholar] [CrossRef]
- Sani, Y.M.; Daud, W.M.A.W.; Abdul Aziz, A.R. Activity of solid acid catalysts for biodiesel production: A critical review. Appl. Catal. A Gen. 2014, 470, 140–161. [Google Scholar] [CrossRef]
- Erika, L.P.A.; Ochoa-Herrera, V.; Quintanilla, F.; Egas, D.A.; Mora, J.R. Optimization of a Gas Chromatography Methodology for Biodiesel Analysis. J. Anal. Chem. 2021, 76, 106–111. [Google Scholar] [CrossRef]
- Ramos, K.; Riddell, A.; Tsiagras, H.; Hupp, A.M. Analysis of biodiesel-diesel blends: Does ultrafast gas chromatography provide for similar separation in a fraction of the time? J. Chromatogr. A 2022, 1667, 462903. [Google Scholar] [CrossRef] [PubMed]
- Syed, M.B. Analysis of biodiesel by high performance liquid chromatography using refractive index detector. MethodsX 2017, 4, 256–259. [Google Scholar] [CrossRef]
- Torres, A.; Fuentes, B.; Rodríguez, K.E.; Brito, A.; Díaz, L. Analysis of the Content of Fatty Acid Methyl Esters in Biodiesel by Fourier-Transform Infrared Spectroscopy: Method and Comparison with Gas Chromatography. J. Am. Oil Chem. Soc. 2020, 97, 651–661. [Google Scholar] [CrossRef]
- Bligaard, T.; Bullock, R.M.; Campbell, C.T.; Chen, J.G.; Gates, B.C.; Gorte, R.J.; Jones, C.W.; Jones, W.D.; Kitchin, J.R.; Scott, S.L. Toward Benchmarking in Catalysis Science: Best Practices, Challenges, and Opportunities. ACS Catal. 2016, 6, 2590–2602. [Google Scholar] [CrossRef]
- Schüth, F.; Ward, M.D.; Buriak, J.M. Common Pitfalls of Catalysis Manuscripts Submitted to Chemistry of Materials. Chem. Mater. 2018, 30, 3599–3600. [Google Scholar] [CrossRef]
- Guo, F.; Fang, Z. Biodiesel Production with Solid Catalysts. In Biodiesel—Feedstocks and Processing Technologies; Stoytcheva, M., Ed.; InTech: London, UK, 2011. [Google Scholar]
- Chung, K.-H.; Park, B.-G. Esterification of oleic acid in soybean oil on zeolite catalysts with different acidity. J. Ind. Eng. Chem. 2009, 15, 388–392. [Google Scholar] [CrossRef]
- Chung, K.-H.; Chang, D.-R.; Park, B.-G. Removal of free fatty acid in waste frying oil by esterification with methanol on zeolite catalysts. Bioresour. Technol. 2008, 99, 7438–7443. [Google Scholar] [CrossRef]
- Hassani, M.; Najafpour, G.D.; Mohammadi, M.; Rabiee, M. Preparation, characterization and application of zeolite-based catalyst for production of biodiesel from waste cooking oil. J. Sci. Ind. Res. 2014, 73, 120–133. [Google Scholar]
- Brito, A.; Borges, M.E.; Arvelo, R.; Garcia, F.; Diaz, M.C.; Otero, N. Reuse of Fried Oil to Obtain Biodiesel: Zeolites Y as a Catalyst. Int. J. Chem. React. Eng. 2007, 5. [Google Scholar] [CrossRef]
- Medina-Valtierra, J.; Ramirez-Ortiz, J. Biodiesel production from waste frying oil in sub- and supercritical methanol on a zeolite Y solid acid catalyst. Front. Chem. Sci. Eng. 2013, 7, 401–407. [Google Scholar] [CrossRef]
- Prinsen, P.; Luque, R.; González-Arellano, C. Zeolite catalyzed palmitic acid esterification. Micropor. Mesoporous Mater. 2018, 262, 133–139. [Google Scholar] [CrossRef]
- Septiani, U.; Putri, R.; Jamarun, N. Synthesis of Zeolite ZSM-5 from rice husk ash as cataliyst in vegetable oil transesterification for biodiesel production. Der Pharmacia Lettre 2016, 8, 86–91. [Google Scholar]
- Védrine, J. Heterogeneous Catalysis on Metal Oxides. Catalysts 2017, 7, 341. [Google Scholar] [CrossRef]
- Abreu, W.C.D.; Moura, B.C.V.R.D.; Costa, J.C.S.; Moura, E.M.D. Strontium and Nickel Heterogeneous Catalysts for Biodiesel Production from Macaw Oil. J. Braz. Chem. Soc. 2017, 28, 319–327. [Google Scholar] [CrossRef]
- Khder, A.S.; El-Sharkawy, E.A.; El-Hakam, S.A.; Ahmed, A.I. Surface characterization and catalytic activity of sulfated tin oxide catalyst. Catal. Commun. 2008, 9, 769–777. [Google Scholar] [CrossRef]
- Istadi, I.; Anggoro, D.D.; Buchori, L.; Rahmawati, D.A.; Intaningrum, D. Active Acid Catalyst of Sulphated Zinc Oxide for Transesterification of Soybean Oil with Methanol to Biodiesel. Procedia Environ. Sci. 2015, 23, 385–393. [Google Scholar] [CrossRef]
- Shao, G.N.; Sheikh, R.; Hilonga, A.; Lee, J.E.; Park, Y.-H.; Kim, H.T. Biodiesel production by sulfated mesoporous titania–silica catalysts synthesized by the sol–gel process from less expensive precursors. Chem. Eng. J. 2013, 215–216, 600–607. [Google Scholar] [CrossRef]
- Jitputti, J.; Kitiyanan, B.; Rangsunvigit, P.; Bunyakiat, K.; Attanatho, L.; Jenvanitpanjakul, P. Transesterification of crude palm kernel oil and crude coconut oil by different solid catalysts. Chem. Eng. J. 2006, 116, 61–66. [Google Scholar] [CrossRef]
- Noda, L.K.; Almeida, R.M.D.; Gonçalves, N.S.; Probst, L.F.D.; Sala, O. TiO2 with a high sulfate content—Thermogravimetric analysis, determination of acid sites by infrared spectroscopy and catalytic activity. Catal. Today 2003, 85, 69–74. [Google Scholar] [CrossRef]
- Zhang, H.; Yu, H.; Zheng, A.; Li, S.; Shen, W.; Deng, F. Reactivity Enhancement of 2-Propanol Photocatalysis on SO42−/TiO2: Insights from Solid-State NMR Spectroscopy. Environ. Sci. Technol. 2008, 42, 5316–5321. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Wnetrzak, R.; Kwapinski, W.; Leahy, J.J. Synthesis and Characterization of Sulfated TiO2 Nanorods and ZrO2/TiO2 Nanocomposites for the Esterification of Biobased Organic Acid. ACS Appl. Mater. Interfaces 2012, 4, 4499–4505. [Google Scholar] [CrossRef]
- Zhao, J.; Yue, Y.; Hua, W.; He, H.; Gao, Z. Catalytic activities and properties of sulfated zirconia supported on mesostructured γ-Al2O3. Appl. Catal. A Gen. 2008, 336, 133–139. [Google Scholar] [CrossRef]
- Wang, J.; Yang, P.; Fan, M.; Yu, W.; Jing, X.; Zhang, M.; Duan, X. Preparation and characterization of novel magnetic ZrO2/TiO2/Fe3O4 solid superacid. Mater. Lett. 2007, 61, 2235–2238. [Google Scholar] [CrossRef]
- Li, C.; Tan, J.; Fan, X.; Zhang, B.; Zhang, H.; Zhang, Q. Magnetically separable one dimensional Fe3O4/P(MAA-DVB)/TiO2 nanochains: Preparation, characterization and photocatalytic activity. Ceram. Int. 2015, 41, 3860–3868. [Google Scholar] [CrossRef]
- Jiang, Y.-X.; Chen, X.-M.; Mo, Y.-F.; Tong, Z.-F. Preparation and properties of Al-PILC supported SO42−/TiO2 superacid catalyst. J. Mol. Catal. A Chem. 2004, 213, 231–234. [Google Scholar] [CrossRef]
- Lai, D.-M.; Deng, L.; Guo, Q.-X.; Fu, Y. Hydrolysis of biomass by magnetic solid acid. Energy Environ. Sci. 2011, 4, 3552–3557. [Google Scholar] [CrossRef]
- Wu, L.; Zhou, Y.; Nie, W.; Song, L.; Chen, P. Synthesis of highly monodispersed teardrop-shaped core–shell SiO2/TiO2 nanoparticles and their photocatalytic activities. Appl. Surf. Sci. 2015, 351, 320–326. [Google Scholar] [CrossRef]
- Shen, J.; Zhu, Y.; Yang, X.; Li, C. Magnetic composite microspheres with exposed {001} faceted TiO2 shells: A highly active and selective visible-light photocatalyst. J. Mater. Chem. 2012, 22, 13341–13347. [Google Scholar] [CrossRef]
- Jacinto, M.J.; Santos, O.H.C.F.; Jardim, R.F.; Landers, R.; Rossi, L.M. Preparation of recoverable Ru catalysts for liquid-phase oxidation and hydrogenation reactions. Appl. Catal. A Gen. 2009, 360, 177–182. [Google Scholar] [CrossRef]
- Lu, A.-H.; Salabas, E.L.; Schüth, F. Magnetic Nanoparticles: Synthesis, Protection, Functionalization, and Application. Angew. Chem. Int. Ed. 2007, 46, 1222–1244. [Google Scholar] [CrossRef]
- Sobhani, S.; Parizi, Z.P.; Razavi, N. Nano n-propylsulfonated γ-Fe2O3 as magnetically recyclable heterogeneous catalyst for the efficient synthesis of β-phosphonomalonates. Appl. Catal. A Gen. 2011, 409–410, 162–166. [Google Scholar] [CrossRef]
- Tang, S.; Wang, L.; Zhang, Y.; Li, S.; Tian, S.; Wang, B. Study on preparation of Ca/Al/Fe3O4 magnetic composite solid catalyst and its application in biodiesel transesterification. Fuel Process. Technol. 2012, 95, 84–89. [Google Scholar] [CrossRef]
- Xin, T.; Ma, M.; Zhang, H.; Gu, J.; Wang, S.; Liu, M.; Zhang, Q. A facile approach for the synthesis of magnetic separable Fe3O4@TiO2, core–shell nanocomposites as highly recyclable photocatalysts. Appl. Surf. Sci. 2014, 288, 51–59. [Google Scholar] [CrossRef]
- Moghanian, H.; Mobinikhaledi, A.; Blackman, A.G.; Sarough-Farahani, E. Sulfanilic acid-functionalized silica-coated magnetite nanoparticles as an efficient, reusable and magnetically separable catalyst for the solvent-free synthesis of 1-amido- and 1-aminoalkyl-2-naphthols. RSC Adv. 2014, 4, 28176–28185. [Google Scholar] [CrossRef]
- Qin, S.; Cai, W.; Tang, X.; Yang, L. Sensitively monitoring photodegradation process of organic dye molecules by surface-enhanced Raman spectroscopy based on Fe3O4@SiO2@TiO2@Ag particle. Analyst 2014, 139, 5509–5515. [Google Scholar] [CrossRef]
- Petchmala, A.; Laosiripojana, N.; Jongsomjit, B.; Goto, M.; Panpranot, J.; Mekasuwandumrong, O.; Shotipruk, A. Transesterification of palm oil and esterification of palm fatty acid in near- and super-critical methanol with SO4–ZrO2 catalysts. Fuel 2010, 89, 2387–2392. [Google Scholar] [CrossRef]
- Yee, K.F.; Lee, K.T.; Ceccato, R.; Abdullah, A.Z. Production of biodiesel from Jatropha curcas L. oil catalyzed by SO42−/ZrO2 catalyst: Effect of interaction between process variables. Bioresour. Technol. 2011, 102, 4285–4289. [Google Scholar] [CrossRef]
- Jiménez-Morales, I.; Santamaría-González, J.; Maireles-Torres, P.; Jiménez-López, A. Calcined zirconium sulfate supported on MCM-41 silica as acid catalyst for ethanolysis of sunflower oil. Appl. Catal. B Environ. 2011, 103, 91–98. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, X.-D.; Sun, L.; Xu, M.; Zhou, W.-G.; Liang, X.-H. Solid superacid catalyzed fatty acid methyl esters production from acid oil. Appl. Energy 2010, 87, 2369–2373. [Google Scholar]
- Yan, F.; Yuan, Z.; Lu, P.; Luo, W.; Yang, L.; Deng, L. Fe–Zn double-metal cyanide complexes catalyzed biodiesel production from high-acid-value oil. Renew. Energy 2011, 36, 2026–2031. [Google Scholar] [CrossRef]
- Kafuku, G.; Lam, M.K.; Kansedo, J.; Lee, K.T.; Mbarawa, M. Croton megalocarpus oil: A feasible non-edible oil source for biodiesel production. Bioresour. Technol. 2010, 101, 7000–7004. [Google Scholar] [CrossRef]
- Kafuku, G.; Lam, M.K.; Kansedo, J.; Lee, K.T.; Mbarawa, M. Heterogeneous catalyzed biodiesel production from Moringa oleifera oil. Fuel Process. Technol. 2010, 91, 1525–1529. [Google Scholar] [CrossRef]
- Athar, M.; Zaidi, S.; Hassan, S.Z. Intensification and optimization of biodiesel production using microwave-assisted acid-organo catalyzed transesterification process. Sci. Rep. 2020, 10, 21239. [Google Scholar] [CrossRef]
- Lee, A.F.; Bennett, J.A.; Manayil, J.C.; Wilson, K. Heterogeneous catalysis for sustainable biodiesel production via esterification and transesterification. Chem. Soc. Rev. 2014, 43, 7887–7916. [Google Scholar] [CrossRef]
- Manayil, J.C.; Inocencio, C.V.M.; Lee, A.F.; Wilson, K. Mesoporous sulfonic acid silicas for pyrolysis bio-oil upgrading via acetic acid esterification. Green Chem. 2016, 18, 1387–1394. [Google Scholar] [CrossRef]
- Guldhe, A.; Singh, P.; Ansari, F.A.; Singh, B.; Bux, F. Biodiesel synthesis from microalgal lipids using tungstated zirconia as a heterogeneous acid catalyst and its comparison with homogeneous acid and enzyme catalysts. Fuel 2017, 187, 180–188. [Google Scholar] [CrossRef]
- Gardy, J.; Hassanpour, A.; Lai, X.; Ahmed, M.H.; Rehan, M. Biodiesel production from used cooking oil using a novel surface functionalised TiO2 nano-catalyst. Appl. Catal. B Environ. 2017, 207, 297–310. [Google Scholar] [CrossRef]
- Furuta, S.; Matsuhashi, H.; Arata, K. Biodiesel fuel production with solid amorphous-zirconia catalysis in fixed bed reactor. Biomass Bioenergy 2006, 30, 870–873. [Google Scholar] [CrossRef]
- De Almeida, R.M.; Noda, L.K.; Gonçalves, N.S.; Meneghetti, S.M.P.; Meneghetti, M.R. Transesterification reaction of vegetable oils, using superacid sulfated TiO2–base catalysts. Appl. Catal. A Gen. 2008, 347, 100–105. [Google Scholar] [CrossRef]
- Lam, M.K.; Lee, K.T.; Mohamed, A.R. Sulfated tin oxide as solid superacid catalyst for transesterification of waste cooking oil: An optimization study. Appl. Catal. B Environ. 2009, 93, 134–139. [Google Scholar] [CrossRef]
- Guan, D.; Fan, M.; Wang, J.; Zhang, Y.; Liu, Q.; Jing, X. Synthesis and properties of magnetic solid superacid: SO42−/ZrO2–B2O3–Fe3O4. Mater. Chem. Phys. 2010, 122, 278–283. [Google Scholar] [CrossRef]
- Wen, Z.; Yu, X.; Tu, S.-T.; Yan, J.; Dahlquist, E. Biodiesel production from waste cooking oil catalyzed by TiO2–MgO mixed oxides. Bioresour. Technol. 2010, 101, 9570–9576. [Google Scholar] [CrossRef]
