Fabrication of Two-Dimensional B-Doped C3N4 Nanosheet-Encapsulated One-Dimensional Rod-like Mo-MOF-Derived MoS2 Heterojunctions for Enhanced Photocatalytic Ethanol Conversion and Synergistic Hydrogen Production
Abstract
1. Introduction
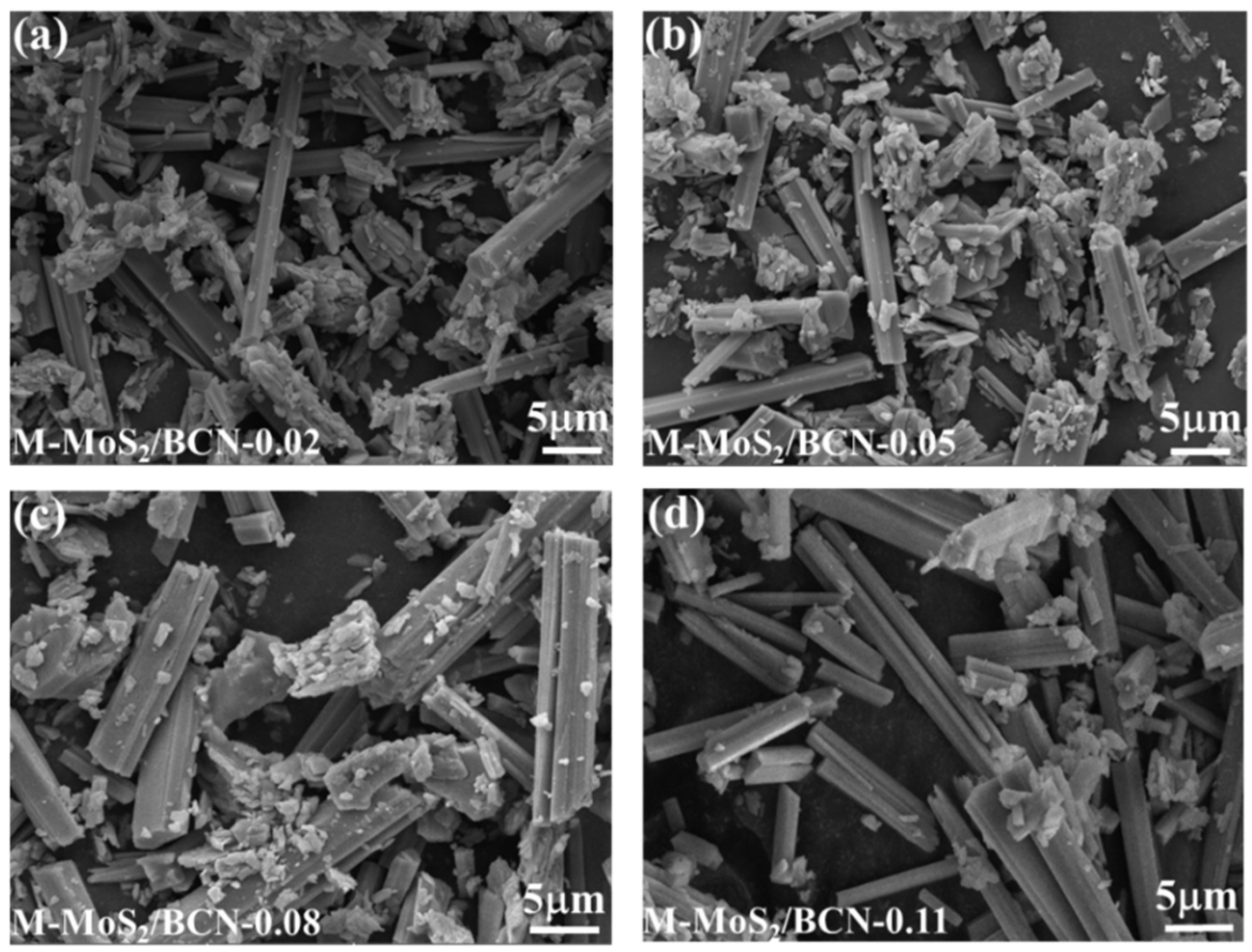
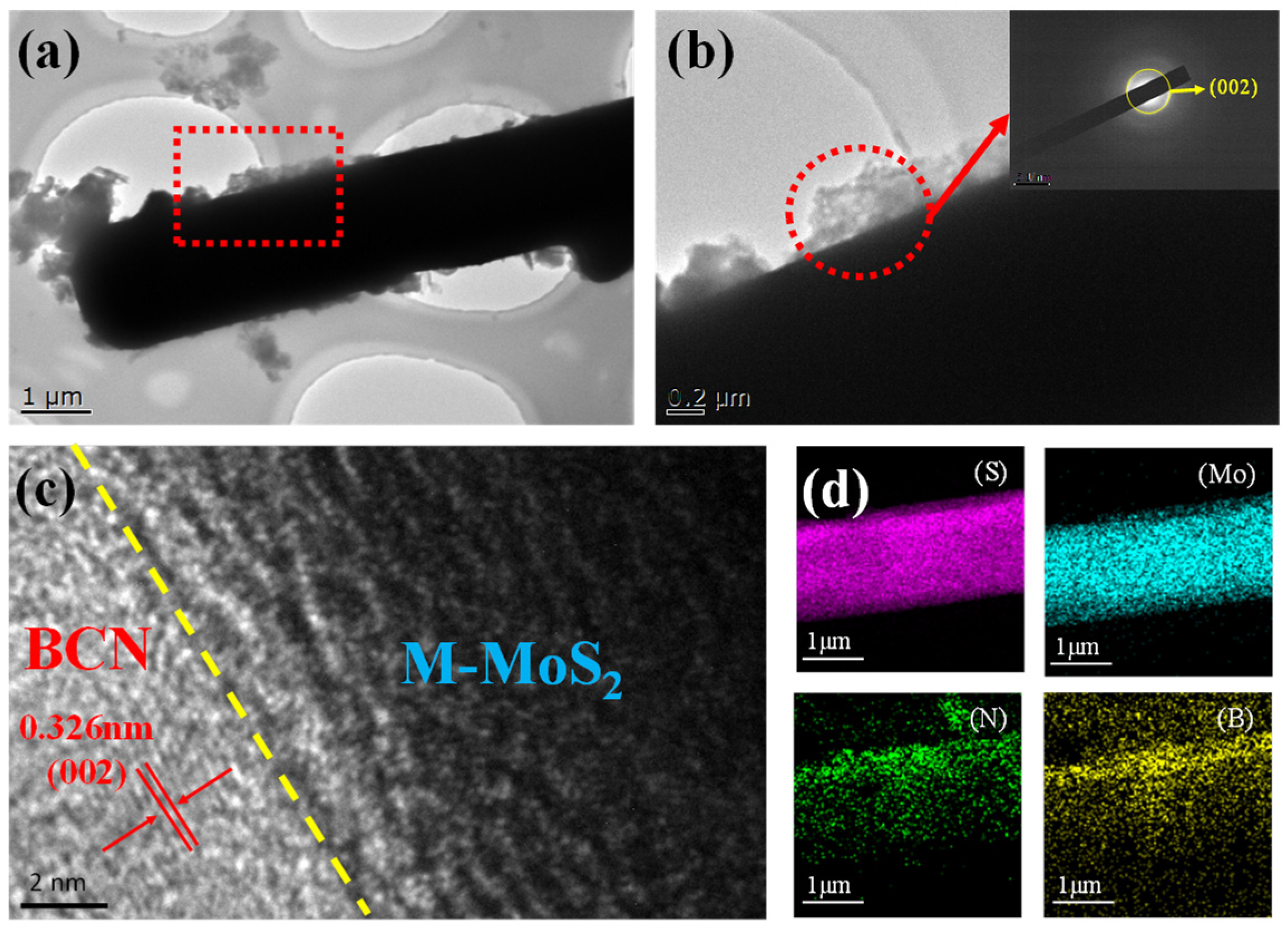
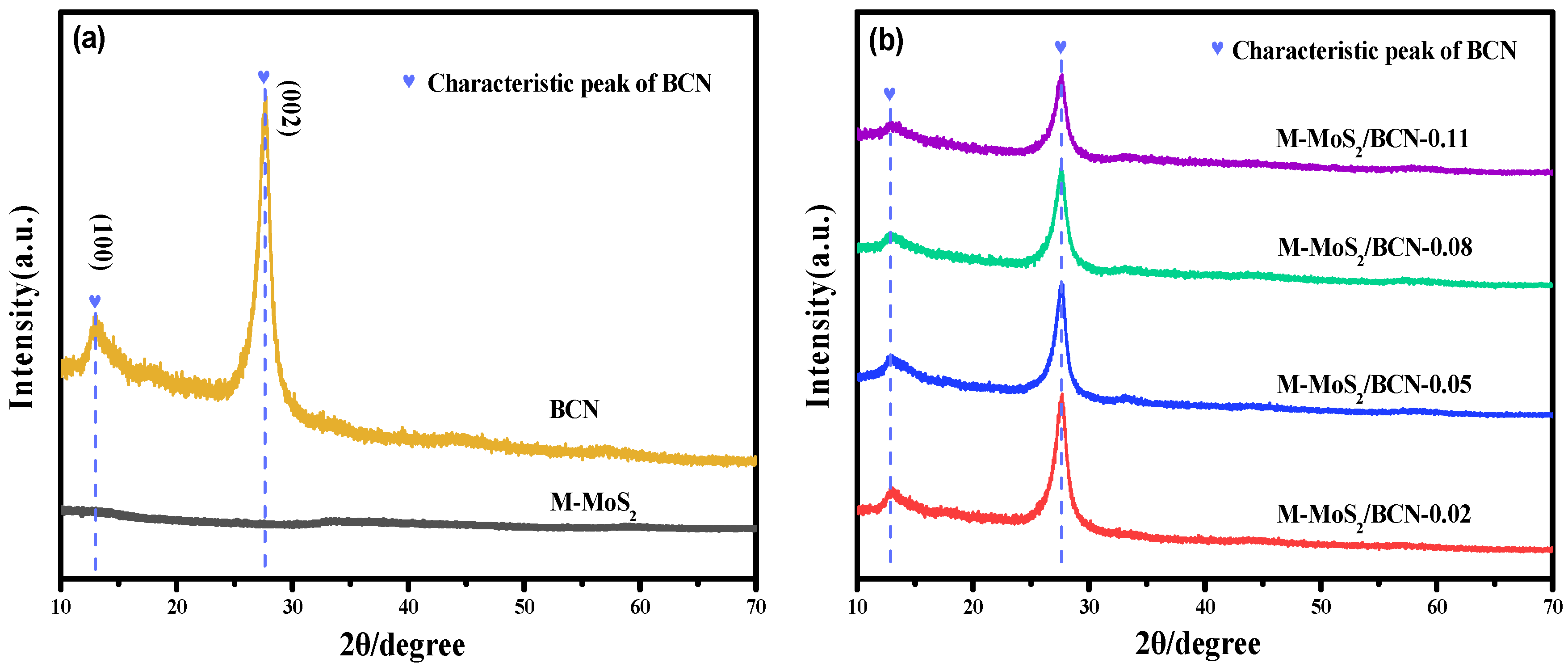
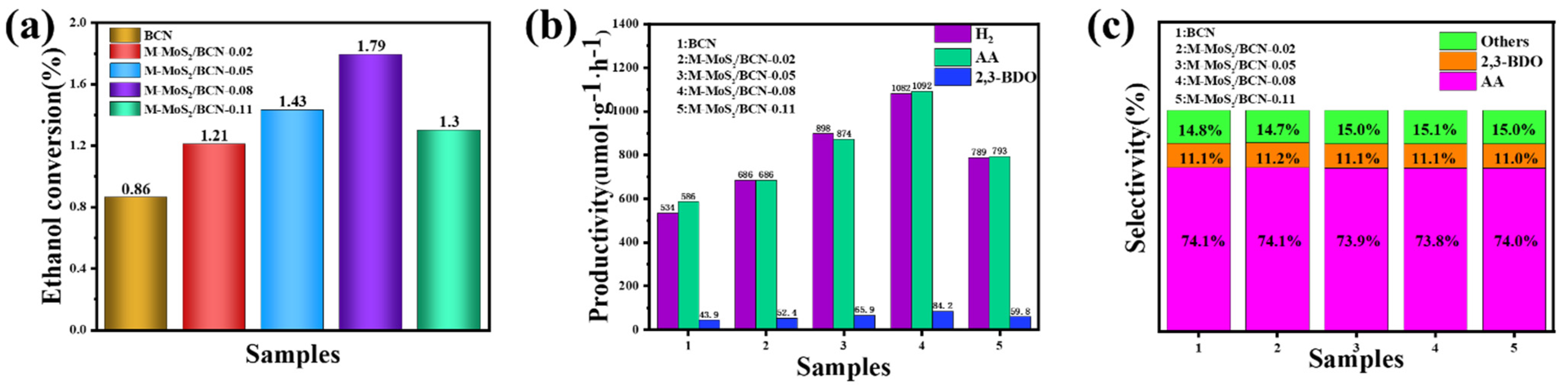
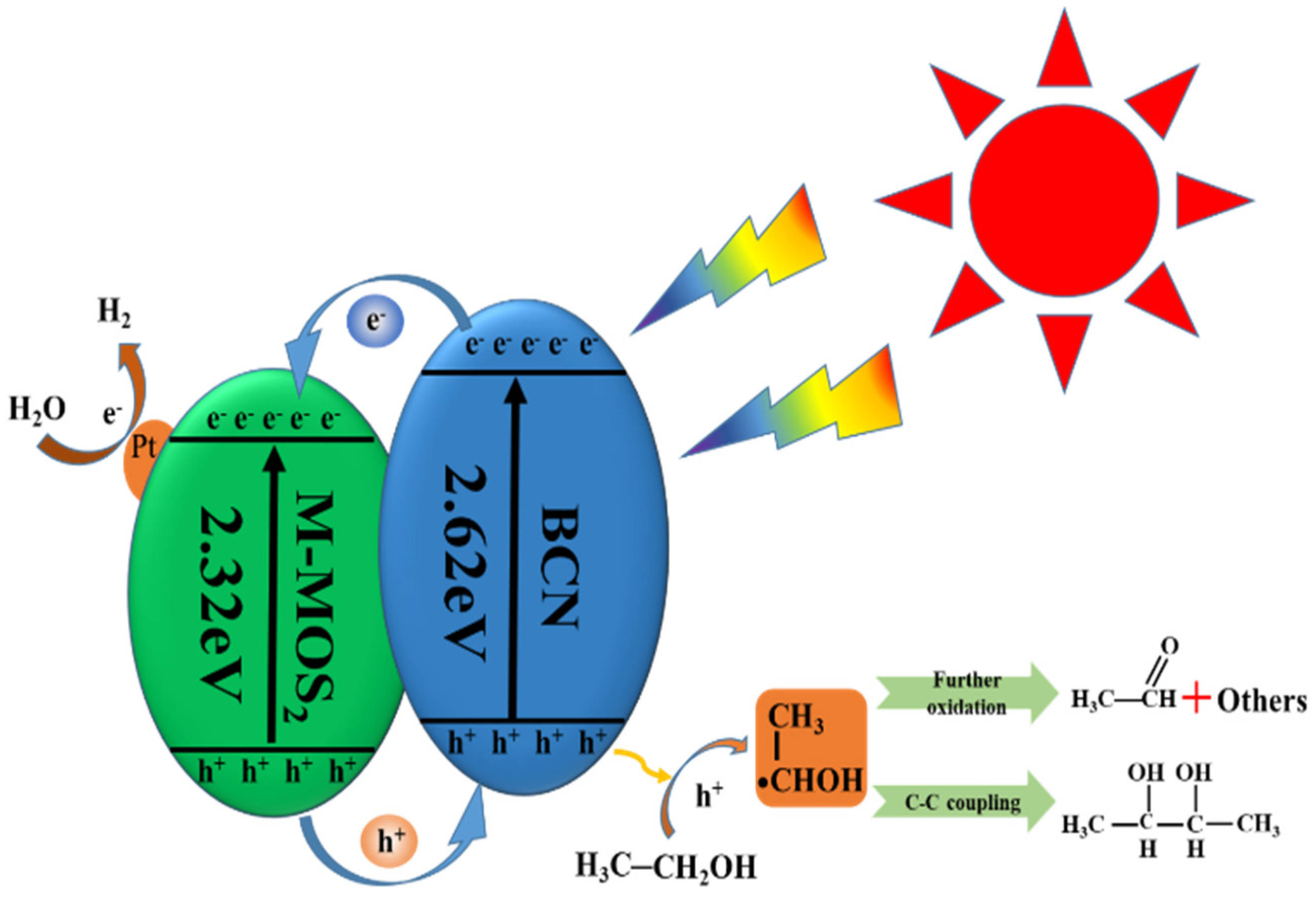
2. Results and Discussion
3. Materials and Methods
3.1. Synthesis of B-Doped g-C3N4 Nanosheets
3.2. Preparation of MoS2 Nanosheets (H-MoS2)
3.3. Preparation of Rod-like Mo-MOF and Derived MoS2 (M-MoS2)
3.4. Preparation of 1D/2D M-MoS2/BCN Heterostructure
3.5. Characterization of the Catalyst
3.6. Photocatalytic Hydrogen Production
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Liu, Y.; Zheng, X.; Yang, Y.; Li, J.; Liu, W.; Shen, Y.; Tian, X. Photocatalytic hydrogen evolution using ternary-metal-sulfide/TiO2 heterojunction photocatalysts. Chemcatchem 2022, 14, e202101439. [Google Scholar] [CrossRef]
- Lu, H.Q.; Zhao, J.H.; Li, L.; Gong, L.M.; Zheng, J.F.; Zhang, L.X.; Wang, Z.J.; Zhang, J.; Zhu, Z.P. Selective oxidation of sacrificial ethanol over TiO2-based photocatalysts during water splitting. Energy Environ. Sci. 2011, 4, 3384–3388. [Google Scholar] [CrossRef]
- Gao, Z.Y.; Mu, J.J.; Zhang, J.; Huang, Z.P.; Lin, X.S.; Luo, N.C.; Wang, F. Hydrogen bonding promotes alcohol C-C coupling. J. Am. Chem. Soc. 2022, 144, 18986–18994. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.K.; Xie, S.J.; Hu, J.Y.; Wu, X.J.; Zhang, Q.H.; Cheng, J.; Wang, Y. C-H activations of methanol and ethanol and C-C couplings into diols by zinc-indium-sulfide under visible light. Chem. Commun. 2020, 56, 1776–1779. [Google Scholar] [CrossRef] [PubMed]
