Abstract
Formolase, a thiamine pyrophosphate (TPP)-dependent enzyme, catalyzes the carboligation of three one-carbon formaldehyde molecules into one three-carbon dihydroxyacetone molecule. It has many important functions in the biosynthesis of carbon-based compounds and utilization of CO2. However, the enzyme has low activity and stability in the catalytic process, resulting in high cost in the applications. To improve the stability, formolase was immobilized onto magnetic nanoparticles, which were designed to have functional epoxy groups for covalently binding the enzyme. In the immobilization, effects of pH, temperature, and cofactor TPP on the immobilization were investigated and optimized. The results showed that the retention activity of immobilized formolase was highly related to TPP. In the presence of TPP, the specific activity of the immobilized formolase was 6.8 times higher than that without TPP. The optimal immobilization conditions were as follows: a temperature of 20 °C, a pH of 7.0, an immobilization time of 8 h, and an enzyme loading of 20 mg/g. Molecular docking was used to analyze the effect of TPP on the stabilization of the enzyme in the immobilization, which indicated that TTP could stabilize the enzyme structure during the immobilization. The stabilization effect of TPP could be a reference in the immobilization of other enzymes with TPP as the cofactor.
1. Introduction
As highly efficient biocatalysts, enzymes can catalyze many types of chemical reactions with high catalytic efficiency and stereoselectivity under mild reaction conditions [1,2,3]. At present, many enzymes have been applied in industrial applications including organic synthesis, medicine, cosmetics, detergent, and food manufacture [4,5]. However, the low stability and short life of enzymes limit their applications due to the high cost [6]. In order to improve the enzyme performance and lower the cost, protein engineering, chemical modification, or immobilization are employed to improve the stability [7]. Among these strategies, immobilization is one of the most efficient methods to improve the stability [8].
Compared with free enzymes, immobilized enzymes can be used repeatedly and continuously [9], and they also have some advantages, including being easy to separate from the reaction solution and having high mechanical stability [10]. Generally, the immobilized enzyme is defined as the enzyme immobilized on/in carriers. The interactions between enzymes and carriers may include Van der Waals forces, hydrophobic interactions, ionic interactions, hydrogen bonds, covalent bonds, and other combinations thereof [11]. The binding mode is the main factor for the stability of the immobilized enzymes and the first priority for immobilization of enzymes. Among all the immobilization methods, including adsorption, entrapment, covalent bonding, and cross-linking [12], covalent bonding and cross-linking are the most preferred because covalent bonds are the most stable bonds.
Formolase is a computationally designed enzyme that can catalyze the carboligation of three one-carbon formaldehyde molecules into one three-carbon dihydroxyacetone molecule. Siegel et al. designed a computational enzyme for the formolase pathway, which was a new carbon fixation pathway in 2015 [13]. Several groups employed formolase in the transformation of formaldehyde into functional sugars and upgrading ethanol to higher-order alcohols with multiple enzyme cascade catalysis because formolase has a wide range of industrial applications [14]. The enzyme was reported to be used in the synthesis of acetoin in vivo [15]. However, the expression of the recombinant formolase was very low; even the titer of acetoin was improved 40-fold after codon optimization in a whole-cell biocatalytic system. To reduce the cost, immobilization could be a good choice for recycling the enzyme.
Li et al. immobilized alcohol dehydrogenase, acetaldehyde lyase, and formolase onto magnetic nanomaterials for production of acetoin [16]. Kondaveeti et al. developed an immobilization support of conductive metal-alloy nanoparticles with carbon cloth for formolase [17]. Gupta et al. encapsulated three enzymes, including formolase, for the production of acetoin in a cascade enzymatic reaction system [18]. These approaches are considered environmentally friendly and promising synthetic ways to produce acetoin [19] because the immobilized formolase could be recycled and reused.
In the present work, formolase was immobilized on magnetic nanomaterials. The main factors that influence the immobilization were investigated, and the properties of the immobilized enzyme were evaluated. The immobilization conditions, including pH, temperature, and the ratio between enzymes and carriers were optimized. The stabilization effect of cofactor TPP in the immobilization and the possible mechanism were explored with molecular docking.
2. Results and Discussion
2.1. Preparation of the Purified Formolase and Nanocarriers
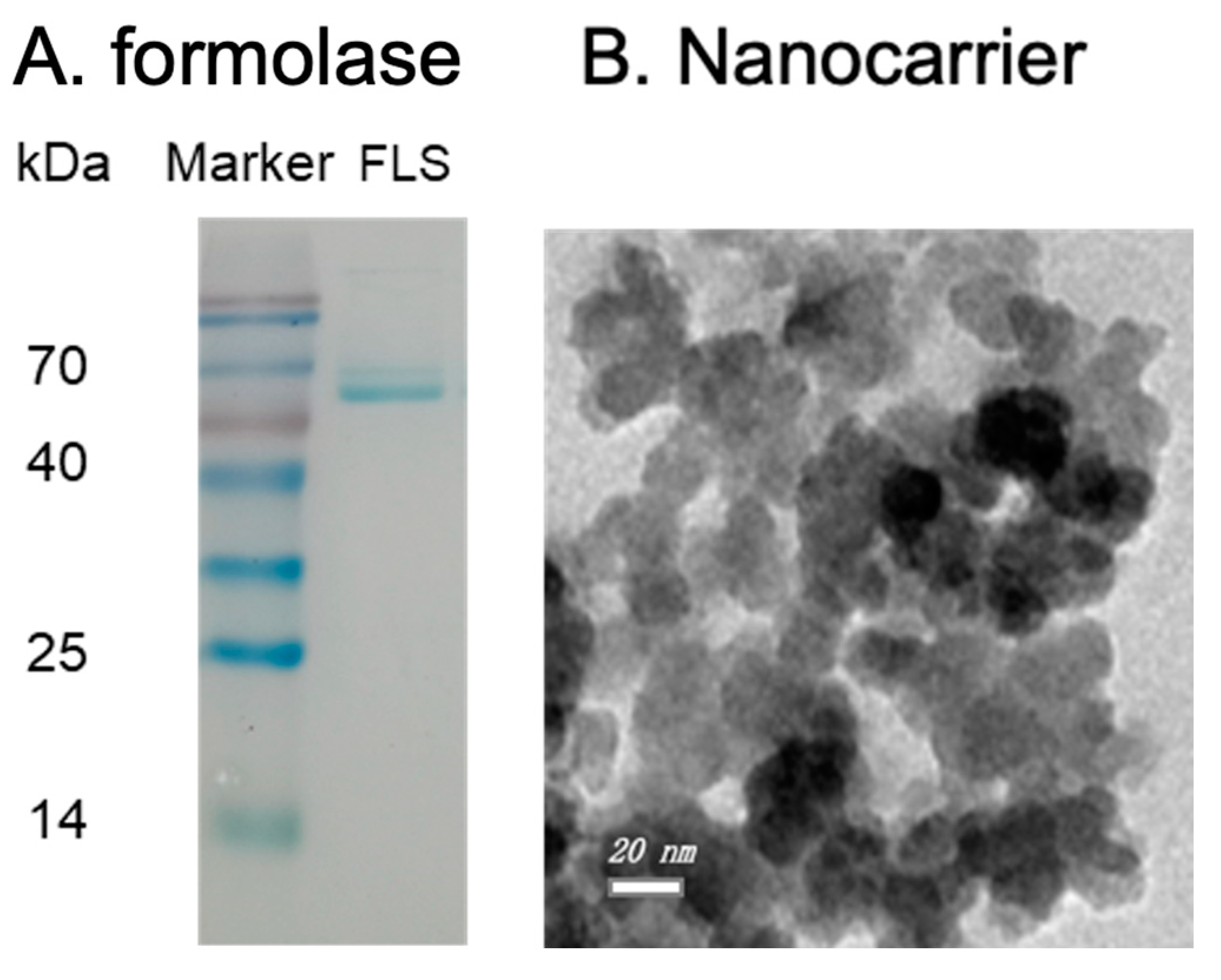
The SDS-PAGE analysis of the purified formolase is shown in Figure 1A. It is clear that the molecular weight of formolase is about 60 kDa, and the purity was higher than 90%. From the enzyme assay, it is confirmed that the formolase was successfully expressed and purified. The epoxy-functionalized nanoparticles were prepared and characterized, and the dispersion of the nanocarriers was not uniform, and they tend to form aggregates because of the magnetism. The diameter of the particles was around 26 nm (Figure 1B). The properties were characterized and were similar to the previous report. The grafted epoxy group on the nanoparticles was around 0.2 mmol/g particles, and the coating epoxide did not change the size distribution significantly; the saturated magnetization was 29 emu/g. With the prepared nanoparticles, a high immobilization efficiency of 92% could be obtained for the immobilization of alcohol dehydrogenase [10].
