Lewis Acid-Base Site-Assisted In Situ Transesterification Catalysis to Produce Biodiesel
Abstract
1. Introduction
2. Transesterification of Biodiesel
2.1. Fatty Acid Composition and Properties of Biodiesel
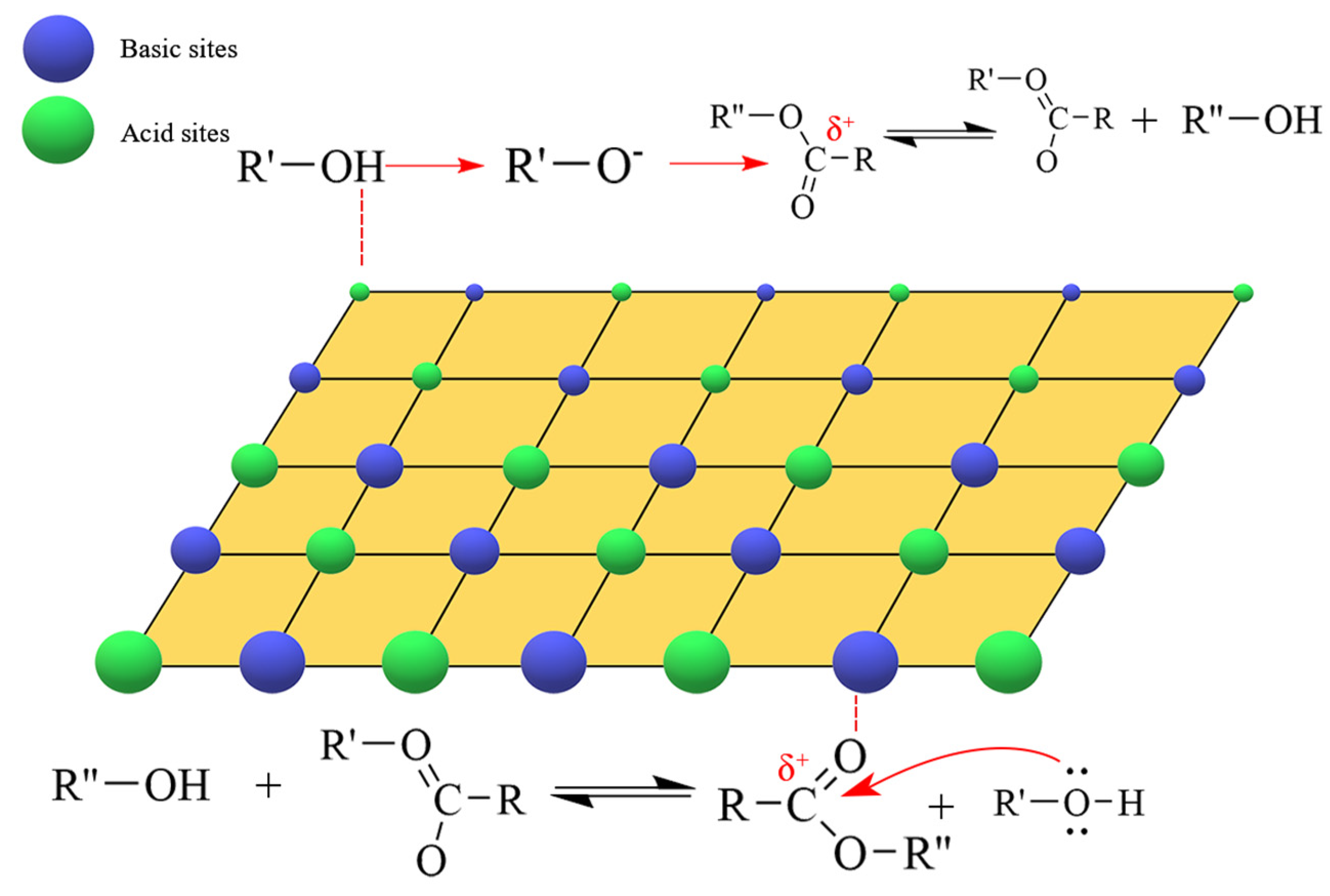
2.2. Research Status of Acid-Base Catalyzed Transesterification
3. Lewis Acidic Catalyst
3.1. Research Status of Lewis Acidic Catalyst
3.2. Heterogeneous Lewis Acidic Catalysis
3.3. Lewis Acidic Ionic Liquids and Deep Eutectic Solvents
3.4. The Research Gaps and Directions of Lewis Acidic Catalysts
4. Lewis Basic Catalyst
4.1. Research Status of Lewis Basic Catalysis
4.2. A Solid Alkali Metal Catalyst
4.3. Alkaline Earth Metal Oxide Catalyst
4.4. Lewis Alkaline Sites Loaded on Hydrotalcite
4.5. Alkaline Metal-Based Nanomaterials
4.6. The Research Gaps and Directions of Lewis Base Catalysts
5. Lewis Acid-Base Bifunctional Catalyst
5.1. Metal Oxide Catalysts Containing Lewis Acid-Base Sites
5.2. Heteropoly Acid Catalysts with Acid-Base Bifunctional Groups
5.3. Sulfated Solid Acid-Base Amphoteric Catalyst
5.4. Magnetic Functionalized Double Lewis Acid-Base Sites
5.5. The Research Gaps and Directions of Lewis Acid-Base Catalysts
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Tien Thanh, N.; Mostapha, M.; Lam, M.K.; Ishak, S.; Kanna Dasan, Y.; Lim, J.W.; Tan, I.S.; Lau, S.Y.; Chin, B.L.F.; Hadibarata, T. Fundamental understanding of in-situ transesterification of microalgae biomass to biodiesel: A critical review. Energy Convers. Manag. 2022, 270, 116212. [Google Scholar] [CrossRef]
- Park, J.; Kim, B.; Lee, J.W. In-situ transesterification of wet spent coffee grounds for sustainable biodiesel production. Bioresour. Technol. 2016, 221, 55–60. [Google Scholar] [CrossRef]
- Kadir, W.N.A.; Lam, M.K.; Uemura, Y.; Lim, J.W.; Lee, K.T. Harvesting and pre-treatment of microalgae cultivated in wastewater for biodiesel production: A review. Energy Convers. Manag. 2018, 171, 1416–1429. [Google Scholar] [CrossRef]
- Tuntiwiwattanapun, N.; Tongcumpou, C. Sequential extraction and reactive extraction processing of spent coffee grounds: An alternative approach for pretreatment of biodiesel feedstocks and biodiesel production. Ind. Crops Prod. 2018, 117, 359–365. [Google Scholar] [CrossRef]
- Mat Husin, M.A.; Mohd Yasin, N.H.; Takriff, M.S.; Jamar, N.H. A review on pretreatment methods for lipid extraction from microalgae biomass. Prep. Biochem. Biotechnol. 2024, 54, 159–174. [Google Scholar] [CrossRef]
- Bauer, G.; Lima, S.; Chenevard, J.; Sugnaux, M.; Fischer, F. Biodiesel via in Situ Wet Microalgae Biotransformation: Zwitter-Type Ionic Liquid Supported Extraction and Transesterification. ACS Sustain. Chem. Eng. 2017, 5, 1931–1937. [Google Scholar] [CrossRef]
- Chiang, C.-L.; Lin, K.-S.; Shu, C.-W.; Wu, J.C.-S.; Wu, K.C.-W.; Huang, Y.-T. Enhancement of biodiesel production via sequential esterification/tran sesterification over solid superacidic and superbasic catalysts. Catal. Today 2020, 348, 257–269. [Google Scholar] [CrossRef]
- Jin, B.; Duan, P.; Xu, Y.; Wang, B.; Wang, F.; Zhang, L. Lewis acid-catalyzed in situ transesterification/esterification of microalgae in supercritical ethanol. Bioresour. Technol. 2014, 162, 341–349. [Google Scholar] [CrossRef]
- Encinar, J.M.; Pardal, A.; Sánchez, N.; Nogales, S. Biodiesel by Transesterification of Rapeseed Oil Using Ultrasound: A Kinetic Study of Base-Catalysed Reactions. Energies 2018, 11, 2229. [Google Scholar] [CrossRef]
- Velasquez-Orta, S.B.; Lee, J.G.M.; Harvey, A. Alkaline in situ transesterification of Chlorella vulgaris. Fuel 2012, 94, 544–550. [Google Scholar] [CrossRef]
- Lam, M.K.; Lee, K.T.; Mohamed, A.R. Homogeneous, heterogeneous and enzymatic catalysis for transesterification of high free fatty acid oil (waste cooking oil) to biodiesel: A review. Biotechnol. Adv. 2010, 28, 500–518. [Google Scholar] [CrossRef] [PubMed]
- EN 14214; Liquid Petroleum Products. Fatty Acid Methyl Esters (FAME) for Use in Diesel Engines and Heating Applications. Requirements and Test Methods. The British Standards Institution: London, UK, 2013. [CrossRef]
- Fadhil, A.B.; Al-Tikrity, E.T.B.; Khalaf, A.M. Transesterification of non-edible oils over potassium acetate impregnated CaO solid base catalyst. Fuel 2018, 234, 81–93. [Google Scholar] [CrossRef]
- Ott, L.S.; Riddell, M.M.; O’Neill, E.L.; Carini, G.S. From orchids to biodiesel: Coco coir as an effective drywash material for biodiesel fuel. Fuel Process. Technol. 2018, 176, 1–6. [Google Scholar] [CrossRef]
- Veljković, V.B.; Stamenković, O.S.; Tasić, M.B. The wastewater treatment in the biodiesel production with alkali-catalyzed transesterification. Renew. Sustain. Energy Rev. 2014, 32, 40–60. [Google Scholar] [CrossRef]
- Yang, X.-X.; Wang, Y.-T.; Yang, Y.-T.; Feng, E.-Z.; Luo, J.; Zhang, F.; Yang, W.-J.; Bao, G.-R. Catalytic transesterification to biodiesel at room temperature over several solid bases. Energy Convers. Manag. 2018, 164, 112–121. [Google Scholar] [CrossRef]
- Denmark, S.E.; Beutner, G.L. Lewis base catalysis in organic synthesis. Angew. Chem. Int. Ed. Engl. 2008, 47, 1560–1638. [Google Scholar] [CrossRef]
- Zapelini, I.W.; Silva, L.L.; Cardoso, D. The role of alcohols on the accessibility of basic ≡SiO− sites in hybrid silicas for catalytic transesterification. Braz. J. Chem. Eng. 2023, 41, 409–416. [Google Scholar] [CrossRef]
- She, Q.; Qiu, M.; Li, K.; Liu, J.; Zhou, C. Acidic and basic sites on the surface of sodium montmorillonite active for catalytic transesterification of glycerol to glycerol carbonate. Appl. Clay Sci. 2023, 238, 106916. [Google Scholar] [CrossRef]
- Eid, J.G.; de Paula, G.M.; Cardoso, D. Heterogeneous transesterification catalyzed by silicas containing basic sites. Mol. Catal. 2022, 531, 112631. [Google Scholar] [CrossRef]
