Insights into the Role of Pt Promoter in Co/TiO2 Catalysts for CO Hydrogenation
Abstract
:1. Introduction
2. Results and Discussion
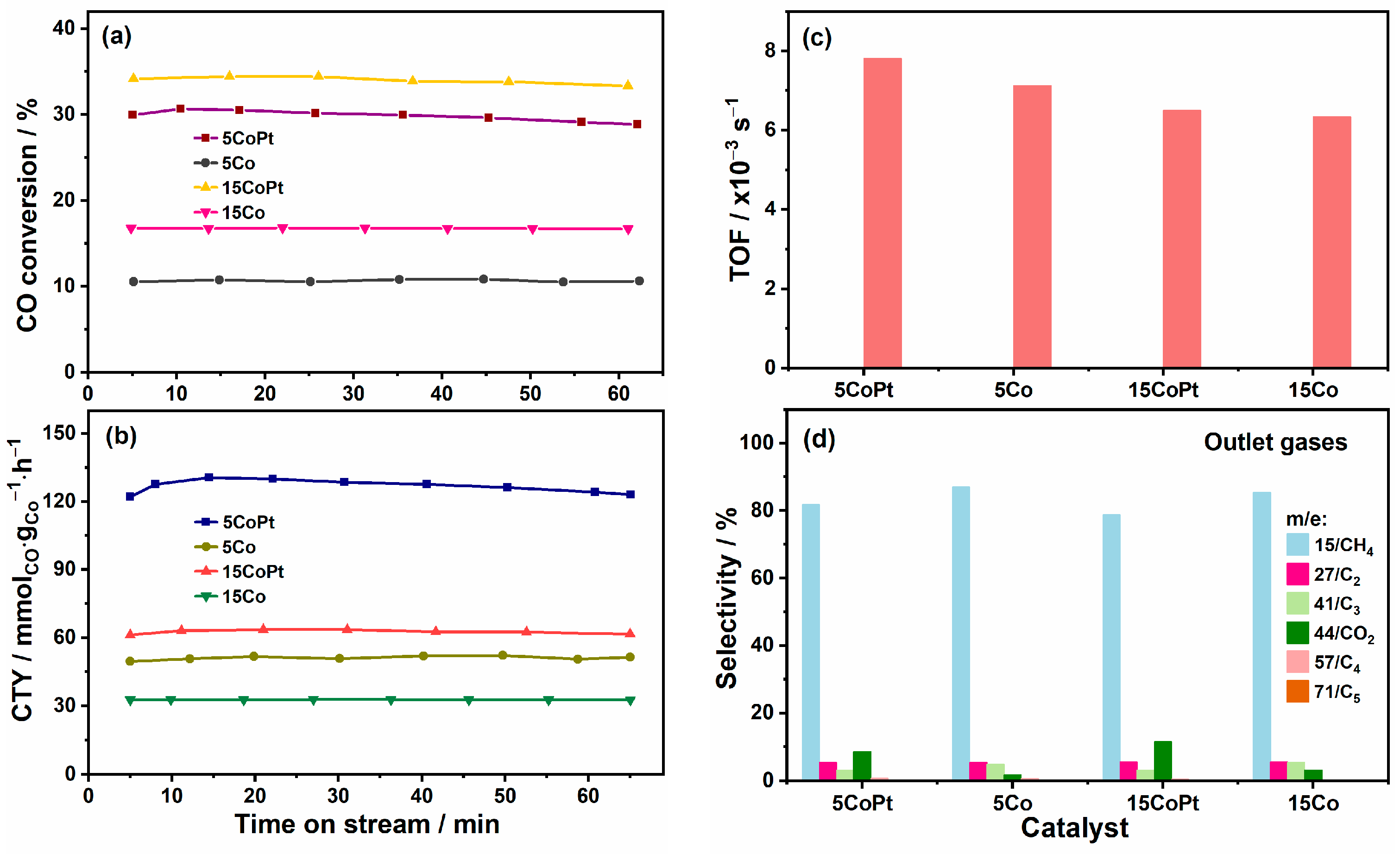
2.1. Improved Activities of CO Hydrogenation on Platinum-Promoted Catalysts
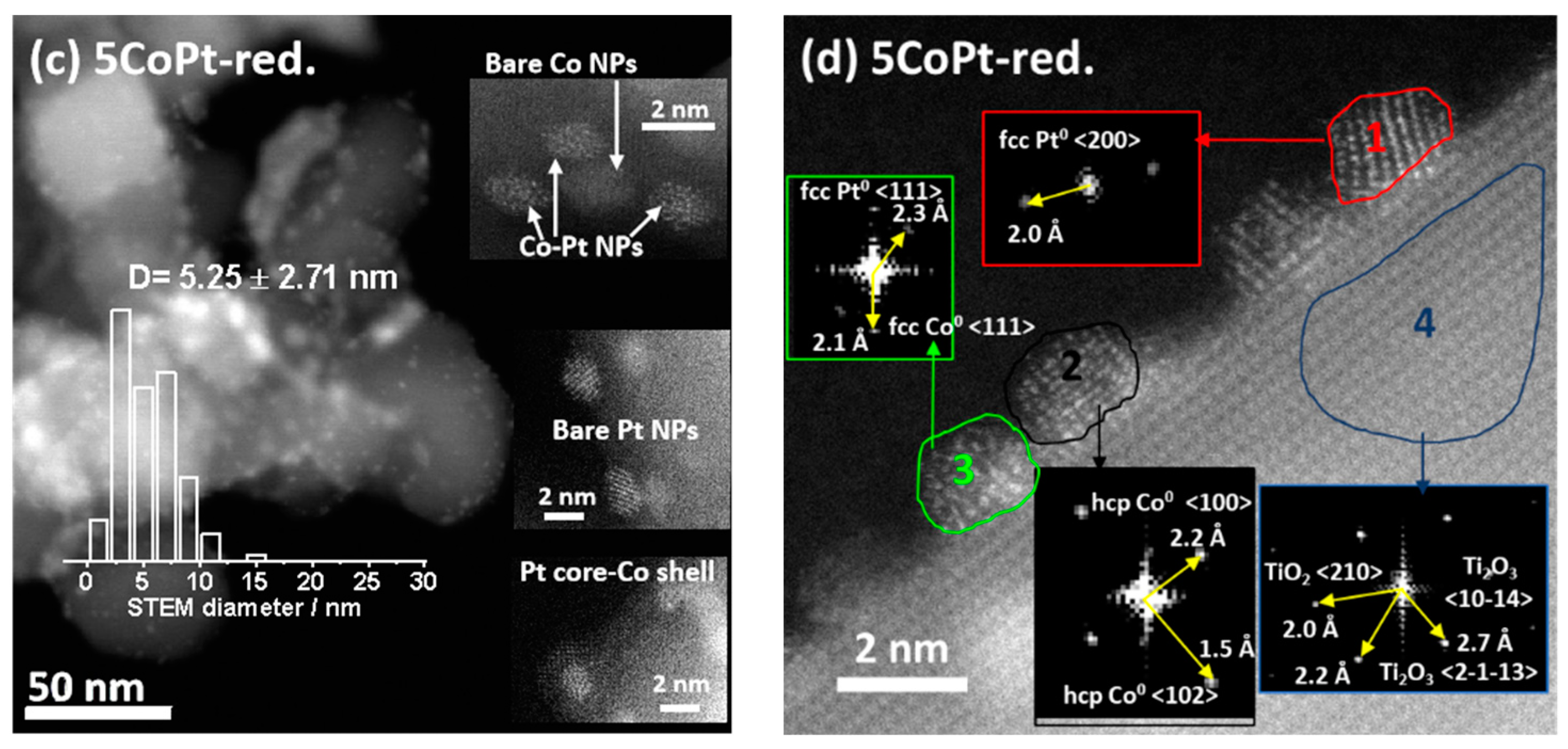
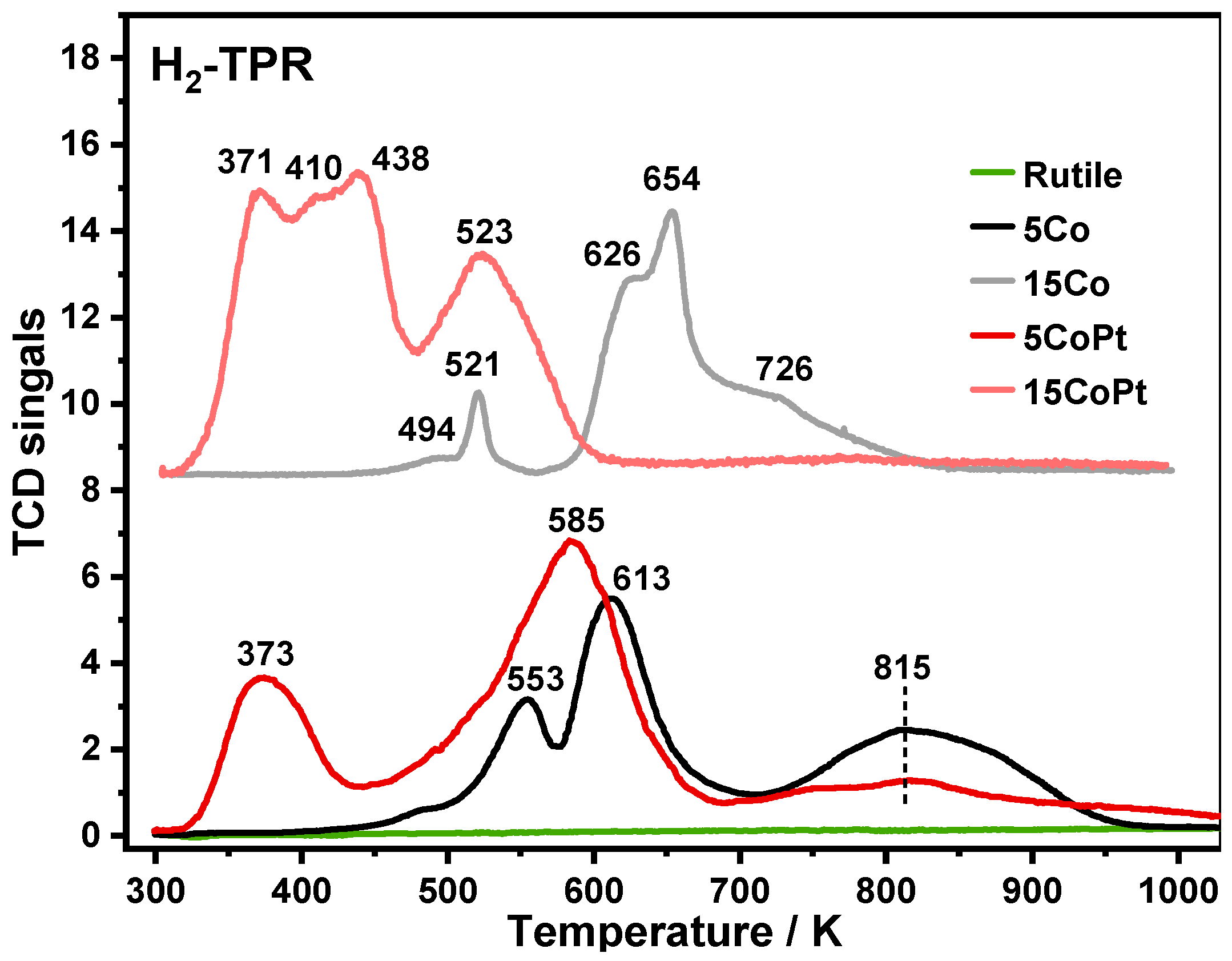
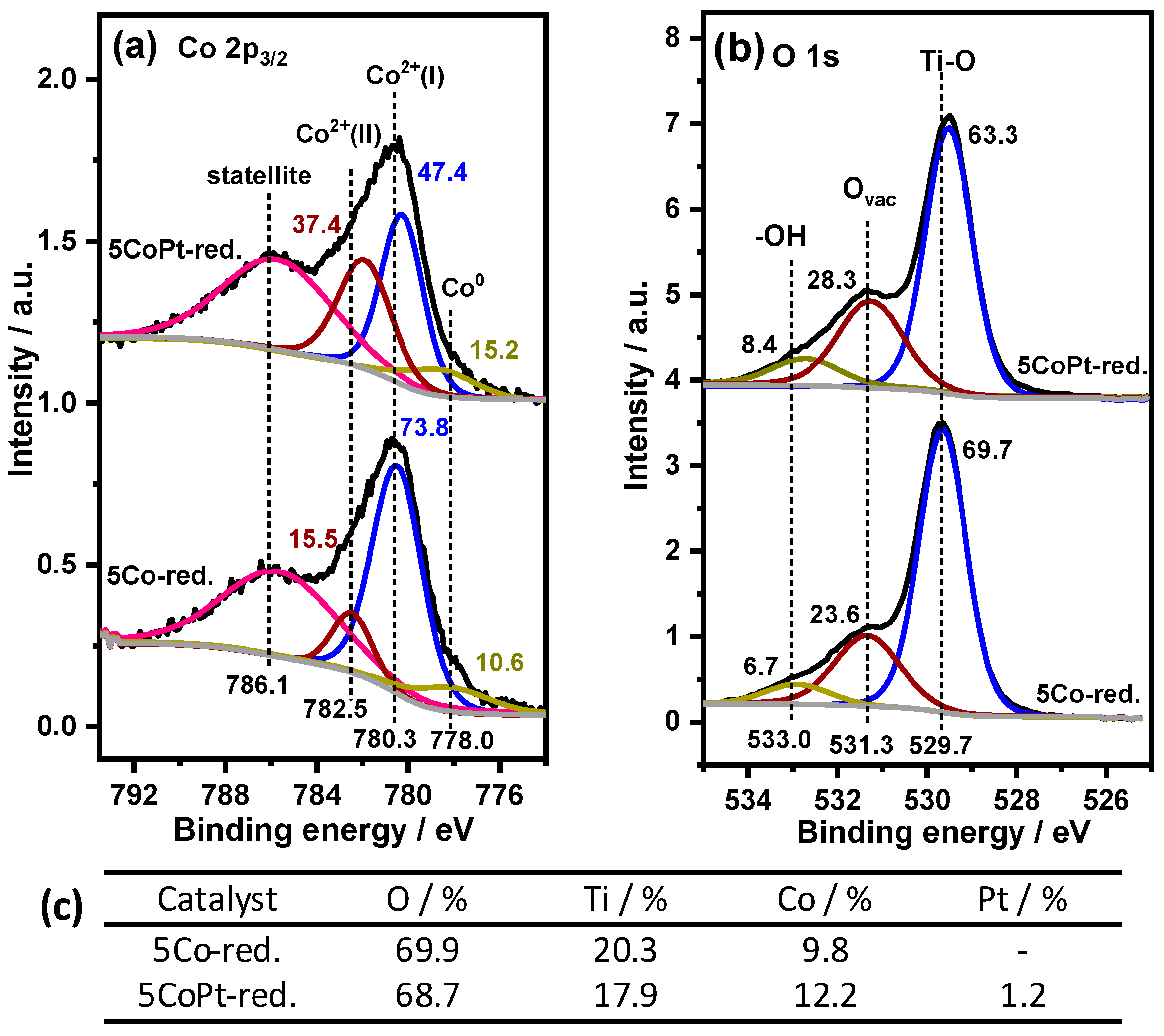
2.2. Identifying Surface Properties, Nanoparticle Sizes, and Active Components of the Catalysts
2.3. Rationalizing Reaction Performance with Catalyst’s Properties
3. Experimental Section
3.1. Catalyst Preparation
3.2. Catalyst Characterization
3.3. CO Hydrogenation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Storsæter, S.; Tøtdal, B.; Walmsley, J.C.; Tanem, B.S.; Holmen, A. Characterization of alumina-, silica-, and titania-supported cobalt Fischer-Tropsch catalysts. J. Catal. 2005, 236, 139–152. [Google Scholar] [CrossRef]
- Gnanamani, M.K.; Ribeiro, M.C.; Ma, W.; Shafer, W.D.; Jacobs, G.; Graham, U.M.; Davis, B.H. Fischer-Tropsch synthesis: Metal-support interfacial contact governs oxygenates selectivity over CeO2 supported Pt-Co catalysts. Appl. Catal. A Gen. 2011, 393, 17–23. [Google Scholar] [CrossRef]
- Lee, J.H.; Bonte, W.; Corthals, S.; Krumeich, F.; Ruitenbeek, M.; van Bokhoven, J.A. Zeolite nanoreactor for investigating sintering effects of cobalt-catalyzed Fischer-Tropsch synthesis. Ind. Eng. Chem. Res. 2019, 58, 5140–5145. [Google Scholar] [CrossRef]
- Voß, M.; Borgmann, D.; Wedler, G. Characterization of alumina, silica, and titania supported cobalt catalysts. J. Catal. 2002, 212, 10–21. [Google Scholar] [CrossRef]
- Galván, M.C.Á.; Prats, A.E.P.; Campos-Martin, J.M.; Fierro, J.L. Direct evidence of the SMSI decoration effect: The case of Co/TiO2 catalyst. Chem. Commun. 2011, 47, 7131–7133. [Google Scholar]
- Hernández Mejía, C.; van Deelen, T.W.; de Jong, K.P. Activity enhancement of cobalt catalysts by tuning metal-support interactions. Nat. Commun. 2018, 9, 4459. [Google Scholar] [CrossRef]
- van Deelen, T.W.; Hernández Mejía, C.; de Jong, K.P. Control of metal-support interactions in heterogeneous catalysts to enhance activity and selectivity. Nat. Catal. 2019, 2, 955–970. [Google Scholar] [CrossRef]
- Tang, H.; Su, Y.; Zhang, B.; Lee, A.F.; Isaacs, M.A.; Wilson, K.; Li, L.; Ren, Y.; Huang, J.; Haruta, M. Classical strong metal-support interactions between gold nanoparticles and titanium dioxide. Sci. Adv. 2017, 3, e1700231. [Google Scholar] [CrossRef]
