Effect of α-FeOOH in KOH Electrolytes on the Activity of NiO Electrodes in Alkaline Water Electrolysis for the Oxygen Evolution Reaction
Abstract
1. Introduction
2. Results
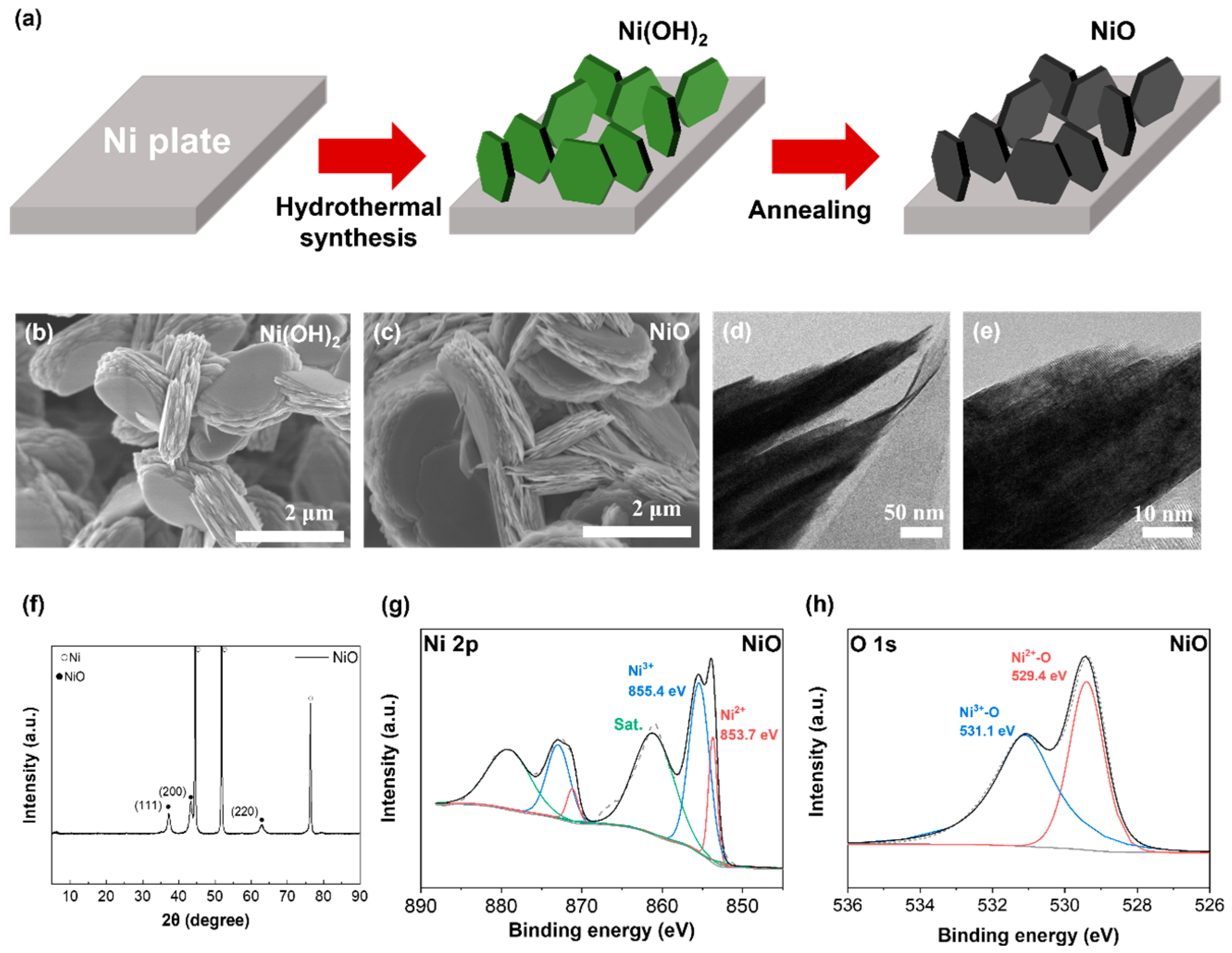
2.1. Preparation and Characterization of the NiO Electrode
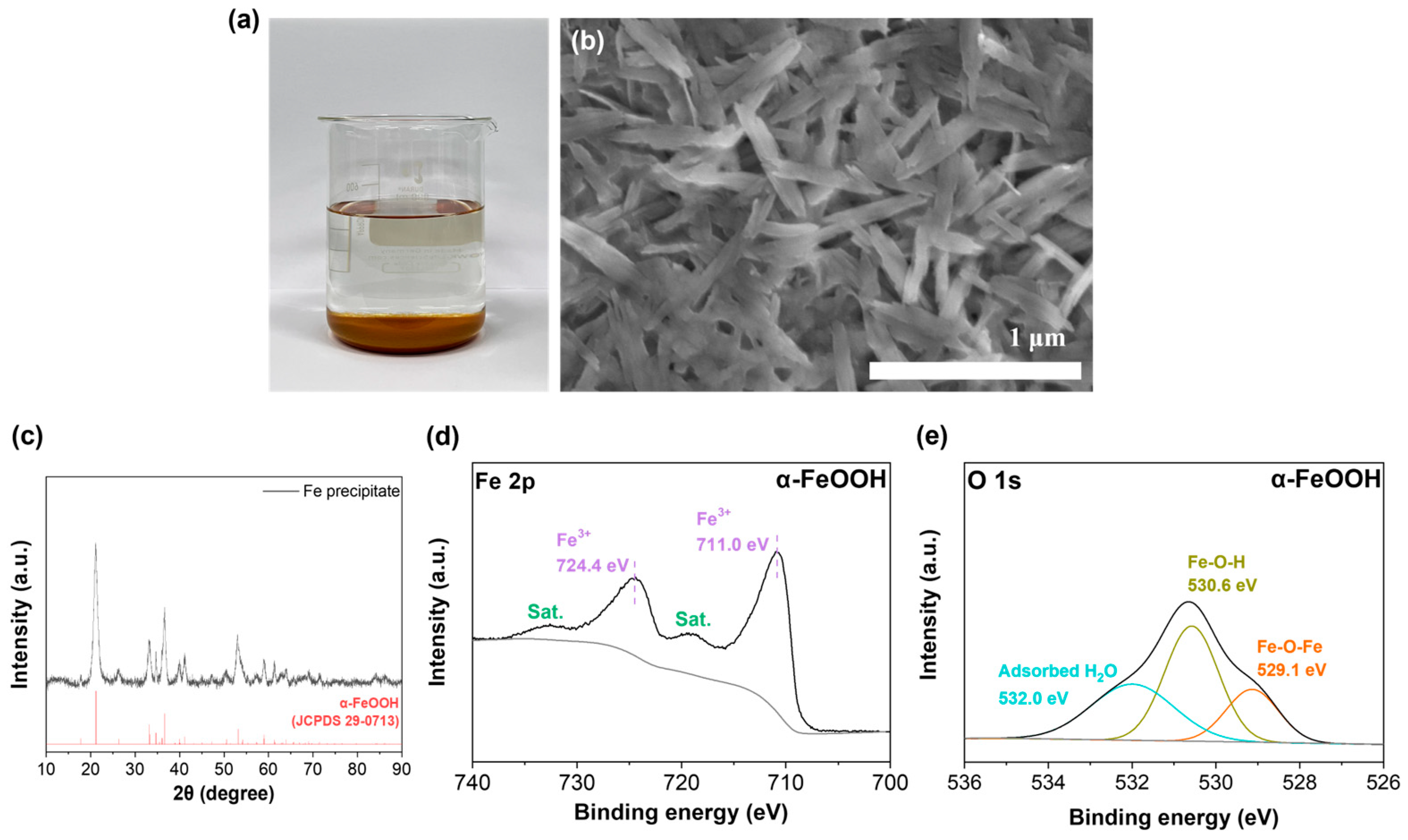
2.2. Fabrication of α-FeOOH (Fe Salt) in KOH Electrolyte
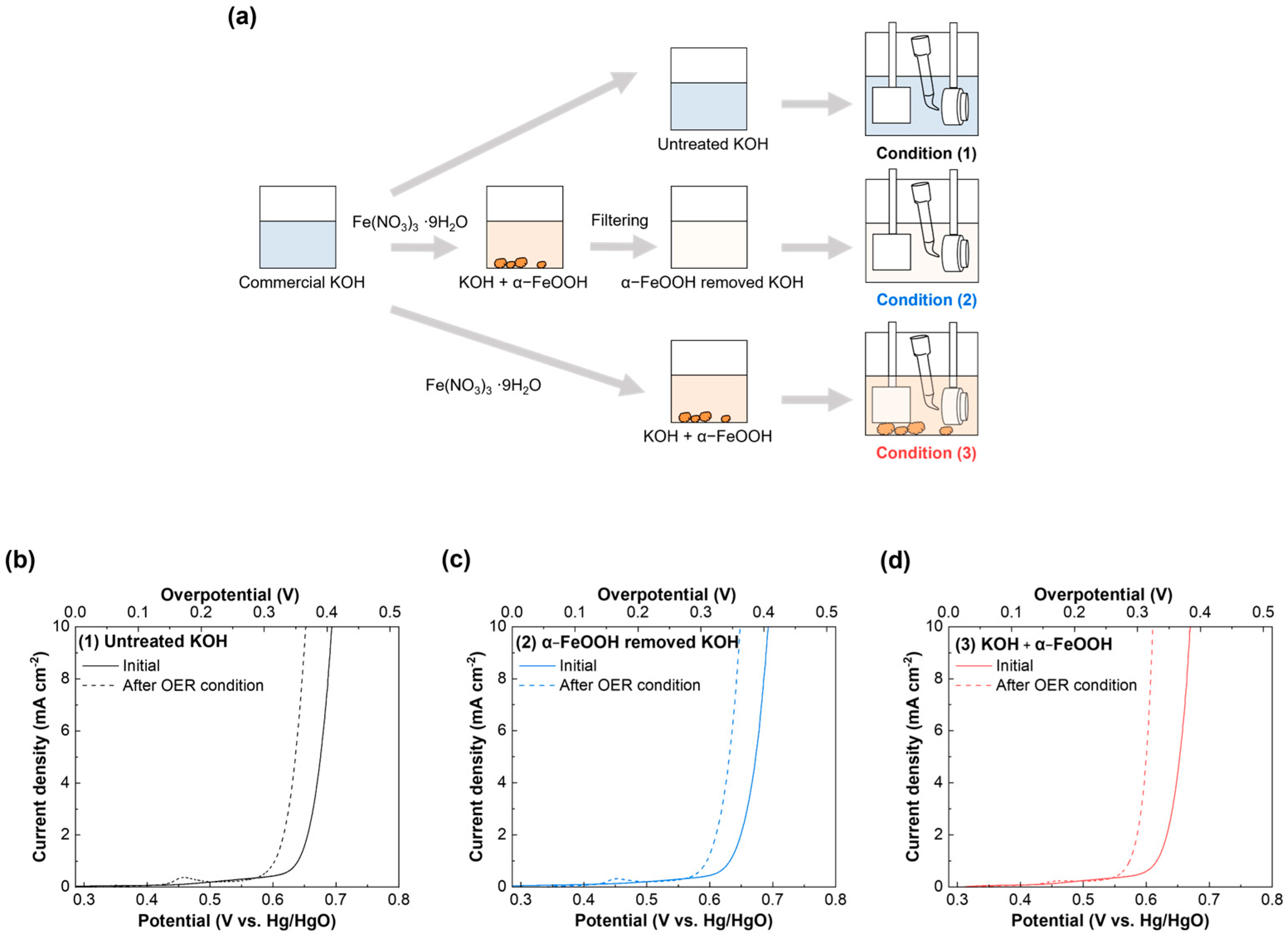
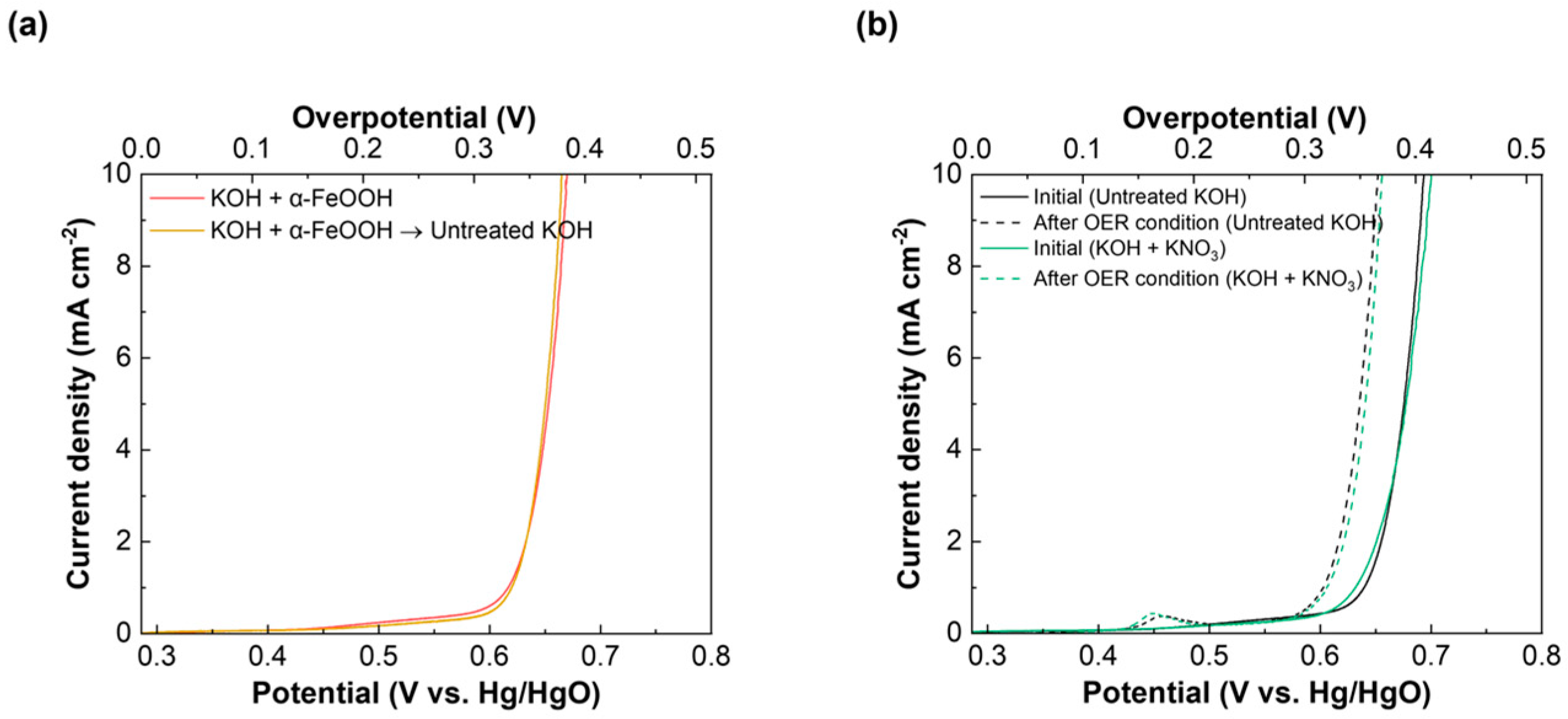
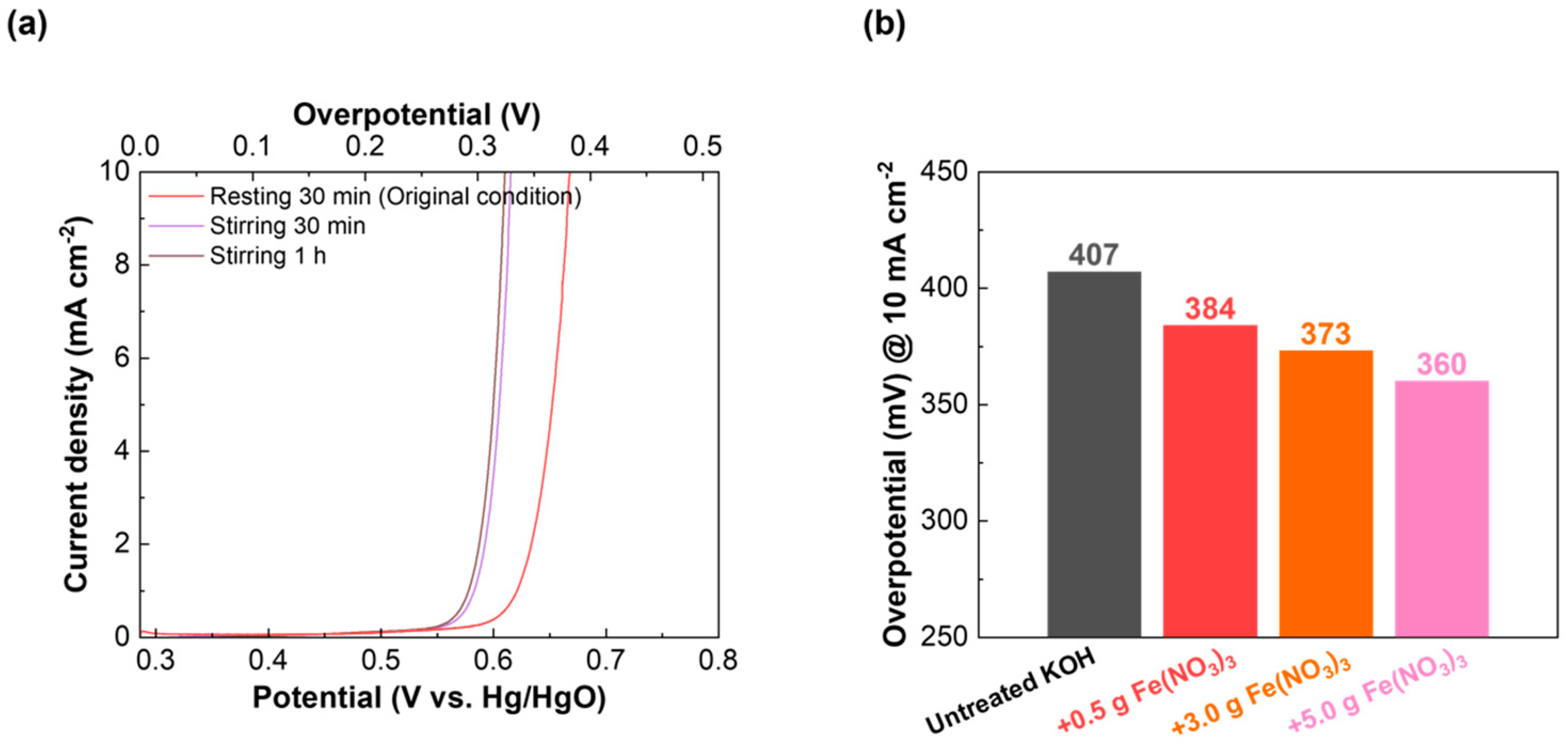
2.3. Influence of the Ionic Fe Impurity and α-FeOOH in KOH on the Electrochemical Performance
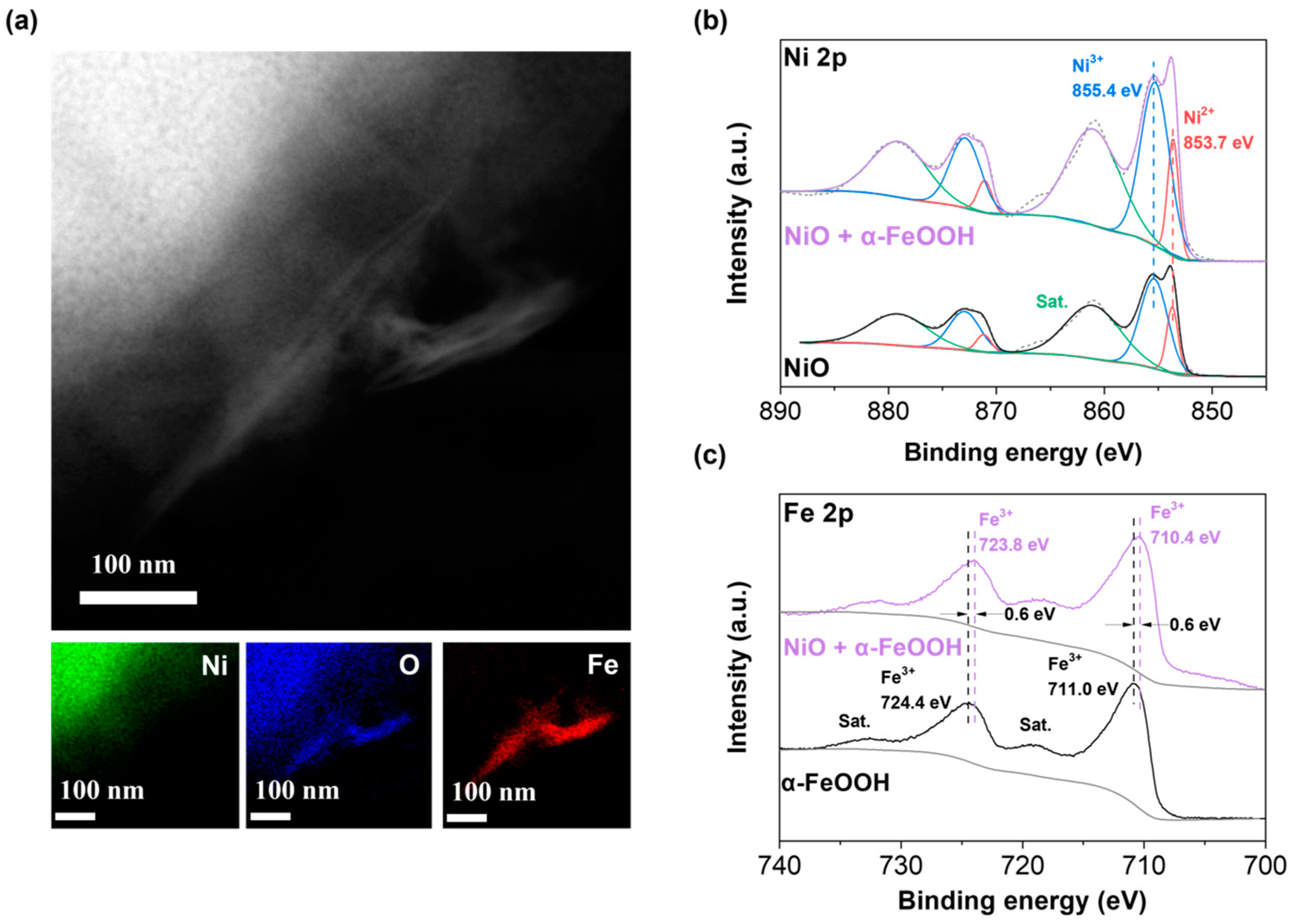
2.4. Confirmation of α-FeOOH on the NiO Electrode
3. Materials and Methods
3.1. Fabrication of NiO Electrode
3.2. Electrolyte Modification
3.3. Incorporating Ionic Fe Impurities into NiO
3.4. Electrochemical Measurement
3.5. Characterization
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| OER | Oxygen evolution reaction |
| AWE | Alkaline water electrolysis |
| HER | Hydrogen evolution reaction |
| CC | Constant current |
| EIS | Electrochemical impedance spectroscopy |
| SEM | Scanning electron microscopy |
| EDS | Energy-dispersive X-ray spectroscopy |
| TEM | Transmission electron microscopy |
| XRD | X-ray diffraction |
| XPS | X-ray photoelectron spectroscopy |
| CV | Cyclic voltammetry |
References
