Spirobifluorene-Based D-A Type Conjugated Polymer Photocatalysts for Water Splitting
Abstract
1. Introduction
2. Results and Discussion
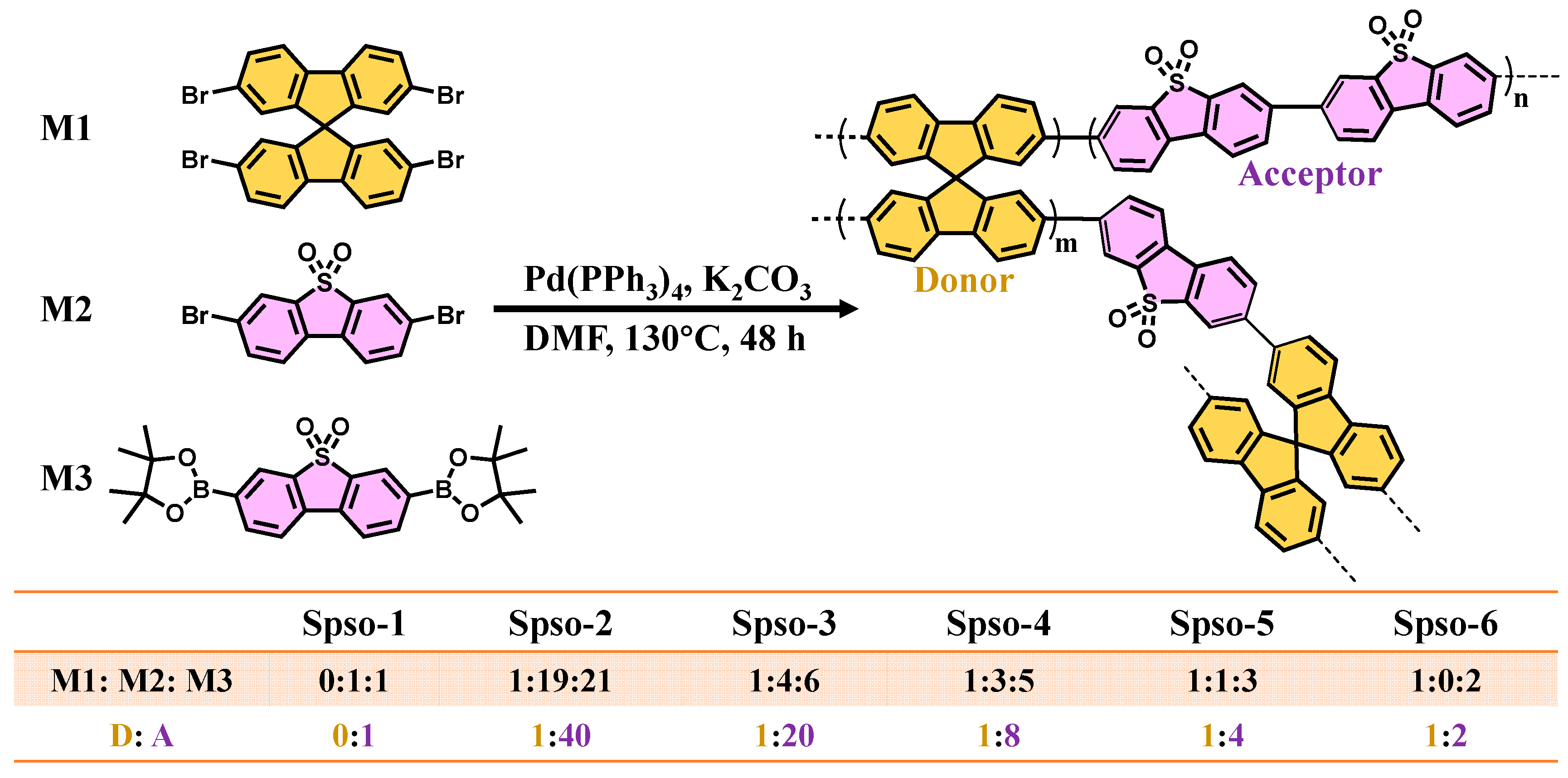
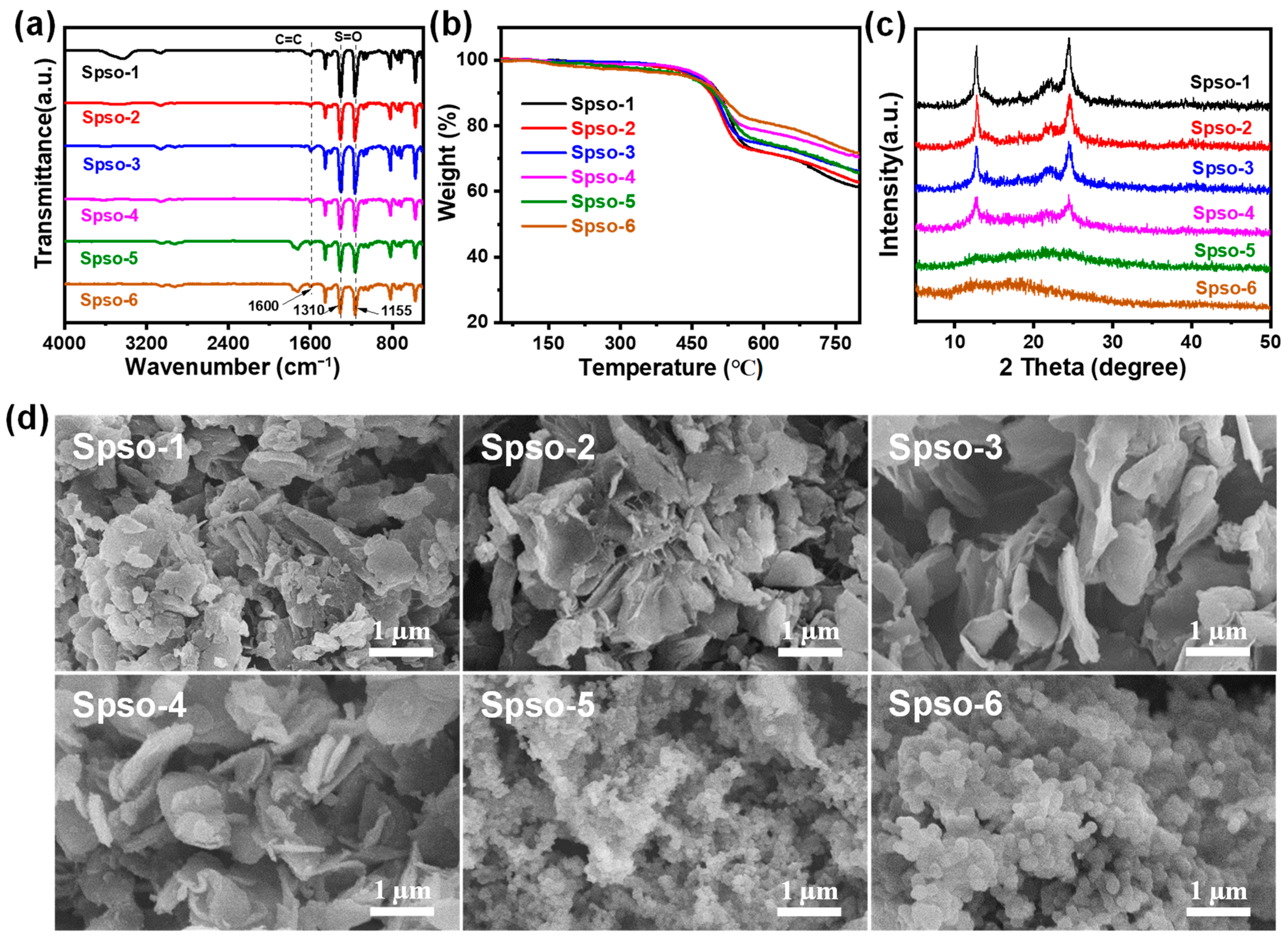
2.1. Structure and Morphology Analyses
2.2. Energy Band and Photophysical Property
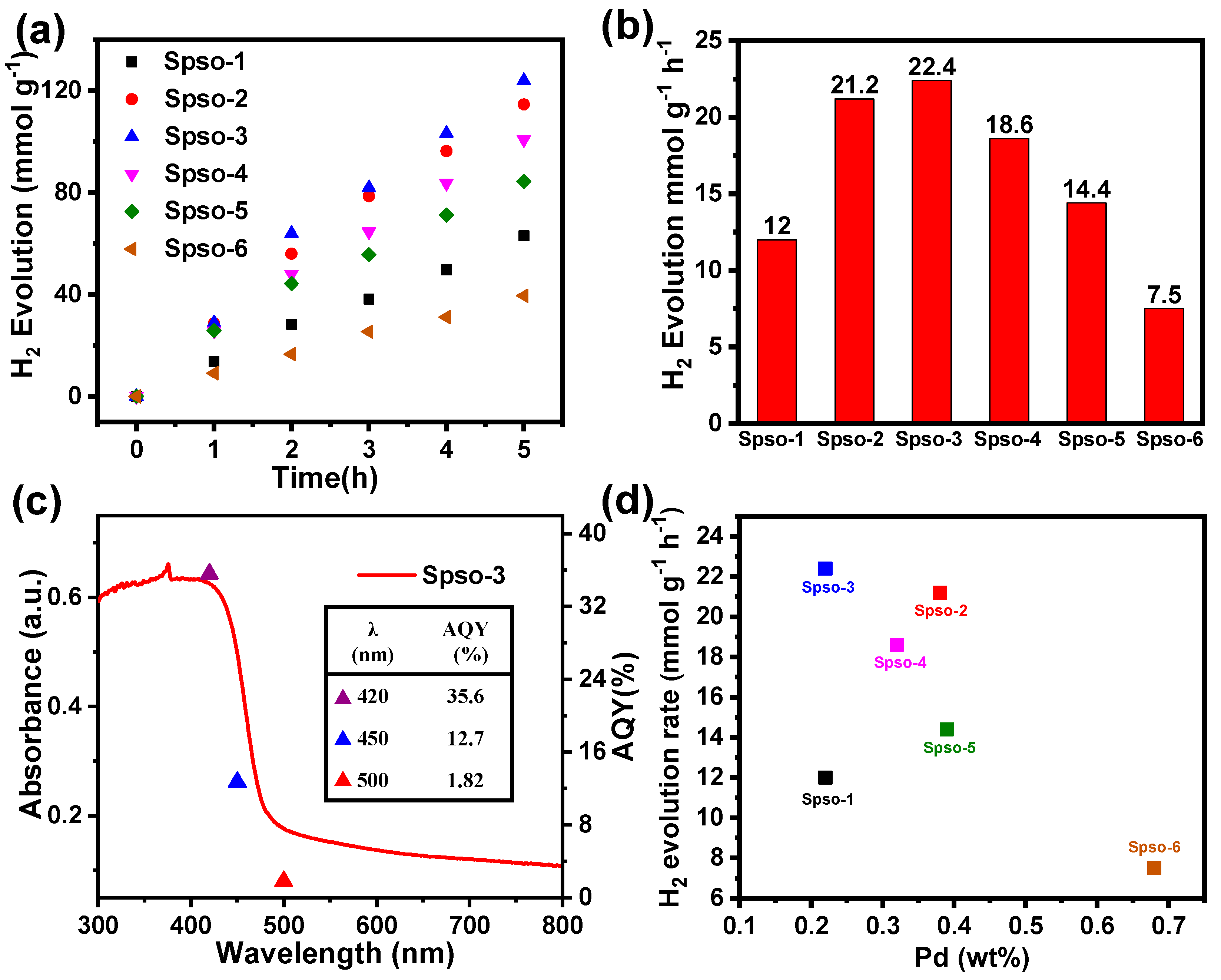
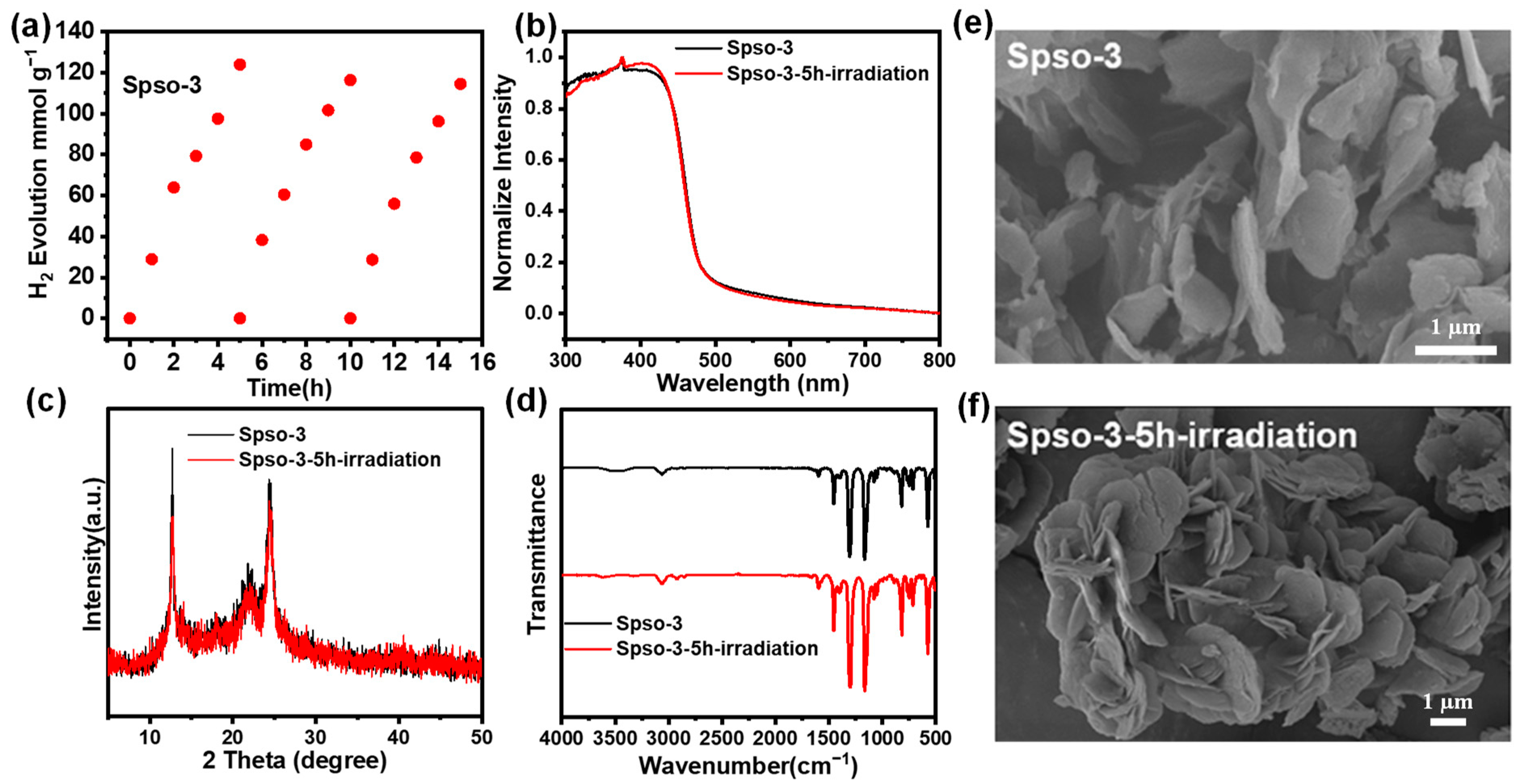
2.3. Photocatalytic Performance
3. Materials and Methods
3.1. Materials and Reagents
3.2. Polymer Synthesis Methods
3.2.1. Synthesis of 3,7-Dibromo-dibenzothiophene Sulfone (M2)
3.2.2. Synthesis of 3,7-Diborate Ester-dibenzothiophene Sulfone (M3)
3.2.3. Typical Procedure of Suzuki–Miyaura Coupling Polymerization
3.2.4. Synthesis of Spso-1
3.2.5. Synthesis of Spso-2
3.2.6. Synthesis of Spso-3
3.2.7. Synthesis of Spso-4
3.2.8. Synthesis of Spso-5
3.2.9. Synthesis of Spso-6
3.3. Instrumentation/General Methods
3.4. Photocatalytic Activity Measurements
3.5. AQY Measurements
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lubitz, W.; Tumas, W. Hydrogen: An Overview. Chem. Rev. 2007, 107, 3900–3903. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Shen, S.; Guo, L.; Mao, S.S. Semiconductor-based Photocatalytic Hydrogen Generation. Chem. Rev. 2010, 110, 6503–6570. [Google Scholar] [CrossRef] [PubMed]
- Dawood, F.; Anda, M.; Shafiullah, G.M. Hydrogen production for energy: An overview. Int. J. Hydrogen Energy 2020, 45, 3847–3869. [Google Scholar] [CrossRef]
- Colón, G. Towards the hydrogen production by photocatalysis. Appl. Catal. A-Gen. 2016, 518, 48–59. [Google Scholar] [CrossRef]
- Wang, Q.; Domen, K. Particulate Photocatalysts for Light-Driven Water Splitting: Mechanisms, Challenges, and Design Strategies. Chem. Rev. 2020, 120, 919–985. [Google Scholar] [CrossRef]
- Hisatomi, T.; Domen, K. Overall water splitting: What’s next? Next Energy 2023, 1, 100006. [Google Scholar] [CrossRef]
- Kudo, A.; Miseki, Y. Heterogeneous photocatalyst materials for water splitting. Chem. Soc. Rev. 2009, 38, 253–278. [Google Scholar] [CrossRef]
- Wang, X.; Maeda, K.; Thomas, A.; Takanabe, K.; Xin, G.; Carlsson, J.M.; Domen, K.; Antonietti, M. A metal-free polymeric photocatalyst for hydrogen production from water under visible light. Nat. Mater. 2009, 8, 76–80. [Google Scholar] [CrossRef]
- Ong, W.-J.; Tan, L.-L.; Ng, Y.H.; Yong, S.-T.; Chai, S.-P. Graphitic Carbon Nitride (g-C3N4)-Based Photocatalysts for Artificial Photosynthesis and Environmental Remediation: Are We a Step Closer to Achieving Sustainability? Chem. Rev. 2016, 116, 7159–7329. [Google Scholar] [CrossRef]