- Abdelgaid, M.; Mpourmpakis, G. Structure–Activity Relationships in Lewis Acid–Base Heterogeneous Catalysis. ACS Catal. 2022, 12, 4268–4289. [Google Scholar] [CrossRef]
- Xia, Y.; Chen, L.; Liang, R.; Liu, X.; Yan, G.; Zhu, S.; Wang, X. Bifunctional Lewis acid-base nanocatalysts with dual active sites for strengthened coupling of alcohol conversion and H2 evolution. Int. J. Hydrogen Energy 2024, 51, 1598–1607. [Google Scholar] [CrossRef]
- Munyentwali, A.; Li, H.; Yang, Q. Review of advances in bifunctional solid acid/base catalysts for sustainable biodiesel production. Appl. Catal. A Gen. 2022, 633, 118525. [Google Scholar] [CrossRef]
- Rezki, B.; Essamlali, Y.; Amadine, O.; Sair, S.; Aadil, M.; Len, C.; Zahouily, M. A comprehensive review on apatite-derived catalysts for sustainable biodiesel production: Classification, features and challenges. J. Environ. Chem. Eng. 2024, 12, 111913. [Google Scholar] [CrossRef]
- Sneha, R.; Madhumitha, G. Application of organo-tin based complexes in transesterification reaction for biodiesel production—A review. J. Organomet. Chem. 2023, 1001, 122870. [Google Scholar] [CrossRef]
- Jaroszewska, K.; Nowicki, J.; Nosal-Kovalenko, H.; Grzechowiak, J.; Pstrowska, K.; Łużny, R.; Lewandowski, M.; Kaczmarczyk, J.; Witek-Krowiak, A.; Moustakas, K.; et al. Selected acid and basic functionalized ordered mesoporous materials as solid catalysts for transesterification of canola oil: A comparative study. Fuel 2022, 325, 124902. [Google Scholar] [CrossRef]
- Ma, X.; Liu, F.; Helian, Y.; Li, C.; Wu, Z.; Li, H.; Chu, H.; Wang, Y.; Wang, Y.; Lu, W.; et al. Current application of MOFs based heterogeneous catalysts in catalyzing transesterification/esterification for biodiesel production: A review. Energy Convers. Manag. 2021, 229, 113760. [Google Scholar] [CrossRef]
- Salam, K.A.; Velasquez-Orta, S.B.; Harvey, A.P. A sustainable integrated in situ transesterification of microalgae for biodiesel production and associated co-product-a review. Renew. Sustain. Energy Rev. 2016, 65, 1179–1198. [Google Scholar] [CrossRef]
- Zhang, Y.; Sun, S. A review on biodiesel production using basic ionic liquids as catalysts. Ind. Crops Prod. 2023, 202, 117099. [Google Scholar] [CrossRef]
- Troter, D.Z.; Todorović, Z.B.; Đokić-Stojanović, D.R.; Stamenković, O.S.; Veljković, V.B. Application of ionic liquids and deep eutectic solvents in biodiesel production: A review. Renew. Sustain. Energy Rev. 2016, 61, 473–500. [Google Scholar] [CrossRef]
- Kedia, A.G.; Dutta, A.; Kumar, P. Dimethyl Carbonate as a Cost-Effective Substitute of Methanol for Biodiesel Production via Transesterification of Nonedible Oil. BioEnergy Res. 2023, 16, 1134–1142. [Google Scholar] [CrossRef]
- Vicente, G.; Carrero, A.; Rodríguez, R.; del Peso, G.L. Heterogeneous-catalysed direct transformation of microalga biomass into Biodiesel-Grade FAMEs. Fuel 2017, 200, 590–598. [Google Scholar] [CrossRef]
- Bouaid, A.; Vázquez, R.; Martinez, M.; Aracil, J. Effect of free fatty acids contents on biodiesel quality. Pilot plant studies. Fuel 2016, 174, 54–62. [Google Scholar] [CrossRef]
- Supeno, M.; Sihotang, J.P.; Panjaitan, Y.V.; Damanik, D.S.Y.; Tarigan, J.B.; Sitepu, E.K. Room temperature esterification of high-free fatty acid feedstock into biodiesel. RSC Adv. 2023, 13, 33107–33113. [Google Scholar] [CrossRef] [PubMed]
- Manikandan, G.; Kanna, P.R.; Taler, D.; Sobota, T. Review of Waste Cooking Oil (WCO) as a Feedstock for Biofuel—Indian Perspective. Energies 2023, 16, 1739. [Google Scholar] [CrossRef]
- Yu, H.; Niu, S.; Lu, C.; Li, J.; Yang, Y. Preparation and esterification performance of sulfonated coal-based heterogeneous acid catalyst for methyl oleate production. Energy Convers. Manag. 2016, 126, 488–496. [Google Scholar] [CrossRef]
- Marchetti, J.M.; Miguel, V.U.; Errazu, A.F. Heterogeneous esterification of oil with high amount of free fatty acids. Fuel 2007, 86, 906–910. [Google Scholar] [CrossRef]
- Ganesan, S.; Nadarajah, S.; Chee, X.Y.; Khairuddean, M.; Teh, G.B. Esterification of free fatty acids using ammonium ferric sulphate-calcium silicate as a heterogeneous catalyst. Renew. Energy 2020, 153, 1406–1417. [Google Scholar] [CrossRef]
- Bi, Z.; He, B.B. Phospholipid transesterification in sub-/super-critical methanol with the presence of free fatty acids. Fuel 2016, 166, 461–466. [Google Scholar] [CrossRef]
- Law, S.Q.K.; Halim, R.; Scales, P.J.; Martin, G.J.O. Conversion and recovery of saponifiable lipids from microalgae using a nonpolar solvent via lipase-assisted extraction. Bioresour. Technol. 2018, 260, 338–347. [Google Scholar] [CrossRef]
- Ahmad, A.F.; Zulkurnain, N.; Rosid, S.J.M.; Azid, A.; Endut, A.; Toemen, S.; Ismail, S.; Abdullah, W.N.W.; Aziz, S.M.; Yusoff, N.M.; et al. Catalytic Transesterification of Coconut Oil in Biodiesel Production: A Review. Catal. Surv. Asia 2022, 26, 129–143. [Google Scholar] [CrossRef]
- Huang, J.; Lu, Y.; Jin, J.; Song, Z.; Tang, J. Chemical transesterification of flaxseed oil and medium-chain triacylglycerols: MLCT yield, DAG content, physicochemical properties, minor compounds and oxidation stability. Int. J. Food Sci. Technol. 2021, 56, 5160–5167. [Google Scholar] [CrossRef]
- Liu, J.; Zheng, Y.; Zhang, R.; Yu, Y.; Wang, F.; Deng, L.; Wu, K. A novel synthesis method of medium- and long-chain triglyceride lipids from rubber seed oil catalyzed by enzymatic interesterification and its metabolism mechanism. Food Funct. 2024, 15, 9903–9915. [Google Scholar] [CrossRef] [PubMed]
- Encinar, J.M.; González, J.F.; Martínez, G.; Nogales-Delgado, S. Transesterification of Soybean Oil through Different Homogeneous Catalysts: Kinetic Study. Catalysts 2022, 12, 146. [Google Scholar] [CrossRef]
- Johnson, M.B.; Wen, Z. Production of Biodiesel Fuel from the Microalga Schizochytrium limacinum by Direct Transesterification of Algal Biomass. Energy Fuels 2009, 23, 5179–5183. [Google Scholar] [CrossRef]