- Hu, S.; Guo, B.; Ding, S.; Yang, F.; Dang, J.; Liu, B.; Gu, J.; Ma, J.; Ouyang, M. A comprehensive review of alkaline water electrolysis mathematical modeling. Appl. Energy 2022, 327, 120099. [Google Scholar] [CrossRef]
- Wan, L.; Xu, Z.; Xu, Q.; Pang, M.; Lin, D.; Liu, J.; Wang, B. Key components and design strategy of the membrane electrode assembly for alkaline water electrolysis. Energy Environ. Sci. 2023, 16, 1384–1430. [Google Scholar] [CrossRef]
- Sebbahi, S.; Assila, A.; Alaoui Belghiti, A.; Laasri, S.; Kaya, S.; Hlil, E.K.; Rachidi, S.; Hajjaji, A. A comprehensive review of recent advances in alkaline water electrolysis for hydrogen production. Int. J. Hydrogen Energy 2024, 82, 583–599. [Google Scholar] [CrossRef]
- Zamanizadeh, H.R.; Sunde, S.; Pollet, B.G.; Seland, F. Tailoring the oxide surface composition of stainless steel for improved OER performance in alkaline water electrolysis. Electrochim. Acta 2022, 424, 140561. [Google Scholar] [CrossRef]
- Hoang, A.L.; Balakrishnan, S.; Hodges, A.; Tsekouras, G.; Al-Musawi, A.; Wagner, K.; Lee, C.-Y.; Swiegers, G.F.; Wallace, G.G. High-performing catalysts for energy-efficient commercial alkaline water electrolysis. Sustain. Energy Fuels 2023, 7, 31–60. [Google Scholar] [CrossRef]
- Wang, J.; Gao, Y.; Kong, H.; Kim, J.; Choi, S.; Ciucci, F.; Hao, Y.; Yang, S.; Shao, Z.; Lim, J. Non-precious-metal catalysts for alkaline water electrolysis: Operando characterizations, theoretical calculations, and recent advances. Chem. Soc. Rev. 2020, 49, 9154–9196. [Google Scholar] [CrossRef]