- Valle-Vigón, P.; Sevilla, M.; Fuertes, A.B. Sulfonated mesoporous silica–carbon composites and their use as solid acid catalysts. Appl. Surf. Sci. 2012, 261, 574–583. [Google Scholar] [CrossRef]
- Madhuvilakku, R.; Piraman, S. Biodiesel synthesis by TiO2–ZnO mixed oxide nanocatalyst catalyzed palm oil transesterification process. Bioresour. Technol. 2013, 150, 55–59. [Google Scholar] [CrossRef]
- Osatiashtiani, A.; Durndell, L.J.; Manayil, J.C.; Lee, A.F.; Wilson, K. Influence of alkyl chain length on sulfated zirconia catalysed batch and continuous esterification of carboxylic acids by light alcohols. Green Chem. 2016, 18, 5529–5535. [Google Scholar] [CrossRef]
- Alhassan, F.H.; Rashid, U.; Taufiq-Yap, Y.H. Synthesis of waste cooking oil-based biodiesel via effectual recyclable bi-functional Fe2O3MnOSO42−/ZrO2 nanoparticle solid catalyst. Fuel 2015, 142, 38–45. [Google Scholar] [CrossRef]
- Raia, R.Z.; Da Silva, L.S.; Marcucci, S.M.P.; Arroyo, P.A. Biodiesel production from Jatropha curcas L. oil by simultaneous esterification and transesterification using sulphated zirconia. Catal. Today 2017, 289, 105–114. [Google Scholar] [CrossRef]
- Fu, X.-B.; Chen, J.; Song, X.-L.; Zhang, Y.-M.; Zhu, Y.; Yang, J.; Zhang, C.-W. Biodiesel Production Using a Carbon Solid Acid Catalyst Derived from β-Cyclodextrin. J. Am. Oil Chem. Soc. 2015, 92, 495–502. [Google Scholar] [CrossRef]
- Dehkhoda, A.M.; West, A.H.; Ellis, N. Biochar based solid acid catalyst for biodiesel production. Appl. Catal. A Gen. 2010, 382, 197–204. [Google Scholar] [CrossRef]
- Zhang, D.-Y.; Duan, M.-H.; Yao, X.-H.; Fu, Y.-J.; Zu, Y.-G. Preparation of a novel cellulose-based immobilized heteropoly acid system and its application on the biodiesel production. Fuel 2016, 172, 293–300. [Google Scholar] [CrossRef]
- Ryu, Y.-J.; Kim, Z.-H.; Lee, S.G.; Yang, J.-H.; Shin, H.-Y.; Lee, C.-G. Development of Carbon-Based Solid Acid Catalysts Using a Lipid-Extracted Alga, Dunaliella tertiolecta, for Esterification. J. Microbiol. Biotechnol. 2018, 28, 732–738. [Google Scholar] [CrossRef]
- Shu, Q.; Gao, J.; Nawaz, Z.; Liao, Y.; Wang, D.; Wang, J. Synthesis of biodiesel from waste vegetable oil with large amounts of free fatty acids using a carbon-based solid acid catalyst. Appl. Energy 2010, 87, 2589–2596. [Google Scholar] [CrossRef]
- Gupta, J.; Agarwal, M.; Dalai, A.K. An overview on the recent advancements of sustainable heterogeneous catalysts and prominent continuous reactor for biodiesel production. J. Ind. Eng. Chem. 2020, 88, 58–77. [Google Scholar] [CrossRef]
- Cao, F.; Chen, Y.; Zhai, F.; Li, J.; Wang, J.; Wang, X.; Wang, S.; Zhu, W. Biodiesel production from high acid value waste frying oil catalyzed by superacid heteropolyacid. Biotechnol. Bioeng. 2008, 101, 93–100. [Google Scholar] [CrossRef]
- Chai, F.; Cao, F.; Zhai, F.; Chen, Y.; Wang, X.; Su, Z. Transesterification of Vegetable Oil to Biodiesel using a Heteropolyacid Solid Catalyst. Adv. Synth. Catal. 2007, 349, 1057–1065. [Google Scholar] [CrossRef]
- Alcañiz-Monge, J.; Bakkali, B.E.; Trautwein, G.; Reinoso, S. Zirconia-supported tungstophosphoric heteropolyacid as heterogeneous acid catalyst for biodiesel production. Appl. Catal. B Environ. 2018, 224, 194–203. [Google Scholar] [CrossRef]
- Thangaraj, B.; Solomon, P.R.; Muniyandi, B.; Ranganathan, S.; Lin, L. Catalysis in biodiesel production—A review. Clean Energy 2019, 3, 2–23. [Google Scholar] [CrossRef]
- Marwaha, A.; Dhir, A.; Mahla, S.K.; Mohapatra, S.K. An overview of solid base heterogeneous catalysts for biodiesel production. Catal. Rev. 2018, 60, 594–628. [Google Scholar] [CrossRef]
- Di Serio, M.; Tesser, R.; Pengmei, L.; Santacesaria, E. Heterogeneous Catalysts for Biodiesel Production. Energy Fuels 2008, 22, 207–217. [Google Scholar] [CrossRef]
- Li, Z.; Ding, S.; Chen, C.; Qu, S.; Du, L.; Lu, J.; Ding, J. Recyclable Li/NaY zeolite as a heterogeneous alkaline catalyst for biodiesel production: Process optimization and kinetics study. Energy Convers. Manag. 2019, 192, 335–345. [Google Scholar] [CrossRef]
- Baskar, G.; Aiswarya, R. Trends in catalytic production of biodiesel from various feedstocks. Renew. Sustain. Energy Rev. 2016, 57, 496–504. [Google Scholar] [CrossRef]
- Refaat, A.A. Biodiesel production using solid metal oxide catalysts. Int. J. Environ. Sci. Technol. 2011, 8, 203–221. [Google Scholar] [CrossRef]
- MacLeod, C.S.; Harvey, A.P.; Lee, A.F.; Wilson, K. Evaluation of the activity and stability of alkali-doped metal oxide catalysts for application to an intensified method of biodiesel production. Chem. Eng. J. 2008, 135, 63–70. [Google Scholar] [CrossRef]
- Jothiramalingam, R.; Wang, M.K. Review of Recent Developments in Solid Acid, Base, and Enzyme Catalysts (Heterogeneous) for Biodiesel Production via Transesterification. Ind. Eng. Chem. Res. 2009, 48, 6162–6172. [Google Scholar] [CrossRef]
- Yang, G.; Yu, J. Advancements in Basic Zeolites for Biodiesel Production via Transesterification. Chemistry 2023, 5, 438–451. [Google Scholar] [CrossRef]
- Shan, R.; Lu, L.; Shi, Y.; Yuan, H.; Shi, J. Catalysts from renewable resources for biodiesel production. Energy Convers. Manag. 2018, 178, 277–289. [Google Scholar] [CrossRef]
- Borges, M.E.; Díaz, L. Recent developments on heterogeneous catalysts for biodiesel production by oil esterification and transesterification reactions: A review. Renew. Sustain. Energy Rev. 2012, 16, 2839–2849. [Google Scholar] [CrossRef]
- Viriya-empikul, N.; Krasae, P.; Puttasawat, B.; Yoosuk, B.; Chollacoop, N.; Faungnawakij, K. Waste shells of mollusk and egg as biodiesel production catalysts. Bioresour. Technol. 2010, 101, 3765–3767. [Google Scholar] [CrossRef]
- Zhu, H.; Wu, Z.; Chen, Y.; Zhang, P.; Duan, S.; Liu, X.; Mao, Z. Preparation of Biodiesel Catalyzed by Solid Super Base of Calcium Oxide and Its Refining Process. Chin. J. Chem. 2006, 27, 391–396. [Google Scholar] [CrossRef]
- Kouzu, M.; Kasuno, T.; Tajika, M.; Sugimoto, Y.; Yamanaka, S.; Hidaka, J. Calcium oxide as a solid base catalyst for transesterification of soybean oil and its application to biodiesel production. Fuel 2008, 87, 2798–2806. [Google Scholar] [CrossRef]
- Marinković, D.M.; Stanković, M.V.; Veličković, A.V.; Avramović, J.M.; Miladinović, M.R.; Stamenković, O.O.; Veljković, V.B.; Jovanović, D.M. Calcium oxide as a promising heterogeneous catalyst for biodiesel production: Current state and perspectives. Renew. Sustain. Energy Rev. 2016, 56, 1387–1408. [Google Scholar] [CrossRef]
- Liu, X.; He, H.; Wang, Y.; Zhu, S. Transesterification of soybean oil to biodiesel using SrO as a solid base catalyst. Catal. Commun. 2007, 8, 1107–1111. [Google Scholar] [CrossRef]
- López, D.E.; Goodwin, J.G.; Bruce, D.A.; Lotero, E. Transesterification of triacetin with methanol on solid acid and base catalysts. Appl. Catal. A Gen. 2005, 295, 97–105. [Google Scholar] [CrossRef]
- Meher, L.C.; Kulkarni, M.G.; Dalai, A.K.; Naik, S.N. Transesterification of karanja (Pongamia pinnata) oil by solid basic catalysts. Eur. J. Lipid Sci. Technol. 2006, 108, 389–397. [Google Scholar] [CrossRef]
- Gurunathan, B.; Ravi, A. Process optimization and kinetics of biodiesel production from neem oil using copper doped zinc oxide heterogeneous nanocatalyst. Bioresour. Technol. 2015, 190, 424–428. [Google Scholar] [CrossRef]
- Torres-Rodríguez, D.A.; Romero-Ibarra, I.C.; Ibarra, I.A.; Pfeiffer, H. Biodiesel production from soybean and Jatropha oils using cesium impregnated sodium zirconate as a heterogeneous base catalyst. Renew. Energy 2016, 93, 323–331. [Google Scholar] [CrossRef]
- Kaur, M.; Ali, A. An efficient and reusable Li/NiO heterogeneous catalyst for ethanolysis of waste cottonseed oil. Eur. J. Lipid Sci. Technol. 2015, 117, 550–560. [Google Scholar] [CrossRef]
- Taufiq-Yap, Y.H.; Lee, H.V.; Hussein, M.Z.; Yunus, R. Calcium-based mixed oxide catalysts for methanolysis of Jatropha curcas oil to biodiesel. Biomass Bioenergy 2011, 35, 827–834. [Google Scholar] [CrossRef]
- Xie, W.; Peng, H.; Chen, L. Calcined Mg–Al hydrotalcites as solid base catalysts for methanolysis of soybean oil. J. Mol. Catal. A Chem. 2006, 246, 24–32. [Google Scholar] [CrossRef]
- Wang, Y.-T.; Fang, Z.; Zhang, F.; Xue, B.-J. One-step production of biodiesel from oils with high acid value by activated Mg–Al hydrotalcite nanoparticles. Bioresour. Technol. 2015, 193, 84–89. [Google Scholar] [CrossRef] [PubMed]
- Navajas, A.; Campo, I.; Moral, A.; Echave, J.; Sanz, O.; Montes, M.; Odriozola, J.A.; Arzamendi, G.; Gandía, L.M. Outstanding performance of rehydrated Mg-Al hydrotalcites as heterogeneous methanolysis catalysts for the synthesis of biodiesel. Fuel 2018, 211, 173–181. [Google Scholar] [CrossRef]
- Brito, A.; Borges, M.E.; Garín, M.; Hernández, A. Biodiesel Production from Waste Oil Using Mg–Al Layered Double Hydroxide Catalysts. Energy Fuels 2009, 23, 2952–2958. [Google Scholar] [CrossRef]
- Cantrell, D.G.; Gillie, L.J.; Lee, A.F.; Wilson, K. Structure-reactivity correlations in MgAl hydrotalcite catalysts for biodiesel synthesis. Appl. Catal. A Gen. 2005, 287, 183–190. [Google Scholar] [CrossRef]
- Tajuddin, N.A.; Manayil, J.C.; Lee, A.F.; Wilson, K. Alkali-Free Hydrothermally Reconstructed NiAl Layered Double Hydroxides for Catalytic Transesterification. Catalysts 2022, 12, 286. [Google Scholar] [CrossRef]
- Tajuddin, N.A.; Manayil, J.C.; Isaacs, M.A.; Parlett, C.M.A.; Lee, A.F.; Wilson, K. Alkali-Free Zn–Al Layered Double Hydroxide Catalysts for Triglyceride Transesterification. Catalysts 2018, 8, 667. [Google Scholar] [CrossRef]
- Du, L.; Ding, S.; Li, Z.; Lv, E.; Lu, J.; Ding, J. Transesterification of castor oil to biodiesel using NaY zeolite-supported La2O3 catalysts. Energy Convers. Manag. 2018, 173, 728–734. [Google Scholar] [CrossRef]
- Babajide, O.; Musyoka, N.; Petrik, L.; Ameer, F. Novel zeolite Na-X synthesized from fly ash as a heterogeneous catalyst in biodiesel production. Catal. Today 2012, 190, 54–60. [Google Scholar] [CrossRef]
- Al-Jammal, N.; Al-Hamamre, Z.; Alnaief, M. Manufacturing of zeolite based catalyst from zeolite tuft for biodiesel production from waste sunflower oil. Renew. Energy 2016, 93, 449–459. [Google Scholar] [CrossRef]
- Xie, J.; Zheng, X.; Dong, A.; Xiao, Z.; Zhang, J. Biont shell catalyst for biodiesel production. Green Chem. 2009, 11, 355–364. [Google Scholar] [CrossRef]
- Yang, L.; Zhang, A.; Zheng, X. Shrimp Shell Catalyst for Biodiesel Production. Energy Fuels 2009, 23, 3859–3865. [Google Scholar] [CrossRef]
- Rezaei, R.; Mohadesi, M.; Moradi, G.R. Optimization of biodiesel production using waste mussel shell catalyst. Fuel 2013, 109, 534–541. [Google Scholar] [CrossRef]
- Nakatani, N.; Takamori, H.; Takeda, K.; Sakugawa, H. Transesterification of soybean oil using combusted oyster shell waste as a catalyst. Bioresour. Technol. 2009, 100, 1510–1513. [Google Scholar] [CrossRef]
- Chakraborty, R.; Bepari, S.; Banerjee, A. Transesterification of soybean oil catalyzed by fly ash and egg shell derived solid catalysts. Chem. Eng. J. 2010, 165, 798–805. [Google Scholar] [CrossRef]
- Nair, P.; Singh, B.; Upadhyay, S.N.; Sharma, Y.C. Synthesis of biodiesel from low FFA waste frying oil using calcium oxide derived from Mereterix mereterix as a heterogeneous catalyst. J. Clean. Prod. 2012, 29–30, 82–90. [Google Scholar] [CrossRef]
- Liu, X.H.; Bai, H.X.; Zhu, D.J.; Cao, G. Green Catalyzing Transesterification of Soybean Oil with Methanol for Biodiesel Based on the Reuse of Waste River-Snail Shell. Adv. Mater. Res. 2010, 148–149, 794–798. [Google Scholar]
- Gupta, J.; Agarwal, M. Preparation and characterization of highly active solid base catalyst from snail shell for biodiesel production. Biofuels 2019, 10, 315–324. [Google Scholar] [CrossRef]
- Ngamcharussrivichai, C.; Nunthasanti, P.; Tanachai, S.; Bunyakiat, K. Biodiesel production through transesterification over natural calciums. Fuel Process. Technol. 2010, 91, 1409–1415. [Google Scholar] [CrossRef]
- Aktas, A.; Ozer, S. Biodiesel production from leftover olive cake. Parameters 2012, 10, 11. [Google Scholar]
- Al-Hamamre, Z. Potential of Utilizing Olive Cake Oil for Biodiesel Manufacturing. Energy Sources Part A Recovery Util. Environ. Eff. 2015, 37, 2609–2615. [Google Scholar] [CrossRef]
- Sandouqa, A.; Al-Hamamre, Z.; Asfar, J. Preparation and performance investigation of a lignin-based solid acid catalyst manufactured from olive cake for biodiesel production. Renew. Energy 2019, 132, 667–682. [Google Scholar] [CrossRef]
