Review of Bio-Inspired Green Synthesis of Titanium Dioxide for Photocatalytic Applications
Abstract
:1. Introduction
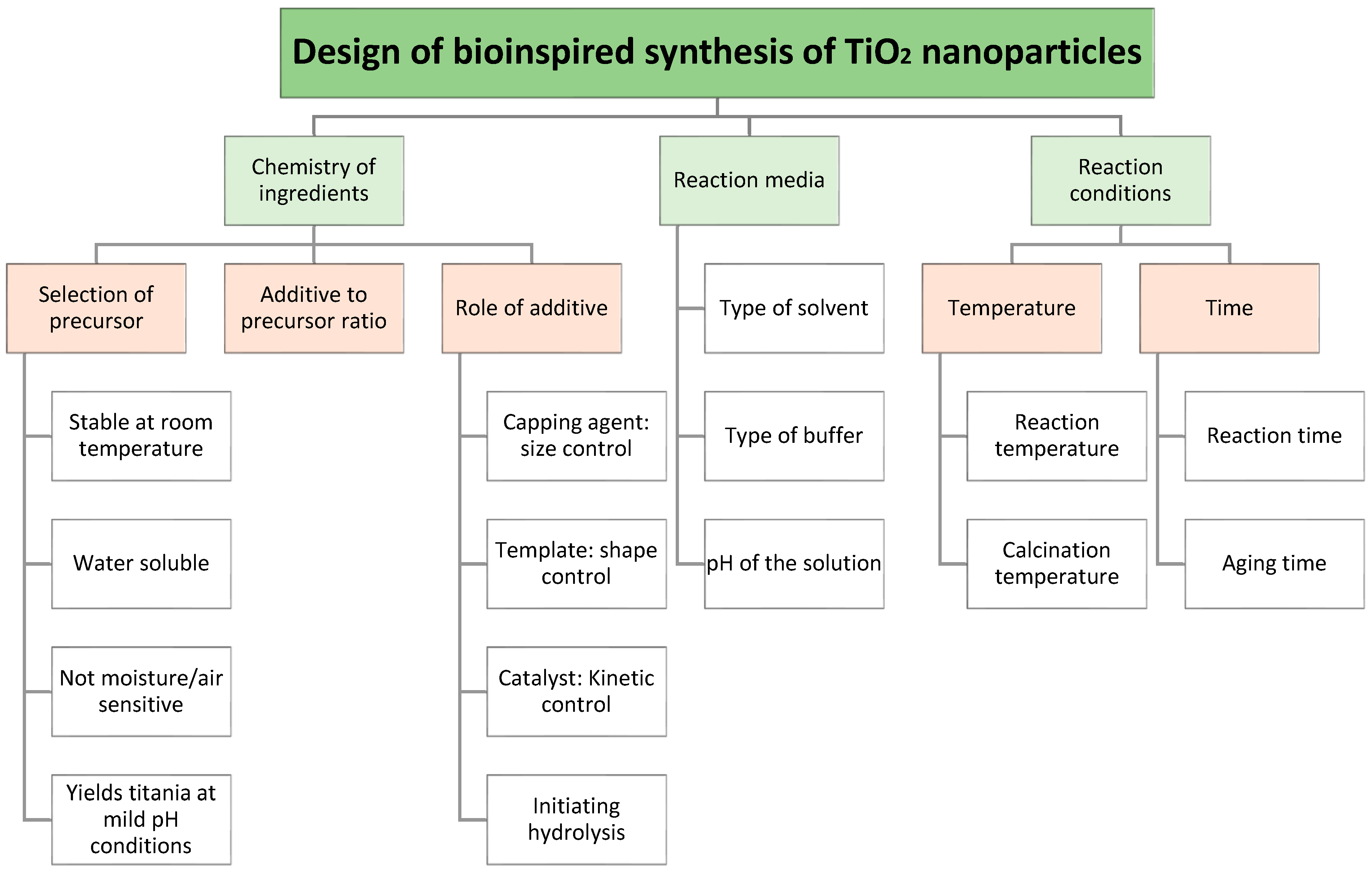
2. Choice of Synthesis Parameters
2.1. Selection of Precursor
| Solvent | Additives | Reaction Temperature | Reaction Time | Calcination Temperature | Product | Ref. |
|---|---|---|---|---|---|---|
| Deionized water | Urea | RT, then 100 °C | 1 h at RT, 20 h at 100 °C | 500 °C | Amorphous thin-film coating | [64] |
| Aqueous solution | Urea | 160 °C | Overnight | 300–550 °C | Pure anatase, pure brookite or biphasic anatase/brookite mixtures | [69] |
| Water | Urea | 95 °C | 24 h | - | Anatase TiO2 sol | [70] |
| Water | L-arginine | RT | 30 min | 480 °C | Anatase | [71] |
| Tris–HCl buffer | Arginine | RT | 0.5–10.0 min | - | Anatase | [72] |
| Water, phosphate buffer | Spermidine or spermine | RT | Overnight | 200, 400, 600, 800 °C | Anatase after annealing at 800 °C | [73] |
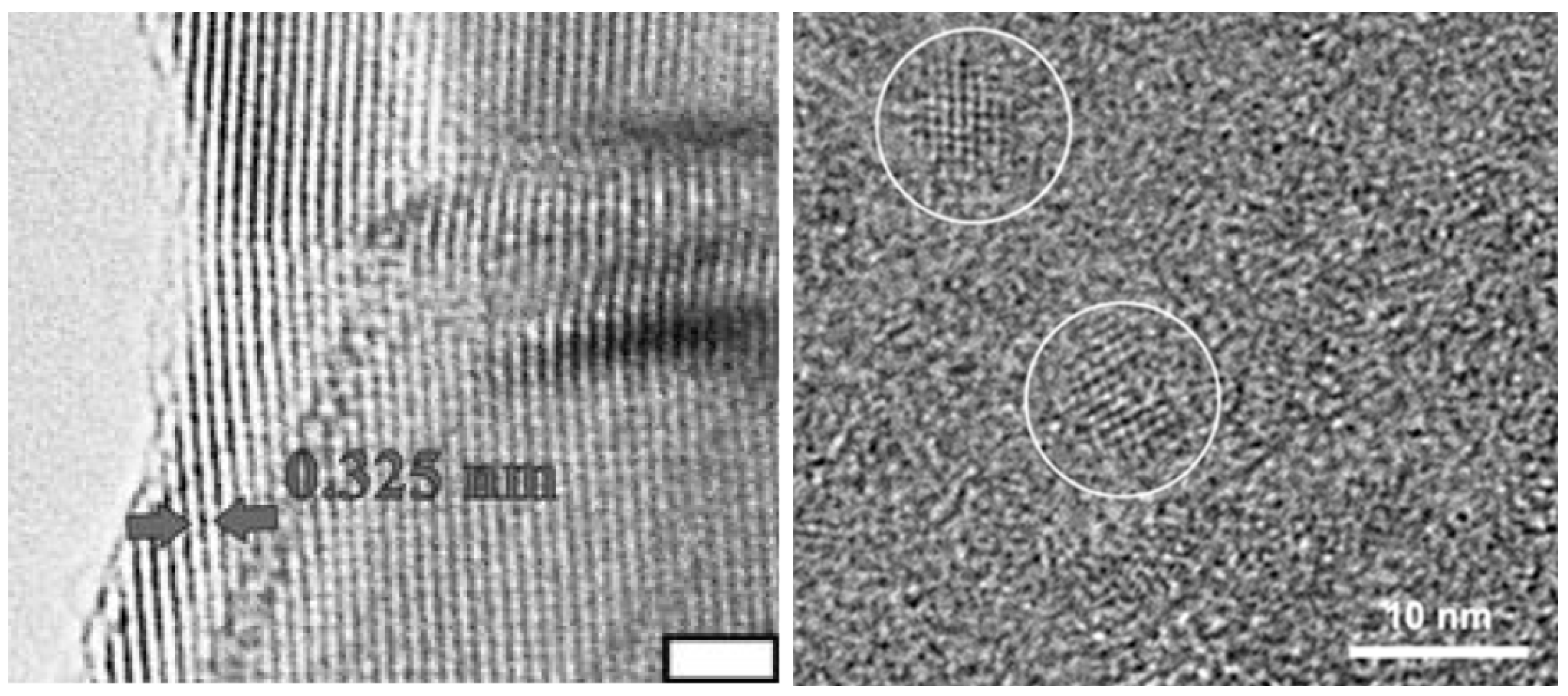
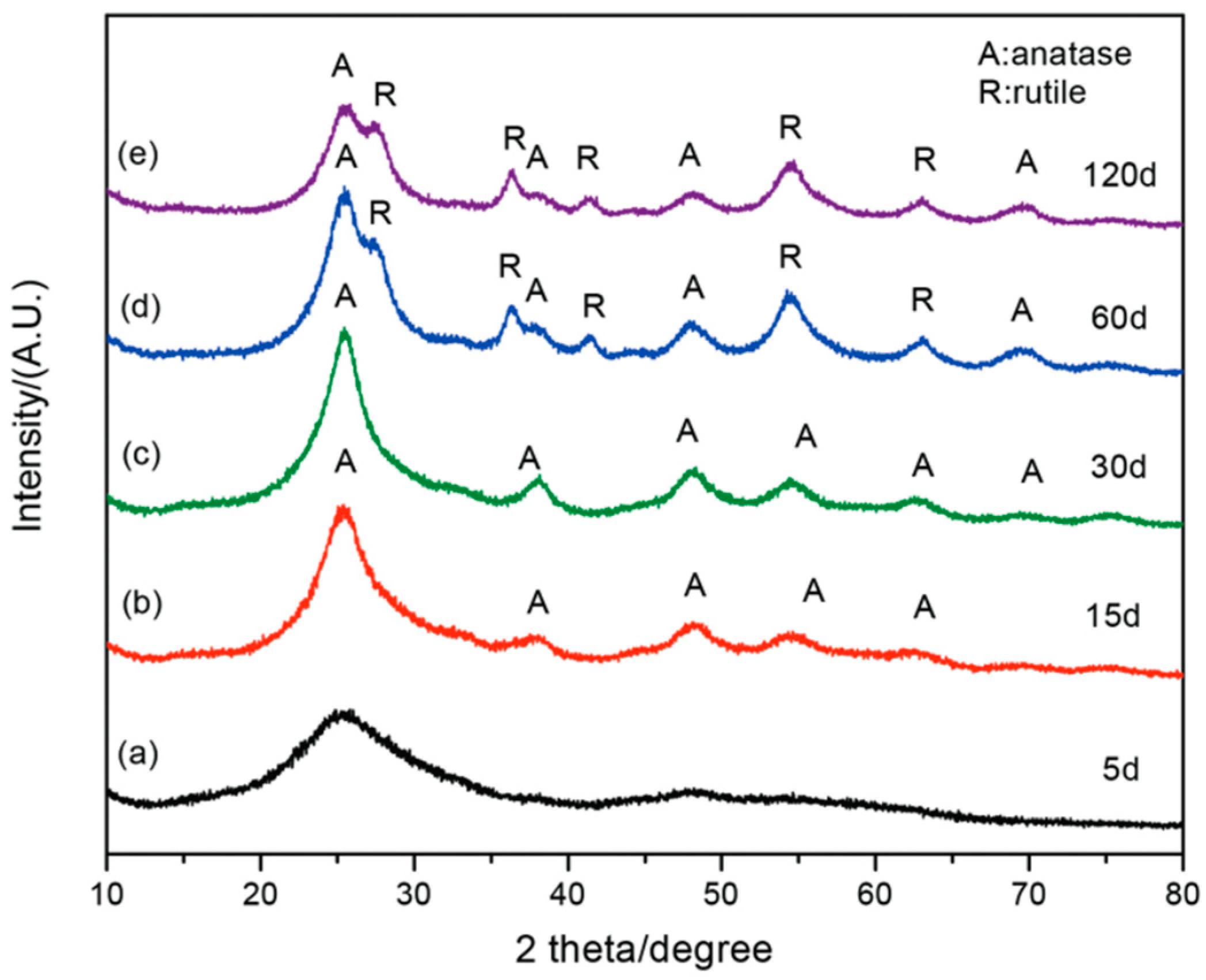
| Aqueous solution | Poly(allylamine hydrochloride), poly(diallyldimethyl-ammonium chloride) | RT | 5–60 days | - | Aggregated nanoparticles of anatase (anatase was observed after 30 days) | [74] |
| Phosphate-citrate buffer solution | c-terminal tetra peptide Gly-Gly-Gly-Trp | RT | 10 min | - | Nanoparticles <50 nm in size contained very fine (<10 nm) anatase and monoclinic TiO2 domains | [75] |
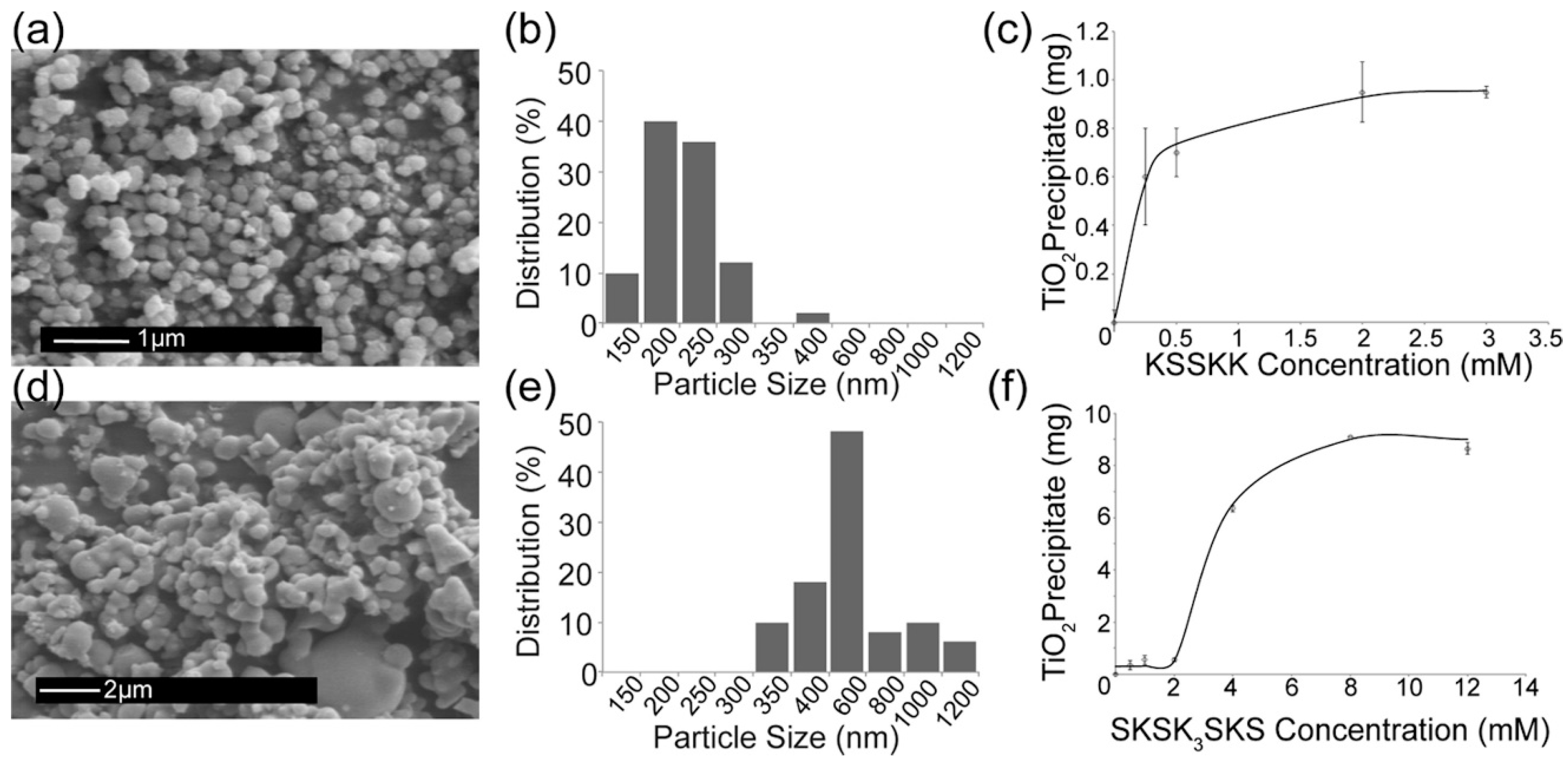
| Tris buffer | Serine-lysine (S-K) peptides KSSKK, SKSK3SKS | RT | 24 h | - | Amorphous or crystalline particles, 150–1200 nm diameter | [76] |
| Water | KIIIIKYWYAF peptide | 70 °C | 48 h | 580 °C | Anatase after 580 °C | [77] |
| Phosphate buffer or water | R5 peptide or poly-L-lysine-hydrobromide | RT | 5 min | 600–900 °C | Anatase at 600 °C. Anatase to rutile transition was at 700 °C | [78] |
| Tris or phosphate buffer | R5 peptide and its truncated analogues | RT | 24 h | 600 °C | Amorphous TiO2 at RT; anatase formed after annealing at 600 °C | [79] |
| Tris buffer, phosphate buffer or distilled water | Titanium dioxide binding peptides Ti-1, Ti-2 and R5 | RT | 2–72 h | - | <10 nm TiO2 sols, mostly amorphous with some anatase and monoclinic phases | [41] |
| Phosphate buffer | R5 peptide | RT | - | TiO2 nanosheets several μm in size, amorphous with <10 nm anatase domains | [80] | |
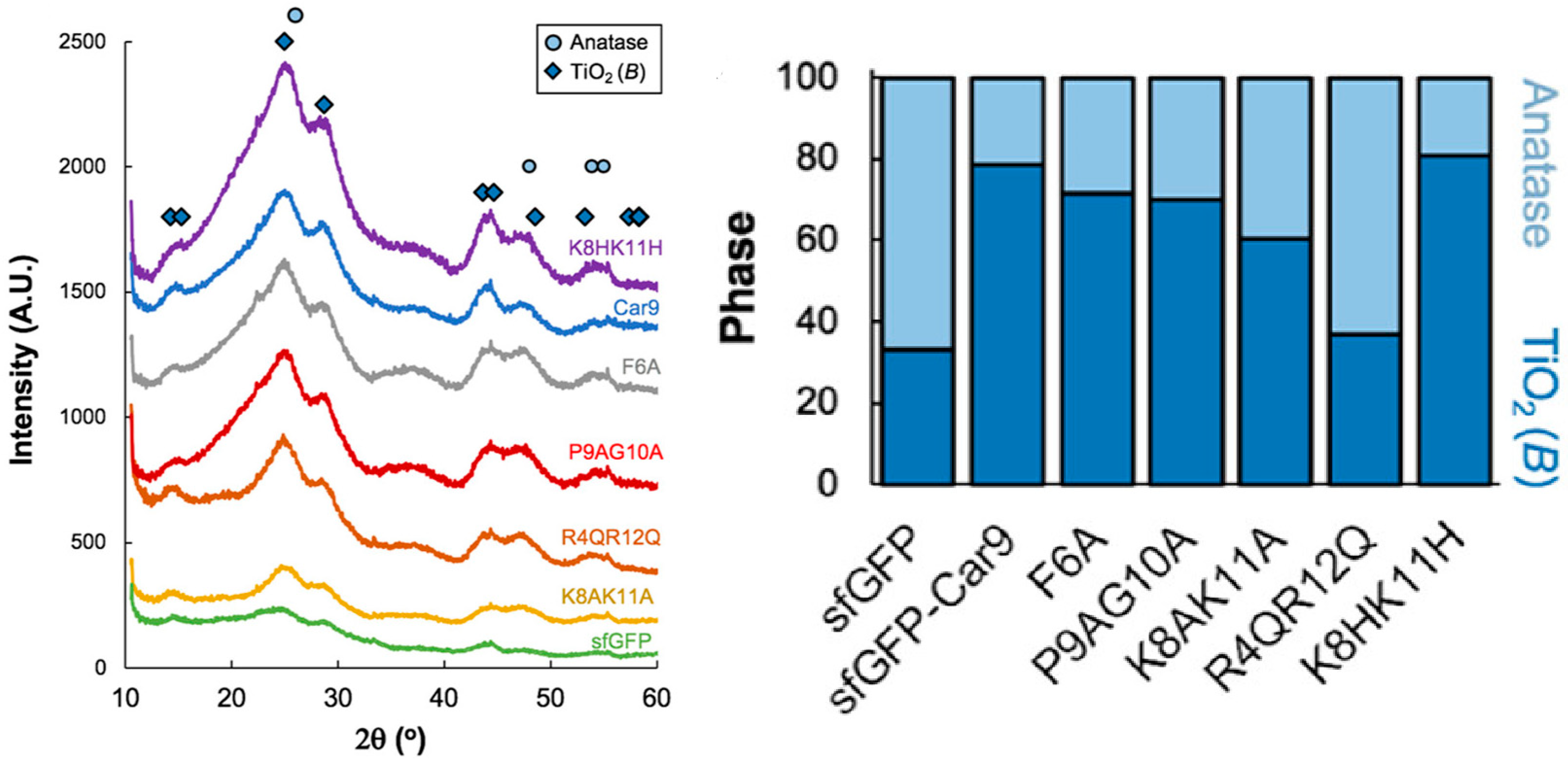
| Citrate buffer | Car9 peptide fused to superfolder green fluorescent protein (sfGFP) | RT | 120 min | - | Mixture of amorphous, anatase and monoclinic (bronze) TiO2 phases | [81] |
| Deionized water | Silicatein protein | 20 °C | 24 h (at 20 °C), 1 h (calcination) | 27–927 °C in steps of 100 °C | Mixture of amorphous and nanocrystalline anatase; transition to rutile was at 850 °C | [37] |
| Tris-HCl buffer | Proteins protamine, lysozyme, gelatin, haemoglobin, yeast alcohol dehydrogenase and bovine serum albumin | RT | 5 min | 600–700 °C | Amorphous at RT; transition to anatase at 600–700 °C and to rutile at 800 °C | [82] |
| Phosphate-buffered saline (PBS) | Bioengineered silicatein α and β and scaffold protein silintaphin-1 | RT | 12 h | - | Amorphous and anatase phases | [83] |
| Water, phosphate/ citrate buffer | Silaffin protein | RT | 20 min | - | Rutile | [40] |
2.2. Role of Solvent, pH and Buffer
3. Bio-Inspired Additives
| Ti Precursor | Types of Amino Acids | Process | Calcination | TiO2 Phase | TiO2 Morphology | Ref. |
|---|---|---|---|---|---|---|
| Titanium n-butoxide (Ti(OBu)4 | Glycine | Hydrothermal synthesis at 120 °C for 48 h | 500 °C; 3.5 h | Anatase | Flower-like hierarchical spheres with a 2 μm diameter assembled on 20 nm thick nanosheet | [102] |
| Titanium isopropoxide | Glycine, DL-alanine, β-alanine, DL-valine, proline, serine, DL-aspartic acid, L-glutamic acid | Gel formation after 12 h at room temperature, drying at 100 °C | 500 °C; 3 h | Anatase | 10–15 nm cubic particles | [101] |
| TiBALDH | Arginine | g-C3N4 + distilled water; 30 min at room temperature | 480 °C; 2 h | Anatase | Uniformly distributed TiO2 nanoparticles, d < 10 nm on g-C3N4 nanosheets | [71] |
| Titanium n-butoxide (Ti(OBu)4 | Glycine | 200 °C for 20 h | 450 °C; 5 h | Anatase | Hollow microspheres, with a crystallite size of 4.8 nm | [103] |
| TiCl4 | Glycine, alanine, serine, threonine, β-alanine | Seeded growth of TiO2 nanorods in HCl on pre-annealed FTO glass. Seeds grown at 95 °C | 450 °C; 1 h | Rutile | 300–900 nm nanorods on FTO glass | [100] |
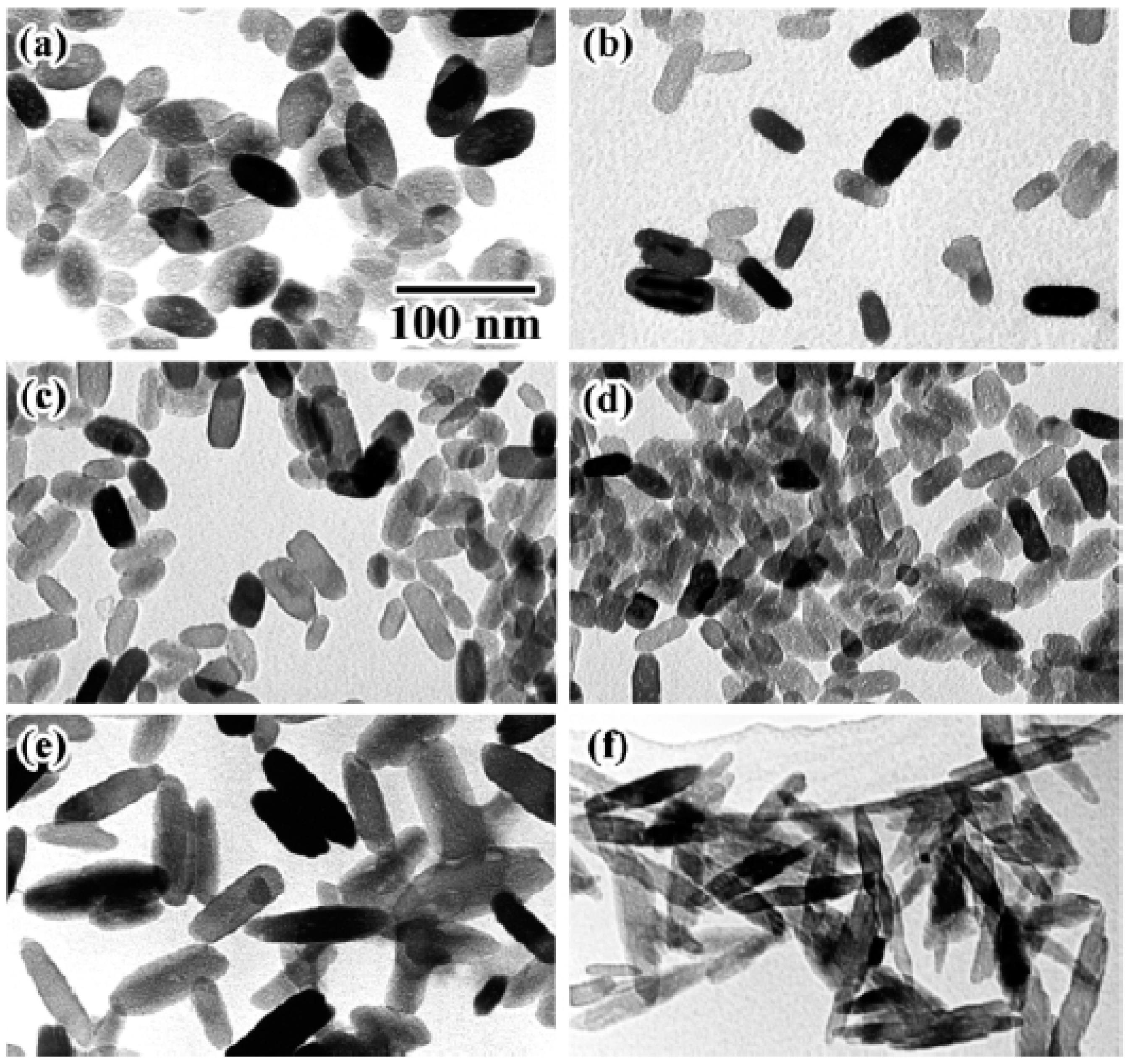
| Titanium isopropoxide (TTIP) | L-alanine | TTIP, L-alanine and dodecylamine in ethyl alcohol reacted at 60 °C for 24 h | 400 °C; 4 h | Anatase | 200 nm nanoparticles | [112] |
| Titanium isobutoxide | L-lysine | 60 °C 20 h; 100 °C 24 h | 350 °C | Mixed phase anatase + brookite | Mesoporous nanocrystals | [113] |
| Titanium (IV) oxysulfate | Lysine | Solvothermal synthesis in precursor in diluted H2SO4 at 160 °C 24 h | No further calcination | Anatase with exposed {101} and {111} facets | Single-crystal-like hierarchical spheres | [60] |
| TiCl4 | Glycine, glutamic acid, aspartic acid, serine, histidine, proline, lysine, arginine | Thermo-hydrolysis at 60 °C, from 1 day to 1 week, at a pH of 1 to 8 | No further calcination, but long reaction time | Anatase, brookite, anatase + brookite, anatase + rutile, amorphous | Nanoparticles with controlled shapes and sizes | [87] |
| TiBALDH | Arginine, serine, lysine, histidine, glycine | 10 min at room temperature | No further calcination | Surface functionalised anatase only with arginine | 35–350 nm nanoparticles | [72] |
3.1. Influence of Bio-Inspired Additives on Reaction Kinetics and Phase Control
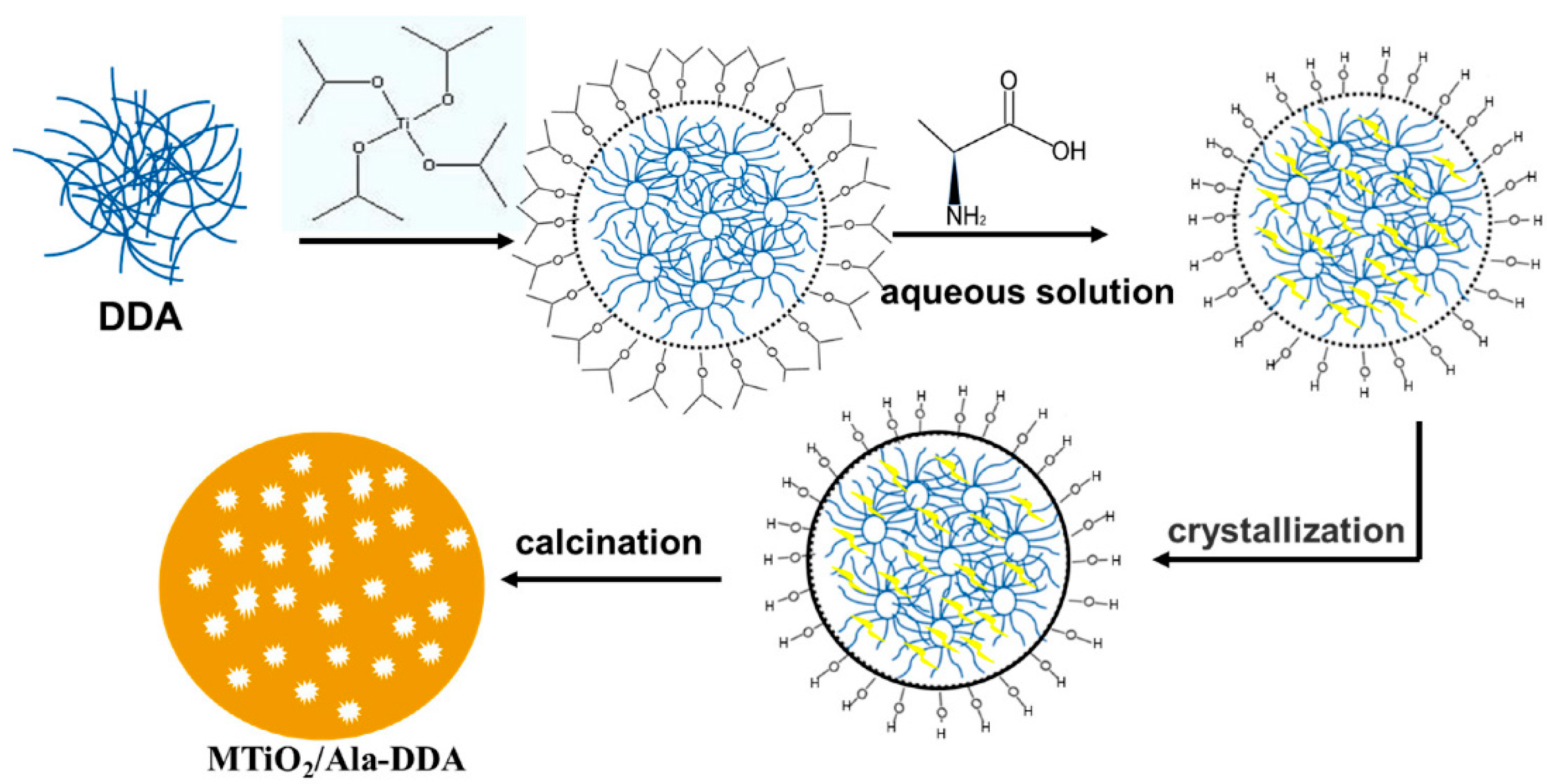
3.2. Role of Bio-Inspired Additives as Templates and Capping Agents: Effect on Morphology
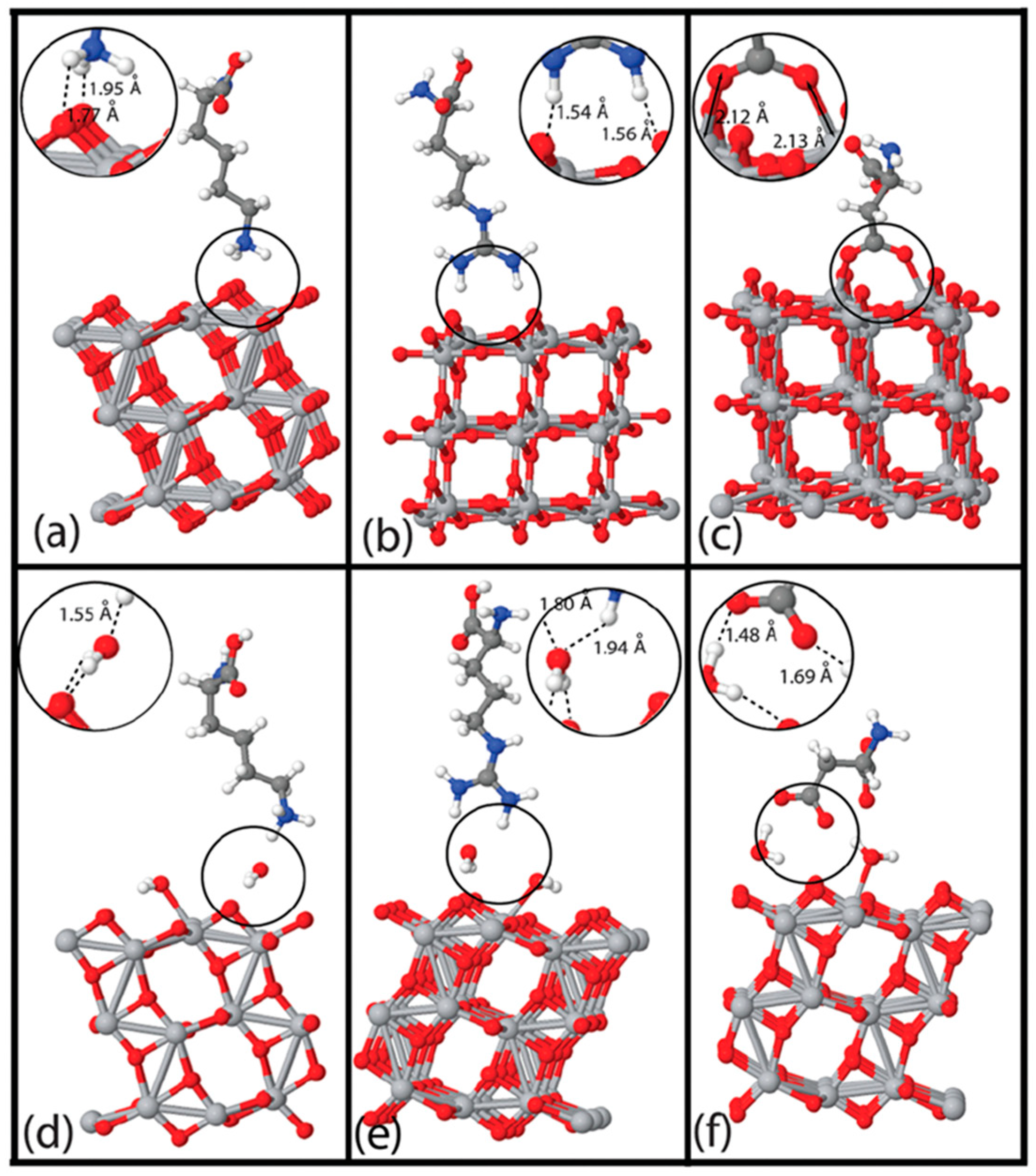
3.3. Interactions of Bio-Additives with TiO2
4. Photocatalytic Performance of TiO2 Synthesised via Bio-Inspired Route
| Material | Precursor | Additive | Photocatalytic Process | Photocatalytic Performance | Ref. |
|---|---|---|---|---|---|
| TiO2 nanofibers | TiCl4 | Pomelo peel | Degradation of methyl orange (MO), rhodamine B, reactive brilliant blue, malachite green | Better photocatalytic activity than commercial P25 TiO2. Up to 99% degradation of MO in 30 min | [88] |
| TiO2 nanoparticles | TiCl4 | Jatropha leaf extract | Degradation of tannery wastewater | 82% removal of chemical oxygen demand COD; 76% removal of Cr+6 | [137] |
