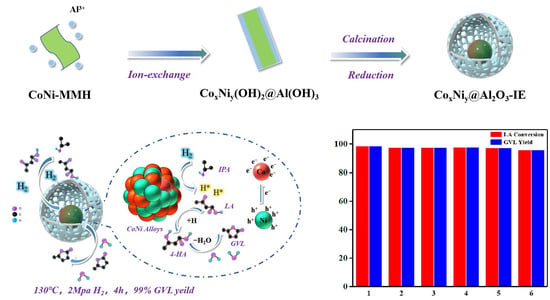
Ion-Exchange Synthesis of Surrounded CoNi@Al2O3 Catalyst for Levulinic Acid Hydrogenation to γ-Valerolactone under Mild Conditions
Abstract
:1. Introduction
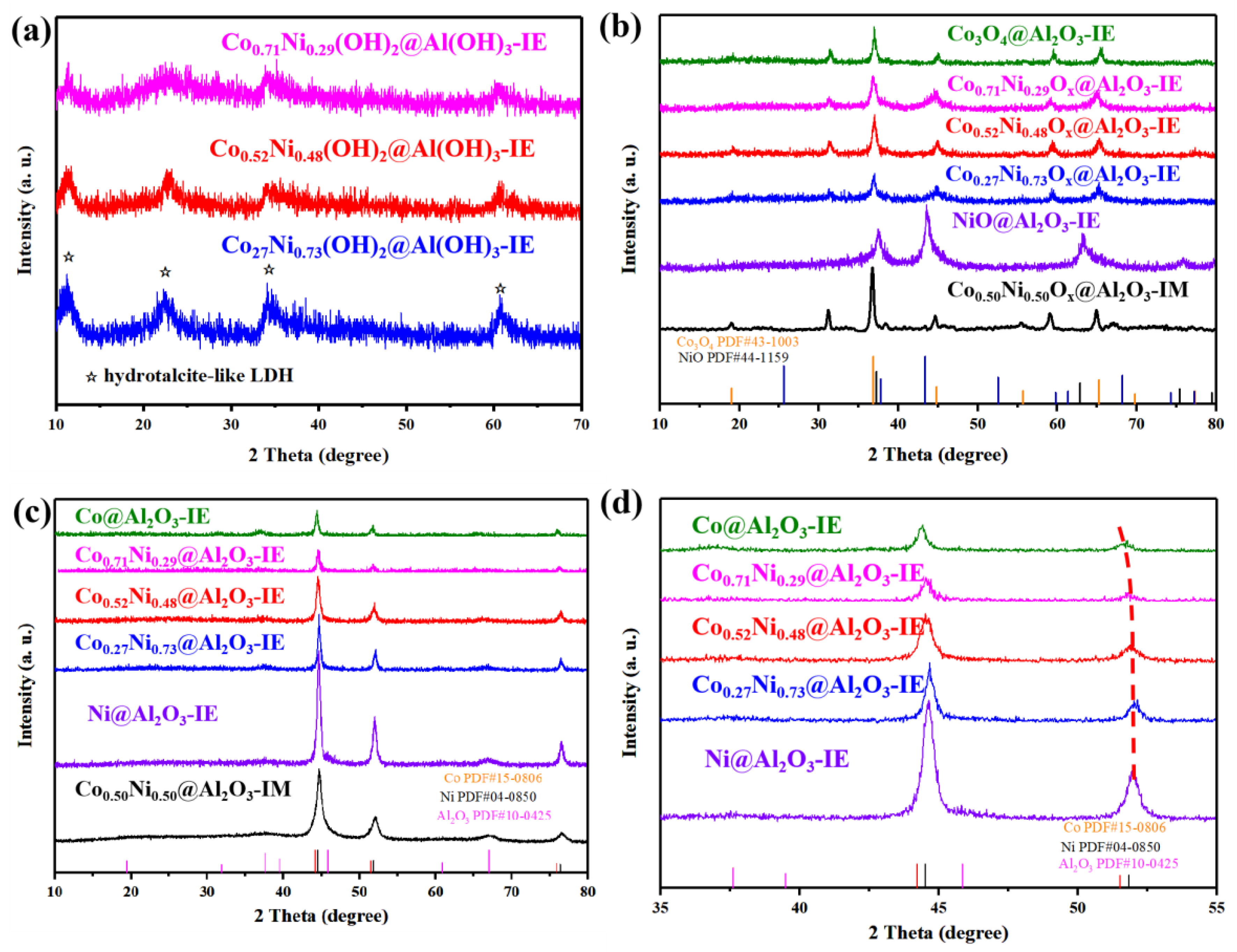
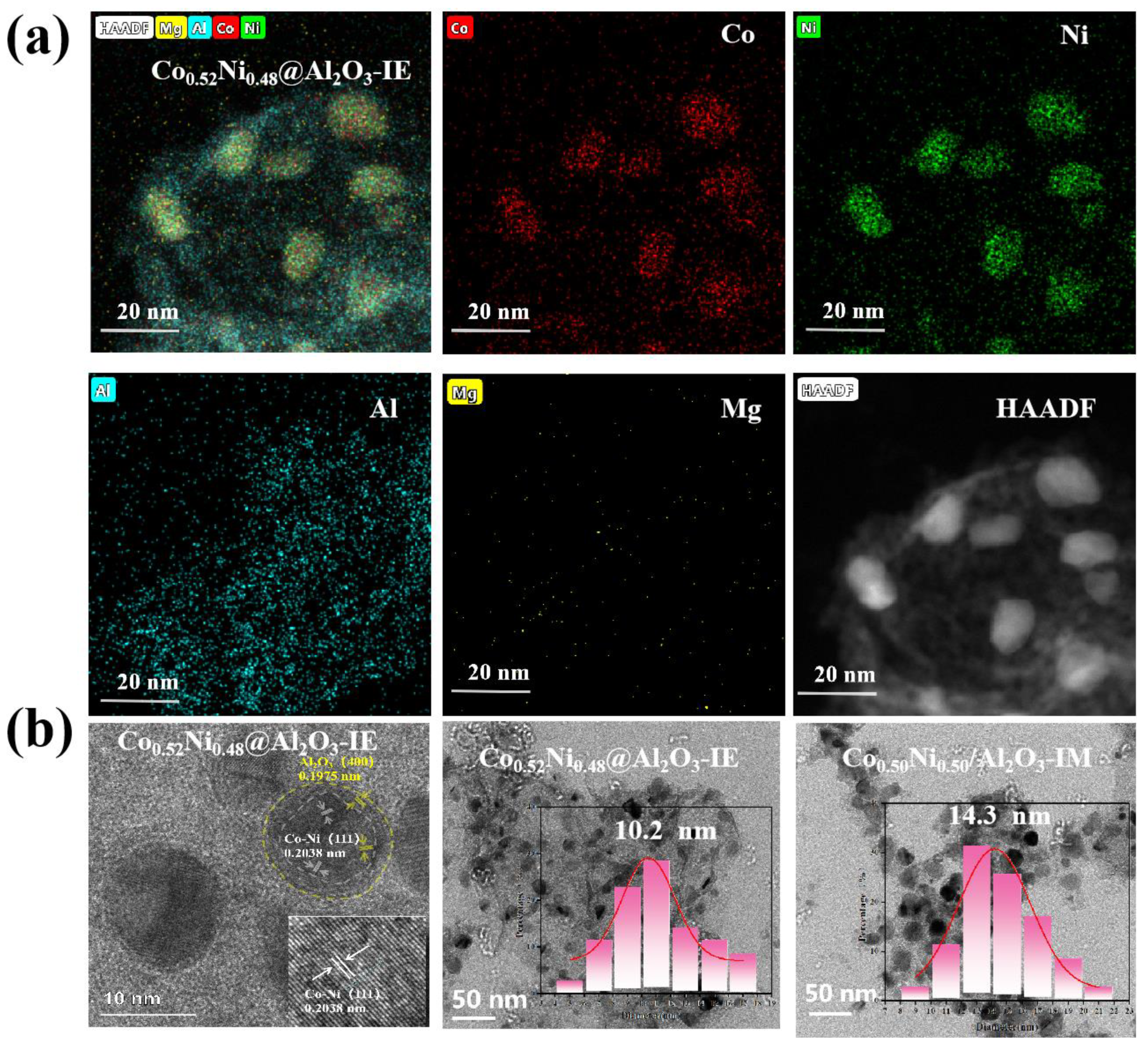
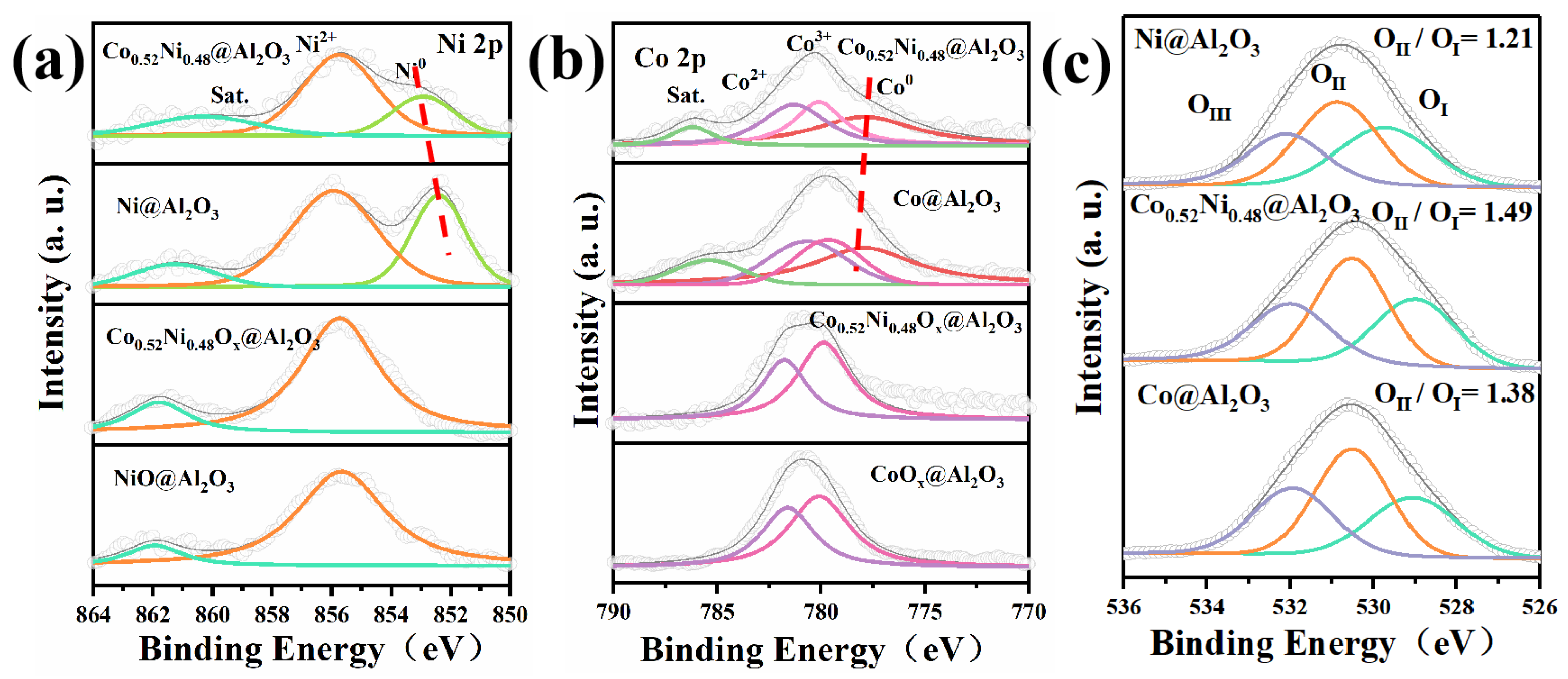
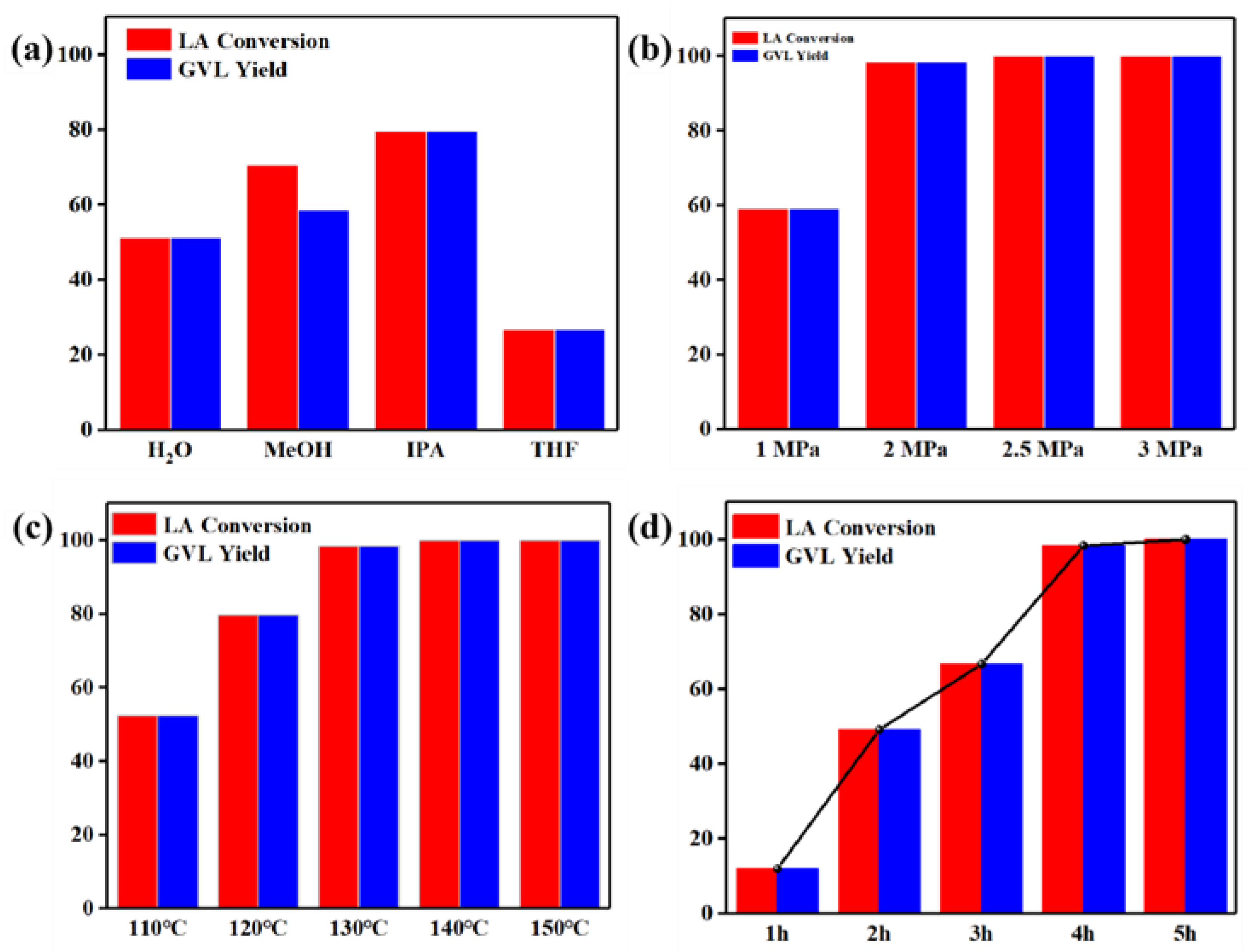
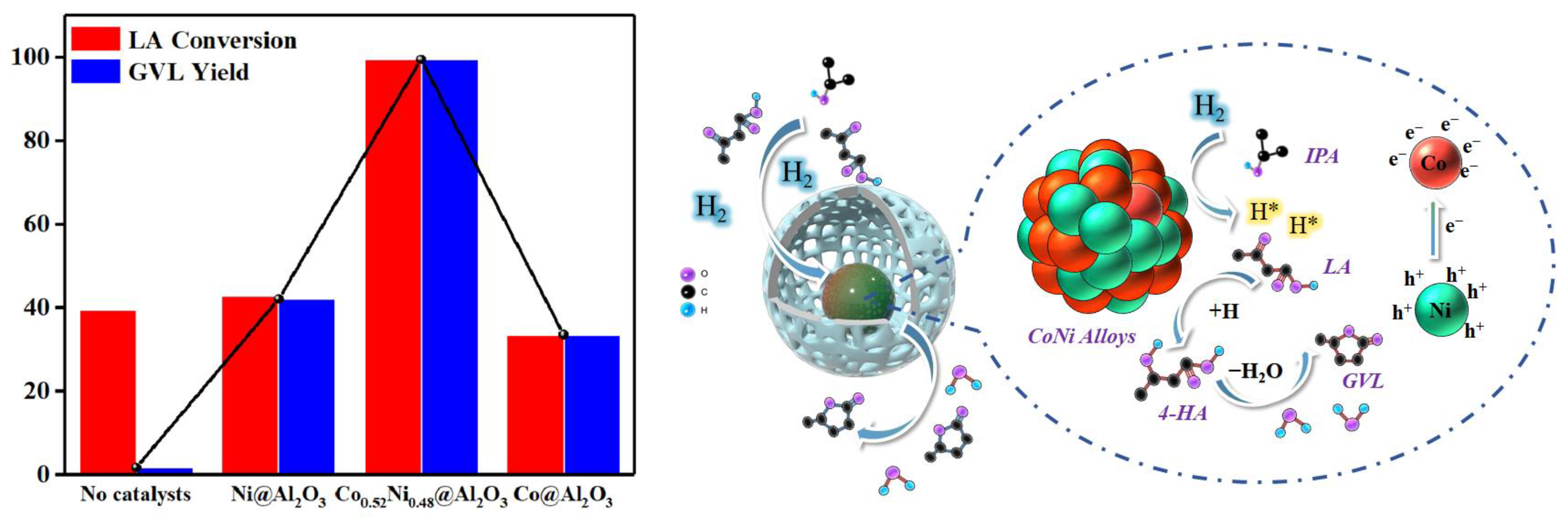
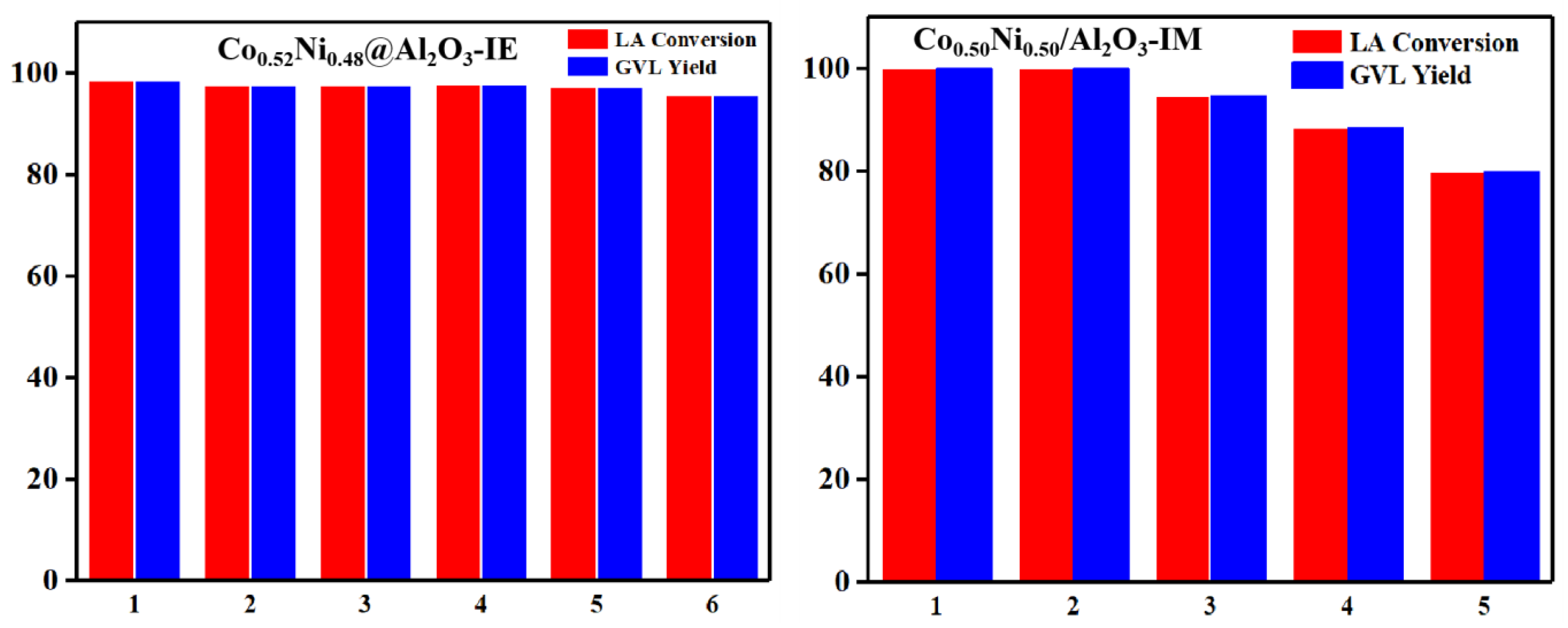
2. Results and Discussion
3. Materials and Methods
3.1. Materials
3.2. Catalyst Preparation
3.2.1. Synthesis of CoNi-MMH Precursors
3.2.2. Synthesis of CoxNiy(OH)2@Al(OH)3
3.2.3. Synthesis of CoxNiy@Al2O3-IE
3.2.4. Synthesis of Comparative Catalysts
3.3. Catalyst Characterization
3.4. Catalytic Test
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Huang, Y.-B.; Liu, A.-F.; Zhang, Q.; Li, K.-M.; Porterfield, W.B.; Li, L.-C.; Wang, F. Mechanistic Insights into the Solvent-Driven Adsorptive Hydrodeoxygenation of Biomass Derived Levulinate Acid/Ester to 2-Methyltetrahydrofuran over Bimetallic Cu–Ni Catalysts. ACS Sustain. Chem. Eng. 2020, 8, 11477–11490. [Google Scholar] [CrossRef]
- Zhang, L.; Mao, J.; Li, S.; Yin, J.; Sun, X.; Guo, X.; Song, C.; Zhou, J. Hydrogenation of levulinic acid into gamma-valerolactone over in situ reduced CuAg bimetallic catalyst: Strategy and mechanism of preventing Cu leaching. Appl. Catal. B Environ. 2018, 232, 1–10. [Google Scholar] [CrossRef]
- Bai, J.; Cheng, C.; Liu, Y.; Wang, C.; Liao, Y.; Chen, L.; Ma, L. Selective hydrogenation of levulinic acid to γ-valerolactone on Ni-based catalysts. Mol. Catal. 2021, 516, 112000. [Google Scholar] [CrossRef]
- Cai, B.; Zhou, X.-C.; Miao, Y.-C.; Luo, J.-Y.; Pan, H.; Huang, Y.-B. Enhanced Catalytic Transfer Hydrogenation of Ethyl Levulinate to γ-Valerolactone over a Robust Cu–Ni Bimetallic Catalyst. ACS Sustain. Chem. Eng. 2016, 5, 1322–1331. [Google Scholar] [CrossRef]
- Sakakibara, K.; Endo, K.; Osawa, T. Facile synthesis of γ-valerolactone by transfer hydrogenation of methyl levulinate and levulinic acid over Ni/ZrO2. Catal. Commun. 2019, 125, 52–55. [Google Scholar] [CrossRef]