- Elnasr, T.A.S.; Al-Enezi, A.T.; Hussein, M.F.; Bielal, H.; Alhumaimess, M.S.; El-Ossaily, Y.A.; Hassan, H.M.A.; AlNahwa, L.H.M.; Aldawsari, A.M.; Alsohaimi, I.H. Sustainable biodiesel production from waste olive oil: Utilizing olive pulp-derived catalysts for environmental and economic benefits. Sustain. Chem. Pharm. 2024, 37, 101426. [Google Scholar] [CrossRef]
- Behzadi, S.; Farid, M.M. Production of biodiesel using a continuous gas–liquid reactor. Bioresour. Technol. 2009, 100, 683–689. [Google Scholar] [CrossRef]
- Chen, Y.-H.; Wang, L.-C.; Tsai, C.-H.; Shang, N.-C. Continuous-flow Esterification of Free Fatty Acids in a Rotating Packed Bed. Ind. Eng. Chem. Res. 2010, 49, 4117–4122. [Google Scholar] [CrossRef]
- Veljković, V.B.; Avramović, J.M.; Stamenković, O.S. Biodiesel production by ultrasound-assisted transesterification: State of the art and the perspectives. Renew. Sustain. Energy Rev. 2012, 16, 1193–1209. [Google Scholar] [CrossRef]
- Qiu, Z.; Zhao, L.; Weatherley, L. Process intensification technologies in continuous biodiesel production. Chem. Eng. Process. 2010, 49, 323–330. [Google Scholar] [CrossRef]
- Perego, C.; Ricci, M. Diesel fuel from biomass. Catal. Sci. Technol. 2012, 2, 1776–1786. [Google Scholar] [CrossRef]
- Badday, A.S.; Abdullah, A.Z.; Lee, K.T.; Khayoon, M.S. Intensification of biodiesel production via ultrasonic-assisted process: A critical review on fundamentals and recent development. Renew. Sustain. Energy Rev. 2012, 16, 4574–4587. [Google Scholar] [CrossRef]
- Knothe, G. Improving biodiesel fuel properties by modifying fatty ester composition. Energy Environ. Sci. 2009, 2, 759–766. [Google Scholar] [CrossRef]
- Srivastava, A.; Prasad, R. Triglycerides-based diesel fuels. Renew. Sustain. Energy Rev. 2000, 4, 111–133. [Google Scholar] [CrossRef]
- Gerpen, J.V. Biodiesel processing and production. Fuel Process. Technol. 2005, 86, 1097–1107. [Google Scholar] [CrossRef]
- Gole, V.L.; Gogate, P.R. Intensification of synthesis of biodiesel from non-edible oil using sequential combination of microwave and ultrasound. Fuel Process. Technol. 2013, 106, 62–69. [Google Scholar] [CrossRef]
- Gole, V.L.; Gogate, P.R. Intensification of glycerolysis reaction of higher free fatty acid containing sustainable feedstock using microwave irradiation. Fuel Process. Technol. 2014, 118, 110–116. [Google Scholar] [CrossRef]
- Eze, V.C.; Phan, A.N.; Pirez, C.; Harvey, A.P.; Lee, A.F.; Wilson, K. Heterogeneous catalysis in an oscillatory baffled flow reactor. Catal. Sci. Technol. 2013, 3, 2373–2379. [Google Scholar] [CrossRef]
- Ghayal, D.; Pandit, A.B.; Rathod, V.K. Optimization of biodiesel production in a hydrodynamic cavitation reactor using used frying oil. Ultrason. Sonochem. 2013, 20, 322–328. [Google Scholar] [CrossRef]
- Santacesaria, E.; Di Serio, M.; Tesser, R.; Turco, R.; Tortorelli, M.; Russo, V. Biodiesel process intensification in a very simple microchannel device. Chem. Eng. Process. 2012, 52, 47–54. [Google Scholar] [CrossRef]
- Wen, Z.; Yu, X.; Tu, S.-T.; Yan, J.; Dahlquist, E. Intensification of biodiesel synthesis using zigzag micro-channel reactors. Bioresour. Technol. 2009, 100, 3054–3060. [Google Scholar] [CrossRef]
- Dimian, A.C.; Bildea, C.S.; Omota, F.; Kiss, A.A. Innovative process for fatty acid esters by dual reactive distillation. Comput. Chem. Eng. 2009, 33, 743–750. [Google Scholar] [CrossRef]
- Demirbas, A. Biodiesel from Vegetable Oils with MgO Catalytic Transesterification in Supercritical Methanol. Energy Sources Part A Recovery Util. Environ. Eff. 2008, 30, 1645–1651. [Google Scholar] [CrossRef]
- Lim, S.; Lee, K.T. Process intensification for biodiesel production from Jatropha curcas L. seeds: Supercritical reactive extraction process parameters study. Appl. Energy 2013, 103, 712–720. [Google Scholar] [CrossRef]
- Kobayashi, J.; Mori, Y.; Kobayashi, S. Multiphase Organic Synthesis in Microchannel Reactors. Chem. Asian J. 2006, 1, 22–35. [Google Scholar] [CrossRef] [PubMed]
- Kockmann, N. Process Engineering Methods and Microsystem Technology. In Advanced Micro and Nanosystems, 1st ed.; Kockmann, N., Ed.; Wiley: Hoboken, NJ, USA, 2006; pp. 1–45. [Google Scholar]
- Dessimoz, A.-L.; Cavin, L.; Renken, A.; Kiwi-Minsker, L. Liquid–liquid two-phase flow patterns and mass transfer characteristics in rectangular glass microreactors. Chem. Eng. Sci. 2008, 63, 4035–4044. [Google Scholar] [CrossRef]
- Mazubert, A.; Poux, M.; Aubin, J. Intensified processes for FAME production from waste cooking oil: A technological review. Chem. Eng. J. 2013, 233, 201–223. [Google Scholar] [CrossRef]
- Kalu, E.E.; Chen, K.S.; Gedris, T. Continuous-flow biodiesel production using slit-channel reactors. Bioresour. Technol. 2011, 102, 4456–4461. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Ju, J.; Ji, L.; Zhang, L.; Xu, N. Synthesis of Biodiesel in Capillary Microreactors. Ind. Eng. Chem. Res. 2008, 47, 1398–1403. [Google Scholar] [CrossRef]
- Xie, T.; Zhang, L.; Xu, N. Biodiesel synthesis in microreactors. Green Process. Synth. 2012, 1, 61–70. [Google Scholar] [CrossRef]
- Fernandez Rivas, D.; Kuhn, S. Synergy of Microfluidics and Ultrasound: Process Intensification Challenges and Opportunities. Top. Curr. Chem. 2016, 374, 70. [Google Scholar] [CrossRef]
- Santana, H.S.; Silva, J.L.; Taranto, O.P. Development of microreactors applied on biodiesel synthesis: From experimental investigation to numerical approaches. J. Ind. Eng. Chem. 2019, 69, 1–12. [Google Scholar] [CrossRef]
- Tiwari, A.; Rajesh, V.M.; Yadav, S. Biodiesel production in micro-reactors: A review. Energy Sustain. Dev. 2018, 43, 143–161. [Google Scholar] [CrossRef]
- Chueluecha, N.; Kaewchada, A.; Jaree, A. Biodiesel synthesis using heterogeneous catalyst in a packed-microchannel. Energy Convers. Manag. 2017, 141, 145–154. [Google Scholar] [CrossRef]
- Madhawan, A.; Arora, A.; Das, J.; Kuila, A.; Sharma, V. Microreactor technology for biodiesel production: A review. Biomass Convers. Biorefin. 2018, 8, 485–496. [Google Scholar] [CrossRef]
- Gopi, R.; Thangarasu, V.; Ramanathan, A. A critical review of recent advancements in continuous flow reactors and prominent integrated microreactors for biodiesel production. Renew. Sustain. Energy Rev. 2022, 154, 111869. [Google Scholar]
- Abdulla Yusuf, H.; Hossain, S.M.Z.; Aloraibi, S.; Alzaabi, N.J.; Alfayhani, M.A.; Almedfaie, H.J. Fabrication of novel microreactors in-house and their performance analysis via continuous production of biodiesel. Chem. Eng. Process. 2022, 172, 108792. [Google Scholar] [CrossRef]
- Gole, V.L.; Gogate, P.R. A review on intensification of synthesis of biodiesel from sustainable feed stock using sonochemical reactors. Chem. Eng. Process. 2012, 53, 1–9. [Google Scholar] [CrossRef]
- Gogate, P.R. Cavitational reactors for process intensification of chemical processing applications: A critical review. Chem. Eng. Process. 2008, 47, 515–527. [Google Scholar] [CrossRef]
- Gole, V.L.; Gogate, P.R. Intensification of Synthesis of Biodiesel from Nonedible Oils Using Sonochemical Reactors. Ind. Eng. Chem. Res. 2012, 51, 11866–11874. [Google Scholar] [CrossRef]
- Santos, F.F.P.; Malveira, J.Q.; Cruz, M.G.A.; Fernandes, F.A.N. Production of biodiesel by ultrasound assisted esterification of Oreochromis niloticus oil. Fuel 2010, 89, 275–279. [Google Scholar] [CrossRef]
- Boffito, D.C.; Mansi, S.; Leveque, J.-M.; Pirola, C.; Bianchi, C.L.; Patience, G.S. Ultrafast Biodiesel Production Using Ultrasound in Batch and Continuous Reactors. ACS Sustain. Chem. Eng. 2013, 1, 1432–1439. [Google Scholar] [CrossRef]
- Mahamuni, N.N.; Adewuyi, Y.G. Optimization of the Synthesis of Biodiesel via Ultrasound-Enhanced Base-Catalyzed Transesterification of Soybean Oil Using a Multifrequency Ultrasonic Reactor. Energy Fuels 2009, 23, 2757–2766. [Google Scholar] [CrossRef]
- Boffito, D.C.; Galli, F.; Pirola, C.; Bianchi, C.L.; Patience, G.S. Ultrasonic free fatty acids esterification in tobacco and canola oil. Ultrason. Sonochem. 2014, 21, 1969–1975. [Google Scholar] [CrossRef]
- Pirola, C.; Bianchi, C.L.; Boffito, D.C.; Carvoli, G.; Ragaini, V. Vegetable Oil Deacidification by Amberlyst: Study of the Catalyst Lifetime and a Suitable Reactor Configuration. Ind. Eng. Chem. Res. 2010, 49, 4601–4606. [Google Scholar] [CrossRef]
- Pirola, C.; Galli, F.; Bianchi, C.L.; Boffito, D.C.; Comazzi, A.; Manenti, F. Vegetable Oil Deacidification by Methanol Heterogeneously Catalyzed Esterification in (Monophasic Liquid)/Solid Batch and Continuous Reactors. Energy Fuels 2014, 28, 5236–5240. [Google Scholar] [CrossRef]
- Kardos, N.; Luche, J.-L. Sonochemistry of carbohydrate compounds. Carbohydr. Res. 2001, 332, 115–131. [Google Scholar] [CrossRef]
- Thompson, J.C.; He, B.B. Biodiesel Production Using Static Mixers. Trans. ASABE 2007, 50, 161–165. [Google Scholar] [CrossRef]
- Avramović, J.M.; Stamenković, O.S.; Todorović, Z.B.; Lazić, M.L.; Veljković, V.B. The optimization of the ultrasound-assisted base-catalyzed sunflower oil methanolysis by a full factorial design. Fuel Process. Technol. 2010, 91, 1551–1557. [Google Scholar] [CrossRef]
- Kumar, D.; Kumar, G.; Poonam; Singh, C.P. Fast, easy ethanolysis of coconut oil for biodiesel production assisted by ultrasonication. Ultrason. Sonochem. 2010, 17, 555–559. [Google Scholar] [CrossRef]
- Thanh, L.T.; Okitsu, K.; Sadanaga, Y.; Takenaka, N.; Maeda, Y.; Bandow, H. Ultrasound-assisted production of biodiesel fuel from vegetable oils in a small scale circulation process. Bioresour. Technol. 2010, 101, 639–645. [Google Scholar] [CrossRef] [PubMed]
- Colucci, J.A.; Borrero, E.E.; Alape, F. Biodiesel from an alkaline transesterification reaction of soybean oil using ultrasonic mixing. J. Am. Oil Chem. Soc. 2005, 82, 525–530. [Google Scholar] [CrossRef]
- Chen, X.; Qian, W.-W.; Lu, X.-P.; Han, P.-F. Preparation of biodiesel catalysed by KF/CaO with ultrasound. Nat. Prod. Res. 2012, 26, 1249–1256. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Z.; Petera, J.; Weatherley, L.R. Biodiesel synthesis in an intensified spinning disk reactor. Chem. Eng. J. 2012, 210, 597–609. [Google Scholar] [CrossRef]
- Pask, S.D.; Nuyken, O.; Cai, Z. The spinning disk reactor: An example of a process intensification technology for polymers and particles. Polym. Chem. 2012, 3, 2698–2707. [Google Scholar] [CrossRef]
- Chanthon, N.; Ngaosuwan, K.; Kiatkittipong, W.; Wongsawaeng, D.; Appamana, W.; Quitain, A.T.; Assabumrungrat, S. High-efficiency biodiesel production using rotating tube reactor: New insight of operating parameters on hydrodynamic regime and biodiesel yield. Renew. Sustain. Energy Rev. 2021, 151, 111430. [Google Scholar] [CrossRef]
- Tabatabaei, M.; Aghbashlo, M.; Dehhaghi, M.; Panahi, H.K.S.; Mollahosseini, A.; Hosseini, M.; Soufiyan, M.M. Reactor technologies for biodiesel production and processing: A review. Prog. Energy Combust. Sci. 2019, 74, 239–303. [Google Scholar] [CrossRef]
- Chen, K.-J.; Chen, Y.-S. Intensified production of biodiesel using a spinning disk reactor. Chem. Eng. Process. 2014, 78, 67–72. [Google Scholar] [CrossRef]
- Motasemi, F.; Ani, F.N. A review on microwave-assisted production of biodiesel. Renew. Sustain. Energy Rev. 2012, 16, 4719–4733. [Google Scholar] [CrossRef]
- Azcan, N.; Danisman, A. Alkali catalyzed transesterification of cottonseed oil by microwave irradiation. Fuel 2007, 86, 2639–2644. [Google Scholar] [CrossRef]
- Lin, Y.-C.; Hsu, K.-H.; Lin, J.-F. Rapid palm-biodiesel production assisted by a microwave system and sodium methoxide catalyst. Fuel 2014, 115, 306–311. [Google Scholar] [CrossRef]
- Liao, C.-C.; Chung, T.-W. Optimization of process conditions using response surface methodology for the microwave-assisted transesterification of Jatropha oil with KOH impregnated CaO as catalyst. Chem. Eng. Res. Des. 2013, 91, 2457–2464. [Google Scholar] [CrossRef]
- Boucher, M.B.; Weed, C.; Leadbeater, N.E.; Wilhite, B.A.; Stuart, J.D.; Parnas, R.S. Pilot Scale Two-Phase Continuous Flow Biodiesel Production via Novel Laminar Flow Reactor–Separator. Energy Fuels 2009, 23, 2750–2756. [Google Scholar] [CrossRef]
- Frascari, D.; Zuccaro, M.; Pinelli, D.; Paglianti, A. A Pilot-Scale Study of Alkali-Catalyzed Sunflower Oil Transesterification with Static Mixing and with Mechanical Agitation. Energy Fuels 2008, 22, 1493–1501. [Google Scholar] [CrossRef]
- ASTM 6584; Standard Test Method for Determination of Total Monoglycerides, Total Diglycerides, Total Triglycerides, and Free and Total Glycerin in B-100 Biodiesel Methyl Esters by Gas Chromatography. ASTM: West Conshohocken, PA, USA, 2022.
