Impact of Ga, Sr, and Ce on Ni/DSZ95 Catalyst for Methane Partial Oxidation in Hydrogen Production
Abstract
:1. Introduction
2. Results
2.1. N2 Isothermal Adsorption–Desorption
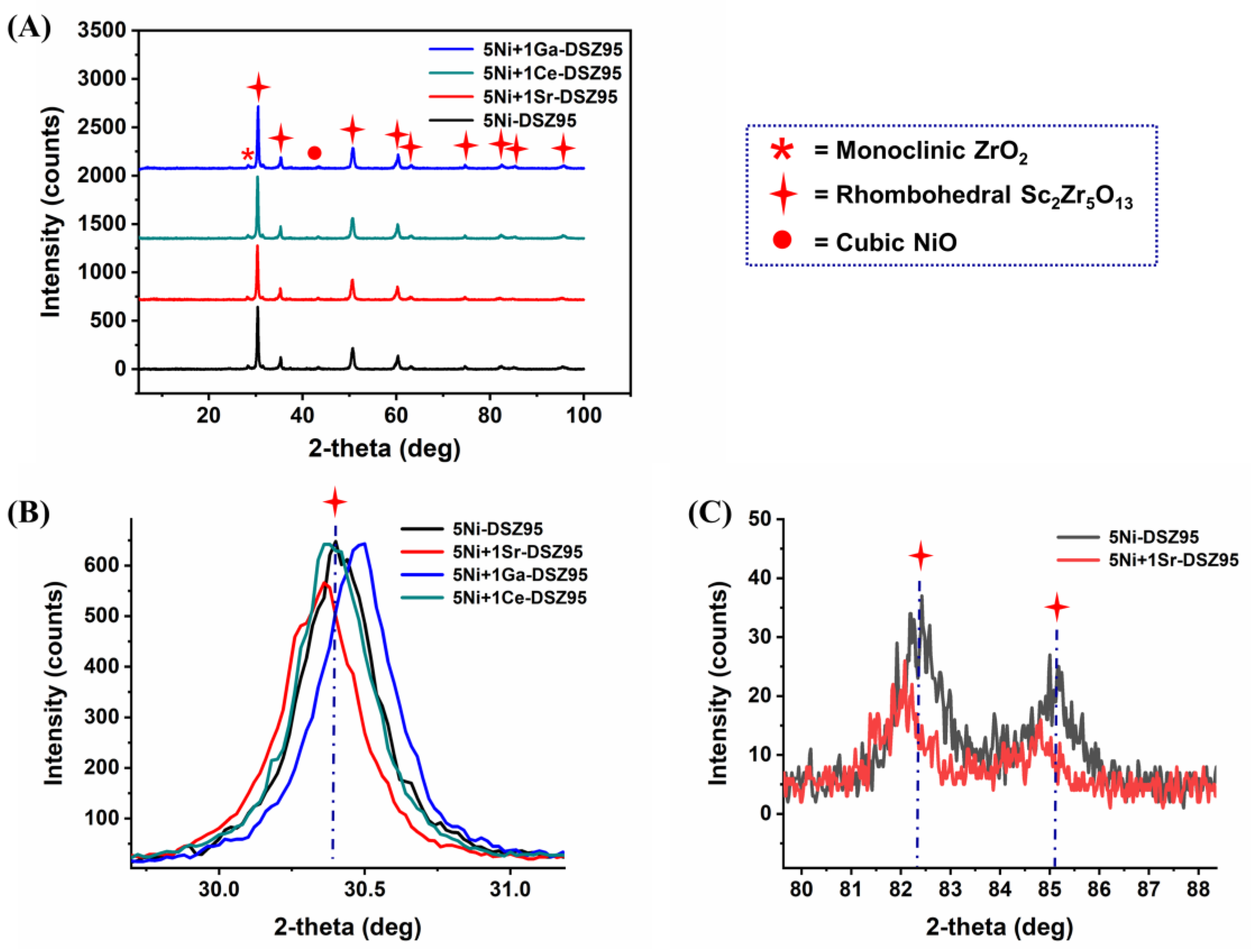
2.2. X-Ray Diffraction (XRD) Analysis
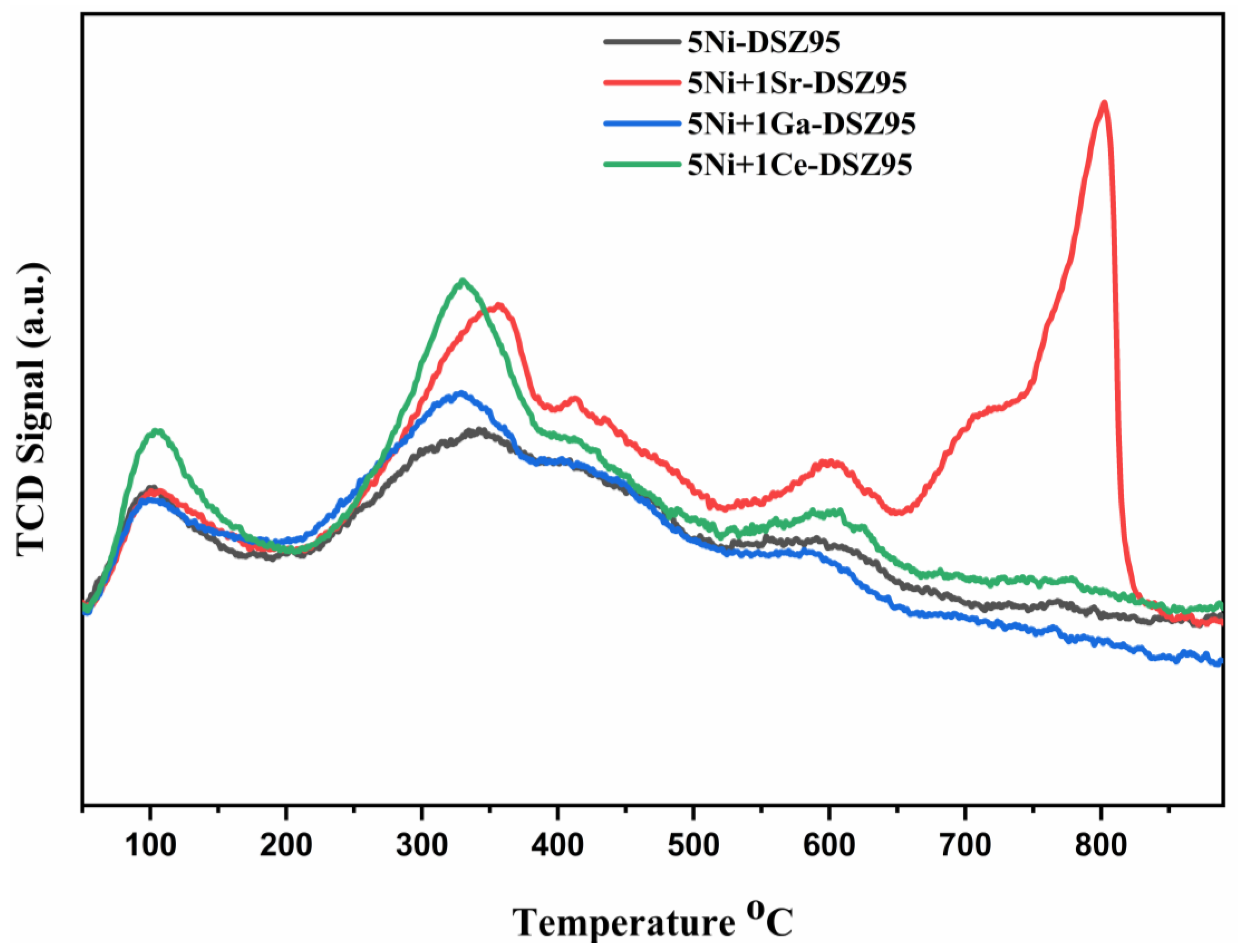
2.3. H2 Temperature Programmed Reduction
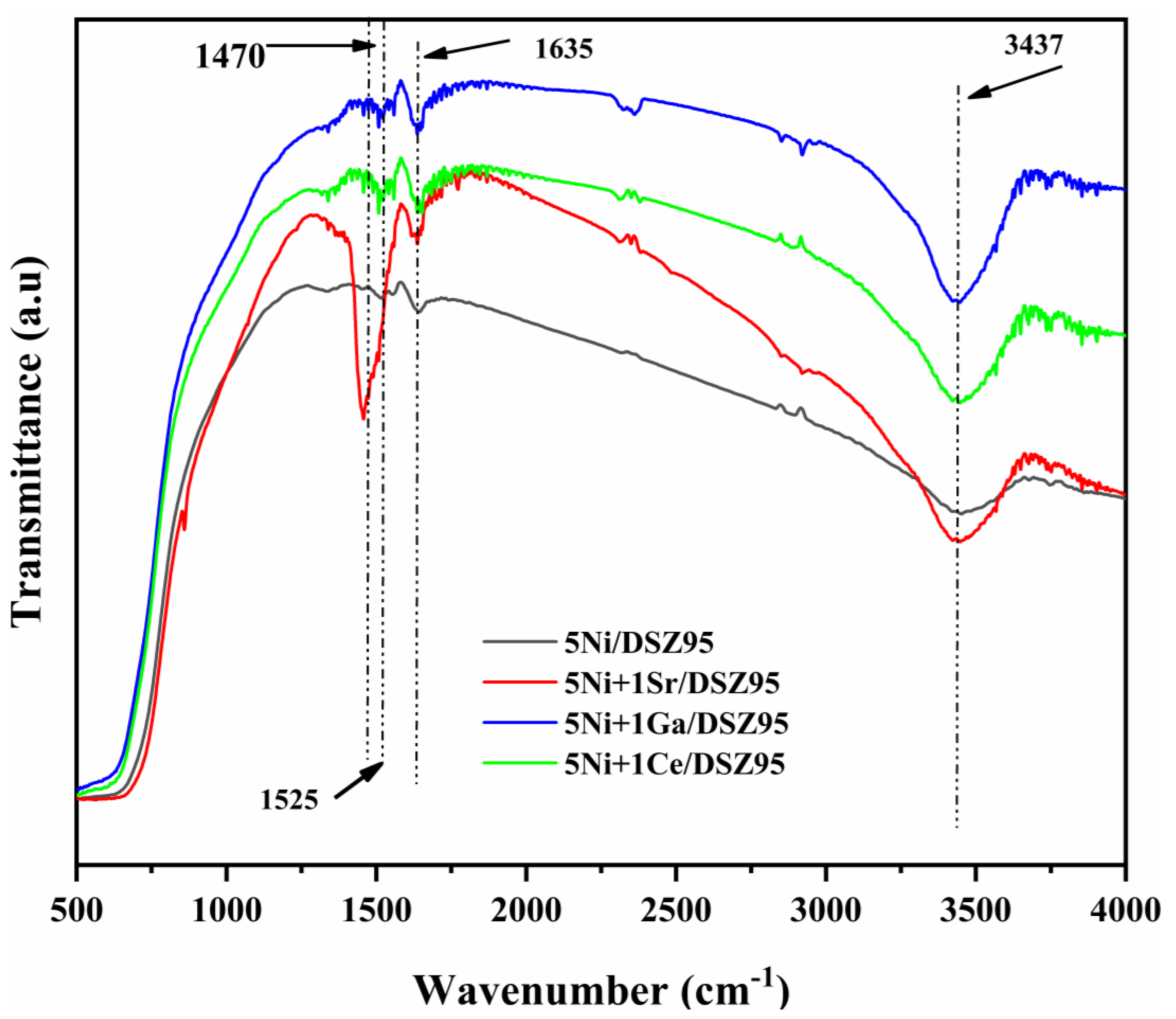
2.4. Fourier-Transform Infrared Spectroscopy (FTIR)
2.5. Temperature Programmed Desorption (CO2-TPD)
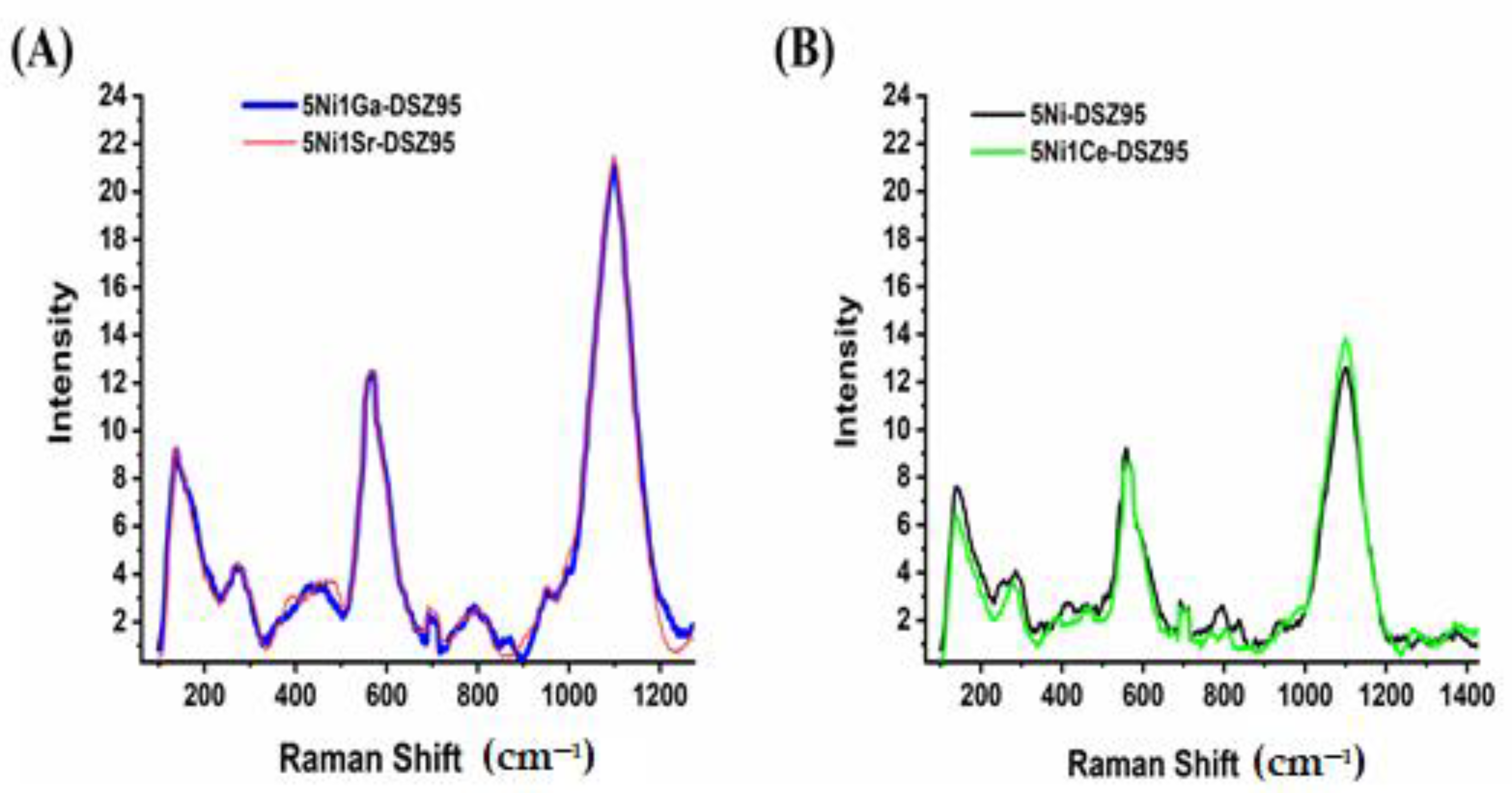
2.6. Raman Spectra of the Fresh Catalysts
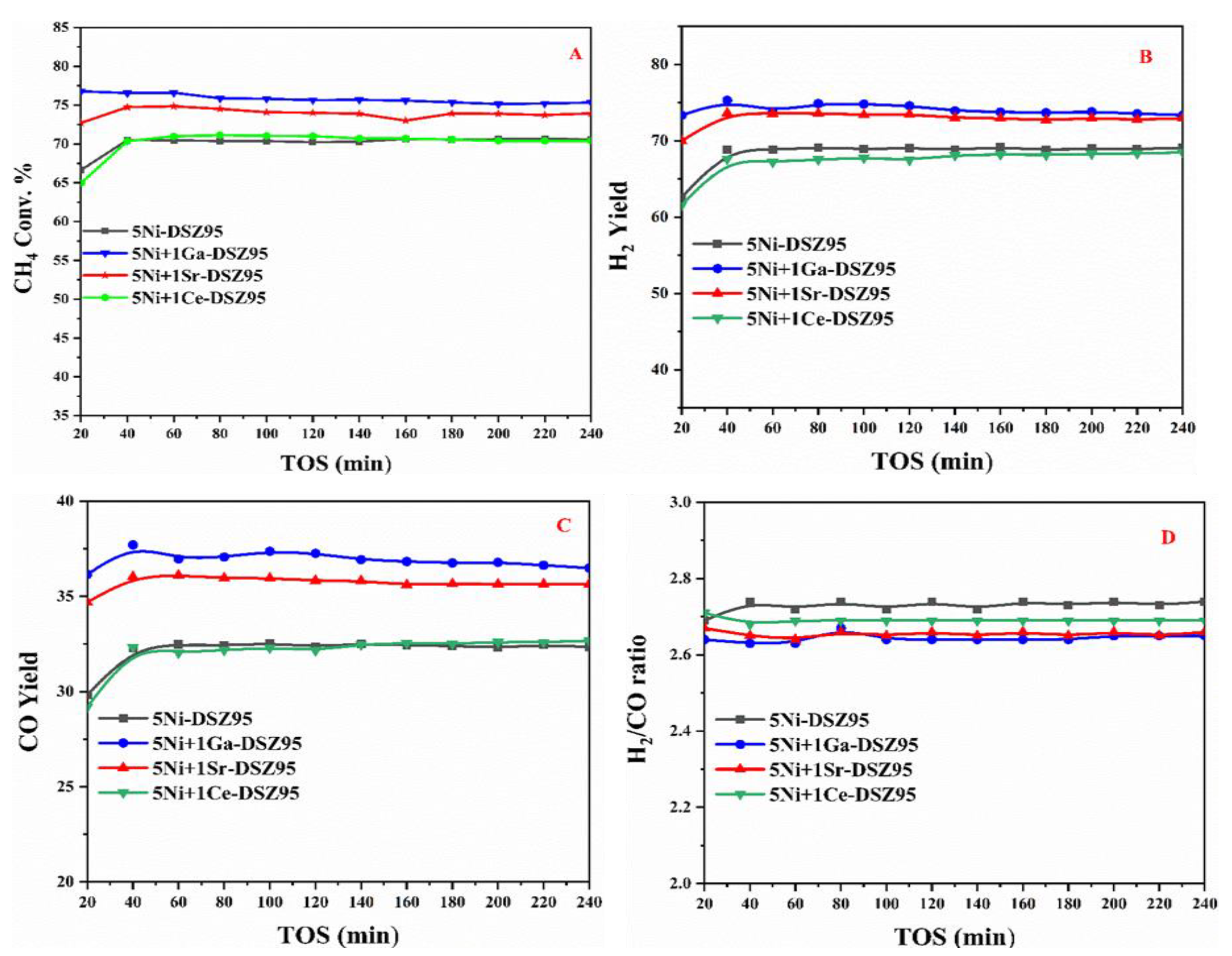
2.7. The Catalytic Activity Evaluation
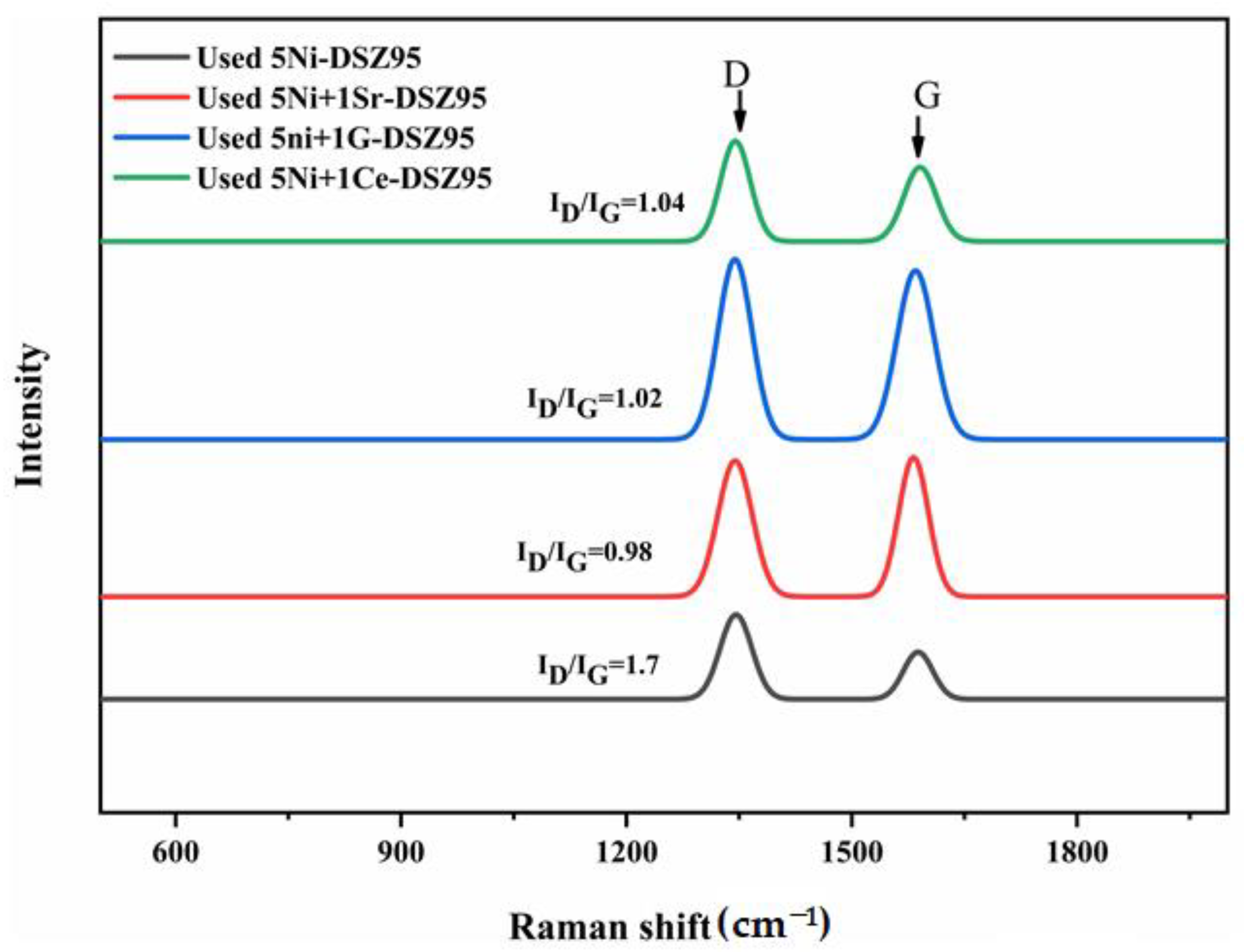
2.8. Raman Spectra for the Spent Catalysts
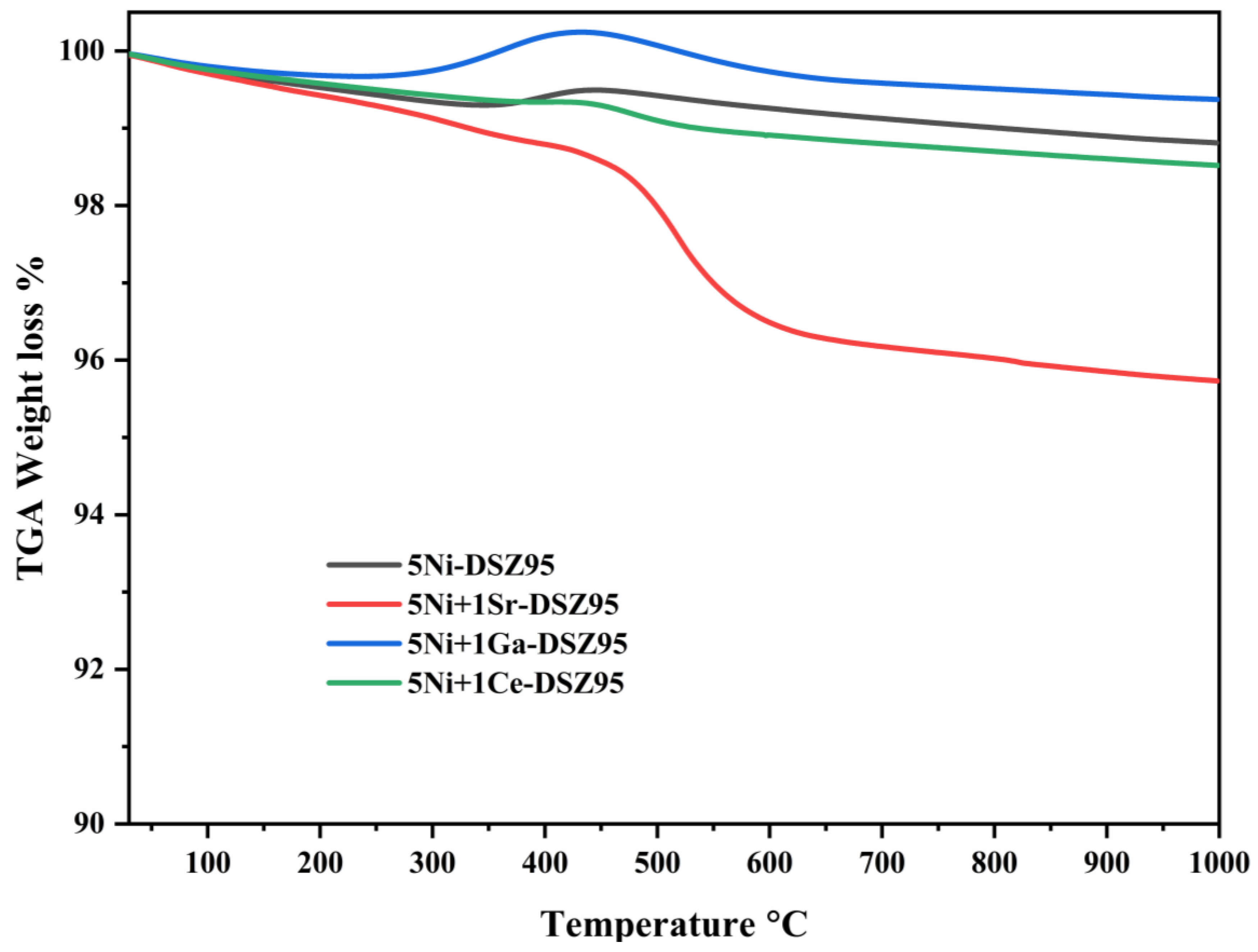
2.9. Thermo-Gravimetric Analysis (TGA)
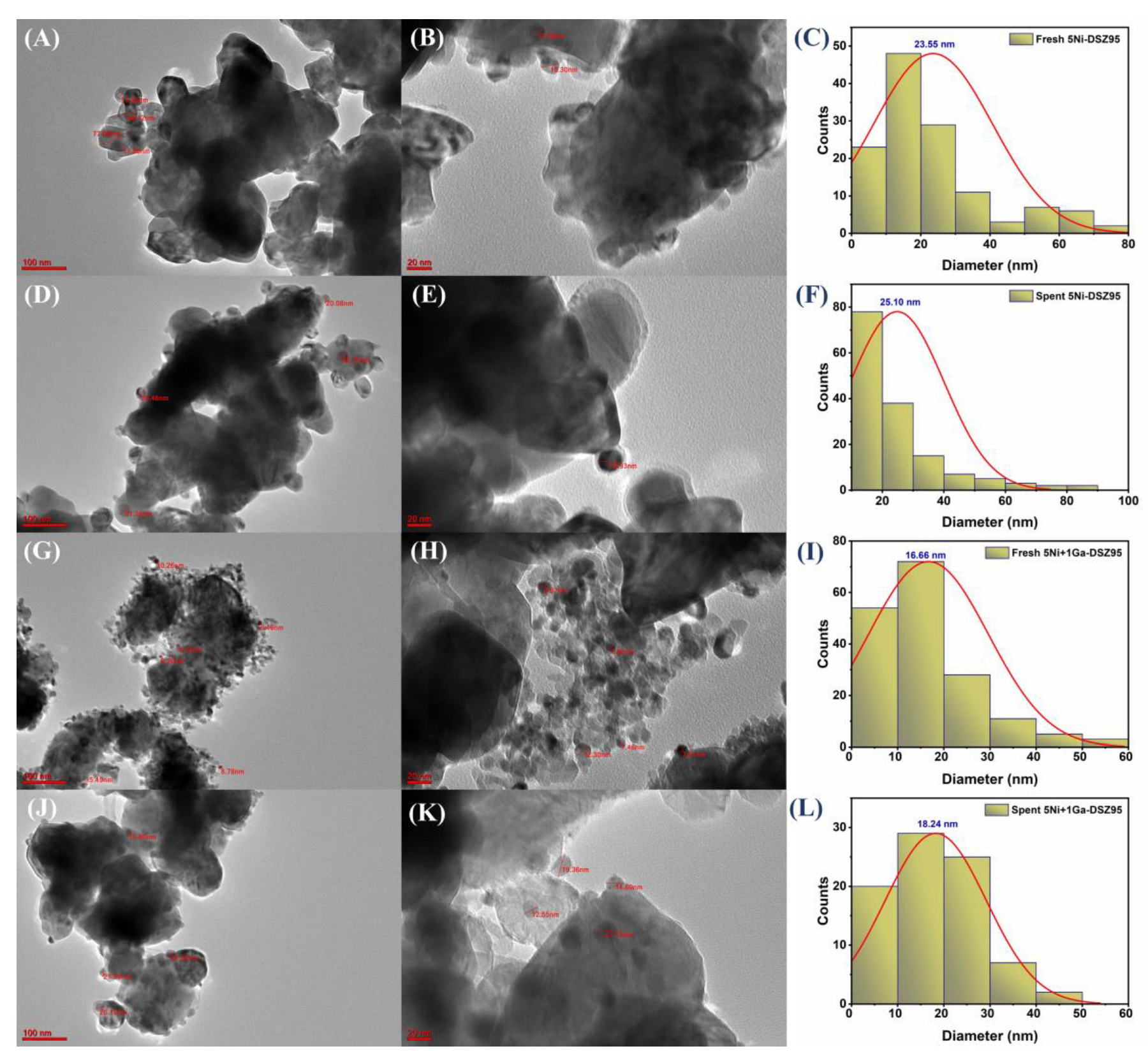
2.10. TEM Images of Selected Catalysts
3. Materials and Methods
Catalyst Preparation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Al-anazi, A.; Bellahwel, O.; Kavitha, C.; Abu-dahrieh, J.; Ibrahim, A.A.; Santhosh, S.; Abasaeed, A.E.; Fakeeha, A.H.; Al-fatesh, A.S. Promoter Impact on 5Ni/SAPO−5 Catalyst for H2 Production via Methane Partial Oxidation. Catalysts 2024, 14, 316. [Google Scholar] [CrossRef]
- Liu, M.; Liu, Y.; Li, Y.; Wang, X.; Pan, Y.; Jin, H. Enhanced Hydrogen Production of Mid-Temperature Chemical Looping Steam Methane Reforming Using Lithium-Based Sorbent Particles. Chem. Eng. J. 2024, 498, 155522. [Google Scholar] [CrossRef]
- Maharaj, L.; Lokhat, D. Dry Reforming of Methane in a Direct Irradiated Solar Powered System: Process Design and Optimisation. Appl. Energy 2025, 377, 124536. [Google Scholar] [CrossRef]
- Romay, M.; Serrano, D.P.; Escola, J.M.; Pizarro, P. Unravelling the Effectiveness of the Small Partial Substitution of Fe by Ni in La0.9Sr0.1Fe1-XNixO3 Perovskites to Improve Their Performance in Dry Reforming of Methane. Chem. Eng. J. 2024, 496, 154039. [Google Scholar] [CrossRef]
- Bahari, M.B.; Mamat, C.R.; Jalil, A.A.; Hassan, N.S.; Hatta, A.H.; Alhassan, M.; Aziz, M.A.; Le, V.G.; Siang, T.J.; Timmiati, S.N. Mitigating Deactivation in Dry Methane Reforming by Lanthanum Catalysts for Enhanced Hydrogen Production: A Review. Int. J. Hydrogen Energy, 2024; in press. [Google Scholar] [CrossRef]
