Deep Eutectic Solvents as Catalysts in the Synthesis of Active Pharmaceutical Ingredients and Precursors
Abstract
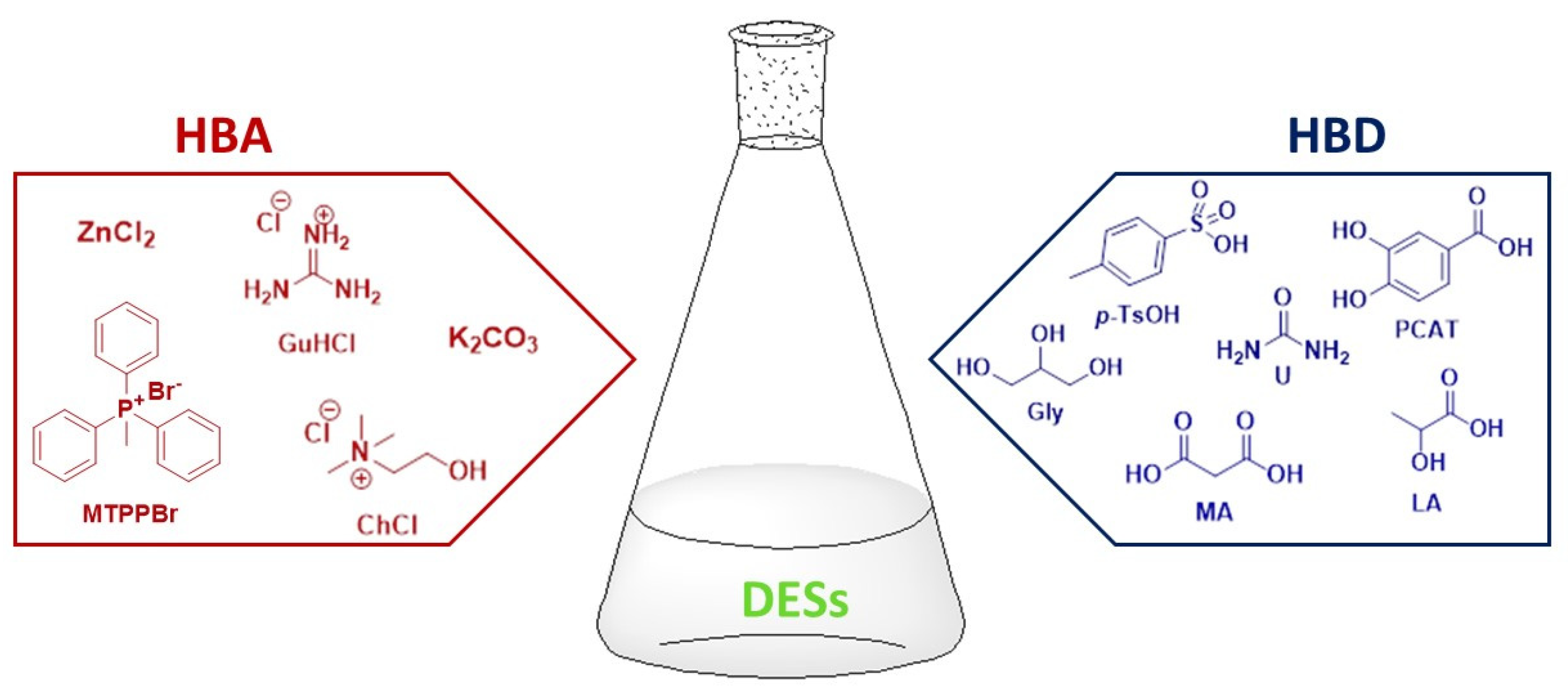
:1. Introduction
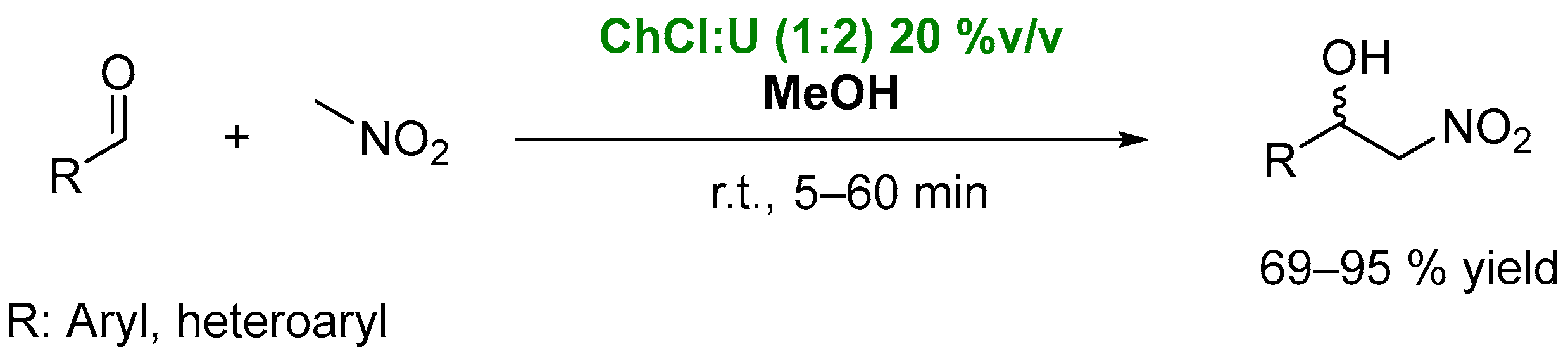
2. DESs as Catalysts in Condensation Processes
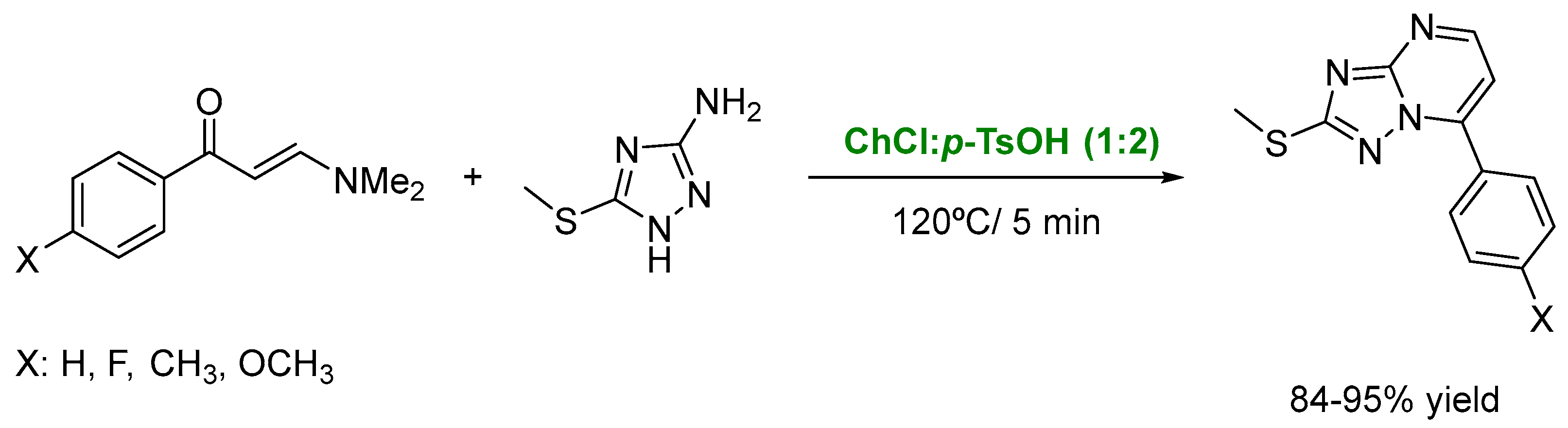
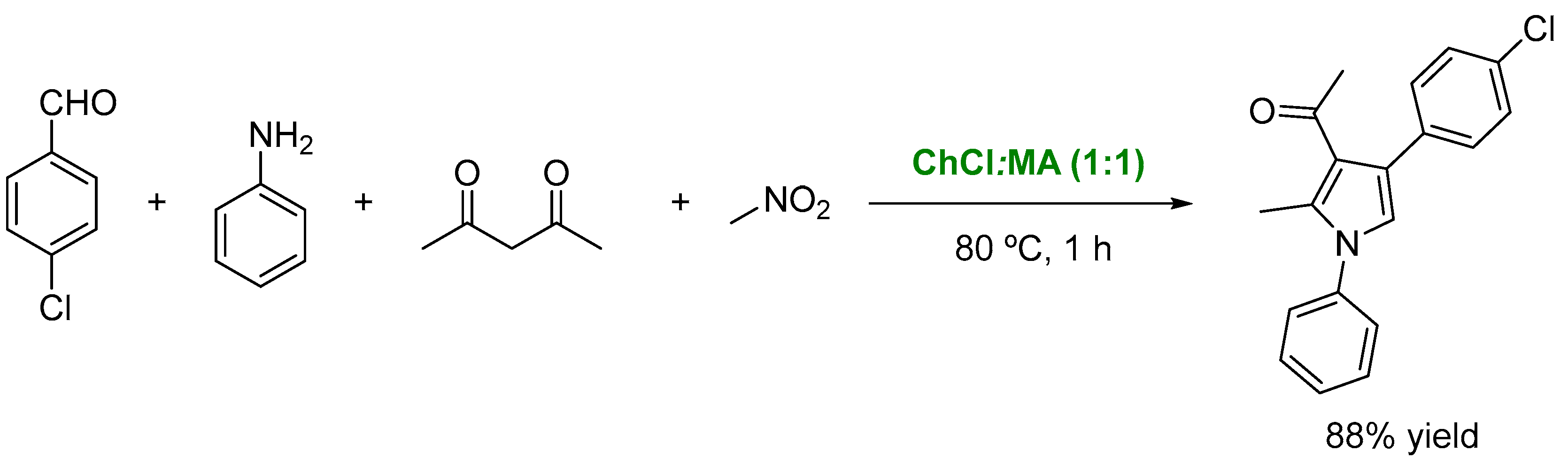
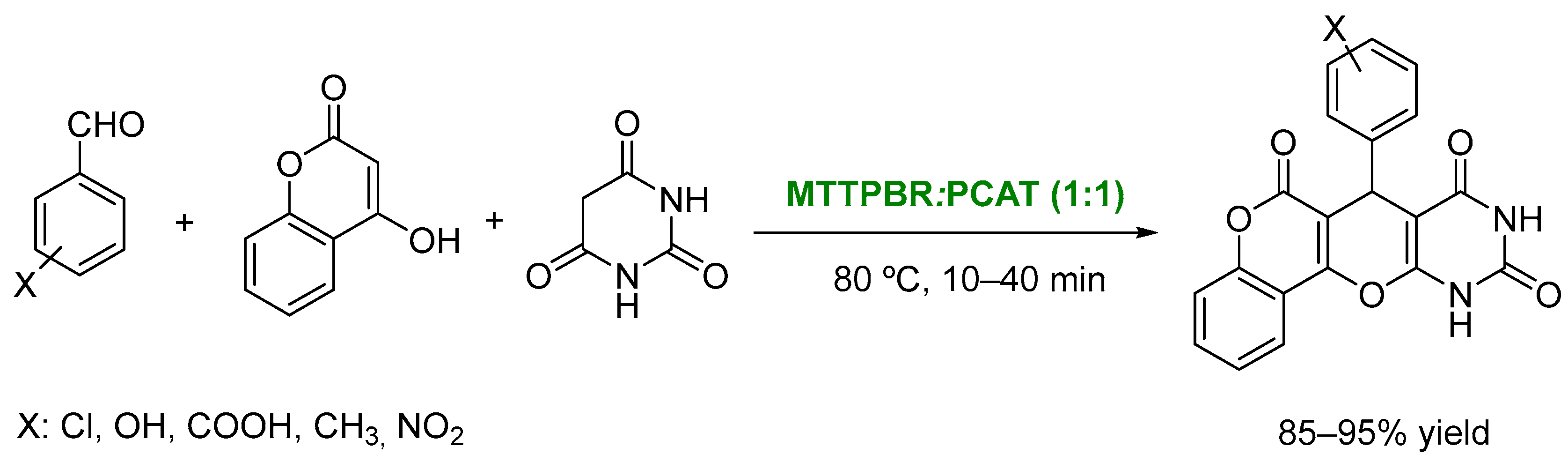
3. DESs as Catalysts in Multicomponent Reactions
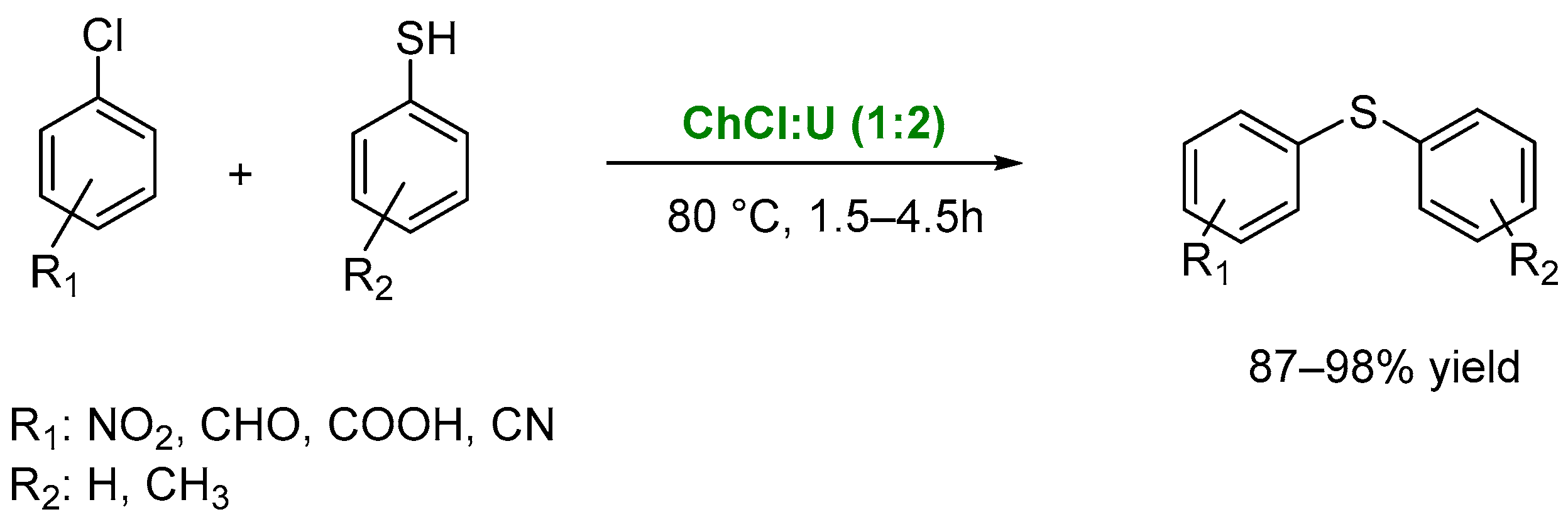
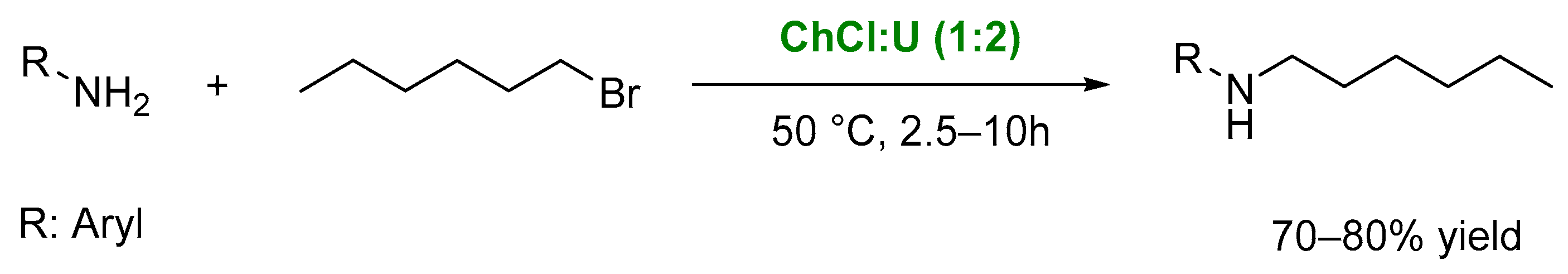
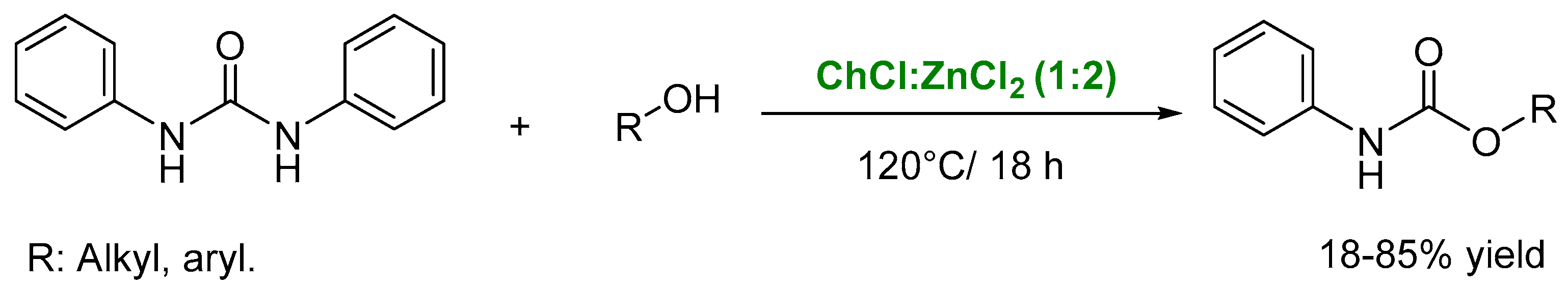
4. Other Reactions Involving Carbon-Heteroatom Bond Formation
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Winterton, N. The green solvent: A critical perspective. Clean Technol. Environ. Policy 2021, 23, 2499–2522. [Google Scholar] [CrossRef] [PubMed]
- Sheldon, R.A. The E factor 25 years on: The rise of green chemistry and sustainability. Green Chem. 2017, 19, 18–43. [Google Scholar] [CrossRef]
- Sheldon, R.A. The E factor at 30: A passion for pollution. Green Chem. 2023, 25, 1704–1728. [Google Scholar] [CrossRef]
- Henderson, R.K.; Jiménez-González, C.; Constable, D.J.C.; Alston, S.R.; Inglis, G.G.A.; Fisher, G.; Sherwood, J.; Binks, S.P.; Curzons, A.D. Expanding GSK’s solvent selection guide—Embedding sustainability into solvent selection starting at medicinal chemistry. Green Chem. 2011, 13, 854–862. [Google Scholar] [CrossRef]
- Ciriminna, R.; Pagliaro, M. Green Chemistry in the Fine Chemicals and Pharmaceutical Industries. Org. Process Res. Dev. 2013, 17, 1479–1484. [Google Scholar] [CrossRef]
- Swati; Pathania, S.; Rawal, R.K. Current prospective of green chemistry in the pharmaceutical industry. In Green Sustainable Process for Chemical and Environmental Engineering and Science; Elsevier: Amsterdam, The Netherlands, 2023; pp. 419–450. [Google Scholar]
- Clarke, C.J.; Tu, W.C.; Levers, O.; Bröhl, A.; Hallett, J.P. Green and Sustainable Solvents in Chemical Processes. Chem Rev. 2018, 118, 747–800. [Google Scholar] [CrossRef]
- de Gonzalo, G.; Alcántara, A.R.; Domínguez de María, P. Cyclopentyl Methyl Ether (CPME): A Versatile Eco-Friendly Solvent for Applications in Biotechnology and Biorefineriesed. ChemSusChem 2018, 10, 2083–2097. [Google Scholar] [CrossRef] [PubMed]
- Miele, M.; Pillari, V.; Pace, V.; Alcántara, A.R.; de Gonzalo, G. Application of Biobased Solvents in Asymmetric Catalysis. Molecules 2022, 27, 6701. [Google Scholar] [CrossRef]
- de Gonzalo, G. Biocatalysed reductions of α-ketoesters employing CyreneTM as cosolvent. Biocat. Biotrans. 2022, 40, 252–257. [Google Scholar] [CrossRef]
- Hoang, H.N.; Nagashima, Y.; Mori, S.; Kagechika, H.; Matsuda, T. CO2-expanded bio-based liquids as novel solvents for enantioselective biocatalysis. Tetrahedron 2017, 73, 2984–2989. [Google Scholar] [CrossRef]