- Bertella, F.; Concepción, P.; Martínez, A. TiO2 polymorph dependent SMSI effect in Co-Ru/TiO2 catalysts and its relevance to Fischer-Tropsch synthesis. Catal. Today 2017, 289, 181–191. [Google Scholar] [CrossRef]
- Du, X.; Huang, Y.; Pan, X.; Han, B.; Su, Y.; Jiang, Q.; Li, M.; Tang, H.; Li, G.; Qiao, B. Size-dependent strong metal-support interaction in TiO2 supported Au nanocatalysts. Nat. Commun. 2020, 11, 5811. [Google Scholar] [CrossRef]
- Jongsomjit, B.; Sakdamnuson, C.; Goodwin, J.G.; Praserthdam, P. Co-support compound formation in titania-supported cobalt catalyst. Catal. Lett. 2004, 94, 209–215. [Google Scholar] [CrossRef]
- Wolf, M.; Gibson, E.K.; Olivier, E.J.; Neethling, J.H.; Catlow, C.R.A.; Fischer, N.; Claeys, M. Water-induced formation of cobalt-support compounds under simulated high conversion Fischer-Tropsch environment. ACS Catal. 2019, 9, 4902–4918. [Google Scholar] [CrossRef]
- Qiu, C.; Odarchenko, Y.; Meng, Q.; Cong, P.; Schoen, M.A.; Kleibert, A.; Forrest, T.; Beale, A.M. Direct observation of the evolving metal-support interaction of individual cobalt nanoparticles at the titania and silica interface. Chem. Sci. 2020, 11, 13060–13070. [Google Scholar] [CrossRef]
- Cats, K.; Andrews, J.; Stéphan, O.; March, K.; Karunakaran, C.; Meirer, F.; De Groot, F.; Weckhuysen, B. Active phase distribution changes within a catalyst particle during Fischer-Tropsch synthesis as revealed by multi-scale microscopy. Catal. Sci. Technol. 2016, 6, 4438–4449. [Google Scholar] [CrossRef]
- Qiu, C.; Odarchenko, Y.; Meng, Q.; Xu, S.; Lezcano-Gonzalez, I.; Olalde-Velasco, P.; Maccherozzi, F.; Zanetti-Domingues, L.; Martin-Fernandez, M.; Beale, A.M. Resolving the effect of oxygen vacancies on Co nanostructures using soft XAS/X-PEEM. ACS Catal. 2022, 12, 9125–9134. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; He, Y.; Wei, L.; Zhao, Y.; Zhang, Y.; Zhao, F.; Lyu, S.; Chen, S.; Hong, J.; Li, J. Effect of TiO2 surface engineering on the performance of cobalt-based catalysts for Fischer-Tropsch synthesis. Ind. Eng. Chem. Res. 2018, 58, 1095–1104. [Google Scholar] [CrossRef]
- Eschemann, T.O.; Oenema, J.; de Jong, K.P. Effects of noble metal promotion for Co/TiO2 Fischer-Tropsch catalysts. Catal. Today 2016, 261, 60–66. [Google Scholar] [CrossRef]
- Yang, J.; Qi, Y.; Zhu, J.; Zhu, Y.-A.; Chen, D.; Holmen, A. Reaction mechanism of CO activation and methane formation on Co Fischer-Tropsch catalyst: A combined DFT, transient, and steady-state kinetic modeling. J. Catal. 2013, 308, 37–49. [Google Scholar] [CrossRef]