- Brunold, C.R.; Hunns, J.C.B.; Mackley, M.R.; Thompson, J.W. Experimental observations on flow patterns and energy losses for oscillatory flow in ducts containing sharp edges. Chem. Eng. Sci. 1989, 44, 1227–1244. [Google Scholar] [CrossRef]
- Dickens, A.W.; Mackley, M.R.; Williams, H.R. Experimental residence time distribution measurements for unsteady flow in baffled tubes. Chem. Eng. Sci. 1989, 44, 1471–1479. [Google Scholar] [CrossRef]
- Howes, T.; Mackley, M.R.; Roberts, E.P.L. The simulation of chaotic mixing and dispersion for periodic flows in baffled channels. Chem. Eng. Sci. 1991, 46, 1669–1677. [Google Scholar] [CrossRef]
- Ni, X.; Jian, H.; Fitch, A.W. Computational fluid dynamic modelling of flow patterns in an oscillatory baffled column. Chem. Eng. Sci. 2002, 57, 2849–2862. [Google Scholar] [CrossRef]
- Harvey, A.P.; Mackley, M.R.; Stonestreet, P. Operation and Optimization of an Oscillatory Flow Continuous Reactor. Ind. Eng. Chem. Res. 2001, 40, 5371–5377. [Google Scholar] [CrossRef]
- Harvey, A.P.; Mackley, M.R.; Seliger, T. Process intensification of biodiesel production using a continuous oscillatory flow reactor. J. Chem. Technol. Biotechnol. 2003, 78, 338–341. [Google Scholar] [CrossRef]
- Zheng, M.; Skelton, R.L.; Mackley, M.R. Biodiesel Reaction Screening Using Oscillatory Flow Meso Reactors. Process Saf. Environ. Prot. 2007, 85, 365–371. [Google Scholar] [CrossRef]
- Phan, A.N.; Harvey, A.P.; Rawcliffe, M. Continuous screening of base-catalysed biodiesel production using New designs of mesoscale oscillatory baffled reactors. Fuel Process. Technol. 2011, 92, 1560–1567. [Google Scholar] [CrossRef]
- Phan, A.N.; Harvey, A.P.; Eze, V. Rapid Production of Biodiesel in Mesoscale Oscillatory Baffled Reactors. Chem. Eng. Technol. 2012, 35, 1214–1220. [Google Scholar] [CrossRef]
- Eze, V.C.; Phan, A.N.; Harvey, A.P. A more robust model of the biodiesel reaction, allowing identification of process conditions for significantly enhanced rate and water tolerance. Bioresour. Technol. 2014, 156, 222–231. [Google Scholar] [CrossRef] [PubMed]
- Eze, V.C.; Phan, A.N.; Harvey, A.P. Intensified one-step biodiesel production from high water and free fatty acid waste cooking oils. Fuel 2018, 220, 567–574. [Google Scholar] [CrossRef]
- Bianchi, P.; Williams, J.D.; Kappe, C.O. Oscillatory flow reactors for synthetic chemistry applications. J. Flow Chem. 2020, 10, 475–490. [Google Scholar] [CrossRef]
- Moser, B.R. Biodiesel Production, Properties, and Feedstocks. In Biofuels: Global Impact on Renewable Energy, Production Agriculture, and Technological Advancements; Tomes, D., Lakshmanan, P., Songstad, D., Eds.; Springer: New York, NY, USA, 2011; pp. 285–347. [Google Scholar]
- Lin, C.-Y.; Ma, L. Influences of Water Content in Feedstock Oil on Burning Characteristics of Fatty Acid Methyl Esters. Processes 2020, 8, 1130. [Google Scholar] [CrossRef]
- Kumar, S.; Singhal, M.K.; Sharma, M.P. Predictability of Biodiesel Fuel Properties from the Fatty Acid Composition of the Feedstock Oils. Arab. J. Sci. Eng. 2022, 47, 5671–5691. [Google Scholar] [CrossRef]
- Özer, S. The effect of diesel fuel-tall oil/ethanol/methanol/isopropyl/n-butanol/fusel oil mixtures on engine performance and exhaust emissions. Fuel 2020, 281, 118671. [Google Scholar] [CrossRef]
- Aketo, T.; Waga, K.; Yabu, Y.; Maeda, Y.; Yoshino, T.; Hanada, A.; Sano, K.; Kamiya, T.; Takano, H.; Tanaka, T. Algal biomass production by phosphorus recovery and recycling from wastewater using amorphous calcium silicate hydrates. Bioresour. Technol. 2021, 340, 125678. [Google Scholar] [CrossRef]
- Im, K.; Choi, K.H.; Park, B.J.; Yoo, S.J.; Kim, J. Tofu-derived heteroatom-doped carbon for oxygen reduction reaction in an anion exchange membrane–fuel cell. Energy Convers. Manag. 2022, 265, 115754. [Google Scholar] [CrossRef]
- Tomić, M.; Đurišić-Mladenović, N.; Mićić, R.; Simikić, M.; Savin, L. Effects of accelerated oxidation on the selected fuel properties and composition of biodiesel. Fuel 2019, 235, 269–276. [Google Scholar] [CrossRef]
- Masudi, A.; Muraza, O.; Jusoh, N.W.C.; Ubaidillah, U. Improvements in the stability of biodiesel fuels: Recent progress and challenges. Environ. Sci. Pollut. Res. 2023, 30, 14104–14125. [Google Scholar] [CrossRef]
- Verma, T.N.; Shrivastava, P.; Rajak, U.; Dwivedi, G.; Jain, S.; Zare, A.; Shukla, A.K.; Verma, P. A comprehensive review of the influence of physicochemical properties of biodiesel on combustion characteristics, engine performance and emissions. J. Traffic Transp. Eng. (Engl. Ed.) 2021, 8, 510–533. [Google Scholar] [CrossRef]
- Saluja, R.K.; Kumar, V.; Sham, R. Stability of biodiesel—A review. Renew. Sustain. Energy Rev. 2016, 62, 866–881. [Google Scholar] [CrossRef]
- Wang, Y.; Wu, B.; Ma, T.; Mi, Y.; Jiang, H.; Yan, H.; Zhao, P.; Zhang, S.; Wu, L.; Chen, L.; et al. Efficient conversion of hemicellulose into 2,3-butanediol by engineered psychrotrophic Raoultella terrigena: Mechanism and efficiency. Bioresour. Technol. 2022, 359, 127453. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, L.; Da Silva, M. Relationship between cetane number and calorific value of biodiesel from Tilapia visceral oil blends with mineral diesel. In Proceedings of the International Conference on Renewable Energies and Power Quality (ICREPQ’13), Bilbao, Spain, 20–22 March 2013; pp. 20–23. [Google Scholar]
- Mekonnen, K.D.; Endris, Y.A.; Abdu, K.Y. Alternative Methods for Biodiesel Cetane Number Valuation: A Technical Note. ACS Omega 2024, 9, 6296–6304. [Google Scholar] [CrossRef]
- Lin, C.-Y.; Wu, X.-E. Determination of Cetane Number from Fatty Acid Compositions and Structures of Biodiesel. Processes 2022, 10, 1502. [Google Scholar] [CrossRef]
- Sharma, A.K.; Sharma, P.K.; Chintala, V.; Khatri, N.; Patel, A. Environment-Friendly Biodiesel/Diesel Blends for Improving the Exhaust Emission and Engine Performance to Reduce the Pollutants Emitted from Transportation Fleets. Int. J. Environ. Res. Public Health 2020, 17, 3896. [Google Scholar] [CrossRef]
- de Menezes, L.C.; de Sousa, E.R.; da Silva, G.S.; Marques, A.L.B.; Viegas, H.D.C.; dos Santos, M.J.C. Investigations on Storage and Oxidative Stability of Biodiesel from Different Feedstocks Using the Rancimat Method, Infrared Spectroscopy, and Chemometry. ACS Omega 2022, 7, 30746–30755. [Google Scholar] [CrossRef]
- Knothe, G. Some aspects of biodiesel oxidative stability. Fuel Process. Technol. 2007, 88, 669–677. [Google Scholar] [CrossRef]
- Longanesi, L.; Pereira, A.P.; Johnston, N.; Chuck, C.J. Oxidative stability of biodiesel: Recent insights. Biofuels Bioprod. Biorefin. 2022, 16, 265–289. [Google Scholar] [CrossRef]
- Neves, C.V.; Módenes, A.N.; Scheufele, F.B.; Rocha, R.P.; Pereira, M.F.R.; Figueiredo, J.L.; Borba, C.E. Dibenzothiophene adsorption onto carbon-based adsorbent produced from the coconut shell: Effect of the functional groups density and textural properties on kinetics and equilibrium. Fuel 2021, 292, 120354. [Google Scholar] [CrossRef]
- ASTM D6751; Standard Specification for Biodiesel Fuel Blend Stock (B100) for Middle Distillate Fuels. ASTM: West Conshohocken, PA, USA, 2023.
- Sakthivel, R.; Ramesh, K.; Purnachandran, R.; Mohamed Shameer, P. A review on the properties, performance and emission aspects of the third generation biodiesels. Renew. Sustain. Energy Rev. 2018, 82, 2970–2992. [Google Scholar] [CrossRef]
- EU Ban on the Sale of New Petrol and Diesel Cars from 2035 Explained. Available online: https://www.europarl.europa.eu/topics/en/article/20221019STO44572/eu-ban-on-sale-of-new-petrol-and-diesel-cars-from-2035-explained (accessed on 17 August 2024).
- Ang, R. Malaysia Targets B30 Mandate for Heavy Vehicles by 2030. Available online: https://www.argusmedia.com/en/news-and-insights/latest-market-news/2484262-malaysia-targets-b30-mandate-for-heavy-vehicles-by-2030 (accessed on 17 August 2024).
- Pakulski, L. Brazil’s Chamber of Deputies Approves Proposal to Raise Biodiesel Mandate to 20% in 2030. Available online: https://www.fastmarkets.com/insights/brazil-approves-proposal-to-raise-biodiesel-mandate/ (accessed on 17 August 2024).
- Stamatopoulos, B. The Role of Biofuels in the New Regulations Landscape. Available online: https://safety4sea.com/cm-the-role-of-biofuels-in-the-new-regulations-landscape/ (accessed on 17 August 2024).
- U.S. Department of Agriculture. Waste Management in Biodiesel Production. Available online: https://farm-energy.extension.org/waste-management-in-biodiesel-production/ (accessed on 17 August 2024).