- Varyambath, A.; Kim, M.-R.; Kim, I. Sulfonic acid-functionalized organic knitted porous polyaromatic microspheres as heterogeneous catalysts for biodiesel production. New J. Chem. 2018, 42, 12745–12753. [Google Scholar] [CrossRef]
- Tiwari, P.; Garg, S. Study of reversible kinetic models for alkali-catalyzed Jatropha curcas transesterification. Biomass Convers. Biorefinery 2016, 6, 61–70. [Google Scholar] [CrossRef]
- Liu, Y.; Lotero, E.; Goodwin, J.G.; Lu, C. Transesterification of triacetin using solid Brønsted bases. J. Catal. 2007, 246, 428–433. [Google Scholar] [CrossRef]
- Georgogianni, K.G.; Katsoulidis, A.K.; Pomonis, P.J.; Manos, G.; Kontominas, M.G. Transesterification of rapeseed oil for the production of biodiesel using homogeneous and heterogeneous catalysis. Fuel Process. Technol. 2009, 90, 1016–1022. [Google Scholar] [CrossRef]
- Stojković, I.J.; Stamenković, O.S.; Povrenović, D.S.; Veljković, V.B. Purification technologies for crude biodiesel obtained by alkali-catalyzed transesterification. Renew. Sustain. Energy Rev. 2014, 32, 1–15. [Google Scholar] [CrossRef]
- Liu, X.; Piao, X.; Wang, Y.; Zhu, S.; He, H. Calcium methoxide as a solid base catalyst for the transesterification of soybean oil to biodiesel with methanol. Fuel 2008, 87, 1076–1082. [Google Scholar] [CrossRef]
- Zhao, Z.; Wu, W.; Jia, L.; Guo, X. Sodium phosphate solid base catalysts for production of novel biodiesel by transesterification reaction. RSC Adv. 2023, 13, 26700–26708. [Google Scholar] [CrossRef] [PubMed]
- Kouider Elouahed, S.; Asikin-Mijan, N.; Alsultan, G.A.; Kaddour, O.; Yusop, M.R.; Mimoun, H.; Samidin, S.; Mansir, N.; Taufiq-Yap, Y.H. Optimization of the activity of Mo7-Zn3/CaO catalyst in the transesterification of waste cooking oil into sustainable biodiesel via response surface methodology. Energy Convers. Manag. 2024, 303, 118185. [Google Scholar] [CrossRef]
- Xie, W.; Zhao, L. Production of biodiesel by transesterification of soybean oil using calcium supported tin oxides as heterogeneous catalysts. Energy Convers. Manag. 2013, 76, 55–62. [Google Scholar] [CrossRef]
- Shahidul Islam, M.; Robin Hart, C.; Casadonte, D. Ultrasound-assisted solid Lewis acid-catalyzed transesterification of Lesquerella fendleri oil for biodiesel synthesis. Ultrason. Sonochem. 2022, 88, 106082. [Google Scholar] [CrossRef]
- Casas, A.; Ramos, M.J.; Rodríguez, J.F.; Pérez, Á. Tin compounds as Lewis acid catalysts for esterification and transesterification of acid vegetable oils. Fuel Process. Technol. 2013, 106, 321–325. [Google Scholar] [CrossRef]
- Guan, Q.; Shang, H.; Liu, J.; Gu, J.; Li, B.; Miao, R.; Chen, Q.; Ning, P. Biodiesel from transesterification at low temperature by AlCl3 catalysis in ethanol and carbon dioxide as cosolvent: Process, mechanism and application. Appl. Energy 2016, 164, 380–386. [Google Scholar] [CrossRef]
- Reinoso, D.M.; Damiani, D.E.; Tonetto, G.M. Zinc carboxylic salts used as catalyst in the biodiesel synthesis by esterification and transesterification: Study of the stability in the reaction medium. Appl. Catal. A Gen. 2012, 449, 88–95. [Google Scholar] [CrossRef]
- Interrante, L.; Bensaid, S.; Galletti, C.; Pirone, R.; Schiavo, B.; Scialdone, O.; Galia, A. Interesterification of rapeseed oil catalysed by a low surface area tin (II) oxide heterogeneous catalyst. Fuel Process. Technol. 2018, 177, 336–344. [Google Scholar] [CrossRef]
- Sankaranarayanan, T.M.; Pandurangan, A.; Banu, M.; Sivasanker, S. Transesterification of sunflower oil over MoO3 supported on alumina. Appl. Catal. A Gen. 2011, 409–410, 239–247. [Google Scholar] [CrossRef]
- Aziz, M.A.A.; Puad, K.; Triwahyono, S.; Jalil, A.A.; Khayoon, M.S.; Atabani, A.E.; Ramli, Z.; Majid, Z.A.; Prasetyoko, D.; Hartanto, D. Transesterification of croton megalocarpus oil to biodiesel over WO3 supported on silica mesoporous-macroparticles catalyst. Chem. Eng. J. 2017, 316, 882–892. [Google Scholar] [CrossRef]
- Shu, Q.; Tang, G.; Liu, F.; Zou, W.; He, J.; Zhang, C.; Zou, L. Study on the preparation, characterization of a novel solid Lewis acid Al3+-SO42−/MWCNTs catalyst and its catalytic performance for the synthesis of biodiesel via esterification reaction of oleic acid and methanol. Fuel 2017, 209, 290–298. [Google Scholar] [CrossRef]
- Hasan, Z.; Jun, J.W.; Jhung, S.H. Sulfonic acid-functionalized MIL-101(Cr): An efficient catalyst for esterification of oleic acid and vapor-phase dehydration of butanol. Chem. Eng. J. 2015, 278, 265–271. [Google Scholar] [CrossRef]
- Panchal, B.; Chang, T.; Qin, S.; Sun, Y.; Wang, J.; Bian, K. Optimization of soybean oil transesterification using an ionic liquid and methanol for biodiesel synthesis. Energy Rep. 2020, 6, 20–27. [Google Scholar] [CrossRef]
- Liu, W.; Wang, F. p-Toluenesulfonic Acid-based Deep Eutectic Solvent as Transesterification Catalyst for Biodiesel Production. J. Oleo Sci. 2018, 67, 1163–1169. [Google Scholar] [CrossRef] [PubMed]
- Soriano, N.U.; Venditti, R.; Argyropoulos, D.S. Biodiesel synthesis via homogeneous Lewis acid-catalyzed transesterification. Fuel 2009, 88, 560–565. [Google Scholar] [CrossRef]
- Chin, S.Y.; Ahmad, A.L.; Mohamed, A.R.; Bhatia, S. Characterization and activity of zinc acetate complex supported over functionalized silica as a catalyst for the production of isopropyl palmitate. Appl. Catal. A Gen. 2006, 297, 8–17. [Google Scholar] [CrossRef]
- Di Serio, M.; Tesser, R.; Dimiccoli, M.; Cammarota, F.; Nastasi, M.; Santacesaria, E. Synthesis of biodiesel via homogeneous Lewis acid catalyst. J. Mol. Catal. A Chem. 2005, 239, 111–115. [Google Scholar] [CrossRef]
- Kolet, M.; Atrash, M.; Molina, K.; Zerbib, D.; Albo, Y.; Nakonechny, F.; Nisnevitch, M. Sol–Gel Entrapped Lewis Acids as Catalysts for Biodiesel Production. Molecules 2020, 25, 5936. [Google Scholar] [CrossRef]