- Hoang, H.N.; Granero-Fernández, E.; Yamada, S.; Mori, S.; Kagechika, H.; Medina-González, Y.; Matsuda, T. Modulating biocatalytic activity toward sterically bulky substrates in CO2-expanded biobased liquids by tuning the physico-chemical properties. ACS Sustain. Chem. Eng. 2017, 5, 11051–11059. [Google Scholar] [CrossRef]
- Vekariya, R.L. A review of ionic liquids: Applications towards catalytic organic transformations. J. Mol. Liq. 2017, 227, 44–60. [Google Scholar] [CrossRef]
- Greer, A.J.; Jacquemin, J.; Hardcare, C. Industrial Applications of Ionic Liquids. Molecules 2020, 25, 5207. [Google Scholar] [CrossRef] [PubMed]
- Hallett, J.P.; Welton, T. Room-Temperature Ionic Liquids: Solvents for Synthesis and Catalysis. 2. Chem. Rev. 2011, 111, 3508–3576. [Google Scholar] [CrossRef] [PubMed]
- Smith, E.L.; Abbott, A.P.; Ryder, K.S. Deep Eutectic Solvents (DESs) and Their Applications. Chem. Rev. 2014, 114, 11060–11082. [Google Scholar] [CrossRef]
- Hansen, B.B.; Spittle, S.; Chen, B.; Poe, D.; Zhang, Y.; Klein, J.M.; Horton, A.; Adhikari, L.; Zelovich, T.; Doherty, B.W.; et al. Deep Eutectic Solvents: A Review of Fundamentals and Applications. Chem. Rev. 2021, 121, 1232–1285. [Google Scholar] [CrossRef] [PubMed]
- Mu, L.; Gao, J.; Zhang, Q.; Kong, F.; Zhang, Y.; Ma, Z.; Sun, C.; Lv, S. Research Progress on Deep Eutectic Solvents and Recent Applications. Processes 2023, 11, 1986. [Google Scholar] [CrossRef]
- El Achkar, T.; Greige-Gerges, H.; Fourmentin, S. Basics and properties of Deep Eutectic Solvents: A review. Environ. Chem. Lett. 2021, 19, 3397–3408. [Google Scholar] [CrossRef]
- De Oliveira Vigier, K.; Fracois, J. Synthesis and properties. In Deep Eutectic Solvents: Synthesis, Properties and Applications; Ramón, D.J., Guillena, G., Eds.; Wiley-VCH: Weinheim, Germany, 2020; pp. 1–24. [Google Scholar]
- Abbot, A.P.; Capper, G.; Davies, D.L.; Rasheed, R.K.; Tambyrajah, V. Novel solvent properties of choline chloride/urea mixtures. Chem. Commun. 2003, 70–71. [Google Scholar] [CrossRef]
- Plotka-Wasylka, J.; de la Guardia, M.; Andruch, V.; Vilková, M. Deep eutectic solvents vs ionic liquids: Similarities and differences. Microchem. J. 2020, 159, 105539. [Google Scholar] [CrossRef]
- Paiva, A.; Craveiro, R.; Aroso, I.; Martins, M.; Reis, R.L.; Duarte, A.R.C. Natural Deep Eutectic Solvents—Solvents for the 21st Century. ACS Sustain. Chem. Eng. 2014, 2, 1063–1071. [Google Scholar] [CrossRef]
- Liu, Y.; Friesen, J.B.; McAlpine, J.B.; Lankin, D.C.; Chen, S.N.; Pauli, G.F. Natural Deep Eutectic Solvents: Properties, Applications, and Perspectives. J. Nat. Prod. 2018, 81, 679–690. [Google Scholar] [CrossRef] [PubMed]
- van Osch, D.J.G.P.; Dietz, C.H.J.T.; van Spronsen, J.; Kroon, M.C.; Gallucci, F.; van Sint Annaland, M.; Tuinier, R. A Search for Natural Hydrophobic Deep Eutectic Solvents Based on Natural Components. ACS Sustain. Chem. Eng. 2019, 7, 2933–2942. [Google Scholar] [CrossRef]