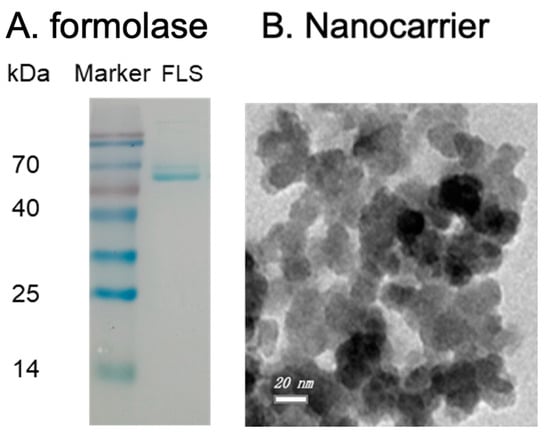
Figure 1.
The SDS-PAGE image of the purified formolase (A) and TEM of the prepared Fe3O4@SiO2-Epoxy nanocarriers (B).
2.2. The Stabilization Effect of TPP on the Immobilization of Formolase
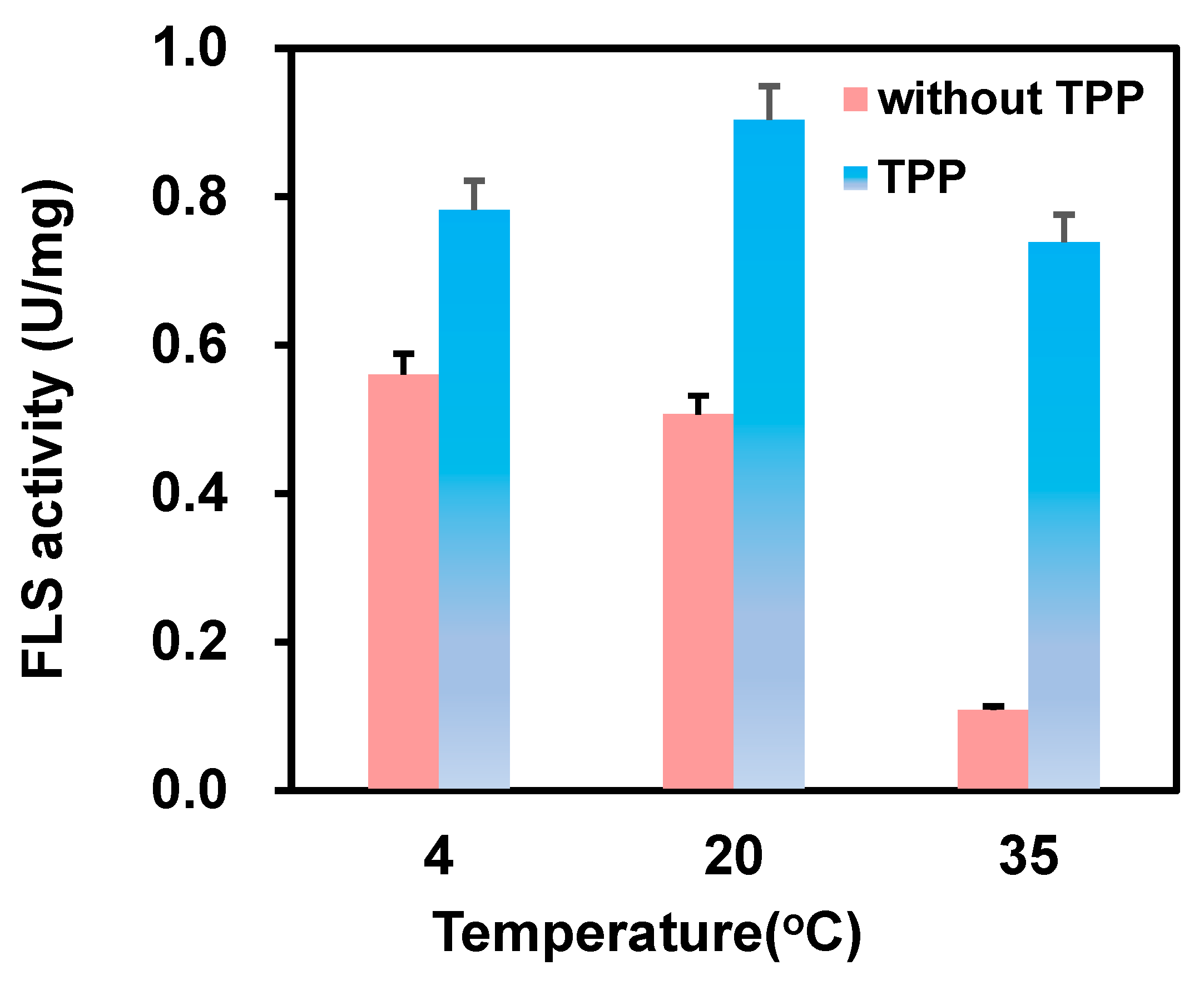
Formolase, a TPP-dependent enzyme, has previously been reported to have lost 50% of enzyme activity at 30 °C for 3 h in TPP-deficient conditions [20]. Thus, the effect of TPP on immobilization was investigated first. In the absence of TPP, formolase lost around 62% activity after 4 h of incubation, but it could retain more than 81% activity in the presence of 1 mM TPP. Then, we investigated the effect of TPP on the immobilization at 4, 20, and 35 °C. The data in Figure 2 showed that the activity of immobilized formolase in the presence of TPP supplementation was much higher than that without TPP. Especially at 35 °C, the specific activity of the immobilized formolase in the presence of TPP was 6.8 times higher compared to that without TPP in the immobilization. The higher activity of the immobilized enzyme at 20 °C than that at 4 °C could be attributed to the fact that more enzyme was immobilized on the carrier. It is noted that the specific activities of the immobilized formolase in the presence of 1 mM TPP were 0.78, 0.90, and 0.74 U/mg, respectively. However, the activity of the immobilized formolase decreased significantly when the temperature increased from 4 to 35 °C; the specific activities were 0.56, 0.51, and 0.10 U/mg, respectively. The stabilization effect of TPP in the immobilization of formolase could be observed clearly. This could be explained by the fact that the binding of TPP with enzymes can induce the protective effect, preventing the unfavorable reaction between some residues like lysine and the epoxy group on the carriers.
