Deep Eutectic Solvents as Catalysts in the Synthesis of Active Pharmaceutical Ingredients and Precursors
Abstract
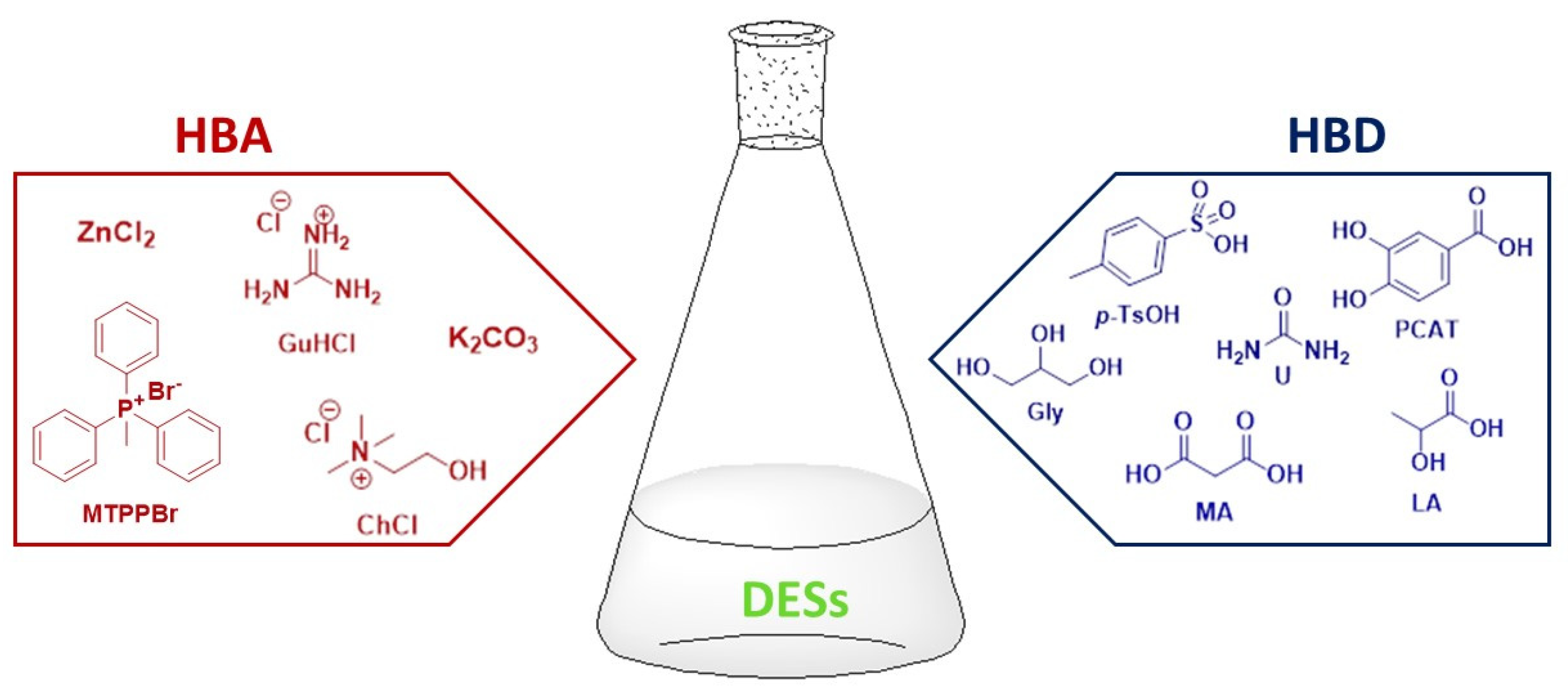
1. Introduction
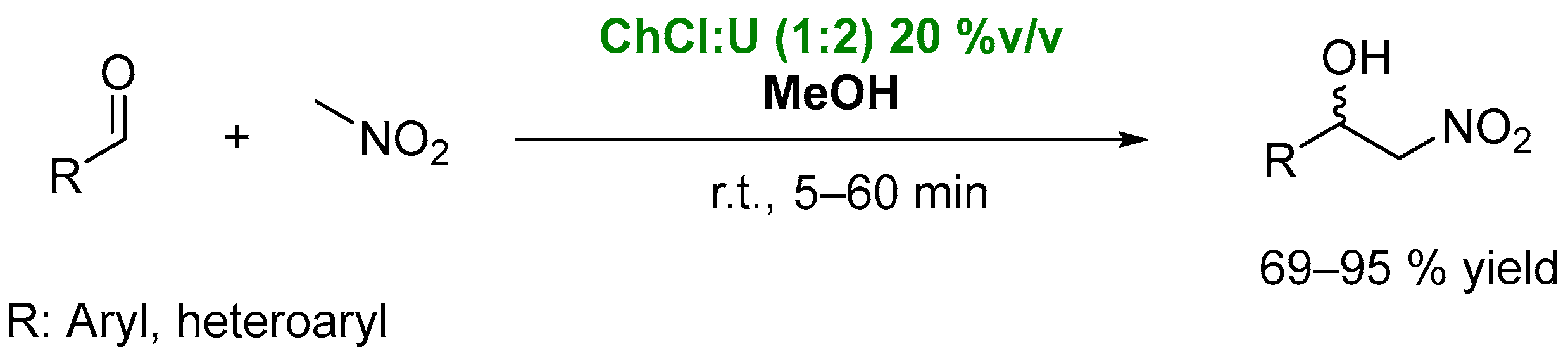
2. DESs as Catalysts in Condensation Processes
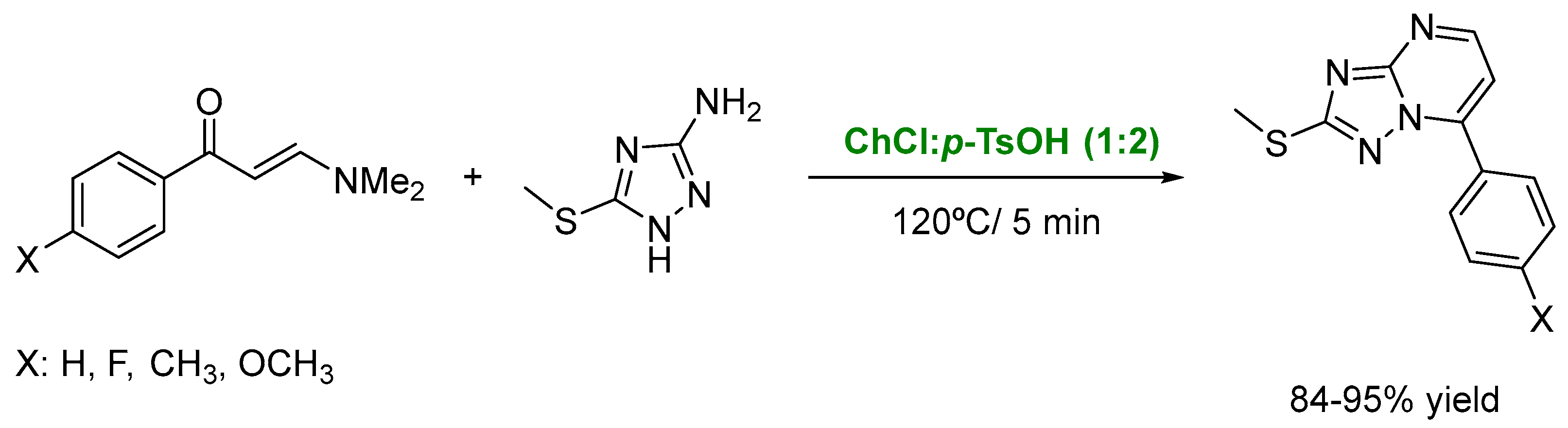
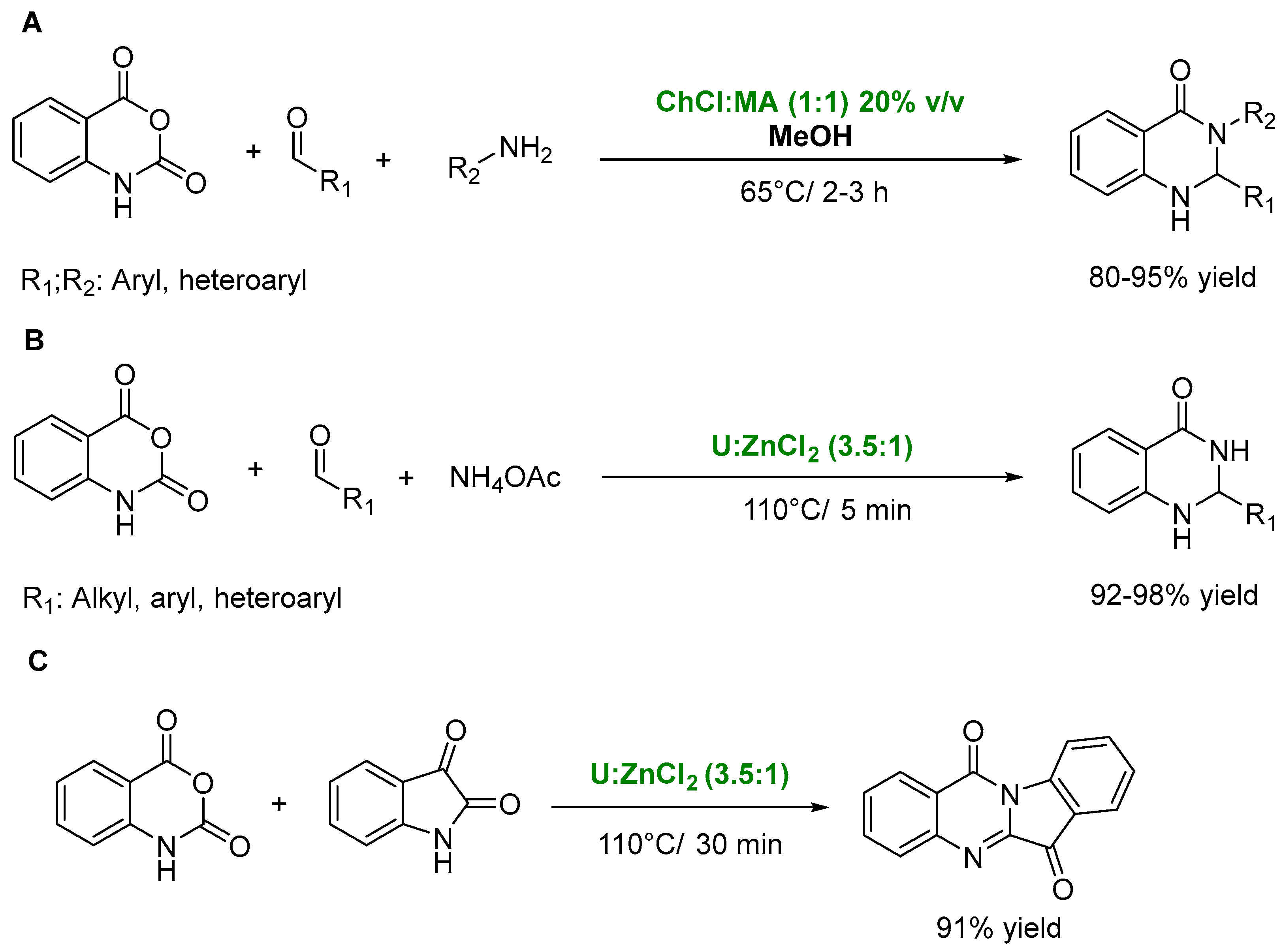
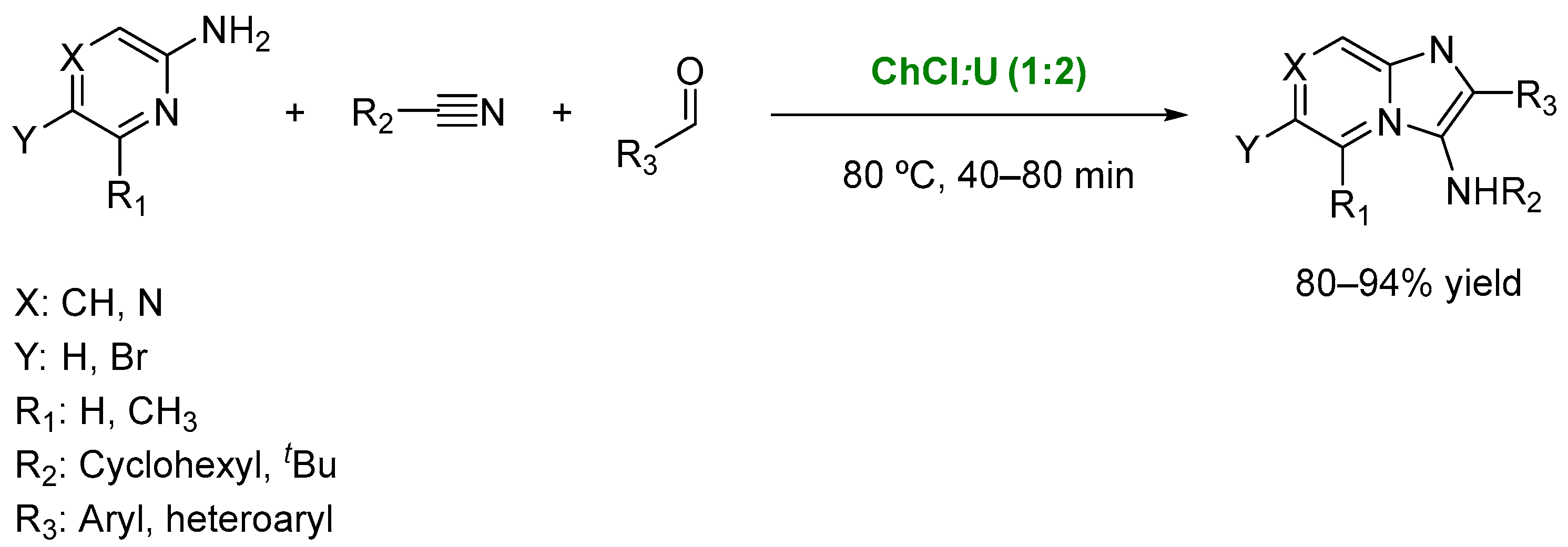
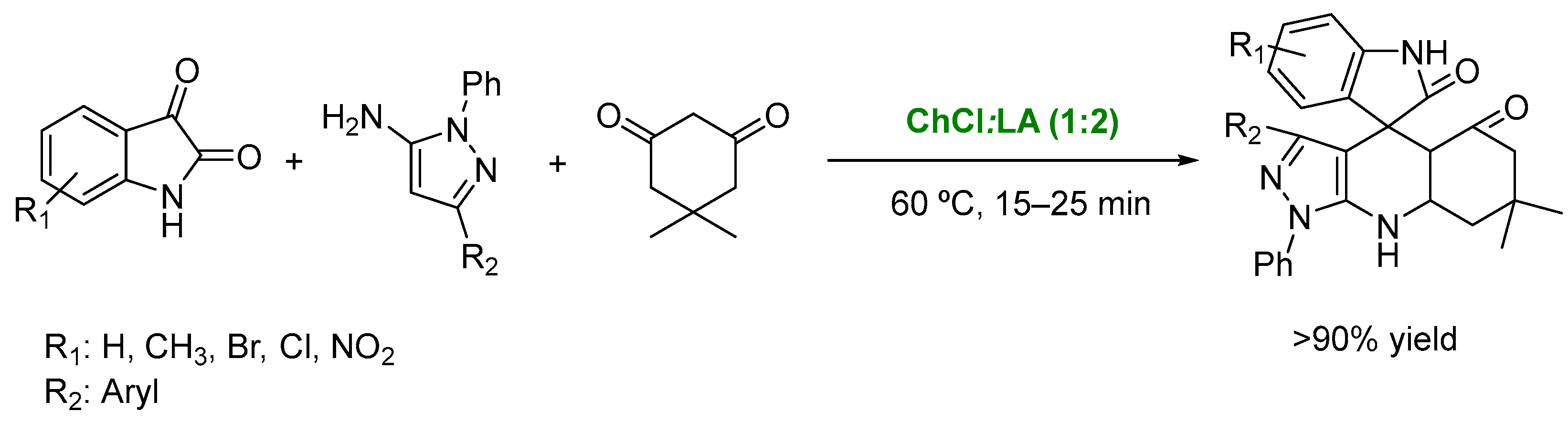
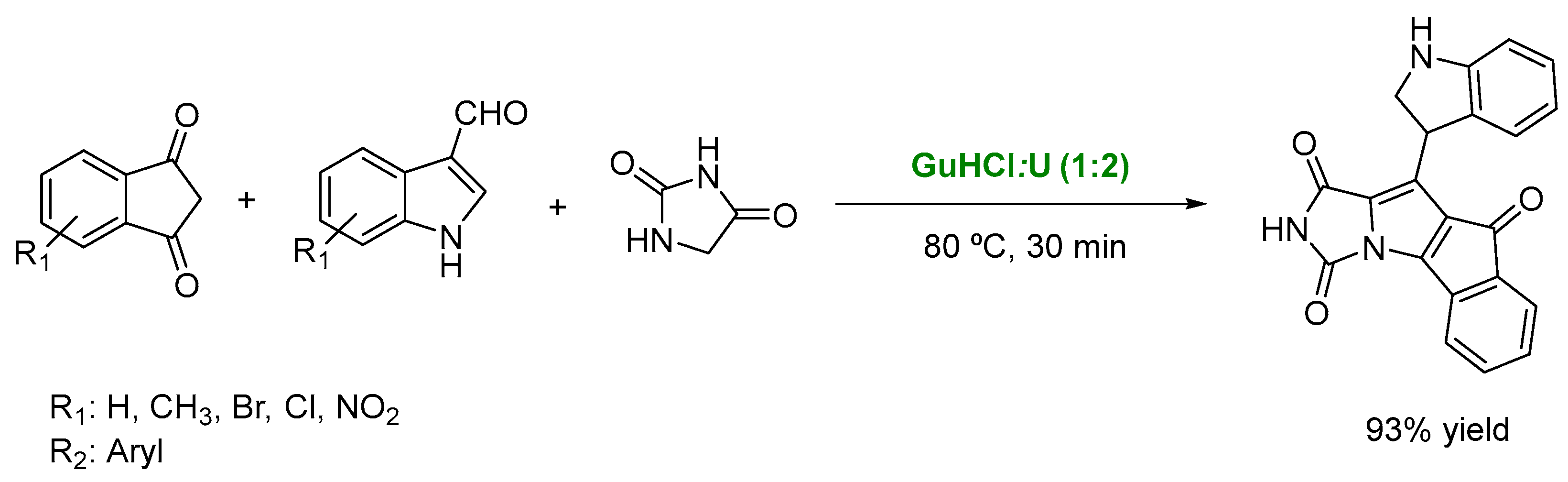
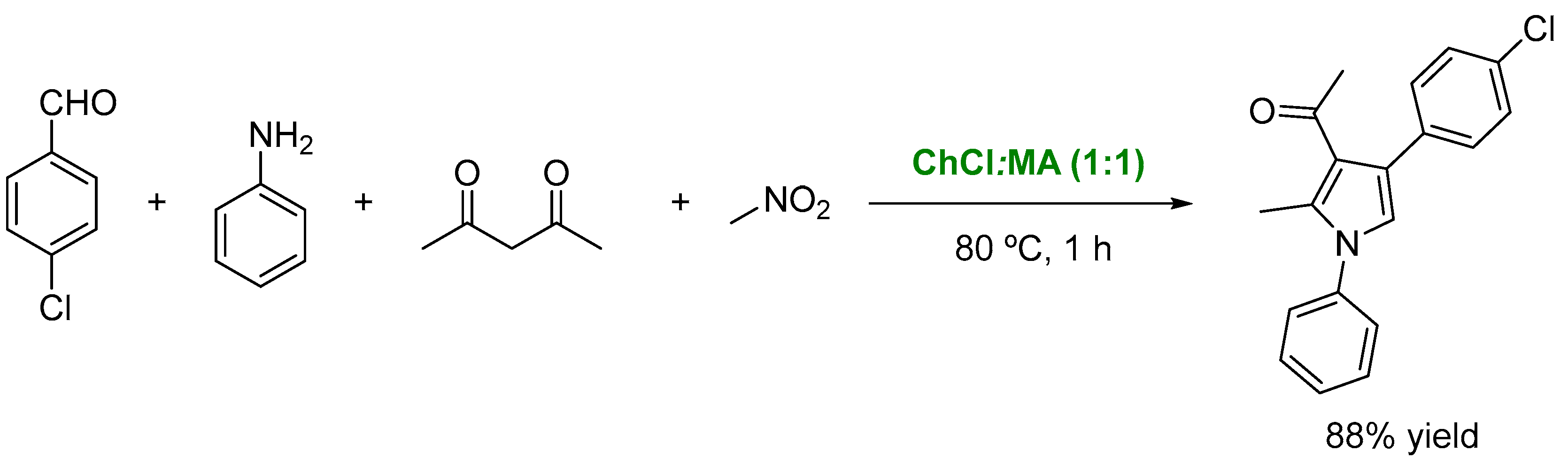
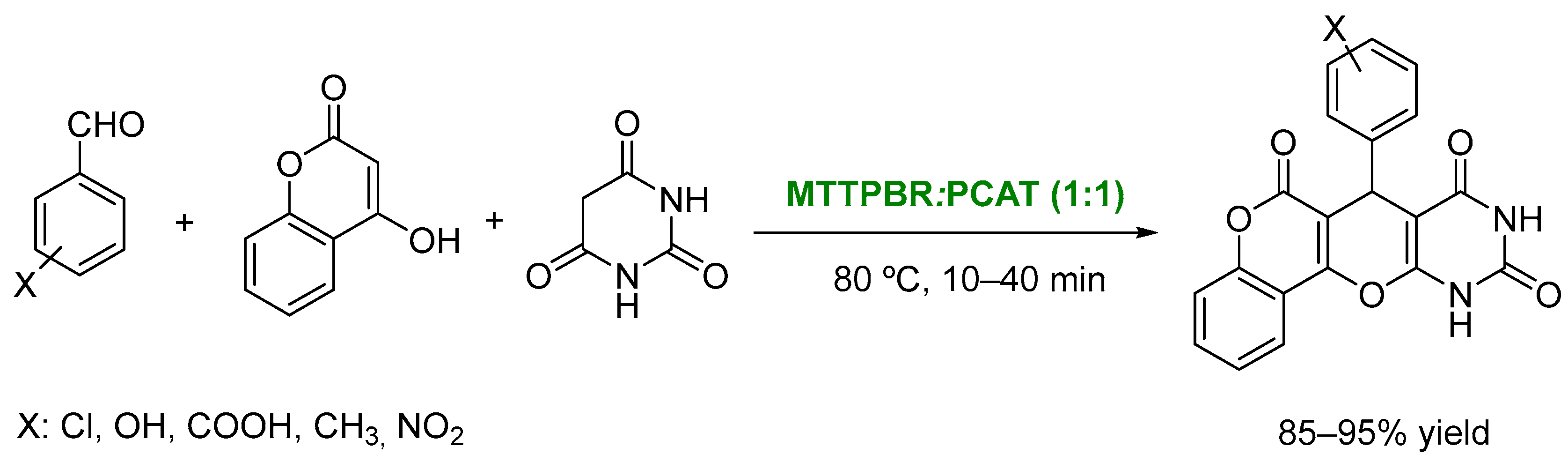
3. DESs as Catalysts in Multicomponent Reactions
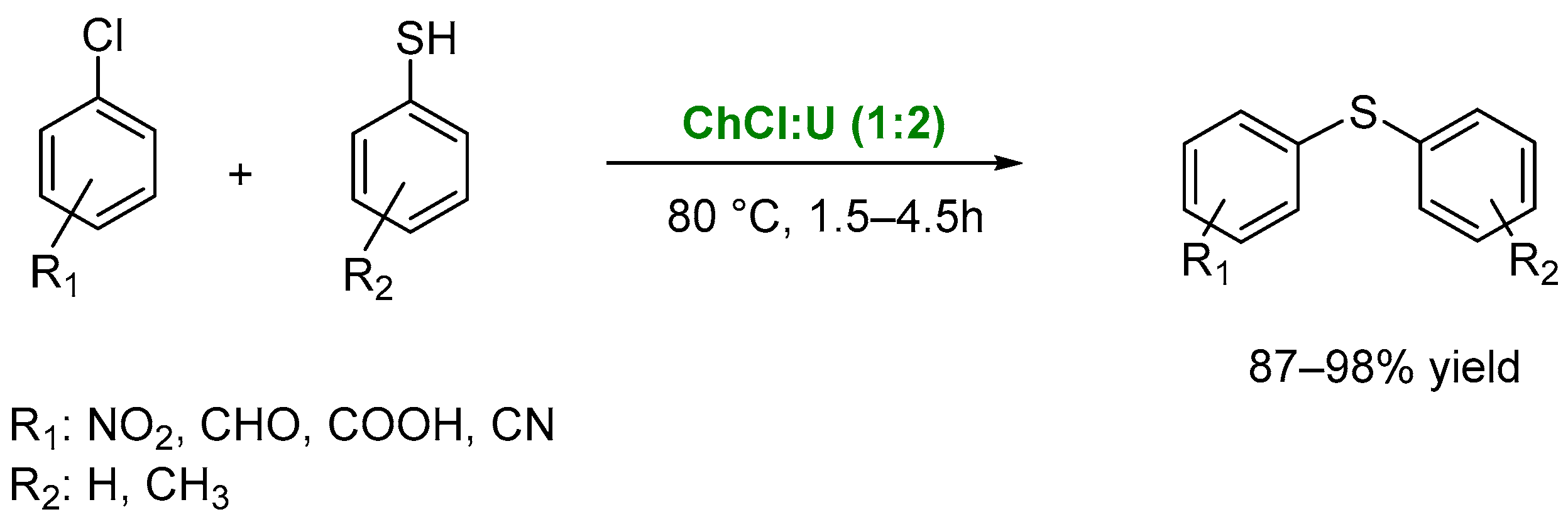
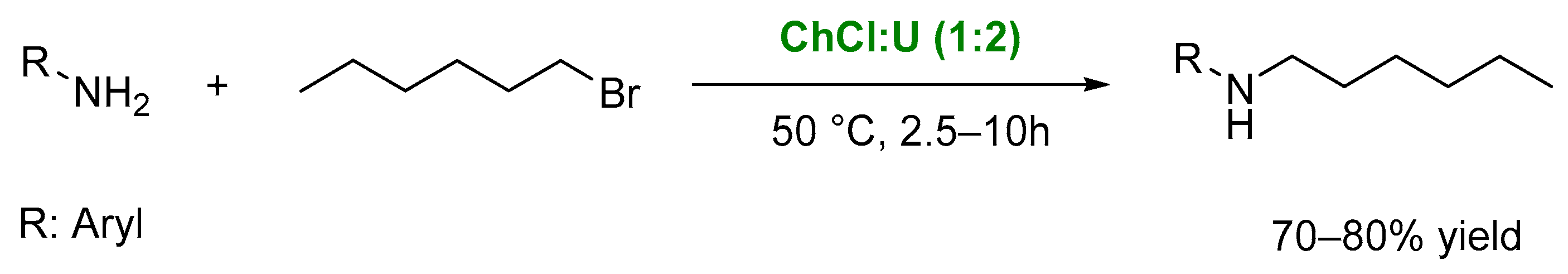