- Fateminia, S.M.A.; Mao, Z.; Xu, S.; Yang, Z.; Chi, Z.; Liu, B. Organic Nanocrystals with Bright Red Persistent Room-Temperature Phosphorescence for Biological Applications. Angew. Chem. Int. Ed. 2017, 56, 12160–12164. [Google Scholar] [CrossRef]
- Cai, S.; Shi, H.; Zhang, Z.; Wang, X.; Ma, H.; Gan, N.; Wu, Q.; Cheng, Z.; Ling, K.; Gu, M.; et al. Hydrogen-Bonded Organic Aromatic Frameworks for Ultralong Phosphorescence by Intralayer π–π Interactions. Angew. Chem. Int. Ed. 2018, 57, 4005–4009. [Google Scholar] [CrossRef] [PubMed]
- Sprick, R.S.; Bonillo, B.; Clowes, R.; Guiglion, P.; Brownbill, N.J.; Slater, B.J.; Blanc, F.; Zwijnenburg, M.A.; Adams, D.J.; Cooper, A.I. Visible-Light-Driven Hydrogen Evolution Using Planarized Conjugated Polymer Photocatalysts. Angew. Chem. Int. Ed. 2016, 55, 1792–1796. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Lan, Z.-A.; Wang, X. Conjugated Polymers: Catalysts for Photocatalytic Hydrogen Evolution. Angew. Chem. Int. Ed. 2016, 55, 15712–15727. [Google Scholar] [CrossRef] [PubMed]
- Bai, Y.; Wilbraham, L.; Slater, B.J.; Zwijnenburg, M.A.; Sprick, R.S.; Cooper, A.I. Accelerated Discovery of Organic Polymer Photocatalysts for Hydrogen Evolution from Water through the Integration of Experiment and Theory. J. Am. Chem. Soc. 2019, 141, 9063–9071. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Chen, Z.; Shi, R.; Yang, X.; Zhang, T. Recent Advances in Conjugated Polymers for Visible-Light-Driven Water Splitting. Adv. Mater. 2020, 32, 1907296. [Google Scholar] [CrossRef] [PubMed]
- Yin, X.; Guo, F.; Lalancette, R.A.; Jäkle, F. Luminescent Main-Chain Organoborane Polymers: Highly Robust, Electron-Deficient Poly(oligothiophene borane)s via Stille Coupling Polymerization. Macromolecules 2016, 49, 537–546. [Google Scholar] [CrossRef]
- Li, L.; Hadt, R.G.; Yao, S.; Lo, W.-Y.; Cai, Z.; Wu, Q.; Pandit, B.; Chen, L.X.; Yu, L. Photocatalysts Based on Cobalt-Chelating Conjugated Polymers for Hydrogen Evolution from Water. Chem. Mater. 2016, 28, 5394–5399. [Google Scholar] [CrossRef]
- Lan, Z.-A.; Ren, W.; Chen, X.; Zhang, Y.; Wang, X. Conjugated donor-acceptor polymer photocatalysts with electron-output “tentacles” for efficient hydrogen evolution. Appl. Catal. B Environ. 2019, 245, 596–603. [Google Scholar] [CrossRef]
- Lee, J.-S.M.; Cooper, A.I. Advances in Conjugated Microporous Polymers. Chem. Rev. 2020, 120, 2171–2214. [Google Scholar] [CrossRef]
- Han, C.; Dong, P.; Tang, H.; Zheng, P.; Zhang, C.; Wang, F.; Huang, F.; Jiang, J.-X. Realizing high hydrogen evolution activity under visible light using narrow band gap organic photocatalysts. Chem. Sci. 2021, 12, 1796–1802. [Google Scholar] [CrossRef]