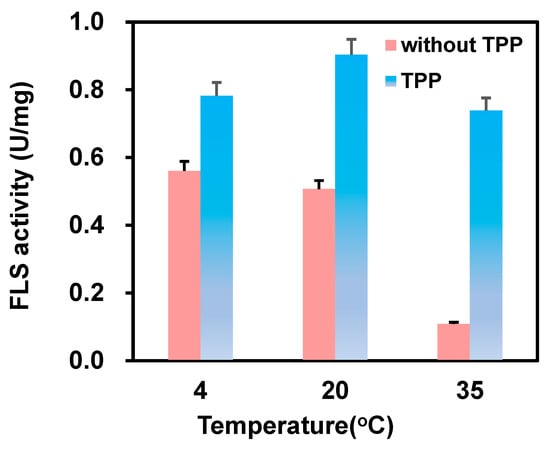
Figure 2.
Effect of cofactor TPP on the immobilization (blue column: in the presence of 1 mM TPP; red: absence of TPP).
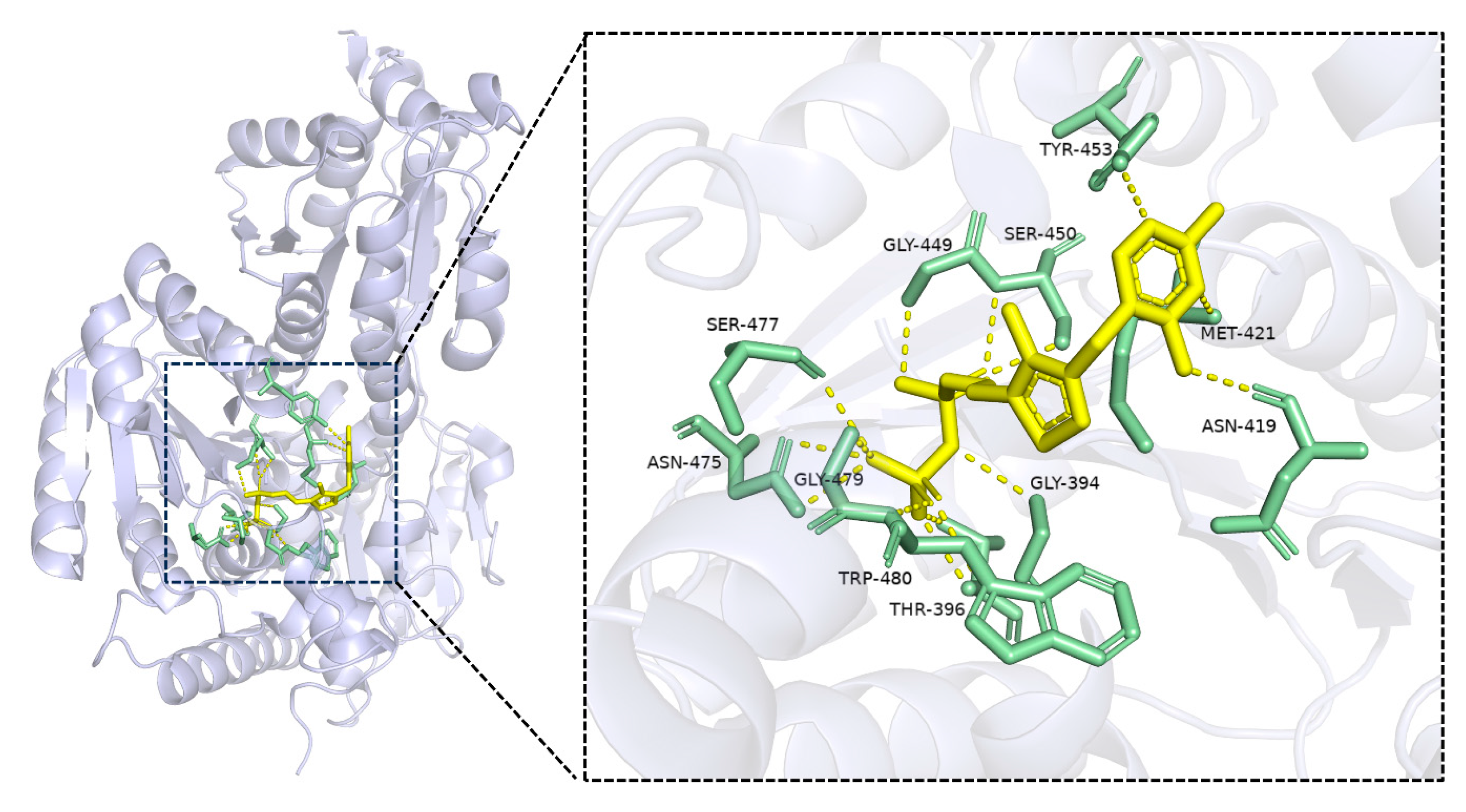
It is known that TPP binds the interface of the conserved pyrophosphate and pyrimidine domains of the TPP-dependent enzymes [21]. To explain the possible reasons for the stabilization effect of TPP in the immobilization, multiple TPP-dependent enzymes were selected for alignment of the primary sequences. Although the similarity of the TPP-dependent enzymes’ primary sequences is low, the TPP binding domain is highly conserved, including a -GDG-E-NN- sequence, and is located in the random coil area of the joint sequence linking the alpha helix and beta sheet. It could be deduced that the TPP in the binding domain could stabilize the spatial structure of the TPP-dependent enzymes. To further prove this point, molecular docking of TPP-dependent formolase was performed as shown in Figure 3. Also, it can be seen that the TPP domain is located inside the enzyme molecules and links the alpha helix and beta sheet, playing a significant role in maintaining the structure and enhancing the rigidity of the enzyme. The thiazole ring at the end of TPP can form hydrogen bonds with the residues Asn419, Met421, and Tyr453 of formolase. These hydrogen bonds help to maintain the spatial structure of formolase, thus playing an enhanced stabilization role in the immobilization of formolase.
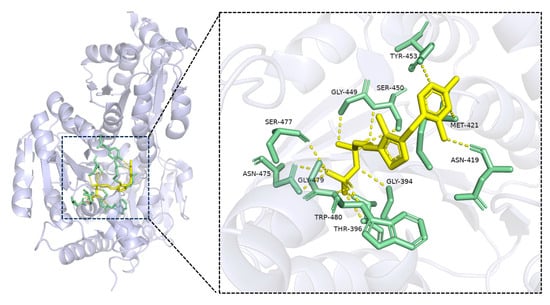
Figure 3.
The interactions of TPP and formolase (4QQ8). TPP and the binding sites were colored yellow and green, respectively.