- Huang, X.; Liu, K.; Vrijburg, W.L.; Ouyang, X.; Iulian Dugulan, A.; Liu, Y.; Tiny Verhoeven, M.W.G.M.; Kosinov, N.A.; Pidko, E.A.; Hensen, E.J.M. Hydrogenation of levulinic acid to γ-valerolactone over Fe-Re/TiO2 catalysts. Appl. Catal. B Environ. 2020, 278, 119314. [Google Scholar] [CrossRef]
- Raguindin, R.Q.; Desalegn, B.Z.; Vishwanath, H.; Gebresillase, M.N.; Seo, J.G. Enhanced Hydrogenation of Levulinic Acid over Ordered Mesoporous Alumina-Supported Catalysts: Elucidating the Effect of Fabrication Strategy. ChemSusChem 2022, 15, e202102662. [Google Scholar] [CrossRef]
- Di Menno Di Bucchianico, D.; Wang, Y.; Buvat, J.-C.; Pan, Y.; Casson Moreno, V.; Leveneur, S. Production of levulinic acid and alkyl levulinates: A process insight. Green Chem. 2022, 24, 614–646. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, C.; Gong, W.; Song, J.; Zhang, H.; Zhang, Y.; Wang, G.; Zhao, H. Self-assembled Pd/CeO2 catalysts by a facile redox approach for high-efficiency hydrogenation of levulinic acid into gamma-valerolactone. Catal. Commun. 2017, 93, 10–14. [Google Scholar] [CrossRef]
- Luo, W.; Deka, U.; Beale, A.M.; van Eck, E.R.H.; Bruijnincx, P.C.A.; Weckhuysen, B.M. Ruthenium-catalyzed hydrogenation of levulinic acid: Influence of the support and solvent on catalyst selectivity and stability. J. Catal. 2013, 301, 175–186. [Google Scholar] [CrossRef]
- Cao, W.; Lin, L.; Qi, H.; He, Q.; Wu, Z.; Wang, A.; Luo, W.; Zhang, T. In-situ synthesis of single-atom Ir by utilizing metal-organic frameworks: An acid-resistant catalyst for hydrogenation of levulinic acid to γ-valerolactone. J. Catal. 2019, 373, 161–172. [Google Scholar] [CrossRef]
- Yan, K.; Lafleur, T.; Wu, G.; Liao, J.; Ceng, C.; Xie, X. Highly selective production of value-added γ-valerolactone from biomass-derived levulinic acid using the robust Pd nanoparticles. Appl. Catal. A Gen. 2013, 468, 52–58. [Google Scholar] [CrossRef]
- Li, S.; Wang, Y.; Yang, Y.; Chen, B.; Tai, J.; Liu, H.; Han, B. Conversion of levulinic acid to γ-valerolactone over ultra-thin TiO2 nanosheets decorated with ultrasmall Ru nanoparticle catalysts under mild conditions. Green Chem. 2019, 21, 770–774. [Google Scholar] [CrossRef]