- Xu, F.; Yang, S.; Chen, X.; Liu, Q.; Li, H.; Wang, H.; Wei, B.; Jiang, D. Energy-storage covalent organic frameworks: Improving performance via engineering polysulfide chains on walls. Chem. Sci. 2019, 10, 6001–6006. [Google Scholar] [CrossRef] [PubMed]
- Pachfule, P.; Acharjya, A.; Roeser, J.; Langenhahn, T.; Schwarze, M.; Schomacker, R.; Thomas, A.; Schmidt, J. Diacetylene Functionalized Covalent Organic Framework (COF) for Photocatalytic Hydrogen Generation. J. Am. Chem. Soc. 2018, 140, 1423–1427. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Chen, L.; Chong, S.Y.; Little, M.A.; Wu, Y.; Zhu, W.-H.; Clowes, R.; Yan, Y.; Zwijnenburg, M.A.; Sprick, R.S.; et al. Sulfone-containing covalent organic frameworks for photocatalytic hydrogen evolution from water. Nat. Chem. 2018, 10, 1180–1189. [Google Scholar] [CrossRef] [PubMed]
- Kochergin, Y.S.; Schwarz, D.; Acharjya, A.; Ichangi, A.; Kulkarni, R.; Eliášová, P.; Vacek, J.; Schmidt, J.; Thomas, A.; Bojdys, M.J. Exploring the “Goldilocks Zone” of Semiconducting Polymer Photocatalysts by Donor–Acceptor Interactions. Angew. Chem. Int. Ed. 2018, 57, 14188–14192. [Google Scholar] [CrossRef] [PubMed]
- Lan, Z.-A.; Zhang, G.; Chen, X.; Zhang, Y.; Zhang, K.A.I.; Wang, X. Reducing the Exciton Binding Energy of Donor–Acceptor-Based Conjugated Polymers to Promote Charge-Induced Reactions. Angew. Chem. Int. Ed. 2019, 58, 10236–10240. [Google Scholar] [CrossRef]
- Huang, W.; He, Q.; Hu, Y.; Li, Y. Molecular Heterostructures of Covalent Triazine Frameworks for Enhanced Photocatalytic Hydrogen Production. Angew. Chem. Int. Ed. 2019, 58, 8676–8680. [Google Scholar] [CrossRef]
- Fukuzumi, S.; Ohkubo, K. Selective photocatalytic reactions with organic photocatalysts. Chem. Sci. 2013, 4, 561–574. [Google Scholar] [CrossRef]
- Ru, C.; Chen, P.; Wu, X.; Chen, C.; Zhang, J.; Zhao, H.; Wu, J.; Pan, X. Enhanced Built-in Electric Field Promotes Photocatalytic Hydrogen Performance of Polymers Derived from the Introduction of B ← N Coordination Bond. Adv. Sci. 2022, 9, 2204055. [Google Scholar] [CrossRef]
- Wu, X.; Zhang, M.; Xia, Y.; Ru, C.; Chen, P.; Zhao, H.; Zhou, L.; Gong, C.; Wu, J.; Pan, X. Arylboron functional covalent organic frameworks for synergistic photocatalytic hydrogen evolution. J. Mater. Chem. A 2022, 10, 17691–17698. [Google Scholar] [CrossRef]