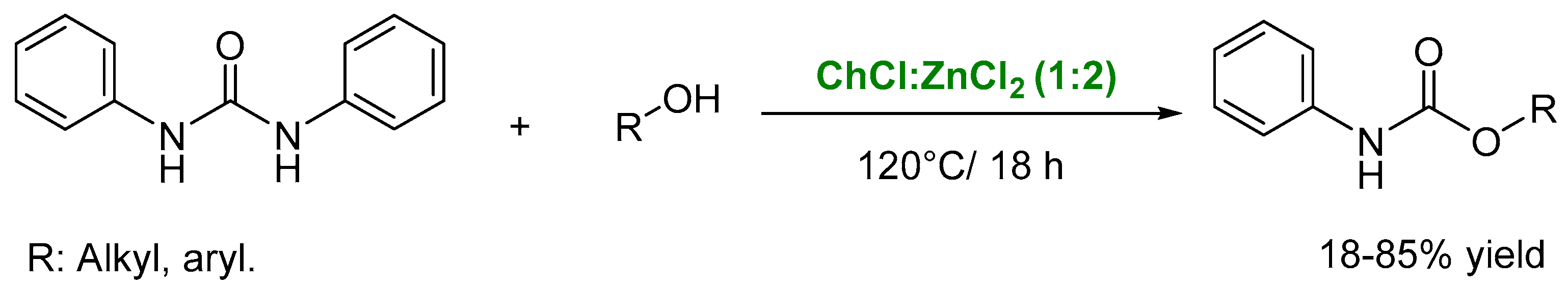
4. Other Reactions Involving Carbon-Heteroatom Bond Formation
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Winterton, N. The green solvent: A critical perspective. Clean Technol. Environ. Policy 2021, 23, 2499–2522. [Google Scholar] [CrossRef] [PubMed]
- Sheldon, R.A. The E factor 25 years on: The rise of green chemistry and sustainability. Green Chem. 2017, 19, 18–43. [Google Scholar] [CrossRef]
- Sheldon, R.A. The E factor at 30: A passion for pollution. Green Chem. 2023, 25, 1704–1728. [Google Scholar] [CrossRef]
- Henderson, R.K.; Jiménez-González, C.; Constable, D.J.C.; Alston, S.R.; Inglis, G.G.A.; Fisher, G.; Sherwood, J.; Binks, S.P.; Curzons, A.D. Expanding