- Jamsaz, A.; Pham-Ngoc, N.; Wang, M.; Jeong, D.H.; Oh, E.S.; Shin, E.W. Synergistic Effect of Macroporosity and Crystallinity on Catalyst Deactivation Behavior over Macroporous Ni/CexZr1-XO2–Al2O3 for Dry Reforming of Methane. Chem. Eng. J. 2023, 476, 146821. [Google Scholar] [CrossRef]
- Zagaynov, I.V.; Loktev, A.S.; Konovalov, A.A.; Klimashin, A.A.; Antonova, O.S.; Dedov, A.G. NiCo-Gd0.1Ti0.1Zr0.1Ce0.7O2 Catalyst for Dry Reforming and Partial Oxidation of Methane: Effect of NiCo Applying Method on the Conversion of Methane to Synthesis Gas. Mendeleev Commun. 2024, 34, 572–575. [Google Scholar] [CrossRef]
- Yin, X.; Zhang, R.; Zhang, Y.; Wang, S.; Shen, L. Enhanced Reactivity of Methane Partial Oxidation of Nickel Doped LaMnO3+δ Perovskites for Chemical Looping Process. Int. J. Hydrogen Energy 2024, 71, 481–492. [Google Scholar] [CrossRef]
- Christian Enger, B.; Lødeng, R.; Holmen, A. A Review of Catalytic Partial Oxidation of Methane to Synthesis Gas with Emphasis on Reaction Mechanisms over Transition Metal Catalysts. Appl. Catal. A Gen. 2008, 346, 1–27. [Google Scholar] [CrossRef]
- Abahussain, A.A.M.; Al-Fatesh, A.S.; Vadodariya, D.M.; Abu-Dahrieh, J.K.; Banabdwin, K.M.; Alarifi, N.; Ibrahim, A.A.; Fakeeha, A.H.; Abasaeed, A.E.; Kumar, R. The Role of Ceria in Promoting Ni Catalysts Supported on Phosphate-Modified Zirconia for the Partial Oxidation of Methane. Energy Sci. Eng. 2024, 12, 3379–3389. [Google Scholar] [CrossRef]
- Alvarez-Galvan, C.; Melian, M.; Ruiz-Matas, L.; Eslava, J.L.; Navarro, R.M.; Ahmadi, M.; Roldan Cuenya, B.; Fierro, J.L.G. Partial Oxidation of Methane to Syngas Over Nickel-Based Catalysts: Influence of Support Type, Addition of Rhodium, and Preparation Method. Front. Chem. 2019, 7, 104. [Google Scholar] [CrossRef]
- Fleys, M.; Simon, Y.; Swierczynski, D.; Kiennemann, A.; Marquaire, P.M. Investigation of the Reaction of Partial Oxidation of Methane over Ni/La2O3 Catalyst. Energy Fuels 2006, 20, 2321–2329. [Google Scholar] [CrossRef]
- Pauletto, G.; Mendil, M.; Libretto, N.; Mocellin, P.; Miller, J.T.; Patience, G.S. Short Contact Time CH4 Partial Oxidation over Ni Based Catalyst at 1.5 MPa. Chem. Eng. J. 2021, 414, 128831. [Google Scholar] [CrossRef]
- Hasani Estalkhi, M.; Yousefpour, M.; Koohestan, H.; Taherian, Z. Catalytic Evaluation of Ni–3%Sr-/MCM-41 in Dry and Steam Reforming of Methane. Int. J. Hydrogen Energy 2024, 68, 1344–1351. [Google Scholar] [CrossRef]