- Mello, V.M.; Pousa, G.P.A.G.; Pereira, M.S.C.; Dias, I.M.; Suarez, P.A.Z. Metal oxides as heterogeneous catalysts for esterification of fatty acids obtained from soybean oil. Fuel Process. Technol. 2011, 92, 53–57. [Google Scholar] [CrossRef]
- Raia, R.Z.; da Silva, L.S.; Marcucci, S.M.P.; Arroyo, P.A. Biodiesel production from Jatropha curcas L. oil by simultaneous esterification and transesterification using sulphated zirconia. Catal. Today 2017, 289, 105–114. [Google Scholar] [CrossRef]
- Du, Y.; Liu, S.; Ji, Y.; Zhang, Y.; Wei, S.; Liu, F.; Xiao, F.-S. Synthesis of Sulfated Silica-Doped Tin Oxides and Their High Activities in Transesterification. Catal. Lett. 2008, 124, 133–138. [Google Scholar] [CrossRef]
- Li, D.; Feng, W.; Chen, C.; Chen, S.; Fan, G.; Liao, S.; Wu, G.; Wang, Z. Transesterification of Litsea cubeba kernel oil to biodiesel over zinc supported on zirconia heterogeneous catalysts. Renew. Energy 2021, 177, 13–22. [Google Scholar] [CrossRef]
- Hechelski, M.; Ghinet, A.; Louvel, B.; Dufrénoy, P.; Rigo, B.; Daïch, A.; Waterlot, C. From Conventional Lewis Acids to Heterogeneous Montmorillonite K10: Eco-Friendly Plant-Based Catalysts Used as Green Lewis Acids. ChemSusChem 2018, 11, 1249–1277. [Google Scholar] [CrossRef] [PubMed]
- Shestakova, P.; Popova, M.; Szegedi, Á.; Lazarova, H.; Nga Luong, T.K.; Trendafilova, I.; Mihály, J.; Parac-Vogt, T.N. Hybrid catalyst with combined Lewis and Brønsted acidity based on ZrIV substituted polyoxometalate grafted on mesoporous MCM-41 silica for esterification of renewable levulinic acid. Microporous Mesoporous Mater. 2021, 323, 111203. [Google Scholar] [CrossRef]
- Duan, Y.; Ding, R.; Shi, Y.; Fang, X.; Hu, H.; Yang, M.; Wu, Y. Synthesis of Renewable Diesel Range Alkanes by Hydrodeoxygenation of Palmitic Acid over 5% Ni/CNTs under Mild Conditions. Catalysts 2017, 7, 81. [Google Scholar] [CrossRef]
- Shu, Q.; Zou, W.; He, J.; Lesmana, H.; Zhang, C.; Zou, L.; Wang, Y. Preparation of the F-SO42-/MWCNTs catalyst and kinetic studies of the biodiesel production via esterification reaction of oleic acid and methanol. Renew. Energy 2019, 135, 836–845. [Google Scholar] [CrossRef]
- Ibrahim, M.L.; Nik Abdul Khalil, N.N.A.; Islam, A.; Rashid, U.; Ibrahim, S.F.; Sinar Mashuri, S.I.; Taufiq-Yap, Y.H. Preparation of Na2O supported CNTs nanocatalyst for efficient biodiesel production from waste-oil. Energy Convers. Manag. 2020, 205, 112445. [Google Scholar] [CrossRef]
- Guo, J.; Zheng, Y.; Li, Y.; Wen, Z.; Shen, X.; Zhao, Y. Application of functional metal anionic Lewis acid ionic liquids in the alkylation of chlorobenzene/SOCl2. RSC Adv. 2023, 13, 11635–11641. [Google Scholar] [CrossRef]
- Ullah, Z.; Khan, A.S.; Muhammad, N.; Ullah, R.; Alqahtani, A.S.; Shah, S.N.; Ghanem, O.B.; Bustam, M.A.; Man, Z. A review on ionic liquids as perspective catalysts in transesterification of different feedstock oil into biodiesel. J. Mol. Liq. 2018, 266, 673–686. [Google Scholar] [CrossRef]
- Han, X.; Yan, W.; Hung, C.-T.; He, Y.; Wu, P.-H.; Liu, L.-L.; Huang, S.-J.; Liu, S.-B. Transesterification of soybean oil to biodiesel by tin-based Brønsted-Lewis acidic ionic liquid catalysts. Korean J. Chem. Eng. 2016, 33, 2063–2072. [Google Scholar] [CrossRef]
- Xie, W.; Wan, F. Immobilization of polyoxometalate-based sulfonated ionic liquids on UiO-66-2COOH metal-organic frameworks for biodiesel production via one-pot transesterification-esterification of acidic vegetable oils. Chem. Eng. J. 2019, 365, 40–50. [Google Scholar] [CrossRef]
- Yu, Y.; Yu, F.; Li, L.; Yuan, B.; Xie, C.; Yu, S. Lewis acidic deep eutectic solvents as catalysts for rosin polymerization. New J. Chem. 2023, 47, 20144–20150. [Google Scholar] [CrossRef]
- Bai, Y.; Zhang, X.-F.; Wang, Z.; Zheng, T.; Yao, J. Deep eutectic solvent with bifunctional Brønsted-Lewis acids for highly efficient lignocellulose fractionation. Bioresour. Technol. 2022, 347, 126723. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Sun, C.; Hu, Y.; Xia, C.; Sun, F.; Zhang, Z. Reaction characteristics of metal-salt coordinated deep eutectic solvents during lignocellulosic pretreatment. J. Environ. Chem. Eng. 2023, 11, 109531. [Google Scholar] [CrossRef]
- Potchamyou Ngatcha, A.D.; Zhao, A.; Zhang, S.; Xiong, W.; Sarker, M.; Xu, J.; Alam, M.A. Determination of active sites on the synthesis of novel Lewis acidic deep eutectic solvent catalysts and kinetic studies in microalgal biodiesel production. RSC Adv. 2023, 13, 10110–10122. [Google Scholar] [CrossRef]
- Alam, M.A.; Deng, L.; Ngatcha, A.D.P.; Fouegue, A.D.T.; Wu, J.; Zhang, S.; Zhao, A.; Xiong, W.; Xu, J. Biodiesel production from microalgal biomass by Lewis acidic deep eutectic solvent catalysed direct transesterification. Ind. Crops Prod. 2023, 206, 117725. [Google Scholar] [CrossRef]
- Yang, Z.-J.; Peng, S.-Y.; Wang, H.; Zhang, Z.-Q.; Xu, Y.-P.; Sun, J.; Xu, Z.-N.; Guo, G.-C. Enhanced catalytic activity for CO esterification to dimethyl oxalate via increasing Lewis basic sites in Pd/MgAl-LDO catalyst. Catal. Commun. 2023, 184, 106781. [Google Scholar] [CrossRef]
- Zhang, S.; He, H.; Sun, F.; Zhao, N.; Du, J.; Pan, Q.; Zhu, G. A novel adenine-based zinc(II) metal-organic framework featuring the Lewis basic sites for heterogeneous catalysis. Inorg. Chem. Commun. 2017, 79, 55–59. [Google Scholar] [CrossRef]
- Liu, X.; Fan, W.; Zhang, M.; Li, G.; Liu, H.; Sun, D.; Zhao, L.; Zhu, H.; Guo, W. Enhancing light hydrocarbon storage and separation through introducing Lewis basic nitrogen sites within a carboxylate-decorated copper–organic framework. Mater. Chem. Front. 2018, 2, 1146–1154. [Google Scholar] [CrossRef]
- Naganawa, Y.; Abe, H.; Nishiyama, H. Design of bifunctional chiral phenanthroline ligand with Lewis basic site for palladium-catalyzed asymmetric allylic substitution. Chem. Commun. 2018, 54, 2674–2677. [Google Scholar] [CrossRef]