- Wang, S.; Zhuang, Z.; Chen, X.; Wang, Y.; Li, X.; Yang, M.; Wu, Y.; Peng, Q.; Chen, C.; Li, Y. 3D Oxide-Derived Ru Catalyst for Ultra-Efficient Hydrogenation of Levulinic Acid to γ-Valerolactone. Small 2023. early view. [Google Scholar] [CrossRef]
- Sun, D.; Ohkubo, A.; Asami, K.; Katori, T.; Yamada, Y.; Sato, S. Vapor-phase hydrogenation of levulinic acid and methyl levulinate to γ-valerolactone over non-noble metal-based catalysts. Mol. Catal. 2017, 437, 105–113. [Google Scholar] [CrossRef]
- Shao, Y.; Ba, S.; Sun, K.; Gao, G.; Fan, M.; Wang, J.; Fan, H.; Zhang, L.; Hu, X. Selective production of γ-valerolactone or 1,4-pentanediol from levulinic acid/esters over Co-based catalyst: Importance of the synergy of hydrogenation sites and acidic sites. Chem. Eng. J. 2022, 429, 132433. [Google Scholar] [CrossRef]
- Ren, D.; Zhao, C.; Zhang, N.; Norinaga, K.; Zeng, X.; Huo, Z. Catalytic transfer hydrogenation of ethyl levulinate into γ-valerolactone over air-stable skeletal cobalt catalyst. J. Environ. Chem. Eng. 2022, 10, 107188. [Google Scholar] [CrossRef]
- Lan, F.; Zhang, H.; Zhao, C.; Shu, Y.; Guan, Q.; Li, W. Copper Clusters Encapsulated in Carbonaceous Mesoporous Silica Nanospheres for the Valorization of Biomass-Derived Molecules. ACS Catal. 2022, 12, 5711–5725. [Google Scholar] [CrossRef]
- Zhang, C.; Huo, Z.; Ren, D.; Song, Z.; Liu, Y.; Jin, F.; Zhou, W. Catalytic transfer hydrogenation of levulinate ester into γ-valerolactone over ternary Cu/ZnO/Al2O3 catalyst. J. Energy Chem. 2019, 32, 189–197. [Google Scholar] [CrossRef]
- Hengne, A.M.; Kadu, B.S.; Biradar, N.S.; Chikate, R.C.; Rode, C.V. Transfer hydrogenation of biomass-derived levulinic acid to γ-valerolactone over supported Ni catalysts. RSC Adv. 2016, 6, 59753–59761. [Google Scholar] [CrossRef]
- Xu, H.; Hu, D.; Zhang, M.; Wang, Y.; Zhao, Z.; Jiang, Z.; Garces, H.F.; Yan, K. Bimetallic NiCu Alloy Catalysts for Hydrogenation of Levulinic Acid. ACS Appl. Nano Mater. 2021, 4, 3989–3997. [Google Scholar] [CrossRef]
- Kadu, B.S.; Hengne, A.M.; Biradar, N.S.; Rode, C.V.; Chikate, R.C. Reductive Cyclization of Levulinic Acid to γ-Valerolactone over Non-Noble Bimetallic Nanocomposite. Ind. Eng. Chem. Res. 2016, 55, 13032–13039. [Google Scholar] [CrossRef]