2.3. Immobilization of Formolase on the Fe3O4-SiO2-Epoxy Nanoparticles
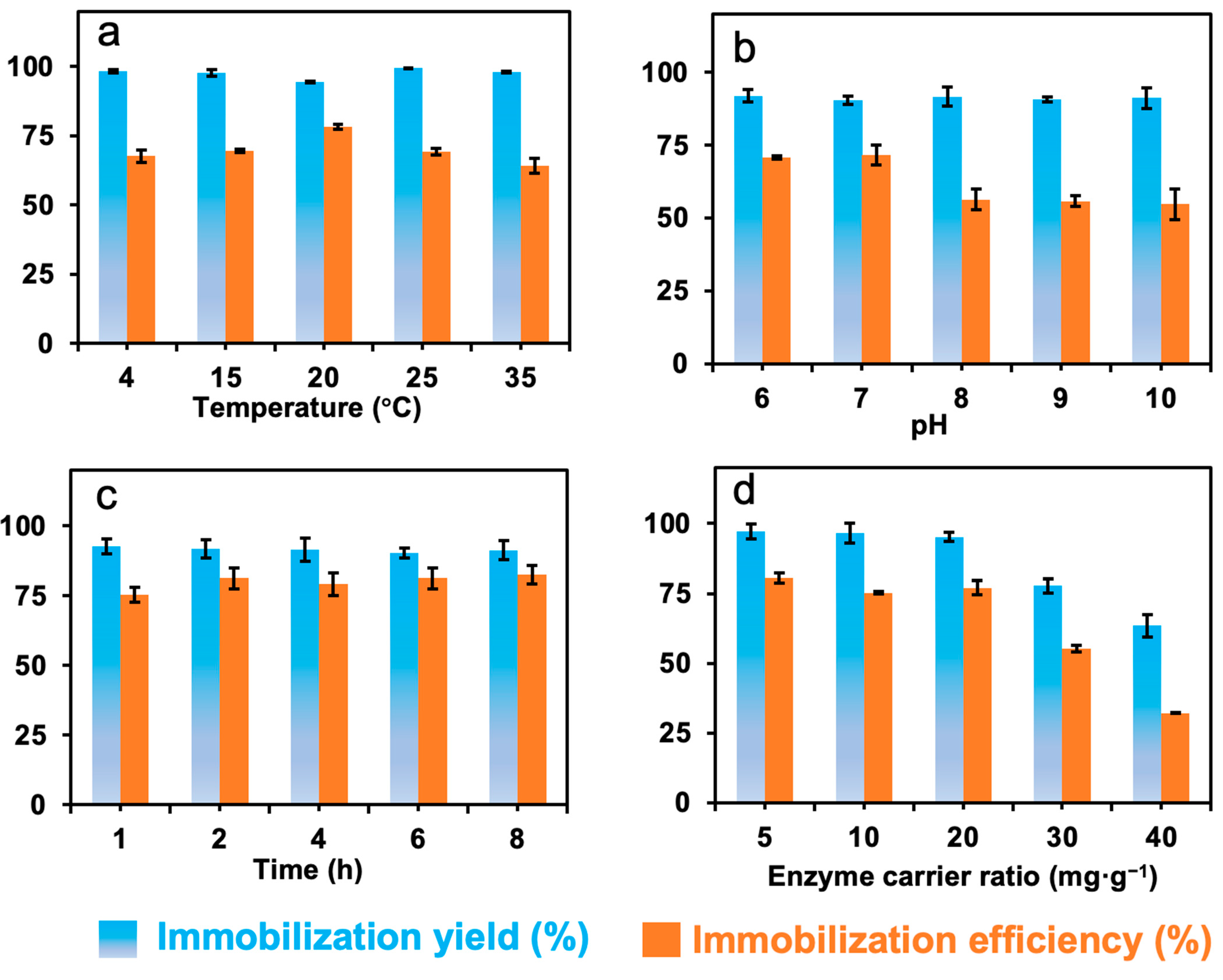
In order to obtain high immobilization yield and efficiency, the factors that influence the immobilization, including temperature, pH, immobilization time, and the ratio of protein to carrier were investigated, and the data are presented in Figure 4.
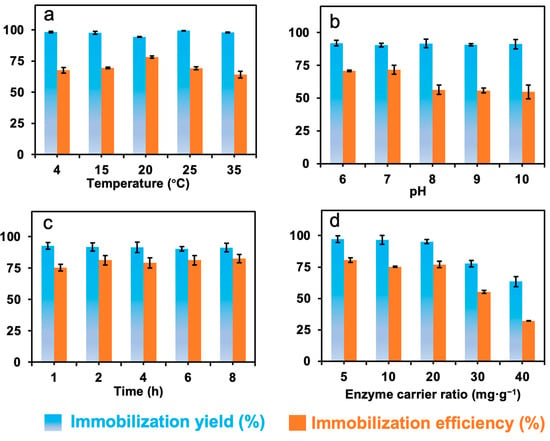
Figure 4.
The effects of (a) temperature, (b) pH, and (c) immobilization time, and (d) the enzyme–carrier ratio on the immobilization of formolase.
Figure 4a shows that the effect of temperature on the immobilization yield was around 93% in the presence of 1 mM TPP, which means most of the enzyme molecules were covalently bound to the carriers. The immobilization efficiency was about 70%, and the highest value of 78% was achieved at 20 °C. In the temperature range from 4 to 35 °C, more than 30% activity loss was observed, and this might be caused by the mass transfer limitations during the immobilization process. Thus, in the following immobilization experiments, 20 °C was selected as the optimal temperature for the immobilization of formolase in the presence of TPP.
It has been reported that pH can affect not only the dissociation of the amino acid residues of enzymes and the conformation of the protein molecule but also the binding of cofactors. The results in Figure 4b showed that when pH was increased from 6.0 to 10.0, the immobilization yield and efficiency varied around 92% and 70%, respectively. This may be related to the high reactivity of the functional epoxy groups on the surface of nanoparticles [22]. The immobilization efficiency of formolase was 71% when the pH was 7.0, which was the highest value under the immobilization conditions.
The effect of the immobilization time on the immobilization of formolase was shown in Figure 4c. The immobilization time can affect the immobilization yield and efficiency in two ways. When the immobilization time is extended, some amino acid residues that are not highly reactive on the surface of the enzyme might react with the epoxy group, affecting the conformation and catalytic activity of the enzyme. Another one is related to the stability of the enzyme, and the enzyme inactivation in the reaction system normally leads to low yield and efficiency [23]. A high immobilization yield and efficiency will be obtained at the optimal immobilization time, resulting in an economical immobilization process. The immobilization yield and efficiency were 92% and 75% at an immobilization time of 1 h, respectively. Although the reaction of amino acid residues of enzymes and epoxy groups is fast, the immobilization efficiency was lower than the highest one due to the insufficient bonding of the enzyme with epoxy groups and the presence of adsorptive immobilization [22]. When the incubation time was 8 h, the immobilization yield of the enzyme was 92%, and the immobilization efficiency was maximal at 82%.
The effect of enzyme-to-support ratio on the immobilization is shown in Figure 4d. When the amount of enzyme is excessive, enzyme molecules might bind on the surface of the support by crowding together, or some enzyme molecules might not be able to approach carriers, leading to a high mass transfer resistance to substrate molecules in the catalytic reaction [24]. While the amount of enzyme is insufficient for the support, the immobilization yield will be high because most of the protein can be immobilized. But the specific activity may be low because the enzyme is not enough for the immobilization. It is difficult to find a suitable ratio of enzyme to carrier [25]. When the ratio of enzyme to carrier was 20 mg g−1, the immobilized efficiency was 77%, while the immobilization yield was as high as 95%.