- Liu, Y.; Wu, Y.; Liu, J.; Wang, W.; Yang, Q.; Yang, G. Deep eutectic solvents: Recent advances in fabrication approaches and pharmaceutical applications. Int. J. Pharm. 2022, 622, 121811. [Google Scholar] [CrossRef] [PubMed]
- Cicco, L.; Dilauro, G.; Perna, F.M.; Vitale, P.; Capriati, V. Advances in deep eutectic solvents and water: Applications in metal- and biocatalyzed processes, in the synthesis of APIs, and other biologically active compounds. Org. Biomol. Chem. 2021, 19, 2558–2577. [Google Scholar] [CrossRef] [PubMed]
- Bazzo, G.C.; Pezzini, B.R.; Stulzer, H.K. Eutectic mixtures as an approach to enhance solubility, dissolution rate and oral bioavailability of poorly water-soluble drugs. Int. J. Pharm. 2020, 588, 119741. [Google Scholar] [CrossRef]
- Abdelquader, M.M.; Li, S.; Andrews, G.P.; Jones, D.S. Therapeutic deep eutectic solvents: A comprehensive review of their thermodynamics, microstructure and drug delivery applications. Eur. J. Pharm. Biopharm. 2023, 186, 85–104. [Google Scholar] [CrossRef]
- Unlü, A.E.; Arikaya, A.; Takac, S. Use of deep eutectic solvents as catalyst: A mini-review. Green Proc. Synth. 2019, 8, 355–372. [Google Scholar] [CrossRef]
- Shahiri-Haghayegh, M.; Azizi, N. DES as catalyst. In Deep Eutectic Solvents: Synthesis, Properties and Applications; Ramón, D.J., Guillena, G., Eds.; Wiley-VCH: Weinheim, Germany, 2020; pp. 135–170. [Google Scholar]
- Amoroso, R.; Hollmann, F.; Maccallini, C. Choline Chloride-Based DES as Solvents/Catalysts/Chemical Donors in Pharmaceutical Synthesis. Molecules 2021, 26, 6286. [Google Scholar] [CrossRef]
- Hooshmand, S.E.; Kumar, S.; Bahadur, I.; Singh, T.; Varma, R.S. Deep eutectic solvents as reusable catalysts and promoter for the greener syntheses of small molecules: Recent advances. J. Mol. Liq. 2023, 271, 121013. [Google Scholar] [CrossRef]
- Shah, P.A.; Chavda, V.; Hirpara, D.; Sharma, V.S.; Shrivastav, P.S.; Kumar, S. Exploring the potential of deep eutectic solvents in pharmaceuticals: Challenges and opportunities. J. Mol. Liq. 2023, 390, 123171. [Google Scholar] [CrossRef]
- Taylor, M.S.; Jacobsen, E.N. Asymmetric Catalysis by Chiral Hydrogen-Bond Donors. Angew. Chem. Int. Ed. 2006, 45, 1520–1543. [Google Scholar] [CrossRef] [PubMed]
- Pihko, P.P. (Ed.) Hydrogen Bonding in Organic Synthesis; Wiley-VCH: Weinheim, Germany, 2009. [Google Scholar]
- Saá, J.M.; Lillo, V.J.; Mansilla, J. Catalysis by Networks of Cooperative Hydrogen Bonds. In Noncovalent Interactions in Catalysis; Mahmudov, K.T., Kopylovich, M.N., Guedes da Silva, M.F.C., Pombeiro, A.J.L., Eds.; Royal Society of Chemistry: Cambridge, UK, 2019; pp. 66–93. [Google Scholar]