| Mesoporous TiO2 photocatalysts | Tetra butyl titanate (Ti(OBu)4) | Pollen grains | Degradation of rhodamine B | 95% degradation after 120 min | [90] |
| TiO2 nanoparticles | Ti isopropoxide (Ti(Oi-Pr)4) | Aloe vera gel | Degradation of picric acid | Complete degradation in 120 min | [91] |
| TiO2 nanohybrids | TiO4 | Parthenium hysterophorus | Degradation of methylene blue (MB), crystal violet (CV), methyl orange (MO), alizarin red (AR) | In 6 h: degradation in (%) 92.5 MB, 81.5 MO, 79.7 CV, 77.3 AR | [93] |
| Indium-modified TiO2 composite with tobacco stem silk | Tetra butyl titanate | Tobacco stem silk | Degradation of tetracycline hydrochloride (TCH) | 92.9% removal efficiency in 90 min under visible light | [138] |
| Graphene-supported g-C3N4/TiO4 hetero-aerogels | Ti-BALDH | KIIIIKYWYAF peptide | Degradation of methylene blue, rhodamine B (RhB) | MB: 97% in 120 min; RhB: 60% in 120 min | [77] |
| g-C3N4/TiO2 | Ti-BALDH | Arginine | Degradation of rhodamine B, phenol | Rhodamine B 84% degraded in 5 h; Phenol 76% degradation in 120 min | [71] |
| Mesoporous nano TiO2 | Ti isopropoxide | Various amino acids | Degradation of methylene blue, calmagite | Almost complete degradation. Samples prepared with proline, valine and aspartic acid resulted in better degradation activity than P25 TiO2. | [101] |
| TiO2 hierarchical spheres | Tetra butyl titanate | Glycine | Degradation of methyl orange (MO) | 98% degradation of MO in 30 min | [102] |
| L-hydroproline modified TiO2 | TiCl4 | L-hydroproline | Degradation of rhodamine B | 97% degradation in 4 h, better performance than pure TiO2, visible light activity | [135] |
| Amino-acid-modified TiO2 | Tetra butyl titanate | L-proline, L-arginine, L-methionine | Degradation of methyl orange (MO), direct red 16 DR16 | MO removal: 95% DR16 removal: 97% in 60 min | [139] |
| Amino-acid-modified TiO2 | Tetra butyl titanate | L-proline, L-arginine, L-methionine | Degradation of metronidazole, cephalexin | Metronidazole removal: 99.9% (TOC removal: 81%) Cephalexin removal: 97.2% (TOC removal: 75%) | [140] |
| CdS/Au/N-doped TiO2 heterostructure | TiCl3 | Cherry blossom leaves | Hydrogen production | H2 evolution activity higher than P25 or TiO2 synthesized without template | [141] |
| Templated TiO2 | TiCl3 | Olive leaves | Hydrogen production | H2 evolution activity 64% higher than P25 under solar light and 144% higher under UV light | [65] |
| Templated mesoporous TiO2 | TiCl3 | Camellia tree leaves | CO2 reduction | Higher yield of CO + CH4, higher selectivity towards CH4 than towards P25 | [66] |
| TiO2 rutile and brookite nanoparticles | Peroxo-titanic acid | Various amino acids | CO2 reduction | Brookite synthesised in the presence of Lys showed the highest photocatalytic activity | [68] |
5. Conclusions
- Obtaining desired critical quality attributes (CQAs) such as the crystallinity and morphology of titanium dioxide suitable for desired applications, such as photocatalysis;
- Carrying out synthesis under mild conditions, ideally at room temperature;
- Assessing the economics and sustainability of bio-inspired synthesis methods;
- Up-scaling the methods of the green synthesis of TiO2 for industrial production.
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kudo, A.; Miseki, Y. Heterogeneous photocatalyst materials for water splitting. Chem. Soc. Rev. 2009, 38, 253–278. [Google Scholar] [CrossRef] [PubMed]
- Friedmann, D.; Hakki, A.; Kim, H.; Choi, W.; Bahnemann, D. Heterogeneous photocatalytic organic synthesis: State-of-the-art and future perspectives. Green Chem. 2016, 18, 5391–5411. [Google Scholar] [CrossRef]
- Fang, S.; Rahaman, M.; Bharti, J.; Reisner, E.; Robert, M.; Ozin, G.A.; Hu, Y.H. Photocatalytic CO2 reduction. Nat. Rev. Methods Primers 2023, 3, 61. [Google Scholar] [CrossRef]
- Melchionna, M.; Fornasiero, P. Updates on the Roadmap for Photocatalysis. ACS Catal. 2020, 10, 5493–5501. [Google Scholar] [CrossRef]
- Chong, M.N.; Jin, B.; Chow, C.W.K.; Saint, C. Recent developments in photocatalytic water treatment technology: A review. Water Res. 2010, 44, 2997–3027. [Google Scholar] [CrossRef] [PubMed]
- Mulay, M.R.; Martsinovich, N. TiO2 Photocatalysts for Degradation of Micropollutants in Water. In Clean Water and Sanitation; Leal Filho, W., Azul, A.M., Brandli, L., Lange Salvia, A., Wall, T., Eds.; Springer International Publishing: Cham, Switzerland, 2021; pp. 1–19. [Google Scholar] [CrossRef]
- Gupta, V.K.; Ali, I.; Saleh, T.A.; Nayak, A.; Agarwal, S. Chemical treatment technologies for waste-water recycling—An overview. Rsc Adv. 2012, 2, 6380–6388. [Google Scholar] [CrossRef]
- Muñoz, I.; Peral, J.; Ayllón, J.A.; Malato, S.; Passarinho, P.; Domènech, X. Life cycle assessment of a coupled solar photocatalytic–biological process for wastewater treatment. Water Res. 2006, 40, 3533–3540. [Google Scholar] [CrossRef]
- Pesqueira, J.F.; Pereira, M.F.R.; Silva, A.M. A life cycle assessment of solar-based treatments (H2O2, TiO2 photocatalysis, circumneutral photo-Fenton) for the removal of organic micropollutants. Sci. Total Environ. 2021, 761, 143258. [Google Scholar] [CrossRef]
- Magdy, M.; Alalm, M.G.; El-Etriby, H.K. Comparative life cycle assessment of five chemical methods for removal of phenol and its transformation products. J. Clean. Prod. 2021, 291, 125923. [Google Scholar] [CrossRef]
- Caramazana-Gonzalez, P.; Dunne, P.W.; Gimeno-Fabra, M.; Zilka, M.; Ticha, M.; Stieberova, B.; Freiberg, F.; McKechnie, J.; Lester, E. Assessing the life cycle environmental impacts of titania nanoparticle production by continuous flow solvo/hydrothermal syntheses. Green Chem. 2017, 19, 1536–1547. [Google Scholar] [CrossRef]
- Wu, F.; Zhou, Z.; Hicks, A.L. Life cycle impact of titanium dioxide nanoparticle synthesis through physical, chemical, and biological routes. Environ. Sci. Technol. 2019, 53, 4078–4087. [Google Scholar] [CrossRef] [PubMed]
- Nakata, K.; Fujishima, A. TiO2 photocatalysis: Design and applications. J. Photochem. Photobiol. C Photochem. Rev. 2012, 13, 169–189. [Google Scholar]
- Armaković, S.J.; Savanović, M.M.; Armaković, S. Titanium Dioxide as the Most Used Photocatalyst for Water Purification: An Overview. Catalysts 2023, 13, 26. [Google Scholar]
- Fujishima, A.; Honda, K. Electrochemical photolysis of water at a semiconductor electrode. Nature 1972, 238, 37. [Google Scholar] [CrossRef]