- ul Hasnain, S.M.W.; Farooqi, A.S.; Ayodele, B.V.; Setiabudi, H.D.; Farooqi, A.S.; Alshareef, R.S.; Abdullah, B. Synthesis, Characterization, and Catalytic Performance of Ni Supported on Sustainable POFA-Derived SBA-15 for Hydrogen-Rich Syngas from CO2 Reforming of Methane. J. Ind. Eng. Chem. 2024; in press. [Google Scholar] [CrossRef]
- Centi, G.; Perathoner, S. Novel Catalyst Design for Multiphase Reactions. Catal. Today 2003, 79–80, 3–13. [Google Scholar] [CrossRef]
- Agarkov, D.A.; Borik, M.A.; Bublik, V.T.; Chislov, A.S.; Kulebyakin, A.V.; Kuritsyna, I.E.; Kolotygin, V.A.; Lomonova, E.E.; Milovich, F.O.; Myzina, V.A.; et al. Phase Stability and Transport Characteristics of (ZrO2)1-x(Sc2O3)x(CeO2)y and (ZrO2)1-x-y-z(Sc2O3)x(CeO2)y(Y2O3)z Solid Solution Crystals. J. Alloys Compd. 2019, 791, 445–451. [Google Scholar] [CrossRef]
- Badwal, S.P.S.; Ciacchi, F.T.; Milosevic, D. Scandia-Zirconia Electrolytes for Intermediate Temperature Solid Oxide Fuel Cell Operation. Solid State Ionics 2000, 136–137, 91–99. [Google Scholar] [CrossRef]
- Dong, W.S.; Jun, K.W.; Roh, H.S.; Liu, Z.W.; Park, S.E. Comparative Study on Partial Oxidation of Methane over Ni/ZrO2, Ni/CeO2 and Ni/Ce-ZrO2 Catalysts. Catal. Lett. 2002, 78, 215–222. [Google Scholar] [CrossRef]
- Pompeo, F.; Nichio, N.N.; Ferretti, O.A.; Resasco, D. Study of Ni Catalysts on Different Supports to Obtain Synthesis Gas. Int. J. Hydrogen Energy 2005, 30, 1399–1405. [Google Scholar] [CrossRef]
- Hussain, A.; Arif, S.M.; Aslam, M. Emerging Renewable and Sustainable Energy Technologies: State of the Art. Renew. Sustain. Energy Rev. 2017, 71, 12–28. [Google Scholar] [CrossRef]
- Al-Fatesh, A.S.; Patel, N.; Fakeeha, A.H.; Alotibi, M.F.; Alreshaidan, S.B.; Kumar, R. Reforming of Methane: Effects of Active Metals, Supports, and Promoters. Catal. Rev. 2024, 66, 2209–2307. [Google Scholar] [CrossRef]
- Alwadai, N.; Abahussain, A.A.M.; Vadodariya, D.M.; Banabdwin, K.M.; Fakeeha, A.H.; Abu-Dahrieh, J.K.; Almuqati, N.S.; Alghamdi, A.M.; Kumar, R.; Al-Fatesh, A.S. Ni–Sr/TiZr for H2 from Methane via POM: Sr Loading & Optimization. RSC Adv. 2024, 14, 25273–25288. [Google Scholar] [CrossRef] [PubMed]
- Al-Fatesh, A.S.; Vadodariya, D.M.; Banabdwin, K.M.; Ibrahim, A.A.; Fakeeha, A.H.; Adil, S.F.; Kumar, R.; Abahussain, A.A.M. Promoted Ni Catalyst Over Titania-Zirconia Support for Partial Oxidation of Methane: Simple and Practical Catalysts. Catal. Lett. 2024, 154, 4625–4635. [Google Scholar] [CrossRef]