- Sasai, H.; Arai, S.; Tahara, Y.; Shibasaki, M. Catalytic Asymmetric Synthesis of α-Amino Phosphonates Using Lanthanoid-Potassium-BINOL Complexes. J. Org. Chem. 1995, 60, 6656–6657. [Google Scholar] [CrossRef]
- Kisanga, P.B.; Verkade, J.G. P(RNCH2CH2)3N: An efficient promoter for the nitroaldol (Henry) reaction. J. Org. Chem. 1999, 64, 4298–4303. [Google Scholar] [CrossRef]
- Milner, S.E.; Moody, T.S.; Maguir, A.R. Biocatalytic approaches to the Henry (nitroaldol) reaction. Eur. J. Org. Chem. 2012, 16, 3059–3067. [Google Scholar] [CrossRef]
- Luzzio, F.A. The Henry reaction: Recent examples. Tetrahedron 2001, 57, 915–945. [Google Scholar] [CrossRef]
- Singh, B.S.; Lobo, H.R.; Shankarling, G.S. Choline chloride based eutectic solvents: Magical catalytic system for carbon-carbon bond formation in the rapid synthesis of β-hydroxy functionalized derivatives. Catal. Commun. 2012, 24, 70–74. [Google Scholar] [CrossRef]
- Dong, S.; Liu, X.; Feng, X. Asymmetric Catalytic Rearrangements with α-Diazocarbonyl Compounds. Acc. Chem. Res. 2022, 55, 415–428. [Google Scholar] [CrossRef] [PubMed]
- Ford, A.; Miel, H.; Ring, A.; Slattery, C.N.; Maguire, A.R.; McKervey, M.A. Modern Organic Synthesis with α-diazocarbonyl compounds. Chem. Rev. 2015, 115, 9981–10080. [Google Scholar] [CrossRef]
- Miraki, M.K.; Mehraban, J.A.; Yazdani, E.; Heydari, A. Deep eutectic solvent (DES) as dual solvent/catalyst for synthesis of α-diazocarbonyl compounds using aldol-type coupling. J. Mol. Liq. 2017, 234, 129–132. [Google Scholar] [CrossRef]
- Liu, Z.; Yang, G.; Qin, X. Syntheses and biological activities of novel diheterocyclic compounds containing 1,2,4-triazolo [1,5-a]pyrimidine and 1,3,4-oxadiazole. J. Chem. Technol. Biotechnol. 2001, 76, 1154–1158. [Google Scholar] [CrossRef]
- Martins, M.A.P.; Paveglio, G.C.; Munchen, T.S.; Meyer, A.R.; Moreira, D.N.; Rodrigues, L.V.; Frizzo, C.P.; Zanatta, N.; Bonacorso, H.G.; Melo, P.A.; et al. Deep eutectic solvent mediated synthesis of thiomethyltriazolo[1,5-a]pyrimidines. J. Mol. Liq. 2016, 223, 934–938. [Google Scholar] [CrossRef]
- Ganem, B. Strategies for innovation in multicomponent reaction design. Acc. Chem. Res. 2009, 42, 463–472. [Google Scholar] [CrossRef] [PubMed]
- Neto, B.A.D.; Rocha, R.O.; Rodrigues, M.O. Catalytic Approaches to Multicomponent Reactions: A Critical Review and Perspectives on the Roles of Catalysis. Molecules 2022, 27, 132. [Google Scholar] [CrossRef] [PubMed]
- Dos Santos, J.A.; de Castro, P.P.; de Oliveira, K.T.; Brocksom, T.J.; Amarante, G.W. Multicomponent Reactions Applied to Total Synthesis of Biologically Active Molecules: A Short Review. Curr. Top. Med. Chem. 2023, 23, 990–1003. [Google Scholar] [CrossRef] [PubMed]
- Selvam, T.P.; Kumar, P.V. Quinazoline Marketed drugs—A Review. Res. Pharm. 2011, 1, 1–21. [Google Scholar]