- Schneider, J.; Matsuoka, M.; Takeuchi, M.; Zhang, J.; Horiuchi, Y.; Anpo, M.; Bahnemann, D.W. Understanding TiO2 Photocatalysis: Mechanisms and Materials. Chem. Rev. 2014, 114, 9919–9986. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Alonso, M.D.; Fresno, F.; Suárez, S.; Coronado, J.M. Development of alternative photocatalysts to TiO2: Challenges and opportunities. Energy Environ. Sci. 2009, 2, 1231–1257. [Google Scholar]
- Kandiel, T.A.; Robben, L.; Alkaima, A.; Bahnemann, D. Brookite versus anatase TiO2 photocatalysts: Phase transformations and photocatalytic activities. Photochem. Photobiol. Sci. 2013, 12, 602–609. [Google Scholar] [CrossRef]
- Zhang, J.; Zhou, P.; Liu, J.; Yu, J. New understanding of the difference of photocatalytic activity among anatase, rutile and brookite TiO2. Phys. Chem. Chem. Phys. 2014, 16, 20382–20386. [Google Scholar] [CrossRef]
- Luttrell, T.; Halpegamage, S.; Tao, J.; Kramer, A.; Sutter, E.; Batzill, M. Why is anatase a better photocatalyst than rutile?—Model studies on epitaxial TiO2 films. Sci. Rep. 2014, 4, 4043. [Google Scholar] [CrossRef]
- Rej, S.; Hejazi, S.H.; Badura, Z.k.; Zoppellaro, G.; Kalytchuk, S.; Kment, S.; Fornasiero, P.; Naldoni, A. Light-induced defect formation and Pt single atoms synergistically boost photocatalytic H2 production in 2D TiO2-bronze nanosheets. ACS Sustain. Chem. Eng. 2022, 10, 17286–17296. [Google Scholar] [CrossRef]
- Katal, R.; Masudy-Panah, S.; Tanhaei, M.; Farahani, M.H.D.A.; Jiangyong, H. A review on the synthesis of the various types of anatase TiO2 facets and their applications for photocatalysis. Chem. Eng. J. 2020, 384, 123384. [Google Scholar] [CrossRef]
- Dong, H.; Zeng, G.; Tang, L.; Fan, C.; Zhang, C.; He, X.; He, Y. An overview on limitations of TiO2-based particles for photocatalytic degradation of organic pollutants and the corresponding countermeasures. Water Res. 2015, 79, 128–146. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.M.; Tripathi, M. A review on the synthesis of TiO2 nanoparticles by solution route. Cent. Eur. J. Chem. 2012, 10, 279–294. [Google Scholar] [CrossRef]
- Han, X.-H.; Li, C.-Q.; Tang, P.; Feng, C.-X.; Yue, X.-Z.; Zhang, W.-L. Solid-Phase Synthesis of Titanium Dioxide Micro-Nanostructures. ACS Omega 2022, 7, 35538–35544. [Google Scholar] [CrossRef]
- Khan, S.; Katsumata, K.-i.; Rodríguez-González, V.; Terashima, C.; Fujishima, A. Gas-Phase Synthesis for Mass Production of TiO2 Nanoparticles for Environmental Applications. In Handbook of Nanomaterials and Nanocomposites for Energy and Environmental Applications; Kharissova, O.V., Torres-Martínez, L.M., Kharisov, B.I., Eds.; Springer International Publishing: Cham, Switzerland, 2021; pp. 953–973. [Google Scholar] [CrossRef]
- Wu, J.-M.; Shih, H.C.; Wu, W.-T.; Tseng, Y.-K.; Chen, I.C. Thermal evaporation growth and the luminescence property of TiO2 nanowires. J. Cryst. Growth 2005, 281, 384–390. [Google Scholar] [CrossRef]
- Samal, S. Synthesis of TiO2 Nanoparticles from Ilmenite Through the Mechanism of Vapor-Phase Reaction Process by Thermal Plasma Technology. J. Mater. Eng. Perform. 2018, 27, 2622–2628. [Google Scholar] [CrossRef]
- Heo, C.H.; Lee, S.-B.; Boo, J.-H. Deposition of TiO2 thin films using RF magnetron sputtering method and study of their surface characteristics. Thin Solid Film. 2005, 475, 183–188. [Google Scholar] [CrossRef]
- Saraf, L.V.; Patil, S.I.; Ogale, S.B.; Sainkar, S.R.; Kshirsager, S.T. Synthesis of nanophase TiO2 by ion beam sputtering and cold condensation technique. Int. J. Mod. Phys. B 1998, 12, 2635–2647. [Google Scholar] [CrossRef]
- Lee, H.; Song, M.Y.; Jurng, J.; Park, Y.-K. The synthesis and coating process of TiO2 nanoparticles using CVD process. Powder Technol. 2011, 214, 64–68. [Google Scholar] [CrossRef]
- Bessergenev, V.G.; Khmelinskii, I.V.; Pereira, R.J.F.; Krisuk, V.V.; Turgambaeva, A.E.; Igumenov, I.K. Preparation of TiO2 films by CVD method and its electrical, structural and optical properties. Vacuum 2002, 64, 275–279. [Google Scholar] [CrossRef]
- Goossens, A.; Maloney, E.L.; Schoonman, J. Gas-phase synthesis of nanostructured anatase TiO2. Chem. Vap. Depos. 1998, 4, 109–114. [Google Scholar] [CrossRef]
- Cargnello, M.; Gordon, T.R.; Murray, C.B. Solution-phase synthesis of titanium dioxide nanoparticles and nanocrystals. Chem. Rev. 2014, 114, 9319–9345. [Google Scholar] [CrossRef] [PubMed]
- Ragadhita, R.; Nandiyanto, A.B.D.; Maulana, A.C.; Oktiani, R.; Sukmafitri, A.; Machmud, A.; Surachman, E. Techno-economic analysis for the production of titanium dioxide nanoparticle produced by liquid-phase synthesis method. J. Eng. Sci. Technol. 2019, 14, 1639–1652. [Google Scholar]
- Anastas, P.T.; Warner, J.C. Principles of green chemistry. In Green Chemistry: Theory and Practice; Oxford University Press: New York, NY, USA, 1998; pp. 29–56. [Google Scholar]
- Sumerel, J.L.; Yang, W.; Kisailus, D.; Weaver, J.C.; Choi, J.H.; Morse, D.E. Biocatalytically templated synthesis of titanium dioxide. Chem. Mater. 2003, 15, 4804–4809. [Google Scholar] [CrossRef]
- Mann, S. Biomineralization: Principles and Concepts in Bioinorganic Materials Chemistry; Oxford University Press: Oxford, UK, 2001. [Google Scholar]
- Patwardhan, S.V.; Manning, J.R.; Chiacchia, M. Bioinspired synthesis as a potential green method for the preparation of nanomaterials: Opportunities and challenges. Curr. Opin. Green Sustain. Chem. 2018, 12, 110–116. [Google Scholar] [CrossRef]
- Kröger, N.; Dickerson, M.B.; Ahmad, G.; Cai, Y.; Haluska, M.S.; Sandhage, K.H.; Poulsen, N.; Sheppard, V.C. Bioenabled synthesis of rutile (TiO2) at ambient temperature and neutral pH. Angew. Chem. Int. Ed. 2006, 45, 7239–7243. [Google Scholar] [CrossRef]
- Puddu, V.; Slocik, J.M.; Naik, R.R.; Perry, C.C. Titania binding peptides as templates in the biomimetic synthesis of stable titania nanosols: Insight into the role of buffers in peptide-mediated mineralization. Langmuir 2013, 29, 9464–9472. [Google Scholar] [CrossRef]
- Banerjee, I.A.; Yu, L.; Matsui, H. Cu nanocrystal growth on peptide nanotubes by biomineralization: Size control of Cu nanocrystals by tuning peptide conformation. Proc. Natl. Acad. Sci. USA 2003, 100, 14678–14682. [Google Scholar] [CrossRef]
- Sano, K.-I.; Sasaki, H.; Shiba, K. Specificity and biomineralization activities of Ti-binding peptide-1 (TBP-1). Langmuir 2005, 21, 3090–3095. [Google Scholar] [CrossRef]
- Patwardhan, S.V.; Staniland, S.S. Green Nanomaterials; IOP Publishing: Bristol, UK, 2019. [Google Scholar]