- Kim, J.; Choi, H.; Kim, D.; Park, J.Y. Operando Surface Studies on Metal-Oxide Interfaces of Bimetal and Mixed Catalysts. ACS Catal. 2021, 11, 8645–8677. [Google Scholar] [CrossRef]
- Nakaya, Y.; Furukawa, S. Catalysis of Alloys: Classification, Principles, and Design for a Variety of Materials and Reactions. Chem. Rev. 2022, 123, 5859–5947. [Google Scholar] [CrossRef] [PubMed]
- Garkul’, I.A.; Zadesenets, A.V.; Plyusnin, P.E.; Filatov, E.Y.; Asanova, T.I.; Kozlov, D.V.; Korenev, S.V. Zinc(II) and Manganese(II) Oxalatopalladates as Precursors of Bimetallic Nanomaterials. Russ. J. Inorg. Chem. 2020, 65, 1571–1576. [Google Scholar] [CrossRef]
- Yudanova, L.I.; Logvinenko, V.A.; Ishchenko, A.V.; Rudina, N.A. Quantum Size Effect in Bimetallic Nanoparticles Obtained via Thermolysis of Solid Solutions of Co(II), Ni(II), Zn(II) Salts of Maleic Acid. Russ. J. Phys. Chem. A 2020, 94, 2108–2114. [Google Scholar] [CrossRef]
- Yang, Y.; Sun, Y.; Luo, X. The Relationship between Structure and Catalytic Activity-Stability of Non-Precious Metal-Based Catalysts towards Levulinic Acid Hydrogenation to γ-Valerolactone: A Review. Energies 2022, 15, 8093. [Google Scholar] [CrossRef]
- Minamihara, H.; Kusada, K.; Wu, D.; Yamamoto, T.; Toriyama, T.; Matsumura, S.; Kumara, L.S.R.; Ohara, K.; Sakata, O.; Kawaguchi, S.; et al. Continuous-Flow Reactor Synthesis for Homogeneous 1 nm-Sized Extremely Small High-Entropy Alloy Nanoparticles. J. Am. Chem. Soc. 2022, 144, 11525–11529. [Google Scholar] [CrossRef]
- Tang, Y.; Fu, J.; Wang, Y.; Guo, H.; Qi, X. Bimetallic Ni-Zn@OMC catalyst for selective hydrogenation of levulinic acid to γ-valerolactone in water. Fuel Process. Technol. 2023, 240, 107559. [Google Scholar] [CrossRef]
- Wang, H.; Chen, C.; Zhang, H.; Wang, G.; Zhao, H. An efficient and reusable bimetallic Ni3Fe NPs@C catalyst for selective hydrogenation of biomass-derived levulinic acid to γ-valerolactone. Chin. J. Catal. 2018, 39, 1599–1607. [Google Scholar] [CrossRef]
- Huo, J.; Tessonnier, J.-P.; Shanks, B.H. Improving Hydrothermal Stability of Supported Metal Catalysts for Biomass Conversions: A Review. ACS Catal. 2021, 11, 5248–5270. [Google Scholar] [CrossRef]