- Wang, Y.; Zhu, Q.; Zhang, P.; Liu, S.; Wang, J. Boosting the electrocatalytic activity and stability of Ni/NiO toward the oxygen evolution reaction by coupling FeOOH nanosheets. New J. Chem. 2024, 48, 9954–9960. [Google Scholar] [CrossRef]
- Zhou, D.; Li, P.; Xu, W.; Jawaid, S.; Mohammed-Ibrahim, J.; Liu, W.; Kuang, Y.; Sun, X. Recent Advances in Non-Precious Metal-Based Electrodes for Alkaline Water Electrolysis. ChemNanoMat 2020, 6, 336–355. [Google Scholar] [CrossRef]
- Demnitz, M.; Lamas, Y.M.; Garcia Barros, R.L.; de Leeuw den Bouter, A.; van der Schaaf, J.; Theodorus de Groot, M. Effect of iron addition to the electrolyte on alkaline water electrolysis performance. iScience 2024, 27, 108695. [Google Scholar] [CrossRef]
- Chang, J.; Chen, L.; Zang, S.; Wang, Y.; Wu, D.; Xu, F.; Jiang, K.; Gao, Z. The effect of Fe(III) cations in electrolyte on oxygen evolution catalytic activity of Ni(OH)2 electrode. J. Colloid Interface Sci. 2020, 569, 50–56. [Google Scholar] [CrossRef]
- Salmanion, M.; Najafpour, M.M. Oxygen-Evolution Reaction Performance of Nickel (Hydr)Oxide in Alkaline Media: Iron and Nickel Impurities. J. Phys. Chem. C 2023, 127, 18340–18349. [Google Scholar] [CrossRef]