- Pilling, R.; Coles, S.R.; Knecht, M.R.; Patwardhan, S.V. Multi-criteria discovery, design and manufacturing to realise nanomaterial potential. Commun. Eng. 2023, 2, 78. [Google Scholar] [CrossRef]
- Langa, C.; Hintsho-Mbita, N.C. Plant and bacteria mediated synthesis of TiO2 NPs for dye degradation in water. A review. Chem. Phys. Impact 2023, 7, 100293. [Google Scholar] [CrossRef]
- Rajaram, P.; Jeice, A.R.; Jayakumar, K. Review of green synthesized TiO2 nanoparticles for diverse applications. Surf. Interfaces 2023, 39, 102912. [Google Scholar] [CrossRef]
- Sagadevan, S.; Imteyaz, S.; Murugan, B.; Lett, J.A.; Sridewi, N.; Weldegebrieal, G.K.; Fatimah, I.; Oh, W.-C. A comprehensive review on green synthesis of titanium dioxide nanoparticles and their diverse biomedical applications. Green Process. Synth. 2022, 11, 44–63. [Google Scholar] [CrossRef]
- Verma, V.; Al-Dossari, M.; Singh, J.; Rawat, M.; Kordy, M.G.; Shaban, M. A review on green synthesis of TiO2 NPs: Photocatalysis and antimicrobial applications. Polymers 2022, 14, 1444. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Gu, B.; Liang, L.; Hamilton, W.A.; Wesolowski, D.J. Synthesis of rutile (α-TiO2) nanocrystals with controlled size and shape by low-temperature hydrolysis: Effects of solvent composition. J. Phys. Chem. B 2004, 108, 14789–14792. [Google Scholar] [CrossRef]
- Collins, A. Nanotechnology Cookbook: Practical, Reliable and Jargon-Free Experimental Procedures; Elsevier: Amsterdam, The Netherlands, 2012. [Google Scholar]
- Gordon, T.R.; Cargnello, M.; Paik, T.; Mangolini, F.; Weber, R.T.; Fornasiero, P.; Murray, C.B. Nonaqueous Synthesis of TiO2 Nanocrystals Using TiF4 to Engineer Morphology, Oxygen Vacancy Concentration, and Photocatalytic Activity. J. Am. Chem. Soc. 2012, 134, 6751–6761. [Google Scholar] [CrossRef]
- Liu, S.; Yu, J.; Jaroniec, M. Anatase TiO2 with dominant high-energy {001} facets: Synthesis, properties, and applications. Chem. Mater. 2011, 23, 4085–4093. [Google Scholar] [CrossRef]
- Brinker, C.J.; Scherer, G.W. Sol-Gel Science: The Physics and Chemistry of Sol-Gel Processing; Academic Press: Cambridge, MA, USA, 2013. [Google Scholar]
- Sanchez, C.; Livage, J.; Henry, M.; Babonneau, F. Chemical modification of alkoxide precursors. J. Non-Cryst. Solids 1988, 100, 65–76. [Google Scholar] [CrossRef]
- Gai, L.; Mei, Q.; Qin, X.; Li, W.; Jiang, H.; Duan, X. Controlled synthesis of anatase TiO2 octahedra with enhanced photocatalytic activity. Mater. Res. Bull. 2013, 48, 4469–4475. [Google Scholar] [CrossRef]
- Yu, J.; Liu, S.; Yu, H. Microstructures and photoactivity of mesoporous anatase hollow microspheres fabricated by fluoride-mediated self-transformation. J. Catal. 2007, 249, 59–66. [Google Scholar] [CrossRef]
- Liao, J.; Luo, R.; Li, Y.B.; Zhang, J. Preparation of highly photocatalytically active rutile titania nanorods decorated with anatase nanoparticles produced by a titanyl-oxalato complex solution. Mater. Sci. Semicond. Process. 2013, 16, 2032–2038. [Google Scholar] [CrossRef]
- Zhang, M.; Chen, T.; Wang, Y. Insights into TiO2 polymorphs: Highly selective synthesis, phase transition, and their polymorph-dependent properties. RSC Adv. 2017, 7, 52755–52761. [Google Scholar] [CrossRef]
- Zheng, Z.; Wang, Z.; Xie, L.; Fang, Z.; Feng, W.; Huang, M.; Liu, P. Synthesis of single-crystal-like TiO2 hierarchical spheres with exposed {1 0 1} and {1 1 1} facets via lysine-inspired method. Appl. Surf. Sci. 2015, 353, 714–722. [Google Scholar] [CrossRef]
- Möckel, H.; Giersig, M.; Willig, F. Formation of uniform size anatase nanocrystals from bis (ammonium lactato) titanium dihydroxide by thermohydrolysis. J. Mater. Chem. 1999, 9, 3051–3056. [Google Scholar] [CrossRef]
- Seisenbaeva, G.A.; Daniel, G.; Nedelec, J.-M.; Kessler, V.G. Solution equilibrium behind the room-temperature synthesis of nanocrystalline titanium dioxide. Nanoscale 2013, 5, 3330–3336. [Google Scholar] [CrossRef]
- Hernández-Gordillo, A.; Hernández-Arana, A.; Campero-Celis, A.; Vera-Robles, L.I. TiBALDH as a precursor for biomimetic TiO2 synthesis: Stability aspects in aqueous media. RSC Adv. 2019, 9, 34559–34566. [Google Scholar] [CrossRef]
- Hanprasopwattana, A.; Rieker, T.; Sault, A.G.; Datye, A.K. Morphology of titania coatings on silica gel. Catal. Lett. 1997, 45, 165–175. [Google Scholar] [CrossRef]
- Hidalgo-Carrillo, J.; Martín-Gómez, J.; Herrera-Beurnio, M.C.; Estévez, R.C.; Urbano, F.J.; Marinas, A. Olive leaves as biotemplates for enhanced solar-light harvesting by a titania-based solid. Nanomaterials 2020, 10, 1057. [Google Scholar] [CrossRef]
- Hashemizadeh, I.; Golovko, V.B.; Choi, J.; Tsang, D.C.; Yip, A.C. Photocatalytic reduction of CO2 to hydrocarbons using bio-templated porous TiO2 architectures under UV and visible light. Chem. Eng. J. 2018, 347, 64–73. [Google Scholar] [CrossRef]
- Billet, J.; Dujardin, W.; De Keukeleere, K.; De Buysser, K.; De Roo, J.; Van Driessche, I. Size tunable synthesis and surface chemistry of metastable TiO2-bronze nanocrystals. Chem. Mater. 2018, 30, 4298–4306. [Google Scholar] [CrossRef]
- Truong, Q.D.; Le, T.H.; Hoa, H.T. Amino acid-assisted controlling the shapes of rutile, brookite for enhanced photocatalytic CO2 reduction. CrystEngComm 2017, 19, 4519–4527. [Google Scholar] [CrossRef]
- Ismail, A.A.; Kandiel, T.A.; Bahnemann, D.W. Novel (and better?) titania-based photocatalysts: Brookite nanorods and mesoporous structures. J. Photochem. Photobiol. A Chem. 2010, 216, 183–193. [Google Scholar] [CrossRef]
- Hao, Y.; Rui, Y.; Li, Y.; Zhang, Q.; Wang, H. Size-tunable TiO2 nanocrystals from titanium (IV) bis (ammonium lactato) dihydroxide and towards enhance the performance of dye-sensitized solar cells. Electrochim. Acta 2014, 117, 268–275. [Google Scholar] [CrossRef]
- Tong, Z.; Yang, D.; Xiao, T.; Tian, Y.; Jiang, Z. Biomimetic fabrication of g-C3N4/TiO2 nanosheets with enhanced photocatalytic activity toward organic pollutant degradation. Chem. Eng. J. 2015, 260, 117–125. [Google Scholar] [CrossRef]
- Shi, J.; Yang, D.; Jiang, Z.; Jiang, Y.; Liang, Y.; Zhu, Y.; Wang, X.; Wang, H. Simultaneous size control and surface functionalization of titania nanoparticles through bioadhesion-assisted bio-inspired mineralization. J. Nanoparticle Res. 2012, 14, 1120. [Google Scholar] [CrossRef]