- Chen, W.; Zijlstra, B.; Filot, I.A.; Pestman, R.; Hensen, E.J. Mechanism of carbon monoxide dissociation on a cobalt Fischer-Tropsch catalyst. ChemCatChem 2018, 10, 136–140. [Google Scholar] [CrossRef]
- Vasiliades, M.A.; Govender, N.S.; Govender, A.; Crous, R.; Moodley, D.; Botha, T.; Efstathiou, A.M. The effect of H2 pressure on the carbon path of methanation reaction on Co/γ-Al2O3: Transient isotopic and operando methodology studies. ACS Catal. 2022, 12, 15110–15129. [Google Scholar] [CrossRef]
- van Helden, P.; van den Berg, J.-A.; Weststrate, C.J. Hydrogen adsorption on Co surfaces: A density functional theory and temperature programmed desorption study. ACS Catal. 2012, 2, 1097–1107. [Google Scholar] [CrossRef]
- Beaumont, S.K.; Alayoglu, S.; Specht, C.; Michalak, W.D.; Pushkarev, V.V.; Guo, J.; Kruse, N.; Somorjai, G.A. Combining in situ NEXAFS spectroscopy and CO2 methanation kinetics to study Pt and Co nanoparticle catalysts reveals key insights into the role of platinum in promoted cobalt catalysis. J. Am. Chem. Soc. 2014, 136, 9898–9901. [Google Scholar] [CrossRef]
- Karim, W.; Spreafico, C.; Kleibert, A.; Gobrecht, J.; VandeVondele, J.; Ekinci, Y.; van Bokhoven, J.A. Catalyst support effects on hydrogen spillover. Nature 2017, 541, 68–71. [Google Scholar] [CrossRef] [PubMed]
- Nabaho, D.; Niemantsverdriet, J.H.; Claeys, M.; van Steen, E. Hydrogen spillover in the Fischer-Tropsch synthesis: An analysis of platinum as a promoter for cobalt-alumina catalysts. Catal. Today 2016, 261, 17–27. [Google Scholar] [CrossRef]
- Jia, R.; Yu, F.; Lin, T.; An, Y.; Gong, K.; Zhong, L. Effects of noble metals on a Co2C-based supported catalyst for Fischer-Tropsch to olefins. Ind. Eng. Chem. Res. 2022, 61, 4824–4831. [Google Scholar] [CrossRef]
- Den Otter, J.H.; Nijveld, S.R.; De Jong, K.P. Synergistic promotion of Co/SiO2 Fischer-Tropsch catalysts by niobia and platinum. ACS Catal. 2016, 6, 1616–1623. [Google Scholar] [CrossRef]
- Zhang, H.; Chu, W.; Zou, C.; Huang, Z.; Ye, Z.; Zhu, L. Promotion effects of platinum and ruthenium on carbon nanotube supported cobalt catalysts for Fischer-Tropsch synthesis. Catal. Lett. 2011, 141, 438–444. [Google Scholar] [CrossRef]
- Bezemer, G.L.; Bitter, J.H.; Kuipers, H.P.; Oosterbeek, H.; Holewijn, J.E.; Xu, X.; Kapteijn, F.; Van Dillen, A.J.; de Jong, K.P. Cobalt particle size effects in the Fischer-Tropsch reaction studied with carbon nanofiber supported catalysts. J. Am. Chem. Soc. 2006, 128, 3956–3964. [Google Scholar] [CrossRef] [PubMed]