GSK’s solvent selection guide—Embedding sustainability into solvent selection starting at medicinal chemistry. Green Chem. 2011, 13, 854–862. [Google Scholar] [CrossRef]
- Ciriminna, R.; Pagliaro, M. Green Chemistry in the Fine Chemicals and Pharmaceutical Industries. Org. Process Res. Dev. 2013, 17, 1479–1484. [Google Scholar] [CrossRef]
- Swati; Pathania, S.; Rawal, R.K. Current prospective of green chemistry in the pharmaceutical industry. In Green Sustainable Process for Chemical and Environmental Engineering and Science; Elsevier: Amsterdam, The Netherlands, 2023; pp. 419–450. [Google Scholar]
- Clarke, C.J.; Tu, W.C.; Levers, O.; Bröhl, A.; Hallett, J.P. Green and Sustainable Solvents in Chemical Processes. Chem Rev. 2018, 118, 747–800. [Google Scholar] [CrossRef]
- de Gonzalo, G.; Alcántara, A.R.; Domínguez de María, P. Cyclopentyl Methyl Ether (CPME): A Versatile Eco-Friendly Solvent for Applications in Biotechnology and Biorefineriesed. ChemSusChem 2018, 10, 2083–2097. [Google Scholar] [CrossRef] [PubMed]
- Miele, M.; Pillari, V.; Pace, V.; Alcántara, A.R.; de Gonzalo, G. Application of Biobased Solvents in Asymmetric Catalysis. Molecules 2022, 27, 6701. [Google Scholar] [CrossRef]
- de Gonzalo, G. Biocatalysed reductions of α-ketoesters employing CyreneTM as cosolvent. Biocat. Biotrans. 2022, 40, 252–257. [Google Scholar] [CrossRef]