- Zhao, H.; Dong, Y.; Sun, P.; Bai, Y.; Ru, C.; Wu, X.; Li, Z.; Han, X.; Wu, J.; Pan, X. Effect of D/A Ratio on Photocatalytic Hydrogen Evolution Performance of Conjugated Polymer Photocatalysts. ACS Appl. Energy Mater. 2022, 5, 4631–4640. [Google Scholar] [CrossRef]
- Chen, P.; Ru, C.; Hu, L.; Yang, X.; Wu, X.; Zhang, M.; Zhao, H.; Wu, J.; Pan, X. Construction of Efficient D–A-Type Photocatalysts by B–N Bond Substitution for Water Splitting. Macromolecules 2023, 56, 858–866. [Google Scholar] [CrossRef]
- Fournier, J.-H.; Maris, T.; Wuest, J.D. Molecular Tectonics. Porous Hydrogen-Bonded Networks Built from Derivatives of 9,9‘-Spirobifluorene. J. Org. Chem. 2004, 69, 1762–1775. [Google Scholar] [CrossRef] [PubMed]
- Heredia, D.; Natera, J.; Gervaldo, M.; Otero, L.; Fungo, F.; Lin, C.-Y.; Wong, K.-T. Spirobifluorene-Bridged Donor/Acceptor Dye for Organic Dye-Sensitized Solar Cells. Org. Lett. 2010, 12, 12–15. [Google Scholar] [CrossRef] [PubMed]
- Qiu, N.; Yang, X.; Zhang, H.; Wan, X.; Li, C.; Liu, F.; Zhang, H.; Russell, T.P.; Chen, Y. Nonfullerene Small Molecular Acceptors with a Three-Dimensional (3D) Structure for Organic Solar Cells. Chem. Mater. 2016, 28, 6770–6778. [Google Scholar] [CrossRef]
- Lyu, Y.-Y.; Kwak, J.; Jeon, W.S.; Byun, Y.; Lee, H.S.; Kim, D.; Lee, C.; Char, K. Highly Efficient Red Phosphorescent OLEDs based on Non-Conjugated Silicon-Cored Spirobifluorene Derivative Doped with Ir-Complexes. Adv. Funct. Mater. 2009, 19, 420–427. [Google Scholar] [CrossRef]
- Wu, C.; Liu, Y.; Liu, H.; Duan, C.; Pan, Q.; Zhu, J.; Hu, F.; Ma, X.; Jiu, T.; Li, Z.; et al. Highly Conjugated Three-Dimensional Covalent Organic Frameworks Based on Spirobifluorene for Perovskite Solar Cell Enhancement. J. Am. Chem. Soc. 2018, 140, 10016–10024. [Google Scholar] [CrossRef]
- Han, C.; Xiang, S.; Jin, S.; Luo, L.-W.; Zhang, C.; Yan, C.; Jiang, J.-X. Linear multiple-thiophene-containing conjugated polymer photocatalysts with narrow band gaps for achieving ultrahigh photocatalytic hydrogen evolution activity under visible light. J. Mater. Chem. A 2022, 10, 5255–5261. [Google Scholar] [CrossRef]
- Kotp, M.G.; Torad, N.L.; Nara, H.; Chaikittisilp, W.; You, J.; Yamauchi, Y.; El-Mahdy, A.F.M.; Kuo, S.-W. Tunable thiophene-based conjugated microporous polymers for the disposal of toxic hexavalent chromium. J. Mater. Chem. A 2023, 11, 15022–15032. [Google Scholar] [CrossRef]
- Li, Z.; Zhao, F.; Chu, Y.; Meng, F.; Dong, Y.; Zhang, H.; Zhao, J.; Du, Y.; Wang, S. Fused-Thiophene and Sulfone-Based Donor–Acceptor Conjugated Polymers for Enhanced Hydrogen Production. ACS Sustain. Chem. Eng. 2024, 12, 1072–1083. [Google Scholar] [CrossRef]