- Corrigan, D.A. The Catalysis of the Oxygen Evolution Reaction by Iron Impurities in Thin Film Nickel Oxide Electrodes. J. Electrochem. Soc. 1987, 134, 377–384. [Google Scholar] [CrossRef]
- Trotochaud, L.; Young, S.L.; Ranney, J.K.; Boettcher, S.W. Nickel-iron oxyhydroxide oxygen-evolution electrocatalysts: The role of intentional and incidental iron incorporation. J. Am. Chem. Soc. 2014, 136, 6744–6753. [Google Scholar] [CrossRef] [PubMed]
- Burke, M.S.; Enman, L.J.; Batchellor, A.S.; Zou, S.; Boettcher, S.W. Oxygen Evolution Reaction Electrocatalysis on Transition Metal Oxides and (Oxy)hydroxides: Activity Trends and Design Principles. Chem. Mater. 2015, 27, 7549–7558. [Google Scholar] [CrossRef]
- Klaus, S.; Cai, Y.; Louie, M.W.; Trotochaud, L.; Bell, A.T. Effects of Fe Electrolyte Impurities on Ni(OH)2/NiOOH Structure and Oxygen Evolution Activity. J. Phys. Chem. C 2015, 119, 7243–7254. [Google Scholar] [CrossRef]
- Li, Q.; He, T.; Jiang, X.; Lei, Y.; Liu, Q.; Liu, C.; Sun, Z.; Chen, S.; Zhang, Y. Boosting oxygen evolution activity of nickel iron hydroxide by iron hydroxide colloidal particles. J. Colloid Interface Sci. 2022, 606, 518–525. [Google Scholar] [CrossRef]
- Encina, E.R.; Distaso, M.; Klupp Taylor, R.N.; Peukert, W. Synthesis of Goethite α-FeOOH Particles by Air Oxidation of Ferrous Hydroxide Fe(OH)2 Suspensions: Insight on the Formation Mechanism. Cryst. Growth Des. 2015, 15, 194–203. [Google Scholar] [CrossRef]