- Hoang, H.N.; Nagashima, Y.; Mori, S.; Kagechika, H.; Matsuda, T. CO2-expanded bio-based liquids as novel solvents for enantioselective biocatalysis. Tetrahedron 2017, 73, 2984–2989. [Google Scholar] [CrossRef]
- Hoang, H.N.; Granero-Fernández, E.; Yamada, S.; Mori, S.; Kagechika, H.; Medina-González, Y.; Matsuda, T. Modulating biocatalytic activity toward sterically bulky substrates in CO2-expanded biobased liquids by tuning the physico-chemical properties. ACS Sustain. Chem. Eng. 2017, 5, 11051–11059. [Google Scholar] [CrossRef]
- Vekariya, R.L. A review of ionic liquids: Applications towards catalytic organic transformations. J. Mol. Liq. 2017, 227, 44–60. [Google Scholar] [CrossRef]
- Greer, A.J.; Jacquemin, J.; Hardcare, C. Industrial Applications of Ionic Liquids. Molecules 2020, 25, 5207. [Google Scholar] [CrossRef] [PubMed]
- Hallett, J.P.; Welton, T. Room-Temperature Ionic Liquids: Solvents for Synthesis and Catalysis. 2. Chem. Rev. 2011, 111, 3508–3576. [Google Scholar] [CrossRef] [PubMed]
- Smith, E.L.; Abbott, A.P.; Ryder, K.S. Deep Eutectic Solvents (DESs) and Their Applications. Chem. Rev. 2014, 114, 11060–11082. [Google Scholar] [CrossRef]
- Hansen, B.B.; Spittle, S.; Chen, B.; Poe, D.; Zhang, Y.; Klein, J.M.; Horton, A.; Adhikari, L.; Zelovich, T.; Doherty, B.W.; et al. Deep Eutectic Solvents: A Review of Fundamentals and Applications. Chem. Rev. 2021, 121, 1232–1285. [Google Scholar] [CrossRef] [PubMed]