- Bezemer, G.L.; Radstake, P.; Koot, V.; Van Dillen, A.; Geus, J.; De Jong, K. Preparation of Fischer-Tropsch cobalt catalysts supported on carbon nanofibers and silica using homogeneous deposition-precipitation. J. Catal. 2006, 237, 291–302. [Google Scholar] [CrossRef]
- Vasiliades, M.; Kalamaras, C.; Govender, N.; Govender, A.; Efstathiou, A.M. The effect of preparation route of commercial Co/γ-Al2O3 catalyst on important Fischer-Tropsch kinetic parameters studied by SSITKA and CO-DRIFTS transient hydrogenation techniques. J. Catal. 2019, 379, 60–77. [Google Scholar] [CrossRef]
- Fu, T.; Li, Z. Review of recent development in Co-based catalysts supported on carbon materials for Fischer-Tropsch synthesis. Chem. Eng. Sci. 2015, 135, 3–20. [Google Scholar] [CrossRef]
- Vosoughi, V.; Dalai, A.K.; Abatzoglou, N.; Hu, Y. Performances of promoted cobalt catalysts supported on mesoporous alumina for Fischer-Tropsch synthesis. Appl. Catal. A Gen. 2017, 547, 155–163. [Google Scholar] [CrossRef]
- Chu, W.; Chernavskii, P.A.; Gengembre, L.; Pankina, G.A.; Fongarland, P.; Khodakov, A.Y. Cobalt species in promoted cobalt alumina-supported Fischer-Tropsch catalysts. J. Catal. 2007, 252, 215–230. [Google Scholar] [CrossRef]
- Mehrbod, M.; Martinelli, M.; Martino, A.G.; Cronauer, D.C.; Kropf, A.J.; Marshall, C.L.; Jacobs, G. Fischer-Tropsch synthesis: Direct cobalt nitrate reduction of promoted Co/TiO2 catalysts. Fuel 2019, 245, 488–504. [Google Scholar] [CrossRef]
- Cook, K.M.; Perez, H.D.; Bartholomew, C.H.; Hecker, W.C. Effect of promoter deposition order on platinum-, ruthenium-, or rhenium-promoted cobalt Fischer-Tropsch catalysts. Appl. Catal. A Gen. 2014, 482, 275–286. [Google Scholar] [CrossRef]
- Tsubaki, N.; Sun, S.; Fujimoto, K. Different functions of the noble metals added to cobalt catalysts for Fischer-Tropsch synthesis. J. Catal. 2001, 199, 236–246. [Google Scholar] [CrossRef]
- Weststrate, C.; Saib, A.; Niemantsverdriet, J. Promoter segregation in Pt and Ru promoted cobalt model catalysts during oxidation-reduction treatments. Catal. Today 2013, 215, 2–7. [Google Scholar] [CrossRef]
- Pirola, C.; Scavini, M.; Galli, F.; Vitali, S.; Comazzi, A.; Manenti, F.; Ghigna, P. Fischer-Tropsch synthesis: EXAFS study of Ru and Pt bimetallic Co based catalysts. Fuel 2014, 132, 62–70. [Google Scholar] [CrossRef]