- Hassan, H.M.A.; Alhumaimess, M.S.; Kamel, M.M.; Alsohaimi, I.H.; Aljaddua, H.I.; Aldosari, O.F.; Algamdi, M.S.; Mohamed, R.M.K.; El-Aassar, M.R. Electrospinning NH2-MIL-101/PAN nanofiber mats: A promising catalyst with Lewis acidic and basic bifunctional sites for organic transformation reactions. Colloids Surf. A Physicochem. Eng. Asp. 2022, 642, 128659. [Google Scholar] [CrossRef]
- Zhao, G.; Liu, Y.; Pan, J.; Liu, C.; Hu, Y.; Gao, Z.; Zhuang, X. Flexible nanofibrous membranes of dual metallic metal–organic framework with enhanced Lewis basic sites and high loading mass for efficient CO2 capture. J. Colloid Interface Sci. 2023, 651, 200–210. [Google Scholar] [CrossRef]
- Machorro, J.J.; Lazaro, A.L.; Espejel-Ayala, F.; Coutiño-Gonzalez, E.; Chavarria-Hernandez, J.C.; Godínez, L.A.; Rodríguez-Valadez, F.J. The Roles of the Structure and Basic Sites of Sodium Titanates on Transesterification Reactions to Obtain Biodiesel. Catalysts 2019, 9, 989. [Google Scholar] [CrossRef]
- Cannilla, C.; Bonura, G.; Arena, F.; Rombi, E.; Frusteri, F. How surface and textural properties affect the behaviour of Mn-based catalysts during transesterification reaction to produce biodiesel. Catal. Today 2012, 195, 32–43. [Google Scholar] [CrossRef]
- Lingfeng, C.; Guomin, X.; Bo, X.; Guangyuan, T. Transesterification of Cottonseed Oil to Biodiesel by Using Heterogeneous Solid Basic Catalysts. Energy Fuels 2007, 21, 3740–3743. [Google Scholar] [CrossRef]
- Pampararo, G.; Debecker, D.P. Sodium Aluminate-Catalyzed Biodiesel Synthesis. ACS Sustain. Chem. Eng. 2023, 11, 10413–10421. [Google Scholar] [CrossRef]
- Singh, H.; Ali, A. Esterification as well as transesterification of waste oil using potassium imbued tungstophosphoric acid supported graphene oxide as heterogeneous catalyst: Optimization and kinetic modeling. Renew. Energy 2023, 207, 422–435. [Google Scholar] [CrossRef]
- Tavizón-Pozos, J.A.; Chavez-Esquivel, G.; Suárez-Toriello, V.A.; Santolalla-Vargas, C.E.; Luévano-Rivas, O.A.; Valdés-Martínez, O.U.; Talavera-López, A.; Rodriguez, J.A. State of Art of Alkaline Earth Metal Oxides Catalysts Used in the Transesterification of Oils for Biodiesel Production. Energies 2021, 14, 1031. [Google Scholar] [CrossRef]
- Benjapornkulaphong, S.; Ngamcharussrivichai, C.; Bunyakiat, K. Al2O3-supported alkali and alkali earth metal oxides for transesterification of palm kernel oil and coconut oil. Chem. Eng. J. 2009, 145, 468–474. [Google Scholar] [CrossRef]
- Mootabadi, H.; Salamatinia, B.; Bhatia, S.; Abdullah, A.Z. Ultrasonic-assisted biodiesel production process from palm oil using alkaline earth metal oxides as the heterogeneous catalysts. Fuel 2010, 89, 1818–1825. [Google Scholar] [CrossRef]
- Cabrera-Munguia, D.A.; González, H.; Barreto-Gutiérrez, M.; Gutiérrez-Alejandre, A.; Rico, J.L.; Solís-Casados, D.A. Tuning the Basic Properties of ZnAl Hydrotalcites Modified with Ce Applied to Transesterification of Soybean Oil. Catal. Lett. 2020, 150, 1957–1969. [Google Scholar] [CrossRef]
- Wang, Z.; Zhou, H.; Liu, Z.; Miao, R.; He, L.; Guan, Q. Walnut-shaped calcium oxide-cancrinite spheres for transesterification of waste frying oil. Renew. Energy 2023, 208, 229–239. [Google Scholar] [CrossRef]
- Singh, V.; Hameed, B.H.; Sharma, Y.C. Economically viable production of biodiesel from a rural feedstock from eastern India, P. pinnata oil using a recyclable laboratory synthesized heterogeneous catalyst. Energy Convers. Manag. 2016, 122, 52–62. [Google Scholar] [CrossRef]
- Wang, J.; Yang, L.; Luo, W.; Yang, G.; Miao, C.; Fu, J.; Xing, S.; Fan, P.; Lv, P.; Wang, Z. Sustainable biodiesel production via transesterification by using recyclable Ca2MgSi2O7 catalyst. Fuel 2017, 196, 306–313. [Google Scholar] [CrossRef]
- Alkimim, I.P.; Silva, L.L.; Cardoso, D. Synthesis of hybrid spherical silicas and application in catalytic transesterification reaction. Microporous Mesoporous Mater. 2017, 254, 37–44. [Google Scholar] [CrossRef]
- Kubota, Y.; Nishizaki, Y.; Ikeya, H.; Saeki, M.; Hida, T.; Kawazu, S.; Yoshida, M.; Fujii, H.; Sugi, Y. Organic–silicate hybrid catalysts based on various defined structures for Knoevenagel condensation. Microporous Mesoporous Mater. 2004, 70, 135–149. [Google Scholar] [CrossRef]
- Zhao, S.; Yi, H.; Tang, X.; Kang, D.; Yu, Q.; Gao, F.; Wang, J.; Huang, Y.; Yang, Z. Mechanism of activity enhancement of the Ni based hydrotalcite-derived materials in carbonyl sulfide removal. Mater. Chem. Phys. 2018, 205, 35–43. [Google Scholar] [CrossRef]
- Álvarez, M.G.; Plíšková, M.; Segarra, A.M.; Medina, F.; Figueras, F. Synthesis of glycerol carbonates by transesterification of glycerol in a continuous system using supported hydrotalcites as catalysts. Appl. Catal. B Environ. 2012, 113–114, 212–220. [Google Scholar] [CrossRef]
- Coumans, F.J.A.G.; Mitchell, S.; Schütz, J.; Medlock, J.; Pérez-Ramírez, J. Hydrotalcite-Derived Mixed Oxides for the Synthesis of a Key Vitamin A Intermediate Reducing Waste. ACS Omega 2018, 3, 15293–15301. [Google Scholar] [CrossRef]
- Dahdah, E.; Estephane, J.; Taleb, Y.; El Khoury, B.; El Nakat, J.; Aouad, S. The role of rehydration in enhancing the basic properties of Mg–Al hydrotalcites for biodiesel production. Sustain. Chem. Pharm. 2021, 22, 100487. [Google Scholar] [CrossRef]
- Liu, Q.; Wang, B.; Wang, C.; Tian, Z.; Qu, W.; Ma, H.; Xu, R. Basicities and transesterification activities of Zn–Al hydrotalcites-derived solid bases. Green Chem. 2014, 16, 2604–2613. [Google Scholar] [CrossRef]
- Sánchez Faba, E.M.; Ferrero, G.O.; Dias, J.M.; Eimer, G.A. Thermo-chemically tuning of active basic sites on nanoarchitectured silica for biodiesel production. Mol. Catal. 2020, 481, 110171. [Google Scholar] [CrossRef]