- Jiang, L.; Xu, G.; Fu, Y. A nitrogen-doped carbon modified nickel catalyst for the hydrogenation of levulinic acid under mild conditions. Green Chem. 2021, 23, 7065–7073. [Google Scholar] [CrossRef]
- Xue, Z.; Liu, Q.; Wang, J.; Mu, T. Valorization of levulinic acid over non-noble metal catalysts: Challenges and opportunities. Green Chem. 2018, 20, 4391–4408. [Google Scholar] [CrossRef]
- Zheng, Z.; Lin, L.; Mo, S.; Ou, D.; Tao, J.; Qin, R.; Fang, X.; Zheng, N. Economizing Production of Diverse 2D Layered Metal Hydroxides for Efficient Overall Water Splitting. Small 2018, 14, e1800759. [Google Scholar] [CrossRef]
- Wang, X.; Song, H.; Ma, S.; Li, M.; He, G.; Xie, M.; Guo, X. Template ion-exchange synthesis of Co-Ni composite hydroxides nanosheets for supercapacitor with unprecedented rate capability. Chem. Eng. J. 2022, 432, 134319. [Google Scholar] [CrossRef]
- Wang, X.; Ding, H.; Luo, W.; Yu, Y.; Chen, Q.; Luo, B.; Xie, M.; Guo, X. Morphology evolution of CoNi-LDHs synergistically engineered by precipitant and variable cobalt for asymmetric supercapacitor with superior cycling stability. EcoEnergy 2023, 1, 448–459. [Google Scholar] [CrossRef]
- Hao, P.; Xie, M.; Chen, S.; Li, M.; Bi, F.; Zhang, Y.; Lin, M.; Guo, X.; Ding, W.; Guo, X. Surrounded catalysts prepared by ion-exchange inverse loading. Sci. Adv. 2020, 6, eaay7031. [Google Scholar] [CrossRef]
- Wei, D.; Cao, Y.; Yan, L.; Gang, H.; Wu, B.; Ouyang, B.; Chen, P.; Jiang, Y.; Wang, H. Enhanced Pseudo-Capacitance Process in Nanoarchitectural Layered Double Hydroxide Nanoarrays Hollow Nanocages for Improved Capacitive Deionization Performance. ACS Appl. Mater. Interfaces 2023, 15, 24427–24436. [Google Scholar] [CrossRef] [PubMed]
- Cheng, S.; Liu, Y.; Zhao, Y.; Zhao, X.; Lang, Z.; Tan, H.; Qiu, T.; Wang, Y. Superfine CoNi alloy embedded in Al2O3 nanosheets for efficient tandem catalytic reduction of nitroaromatic compounds by ammonia borane. Dalton Trans. 2019, 48, 17499–17506. [Google Scholar] [CrossRef] [PubMed]
- Gebresillase, M.N.; Raguindin, R.Q.; Kim, H.; Seo, J.G. Supported Bimetallic Catalysts for the Solvent-Free Hydrogenation of Levulinic Acid to γ-Valerolactone: Effect of Metal Combination (Ni-Cu, Ni-Co, Cu-Co). Catalysts 2020, 10, 1354. [Google Scholar] [CrossRef]