2.4. Characterization of the Immobilized Formolase
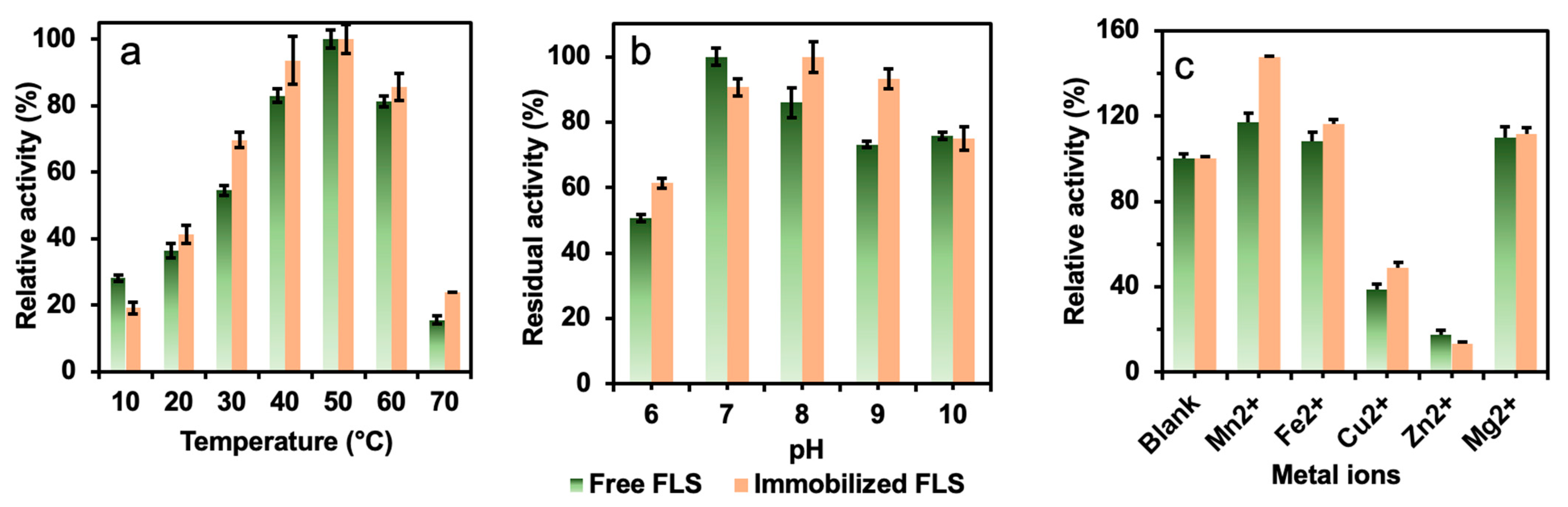
The immobilized formolase was evaluated and compared with the free formolase under different temperature, pH, and metal ions. The effect of temperature on the activity of immobilized and free formolase is shown in Figure 5a. The optimum temperature for both immobilized and free enzymes was 50 °C. The immobilized enzyme displayed higher relative activity than that of free formolase. This was due to the fact that high temperatures caused a large activity loss of the free enzyme [26], and the immobilized formolase had higher resistance to temperature.
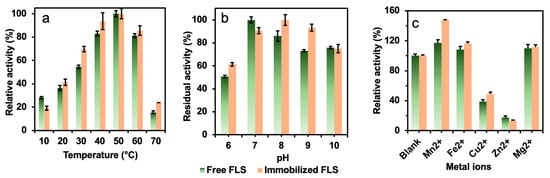
Figure 5.
The effects of (a) temperature, (b) pH, and (c) metal ions on the relative activities of free and immobilized FLS.
The effect of pH on the catalytic activity of enzymes is shown in Figure 5b. The optimum pH of the immobilized formolase was shifted to pH 8.0 from 7.0 of the free enzyme. There is no doubt that the structure of formolase had changed to some extent in the immobilization process because of the covalent bonds formed between the amino acid residues of the enzyme and the epoxide groups on the support [16,27]. The immobilization resulted in an increase in the rigidity of the enzyme and improved the operational stability of the enzyme. The relative activities of the immobilized formolases were higher than those of the free enzyme at a pH range of 8.0–9.0.
Metal ions are essential for many enzymes, especially metal enzymes. In enzymatic reactions, metal ions can bind enzymes in solution, changing the activity of the enzyme. The state and conformation of the substrate can also be affected by metal ions and further induce the conformational change of the enzyme. In the present work, cofactor TPP can form complexes with some metal ions, such as Mg+ [28]. As shown in Figure 5c, it can be seen that Mg2+, Mn2+, and Fe2+ could activate the free and immobilized formolase. The best activation effect was obtained by addition of Mn2+, and the enzyme activity was enhanced by 47% compared to that of the control. This might be attributed to the improved rigidity of enzymes after immobilization [29]. However, Cu2+ and Zn2+ inhibited the activity of the immobilized and free enzymes, and the corresponding apparent activities were 50% and 20% in the presence of 5 mM Zn2+. Obviously, Zn2+ has a stabilization effect or enhancement effect on the activity of formolase. According to the previous report, the pyrophosphate group of TPP could chelate metal ions, stabilizing the specific conformation of the enzyme and further activating the enzyme activity [28].
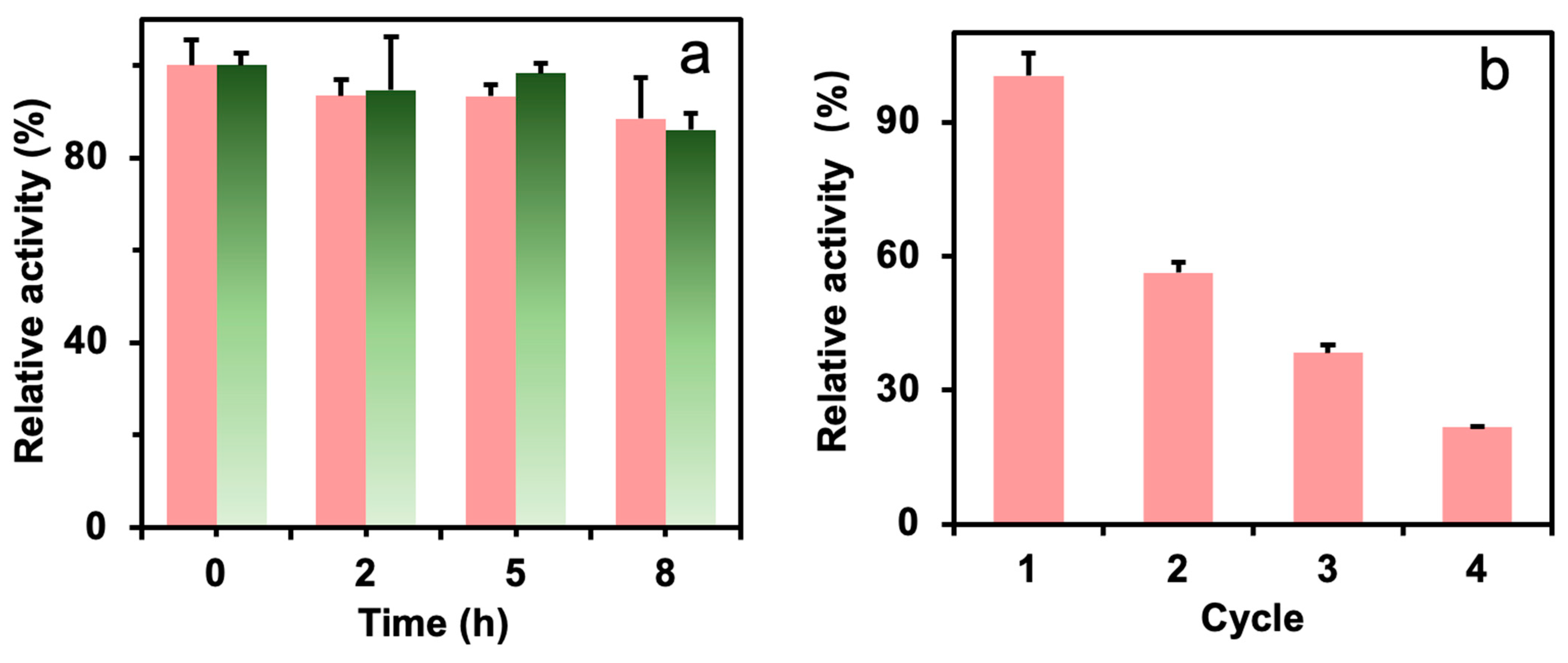
The thermal stability of the immobilized enzyme could influence the enzyme cost in practice. Figure 6a shows the thermal stability of the free and immobilized formolase. The formolase was able to retain high activity (88% for immobilized formolase and 86% for free formolase) at 8 h and 40 °C in the presence of TPP, indicating formolase has acceptable thermal stability. The immobilized formolase did not lose much activity, and this will benefit its applications. The immobilized enzyme did not show significantly higher stability than the free enzyme, and the reason may be attributed to the important stabilization role of TPP [30].