- Dhainaut, J.; Dacquin, J.-P.; Lee, A.F.; Wilson, K. Hierarchical macroporous–mesoporous SBA-15 sulfonic acid catalysts for biodiesel synthesis. Green Chem. 2010, 12, 296–303. [Google Scholar] [CrossRef]
- Pirez, C.; Lee, A.F.; Jones, C.; Wilson, K. Can surface energy measurements predict the impact of catalyst hydrophobicity upon fatty acid esterification over sulfonic acid functionalised periodic mesoporous organosilicas? Catal. Today 2014, 234, 167–173. [Google Scholar] [CrossRef]
- Albuquerque, A.A.; Ng, F.T.T.; Danielski, L.; Stragevitch, L. Phase equilibrium modeling in biodiesel production by reactive distillation. Fuel 2020, 271, 117688. [Google Scholar] [CrossRef]
- He, B.; Shao, Y.; Ren, Y.; Li, J.; Cheng, Y. Continuous biodiesel production from acidic oil using a combination of cation- and anion-exchange resins. Fuel Process. Technol. 2015, 130, 1–6. [Google Scholar] [CrossRef]
- Zhang, G.; Xie, W. ZrMo oxides supported catalyst with hierarchical porous structure for cleaner and sustainable production of biodiesel using acidic oils as feedstocks. J. Clean. Prod. 2023, 384, 135594. [Google Scholar] [CrossRef]
- Li, K.; Xie, W. Enhanced biodiesel production from low-value acidic oils using ordered hierarchical macro–mesoporous MoAl@H-SiO2 catalyst. Fuel 2024, 364, 131105. [Google Scholar] [CrossRef]
- Luque, R.; Clark, J.H. Biodiesel-Like Biofuels from Simultaneous Transesterification/Esterification of Waste Oils with a Biomass-Derived Solid Acid Catalyst. ChemCatChem 2011, 3, 594–597. [Google Scholar] [CrossRef]
- Suwannakarn, K.; Lotero, E.; Ngaosuwan, K.; Goodwin, J.G. Simultaneous Free Fatty Acid Esterification and Triglyceride Transesterification Using a Solid Acid Catalyst with in Situ Removal of Water and Unreacted Methanol. Ind. Eng. Chem. Res. 2009, 48, 2810–2818. [Google Scholar] [CrossRef]
- Morales, G.; Fernando Bautista, L.; Melero, J.A.; Iglesias, J.; Sanchez-Vazquez, R. Low-grade oils and fats: Effect of several impurities on biodiesel production over sulfonic acid heterogeneous catalysts. Bioresour. Technol. 2011, 102, 9571–9578. [Google Scholar] [CrossRef]
- Mata, T.M.; Cardoso, N.; Ornelas, M.; Neves, S.; Caetano, N.S. Evaluation of Two Purification Methods of Biodiesel from Beef Tallow, Pork Lard, and Chicken Fat. Energy Fuels 2011, 25, 4756–4762. [Google Scholar] [CrossRef]
- Aransiola, E.F.; Ojumu, T.V.; Oyekola, O.O.; Madzimbamuto, T.F.; Ikhu-Omoregbe, D.I.O. A review of current technology for biodiesel production: State of the art. Biomass Bioenergy 2014, 61, 276–297. [Google Scholar] [CrossRef]
- Tasić, M.B.; Stamenković, O.S.; Veljković, V.B. Cost analysis of simulated base-catalyzed biodiesel production processes. Energy Convers. Manag. 2014, 84, 405–413. [Google Scholar] [CrossRef]
- Lopes, M.G.M.; Santana, H.S.; Andolphato, V.F.; Russo, F.N.; Silva, J.L.; Taranto, O.P. 3D printed micro-chemical plant for biodiesel synthesis in millireactors. Energy Convers. Manag. 2019, 184, 475–487. [Google Scholar] [CrossRef]
- Laybourn, A.; López-Fernández, A.M.; Thomas-Hillman, I.; Katrib, J.; Lewis, W.; Dodds, C.; Harvey, A.P.; Kingman, S.W. Combining continuous flow oscillatory baffled reactors and microwave heating: Process intensification and accelerated synthesis of metal-organic frameworks. Chem. Eng. J. 2019, 356, 170–177. [Google Scholar] [CrossRef]