- Cole, K.E.; Valentine, A.M. Spermidine and spermine catalyze the formation of nanostructured titanium oxide. Biomacromolecules 2007, 8, 1641–1647. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.; Hao, B.; Wang, X.; Chen, G. Bio-inspired synthesis of titania with polyamine induced morphology and phase transformation at room-temperature: Insight into the role of the protonated amino group. Dalton Trans. 2013, 42, 12179–12184. [Google Scholar] [CrossRef]
- Dickerson, M.B.; Jones, S.E.; Cai, Y.; Ahmad, G.; Naik, R.R.; Kröger, N.; Sandhage, K.H. Identification and design of peptides for the rapid, high-yield formation of nanoparticulate TiO2 from aqueous solutions at room temperature. Chem. Mater. 2008, 20, 1578–1584. [Google Scholar] [CrossRef]
- Buckle, E.L.; Lum, J.S.; Roehrich, A.M.; Stote, R.E.; Vandermoon, B.; Dracinsky, M.; Filocamo, S.F.; Drobny, G.P. Serine–lysine peptides as mediators for the production of titanium dioxide: Investigating the effects of primary and secondary structures using solid-state NMR spectroscopy and DFT calculations. J. Phys. Chem. B 2018, 122, 4708–4718. [Google Scholar] [CrossRef]
- Yang, G.; Guo, Q.; Kong, H.; Luan, X.; Wei, G. Structural design, biomimetic synthesis, and environmental sustainability of graphene-supported gC3N4/TiO2 hetero-aerogels. Environ. Sci. Nano 2023, 10, 1257–1267. [Google Scholar] [CrossRef]
- Sewell, S.L.; Wright, D.W. Biomimetic Synthesis of Titanium Dioxide Utilizing the R5 Peptide Derived from Cylindrotheca F Usiformis. Chem. Mater. 2006, 18, 3108–3113. [Google Scholar] [CrossRef]
- Stote, R.E.; Filocamo, S.F.; Lum, J.S. Silaffin primary structure and its effects on the precipitation morphology of titanium dioxide. J. Mater. Res. 2016, 31, 1373–1382. [Google Scholar] [CrossRef]
- Bregnhøj, M.; Lutz, H.; Roeters, S.J.; Lieberwirth, I.; Mertig, R.; Weidner, T. The diatom peptide R5 fabricates two-dimensional titanium dioxide nanosheets. J. Phys. Chem. Lett. 2022, 13, 5025–5029. [Google Scholar] [CrossRef] [PubMed]
- Hellner, B.; Stegmann, A.E.; Pushpavanam, K.; Bailey, M.J.; Baneyx, F. Phase control of nanocrystalline inclusions in bioprecipitated titania with a panel of mutant silica-binding proteins. Langmuir 2020, 36, 8503–8510. [Google Scholar] [CrossRef]
- Jiang, Y.; Yang, D.; Zhang, L.; Li, L.; Sun, Q.; Zhang, Y.; Li, J.; Jiang, Z. Biomimetic synthesis of titania nanoparticles induced by protamine. Dalton Trans. 2008, 4165–4171. [Google Scholar] [CrossRef]
- Gardères, J.; Elkhooly, T.A.; Link, T.; Markl, J.S.; Müller, W.E.; Renkel, J.; Korzhev, M.; Wiens, M. Self-assembly and photocatalytic activity of branched silicatein/silintaphin filaments decorated with silicatein-synthesized TiO2 nanoparticles. Bioprocess Biosyst. Eng. 2016, 39, 1477–1486. [Google Scholar] [CrossRef]
- Cheng, H.; Ma, J.; Zhao, Z.; Qi, L. Hydrothermal preparation of uniform nanosize rutile and anatase particles. Chem. Mater. 1995, 7, 663–671. [Google Scholar] [CrossRef]
- Testino, A.; Bellobono, I.R.; Buscaglia, V.; Canevali, C.; D’Arienzo, M.; Polizzi, S.; Scotti, R.; Morazzoni, F. Optimizing the photocatalytic properties of hydrothermal TiO2 by the control of phase composition and particle morphology. A systematic approach. J. Am. Chem. Soc. 2007, 129, 3564–3575. [Google Scholar] [CrossRef]
- Shin, H.; Jung, H.S.; Hong, K.S.; Lee, J.-K. Crystallization process of TiO2 nanoparticles in an acidic solution. Chem. Lett. 2004, 33, 1382–1383. [Google Scholar] [CrossRef]
- Durupthy, O.; Bill, J.; Aldinger, F. Bioinspired synthesis of crystalline TiO2: Effect of amino acids on nanoparticles structure and shape. Cryst. Growth Des. 2007, 7, 2696–2704. [Google Scholar] [CrossRef]
- Zhang, G.; Zhang, T.; Li, B.; Zhang, X.; Chen, X. Biomimetic synthesis of interlaced mesh structures TiO2 nanofibers with enhanced photocatalytic activity. J. Alloys Compd. 2016, 668, 113–120. [Google Scholar] [CrossRef]
- Gautam, A.; Kshirsagar, A.; Biswas, R.; Banerjee, S.; Khanna, P.K. Photodegradation of organic dyes based on anatase and rutile TiO2 nanoparticles. RSC Adv. 2016, 6, 2746–2759. [Google Scholar] [CrossRef]
- He, Z.; Que, W.; He, Y. Synthesis and characterization of bioinspired hierarchical mesoporous TiO2 photocatalysts. Mater. Lett. 2013, 94, 136–139. [Google Scholar] [CrossRef]
- Hariharan, D.; Christy, A.J.; Mayandi, J.; Nehru, L. Visible light active photocatalyst: Hydrothermal green synthesized TiO2 NPs for degradation of picric acid. Mater. Lett. 2018, 222, 45–49. [Google Scholar] [CrossRef]
- Sundrarajan, M.; Bama, K.; Bhavani, M.; Jegatheeswaran, S.; Ambika, S.; Sangili, A.; Nithya, P.; Sumathi, R. Obtaining titanium dioxide nanoparticles with spherical shape and antimicrobial properties using M. citrifolia leaves extract by hydrothermal method. J. Photochem. Photobiol. B Biol. 2017, 171, 117–124. [Google Scholar]
- Thandapani, K.; Kathiravan, M.; Namasivayam, E.; Padiksan, I.A.; Natesan, G.; Tiwari, M.; Giovanni, B.; Perumal, V. Enhanced larvicidal, antibacterial, and photocatalytic efficacy of TiO2 nanohybrids green synthesized using the aqueous leaf extract of Parthenium hysterophorus. Environ. Sci. Pollut. Res. 2018, 25, 10328–10339. [Google Scholar] [CrossRef]
- Balaji, S.; Guda, R.; Mandal, B.K.; Kasula, M.; Ubba, E.; Khan, F.-R.N. Green synthesis of nano-titania (TiO2 NPs) utilizing aqueous Eucalyptus globulus leaf extract: Applications in the synthesis of 4 H-pyran derivatives. Res. Chem. Intermed. 2021, 47, 3919–3931. [Google Scholar] [CrossRef]
- Zeebaree, A.Y.S.; Zeebaree, S.Y.S.; Rashid, R.F.; Zebari, O.I.H.; Albarwry, A.J.S.; Ali, A.F.; Zebari, A.Y.S. Sustainable engineering of plant-synthesized TiO2 nanocatalysts: Diagnosis, properties and their photocatalytic performance in removing of methylene blue dye from effluent. A review. Curr. Res. Green Sustain. Chem. 2022, 5, 100312. [Google Scholar] [CrossRef]
- Pushpavanam, K.; Hellner, B.; Baneyx, F. Interrogating biomineralization one amino acid at a time: Amplification of mutational effects in protein-aided titania morphogenesis through reaction-diffusion control. Chem. Commun. 2021, 57, 4803–4806. [Google Scholar] [CrossRef]
- Katagiri, K.; Inami, H.; Ishikawa, T.; Koumoto, K. Enzyme-Assisted Synthesis of Titania under Ambient Conditions. J. Am. Ceram. Soc. 2009, 92, S181–S184. [Google Scholar] [CrossRef]
- Nayak, J.; Meher, S.; Begum, G.; Seth, S.; Rana, R.K. Bioinspired Mineralization and Assembly Route to Integrate TiO2 and Carbon Nitride Nanostructures: Designing Microstructures for Photoregeneration of NADH. ACS Appl. Nano Mater. 2023, 6, 13708–13719. [Google Scholar] [CrossRef]