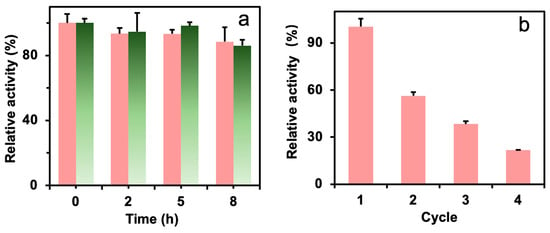
Figure 6.
The thermal stability of free formolase (green column) and immobilized formolase (red column) (a) and the recyclability of the immobilized formolase (b).
The reusability of the immobilized formolase was tested and is shown in Figure 6b. The residual activity of the immobilized enzyme was 56% after one catalytic cycle, which is far below the expectation. The main reason for the loss of enzyme activity is probably that a high concentration of substrate (acetaldehyde) could react with amino acid residues to form Schiff bases and damage the enzyme structure. And acetaldehyde is highly hydrophilic and able to destroy the hydration layer of proteins, leading to a serious loss of enzyme molecule activity [31]. This could be avoided by using multiple enzyme cascade reactions to decrease the concentration of acetaldehydes. In our previous report, the final product acetoin could be achieved with 90% yield and proved the potential applications of the immobilized formolase [16].
3. Materials and Methods
3.1. Materials
Isopropyl-β-D-thiogalactopyranoside (IPTG), yeast extract, tryptone, kanamycin, acetoin, and thiamine pyrophosphate (TPP) were provided by Aladdin (Shanghai, China). Ni-NTA resin was purchased from Sangon Biotech (Shanghai, China). Tryptophan, sodium chloride, sodium hydroxide, 1-naphthol, creatine, dipotassium hydrogen phosphate, trimethylol aminomethane, acetaldehyde, triethylamine, ferrous sulfate, ferric chloride, 3-glycidyloxypropyltrimethoxysilane, magnesium sulfate, and other chemicals were supplied by Sinopharm (Shanghai, China). Unless specifically indicated, all other reagents are of analytical or biological grade.
3.2. Enzyme Assay
The activity of formolase was determined with the method of creatine colorimetry [32], in which the concentration of acetoin could be quantitatively determined. For free enzyme, 1 mL of reaction mixture containing pH 8.0 HEPES (50 mM) buffer, 1 mM Mg2+, 0.1 mM TPP, 100 mM acetaldehyde, and 10 mg mL−1 formolase was incubated for 30 min. After the enzymatic reaction, the mixture was immediately transferred to a boiling-water bath for 5 min to stop the reaction. The concentration of acetoin in the solution was determined at 520 nm [33], and the specific activity of formolase was calculated. For the immobilized enzyme, the reaction system contained 1 mM Mg2+, 100 mM acetaldehyde, and TPP in pH 8.0 HEPES buffer, and the immobilized formolase of 10 mg was used as the catalysts for the biotransformation. After the reaction, the mixture was boiled for 5 min, and the supernatant was collected by centrifugation for calculating the specific activity of the immobilized formolase, and 1 unit of specific activity was defined as the mass of protein producing 1 μmol acetoin per minute.
To calculate the activity recovery of the immobilized formolase and the protein loading on the nanocarriers, the following equations were used:
3.3. Preparation and Characterization of Fe3O4@SiO2-Epoxy Magnetic Nanoparticles
Magnetic nanoparticles functionalized with epoxy groups were prepared according to our previous work [10]. About 2.8 g of FeSO4·7H2O (0.01 mol) and 5.4 g of FeCl3·6H2O (0.02 mol) were mixed in 150 mL of double-distilled water then transferred to a three-neck flask. Then, 10 mL of ammonia solution was added slowly into the mixture at room temperature under the protection of nitrogen flow, and the reaction was continuously stirred vigorously at 70 °C for 30 min. The obtained Fe3O4 nanoparticles were washed with double-distilled water three times and dried in a vacuum dryer (Changcheng, Beijing, China) at 60 °C for 10 h. Then, 1 g of Fe3O4 nanoparticles in ethanol was dispersed by sonication for 10 min, and 50 mL of water and 20 mL of ammonia solution were added into the suspension at room temperature. Afterwards, 3 mL of tetraethyl orthosilicate (TEOS) was added to form the SiO2 layer on the surface of the Fe3O4 nanoparticles. The resulting Fe3O4@SiO2 nanoparticles were washed three times with double-distilled water and dried in a vacuum at 60 °C for 10 h. To modify Fe3O4@SiO2 with epoxy groups, 1 g of Fe3O4@SiO2 was added to 49 mL of toluene and sonicated for 30 min. Afterwards, 0.15 mL of triethylamine and 1 mL of 3-glycidyloxypropyltrimethoxysilane were added to the suspension and refluxed and stirred for 10 h under the protection of nitrogen gas at 115 °C. The obtained Fe3O4@SiO2-Epoxy nanoparticles were washed three times with acetone and dried in a vacuum at 60 °C for 10 h.
3.4. Preparation and Purification of Formolase
The purified recombinant formolase was prepared according to the previous publication, and the details are as follows [34]. The recombinant Escherichia coli BL21 harboring the plasmid containing the formolase gene was cultured at 37 °C in Luria–Bertani broth medium supplemented with kanamycin (50 μg mL−1). When the optical density at 600 nm of the culture reached 0.6–0.8, IPTG was added to the medium at the final concentration of 0.2 mM to induce protein expression for 6 h at 15 °C. Then, the cells were harvested by centrifugation at 4143× g for 5 min and rinsed with HEPES (0.05 M, pH 8.0) buffer. The harvested cells were resuspended with the same HEPES buffer and disrupted with an ultrasonic processor in an ice-water bath for 8 min. The supernatant containing recombinant formolase was obtained by removing the cell debris with centrifuging at 6134× g for 20 min and mixed with Ni-NTA resin for 3 h for binding. The Ni-NTA column was eluted with the elution buffer after removing flowthrough. The eluted solution including formolase was stored at 4 °C for the immobilization. The protein concentration contained in the supernatant was measured with the Bradford method [35].
3.5. Immobilization of Formolase on Fe3O4@SiO2-Epoxy Nanoparticles
The immobilization of formolase on Fe3O4@SiO2-Epoxy nanoparticles was performed at 20 °C for 2 h. Briefly, 10 mg of nanoparticles was gently suspended in a 0.5 mL enzyme solution containing 0.2 mg of formolase and mixed with a rotating vortex (Qilinbeier, Haimen, China). After the immobilization, the immobilized formolase was collected from the reaction solution by centrifugation (4143× g) at 4 °C for 3 min. After washing with HEPES buffer (pH 8.0) 3 times, the immobilized formolase was obtained for further characterization and investigation.
The effects of temperature, pH, immobilization time, the ratio of enzyme to carrier, and cofactors on the immobilization of formolase were investigated. The ranges of pH, immobilization temperature, the ratio of enzyme to carrier, and the time range are set as 6–10, 0–50 °C, 0.005–0.04 mg mg−1, and 0.5–8 h, respectively. To explore the effect of cofactor TPP on immobilization, formolase was immobilized on the nanoparticles at different temperatures (4, 20, and 25 °C) in the presence of 0.1 M TPP, and the control was set as the immobilization without TPP. The protein loading and activity recovery of immobilized enzymes were calculated based on the recovery activity and protein concentration. Each experiment was repeated three times, and the standard deviation was calculated accordingly.