- Li, X.Y.; Pi, Y.H.; Wu, L.Q.; Xia, Q.B.; Wu, J.L.; Li, Z.; Xiao, J. Facilitation of the visible light-induced Fenton-like excitation of H2O2 via heterojunction of g-C3N4/NH2-Iron terephthalate metal-organic framework for MB degradation. Appl. Catal. B Environ. 2017, 202, 653–663. [Google Scholar] [CrossRef]
- Li, S.N.; Dong, G.H.; Hailili, R.; Yang, L.P.; Li, Y.X.; Wang, F.; Zeng, Y.B.; Wang, C.Y. Effective photocatalytic H2O2 production under visible light irradiation at g-C3N4 modulated by carbon vacancies. Appl. Catal. B Environ. 2016, 190, 26–35. [Google Scholar] [CrossRef]
- Ma, Z.J.; Zhang, X.L.; Wu, D.P.; Han, X.Y.; Zhang, L.M.; Wang, H.J.; Xu, F.; Gao, Z.Y.; Jiang, K. Ni and nitrogen-codoped ultrathin carbon nanosheets with strong bonding sites for efficient CO2 electrochemical reduction. J. Colloid Interface Sci. 2020, 570, 31–40. [Google Scholar] [CrossRef]
- Niu, P.; Zhang, L.L.; Liu, G.; Cheng, H.M. Graphene-like carbon nitride nanosheets for improved photocatalytic activities. Adv. Funct. Mater. 2012, 22, 4763–4770. [Google Scholar] [CrossRef]
- Shcherban, N.D.; Diyuk, O.A.; Zazhigalov, V.A.; Murzin, D.Y. Graphitic carbon nitride as a sustainable catalyst for selective ethanol oxidation. ACS Sustain. Chem. Eng. 2021, 9, 5128–5137. [Google Scholar] [CrossRef]
- Li, Z.S.; Lin, R.S.; Liu, Z.S.; Li, D.H.; Wang, H.Q.; Li, Q.Y. Novel graphitic carbon nitride/graphite carbon/palladium nanocomposite as a high-performance electrocatalyst for the ethanol oxidation reaction. Electrochim. Acta 2016, 191, 606–615. [Google Scholar] [CrossRef]