- Lu, Z.; Zhou, H.F.; Liao, J.J.; Yang, Y.Y.; Wang, K.; Che, L.M.; He, N.; Chen, X.D.; Song, R.; Cai, W.F.; et al. A facile dopamine-assisted method for the preparation of antibacterial surfaces based on Ag/TiO2 nanoparticles. Appl. Surf. Sci. 2019, 481, 1270–1276. [Google Scholar] [CrossRef]
- Hayami, Y.; Suzuki, Y.; Sagawa, T.; Yoshikawa, S. TiO2 Rutile Nanorod Arrays Grown on FTO Substrate Using Amino Acid at a Low Temperature. J. Nanosci. Nanotechnol. 2010, 10, 2284–2291. [Google Scholar] [CrossRef]
- Bakre, P.V.; Tilve, S.G.; Ghosh, N.N. Investigation of amino acids as templates for the sol–gel synthesis of mesoporous nano TiO2 for photocatalysis. Monatshefte Für Chem. -Chem. Mon. 2018, 149, 11–18. [Google Scholar] [CrossRef]
- Tao, Y.-G.; Xu, Y.-Q.; Pan, J.; Gu, H.; Qin, C.-Y.; Zhou, P. Glycine assisted synthesis of flower-like TiO2 hierarchical spheres and its application in photocatalysis. Mater. Sci. Eng. B 2012, 177, 1664–1671. [Google Scholar] [CrossRef]
- Ding, S.; Huang, F.; Mou, X.; Wu, J.; Lü, X. Mesoporous hollow TiO2 microspheres with enhanced photoluminescence prepared by a smart amino acid template. J. Mater. Chem. 2011, 21, 4888–4892. [Google Scholar] [CrossRef]
- Bakre, P.V.; Volvoikar, P.S.; Vernekar, A.A.; Tilve, S. Influence of acid chain length on the properties of TiO2 prepared by sol-gel method and LC-MS studies of methylene blue photodegradation. J. Colloid Interface Sci. 2016, 474, 58–67. [Google Scholar] [CrossRef]
- Guo, Y.; Lin, S.; Li, X.; Liu, Y. Amino acids assisted hydrothermal synthesis of hierarchically structured ZnO with enhanced photocatalytic activities. Appl. Surf. Sci. 2016, 384, 83–91. [Google Scholar] [CrossRef]
- Lin, S.; Guo, Y.; Li, X.; Liu, Y. Glycine acid-assisted green hydrothermal synthesis and controlled growth of WO3 nanowires. Mater. Lett. 2015, 152, 102–104. [Google Scholar] [CrossRef]
- Wu, S.; Cao, H.; Yin, S.; Liu, X.; Zhang, X. Amino acid-assisted hydrothermal synthesis and photocatalysis of SnO2 nanocrystals. J. Phys. Chem. C 2009, 113, 17893–17898. [Google Scholar] [CrossRef]
- Jancik Prochazkova, A.; Demchyshyn, S.; Yumusak, C.; Masilko, J.; Bruggemann, O.; Weiter, M.; Kaltenbrunner, M.; Sariciftci, N.S.; Krajcovic, J.; Salinas, Y. Proteinogenic amino acid assisted preparation of highly luminescent hybrid perovskite nanoparticles. ACS Appl. Nano Mater. 2019, 2, 4267–4274. [Google Scholar] [CrossRef]
- Kang, L.; Xu, P.; Chen, D.; Zhang, B.; Du, Y.; Han, X.; Li, Q.; Wang, H.-L. Amino acid-assisted synthesis of hierarchical silver microspheres for single particle surface-enhanced Raman spectroscopy. J. Phys. Chem. C 2013, 117, 10007–10012. [Google Scholar] [CrossRef]
- Hong, C.-B.; Zhu, D.-J.; Ma, D.-D.; Wu, X.-T.; Zhu, Q.-L. An effective amino acid-assisted growth of ultrafine palladium nanocatalysts toward superior synergistic catalysis for hydrogen generation from formic acid. Inorg. Chem. Front. 2019, 6, 975–981. [Google Scholar] [CrossRef]
- Duan, W.; Li, A.; Chen, Y.; Zhang, J.; Zhuo, K. Amino acid-assisted preparation of reduced graphene oxide-supported PtCo bimetallic nanospheres for electrocatalytic oxidation of methanol. J. Appl. Electrochem. 2019, 49, 413–421. [Google Scholar] [CrossRef]
- Chen, Y.; Luo, X.; Luo, Y.; Xu, P.; He, J.; Jiang, L.; Li, J.; Yan, Z.; Wang, J. Efficient charge carrier separation in l-alanine acids derived N-TiO2 nanospheres: The role of oxygen vacancies in tetrahedral Ti4+ sites. Nanomaterials 2019, 9, 698. [Google Scholar] [CrossRef]
- Wu, X.; Liu, J.; Chen, Z.; Yang, Q.; Li, C.; Lu, G.; Wang, L. Amino acid assisted synthesis of mesoporous TiO2 nanocrystals for high performance dye-sensitized solar cells. J. Mater. Chem. 2012, 22, 10438–10440. [Google Scholar] [CrossRef]
- Liu, C.; Jiang, Z.; Tong, Z.; Li, Y.; Yang, D. Biomimetic synthesis of inorganic nanocomposites y a de novo designed peptide. RSC Adv. 2014, 4, 434–441. [Google Scholar] [CrossRef]
- Kanie, K.; Sugimoto, T. Shape control of anatase TiO2 nanoparticles by amino acids in a gel–sol system. Chem. Commun. 2004, 1584–1585. [Google Scholar] [CrossRef]
- Ayorinde, T.; Sayes, C.M. An updated review of industrially relevant titanium dioxide and its environmental health effects. J. Hazard. Mater. Lett. 2023, 4, 100085. [Google Scholar] [CrossRef]
- Dolai, J.; Mandal, K.; Jana, N.R. Nanoparticle Size Effects in Biomedical Applications. ACS Appl. Nano Mater. 2021, 4, 6471–6496. [Google Scholar] [CrossRef]
- Xie, Y.; Kocaefe, D.; Chen, C.; Kocaefe, Y. Review of Research on Template Methods in Preparation of Nanomaterials. J. Nanomater. 2016, 2016, 2302595. [Google Scholar] [CrossRef]
- Ghaedi, H.; Zhao, M. Review on Template Removal Techniques for Synthesis of Mesoporous Silica Materials. Energy Fuels 2022, 36, 2424–2446. [Google Scholar] [CrossRef]
- Tran, T.H.; Nosaka, A.Y.; Nosaka, Y. Adsorption and photocatalytic decomposition of amino acids in TiO2 photocatalytic systems. J. Phys. Chem. B 2006, 110, 25525–25531. [Google Scholar] [CrossRef] [PubMed]
- Fleming, G.J.; Adib, K.; Rodriguez, J.A.; Barteau, M.A.; White, J.M.; Idriss, H. The adsorption and reactions of the amino acid proline on rutile TiO2(010) surfaces. Surf. Sci. 2008, 602, 2029–2038. [Google Scholar] [CrossRef]
- Shchelokov, A.; Palko, N.; Potemkin, V.; Grishina, M.; Morozov, R.; Korina, E.; Uchaev, D.; Krivtsov, I.; Bol’shakov, O. Adsorption of Native Amino Acids on Nanocrystalline TiO2: Physical Chemistry, QSPR, and Theoretical Modeling. Langmuir 2019, 35, 538–550. [Google Scholar] [CrossRef]
- Pászti, Z.; Guczi, L. Amino acid adsorption on hydrophilic TiO2: A sum frequency generation vibrational spectroscopy study. Vib. Spectrosc. 2009, 50, 48–56. [Google Scholar] [CrossRef]
- Lee, N.; Sverjensky, D.A.; Hazen, R.M. Cooperative and Competitive Adsorption of Amino Acids with Ca2+ on Rutile (α-TiO2). Environ. Sci. Technol. 2014, 48, 9358–9365. [Google Scholar] [CrossRef]
- Brandt, E.G.; Lyubartsev, A.P. Molecular Dynamics Simulations of Adsorption of Amino Acid Side Chain Analogues and a Titanium Binding Peptide on the TiO2 (100) Surface. J. Phys. Chem. C 2015, 119, 18126–18139. [Google Scholar] [CrossRef]
- Ustunol, I.B.; Gonzalez-Pech, N.I.; Grassian, V.H. pH-dependent adsorption of α-amino acids, lysine, glutamic acid, serine and glycine, on TiO2 nanoparticle surfaces. J. Colloid Interface Sci. 2019, 554, 362–375. [Google Scholar] [CrossRef]