- He, Y.; Abedi, Z.; Ni, C.; Milliken, S.; O’Connor, K.M.; Ivey, D.G.; Veinot, J.G.C. CoNi Nanoparticle-Decorated ZIF-67-Derived Hollow Carbon Cubes as a Bifunctional Electrocatalyst for Zn–Air Batteries. ACS Appl. Nano Mater. 2022, 5, 12496–12505. [Google Scholar] [CrossRef]
- Wu, J.; Yan, X.; Wang, W.; Jin, M.; Xie, Y.; Wang, C. Highly Dispersed CoNi Alloy Embedded in N-doped Graphitic Carbon for Catalytic Transfer Hydrogenation of Biomass-derived Furfural. Chem.–Asian J. 2021, 16, 3194–3201. [Google Scholar] [CrossRef] [PubMed]
- Ma, R.; Gao, J.; Kou, J.; Dean, D.P.; Breckner, C.J.; Liang, K.; Zhou, B.; Miller, J.T.; Zou, G. Insights into the Nature of Selective Nickel Sites on Ni/Al2O3 Catalysts for Propane Dehydrogenation. ACS Catal. 2022, 12, 12607–12616. [Google Scholar] [CrossRef]
- Son, I.H.; Lee, S.J.; Roh, H.-S. Hydrogen production from carbon dioxide reforming of methane over highly active and stable MgO promoted Co–Ni/γ-Al2O3 catalyst. Int. J. Hydrogen Energy 2014, 39, 3762–3770. [Google Scholar] [CrossRef]
- Pardo-Tarifa, F.; Cabrera, S.; Sanchez-Dominguez, M.; Boutonnet, M. Ce-promoted Co/Al2O3 catalysts for Fischer–Tropsch synthesis. Int. J. Hydrogen Energy 2017, 42, 9754–9765. [Google Scholar] [CrossRef]
- Yang, X.; Yang, J.; Zhao, T.; Qian, W.; Wang, Y.; Holmen, A.; Jiang, W.; Chen, D.; Ben, H. Kinetic insights into the effect of promoters on Co/Al2O3 for Fischer-Tropsch synthesis. Chem. Eng. J. 2022, 445, 136655. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, Y.; Qian, K.; Huang, W. Metal–Support Interactions in Metal/Oxide Catalysts and Oxide–Metal Interactions in Oxide/Metal Inverse Catalysts. ACS Catal. 2022, 12, 1268–1287. [Google Scholar] [CrossRef]
- Liu, M.; Zhang, J.; Zheng, L.; Fan, G.; Yang, L.; Li, F. Significant Promotion of Surface Oxygen Vacancies on Bimetallic CoNi Nanocatalysts for Hydrodeoxygenation of Biomass-derived Vanillin to Produce Methylcyclohexanol. ACS Sustain. Chem. Eng. 2020, 8, 6075–6089. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, M.; Zhang, K.; Wu, H.; Wang, J.; Cheng, Y.; Liu, Y.; Wei, Z. CoNi Alloy Nanoparticles Confined in N-Doped Porous Carbon as an Efficient and Versatile Catalyst for Reductive Amination of Levulinic Acid/Esters to N-Substituted Pyrrolidones. ACS Catal. 2023, 13, 12601–12616. [Google Scholar] [CrossRef]