- Ni, X.; Mackley, M.R.; Harvey, A.P.; Stonestreet, P.; Baird, M.H.I.; Rama Rao, N.V. Mixing Through Oscillations and Pulsations—A Guide to Achieving Process Enhancements in the Chemical and Process Industries. Chem. Eng. Res. Des. 2003, 81, 373–383. [Google Scholar] [CrossRef]
- Taherian, T.; Hemmati, A. Synthesis of the novel catalytic membrane of KOH/ Fe3O4-graphene oxide/PVDF for the production of biodiesel in a membrane reactor: Optimization with response surface methodology (RSM). Renew. Energy 2024, 225, 120219. [Google Scholar] [CrossRef]
- Wandscher Busanello, F.; Sérgi Gomes, M.C.; Paschoal, S.M.; Rodrigues da Silva Baumgärtner, T.; Barp, G.; Fiorentin-Ferrari, L.D. The influence of membrane separation technique in the biodiesel and bioethanol production process: A review. Biofuels 2024, 15, 903–928. [Google Scholar] [CrossRef]
- Lau, J.I.C.; Wang, Y.S.; Ang, T.; Seo, J.C.F.; Khadaroo, S.N.B.A.; Chew, J.J.; Ng Kay Lup, A.; Sunarso, J. Emerging technologies, policies and challenges toward implementing sustainable aviation fuel (SAF). Biomass Bioenergy 2024, 186, 107277. [Google Scholar] [CrossRef]
- Fiorini, A.C.O.; Angelkorte, G.; Maia, P.L.; Bergman-Fonte, C.; Vicente, C.; Morais, T.; Carvalho, L.; Zanon-Zotin, M.; Szklo, A.; Schaeffer, R.; et al. Sustainable aviation fuels must control induced land use change: An integrated assessment modelling exercise for Brazil. Environ. Res. Lett. 2023, 18, 014036. [Google Scholar] [CrossRef]

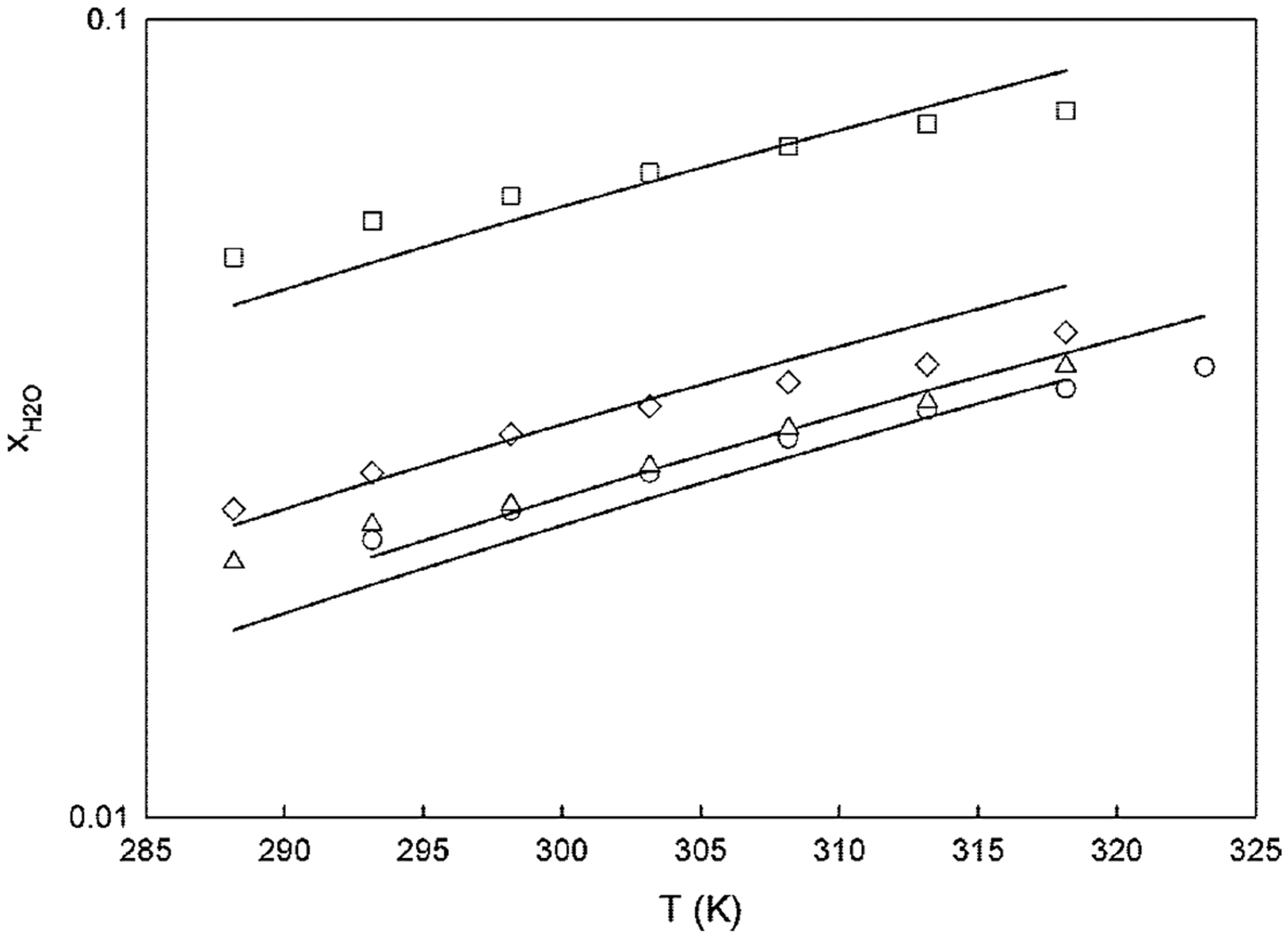
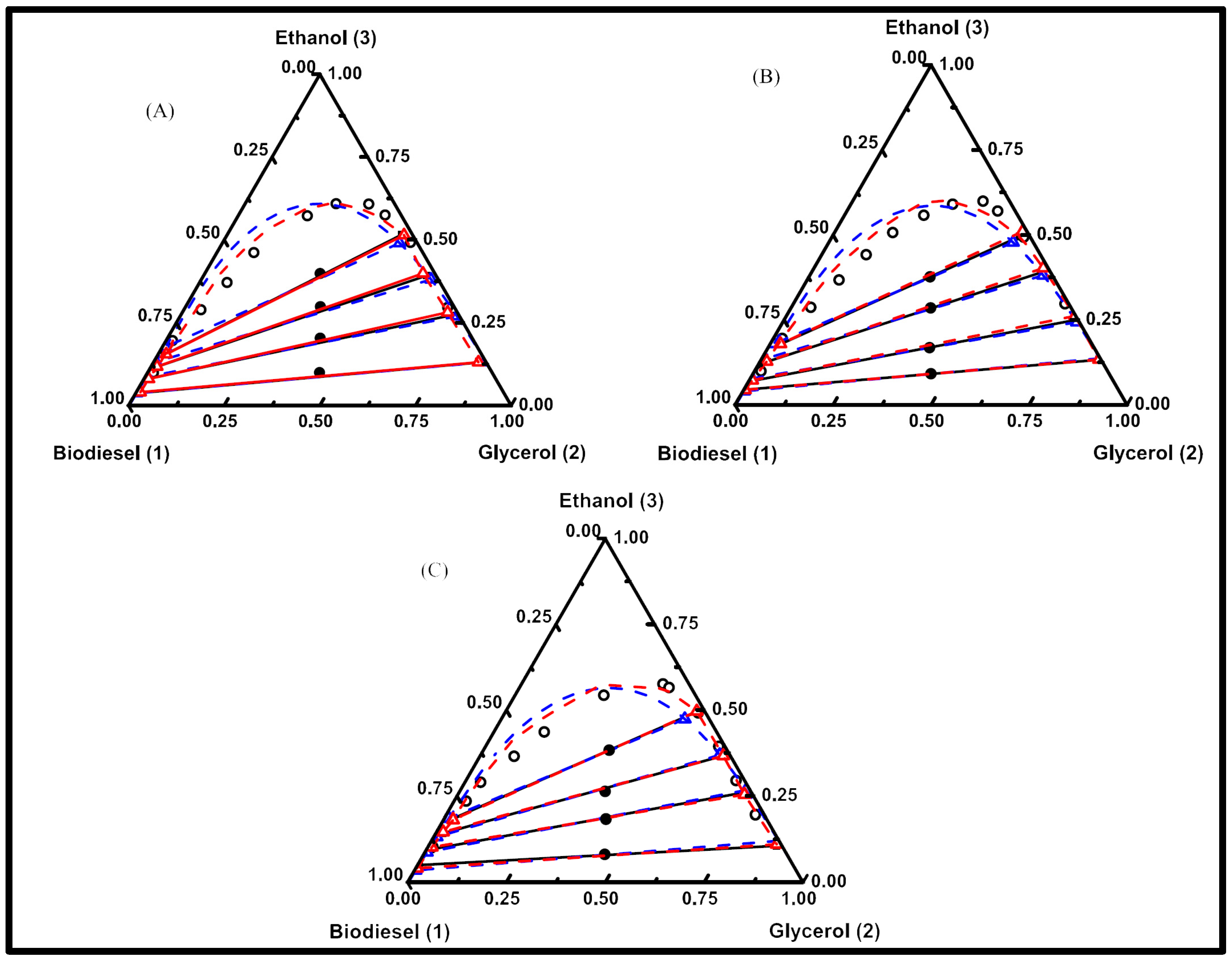

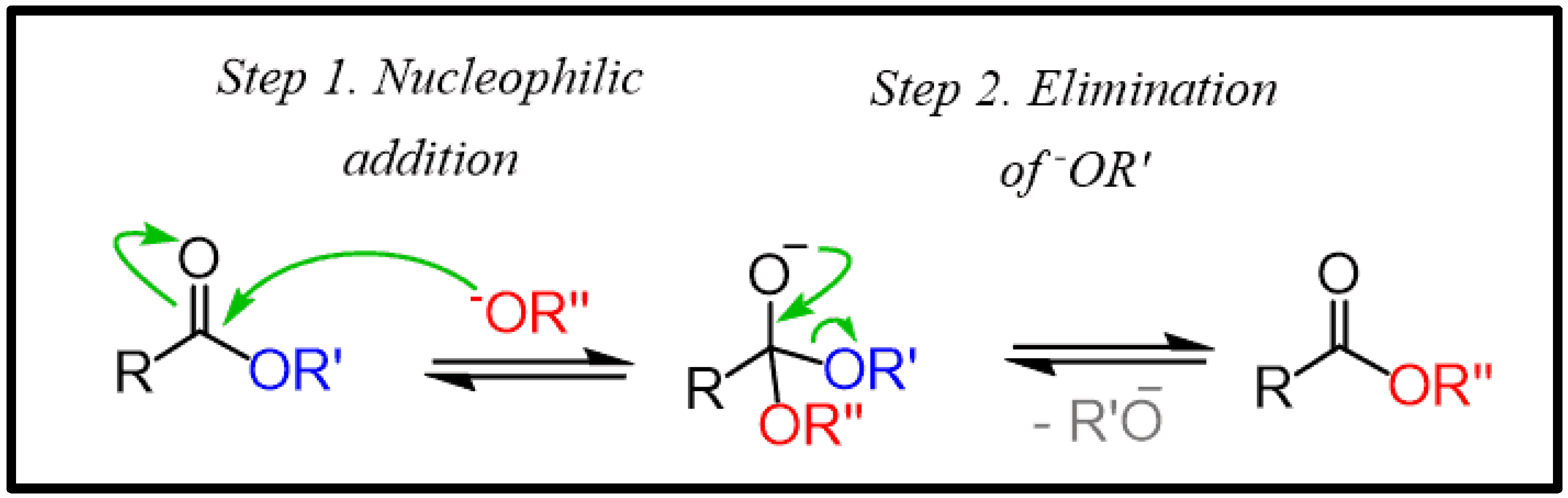
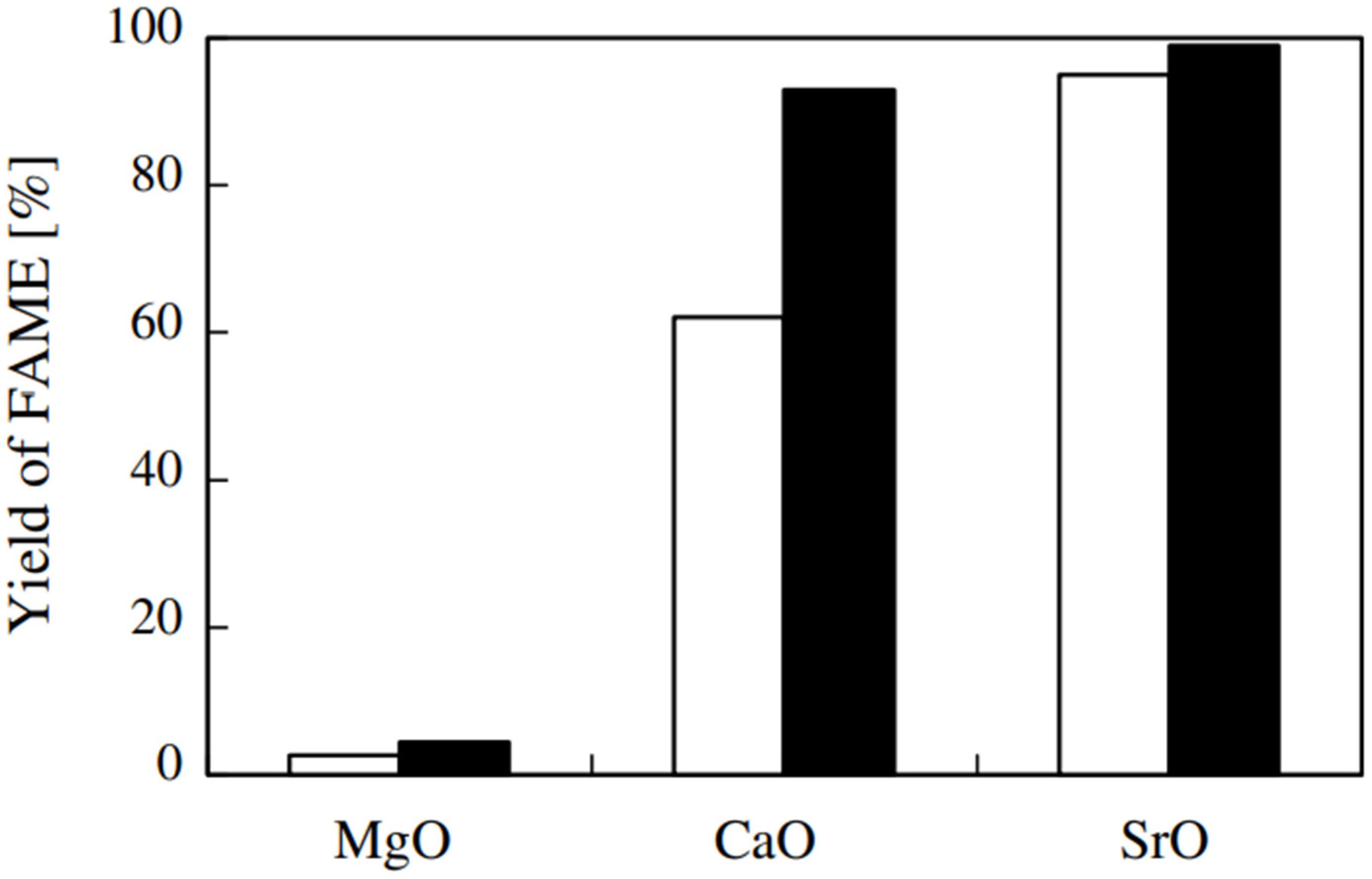
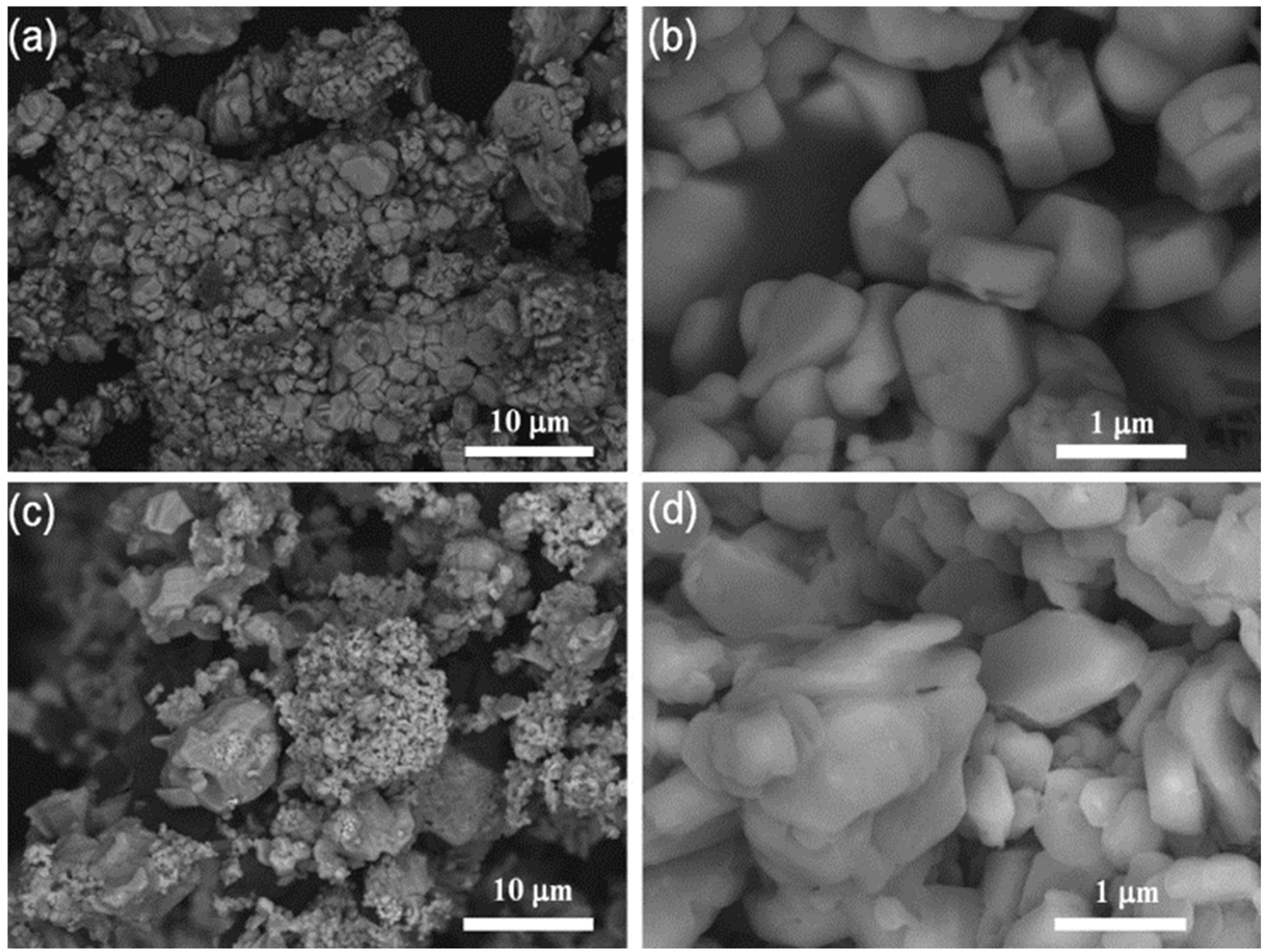

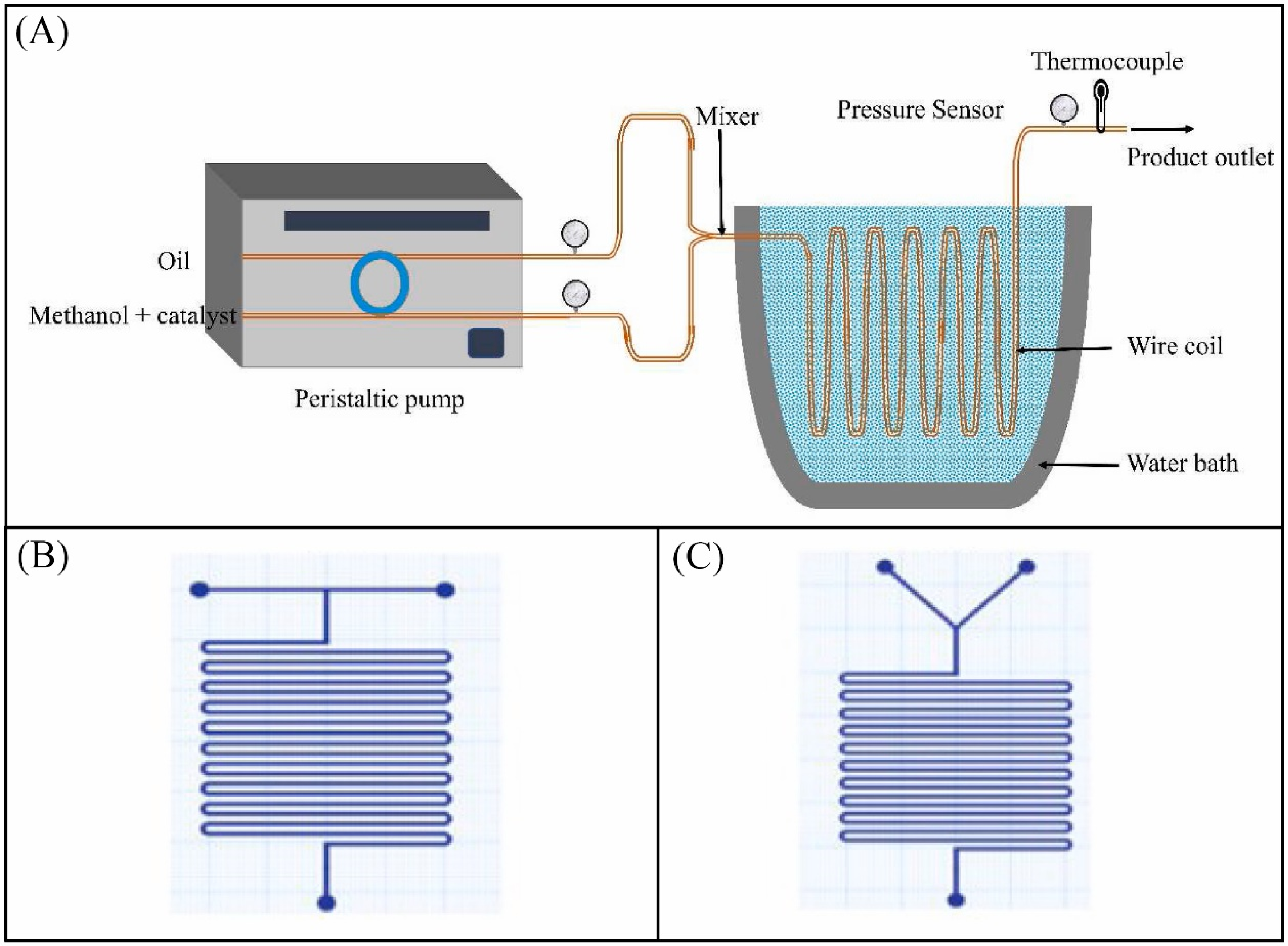
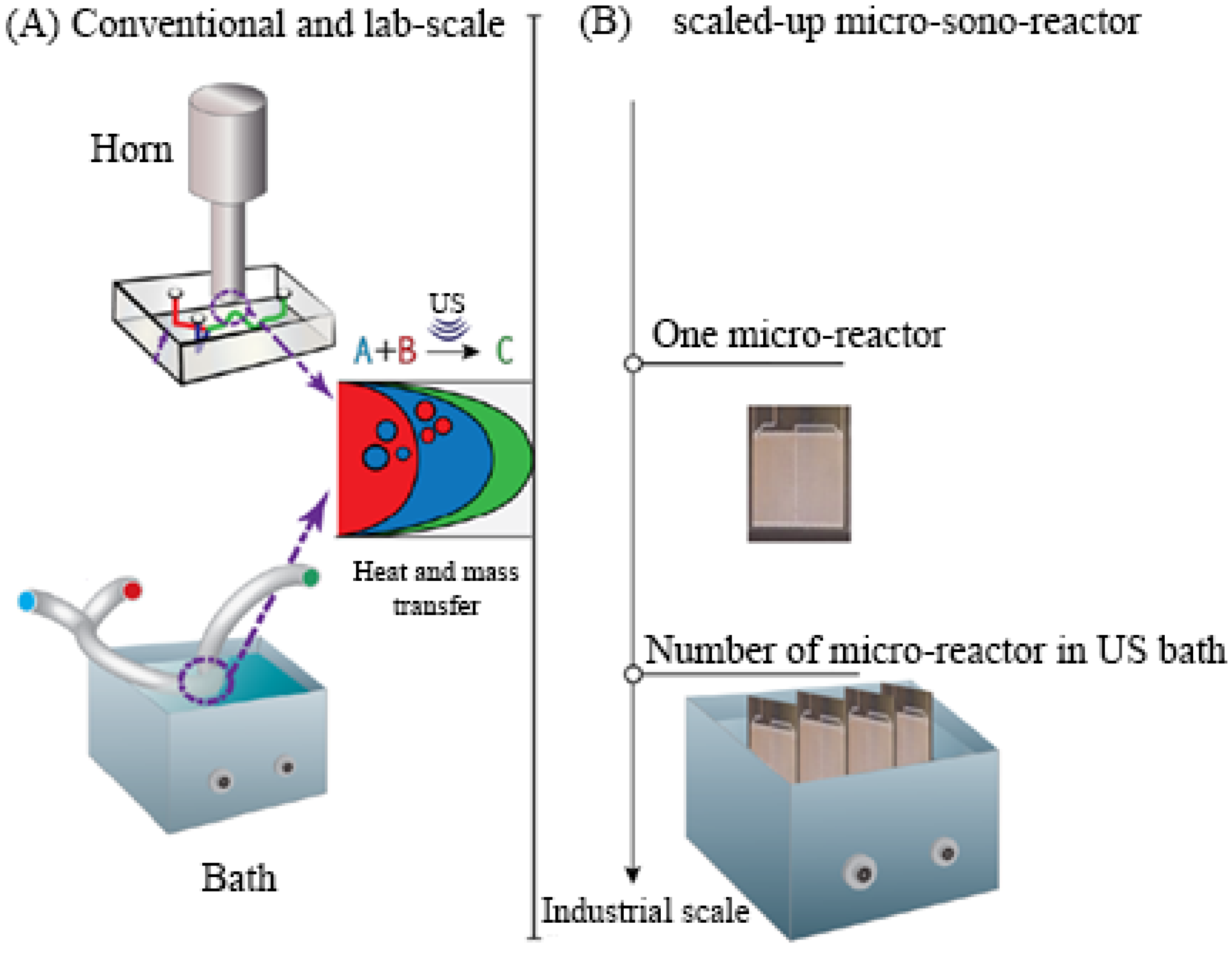
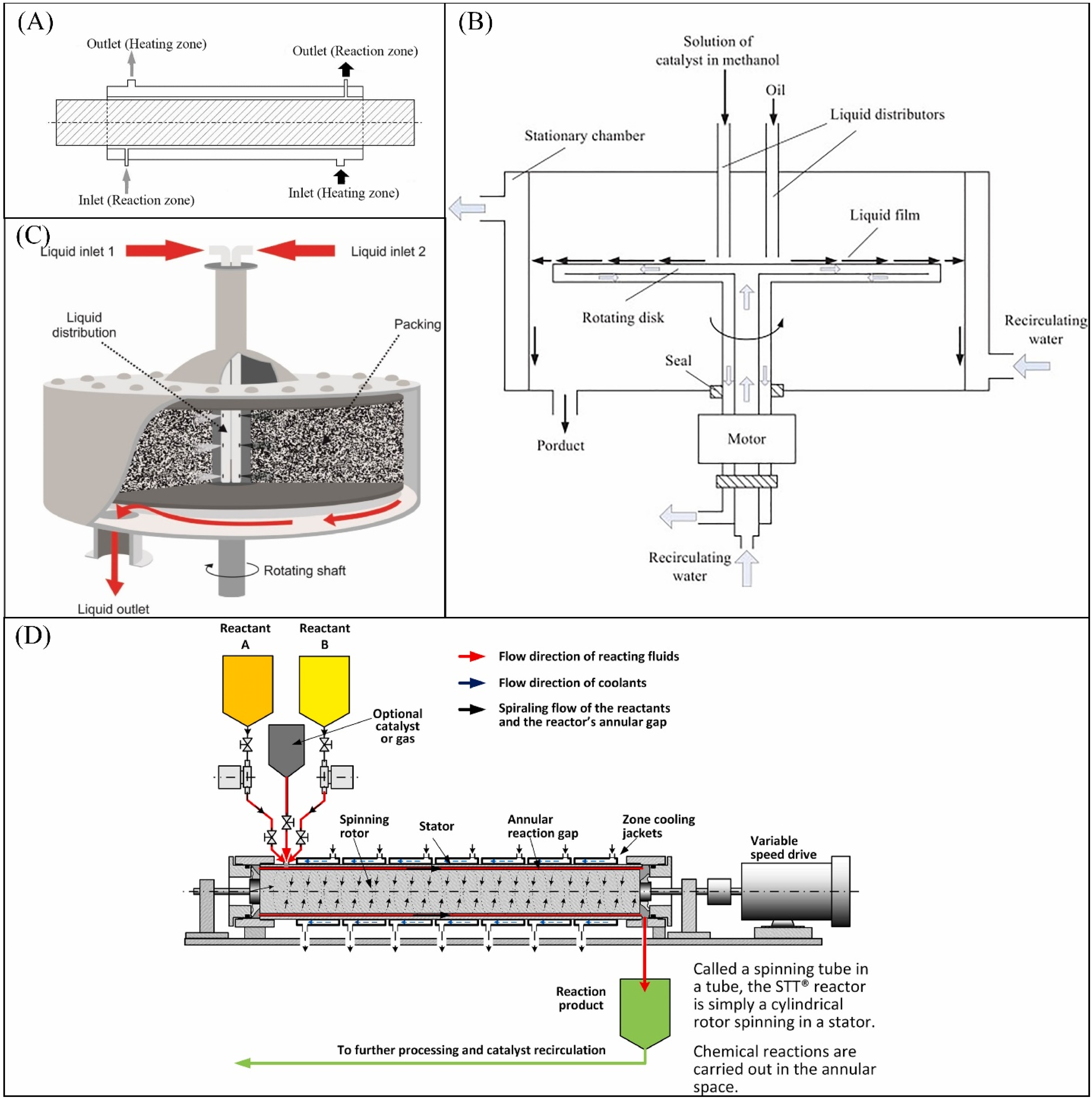
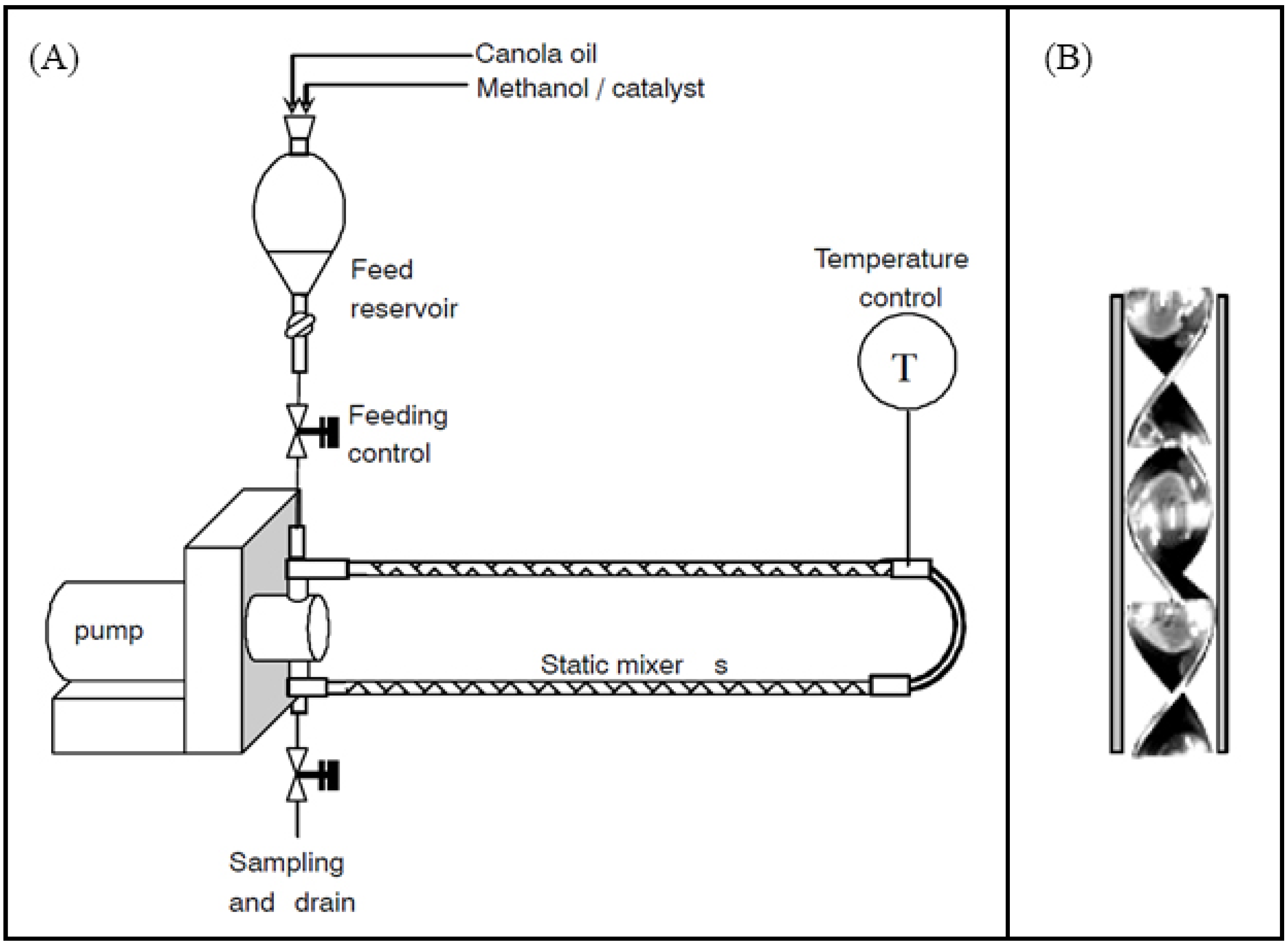
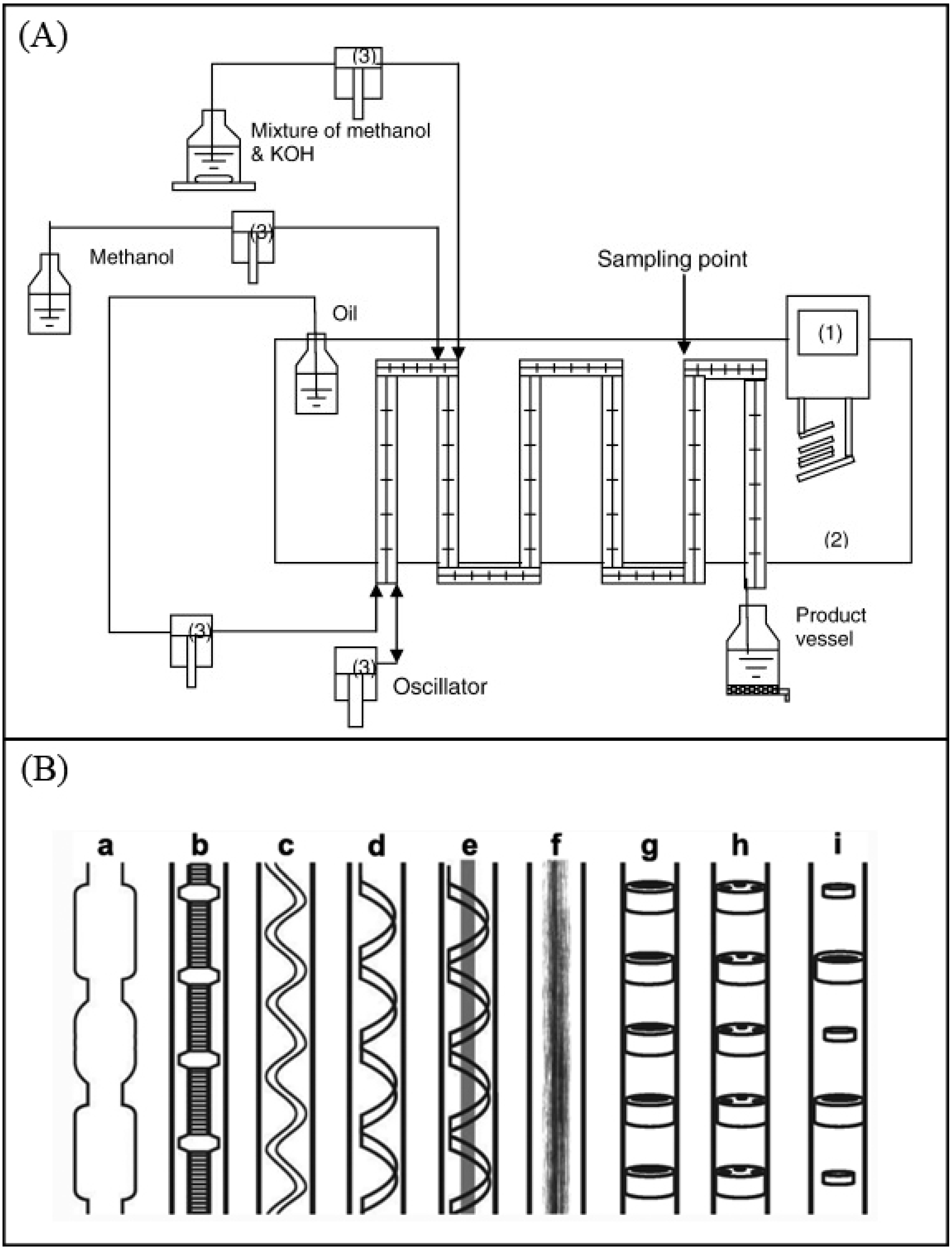
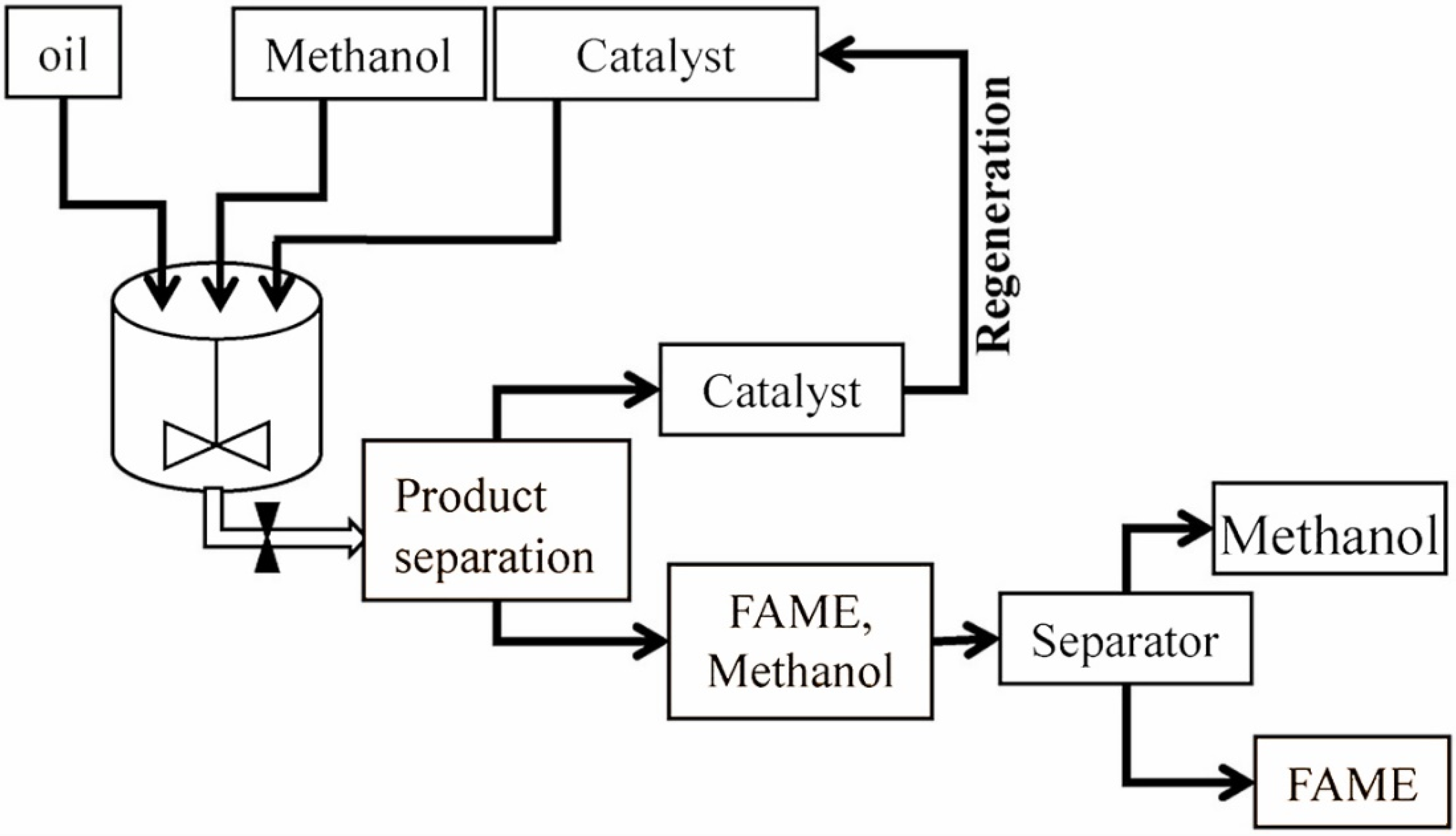
| Fresh oils | |||
| Oil crop | Average oil yield /L·hectare−1·year−1 | Average oilseed price /$·tonne−1 | Average oil price /$·tonne−1 |
| Palm | 5950–1900 | - | 900 |
| Soybean | 446–12,258 | 420 | 900 |
| Rapeseed | 1190 | 400 | 1050 |
| Corn | 172 | 177 | 1200 |
| Sunflower | 952 | 340 | 890 |
| Algae | 58,700–136,900 | - | 0.43–24.60 $/L |
| Waste cooking oil | |||
| Country | Waste cooking oil average FOB price /$·tonne−1 | Waste cooking oil average production /kton·year−1 | |
| USA | 990 | 10,000 | |
| China | 1000 | 5000 | |
| India | 1050 | 3000 | |
| UK | 1050 | 200 | |
| Biodiesel | |||
| B99–B100 price /$·tonne−1 | B20 price /$·tonne−1 | Algae biodiesel /$·tonne−1 | |
| 1183 | 1033 | 2500 | |
| Type of Oil | FFA Content/wt% |
|---|---|
| Palm kernel oil | 2–5 |
| Soybean oil | 0.3–0.7 |
| Corn oil | 0.1–0.2 |
| Sunflower oil | 0.1–0.2 |
| Waste cooking oil | 1.2–20 |
| Olive | ≤0.8% for extra virgin olive oil |
| Coconut | 0.1–0.5 |
| Avocado | 0.1–0.2 |
| Canola | 0.1–0.2 |
| Peanut | 0.1–0.2 |
| Sesame | 0.1–0.2 |
| Feedstock | Temperature Range /°C | /kJ·mol−1 | /kJ·mol−1 | /kJ·mol−1 | Maximum Conversion /% | Kinetic Parameters | Methanol:Oil Molar Ratio | Ref. |
|---|---|---|---|---|---|---|---|---|