3.6. Characterization of the Immobilized Formolase
To characterize the immobilized formolase, the effects of temperature and pH were investigated. For the free and immobilized formolase, the relative activity was investigated in the temperature range of 10 to 70 °C in HEPES (50 mM pH 8.0). The effects of pH on the activities of free and immobilized enzymes were determined in PBS buffers (pH 6.0–9.0). Then, the effects of metal ions such as Mn2+, Fe2+, Cu2+, Zn2+, and Mg2+ on the immobilized and free formolase were evaluated in HEPES buffer (50 mM pH 8.0), and the concentration of metal ions was set as 5 mM.
The thermal stability of the immobilized formolase was tested at 40 °C in HEPES buffer (50 mM, pH 8.0); the enzyme was incubated, and samples were withdrawn to check the stability and activity. The reusability of the immobilized formolase was assessed by reusing the immobilized enzyme to catalyze acetaldehyde in HEPES (pH 8.0) at 40 °C. Briefly, the immobilized enzyme was harvested by centrifugation after each reaction cycle, and the residual activity of the enzyme was determined to calculate the relative activity.
3.7. Molecular Docking of TPP into Formolase
Molecular docking of TPP and formolase (PDB ID: 4QQ8) was performed with AutoDock (version 4.2.6), and the parameters were set as default. All the interactions between enzymes and TPP were visualized with PyMOL (version 3.0.4).
4. Conclusions
In the present work, magnetic nanoparticles functionalized with epoxy groups were prepared as carriers for immobilization of formolase. The diameter of the magnetic nanoparticles was around 26 nm, and the enzyme loading could be 20 mg/g. The effects of temperature, pH, time, and enzyme loading on the immobilization efficiency and yield were investigated. Under the optimal conditions, the immobilization yield and efficiency were around 95% and 77%, respectively. The thermal stability and reusability of the immobilized formolase were enhanced compared with the free enzyme. In the immobilization, cofactor TPP showed significant stabilization effect of the enzyme, and the activity of the immobilized formolase in the presence of TPP was 6.8 times higher compared to that in the absence of TPP. This work elucidated the noncovalent binding of cofactor TPP inside of the enzyme and enhanced the rigidity of formolase, which will stabilize the conformation of formolase in the immobilization.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/catal14120911/s1. The protein sequence of FLS.
Author Contributions
Conceptualization, Y.-W.Z.; methodology, S.Y. and X.-Y.L.; validation, W.-J.S., investigation, S.Y. and X.-Y.L.; writing—original draft preparation, S.Y.; writing—review and editing, Y.-W.Z.; supervision, Y.-W.Z. All authors have read and agreed to the published version of the manuscript.
Funding
The financial support from National Natural Science Foundation of China (No. 22068001) was appreciated.
Data Availability Statement
The original data presented in this work are included in the article and Supplementary Material. Further inquiries can be directed to the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
| TPP | Thiamine pyrophosphate |
| FLS | Formolase |
| SDS-PAGE | Sodium dodecyl sulfate-polyacrylamide gel electrophoresis |
| IPTG | Isopropyl-β-D-thiogalactopyranoside |
| HEPES | 2-[4-(2-hydroxyethyl)piperazin-1-yl]ethanesulfonic acid |
| TEOS | Tetraethyl orthosilicate |
| PDB | Protein data bank |
References
- Meghwanshi, G.K.; Kaur, N.; Verma, S.; Dabi, N.K.; Vashishtha, A.; Charan, P.D.; Purohit, P.; Bhandari, H.S.; Bhojak, N.; Kumar, R. Enzymes for Pharmaceutical and Therapeutic Applications. Biotechnol. Appl. Biochem. 2020, 67, 586–601. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.; Miton, C.M.; Tokuriki, N. A Mechanistic View of Enzyme Evolution. Protein Sci. 2020, 29, 1724–1747. [Google Scholar] [CrossRef] [PubMed]
- Vishnu Priya, B.; Sreenivasa Rao, D.H.; Gilani, R.; Lata, S.; Rai, N.; Akif, M.; Kumar Padhi, S. Enzyme Engineering Improves Catalytic Efficiency and Enantioselectivity of Hydroxynitrile Lyase for Promiscuous Retro-Nitroaldolase Activity. Bioorganic Chem. 2022, 120, 105594. [Google Scholar] [CrossRef] [PubMed]
- Eş, I.; Vieira, J.D.G.; Amaral, A.C. Principles, Techniques, and Applications of Biocatalyst Immobilization for Industrial Application. Appl. Microbiol. Biotechnol. 2015, 99, 2065–2082. [Google Scholar] [CrossRef] [PubMed]
- Bilal, M.; Rasheed, T.; Zhao, Y.; Iqbal, H.M.N.; Cui, J. “Smart” Chemistry and Its Application in Peroxidase Immobilization Using Different Support Materials. Int. J. Biol. Macromol. 2018, 119, 278–290. [Google Scholar] [CrossRef]
- Hartmann, M.; Jung, D. Biocatalysis with Enzymes Immobilized on Mesoporous Hosts: The Status Quo and Future Trends. J. Mater. Chem. 2010, 20, 844–857. [Google Scholar] [CrossRef]
- Teufl, M.; Zajc, C.U.; Traxlmayr, M.W. Engineering Strategies to Overcome the Stability–Function Trade-Off in Proteins. ACS Synth. Biol. 2022, 11, 1030–1039. [Google Scholar] [CrossRef]
- Hanefeld, U.; Gardossi, L.; Magner, E. Understanding Enzyme Immobilisation. Chem. Soc. Rev. 2009, 38, 453–468. [Google Scholar] [CrossRef]
- Zhu, C.-Y.; Li, F.-L.; Zhang, Y.-W.; Gupta, R.K.; Patel, S.K.S.; Lee, J.-K. Recent Strategies for the Immobilization of Therapeutic Enzymes. Polymers 2022, 14, 1409. [Google Scholar] [CrossRef]
- Jiang, X.-P.; Lu, T.-T.; Liu, C.-H.; Ling, X.-M.; Zhuang, M.-Y.; Zhang, J.-X.; Zhang, Y.-W. Immobilization of Dehydrogenase onto Epoxy-Functionalized Nanoparticles for Synthesis of (R)-Mandelic Acid. Int. J. Biol. Macromol. 2016, 88, 9–17. [Google Scholar] [CrossRef]
- Wang, K.; Zhao, L.; Li, T.; Wang, Q.; Ding, Z.; Dong, W. Selective Immobilization of His-Tagged Enzyme on Ni-Chelated Ion Exchange Resin and Its Application in Protein Purification. Int. J. Mol. Sci. 2023, 24, 3864. [Google Scholar] [CrossRef] [PubMed]