- Yan, H.; Yao, S.; Zhang, T.; Li, D.; Tang, X.; Chen, M.; Zhou, Y.; Zhang, M.; Liu, Y.; Zhou, X.; et al. Promoting catalytic transfer hydrodecarbonylation of methyl stearate over bimetallic CoNi/HAP catalysts with strong electronic coupling effect. Appl. Catal. B Environ. 2022, 306, 121138. [Google Scholar] [CrossRef]
- Hao, X.; Liu, Y.; Deng, J.; Jing, L.; Wang, J.; Pei, W.; Dai, H. Bimetallic CoNi single atoms supported on three-dimensionally ordered mesoporous chromia: Highly active catalysts for n-hexane combustion. Green Energy Environ. 2022, in press. [CrossRef]
- Wu, Y.; Wang, H.; Peng, J.; Zhang, J.; Ding, M. Single-atom Cu catalyst in a zirconium-based metal–organic framework for biomass conversion. Chem. Eng. J. 2023, 454, 140156. [Google Scholar] [CrossRef]
- Shimizu, K.-i.; Kanno, S.; Kon, K. Hydrogenation of levulinic acid to γ-valerolactone by Ni and MoOx co-loaded carbon catalysts. Green Chem. 2014, 16, 3899–3903. [Google Scholar] [CrossRef]
- Rodiansono, R.; Astuti, M.D.; Hara, T.; Ichikuni, N.; Shimazu, S. Efficient hydrogenation of levulinic acid in water using a supported Ni–Sn alloy on aluminium hydroxide catalysts. Catal. Sci. Technol. 2016, 6, 2955–2961. [Google Scholar] [CrossRef]
- Gundekari, S.; Srinivasan, K. In situ generated Ni(0)@boehmite from NiAl-LDH: An efficient catalyst for selective hydrogenation of biomass derived levulinic acid to γ-valerolactone. Catal. Commun. 2017, 102, 40–43. [Google Scholar] [CrossRef]
- Yi, Z.; Hu, D.; Xu, H.; Wu, Z.; Zhang, M.; Yan, K. Metal regulating the highly selective synthesis of gamma-valerolactone and valeric biofuels from biomass-derived levulinic acid. Fuel 2020, 259, 116208. [Google Scholar] [CrossRef]
- Shao, S.; Ding, Z.; Shang, C.; Zhang, S.; Ke, Y.; Zhu, G.; Yang, Y. Yolk-shell Co catalysts with controlled nanoparticle/single-atom ratio for aqueous levulinic acid hydrogenation to γ-valerolactone. Chem. Eng. J. 2022, 450, 138153. [Google Scholar] [CrossRef]