| S. triguga oil | 30–60 | 82.4–85.6 | 50.6 | −0.10 | 97 (at 50 °C) | 2nd order, k = 0.04–0.23 L·mol−1·min−1 | 3–12 | [43] |
| Waste cooking oil | 50–100 | 93.8 | 54.1 | −0.11 | 99 (at 100 °C) | 1st order, k = 0.059–0.94 min−1 | 10–40 | [44] |
| Castor oil | 45–75 | 90.9–92.7 | 47.4–46.0 | −0.13–−0.14 | 98 (at 65 °C) | pseudo-1st order | 8–18 | [45] |
| Spirulina platensis algal biomass | 35–75 | 92.7 | 16.4 | −0.23 | 75 (at 55 °C) | 1st order, k = 0.001 min−1 | 1–6 (mass:volume) | [46] |
| Soybean oil | 40–65 | 83.3–87.7 | 28.3 | −0.18 | 98 (at 57.8 °C) | pseudo-1st order, k = 0.085–0.208 min−1 | 4.5–9 | [47] |
| Rapeseed oil | 20–40 | 75.3–79.1 | 19.6 | −0.19 | 98 (at 30 °C) | pseudo-1st order, k = 0.081 min−1 | 6–12 | [48] |
| Schleichera triguga oil | 55–65 | 82.4–85.6 | 50.6 | −0.11 | 90 (at 65 °C) | pseudo-1st order, k = 0.0237–0.0512 min−1 | 6–12 | [49] |
| Refined jatropha oil | 100–160 | 140.0 | 16.7 | −0.28 | 91 (at 160 °C) | pseudo-1st order, k = 0.0029–0.0072 min−1 | 30 | [50] |
| T/K | (xH2O ± σa) | T/K | (xH2O ± σa) | T/K | (xH2O ± σa) |
|---|---|---|---|---|---|
| ethyl butanoate | Methyl tetradecanoate | Ethyl decanoate | |||
| 288.2 | 0.0504 ± 0.0003 | 293.2 | 0.0223 ± 0.0002 | 288.2 | 0.0243 ± 0.0005 |
| 293.2 | 0.056 ± 0.001 | 298.2 | 0.0242 ± 0.0003 | 293.2 | 0.0270 ± 0.0011 |
| 298.2 | 0.060 ± 0.002 | 303.2 | 0.0270 ± 0.0002 | 298.2 | 0.0302 ± 0.0004 |
| 303.2 | 0.0644 ± 0.0004 | 308.2 | 0.0298 ± 0.0004 | 303.2 | 0.0327 ± 0.0004 |
| 308.2 | 0.0694 ± 0.0001 | 313.2 | 0.0324 ± 0.0003 | 308.2 | 0.0351 ± 0.0002 |
| 313.2 | 0.0741 ± 0.0004 | 318.2 | 0.0344 ± 0.0002 | 313.2 | 0.0370 ± 0.0008 |
| 318.2 | 0.0770 ± 0.0005 | 323.2 | 0.0367 ± 0.0004 | 318.2 | 0.0405 ± 0.0008 |
| Feedstock | Catalyst | Model | Pre-Exponential /s−1 | /kJ·mol−1 | Ref. |
|---|---|---|---|---|---|
| Waste cooking oil, Methanol | K3PO4/AC | pseudo-1st order | 28.68 | 34.2 | [69] |
| WCO, Methanol | Waste mussel shells (CaO) | pseudo-1st order | 7.8 × 107 | 79.8 | [70] |
| Waste cotton seed oil, Methanol | Zn/CaO | pseudo-1st order | 0.0275 × 107 | 43.0 | [71] |
| Jatropha oil, Ethanol | Molybdenum impregnated calcium oxide | pseudo-1st order | 0.26 × 107 | 66.0 | [72] |
| Waste cotton seed, Methanol | Li/ZrO2 | pseudo-1st order | 1.35 × 103 | 40.8 and 43.1 | [73] |
| Algae, Methanol | H2SO4 | 1st order | 0.17 × 10−3 | 14.5 | [46] |
| Waste cooking oil, Methanol | Bentonite-CH3ONa | 1st order | 1.4 × 105 | 41.0 | [74] |
| Sunflower oil, Methanol | Al-Sr (nano) | pseudo-1st order | 3.38 × 107 | 72.9 | [75] |
| Karanja oil, Methanol | BaCeO3 | pseudo-1st order | 0.228 × 103 | 36.2 | [76] |
| Reaction | Equation |
|---|---|
| (20) | |
| (21) | |
| (22) | |
| (23) | |
| (24) | |
| (25) | |
| (26) |
| Reaction | Rate Expression | Equation | |
|---|---|---|---|
| SR | (27) | ||
| (28) | |||
| (29) | |||
| (30) | |||
| AD/DE | (31) | ||
| (32) | |||
| (33) |
| Reaction | Rate Expression | Equation |
|---|---|---|
| (34) | ||
| (35) | ||
| (36) |
| Reaction | Equation |
|---|---|
| (37) | |
| (38) | |
| (39) | |
| (40) | |
| (41) | |
| (42) | |
| (24) | |
| (43) | |
| (44) |
| Reaction | Rate Expression | Equation |
|---|---|---|
| (45) | ||
| (46) | ||
| (47) | ||
| (48) | ||
| (49) | ||
| (50) | ||
| (51) | ||
| (52) | ||
| (53) |