- Imam, H.T.; Marr, P.C.; Marr, A.C. Enzyme Entrapment, Biocatalyst Immobilization without Covalent Attachment. Green Chem. 2021, 23, 4980–5005. [Google Scholar] [CrossRef]
- Siegel, J.B.; Smith, A.L.; Poust, S.; Wargacki, A.J.; Bar-Even, A.; Louw, C.; Shen, B.W.; Eiben, C.B.; Tran, H.M.; Noor, E.; et al. Computational Protein Design Enables a Novel One-Carbon Assimilation Pathway. Proc. Natl. Acad. Sci. USA 2015, 112, 3704. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Wang, Y.; Liu, J.; Li, Q.; Zhang, Z.; Zheng, P.; Lu, F.; Sun, J. Biological Conversion of Methanol by Evolved Escherichia coli Carrying a Linear Methanol Assimilation Pathway. Bioresour. Bioprocess. 2017, 4, 41. [Google Scholar] [CrossRef]
- Peng, K.; Guo, D.; Lou, Q.; Lu, X.; Cheng, J.; Qiao, J.; Lu, L.; Cai, T.; Liu, Y.; Jiang, H. Synthesis of Ligustrazine from Acetaldehyde by a Combined Biological-Chemical Approach. ACS Synth. Biol. 2020, 9, 2902–2908. [Google Scholar] [CrossRef]
- Li, X.-Y.; Huang, J.-Y.; Zhou, Q.; Xu, Y.-Y.; Prabhu, P.; Zhang, Y.-W. Immobilization of Alcohol Dehydrogenase, Acetaldehyde Lyase, and NADH Oxidase for Cascade Enzymatic Conversion of Ethanol to Acetoin. Energies 2022, 15, 4242. [Google Scholar] [CrossRef]
- Kondaveeti, S.; Park, G.D.; Shanmugam, R.; Pagolu, R.; Patel, S.K.S.; Bisht, A.; Kim, D.R.; Kang, Y.C.; Lee, J.-K. Investigating the Role of Metals Loaded on Nitrogen-Doped Carbon-Nanotube Electrodes in Electroenzymatic Alcohol Dehydrogenation. Appl. Catal. B Environ. 2022, 307, 121195. [Google Scholar] [CrossRef]
- Gupta, R.K.; Patel, S.K.S.; Lee, J.-K. Novel Cofactor Regeneration-Based Magnetic Metal-Organic Framework for Cascade Enzymatic Conversion of Biomass-Derived Bioethanol to Acetoin. Bioresour. Technol. 2024, 408, 131175. [Google Scholar] [CrossRef]
- Hu, G.; Li, Z.; Ma, D.; Ye, C.; Zhang, L.; Gao, C.; Liu, L.; Chen, X. Light-Driven CO2 Sequestration in Escherichia coli to Achieve Theoretical Yield of Chemicals. Nat. Catal. 2021, 4, 395–406. [Google Scholar] [CrossRef]
- Janzen, E.; Müller, M.; Kolter-Jung, D.; Kneen, M.M.; McLeish, M.J.; Pohl, M. Characterization of Benzaldehyde Lyase from Pseudomonas fluorescens: A Versatile Enzyme for Asymmetric C–C Bond Formation. Bioorganic Chem. 2006, 34, 345–361. [Google Scholar] [CrossRef]
- Costelloe, S.; Ward, J.; Dalby, P. Evolutionary Analysis of the TPP-Dependent Enzyme Family. J. Mol. Evol. 2008, 66, 36–49. [Google Scholar] [CrossRef] [PubMed]
- Mateo, C.; Grazu, V.; Palomo, J.M.; Lopez-Gallego, F.; Fernandez-Lafuente, R.; Guisan, J.M. Immobilization of Enzymes on Heterofunctional Epoxy Supports. Nat. Protoc. 2007, 2, 1022–1033. [Google Scholar] [CrossRef] [PubMed]
- Serra, I.; Benucci, I.; Robescu, M.; Lombardelli, C.; Esti, M.; Calvio, C.; Pregnolato, M.; Terreni, M.; Bavaro, T. Developing a Novel Enzyme Immobilization Process by Activation of Epoxy Carriers with Glucosamine for Pharmaceutical and Food Applications. Catalysts 2019, 9, 843. [Google Scholar] [CrossRef]
- Xu, K.; Chen, X.; Zheng, R.; Zheng, Y. Immobilization of Multi-Enzymes on Support Materials for Efficient Biocatalysis. Front. Bioeng. Biotechnol. 2020, 8, 660. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Núñez, K.; Bernal, C.; Bolívar, J.M. Chapter 1—The Enzyme, the Support, and the Immobilization Strategy: The Key Findings to a Desirable Biocatalyst. In Biocatalyst Immobilization; Ferreira, M.L., Ed.; Foundations and Frontiers in Enzymology; Academic Press: Cambridge, MA, USA, 2023; pp. 1–16. ISBN 978-0-323-91317-1. [Google Scholar]
- Guisan, J.M.; López-Gallego, F.; Bolivar, J.M.; Rocha-Martín, J.; Fernandez-Lorente, G. The Science of Enzyme Immobilization. In Immobilization of Enzymes and Cells: Methods and Protocols; Guisan, J.M., Bolivar, J.M., López-Gallego, F., Rocha-Martín, J., Eds.; Springer: New York, NY, USA, 2020; pp. 1–26. ISBN 978-1-07-160215-7. [Google Scholar]
- Ulu, A.; Ozcan, I.; Koytepe, S.; Ates, B. Design of Epoxy-Functionalized Fe3O4@MCM-41 Core-Shell Nanoparticles for Enzyme Immobilization. Int. J. Biol. Macromol. 2018, 115, 1122–1130. [Google Scholar] [CrossRef]
- Hawkins, C.F.; Borges, A.; Perham, R.N. A Common Structural Motif in Thiamin Pyrophosphate-Binding Enzymes. FEBS Lett. 1989, 255, 77–82. [Google Scholar] [CrossRef]
- Thangaraj, B.; Jia, Z.; Dai, L.; Liu, D.; Du, W. Effect of Silica Coating on Fe3O4 Magnetic Nanoparticles for Lipase Immobilization and Their Application for Biodiesel Production. Arab. J. Chem. 2019, 12, 4694–4706. [Google Scholar] [CrossRef]
- Demir, A.S.; Seşenoglu, O.; Dünkelmann, P.; Müller, M. Benzaldehyde Lyase-Catalyzed Enantioselective Carboligation of Aromatic Aldehydes with Mono- and Dimethoxy Acetaldehyde. Org. Lett. 2003, 5, 2047–2050. [Google Scholar] [CrossRef][Green Version]
- Solís-Calero, C.; Ortega-Castro, J.; Hernández-Laguna, A.; Muñoz, F. A Comparative DFT Study of the Schiff Base Formation from Acetaldehyde and Butylamine, Glycine and Phosphatidylethanolamine. Theor. Chem. Acc. 2012, 131, 1263. [Google Scholar] [CrossRef]
- Zhang, L.; Singh, R.; Sivakumar, D.; Guo, Z.; Li, J.; Chen, F.; He, Y.; Guan, X.; Kang, Y.C.; Lee, J.K. An Artificial Synthetic Pathway for Acetoin, 2,3-Butanediol, and 2-Butanol Production from Ethanol Using Cell Free Multi-Enzyme Catalysis. Green Chem. 2018, 20, 230–242. [Google Scholar] [CrossRef]
- Taiwo, A.E.; Madzimbamuto, T.N.; Ojumu, T.V. Optimization of Process Variables for Acetoin Production in a Bioreactor Using Taguchi Orthogonal Array Design. Heliyon 2020, 6, e05103. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.-Y.; Yu, S.; Li, R.-F.; Han, K.-K.; Zhang, Y.-W. Fabrication of Fe3O4@SiO2@PDA-Ni2+ Nanoparticles for One-Step Affinity Immobilization and Purification of His-Tagged Glucose Dehydrogenase. Process Biochem. 2023, 128, 106–115. [Google Scholar] [CrossRef]
- Bradford, M.M. A Rapid and Sensitive Method for the Quantitation of Microgram Quantities of Protein Utilizing the Principle of Protein-Dye Binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).