- Xu, J.; Long, K.Z.; Chen, T.; Xue, B.; Li, Y.X.; Cao, Y. Mesostructured graphitic carbon nitride as a new base catalyst for the efficient synthesis of dimethyl carbonate by transesterification. Catal. Sci. Technol. 2013, 3, 3192–3199. [Google Scholar] [CrossRef]
- Mao, Z.Y.; Chen, J.J.; Yang, Y.F.; Wang, D.J.; Bie, L.J.; Fahlman, B.D. Novel g-C3N4/CoO nanocomposites with significantly enhanced visible-light photocatalytic activity for H2 evolution. ACS Appl. Mater. Interfaces 2017, 9, 12427–12435. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.H.; Wang, X.F.; Wang, F.Z.; Yu, H.G. Soluble g-C3N4 nanosheets: Facile synthesis and application in photocatalytic hydrogen evolution. Appl. Catal. B Environ. 2019, 247, 70–77. [Google Scholar] [CrossRef]
- Liao, G.F.; Gong, Y.; Zhang, L.; Gao, H.Y.; Yang, G.J.; Fang, B.Z. Semiconductor polymeric graphitic carbon nitride photocatalysts: The “holy grail” for the photocatalytic hydrogen evolution reaction under visible light. Energy Environ. Sci. 2019, 12, 2080–2147. [Google Scholar] [CrossRef]
- Wang, S.J.; Zhang, J.Q.; Li, B.; Sun, H.Q.; Wang, S.B. Engineered graphitic carbon nitride-based photocatalysts for visible-light-driven water splitting: A review. Energy Fuels 2021, 35, 6504–6526. [Google Scholar] [CrossRef]
- Guo, S.E.; Deng, Z.P.; Li, M.X.; Jiang, B.J.; Tian, C.G.; Pan, Q.J.; Fu, H.G. Phosphorus-doped carbon nitride tubes with a layered micro-nanostructure for enhanced visible-light photocatalytic hydrogen evolution. Angew. Chem. Int. Ed. 2016, 55, 1830–1834. [Google Scholar] [CrossRef] [PubMed]