- Valadez Huerta, G.; Reus, L.; Kabelac, S. A Diffusivity Study of (Sc2O3)0.1(CeO2)0.01(ZrO2)0.89 between 1100 and 1500 K at Zero Pressure with Molecular Dynamics. J. Chem. Eng. Data 2018, 63, 1955–1960. [Google Scholar] [CrossRef]
- Liu, M.; He, C.; Wang, J.; Wang, W.G.; Wang, Z. Investigation of (CeO2)x(Sc2O 3)(0.11-x)(ZrO2)0.89 (x = 0.01-0.10) Electrolyte Materials for Intermediate-Temperature Solid Oxide Fuel Cell. J. Alloys Compd. 2010, 502, 319–323. [Google Scholar] [CrossRef]
- Smal, E.; Bespalko, Y.; Arapova, M.; Fedorova, V.; Valeev, K.; Eremeev, N.; Sadovskaya, E.; Krieger, T.; Glazneva, T.; Sadykov, V.; et al. Carbon Formation during Methane Dry Reforming over Ni-Containing Ceria-Zirconia Catalysts. Nanomaterials 2022, 12, 3676. [Google Scholar] [CrossRef]
- Ojeda-Niño, O.; Gallego, J.; Daza, C.E. Pr-Promoted Ni Exsolution from Ni–Mg–Al (O) as Catalysts for Syngas Production by Dry Reforming of Methane. Results Eng. 2023, 17, 100821. [Google Scholar] [CrossRef]
- Cipriani, G.; Di Dio, V.; Genduso, F.; La Cascia, D.; Liga, R.; Miceli, R.; Ricco Galluzzo, G. Perspective on Hydrogen Energy Carrier and Its Automotive Applications. Int. J. Hydrogen Energy 2014, 39, 8482–8494. [Google Scholar] [CrossRef]
- Acharya, K.; Al-Fatesh, A.S.; Almutairi, G.; Fakeeha, A.H.; Ibrahim, A.A.; Abasaeed, A.E.; Siddiqui, M.R.H.; Kumar, R. The Role of Strontium as an Economic Promoter Over WO3 + ZrO2 Supported Ni Catalyst for H2 Production Through Dry Reforming of Methane. Catal. Lett. 2024, 154, 2023–2035. [Google Scholar] [CrossRef]
- de Falco, M.; Iaquaniello, G.; Centi, G. CO2: A Valuable Source of Carbon; Springer: Berlin/Heidelberg, Germany, 2013; Volulme 137, ISBN 9781447151180. [Google Scholar]
- Li, S.; Zhang, H.; Xu, J.; Yang, D. Hydrothermal Synthesis of Flower-like SrCO3 Nanostructures. Mater. Lett. 2005, 59, 420–422. [Google Scholar] [CrossRef]
- Du, F.; Shi, L. Solvothermal Growth of Single-Crystal Hexagonal Prismatic SrCO3 Microrods. Cryst. Res. Technol. 2007, 42, 216–220. [Google Scholar] [CrossRef]
- Pérez-Ramírez, J.; Mul, G.; Moulijn, J.A. In Situ Fourier Transform Infrared and Laser Raman Spectroscopic Study of the Thermal Decomposition of Co-Al and Ni-Al Hydrotalcites. Vib. Spectrosc. 2001, 27, 75–88. [Google Scholar] [CrossRef]