- Yu, C.; Zhou, W.; Yu, J.; Cao, F.; Li, X. Thermal Stability, Microstructure and Photocatalytic Activity of the Bismuth Oxybromide Photocatalyst. Chin. J. Chem. 2012, 30, 721–726. [Google Scholar] [CrossRef]
- Chen, H.-Y.; Yeh, S.-C.; Chen, C.-T.; Chen, C.-T. Comparison of thiophene- and selenophene-bridged donor–acceptor low band-gap copolymers used in bulk-heterojunction organic photovoltaics. J. Mater. Chem. 2012, 22, 21549–21559. [Google Scholar] [CrossRef]
- He, Y.; Liu, Y.; Li, C.; Chen, X.-B.; Shi, Z.; Feng, S. Origin of the Photocatalytic Activity of Crystalline Phase Structures. ACS Appl. Energy Mater. 2022, 5, 8923–8929. [Google Scholar] [CrossRef]
- Li, S.; Liu, W.; Shi, M.; Mai, J.; Lau, T.-K.; Wan, J.; Lu, X.; Li, C.-Z.; Chen, H. A spirobifluorene and diketopyrrolopyrrole moieties based non-fullerene acceptor for efficient and thermally stable polymer solar cells with high open-circuit voltage. Energy Environ. Sci. 2016, 9, 604–610. [Google Scholar] [CrossRef]
- Otero, L.; Sereno, L.; Fungo, F.; Liao, Y.-L.; Lin, C.-Y.; Wong, K.-T. Synthesis and Properties of a Novel Electrochromic Polymer Obtained from the Electropolymerization of a 9,9'-Spirobifluorene-Bridged Donor−Acceptor (D−A) Bichromophore System. Chem. Mater. 2006, 18, 3495–3502. [Google Scholar] [CrossRef]
- Modak, A.; Maegawa, Y.; Goto, Y.; Inagaki, S. Synthesis of 9,9′-spirobifluorene-based conjugated microporous polymers by FeCl3-mediated polymerization. Poly. Chem. 2016, 7, 1290–1296. [Google Scholar] [CrossRef]
- Wu, F.-I.; Dodda, R.; Reddy, D.S.; Shu, C.-F. Synthesis and characterization of spiro-linked poly(terfluorene): A blue-emitting polymer with controlled conjugated length. J. Mater. Chem. 2002, 12, 2893–2897. [Google Scholar] [CrossRef]
- Das, S.; Chowdhury, I.H.; Chakraborty, A.; Naskar, M.K.; Sarkar, M.; Manirul Islam, S.K. Porous organic polymer (POP) nanosheets: An efficient photo-catalyst for visible-light assisted CO2 reduction. Mater. Adv. 2022, 3, 3165–3173. [Google Scholar] [CrossRef]
- Liu, R.; Yu, Z.; Zhang, R.; Xiong, J.; Qiao, Y.; Liu, X.; Lu, X. Hollow Nanoreactors for Controlled Photocatalytic Behaviors: Fundamental Theory, Structure–Performance Relationship, and Catalytic Advantages. Small 2024, 20, 2308142. [Google Scholar] [CrossRef]
- Niu, P.; Zhang, L.; Liu, G.; Cheng, H.-M. Graphene-Like Carbon Nitride Nanosheets for Improved Photocatalytic Activities. Adv. Funct. Mater. 2012, 22, 4763–4770. [Google Scholar] [CrossRef]
- Zhou, M.; Lou, X.W.; Xie, Y. Two-dimensional nanosheets for photoelectrochemical water splitting: Possibilities and opportunities. Nano Today 2013, 8, 598–618. [Google Scholar] [CrossRef]