- Montes-Hernandez, G.; Beck, P.; Renard, F.; Quirico, E.; Lanson, B.; Chiriac, R.; Findling, N. Fast Precipitation of Acicular Goethite from Ferric Hydroxide Gel under Moderate Temperature (30 and 70 °C). Cryst. Growth Des. 2011, 11, 2264–2272. [Google Scholar] [CrossRef]
- Heath, M.M.; Poureshghi, F.; Seland, F.; Sunde, S.; Kriek, R.J. The Enhancing Effect of α-FeOOH on Ni Surfaces Toward Electrolytic Water Splitting. Energy Technol. 2023, 11, 2300313. [Google Scholar] [CrossRef]
- Kim, T.-H.; Koo, K.-Y.; Park, C.-S.; Jeong, S.-U.; Kim, J.-E.; Lee, S.-H.; Kim, Y.-H.; Kang, K.-S. Effect of Fe on Calcined Ni(OH)2 Anode in Alkaline Water Electrolysis. Catalysts 2023, 13, 496. [Google Scholar] [CrossRef]
- Moreno Fernández, H.; Gallenberger, J.; Mempin, C.; Khalek, I.; Neumann, M.; Lotfi, S.; Kim, S.M.; Li, M.; Tian, C.; Hofmann, J.P. Phase transitions in NiO during the oxygen evolution reaction assessed via electrochromic phenomena through operando UV–Vis spectroscopy. Electrochim. Acta 2024, 498, 144626. [Google Scholar] [CrossRef]
- Stern, L.-A.; Hu, X. Enhanced oxygen evolution activity by NiOx and Ni(OH)2 nanoparticles. Faraday Discuss. 2014, 176, 363–379. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Zhai, Z.-Y.; Chen, Z.; Zhang, L.-Z.; Zhao, X.-F.; Si, F.-Z.; Li, J.-H. Engineering Mesoporous NiO with Enriched Electrophilic Ni3+ and O− toward Efficient Oxygen Evolution. Catalysts 2018, 8, 310. [Google Scholar] [CrossRef]