- Wiyantoko, B.; Kurniawati, P.; Purbaningtias, T.E.; Fatimah, I. Synthesis and Characterization of Hydrotalcite at Different Mg/Al Molar Ratios. Procedia Chem. 2015, 17, 21–26. [Google Scholar] [CrossRef]
- Cavani, F.; Trifirò, F.; Vaccari, A. Hydrotalcite-Type Anionic Clays: Preparation, Properties and Applications. Catal. Today 1991, 11, 173–301. [Google Scholar] [CrossRef]
- Dębek, R.; Zubek, K.; Motak, M.; Da Costa, P.; Grzybek, T. Effect of Nickel Incorporation into Hydrotalcite-Based Catalyst Systems for Dry Reforming of Methane. Res. Chem. Intermed. 2015, 41, 9485–9495. [Google Scholar] [CrossRef]
- Syeitkhajy, A.; Boz, I.; Boroglu, M.S. Catalytic Dehydration of Lactic Acid to Acrylic Acid over Phosphorus Promoted and Alkali Modi Ed Catalytic Dehydration of Lactic Acid to Acrylic Acid over Phosphorus Promoted and Alkali Modified ZSM-5. 28 March 2024. [Google Scholar] [CrossRef]
- Patel, R.; Al-Fatesh, A.S.; Fakeeha, A.H.; Arafat, Y.; Kasim, S.O.; Ibrahim, A.A.; Al-Zahrani, S.A.; Abasaeed, A.E.; Srivastava, V.K.; Kumar, R. Impact of Ceria over WO3–ZrO2 Supported Ni Catalyst towards Hydrogen Production through Dry Reforming of Methane. Int. J. Hydrogen Energy 2021, 46, 25015–25028. [Google Scholar] [CrossRef]
- Zhao, Z.; Liu, L.; Zhang, X.; Tu, B.; Ou, D.; Cheng, M. Carbonates Formed during BSCF Preparation and Their Effects on Performance of SOFCs with BSCF Cathode. Int. J. Hydrogen Energy 2012, 37, 19036–19044. [Google Scholar] [CrossRef]
- Xu, G.; Zhang, Y.W.; Liao, C.S.; Yan, C.H. Doping and Grain Size Effects in Nanocrystalline ZrO2-Sc2O3 System with Complex Phase Transitions: XRD and Raman Studies. Phys. Chem. Chem. Phys. 2004, 6, 5410–5418. [Google Scholar] [CrossRef]
- McBride, J.R.; Hass, K.C.; Poindexter, B.D.; Weber, W.H. Raman and X-Ray Studies of Ce1-XRExO2-y, Where RE=La, Pr, Nd, Eu, Gd, and Tb. J. Appl. Phys. 1994, 76, 2435–2441. [Google Scholar] [CrossRef]
- Spanier, J.E.; Robinson, R.D.; Zhang, F.; Chan, S.W.; Herman, I.P. Size-Dependent Properties of (Formula Presented) Nanoparticles as Studied by Raman Scattering. Phys. Rev. B Condens. Matter Mater. Phys. 2001, 64, 245407. [Google Scholar] [CrossRef]
- Pu, Z.Y.; Liu, X.S.; Jia, A.P.; Xie, Y.L.; Lu, J.Q.; Luo, M.F. Enhanced Activity for CO Oxidation over Pr- and Cu-Doped CeO2 Catalysts: Effect of Oxygen Vacancies. J. Phys. Chem. C 2008, 112, 15045–15051. [Google Scholar] [CrossRef]