- Narsaria, A.K.; Poater, J.; Guerra, C.F.; Ehlers, A.W.; Hamlin, T.A.; Lammertsma, K.; Bickelhaupt, F.M. Distortion-Controlled Redshift of Organic Dye Molecules. Chem. Eur. J. 2020, 26, 2080–2093. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Zhang, J.; Ye, R.; Xu, S.; Bai, G. Color modulation of cerium sulfide colorant powders through chemical doping engineering. J. Mater. Chem. C 2023, 11, 9215–9222. [Google Scholar] [CrossRef]
- Ravishankar, S.; Bisquert, J.; Kirchartz, T. Interpretation of Mott–Schottky plots of photoanodes for water splitting. Chem. Sci. 2022, 13, 4828–4837. [Google Scholar] [CrossRef] [PubMed]
- Berezin, M.Y.; Achilefu, S. Fluorescence Lifetime Measurements and Biological Imaging. Chem. Rev. 2010, 110, 2641–2684. [Google Scholar] [CrossRef] [PubMed]
- Shang, Y.; Li, W.; Ma, Y.; Li, B.; Xu, Q.; Du, Y.; Peng, Y.; Wang, Y.; Zhu, Y. Rapid Hole Generation via D–A Structure-Dependent Built-In Electric Field of Deacetylated Chitin-PDI for Efficient Photocatalytic Oxidation. Adv. Funct. Mater. 2024, 2406533. [Google Scholar] [CrossRef]
- Wang, Z.; Yang, X.; Yang, T.; Zhao, Y.; Wang, F.; Chen, Y.; Zeng, J.H.; Yan, C.; Huang, F.; Jiang, J.-X. Dibenzothiophene Dioxide Based Conjugated Microporous Polymers for Visible-Light-Driven Hydrogen Production. ACS Catal. 2018, 8, 8590–8596. [Google Scholar] [CrossRef]
- Zhao, Y.; Ma, W.; Xu, Y.; Zhang, C.; Wang, Q.; Yang, T.; Gao, X.; Wang, F.; Yan, C.; Jiang, J.-X. Effect of Linking Pattern of Dibenzothiophene-S,S-dioxide-Containing Conjugated Microporous Polymers on the Photocatalytic Performance. Macromolecules 2018, 51, 9502–9508. [Google Scholar] [CrossRef]
- Sprick, R.S.; Bai, Y.; Guilbert, A.A.Y.; Zbiri, M.; Aitchison, C.M.; Wilbraham, L.; Yan, Y.; Woods, D.J.; Zwijnenburg, M.A.; Cooper, A.I. Photocatalytic Hydrogen Evolution from Water Using Fluorene and Dibenzothiophene Sulfone-Conjugated Microporous and Linear Polymers. Chem. Mater. 2019, 31, 305–313. [Google Scholar] [CrossRef]
- Meier, C.B.; Clowes, R.; Berardo, E.; Jelfs, K.E.; Zwijnenburg, M.A.; Sprick, R.S.; Cooper, A.I. Structurally Diverse Covalent Triazine-Based Framework Materials for Photocatalytic Hydrogen Evolution from Water. Chem. Mater. 2019, 31, 8830–8838. [Google Scholar] [CrossRef]
- Chen, W.; Wang, L.; Mo, D.; He, F.; Wen, Z.; Wu, X.; Xu, H.; Chen, L. Modulating Benzothiadiazole-Based Covalent Organic Frameworks via Halogenation for Enhanced Photocatalytic Water Splitting. Angew. Chem. Int. Ed. 2020, 59, 16902–16909. [Google Scholar] [CrossRef]