- Agosta, L.; Zollo, G.; Arcangeli, C.; Buonocore, F.; Gala, F.; Celino, M. Water driven adsorption of amino acids on the (101) anatase TiO2 surface: An ab initio study. Phys. Chem. Chem. Phys. 2015, 17, 1556–1561. [Google Scholar] [CrossRef]
- Xue, M.J.; Sampath, J.; Gebhart, R.N.; Haugen, H.J.; Lyngstadaas, S.P.; Pfaendtner, J.; Drobny, G. Studies of Dynamic Binding of Amino Acids to TiO2 Nanoparticle Surfaces by Solution NMR and Molecular Dynamics Simulations. Langmuir 2020, 36, 10341–10350. [Google Scholar] [CrossRef] [PubMed]
- Sampath, J.; Kullman, A.; Gebhart, R.; Drobny, G.; Pfaendtner, J. Molecular recognition and specificity of biomolecules to titanium dioxide from molecular dynamics simulations. npj Comput. Mater. 2020, 6, 34. [Google Scholar] [CrossRef]
- Pantaleone, S.; Rimola, A.; Sodupe, M. Canonical, deprotonated, or zwitterionic? II. A computational study on amino acid interaction with the TiO2(110) rutile surface: Comparison with the anatase (101) surface. Phys. Chem. Chem. Phys. 2020, 22, 16862–16876. [Google Scholar] [CrossRef]
- Sano, K.-I.; Shiba, K. A hexapeptide motif that electrostatically binds to the surface of titanium. J. Am. Chem. Soc. 2003, 125, 14234–14235. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.; Liu, X.; Cai, A.; Xing, S.; Ma, Z. Glycine-assisted synthesis of mesoporous TiO2 nanostructures with improved photocatalytic activity. Ceram. Int. 2014, 40, 14765–14768. [Google Scholar] [CrossRef]
- Senna, M.; Myers, N.; Aimable, A.; Laporte, V.; Pulgarin, C.; Baghriche, O.; Bowen, P. Modification of titania nanoparticles for photocatalytic antibacterial activity via a colloidal route with glycine and subsequent annealing. J. Mater. Res. 2013, 28, 354–361. [Google Scholar] [CrossRef]
- Payan, A.; Fattahi, M.; Roozbehani, B. Synthesis, characterization and evaluations of TiO2 nanostructures prepared from different titania precursors for photocatalytic degradation of 4-chlorophenol in aqueous solution. J. Environ. Health Sci. Eng. 2018, 16, 41–54. [Google Scholar] [CrossRef]
- Jia, H.; Xiao, W.-J.; Zhang, L.; Zheng, Z.; Zhang, H.; Deng, F. In situ L-hydroxyproline functionalization and enhanced photocatalytic activity of TiO2 nanorods. J. Phys. Chem. C 2008, 112, 11379–11384. [Google Scholar] [CrossRef]
- Sarigul, G.; Chamorro-Mena, I.; Linares, N.; García-Martínez, J.; Serrano, E. Hybrid Amino Acid-TiO2 Materials with Tuneable Crystalline Structure and Morphology for Photocatalytic Applications. Adv. Sustain. Syst. 2021, 5, 2100076. [Google Scholar] [CrossRef]
- Goutam, S.P.; Saxena, G.; Singh, V.; Yadav, A.K.; Bharagava, R.N.; Thapa, K.B. Green synthesis of TiO2 nanoparticles using leaf extract of Jatropha curcas L. for photocatalytic degradation of tannery wastewater. Chem. Eng. J. 2018, 336, 386–396. [Google Scholar] [CrossRef]
- Leng, J.; Zhao, Y.; Zhang, J.; Bai, X.; Zhang, A.; Li, Q.; Huang, M.; Wang, J. Synthesis of In-Modified TiO2 Composite Materials from Waste Tobacco Stem Silk and Study of Their Catalytic Performance under Visible Light. Catalysts 2024, 14, 615. [Google Scholar] [CrossRef]
- Zangeneh, H.; Mousavi, S.A.; Eskandari, P. Comparison the visible photocatalytic activity and kinetic performance of amino acids (non-metal doped) TiO2 for degradation of colored wastewater effluent. Mater. Sci. Semicond. Process. 2022, 140, 106383. [Google Scholar] [CrossRef]
- Zangeneh, H.; Mousavi, S.A.; Eskandari, P.; Amarloo, E.; Farghelitiyan, J.; Mohammadi, S. Comparative Study on Photocatalytic Performance of TiO2 Doped with Different Amino Acids in Degradation of Antibiotics. Water 2023, 15, 535. [Google Scholar] [CrossRef]
- Zhou, H.; Ding, L.; Fan, T.; Ding, J.; Zhang, D.; Guo, Q. Leaf-inspired hierarchical porous CdS/Au/N-TiO2 heterostructures for visible light photocatalytic hydrogen evolution. Appl. Catal. B Environ. 2014, 147, 221–228. [Google Scholar] [CrossRef]
- Mallakpour, S.; Aalizadeh, R. A simple and convenient method for the surface coating of TiO2 nanoparticles with bioactive chiral diacids containing different amino acids as the coupling agent. Prog. Org. Coat. 2013, 76, 648–653. [Google Scholar] [CrossRef]
- Stavert, T.; Patwardhan, S.V.; Pilling, R.; Jorge, M. Unlocking the holy grail of sustainable and scalable mesoporous silica using computational modelling. RSC Sustain. 2023, 1, 432–438. [Google Scholar] [CrossRef]
- Dewulf, L.; Chiacchia, M.; Yeardley, A.S.; Milton, R.A.; Brown, S.F.; Patwardhan, S.V. Designing bioinspired green nanosilicas using statistical and machine learning approaches. Mol. Syst. Des. Eng. 2021, 6, 293–307. [Google Scholar] [CrossRef]
- Williamson, E.M.; Sun, Z.; Mora-Tamez, L.; Brutchey, R.L. Design of Experiments for Nanocrystal Syntheses: A How-To Guide for Proper Implementation. Chem. Mater. 2022, 34, 9823–9835. [Google Scholar] [CrossRef]
- Norfolk, L.; Dewulf, L.; Chiacchia, M.; Patwardhan, S.V.; Staniland, S.S. Simultaneous optimisation of shape and magnetisation of nanoparticles synthesised using a green bioinspired route. Mol. Syst. Des. Eng. 2024, 9, 300–310. [Google Scholar] [CrossRef]
- Coley, C.W.; Green, W.H.; Jensen, K.F. Machine learning in computer-aided synthesis planning. Acc. Chem. Res. 2018, 51, 1281–1289. [Google Scholar] [CrossRef]
- Tao, H.; Wu, T.; Aldeghi, M.; Wu, T.C.; Aspuru-Guzik, A.; Kumacheva, E. Nanoparticle synthesis assisted by machine learning. Nat. Rev. Mater. 2021, 6, 701–716. [Google Scholar] [CrossRef]
- Katoueizadeh, E.; Zebarjad, S.M.; Janghorban, K. Optimization of synthesis conditions of N-doped TiO2 nanoparticles using Taguchi robust design. Mater. Chem. Phys. 2017, 201, 69–77. [Google Scholar] [CrossRef]
- Sun, L.; An, T.; Wan, S.; Li, G.; Bao, N.; Hu, X.; Fu, J.; Sheng, G. Effect of synthesis conditions on photocatalytic activities of nanoparticulate TiO2 thin films. Sep. Purif. Technol. 2009, 68, 83–89. [Google Scholar] [CrossRef]
- Gao, B.; Sun, M.; Ding, Z.; Liu, W. Machine learning-optimized synthesis of doped TiO2 with improved photocatalytic performance: A multi-step workflow supported by designed wet-lab experiments. J. Alloys Compd. 2021, 881, 160534. [Google Scholar] [CrossRef]
- Pellegrino, F.; Isopescu, R.; Pellutiè, L.; Sordello, F.; Rossi, A.M.; Ortel, E.; Martra, G.; Hodoroaba, V.-D.; Maurino, V. Machine learning approach for elucidating and predicting the role of synthesis parameters on the shape and size of TiO2 nanoparticles. Sci. Rep. 2020, 10, 18910. [Google Scholar] [CrossRef]