- Fujimori, H.; Yashima, M.; Kakihana, M.; Yoshimura, M. Structural Changes of Scandia-Doped Zirconia Solid Solutions: Rietveld Analysis and Raman Scattering. J. Am. Ceram. Soc. 1998, 81, 2885–2893. [Google Scholar] [CrossRef]
- Li, L.; Song, L.; Chen, C.; Zhang, Y.; Zhan, Y.; Lin, X.; Zheng, Q.; Wang, H.; Ma, H.; Ding, L.; et al. Modified Precipitation Processes and Optimized Copper Content of Cuo-Ceo2 Catalysts for Wateregas Shift Reaction. Int. J. Hydrogen Energy 2014, 39, 19570–19582. [Google Scholar] [CrossRef]
- Tao, F.F.; Shan, J.J.; Nguyen, L.; Wang, Z.; Zhang, S.; Zhang, L.; Wu, Z.; Huang, W.; Zeng, S.; Hu, P. Understanding Complete Oxidation of Methane on Spinel Oxides at a Molecular Level. Nat. Commun. 2015, 6, 7798. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Ma, J.; Yang, L.; He, G.; Zhang, C.; Zhang, R.; He, H. Oxygen Vacancies Induced by Transition Metal Doping in γ-MnO 2 for Highly Efficient Ozone Decomposition. Environ. Sci. Technol. 2018, 52, 12685–12696. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.K.; Sun, B.Z.; Wang, X.; Lu, C.H. The Role of Surface Oxygen Vacancy in 2O Decomposition on Cu2O(111) Surface: A DFT Study. J. Theor. Comput. Chem. 2008, 7, 263–276. [Google Scholar] [CrossRef]
- Song, Y.Q.; He, D.H.; Xu, B.Q. Effects of Preparation Methods of ZrO2 Support on Catalytic Performances of Ni/ZrO2 Catalysts in Methane Partial Oxidation to Syngas. Appl. Catal. A Gen. 2008, 337, 19–28. [Google Scholar] [CrossRef]
- Song, P.; Wen, D.; Guo, Z.X.; Korakianitis, T. Oxidation Investigation of Nickel Nanoparticles. Phys. Chem. Chem. Phys. 2008, 10, 5057–5065. [Google Scholar] [CrossRef]
- Shin, S.A.; Eslami, A.A.; Noh, Y.S.; Song, H.T.; Kim, H.D.; Saeidabad, N.G.; Moon, D.J. Preparation and Characterization of Ni/Zrtialox Catalyst via Sol-Gel and Impregnation Methods for Low Temperature Dry Reforming of Methane. Catalysts 2020, 10, 1335. [Google Scholar] [CrossRef]
- Fu, Z.; Qi, P.; Liu, H.; Zhang, Q.; Zhao, Y.; Feng, X. Influence of Oxidative Properties of CexZr1−xO2 Catalyst on Partial Oxidation of Dimethyl Ether. Catalysts 2022, 12, 1536. [Google Scholar] [CrossRef]
- Zhu, J.; Van Ommen, J.G.; Lefferts, L. Reaction Scheme of Partial Oxidation of Methane to Synthesis Gas over Yttrium-Stabilized Zirconia. J. Catal. 2004, 225, 388–397. [Google Scholar] [CrossRef]