- Wang, H.; Yan, S.; Salley, S.O.; Simon Ng, K.Y. Support effects on hydrotreating of soybean oil over NiMo carbide catalyst. Fuel 2013, 111, 81–87. [Google Scholar] [CrossRef]
- Szkudlarek, Ł.; Chałupka-Śpiewak, K.; Maniukiewicz, W.; Nowosielska, M.; Szynkowska-Jóźwik, M.I.; Mierczyński, P. Biodiesel Production by Methanolysis of Rapeseed Oil—Influence of SiO2/Al2O3 Ratio in BEA Zeolite Structure on Physicochemical and Catalytic Properties of Zeolite Systems with Alkaline Earth Oxides (MgO, CaO, SrO). Int. J. Mol. Sci. 2024, 25, 3570. [Google Scholar] [CrossRef]
- Lee, S.; Lee, H.J.; Chung, S.H.; Lee, J.S.; Lim, S.Y. Synthesis of Ethylene Carbonate by Urea Transesterification Using Zeolitic Imidazolate Framework Derived Fe-Doped ZnO Catalysts. ACS Omega 2023, 8, 48704–48710. [Google Scholar] [CrossRef]
- Lani, N.S.; Ngadi, N. Highly efficient CaO–ZSM-5 zeolite/Fe3O4 as a magnetic acid–base catalyst upon biodiesel production from used cooking oil. Appl. Nanosci. 2022, 12, 3755–3769. [Google Scholar] [CrossRef]
- Ryoo, R. Birth of a class of nanomaterial. Nature 2019, 575, 40–41. [Google Scholar] [CrossRef]
- Davoodbasha, M.; Pugazhendhi, A.; Kim, J.-W.; Lee, S.-Y.; Nooruddin, T. Biodiesel production through transesterification of Chlorella vulgaris: Synthesis and characterization of CaO nanocatalyst. Fuel 2021, 300, 121018. [Google Scholar] [CrossRef]
- Kumari, N.; Aulakh, M.K.; Sareen, S.; Sharma, A.; Sohal, H.S.; Verma, M.; Mehta, S.K.; Mutreja, V. Greener Synthesis of Zirconium-Based Nanocatalyst for Transesterification. Top. Catal. 2022, 65, 1811–1820. [Google Scholar] [CrossRef]
- Al-Abbasi, A.; Almahdi, F.; Almaky, M.; Izriq, R.; Milad, A.; Salim, S.; Najar, A. BaO as a heterogeneous nanoparticle catalyst in oil transesterification for the production of FAME fuel. Inorg. Chem. Commun. 2023, 158, 111620. [Google Scholar] [CrossRef]
- Silva, I.F.; Rios, R.D.F.; Savateev, O.; Teixeira, I.F. Carbon Nitride-Based Nanomaterials as a Sustainable Catalyst for Biodiesel Production. ACS Appl. Nano Mater. 2023, 6, 9718–9727. [Google Scholar] [CrossRef]
- Chen, Y.-S.; Yang, C.-M.; Nguyen Hoang, T.T.; Tsai, D.-H. Porous magnesia-alumina composite nanoparticle for biodiesel production. Fuel 2021, 285, 119203. [Google Scholar] [CrossRef]
- Pamatz-Bolaños, T.; Cabrera-Munguia, D.A.; González, H.; Del Río, R.E.; Rico, J.L.; Rodríguez-García, G.; Gutiérrez-Alejandre, A.; Tzompantzi, F.; Gómez-Hurtado, M.A. Transesterification of Caesalpinia eriostachys seed oil using heterogeneous and homogeneous basic catalysts. Int. J. Green Energy 2018, 15, 465–472. [Google Scholar] [CrossRef]
- Sangeetha, B.; Mohana Priya, S.; Pravin, R.; Tamilarasan, K.; Baskar, G. Process optimization and technoeconomic assessment of biodiesel production by one-pot transesterification of Ricinus communis seed oil. Bioresour. Technol. 2023, 376, 128880. [Google Scholar] [CrossRef]
- Kesica, Z.; Lukic, I.; Zdujic, M.; Liu, H.; Skala, D. Mechanochemically Synthesized CaO ZnO Catalyst For Biodiesel Production. Procedia Eng. 2012, 42, 1169–1178. [Google Scholar] [CrossRef]
- Widiarti, N.; Bahruji, H.; Holilah, H.; Ni’mah, Y.L.; Ediati, R.; Santoso, E.; Jalil, A.A.; Hamid, A.; Prasetyoko, D. Upgrading catalytic activity of NiO/CaO/MgO from natural limestone as catalysts for transesterification of coconut oil to biodiesel. Biomass Convers. Biorefinery 2023, 13, 3001–3015. [Google Scholar] [CrossRef]
- Ghasemi, I.; Haghighi, M.; Bekhradinassab, E.; Ebrahimi, A. Ultrasound-assisted dispersion of bifunctional CaO-ZrO2 nanocatalyst over acidified kaolin for production of biodiesel from waste cooking oil. Renew. Energy 2024, 225, 120287. [Google Scholar] [CrossRef]
- Abdulkareem-Alsultan, G.; Asikin-Mijan, N.; Lee, H.V.; Taufiq-Yap, Y.H. A new route for the synthesis of La-Ca oxide supported on nano activated carbon via vacuum impregnation method for one pot esterification-transesterification reaction. Chem. Eng. J. 2016, 304, 61–71. [Google Scholar] [CrossRef]
- Lin, T.; Zhao, S.; Niu, S.; Lyu, Z.; Han, K.; Hu, X. Halloysite nanotube functionalized with La-Ca bimetallic oxides as novel transesterification catalyst for biodiesel production with molecular simulation. Energy Convers. Manag. 2020, 220, 113138. [Google Scholar] [CrossRef]
- Lee, H.V.; Juan, J.C.; Taufiq-Yap, Y.H. Preparation and application of binary acid–base CaO–La2O3 catalyst for biodiesel production. Renew. Energy 2015, 74, 124–132. [Google Scholar] [CrossRef]
- Nuguid, R.J.G.; Ortino-Ghini, L.; Suskevich, V.L.; Yang, J.; Lietti, L.; Kröcher, O.; Ferri, D. Interconversion between Lewis and Brønsted–Lowry acid sites on vanadia-based catalysts. Phys. Chem. Chem. Phys. 2022, 24, 4555–4561. [Google Scholar] [CrossRef] [PubMed]
- Marberger, A.; Ferri, D.; Elsener, M.; Kröcher, O. The Significance of Lewis Acid Sites for the Selective Catalytic Reduction of Nitric Oxide on Vanadium-Based Catalysts. Angew. Chem. Int. Ed. 2016, 55, 11989–11994. [Google Scholar] [CrossRef]
- Mulyatun, M.; Prameswari, J.; Istadi, I.; Widayat, W. Synthesis Method Effect on the Catalytic Performance of Acid–Base Bifunctional Catalysts for Converting Low-Quality Waste Cooking Oil to Biodiesel. Catal. Lett. 2024, 154, 4837–4855. [Google Scholar] [CrossRef]
- Mansir, N.; Hwa Teo, S.; Lokman Ibrahim, M.; Yun Hin, T.-Y. Synthesis and application of waste egg shell derived CaO supported W-Mo mixed oxide catalysts for FAME production from waste cooking oil: Effect of stoichiometry. Energy Convers. Manag. 2017, 151, 216–226. [Google Scholar] [CrossRef]
- Yan, S.; Tong, T.; Li, Y.; Khan, S.U.; Zhao, J.; Wang, S.; Wang, X. Production of Biodiesel Through Esterification Reaction Using Choline Exchanging Polytungstoboronic Acids as Temperature-Responsive Catalysts. Catal. Surv. Asia 2017, 21, 151–159. [Google Scholar] [CrossRef]