- Beaumont, S.K.; Alayoglu, S.; Specht, C.; Kruse, N.; Somorjai, G.A. A nanoscale demonstration of hydrogen atom spillover and surface diffusion across silica using the kinetics of CO2 methanation catalyzed on spatially separate Pt and Co nanoparticles. Nano Lett. 2014, 14, 4792–4796. [Google Scholar] [CrossRef] [PubMed]
- Chakrabarti, D.; de Klerk, A.; Prasad, V.; Gnanamani, M.K.; Shafer, W.D.; Jacobs, G.; Sparks, D.E.; Davis, B.H. Conversion of CO2 over a co-based Fischer-Tropsch catalyst. Ind. Eng. Chem. Res. 2015, 54, 1189–1196. [Google Scholar] [CrossRef]
- Jacobs, G.; Graham, U.M.; Chenu, E.; Patterson, P.M.; Dozier, A.; Davis, B.H. Low-temperature water-gas shift: Impact of Pt promoter loading on the partial reduction of ceria and consequences for catalyst design. J. Catal. 2005, 229, 499–512. [Google Scholar] [CrossRef]
- Xu, M.; Yao, S.; Rao, D.; Niu, Y.; Liu, N.; Peng, M.; Zhai, P.; Man, Y.; Zheng, L.; Wang, B. Insights into interfacial synergistic catalysis over Ni@TiO2−x catalyst toward water-gas shift reaction. J. Am. Chem. Soc. 2018, 140, 11241–11251. [Google Scholar] [CrossRef] [PubMed]
- Xiao, B.; Zhu, W.; Li, Z.; Zhu, J.; Zhu, X.; Pezzotti, G. Tailoring morphology of cobalt-nickel layered double hydroxide via different surfactants for high-performance supercapacitor. R. Soc. Open Sci. 2018, 5, 180867. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.X.; Su, H.Y.; Sun, D.P.; Zhang, B.Y.; Li, W.X. Crystallographic dependence of CO activation on cobalt catalysts: HCP versus FCC. J. Am. Chem. Soc. 2013, 135, 16284–16287. [Google Scholar] [CrossRef]
- Lyu, S.; Wang, L.; Zhang, J.; Liu, C.; Sun, J.; Peng, B.; Wang, Y.; Rappé, K.G.; Zhang, Y.; Li, J. Role of active phase in Fischer-Tropsch synthesis: Experimental evidence of CO activation over single-phase cobalt catalysts. ACS Catal. 2018, 8, 7787–7798. [Google Scholar] [CrossRef]
- Gnanamani, M.K.; Jacobs, G.; Shafer, W.D.; Davis, B.H. Fischer-Tropsch synthesis: Activity of metallic phases of cobalt supported on silica. Catal. Today 2013, 215, 13–17. [Google Scholar] [CrossRef]
- Riva, R.; Miessner, H.; Vitali, R.; Del Piero, G. Metal-support interaction in Co/SiO2 and Co/TiO2. Appl. Catal. A Gen. 2000, 196, 111–123. [Google Scholar] [CrossRef]
- Zhang, Y.; Wei, D.; Hammache, S.; Goodwin Jr, J.G. Effect of water vapor on the reduction of Ru-promoted Co/Al2O3. J. Catal. 1999, 188, 281–290. [Google Scholar] [CrossRef]
- Kim, D.S.; Kim, Y.H.; Yie, J.E.; Park, E.D. The effect of cobalt precursors on NO oxidation over supported cobalt oxide catalysts. Korean J. Chem. Eng. 2010, 27, 822–827. [Google Scholar] [CrossRef]