- Aitchison, M.; Sprick, R.S.; Cooper, A.I. Emulsion polymerization derived organic photocatalysts for improved light-driven hydrogen evolution. J. Mater. Chem. A 2019, 7, 2490–2496. [Google Scholar] [CrossRef]
- Shu, C.; Han, C.; Yang, X.; Zhang, C.; Chen, Y.; Ren, S.; Wang, F.; Huang, F.; Jiang, J.-X. Boosting the Photocatalytic Hydrogen Evolution Activity for D–π–A Conjugated Microporous Polymers by Statistical Copolymerization. Adv. Mater. 2021, 33, 2008498. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.-H.; Wang, X.-P.; Xiao, J.; Wang, S.-Y.; Huang, D.-K.; Ding, X.; Xiang, Y.-G.; Chen, H. Synthesis of 1,4-diethynylbenzene-based conjugated polymer photocatalysts and their enhanced visible/near-infrared-light-driven hydrogen production activity. J. Catal. 2017, 350, 64–71. [Google Scholar] [CrossRef]
- Dai, C.; Xu, S.; Liu, W.; Gong, X.; Panahandeh-Fard, M.; Liu, Z.; Zhang, D.; Xue, C.; Loh, K.P.; Liu, B. Dibenzothiophene-S,S-Dioxide-Based Conjugated Polymers: Highly Efficient Photocatalyts for Hydrogen Production from Water under Visible Light. Small 2018, 14, 1801839. [Google Scholar] [CrossRef] [PubMed]
- Sachs, M.; Sprick, R.S.; Pearce, D.; Hillman, S.A.J.; Monti, A.; Guilbert, A.A.Y.; Brownbill, N.J.; Dimitrov, S.; Shi, X.; Blanc, F.; et al. Understanding structure-activity relationships in linear polymer photocatalysts for hydrogen evolution. Nat. Commun. 2018, 9, 4968. [Google Scholar] [CrossRef] [PubMed]
- Sprick, R.S.; Jiang, J.X.; Bonillo, B.; Ren, S.; Ratvijitvech, T.; Guiglion, P.; Zwijnenburg, M.A.; Adams, D.J.; Cooper, A.I. Tunable organic photocatalysts for visible-light-driven hydrogen evolution. J. Am. Chem. Soc. 2015, 137, 3265–3270. [Google Scholar] [CrossRef] [PubMed]
- Honda, Y.; Watanabe, M.; Hagiwara, H.; Ida, S.; Ishihara, T. Inorganic/whole-cell biohybrid photocatalyst for highly efficient hydrogen production from water. Appl. Catal. B Environ. 2017, 210, 400–406. [Google Scholar] [CrossRef]
- Peng, Q.-X.; Xue, D.; Zhan, S.-Z.; Ni, C.-L. Visible-light-driven photocatalytic system based on a nickel complex over CdS materials for hydrogen production from water. Appl. Catal. B Environ. 2017, 219, 353–361. [Google Scholar] [CrossRef]
- Wang, X.; Chen, B.; Dong, W.; Zhang, X.; Li, Z.; Xiang, Y.; Chen, H. Hydrophilicity-Controlled Conjugated Microporous Polymers for Enhanced Visible-Light-Driven Photocatalytic H2 Evolution. Macromol. Rapid Commun. 2019, 40, 1800494. [Google Scholar] [CrossRef]