- Lapis, A.A.M.; de Oliveira, L.F.; Neto, B.A.D.; Dupont, J. Ionic Liquid Supported Acid/Base-Catalyzed Production of Biodiesel. ChemSusChem 2008, 1, 759–762. [Google Scholar] [CrossRef] [PubMed]
- Wang, A.; Li, H.; Zhang, H.; Pan, H.; Yang, S. Efficient Catalytic Production of Biodiesel with Acid-Base Bifunctional Rod-Like Ca-B Oxides by the Sol-Gel Approach. Materials 2019, 12, 83. [Google Scholar] [CrossRef]
- Cheng, J.; Guo, H.; Yang, X.; Mao, Y.; Qian, L.; Zhu, Y.; Yang, W. Phosphotungstic acid-modified zeolite imidazolate framework (ZIF-67) as an acid-base bifunctional heterogeneous catalyst for biodiesel production from microalgal lipids. Energy Convers. Manag. 2021, 232, 113872. [Google Scholar] [CrossRef]
- Lee, G.; Lee, C.; Kim, H.; Jeon, Y.; Shul, Y.-G.; Park, J. Bifunctional 1,2,4-Triazole/12-Tungstophosphoric Acid Composite Nanoparticles for Biodiesel Production. Nanomaterials 2022, 12, 4022. [Google Scholar] [CrossRef] [PubMed]
- Mansir, N.; Taufiq-Yap, Y.H.; Rashid, U.; Lokman, I.M. Investigation of heterogeneous solid acid catalyst performance on low grade feedstocks for biodiesel production: A review. Energy Convers. Manag. 2017, 141, 171–182. [Google Scholar] [CrossRef]
- Rechnia-Gorący, P.; Malaika, A.; Kozłowski, M. Acidic activated carbons as catalysts of biodiesel formation. Diam. Relat. Mater. 2018, 87, 124–133. [Google Scholar] [CrossRef]
- Rocha, P.D.; Oliveira, L.S.; Franca, A.S. Sulfonated activated carbon from corn cobs as heterogeneous catalysts for biodiesel production using microwave-assisted transesterification. Renew. Energy 2019, 143, 1710–1716. [Google Scholar] [CrossRef]
- Luo, Y.; Mei, Z.; Liu, N.; Wang, H.; Han, C.; He, S. Synthesis of mesoporous sulfated zirconia nanoparticles with high surface area and their applies for biodiesel production as effective catalysts. Catal. Today 2017, 298, 99–108. [Google Scholar] [CrossRef]
- Bora, A.P.; Konda, L.D.N.V.V.; Pasupuleti, S.; Durbha, K.S. Synthesis of MgO/MgSO4 nanocatalyst by thiourea–nitrate solution combustion for biodiesel production from waste cooking oil. Renew. Energy 2022, 190, 474–486. [Google Scholar] [CrossRef]
- Shobhana, G.; Asikin-Mijan, N.; AbdulKareem-Alsultan, G.; Sivasangar, S.; Izham, S.M.; Taufiq-Yap, Y.H. Biodiesel production via simultaneous esterification and transesterification of chicken fat oil by mesoporous sulfated Ce supported activated carbon. Biomass Bioenergy 2020, 141, 105714. [Google Scholar]
- Li, H.; Liu, F.; Ma, X.; Cui, P.; Guo, M.; Li, Y.; Gao, Y.; Zhou, S.; Yu, M. An efficient basic heterogeneous catalyst synthesis of magnetic mesoporous Fe@C support SrO for transesterification. Renew. Energy 2020, 149, 816–827. [Google Scholar] [CrossRef]
- Lani, N.S.; Ngadi, N.; Mohammed Inuwa, I.; Anako Opotu, L.; Zakaria, Z.Y.; Haron, S. A cleaner approach with magnetically assisted reactor setup over CaO-zeolite/Fe3O4 catalyst in biodiesel production: Evaluation of catalytic performance, reusability and life cycle assessment studies. J. Clean. Prod. 2023, 419, 138329. [Google Scholar] [CrossRef]
- Xue, B.-j.; Luo, J.; Zhang, F.; Fang, Z. Biodiesel production from soybean and Jatropha oils by magnetic CaFe2O4–Ca2Fe2O5-based catalyst. Energy 2014, 68, 584–591. [Google Scholar] [CrossRef]
- Zhang, H.; Yu, S.; Cao, S.; Liu, X.; Tang, J.; Zhu, L.; Ji, J.; Wang, J. Stabilizing Triglyceride in Methanol Emulsions via a Magnetic Pickering Interfacial Catalyst for Efficient Transesterification under Static Conditions. ACS Omega 2021, 6, 14138–14147. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Shi, M.; Liu, Y.; Fan, M.; Jiang, P.; Dong, Y. Sr doping magnetic CaO parcel ferrite improving catalytic activity on the synthesis of biodiesel by transesterification. Fuel 2016, 186, 787–791. [Google Scholar] [CrossRef]
- Banerjee, S.; Rout, S.; Banerjee, S.; Atta, A.; Das, D. Fe2O3 nanocatalyst aided transesterification for biodiesel production from lipid-intact wet microalgal biomass: A biorefinery approach. Energy Convers. Manag. 2019, 195, 844–853. [Google Scholar] [CrossRef]
- Xie, W.; Zang, X. Covalent immobilization of lipase onto aminopropyl-functionalized hydroxyapatite-encapsulated-γ-Fe2O3 nanoparticles: A magnetic biocatalyst for interesterification of soybean oil. Food Chem. 2017, 227, 397–403. [Google Scholar] [CrossRef]
- Mansoorsamaei, Z.; Mowla, D.; Esmaeilzadeh, F.; Dashtian, K. Sustainable biodiesel production from waste cooking oil using banana peel biochar-Fe2O3/Fe2K6O5 magnetic catalyst. Fuel 2024, 357, 129821. [Google Scholar] [CrossRef]
- Kouzu, M.; Hidaka, J.-S. Purification to remove leached CaO catalyst from biodiesel with the help of cation-exchange resin. Fuel 2013, 105, 318–324. [Google Scholar] [CrossRef]
- Xia, S.; Li, J.; Chen, G.; Tao, J.; Li, W.; Zhu, G. Magnetic reusable acid-base bifunctional Co doped Fe2O3–CaO nanocatalysts for biodiesel production from soybean oil and waste frying oil. Renew. Energy 2022, 189, 421–434. [Google Scholar] [CrossRef]
- Kannapiran, N.; Muthusamy, A.; Renganathan, B.; Ganesan, A.R.; Savithiri, S.; Meena, S.S. Magnetic, Dielectric and Ethanol Gas Sensing Properties of Poly(o-phenylenediamine)/(MnNi)Fe2O4 Nanocomposites and Quantum Chemical Calculations of (MnNi)Fe2O4. J. Inorg. Organomet. Polym. Mater. 2022, 32, 2173–2191. [Google Scholar] [CrossRef]
- Rahmanivahid, B.; Ajamein, H.; Zakizadeh, T.; Nayebzadeh, H. Fabrication of super basic BaxMg(1-x)Fe2O4 magnetic spinel nanocatalyst toward biodiesel production. Mater. Res. Bull. 2023, 165, 112321. [Google Scholar] [CrossRef]
- Wang, Y.-T.; Fang, Z.; Yang, X.-X.; Yang, Y.-T.; Luo, J.; Xu, K.; Bao, G.-R. One-step production of biodiesel from Jatropha oils with high acid value at low temperature by magnetic acid-base amphoteric nanoparticles. Chem. Eng. J. 2018, 348, 929–939. [Google Scholar] [CrossRef]