- Chinigo, G.M.; Paige, M.; Grindrod, S.; Hamel, E.; Daksanarmurthy, S.; Chruszcz, M.; Minor, W.; Brown, M.L. Asymmetric synthesis of 2,3-dihydro-2-arylquinazolin-4-ones: Methodology and application to a potent fluorescent tubulin inhibitor with anticancer activity. J. Med. Chem. 2008, 51, 4620–4631. [Google Scholar] [CrossRef]
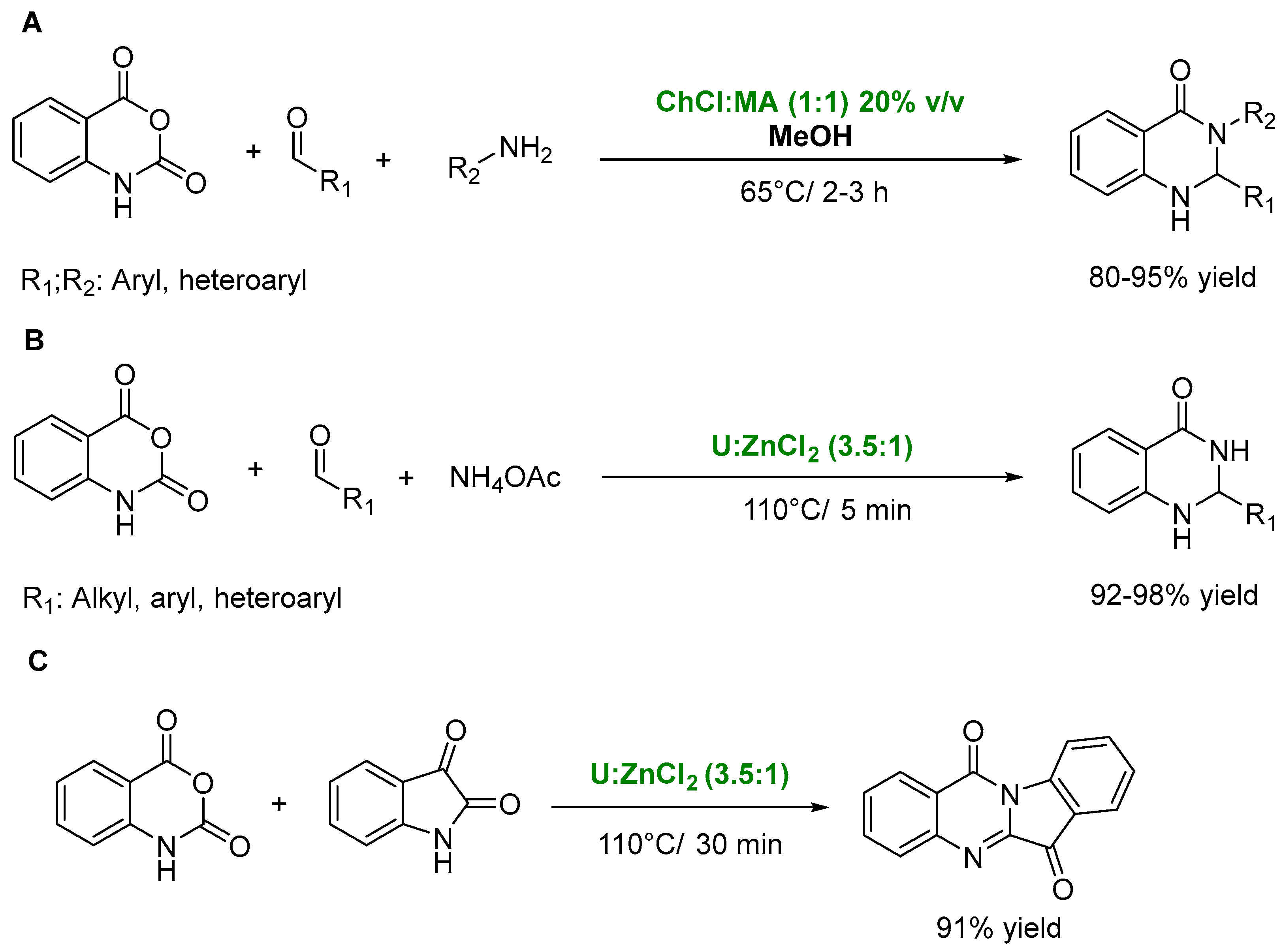
- Lobo, H.R.; Singh, B.S.; Shankarling, G.S. Bio-compatible eutectic mixture for multi-component synthesis: A valuable acidic catalyst for synthesis of novel 2,3-dihydroquinazolin-4(1H)-one derivatives. Catal. Commun. 2012, 27, 179–183. [Google Scholar] [CrossRef]
- Peña-Solórzano, D.; González Guilombo, C.E.; Ochoa-Puentes, C. Rapid and eco-friendly high yield synthesis of dihydroquinazolinones mediated by urea/zinc chloride eutectic mixture. Sustain. Chem. Pharm. 2019, 14, 100167. [Google Scholar] [CrossRef]
- Wang, L.; Zhou, M.; Chen, Q.; He, M.-Y. Brønsted acidic deep eutectic solvent catalysed the one-pot synthesis of 2H-indazolo[2,1-b]phthalazine-triones. J. Chem. Res. 2013, 37, 598–600. [Google Scholar] [CrossRef]
- Asif, M. Some recent approaches of biologically active substituted pyridazine and phthalazine drugs. Curr. Med. Chem. 2012, 19, 2984–2991. [Google Scholar] [CrossRef]
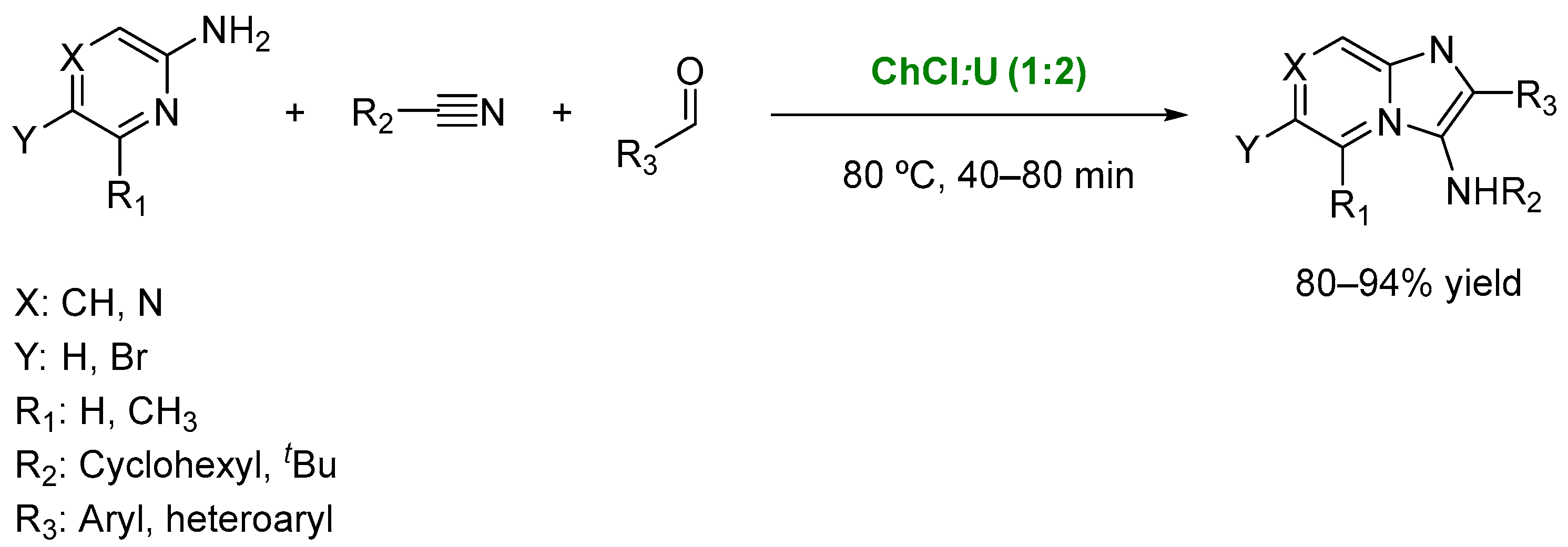
- Devi, N.; Rawal, R.K.; Singh, V. Diversity-oriented synthesis of fused-imidazole derivatives via Groebke–Blackburn–Bienayme reaction: A review. Tetrahedron 2015, 71, 183–232. [Google Scholar] [CrossRef]
- Langer, S.; Arbilla, S.; Benavides, J.; Scatton, B. Zolpidem and alpidem: Two imidazopyridines with selectivity for omega 1- and omega 3-receptor subtypes. Adv. Biochem. Psychopharmacol. 1990, 46, 61. [Google Scholar]
- Shaabani, A.; Hooshmand, S.E. Choline chloride/urea as a deep eutectic solvent/organocatalyst promoted three-component synthesis of 3-aminoimidazo-fused heterocycles via Groebke–Blackburn–Bienayme process. Tetrahedron Lett. 2016, 57, 310–313. [Google Scholar] [CrossRef]
- Cho, H.; Ueda, M.; Shima, K.; Mizuno, A.; Hayashimatsu, M.; Ohnaka, Y. Takeuchi, Y.; Hamaguchi, M.; Aisaka, K.; Hidaka, T.; et al. Dihydropyrimidines: Novel calcium antagonists with potent and long-lasting vasodilative and anti-hypertensive activity. J. Med. Chem. 1989, 32, 2399–2406. [Google Scholar] [CrossRef] [PubMed]
- Biginelli, P. Synthesis of 3,4-dihydropyrimidin-2(1H)-ones. Gazz. Chim. Ital. 1893, 23, 360–413. [Google Scholar]
- Chandravarkar, A.; Aneeja, T.; Anilkumar, G. Advances in Biginelli reaction: A comprehensive review. J. Heterocycl. Chem. 2023, 61, 5–28. [Google Scholar] [CrossRef]
- Cui, Y.; Li, C.; Bao, M. Deep eutectic solvents (DESs) as powerful and recyclable catalysts and solvents for the synthesis of 3,4-dihydropyrimidin-2(1H)-ones/thiones. Green Process Synth. 2019, 8, 568–576. [Google Scholar] [CrossRef]