- Huang, W.; Zuo, Z.; Han, P.; Li, Z.; Zhao, T. XPS and XRD investigation of Co/Pd/TiO2 catalysts by different preparation methods. J. Electron. Spectrosc. Relat. Phenom. 2009, 173, 88–95. [Google Scholar] [CrossRef]
- Jacobs, G.; Chaney, J.A.; Patterson, P.M.; Das, T.K.; Maillot, J.C.; Davis, B.H. Fischer-Tropsch synthesis: Study of the promotion of Pt on the reduction property of Co/Al2O3 catalysts by in situ EXAFS of Co K and Pt LIII edges and XPS. J. Synchrotron Radiat. 2004, 11, 414–422. [Google Scholar] [CrossRef]
- Bagheri, S.; Muhd Julkapli, N.; Bee Abd Hamid, S. Titanium dioxide as a catalyst support in heterogeneous catalysis. Sci. World J. 2014, 2014, 727496. [Google Scholar] [CrossRef]
- Chen, W.; Pestman, R.; Zijlstra, B.; Filot, I.A.; Hensen, E.J. Mechanism of cobalt-catalyzed CO hydrogenation: 1. Methanation. ACS Catal. 2017, 7, 8050–8060. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Filot, I.A.; Pestman, R.; Hensen, E.J. Mechanism of cobalt-catalyzed CO hydrogenation: 2. Fischer-Tropsch synthesis. ACS Catal. 2017, 7, 8061–8071. [Google Scholar] [CrossRef] [PubMed]
- Jamaati, M.; Torkashvand, M.; Sarabadani Tafreshi, S.; de Leeuw, N.H. A review of theoretical studies on carbon monoxide hydrogenation via Fischer-Tropsch synthesis over transition metals. Molecules 2023, 28, 6525. [Google Scholar] [CrossRef] [PubMed]
- Vasiliades, M.A.; Kyprianou, K.K.; Govender, N.S.; Govender, A.; Crous, R.; Moodley, D.; Efstathiou, A.M. The effect of CO partial pressure on important kinetic parameters of methanation reaction on Co-based FTS catalyst studied by SSITKA-MS and operando DRIFTS-MS techniques. Catalysts 2020, 10, 583. [Google Scholar] [CrossRef]
- Tuxen, A.; Carenco, S.; Chintapalli, M.; Chuang, C.H.; Escudero, C.; Pach, E.; Jiang, P.; Borondics, F.; Beberwyck, B.; Alivisatos, A.P. Size-dependent dissociation of carbon monoxide on cobalt nanoparticles. J. Am. Chem. Soc. 2013, 135, 2273–2278. [Google Scholar] [CrossRef]
- Munirathinam, R.; Pham Minh, D.; Nzihou, A. Effect of the support and its surface modifications in cobalt-based Fischer-Tropsch synthesis. Ind. Eng. Chem. Res. 2018, 57, 16137–16161. [Google Scholar] [CrossRef]
- Krylova, A.Y. Products of the Fischer-Tropsch synthesis (a review). Solid Fuel Chem. 2014, 48, 22–35. [Google Scholar] [CrossRef]
- Lizárraga, R.; Pan, F.; Bergqvist, L.; Holmström, E.; Gercsi, Z.; Vitos, L. First principles theory of the hcp-fcc phase transition in cobalt. Sci. Rep. 2017, 7, 3778. [Google Scholar] [CrossRef]