| Reaction | Rate Expression | Equation |
|---|---|---|
| (54) | ||
| (55) | ||
| (56) | ||
| (57) | ||
| (58) |
| Reaction | Equation |
|---|---|
| (59) | |
| (60) | |
| (61) | |
| (62) | |
| (63) | |
| (64) | |
| (65) | |
| (66) | |
| (67) | |
| (68) | |
| (69) |
| Reaction | Rate Expression | Equation |
|---|---|---|
| (70) | ||
| (71) | ||
| (72) | ||
| (73) | ||
| (74) | ||
| (75) | ||
| (76) | ||
| (77) | ||
| (78) | ||
| (79) | ||
| (80) |
| Catalyst Type | Advantages | Disadvantages |
|---|---|---|
| Homogeneous basic catalyst |
| |
| Heterogeneous basic catalyst | ||
| Homogeneous acid catalyst |
| |
| Heterogeneous acid catalyst |
| Source | Catalyst | Calcination T/°C | Calcination Time/h | Reaction T/°C | Reaction Time/h | Methanol:Oil Molar Ratio | Catalyst Loading/wt% | Conversion or Yield/% | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| Biont shell | KF-CaO | 500 | - | 70 | 3 | 9:1 | 3 | Yield: 98 | [232] |
| Shrimp shell | KF-CaO | 450 | - | 65 | 3 | 9:1 | 2.5 | Conversion: 80 | [233] |
| waste mussel shell | CaO | 1050 | 2 | 60 | 8 | 24:1 | 12 | Yield: 94 | [234] |
| combusted oyster shell | CaO | 700 | 3 | 65–70 | 5 | 6:1 | 25 | Yield: 74 | [235] |
| shell of egg | CaO | 800 | 2–4 | 60 | 2 | 18:1 | 10 | Yield: 94 | [210] |
| Coal fly ash loaded with egg shell | CaO-Al2O3 | 1000 | 2 | Room temperature | 5 | 6.9:1 | 1 | Yield: 97 | [236] |
| Clamshell | Calcined clamshell | 900 | 3.5 | 60 | 3 | 6.03:1 | 3 g | Conversion: 97 | [237] |
| river-snail shell | calcined river-snail shell | 800 | 2 | 65 | 3 | 9:1 | 3 | Conversion: 98 | [238] |
| Snail shell | KOH-CaO | 800 | 3 | 65 | 3.5 | 9:1 | 6 | Yield: 96 | [239] |
| Cuttle bone | CaCO3 | 800 | 2 | 60 | 4 | 18:1 | 20 | Conversion: 24 | [240] |
| Dolomite | CaMg(CO3)2 | 800 | 2 | 60 | 3 | 30:1 | 6 | Conversion: 99 | [240] |
| Characteristics | Batch Plant | Microreactor Plant |
|---|---|---|
| Plant volume (tonnes/year) | 20,000 | 20,000 |
| Reactor volume (m3) | 10 | 2.4 × 10−3 |
| Plant footprint (m3) | 149 | 60 |
| Surface area to volume ratio (m2/m3) | 14.9 | 2.5 × 104 |
| Productivity (kg/h/m3) | 250 | 10.4 × 105 |
| Energy consumption (kJ/kg) | 7.1 | 0.4 |
| Mass transfer coefficient (s−1) | 10−2–10 | 2.86 × 106 |
| Reynolds Number for mixing | 7 × 105 | 10 |
| Capital cost (Rm) | 8.6 | 6.5 |
| Manufacturing costs (R/L) | 6.6 | 5.87 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Larimi, A.; Harvey, A.P.; Phan, A.N.; Beshtar, M.; Wilson, K.; Lee, A.F. Aspects of Reaction Engineering for Biodiesel Production. Catalysts 2024, 14, 701. https://doi.org/10.3390/catal14100701
Larimi A, Harvey AP, Phan AN, Beshtar M, Wilson K, Lee AF. Aspects of Reaction Engineering for Biodiesel Production. Catalysts. 2024; 14(10):701. https://doi.org/10.3390/catal14100701
Chicago/Turabian StyleLarimi, Afsanehsadat, Adam P. Harvey, Anh N. Phan, Mehdi Beshtar, Karen Wilson, and Adam F. Lee. 2024. "Aspects of Reaction Engineering for Biodiesel Production" Catalysts 14, no. 10: 701. https://doi.org/10.3390/catal14100701
APA StyleLarimi, A., Harvey, A. P., Phan, A. N., Beshtar, M., Wilson, K., & Lee, A. F. (2024). Aspects of Reaction Engineering for Biodiesel Production. Catalysts, 14(10), 701. https://doi.org/10.3390/catal14100701