- DeVierno Kreuder, A.; House-Knight, T.; Whitford, J.; Ponnusamy, E.; Miller, P.; Jesse, N.; Rodenborn, R.; Sayag, S.; Gebel, M.; Aped, I.; et al. A Method for Assessing Greener Alternatives between Chemical Products Following the 12 Principles of Green Chemistry. ACS Sustain. Chem. Eng. 2017, 5, 2927–2935. [Google Scholar] [CrossRef]
- Brambila, C.; Boyd, P.; Keegan, A.; Sharma, P.; Vetter, C.; Ponnusamy, E.; Patwardhan, S.V. A Comparison of Environmental Impact of Various Silicas Using a Green Chemistry Evaluator. ACS Sustain. Chem. Eng. 2022, 10, 5288–5298. [Google Scholar] [CrossRef]
- Patwardhan, S.V.; Staniland, S.S. Case study 2: Silica. In Green Nanomaterials: From Bioinspired Synthesis to Sustainable Manufacturing of Inorganic Nanomaterials; IOP Publishing: Bristol, UK, 2019; ISBN 978-0-7503-1221-9. [Google Scholar]
- Jundale, R.B.; Sonawane, J.R.; Palghadmal, A.V.; Jaiswal, H.K.; Deore, H.S.; Kulkarni, A.A. Scaling-up continuous production of mesoporous silica particles at kg scale: Design & operational strategies. React. Chem. Eng. 2024, 9, 1914–1923. [Google Scholar] [CrossRef]
- Baba, Y.D.; Chiacchia, M.; Patwardhan, S.V. A Novel Method for Understanding the Mixing Mechanisms to Enable Sustainable Manufacturing of Bioinspired Silica. ACS Eng. Au 2023, 3, 17–27. [Google Scholar] [CrossRef]
- Pilling, R.; Patwardhan, S.V. Recent Advances in Enabling Green Manufacture of Functional Nanomaterials: A Case Study of Bioinspired Silica. ACS Sustain. Chem. Eng. 2022, 10, 12048–12064. [Google Scholar] [CrossRef]
- Drummond, C.; McCann, R.; Patwardhan, S.V. A feasibility study of the biologically inspired green manufacturing of precipitated silica. Chem. Eng. J. 2014, 244, 483–492. [Google Scholar] [CrossRef]
- Yan, M.; Martell, S.; Dasog, M.; Brown, S.; Patwardhan, S.V. Cost-competitive manufacture of porous-silicon anodes via the magnesiothermic reduction: A techno-economic analysis. J. Power Sources 2023, 588, 233720. [Google Scholar] [CrossRef]
- Ashworth, D.J.; Driver, J.; Sasitharan, K.; Prasad, R.R.R.; Nicks, J.; Smith, B.J.; Patwardhan, S.V.; Foster, J.A. Scalable and sustainable manufacturing of ultrathin metal–organic framework nanosheets (MONs) for solar cell applications. Chem. Eng. J. 2023, 477, 146871. [Google Scholar] [CrossRef]
- Pottier, A.; Chaneac, C.; Tronc, E.; Mazerolles, L.; Jolivet, J.P. Synthesis of brookite TiO2 nanoparticles by thermolysis of TiCl4 in strongly acidic aqueous media. J. Mater. Chem. 2001, 11, 1116–1121. [Google Scholar] [CrossRef]
- Chen, K.-Y.; Chen, Y.-W. Synthesis of spherical titanium dioxide particles by homogeneous precipitation in acetone solution. J. Sol-Gel Sci. Technol. 2003, 27, 111–117. [Google Scholar] [CrossRef]
- Zhou, Y.; Antonietti, M. Synthesis of very small TiO2 nanocrystals in a room-temperature ionic liquid and their self-assembly toward mesoporous spherical aggregates. J. Am. Chem. Soc. 2003, 125, 14960–14961. [Google Scholar] [CrossRef]
- Boppella, R.; Basak, P.; Manorama, S.V. Viable Method for the Synthesis of Biphasic TiO2 Nanocrystals with Tunable Phase Composition and Enabled Visible-Light Photocatalytic Performance. ACS Appl. Mater. Interfaces 2012, 4, 1239–1246. [Google Scholar] [CrossRef]
- Yasin, A.; Guo, F.; Demopoulos, G.P. Continuous-reactor, pH-controlled synthesis of multifunctional mesoporous nanocrystalline anatase aggregates. Chem. Eng. J. 2016, 287, 398–409. [Google Scholar] [CrossRef]
- Gopal, M.; Chan, W.M.; De Jonghe, L. Room temperature synthesis of crystalline metal oxides. J. Mater. Sci. 1997, 32, 6001–6008. [Google Scholar] [CrossRef]
- Chemseddine, A.; Moritz, T. Nanostructuring titania: Control over nanocrystal structure, size, shape, and organization. Eur. J. Inorg. Chem. 1999, 1999, 235–245. [Google Scholar] [CrossRef]
- Nadzirah, S.; Foo, K.; Hashim, U. Morphological reaction on the different stabilizers of titanium dioxide nanoparticles. Int. J. Electrochem. Sci. 2015, 10, 5498–5512. [Google Scholar] [CrossRef]
- Patra, S.; Davoisne, C.; Bruyère, S.; Bouyanfif, H.; Cassaignon, S.; Taberna, P.L.; Sauvage, F. Room-Temperature Synthesis of High Surface Area Anatase TiO2 Exhibiting a Complete Lithium Insertion Solid Solution. Part. Part. Syst. Charact. 2013, 30, 1093–1104. [Google Scholar] [CrossRef]
- Gao, Y.; Wang, H.; Wu, J.; Zhao, R.; Lu, Y.; Xin, B. Controlled facile synthesis and photocatalytic activity of ultrafine high crystallinity TiO2 nanocrystals with tunable anatase/rutile ratios. Appl. Surf. Sci. 2014, 294, 36–41. [Google Scholar] [CrossRef]
- Fischer, K.; Grimm, M.; Meyers, J.; Dietrich, C.; Gläser, R.; Schulze, A. Photoactive microfiltration membranes via directed synthesis of TiO2 nanoparticles on the polymer surface for removal of drugs from water. J. Membr. Sci. 2015, 478, 49–57. [Google Scholar] [CrossRef]
- Ahmed, M.; Abou-Gamra, Z.; Medien, H.; Hamza, M. Effect of porphyrin on photocatalytic activity of TiO2 nanoparticles toward Rhodamine B photodegradation. J. Photochem. Photobiol. B Biol. 2017, 176, 25–35. [Google Scholar] [CrossRef]
- Wang, L.; Wu, D.; Guo, Z.; Yan, J.; Hu, Y.; Chang, Z.; Yuan, Q.; Ming, H.; Wang, J. Ultra-thin TiO2 sheets with rich surface disorders for enhanced photocatalytic performance under simulated sunlight. J. Alloys Compd. 2018, 745, 26–32. [Google Scholar] [CrossRef]
- Wu, Z.; Xue, Y.; Zou, Z.; Wang, X.; Gao, F. Single-crystalline titanium dioxide hollow tetragonal nanocones with large exposed (1 0 1) facets for excellent photocatalysis. J. Colloid Interface Sci. 2017, 490, 420–429. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mulay, M.R.; Patwardhan, S.V.; Martsinovich, N. Review of Bio-Inspired Green Synthesis of Titanium Dioxide for Photocatalytic Applications. Catalysts 2024, 14, 742. https://doi.org/10.3390/catal14110742
Mulay MR, Patwardhan SV, Martsinovich N. Review of Bio-Inspired Green Synthesis of Titanium Dioxide for Photocatalytic Applications. Catalysts. 2024; 14(11):742. https://doi.org/10.3390/catal14110742
Chicago/Turabian StyleMulay, Manasi R., Siddharth V. Patwardhan, and Natalia Martsinovich. 2024. "Review of Bio-Inspired Green Synthesis of Titanium Dioxide for Photocatalytic Applications" Catalysts 14, no. 11: 742. https://doi.org/10.3390/catal14110742
APA StyleMulay, M. R., Patwardhan, S. V., & Martsinovich, N. (2024). Review of Bio-Inspired Green Synthesis of Titanium Dioxide for Photocatalytic Applications. Catalysts, 14(11), 742. https://doi.org/10.3390/catal14110742