- Al-Fatesh, A.S.; Bamatraf, N.A.; Alreshaidan, S.B.; Abu-Dahrieh, J.K.; Patel, N.; Ibrahim, A.A.; Fakeeha, A.H.; Jumah, A.B.; Kumar, R. Cost-Effective Single-Step Synthesis of Metal Oxide-Supported Ni Catalyst for H2-Production Through Dry Reforming of Methane. Arab. J. Sci. Eng. 2024, 49, 8031–8047. [Google Scholar] [CrossRef]
- Khatri, J.; Al-Fatesh, A.S.; Fakeeha, A.H.; Ibrahim, A.A.; Abasaeed, A.E.; Kasim, S.O.; Osman, A.I.; Patel, R.; Kumar, R. Ce Promoted Lanthana-Zirconia Supported Ni Catalyst System: A Ternary Redox System for Hydrogen Production. Mol. Catal. 2021, 504, 111498. [Google Scholar] [CrossRef]
- Charisiou, N.D.; Siakavelas, G.; Tzounis, L.; Sebastian, V.; Monzon, A.; Baker, M.A.; Hinder, S.J.; Polychronopoulou, K.; Yentekakis, I.V.; Goula, M.A. An in Depth Investigation of Deactivation through Carbon Formation during the Biogas Dry Reforming Reaction for Ni Supported on Modified with CeO2 and La2O3 Zirconia Catalysts. Int. J. Hydrogen Energy 2018, 43, 18955–18976. [Google Scholar] [CrossRef]
- Kurdi, A.N.; Ibrahim, A.A.; Al-Fatesh, A.S.; Alquraini, A.A.; Abasaeed, A.E.; Fakeeha, A.H. Hydrogen Production from CO2 Reforming of Methane Using Zirconia Supported Nickel Catalyst. RSC Adv. 2022, 12, 10846–10854. [Google Scholar] [CrossRef]
- Kambolis, A.; Matralis, H.; Trovarelli, A.; Papadopoulou, C. Ni/CeO2-ZrO2 Catalysts for the Dry Reforming of Methane. Appl. Catal. A Gen. 2010, 377, 16–26. [Google Scholar] [CrossRef]


| Sample | BET Surface Area (m2/g) | Pore Volume (cm3/g) | Average Pore Size (Å) |
|---|---|---|---|
| 5Ni-DSZ95 | 7.1 | 0.04 | 284 |
| 5N+1Sr-DSZ95 | 6.9 | 0.04 | 306 |
| 5Ni+1Ce-DSZ95 | 8.1 | 0.06 | 338 |
| 5Ni+1Ga-DSZ95 | 8.9 | 0.06 | 334 |
| Sample | Max Peak Temperature (°C) | Quantity (cm3/g STP) | DR a (%) |
|---|---|---|---|
| 5Ni-DSZ95 | 495 | 21.8 | 16.6 |
| 5Ni+1Sr-DSZ95 | 485 | 23.8 | 15.7 |
| 5Ni+1Ga-DSZ95 | 563 | 18.1 | 10.4 |
| 5Ni+1Ce-DSZ95 | 442 | 18.6 | 9.4 |
| Sample | Temperature (°C) | Quantity (cm3/g STP) | Total Quantity (cm3/g STP) |
|---|---|---|---|
| 5Ni-DSZ95 | 101.5 | 0.1527 | 0.9447 |
| 346.1 | 0.6672 | ||
| 589.8 | 0.0706 | ||
| 767.2 | 0.0084 | ||
| 894.3 | 0.0458 | ||
| 5Ni+1Sr-DSZ95 | 103.6 | 0.2026 | 1.5533 |
| 346.0 | 0.2887 | ||
| 413.6 | 0.0185 | ||
| 605.0 | 0.0743 | ||
| 802.7 | 0.9692 | ||
| 5Ni+1Ce-DSZ95 | 105.8 | 0.2642 | 0.7247 |