- Meng, F.; Yang, X.; Zhao, S.; Li, Z.; Zhang, G.; Qi, Y.; Chu, S.; Wang, G.; Zhang, J.; Qin, Y.; et al. Shifting reaction path for levulinic acid aqueous-phase hydrogenation by Pt-TiO2 metal-support interaction. Appl. Catal. B Environ. 2023, 324, 122236. [Google Scholar] [CrossRef]



| SBET (m2/g) | Vtotal (cm3/g) | Pore Size (nm) | |
|---|---|---|---|
| Co0.52Ni0.48@Al2O3-IE | 246.9 | 0.39 | 6.3 |
| Co0.27Ni0.73@Al2O3-IE | 199.8 | 0.40 | 8.1 |
| Co0.71Ni0.29@Al2O3-IE | 176.1 | 0.41 | 9.3 |
| Co0.50Ni0.50/Al2O3-IM | 132.5 | 0.67 | 21.8 |
| γ-Al2O3 | 141.0 | 0.86 | 24.6 |
| Entry | Catalyst | Solvent | T/°C | H2/MPa | t/h | Y/% | Cycles | Ref. |
|---|---|---|---|---|---|---|---|---|
| 1 | Co0.52Ni0.48@Al2O3 | IPA | 130 | 2 | 4 | >99 | 5 | this work |
| 2 | Ni–MoOx/C | — | 250 | 5 | 24 | >99 | — | [53] |
| 3 | Ni–Sn(1.4)/AlOH | H2O | 120 | 4 | 2 | >99 | — | [54] |
| 4 | 25%Fe-25%Ni/MMT | IPA | 200 | 3.5 | 1 | >99 | 6 | [22] |
| 5 | Ni1-Zn1@OMC | H2O | 180 | 2 | 1.5 | >93 | 2 | [29] |
| 6 | Ni(0)@boehmite | H2O | 200 | 3 | 6 | >99 | 2 | [55] |
| 7 | Fe-Re/TiO2 | H2O | 180 | 4 | 4 | 95 | — | [6] |
| 8 | RuHZSM-5 | 1, 4-dioxane | 220 | 3 | 10 | 93.1 | 3 | [56] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ding, H.; Yang, C.; Jiang, C.; Luo, W.; Wang, Q.; Guo, X. Ion-Exchange Synthesis of Surrounded CoNi@Al2O3 Catalyst for Levulinic Acid Hydrogenation to γ-Valerolactone under Mild Conditions. Catalysts 2024, 14, 113. https://doi.org/10.3390/catal14020113
Ding H, Yang C, Jiang C, Luo W, Wang Q, Guo X. Ion-Exchange Synthesis of Surrounded CoNi@Al2O3 Catalyst for Levulinic Acid Hydrogenation to γ-Valerolactone under Mild Conditions. Catalysts. 2024; 14(2):113. https://doi.org/10.3390/catal14020113
Chicago/Turabian StyleDing, Hongzhi, Chenyu Yang, Congyan Jiang, Wei Luo, Qiuyue Wang, and Xuefeng Guo. 2024. "Ion-Exchange Synthesis of Surrounded CoNi@Al2O3 Catalyst for Levulinic Acid Hydrogenation to γ-Valerolactone under Mild Conditions" Catalysts 14, no. 2: 113. https://doi.org/10.3390/catal14020113
APA StyleDing, H., Yang, C., Jiang, C., Luo, W., Wang, Q., & Guo, X. (2024). Ion-Exchange Synthesis of Surrounded CoNi@Al2O3 Catalyst for Levulinic Acid Hydrogenation to γ-Valerolactone under Mild Conditions. Catalysts, 14(2), 113. https://doi.org/10.3390/catal14020113