- Salem, M.S.; Ali, M.A.M. Novel pyrazolo[3,4-b]pyridine derivatives: Synthesis, characterization, antimicrobial and antiproliferative profile. Biol. Pharm. Bull. 2016, 39, 473–483. [Google Scholar] [CrossRef]
- Zhao, B.; Li, Y.; Xu, P.; Dai, Y.; Luo, C.; Sun, Y.; Ai, J.; Geng, M.; Duan, W. Discovery of substituted 1H-Pyrazolo[3,4-b]pyridine derivatives as potent and selective FGFR kinase inhibitors. ACS Med. Chem. Lett. 2016, 7, 629–634. [Google Scholar] [CrossRef]
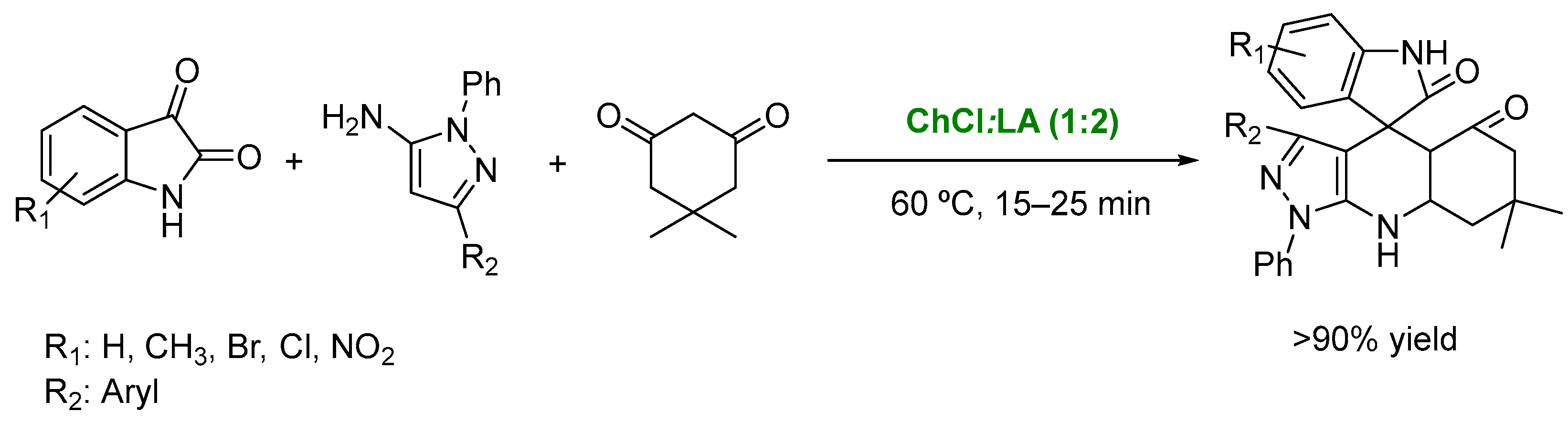
- Santos, M.M.M. Recent advances in the synthesis of biologically active spirooxindoles. Tetrahedron 2014, 70, 9735–9757. [Google Scholar] [CrossRef]
- Zhang, W.-H.; Chen, M.-N.; Hao, Y.; Jiang, X.; Zhou, X.-L.; Zhang, Z.-H. Choline chloride and lactic acid: A natural deep eutectic solvent for one-pot rapid construction of spiro[indoline-3,4′-pyrazolo[3,4-b]pyridines]. J. Mol. Liq. 2019, 278, 124–129. [Google Scholar] [CrossRef]
- Bremner, J.B.; Samosorn, S. Azepines and their Fused-ring Derivatives. In Comprehensive Heterocyclic Chemistry III; Newkome, G.R., Ed.; Elsevier: Amsterdam, The Netherlands, 2008; pp. 1–43. [Google Scholar]
- Miki, T.; Kori, M.; Mabuchi, H.; Tozawa, R.-I.; Nishimoto, T.; Sugiyama, Y.; Teshima, K.; Yukimasa, H. Synthesis of novel 4, 1-benzoxazepine derivatives as squalene synthase inhibitors and their inhibition of cholesterol synthesis. J. Med. Chem. 2002, 45, 4571–4580. [Google Scholar] [CrossRef] [PubMed]
- Shaabani, A.; Hooshmand, S.E.; Nazeri, M.T.; Afshari, R.; Ghasemi, S. Deep eutectic solvent as highly efficient reaction media for the one-pot synthesis of benzo-fused seven-membered heterocycles. Tetrahedron Lett. 2016, 57, 3727–3730. [Google Scholar] [CrossRef]
- Dervan, P.B.; Edelson, B.S. Recognition of the DNA minor groove by pyrrole-imidazole polyamides. Curr. Opin. Struct. Biol. 2003, 13, 284–299. [Google Scholar] [CrossRef]
- He, G.; Bashkin, J.K. What is the antiviral potential of pyrrole-imidazole polyamides? Future Med. Chem. 2015, 7, 1953–1955. [Google Scholar] [CrossRef]
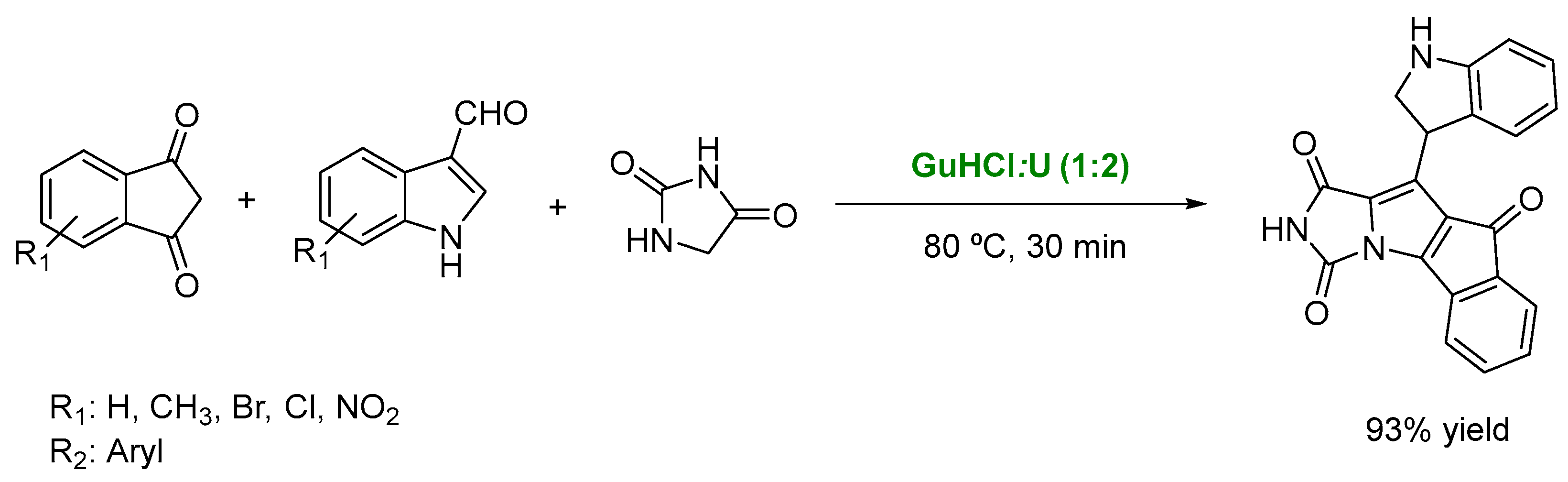
- Rushell, E.; Tailor, Y.K.; Khadewal, S.; Verma, K.; Agarwal, M.; Kumar, M. Deep eutectic solvent promoted synthesis of structurally divers hybrid molecules with privileged heterocyclic structures. New J. Chem. 2019, 43, 12462–12467. [Google Scholar] [CrossRef]
- Hu, H.-C.; Liu, Y.-H.; Li, B.-L.; Cui, Z.-S.; Zhang, Z.-H. Deep eutectic solvent based on choline chloride and malonic acid an efficient and reusable catalytic system for one-pot synthesis of functionalized pyrroles. RSC Adv. 2015, 5, 7720–7728. [Google Scholar] [CrossRef]
- Al-Mourabit, A.; Zancanella, M.A.; Tilvi, S.; Romo, D. Biosynthesis, asymmetric synthesis, and pharmacology, including cellular targets, of the pyrrole-2-aminoimidazole marine alkaloids. Nat. Prod. Rep. 2011, 28, 1229–1260. [Google Scholar] [CrossRef]