- Katanin, A. Magnetic properties of a half metal from the paramagnetic phase: DFT+DMFT study of exchange interactions in CrO2. Phys. Rev. B 2024, 110, 155115. [Google Scholar] [CrossRef]
- Macheli, L.; Leteba, G.M.; Doyle, B.P.; Jewell, L.; van Steen, E. Modulating CO hydrogenation activity through silane functionalization of cobalt catalysts. Appl. Catal. A Gen. 2024, 685, 119874. [Google Scholar] [CrossRef]
- Petit, C.; Rusponi, S.; Brune, H. Magnetic properties of cobalt and cobalt-platinum nanocrystals investigated by magneto-optical Kerr effect. J. Appl. Phys. 2004, 95, 4251–4260. [Google Scholar] [CrossRef]
- Meier, F.; Lounis, S.; Wiebe, J.; Zhou, L.; Heers, S.; Mavropoulos, P.; Dederichs, P.H.; Blügel, S.; Wiesendanger, R. Spin polarization of platinum (111) induced by the proximity to cobalt nanostripes. Phys. Rev. B 2011, 83, 075407. [Google Scholar] [CrossRef]
- van Helden, P.; Ciobîcă, I.M.; Coetzer, R.L. The size-dependent site composition of FCC cobalt nanocrystals. Catal. Today 2016, 261, 48–59. [Google Scholar] [CrossRef]
- Agrawal, R.; Phatak, P.; Spanu, L. Effect of phase and size on surface sites in cobalt nanoparticles. Catal. Today 2018, 312, 174–180. [Google Scholar] [CrossRef]
- Den Breejen, J.; Radstake, P.; Bezemer, G.; Bitter, J.; Frøseth, V.; Holmen, A.; de Jong, K.d. On the origin of the cobalt particle size effects in Fischer-Tropsch catalysis. J. Am. Chem. Soc. 2009, 131, 7197–7203. [Google Scholar] [CrossRef]
- Wolf, M.; Fischer, N.; Claeys, M. Formation of metal-support compounds in cobalt-based Fischer-Tropsch synthesis: A review. Chem Catal. 2021, 1, 1014–1041. [Google Scholar] [CrossRef]
- Tauster, S.; Fung, S.; Garten, R.L. Strong metal-support interactions. Group 8 noble metals supported on titanium dioxide. J. Am. Chem. Soc. 1978, 100, 170–175. [Google Scholar] [CrossRef]
- Tauster, S.; Fung, S.; Baker, R.; Horsley, J. Strong interactions in supported-metal catalysts. Science 1981, 211, 1121–1125. [Google Scholar] [CrossRef] [PubMed]
- Lentz, C.; Jand, S.P.; Melke, J.; Roth, C.; Kaghazchi, P. DRIFTS study of CO adsorption on Pt nanoparticles supported by DFT calculations. J. Mol. Catal. A Chem. 2017, 426, 1–9. [Google Scholar] [CrossRef]
- Martinelli, M.; Gnanamani, M.K.; LeViness, S.; Jacobs, G.; Shafer, W.D. An overview of Fischer-Tropsch Synthesis: XtL processes, catalysts and reactors. Appl. Catal. A Gen. 2020, 608, 117740. [Google Scholar] [CrossRef]
- Fischer, N.; Van Steen, E.; Claeys, M. Preparation of supported nano-sized cobalt oxide and fcc cobalt crystallites. Catal. Today 2011, 171, 174–179. [Google Scholar] [CrossRef]