- Ye, R.Q.; Fang, H.B.; Zheng, Y.Z.; Li, N.; Wang, Y.; Tao, X. Fabrication of CoTiO3/g-C3N4 hybrid photocatalysts with enhanced H2 evolution: Z-scheme photocatalytic mechanism insight. ACS Appl. Mater. Interfaces 2016, 8, 13879–13889. [Google Scholar] [CrossRef]
- Zhang, W.; Hu, Y.L.; Ge, J.; Jiang, H.L.; Yu, S.H. A facile and general coating approach to moisture/water-resistant metal-organic frameworks with intact porosity. J. Am. Chem. Soc. 2014, 136, 16978–16981. [Google Scholar] [CrossRef]
- Kaye, S.S.; Dailly, A.; Yaghi, O.M.; Long, J.R. Impact of preparation and handling on the hydrogen storage properties of Zn4O(1,4-benzenedicarboxylate)3 (MOF-5). J. Am. Chem. Soc. 2007, 129, 14176–14177. [Google Scholar] [CrossRef]
- Xuan, X.X.; Chen, S.Y.; Zhao, S.; Yoon, J.Y.; Boczkaj, G.; Sun, X. Carbon nanomaterials from metal-organic frameworks: A new material horizon for CO2 reduction. Front. Chem. 2020, 8, 573797. [Google Scholar] [CrossRef]
- Shao, P.; Yi, L.C.; Chen, S.M.; Zhou, T.H.; Zhang, J. Metal-organic frameworks for electrochemical reduction of carbon dioxide: The role of metal centers. J. Energy Chem. 2020, 40, 156–170. [Google Scholar] [CrossRef]
- Ren, J.T.; Yuan, K.; Wu, K.; Zhou, L.; Zhang, Y.W. A robust CdS/In2O3 hierarchical heterostructure derived from a metal-organic framework for efficient visible-light photocatalytic hydrogen production. Inorg. Chem. Front. 2019, 6, 366–375. [Google Scholar] [CrossRef]
- Dhakshinamoorthy, A.; Garcia, H. Catalysis by metal nanoparticles embedded on metal-organic frameworks. Chem. Soc. Rev. 2012, 41, 5262–5284. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.Y.; Min, S.X.; Xue, Y.; Tian, L.; Lei, Y.G.; Wang, F. In situ growth and activation of an amorphous MoSx catalyst on Co-containing metal-organic framework nanosheets for highly efficient dye-sensitized H2 evolution. New J. Chem. 2019, 43, 4152–4159. [Google Scholar] [CrossRef]