- Maiti, S.; Biswas, S.; Jana, U. Iron(III)-Catalyzed Four-Component Coupling Reaction of 1,3-Dicarbonyl Compounds, Amines, Aldehydes, and Nitroalkanes: A Simple and Direct Synthesis of Functionalized Pyrroles. J. Org. Chem. 2010, 75, 1674–1683. [Google Scholar] [CrossRef]
- Li, B.L.; Zhang, M.; Hu, H.C.; Du, X.; Zhang, Z.H. Nano-CoFe2O4 supported molybdenum as an efficient and magnetically recoverable catalyst for a one-pot, four-component synthesis of functionalized pyrroles. New J. Chem. 2014, 38, 2435–2442. [Google Scholar] [CrossRef]
- Murthi, P.R.K.; Rambabu, D.; Rao, M.V.B.; Pal, M. Synthesis of substituted pyrroles via Amberlyst-15 mediated MCR under ultrasound. Tetrahedron Lett. 2014, 55, 507–509. [Google Scholar] [CrossRef]
- Kamdar, N.R.; Haveliwala, D.D.; Mistry, P.T.; Patel, S.K. Design, synthesis and in vitro evaluation of antitubercular and anti-microbial activity of some novel pyrano-pyrimidines. Eur. J. Med. Chem. 2010, 45, 5056–5063. [Google Scholar] [CrossRef]
- Valderrama, J.A.; Colonelli, P.; Vásques, D.; González, M.F.; Rodríguez, J.A.; Theoduloz, C. Studies on quinones Part 44: Novel angucyclinone N-heterocyclic analogues endowed with antitumoral activity. Bioorg. Med. Chem. 2008, 16, 10172–10181. [Google Scholar] [CrossRef] [PubMed]
- Alizadeh, A.; Ghanbaripour, R.; Ng, S.W. An efficient synthesis of chromeno[2,3-d]- pyrimidine-2,4-diones with a nitroketene-aminal moiety at C(5) by a one-pot four-component reaction. Helv. Chim. Acta 2015, 98, 663–667. [Google Scholar] [CrossRef]
- Esmaeilia, A.A.; Salehan, F.; Habibi, A.; Fakhari, A.R. Efficient synthesis of novel pyrano[2,3-d]pyrido[1,2-a]pyrimidines via isocyanide-based three-component reaction. Tetrahedron Lett. 2016, 57, 100–102. [Google Scholar] [CrossRef]
- Monem, A.; Habibi, D.; Goudarzi, H. An acid-based DES as a novel catalyst for the synthesis of pyranopyrimidines. Sci. Rep. 2023, 13, 18009. [Google Scholar] [CrossRef] [PubMed]
- Bentley, R. Role of sulfur chirality in the chemical processes of biology. Chem. Soc. Rev. 2005, 34, 609–624. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Lu, G.P.; Cai, C. Facile aromatic nucleophilic substitution (SNAr) reactions in ionic liquids: An electrophile-nucleophile dual activation by [Omim]Br for the reaction. Green Chem. 2016, 18, 5580–5585. [Google Scholar] [CrossRef]
- Argüello, J.E.; Schmidt, L.C.; Peñéñory, A.B. One-Pot’ Two-Step Synthesis of Aryl Sulfur Compounds by Photoinduced Reactions of Thiourea Anion with Aryl Halides. Org. Lett. 2003, 5, 4133–4136. [Google Scholar] [CrossRef]
- Pant, P.L.; Shankarling, G.S. Deep Eutectic Solvent: An Efficient and Recyclable Catalyst for Synthesis of Thioethers. Chem. Select 2017, 2, 7645–7650. [Google Scholar] [CrossRef]
- Nugent, T.C. (Ed.) Chiral Amine Synthesis: Methods, Developments and Applications; Wiley-VCH: Weinheim, Germany, 2010; pp. 1–479. [Google Scholar]