- Kuai, C.; Xi, C.; Hu, A.; Zhang, Y.; Xu, Z.; Nordlund, D.; Sun, C.-J.; Cadigan, C.A.; Richards, R.M.; Li, L.; et al. Revealing the Dynamics and Roles of Iron Incorporation in Nickel Hydroxide Water Oxidation Catalysts. J. Am. Chem. Soc. 2021, 143, 18519–18526. [Google Scholar] [CrossRef]
- Gao, M.; Sheng, W.; Zhuang, Z.; Fang, Q.; Gu, S.; Jiang, J.; Yan, Y. Efficient water oxidation using nanostructured α-nickel-hydroxide as an electrocatalyst. J. Am. Chem. Soc. 2014, 136, 7077–7084. [Google Scholar] [CrossRef]
- Salunkhe, P.; Kekuda, D. Effect of annealing temperature on the physical properties of NiO thin films and ITO/NiO/Al Schottky diodes. J. Mater. Sci. Mater. Electron. 2022, 33, 21060–21074. [Google Scholar] [CrossRef]
- Li, S.; Liu, D.; Wang, G.; Ma, P.; Wang, X.; Wang, J.; Ma, R. Vertical 3D Nanostructures Boost Efficient Hydrogen Production Coupled with Glycerol Oxidation Under Alkaline Conditions. Nanomicro Lett. 2023, 15, 189. [Google Scholar] [CrossRef]
- Hu, J.; Li, S.; Chu, J.; Niu, S.; Wang, J.; Du, Y.; Li, Z.; Han, X.; Xu, P. Understanding the Phase-Induced Electrocatalytic Oxygen Evolution Reaction Activity on FeOOH Nanostructures. ACS Catal. 2019, 9, 10705–10711. [Google Scholar] [CrossRef]
- Padhi, D.K.; Parida, K. Facile fabrication of α-FeOOH nanorod/RGO composite: A robust photocatalyst for reduction of Cr(vi) under visible light irradiation. J. Mater. Chem. A 2014, 2, 10300–10312. [Google Scholar] [CrossRef]