| 329.9 | 0.3761 | ||
| 423.9 | 0.0165 | ||
| 608.6 | 0.0679 | ||
| 5Ni+1Ga-DSZ95 | 96.7 | 0.1508 | 0.4148 |
| 318.8 | 0.2061 | ||
| 439.8 | 0.0216 | ||
| 591.6 | 0.0363 |
| Sample | Catalyst Weight (mg) | CH4/O2 | Reaction Temperature (°C) | CH4 Conversion (%) | H2 Yield (%) | Ref. |
|---|---|---|---|---|---|---|
| Ni/Ce–ZrO2 | 15 | 2:1 | 800 | 27.4 | - | [19] |
| CexZr1–xO2 | 10 | 2:1 | 500 | 40.0 | 20 | [53] |
| YSZ * | 30 | 2:1 | 400–475 | 40.0 | 10 | [54] |
| 14%Ni/ZrO2 | 700 | 44.0 | 36 | [50] | ||
| 10Ni/PO4+ZrO2 | 10 | 2:1 | 600 | 44.0 | 36 | [10] |
| 2wt% Cs, Ce, Sr over Ni/TiZr | 10 | 2:1 | 600 | 45.0 | 40 | [24] |
| Ni/ZrO2 | 10 | 2:1 | 700 | 60.0 | 45 | [55] |
| Ni/ZrO2 | 10 | 3:3 | 700 | -- | 43 | [56] |
| Ni/ZrO2 | 10 | 55:35 | 800 | -- | 10 | [57] |
| Ni/Y2O3-ZrO2 | 10 | 3:3 | 700 | -- | 45 | [58] |
| Ni/La2O3-ZrO2 | - | 55:35 | 800 | -- | 11 | [57] |
| Ni/CeO2-ZrO2 | 15 | 1:1 | 700 | -- | 34 | [59] |
| 5Ni-DSZ95 | 100 | 2:1 | 700 | 69.2 | 70 | [R*] [R*] |
| 5Ni+1Ga-DSZ95 | 100 | 2:1 | 700 | 75.9 | 75 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-Zahrani, S.A.; Bellahwel, O.; Ibrahim, A.A.; Alotibi, M.F.; Masood, N.; Rajeh, S.Y.; Al Otaibi, A.; Al-Enazy, H.D.A.; Al-Fatesh, A.S. Impact of Ga, Sr, and Ce on Ni/DSZ95 Catalyst for Methane Partial Oxidation in Hydrogen Production. Catalysts 2024, 14, 851. https://doi.org/10.3390/catal14120851
Al-Zahrani SA, Bellahwel O, Ibrahim AA, Alotibi MF, Masood N, Rajeh SY, Al Otaibi A, Al-Enazy HDA, Al-Fatesh AS. Impact of Ga, Sr, and Ce on Ni/DSZ95 Catalyst for Methane Partial Oxidation in Hydrogen Production. Catalysts. 2024; 14(12):851. https://doi.org/10.3390/catal14120851
Chicago/Turabian StyleAl-Zahrani, Salma A., Omer Bellahwel, Ahmed Aidid Ibrahim, Mohammed F. Alotibi, Najat Masood, Sahar Y. Rajeh, Ahmed Al Otaibi, Hessah Difallah A. Al-Enazy, and Ahmed S. Al-Fatesh. 2024. "Impact of Ga, Sr, and Ce on Ni/DSZ95 Catalyst for Methane Partial Oxidation in Hydrogen Production" Catalysts 14, no. 12: 851. https://doi.org/10.3390/catal14120851
APA StyleAl-Zahrani, S. A., Bellahwel, O., Ibrahim, A. A., Alotibi, M. F., Masood, N., Rajeh, S. Y., Al Otaibi, A., Al-Enazy, H. D. A., & Al-Fatesh, A. S. (2024). Impact of Ga, Sr, and Ce on Ni/DSZ95 Catalyst for Methane Partial Oxidation in Hydrogen Production. Catalysts, 14(12), 851. https://doi.org/10.3390/catal14120851