- Singh, B.; Lobo, H.; Shankarling, G. Selective N-alkylation of aromatic primary amines catalysed by bio-catalyst of Deep Eutectic Solvent. Catal. Lett. 2011, 141, 178–182. [Google Scholar] [CrossRef]
- Carey, J.S.; Laffan, D.; Thomson, C.; Williams, M.T. Analysis of the reactions used for the preparation of drug candidate molecules. Org. Biomol. Chem. 2006, 4, 2337–2347. [Google Scholar] [CrossRef] [PubMed]
- Montalbetti, C.A.G.N.; Falque, V. Amide bond formation and peptide coupling. Tetrahedron 2005, 61, 10827–10852. [Google Scholar] [CrossRef]
- Kukushkin, V.Y.; Pombeiro, A.J.L. Metal-mediated and metal-catalyzed hydrolysis of nitriles. Inorg. Chim. Acta 2005, 358, 1–21. [Google Scholar] [CrossRef]
- Patil, U.B.; Singh, A.S.; Nagarkar, J.M. Choline chloride based eutectic solvent: An efficient and reusable solvent system for the synthesis of primary amides from aldehydes and from nitriles. RSC Adv. 2014, 4, 1102–1106. [Google Scholar] [CrossRef]
- Ghosh, A.K.; Brindisi, M. Organic Carbamates in Drug Design and Medicinal Chemistry. J. Med. Chem. 2015, 58, 2895–2940. [Google Scholar] [CrossRef] [PubMed]
- Carafa, M.; Iannone, F.; Mele, V.; Quaranta, E. Solventless selective phosgene-free N-carbonylation of N-heteroaromatics (pyrrole, indole, carbazole) under mild conditions. Green Chem. 2012, 14, 3377–3385. [Google Scholar] [CrossRef]
- Riemer, D.; Hirapara, P.; Das, S. Chemoselective synthesis of carbamates using CO2 as carbon source. ChemSusChem 2016, 9, 1916–1920. [Google Scholar] [CrossRef]
- Dindarloo Inaloo, I.; Majnooni, S. Ureas as safe carbonyl sources for the synthesis of carbamates with deep eutectic solvents (DESs) as efficient and recyclable solvent/catalyst systems. New J. Chem. 2018, 42, 13249–13255. [Google Scholar] [CrossRef]
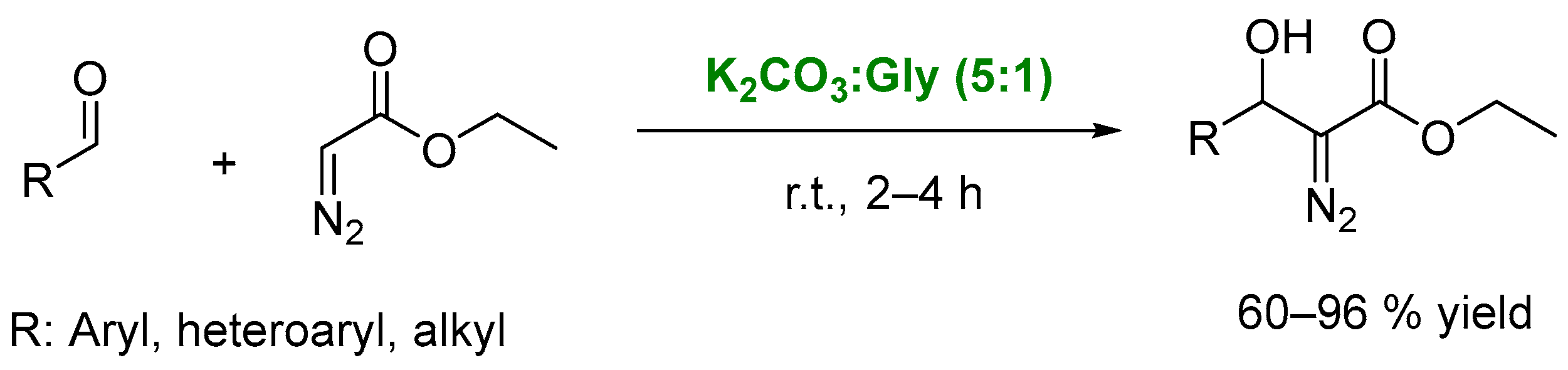



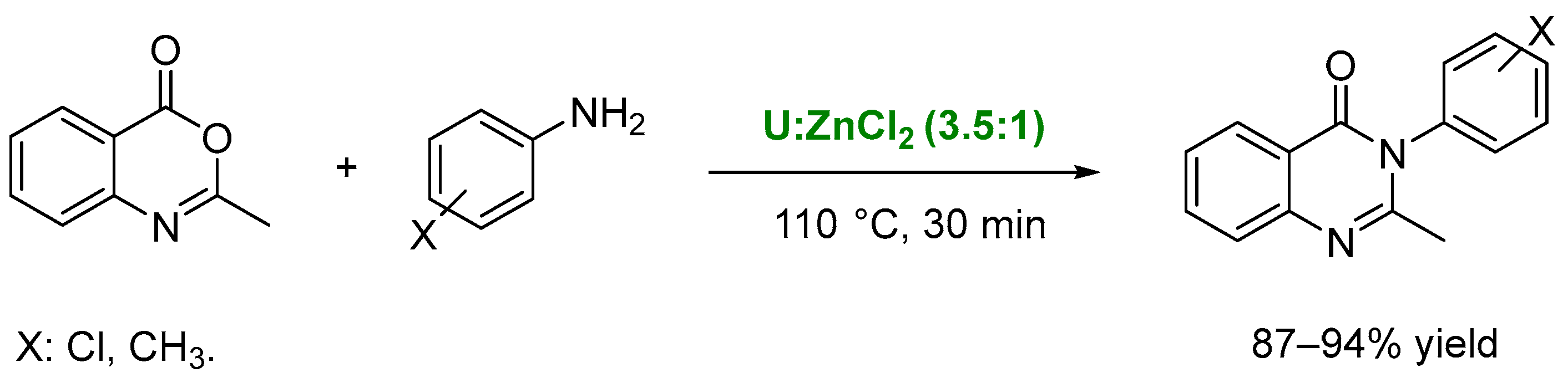
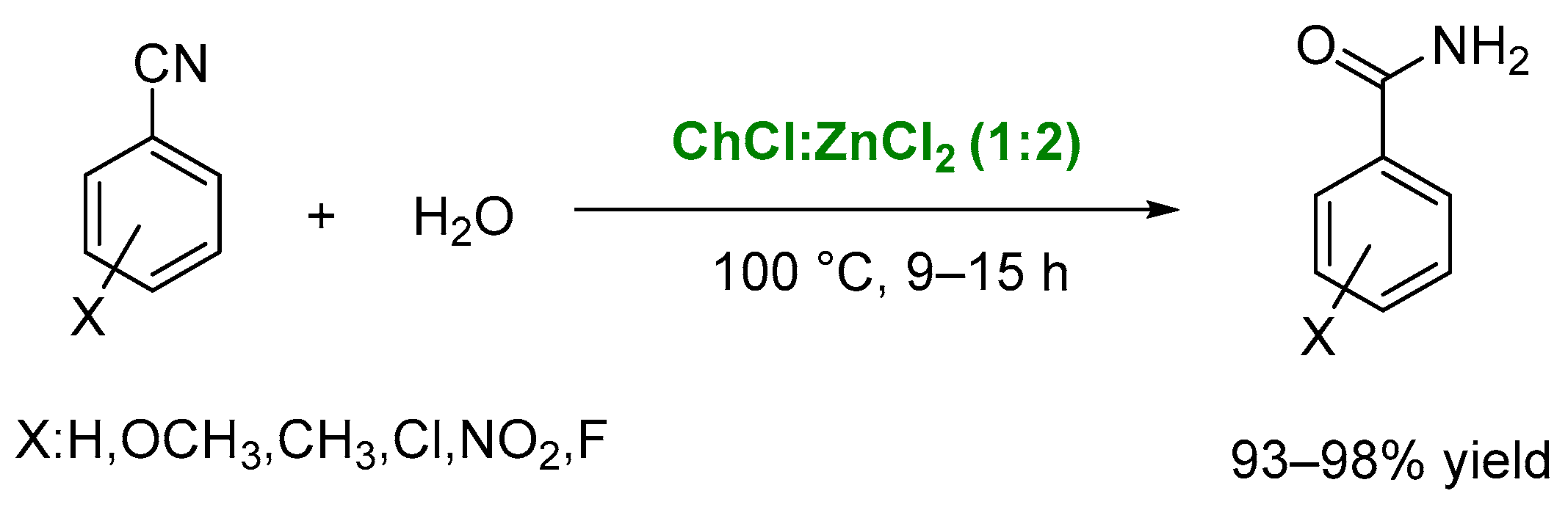
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Falcini, C.; Gonzalo, G.d. Deep Eutectic Solvents as Catalysts in the Synthesis of Active Pharmaceutical Ingredients and Precursors. Catalysts 2024, 14, 120. https://doi.org/10.3390/catal14020120
Falcini C, Gonzalo Gd. Deep Eutectic Solvents as Catalysts in the Synthesis of Active Pharmaceutical Ingredients and Precursors. Catalysts. 2024; 14(2):120. https://doi.org/10.3390/catal14020120
Chicago/Turabian StyleFalcini, Chiara, and Gonzalo de Gonzalo. 2024. "Deep Eutectic Solvents as Catalysts in the Synthesis of Active Pharmaceutical Ingredients and Precursors" Catalysts 14, no. 2: 120. https://doi.org/10.3390/catal14020120
APA StyleFalcini, C., & Gonzalo, G. d. (2024). Deep Eutectic Solvents as Catalysts in the Synthesis of Active Pharmaceutical Ingredients and Precursors. Catalysts, 14(2), 120. https://doi.org/10.3390/catal14020120