- Fan, Z.B.; Guo, X.; Jin, Z.L.; Li, X.; Li, Y.J. Bridging effect of S-C bond for boosting electron transfer over cubic hollow CoS/g-C3N4 heterojunction toward photocatalytic hydrogen production. Langmuir 2022, 38, 3244–3256. [Google Scholar] [CrossRef] [PubMed]
- Qi, Y.; Xu, J.; Zhang, M.; Lin, H.; Wang, L. In situ metal-organic framework-derived c-doped Ni3S4/Ni2P hybrid co-catalysts for photocatalytic H2 production over g-C3N4 via dye sensitization. Int. J. Hydrogen Energy 2019, 44, 16336–16347. [Google Scholar] [CrossRef]
- Hu, C.Y.; Zhou, J.; Sun, C.Y.; Chen, M.M.; Wang, X.L.; Su, Z.M. HKUST-1 derived hollow C-Cu2−xS nanotube/g-C3N4 composites for visible-light CO2 photoreduction with H2O vapor. Chem. Eur. J. 2019, 25, 379–385. [Google Scholar] [CrossRef]
- Parzinger, E.; Miller, B.; Blaschke, B.; Garrido, J.A.; Ager, J.W.; Holleitner, A.; Wurstbauer, U. Photocatalytic stability of single- and few-layer MoS2. ACS Nano 2015, 9, 11302–11309. [Google Scholar] [CrossRef]
- Xia, D.; Gong, F.; Pei, X.D.; Wang, W.B.; Li, H.; Zeng, W.; Wu, M.Q.; Papavassiliou, D.V. Molybdenum and tungsten disulfides-based nanocomposite films for energy storage and conversion: A review. Chem. Eng. J. 2018, 348, 908–928. [Google Scholar] [CrossRef]
- Nagaraja, C.M.; Kaur, M.; Dhingra, S. Enhanced visible-light-assisted photocatalytic hydrogen generation by MoS2/g-C3N4 nanocomposites. Int. J. Hydrogen Energy 2020, 45, 8497–8506. [Google Scholar] [CrossRef]