- Wang, Z.; Mao, N.; Zhao, Y.; Yang, T.; Wang, F.; Jiang, J.-X. Building an electron push–pull system of linear conjugated polymers for improving photocatalytic hydrogen evolution efficiency. Polym. Bull. 2018, 76, 3195–3206. [Google Scholar] [CrossRef]
- Liu, G.; Wang, T.; Zhang, H.; Meng, X.; Hao, D.; Chang, K.; Li, P.; Kako, T.; Ye, J. Nature-Inspired Environmental “Phosphorylation” Boosts Photocatalytic H2 Production over Carbon Nitride Nanosheets under Visible-Light Irradiation. Angew. Chem. Int. Ed. 2015, 54, 13561–13565. [Google Scholar] [CrossRef] [PubMed]


| Sample | λonset (nm) | λem (nm) | Eg (eV) | BET Specific Surface Area (m2 g−1) | LUMO (eV) | HOMO (eV) |
|---|---|---|---|---|---|---|
| Spso-1 | 479 | 511 | 2.59 | 54.3 | −0.60 | 1.99 |
| Spso-2 | 480 | 507 | 2.58 | 177.3 | −0.60 | 1.98 |
| Spso-3 | 495 | 505 | 2.51 | 131.8 | −0.64 | 1.87 |
| Spso-4 | 482 | 503 | 2.57 | 267.4 | −0.62 | 1.95 |
| Spso-5 | 474 | 492 | 2.62 | 384.8 | −0.63 | 1.99 |
| Spso-6 | 469 | 485 | 2.64 | 681.7 | −0.59 | 2.05 |
| Sample | τ1 [ns] (Rel.%) | τ2 [ns] (Rel.%) | τ3 [ns] (Rel.%) | τ [ns] | χ2 |
|---|---|---|---|---|---|
| Spso-1 | 1.12 (44.93) | 3.34 (48.89) | 13.30 (6.18) | 2.95 | 1.155 |
| Spso-2 | 0.61 (33.38) | 2.31 (54.79) | 8.05 (11.83) | 2.42 | 1.070 |
| Spso-3 | 0.91 (53.22) | 2.88 (39.30) | 11.24 (7.39) | 2.45 | 1.139 |
| Spso-4 | 0.39 (79.74) | 1.71 (16.66) | 9.18 (3.61) | 0.93 | 1.140 |
| Spso-5 | 0.59 (61.36) | 2.18 (30.68) | 10.32 (7.96) | 1.85 | 1.287 |
| Spso-6 | 0.75 (55.59) | 1.88 (42.73) | 6.62 (1.68) | 1.33 | 1.130 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, H.; Sun, P.; Xu, H.; Xiao, X.; Kong, Z.; Song, S.; Li, W.; Liu, L.; Wang, J.; Pan, X. Spirobifluorene-Based D-A Type Conjugated Polymer Photocatalysts for Water Splitting. Catalysts 2024, 14, 717. https://doi.org/10.3390/catal14100717
Zhao H, Sun P, Xu H, Xiao X, Kong Z, Song S, Li W, Liu L, Wang J, Pan X. Spirobifluorene-Based D-A Type Conjugated Polymer Photocatalysts for Water Splitting. Catalysts. 2024; 14(10):717. https://doi.org/10.3390/catal14100717
Chicago/Turabian StyleZhao, Hao, Pengyao Sun, Hui Xu, Xinyi Xiao, Zhiyuan Kong, Shige Song, Weihao Li, Luzun Liu, Jiadong Wang, and Xiaobo Pan. 2024. "Spirobifluorene-Based D-A Type Conjugated Polymer Photocatalysts for Water Splitting" Catalysts 14, no. 10: 717. https://doi.org/10.3390/catal14100717
APA StyleZhao, H., Sun, P., Xu, H., Xiao, X., Kong, Z., Song, S., Li, W., Liu, L., Wang, J., & Pan, X. (2024). Spirobifluorene-Based D-A Type Conjugated Polymer Photocatalysts for Water Splitting. Catalysts, 14(10), 717. https://doi.org/10.3390/catal14100717