- Guo, H.; Cheng, J.; Mao, Y.; Qian, L.; Shao, Y.; Yang, W. Fabricating different coordination states of cobalt as magnetic acid-base bifunctional catalyst for biodiesel production from microalgal lipid. Fuel 2022, 322, 124172. [Google Scholar] [CrossRef]
- Zhu, J.; Qu, T.; Niu, S.; Liu, J.; Liu, S.; Geng, J.; Yang, Z.; Abulizi, A. Preparation and characterization of a novel bifunctional heterogeneous Sr–La/wollastonite catalyst for biodiesel production. Mater. Today Sustain. 2024, 26, 100716. [Google Scholar] [CrossRef]
- Kingkam, W.; Nuchdang, S.; Phalakornkule, C.; Suwanmanee, U.; Rattanaphra, D. Synergistic effect of La2O3-Al2O3 based catalysts for efficient biodiesel production. J. Ind. Eng. Chem. 2024. [Google Scholar] [CrossRef]
- Sivaramakrishnan, R.; Incharoensakdi, A. Direct transesterification of Botryococcus sp. catalysed by immobilized lipase: Ultrasound treatment can reduce reaction time with high yield of methyl ester. Fuel 2017, 191, 363–370. [Google Scholar] [CrossRef]
- Yasvanthrajan, N.; Sivakumar, P.; Muthukumar, K.; Murugesan, T.; Arunagiri, A. Production of biodiesel from waste bio-oil through ultrasound assisted transesterification using immobilized lipase. Environ. Technol. Innov. 2021, 21, 101199. [Google Scholar] [CrossRef]
- Dhawane, S.H.; Chowdhury, S.; Halder, G. Lipase immobilised carbonaceous catalyst assisted enzymatic transesterification of Mesua ferrea oil. Energy Convers. Manag. 2019, 184, 671–680. [Google Scholar] [CrossRef]



| Type | Catalyst | Reaction Conditions | Performance | Comments | Ref. |
|---|---|---|---|---|---|
| Metal salt | ZnCl2 | The molar ratio of catalyst to oil is 0.15 mol/mol, and the reaction is carried out at 150 °C for 1 h | 76.7% by weight | It has esterification ability and reduces FFAs by 60% at 150 °C. This catalyst has a low conversion efficiency compared to mature catalysts. | [57] |
| Metal salt | AlCl3 | Using 5.1 MPa CO2 as cosolvent mass ratio of biomass to ethanol of 1:20, 4 wt% AlCl3, 250 °C, 90 min | Over 90% conversion rate | The conversion rate of FFAs is 96%. AlCl3 plays a role in flocculating and suspending biomass in the reaction system, expanding the use of the catalyst, but the reaction conditions are too high | [57] |
| Metal salt | ZnLa, ZnPa, ZnSt and ZnOl | 100 °C, 2 h, 500 rpm | The triglyceride conversion rate is higher than 99%, and the FAME yield is higher than 84% | They have lower mass transfer resistance than heterogeneous catalysts and can be easily separated from the reaction medium without activation or cleaning treatment | [58] |
| Metal oxide | Tin oxide (II) | When the molar ratio of methyl acetate to oil is over 40, 483 K, the reaction time is 4 h | 90% FAME yield | The reaction system requires water and the reaction time is long | [59] |
| Metal oxide | MoO3/γ-Al2O3 | The catalyst to oil ratio is 1:20; 373 K, 2–16 h, | The maximum conversion rate is 95% | Catalyst preparation is complex, requires long reaction times, and there is a conversion bottleneck in optimizing the transesterification process | [60] |
| Metal oxide | WO3/SMP | The 2 wt% WO3 loading, 4.5 wt% catalyst amount, 9:1 methanol to oil molar ratio, 45 min reaction time, and 343 K reaction temperature | 96% biodiesel product | The excellent effect of the catalyst comes from the high Lewis acid sites, and the particle gap of the catalyst also plays a key role | [61] |
| Carbon nanotubes | Al3+-SO42−/MWCNTs | The mass ratio of catalyst to reactant is 0.9 wt%, and the molar ratio of methanol to oleic acid is 12:1, after 7 h | The conversion rate exceeds 95% | The carbon-carbon bond structure of MWCNTs is stable and suitable for loading rich Lewis acid sites. The pore structure and the existence of surface functional groups of MWCNTs enable them to effectively adsorb and immobilize the active components of the catalyst, improving the dispersibility and stability of the catalyst | [62] |
| Metal organic frameworks | MIL-101 (Cr)-SO3H | Microwave irradiation for 20 min | 93% conversion rate | MOFs have a very high specific surface area and adjustable pore size, which provides a large number of active sites and good material transport channels for catalytic reactions, thereby improving catalytic efficiency. Microwave irradiation reduces the reaction time significantly compared to heating. | [63] |
| ILs | The ionic liquid [DDPA] [Tos] | The amount of catalyst was 8% w/v, the ratio of oil to methanol was 1:2 v/v, the reaction temperature was 65 °C, and the reaction time was 4 h | 75% Biodiesel Yield | The catalyst is easy to prepare, has high recyclability, and remains low in reaction rate and final conversion | [64] |
| DES | The p-toluene sulfonate eutectic solvent | The molar ratio of methanol to oil was 8:1, the reaction temperature was 110 °C, and the reaction time was 2 h | The ester exchange rate can reach 98.66 ± 0.17% | It has the characteristics of ionic liquids and a higher reaction rate | [65] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Z.; Meng, P.; Luo, H.; Pei, Z.; Liu, X. Lewis Acid-Base Site-Assisted In Situ Transesterification Catalysis to Produce Biodiesel. Catalysts 2024, 14, 731. https://doi.org/10.3390/catal14100731
Zhang Z, Meng P, Luo H, Pei Z, Liu X. Lewis Acid-Base Site-Assisted In Situ Transesterification Catalysis to Produce Biodiesel. Catalysts. 2024; 14(10):731. https://doi.org/10.3390/catal14100731
Chicago/Turabian StyleZhang, Zhuangzhuang, Pan Meng, Hangyu Luo, Zhengfei Pei, and Xiaofang Liu. 2024. "Lewis Acid-Base Site-Assisted In Situ Transesterification Catalysis to Produce Biodiesel" Catalysts 14, no. 10: 731. https://doi.org/10.3390/catal14100731
APA StyleZhang, Z., Meng, P., Luo, H., Pei, Z., & Liu, X. (2024). Lewis Acid-Base Site-Assisted In Situ Transesterification Catalysis to Produce Biodiesel. Catalysts, 14(10), 731. https://doi.org/10.3390/catal14100731