- Zhu, K.L.; Wang, C.J.; Luan, X.X.; Li, J.; Yang, P. Efficient charge transfer in z-scheme g-C3N4/Cu/MoS2 heterojunctions for enhanced photocatalysis and photoenhanced electrocatalysis. Adv. Mater. Interfaces 2022, 9, 2201114. [Google Scholar] [CrossRef]
- Shi, J.; Yang, L.; Zhang, J.; Wang, Z.; Zhu, W.; Wang, Y.; Zou, Z. Dual MOF-derived MoS2/CdS photocatalysts with rich sulfur vacancies for efficient hydrogen evolution reaction. Chem. Eur. J. 2022, 28, e202202019. [Google Scholar] [CrossRef]
- Sun, J.; Li, X.; Li, J.; Mu, M.; Yin, X. Fabrication of Bi4O5Br2-decorated rod-like MOF-derived MoS2 hierarchical heterostructures for boosting photocatalytic CO2 reduction. Colloids Surfaces A 2022, 653, 129940. [Google Scholar] [CrossRef]
- Huang, H.; Zhang, W.; Wang, H.; Wei, A.; Wang, J.; Liu, Y. The heterojunction construction of hybrid B-doped g-C3N4 nanosheets and ZIF67 by simple mechanical grinding for improved photocatalytic hydrogen evolution. Int. J. Hydrogen Energy 2023, 48, 25366–25378. [Google Scholar] [CrossRef]
- Lu, L.; Xu, X.; An, K.; Wang, Y.; Shi, F.N. Coordination polymer derived NiS@g-C3N4 Composite photocatalyst for sulfur vacancy and photothermal effect synergistic enhanced H2 production. ACS Sustain. Chem. Eng. 2018, 6, 11869–11876. [Google Scholar] [CrossRef]
- Bian, H.; Ji, Y.J.; Yan, J.Q.; Li, P.; Li, L.; Li, Y.Y.; Liu, S.Z. In situ synthesis of few-layered g-C3N4 with vertically aligned MoS2 loading for boosting solar-to-hydrogen generation. Small 2018, 14, 1703003. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.K.; Jin, Z.L. Synergistic enhancement of hydrogen production by ZIF-67 (Co) derived Mo-Co-S modified g-C3N4/rGO photocatalyst. Catal. Lett. 2019, 149, 34–48. [Google Scholar] [CrossRef]