- Cao, Y.; Shen, L.; Hu, X.; Du, Z.; Jiang, L. Low temperature desulfurization on Co-doped α-FeOOH: Tailoring the phase composition and creating the defects. Chem. Eng. J. 2016, 306, 124–130. [Google Scholar] [CrossRef]
- Wang, T.; Jiang, Z.; Chu, K.H.; Wu, D.; Wang, B.; Sun, H.; Yip, H.Y.; An, T.; Zhao, H.; Wong, P.K. X-Shaped α-FeOOH with Enhanced Charge Separation for Visible-Light-Driven Photocatalytic Overall Water Splitting. ChemSusChem 2018, 11, 1365–1373. [Google Scholar] [CrossRef] [PubMed]
- Guo, N.; Lv, X.; Li, Q.; Ren, T.; Song, H.; Yang, Q. Removal of hexavalent chromium from aqueous solution by mesoporous α-FeOOH nanoparticles: Performance and mechanism. Micropor. Mesopor. Mater. 2020, 299, 110101. [Google Scholar] [CrossRef]
- Cai, M.; Zhu, Q.; Wang, X.; Shao, Z.; Yao, L.; Zeng, H.; Wu, X.; Chen, J.; Huang, K.; Feng, S. Formation and Stabilization of NiOOH by Introducing α-FeOOH in LDH: Composite Electrocatalyst for Oxygen Evolution and Urea Oxidation Reactions. Adv. Mater. 2023, 35, e2209338. [Google Scholar] [CrossRef] [PubMed]
- Murphy, E.; Sun, B.; Rüscher, M.; Liu, Y.; Zang, W.; Guo, S.; Chen, Y.-H.; Hejral, U.; Huang, Y.; Ly, A.; et al. Synergizing Fe2O3 Nanoparticles on Single Atom Fe-N-C for Nitrate Reduction to Ammonia at Industrial Current Densities. Adv. Mater. 2024, 36, e2401133. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, T.-H.; Jeon, J.-H.; Kim, J.-E.; Kang, K.-S.; Yoon, J.; Park, C.-S.; Jung, K.; Han, T.; Lee, H.; Joo, H.; et al. Effect of α-FeOOH in KOH Electrolytes on the Activity of NiO Electrodes in Alkaline Water Electrolysis for the Oxygen Evolution Reaction. Catalysts 2024, 14, 870. https://doi.org/10.3390/catal14120870
Kim T-H, Jeon J-H, Kim J-E, Kang K-S, Yoon J, Park C-S, Jung K, Han T, Lee H, Joo H, et al. Effect of α-FeOOH in KOH Electrolytes on the Activity of NiO Electrodes in Alkaline Water Electrolysis for the Oxygen Evolution Reaction. Catalysts. 2024; 14(12):870. https://doi.org/10.3390/catal14120870
Chicago/Turabian StyleKim, Tae-Hyun, Jae-Hee Jeon, Ji-Eun Kim, Kyoung-Soo Kang, Jaekyung Yoon, Chu-Sik Park, Kwangjin Jung, Taeyang Han, Heonjoong Lee, Hyunku Joo, and et al. 2024. "Effect of α-FeOOH in KOH Electrolytes on the Activity of NiO Electrodes in Alkaline Water Electrolysis for the Oxygen Evolution Reaction" Catalysts 14, no. 12: 870. https://doi.org/10.3390/catal14120870
APA StyleKim, T.-H., Jeon, J.-H., Kim, J.-E., Kang, K.-S., Yoon, J., Park, C.-S., Jung, K., Han, T., Lee, H., Joo, H., & Lee, H. (2024). Effect of α-FeOOH in KOH Electrolytes on the Activity of NiO Electrodes in Alkaline Water Electrolysis for the Oxygen Evolution Reaction. Catalysts, 14(12), 870. https://doi.org/10.3390/catal14120870