- Kharisov, B.I.; Kharissova, O.V.; Ortiz-Mendez, U. CRC Concise Encyclopedia of Nanotechnology; CRC Press: Boca Raton, FL, USA, 2016. [Google Scholar]
- Gholami, Z.; Tišler, Z.; Rubáš, V. Recent advances in Fischer-Tropsch synthesis using cobalt-based catalysts: A review on supports, promoters, and reactors. Catal. Rev. 2021, 63, 512–595. [Google Scholar] [CrossRef]
- Liu, X.; Atwater, M.; Wang, J.; Huo, Q. Extinction coefficient of gold nanoparticles with different sizes and different capping ligands. Colloids Surf. B 2007, 58, 3–7. [Google Scholar] [CrossRef] [PubMed]
- Prathibha, V.; Karthika, S.; Cyriac, J.; Sudarasanakumar, C.; Unnikrishnan, N. Synthesis of pure anatase TiO2 nanocrystals in SiO2 host and the determination of crystal planes by ImageJ. Mater. Lett. 2011, 65, 664–666. [Google Scholar] [CrossRef]
- Biesinger, M.C.; Payne, B.P.; Grosvenor, A.P.; Lau, L.W.; Gerson, A.R.; Smart, R.S.C. Resolving surface chemical states in XPS analysis of first row transition metals, oxides and hydroxides: Cr, Mn, Fe, Co and Ni. Appl. Surf. Sci. 2011, 257, 2717–2730. [Google Scholar] [CrossRef]



| Catalyst | BET Surface Area a/m2/g | BJH Pore Volume a/cm3/g | BJH Pore Diameter a/nm | CoNP Size b/nm | |
|---|---|---|---|---|---|
| STEM/TEM | XRD | ||||
| 5Co | 33.64 | 0.19 | 1.94 | 10.08/10.29 | 11.37 |
| 5CoPt | 38.73 | 0.22 | 2.11 | 5.25/5.80 | 6.08 |
| 15Co | 44.72 | 0.15 | 3.81 | -/13.34 | 16.26 |
| 15CoPt | 55.59 | 0.24 | 3.82 | -/7.68 | 10.56 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, C.; Qiu, C.; Zhang, W.; Song, J.; Meng, Q.; Yuan, Q.; Wang, T. Insights into the Role of Pt Promoter in Co/TiO2 Catalysts for CO Hydrogenation. Catalysts 2024, 14, 922. https://doi.org/10.3390/catal14120922
Hu C, Qiu C, Zhang W, Song J, Meng Q, Yuan Q, Wang T. Insights into the Role of Pt Promoter in Co/TiO2 Catalysts for CO Hydrogenation. Catalysts. 2024; 14(12):922. https://doi.org/10.3390/catal14120922
Chicago/Turabian StyleHu, Changsong, Chengwu Qiu, Wenli Zhang, Jinliang Song, Qingwei Meng, Qingchun Yuan, and Tiejun Wang. 2024. "Insights into the Role of Pt Promoter in Co/TiO2 Catalysts for CO Hydrogenation" Catalysts 14, no. 12: 922. https://doi.org/10.3390/catal14120922
APA StyleHu, C., Qiu, C., Zhang, W., Song, J., Meng, Q., Yuan, Q., & Wang, T. (2024). Insights into the Role of Pt Promoter in Co/TiO2 Catalysts for CO Hydrogenation. Catalysts, 14(12), 922. https://doi.org/10.3390/catal14120922