- Yu, J.G.; Wang, S.H.; Cheng, B.; Lin, Z.; Huang, F. Noble metal-free Ni(OH)2-g-C3N4 composite photocatalyst with enhanced visible-light photocatalytic H2-production activity. Catal. Sci. Technol. 2013, 3, 1782–1789. [Google Scholar] [CrossRef]
- Wang, Y.; Li, H.; Yao, J.; Wang, X.; Antonietti, M. Synthesis of boron doped polymeric carbon nitride solids and their use as metal-free catalysts for aliphatic C-H bond oxidation. Chem. Sci. 2011, 2, 446–450. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, Z.Z.; Zhang, L.N.; Luo, Z.B.; Shen, J.N.; Lin, H.X.; Long, J.L.; Wu, J.C.S.; Fu, X.Z.; Wang, X.X.; et al. Visible-light driven overall conversion of CO2 and H2O to CH4 and O2 on 3D-SiC@2D-MoS2 heterostructure. J. Am. Chem. Soc. 2018, 140, 14595–14598. [Google Scholar] [CrossRef]
- Meier, A.J.; Garg, A.; Sutter, B.; Kuhn, J.N.; Bhethanabotla, V.R. MoS2 nanoflowers as a gateway for solar-driven CO2 photoreduction. ACS Sustain. Chem. Eng. 2019, 7, 265–275. [Google Scholar] [CrossRef]
- Che, W.; Cheng, W.R.; Yao, T.; Tang, F.M.; Liu, W.; Su, H.; Huang, Y.Y.; Liu, Q.H.; Liu, J.K.; Hu, F.C.; et al. Fast photoelectron transfer in (Cring)-C3N4 plane heterostructural nanosheets for overall water splitting. J. Am. Chem. Soc. 2017, 139, 3021–3026. [Google Scholar] [CrossRef] [PubMed]
- Bharagav, U.; Ramesh Reddy, N.; Nava Koteswara Rao, V.; Ravi, P.; Sathish, M.; Rangappa, D.; Prathap, K.; Chakra, C.S.; Shankar, M.V.; Appels, L.; et al. Bifunctional g-C3N4/carbon nanotubes/WO3 ternary nanohybrids for photocatalytic energy and environmental applications. Chemosphere 2023, 311, 137030. [Google Scholar] [CrossRef]
- Rao, V.N.; Kwon, H.; Nagaveni, M.; Ravi, P.; Lee, Y.; Lee, S.J.; Kim, K.; Kumari, M.M.; Shankar, M.V.; Yoo, J.H.; et al. Modulating Schottky barriers and active sites of Ag-Ni bi-metallic cluster on mesoporous carbon nitride for enhanced photocatalytic hydrogen evolution. Chem. Eng. J. 2024, 499, 156179. [Google Scholar] [CrossRef]







Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, C.; Wang, J.; Wang, L. Fabrication of Two-Dimensional B-Doped C3N4 Nanosheet-Encapsulated One-Dimensional Rod-like Mo-MOF-Derived MoS2 Heterojunctions for Enhanced Photocatalytic Ethanol Conversion and Synergistic Hydrogen Production. Catalysts 2024, 14, 833. https://doi.org/10.3390/catal14110833
Zhang C, Wang J, Wang L. Fabrication of Two-Dimensional B-Doped C3N4 Nanosheet-Encapsulated One-Dimensional Rod-like Mo-MOF-Derived MoS2 Heterojunctions for Enhanced Photocatalytic Ethanol Conversion and Synergistic Hydrogen Production. Catalysts. 2024; 14(11):833. https://doi.org/10.3390/catal14110833
Chicago/Turabian StyleZhang, Caili, Jian Wang, and Li Wang. 2024. "Fabrication of Two-Dimensional B-Doped C3N4 Nanosheet-Encapsulated One-Dimensional Rod-like Mo-MOF-Derived MoS2 Heterojunctions for Enhanced Photocatalytic Ethanol Conversion and Synergistic Hydrogen Production" Catalysts 14, no. 11: 833. https://doi.org/10.3390/catal14110833
APA StyleZhang, C., Wang, J., & Wang, L. (2024). Fabrication of Two-Dimensional B-Doped C3N4 Nanosheet-Encapsulated One-Dimensional Rod-like Mo-MOF-Derived MoS2 Heterojunctions for Enhanced Photocatalytic Ethanol Conversion and Synergistic Hydrogen Production. Catalysts, 14(11), 833. https://doi.org/10.3390/catal14110833