- Mu, L.; Gao, J.; Zhang, Q.; Kong, F.; Zhang, Y.; Ma, Z.; Sun, C.; Lv, S. Research Progress on Deep Eutectic Solvents and Recent Applications. Processes 2023, 11, 1986. [Google Scholar] [CrossRef]
- El Achkar, T.; Greige-Gerges, H.; Fourmentin, S. Basics and properties of Deep Eutectic Solvents: A review. Environ. Chem. Lett. 2021, 19, 3397–3408. [Google Scholar] [CrossRef]
- De Oliveira Vigier, K.; Fracois, J. Synthesis and properties. In Deep Eutectic Solvents: Synthesis, Properties and Applications; Ramón, D.J., Guillena, G., Eds.; Wiley-VCH: Weinheim, Germany, 2020; pp. 1–24. [Google Scholar]
- Abbot, A.P.; Capper, G.; Davies, D.L.; Rasheed, R.K.; Tambyrajah, V. Novel solvent properties of choline chloride/urea mixtures. Chem. Commun. 2003, 70–71. [Google Scholar] [CrossRef]
- Plotka-Wasylka, J.; de la Guardia, M.; Andruch, V.; Vilková, M. Deep eutectic solvents vs ionic liquids: Similarities and differences. Microchem. J. 2020, 159, 105539. [Google Scholar] [CrossRef]
- Paiva, A.; Craveiro, R.; Aroso, I.; Martins, M.; Reis, R.L.; Duarte, A.R.C. Natural Deep Eutectic Solvents—Solvents for the 21st Century. ACS Sustain. Chem. Eng. 2014, 2, 1063–1071. [Google Scholar] [CrossRef]
- Liu, Y.; Friesen, J.B.; McAlpine, J.B.; Lankin, D.C.; Chen, S.N.; Pauli, G.F. Natural Deep Eutectic Solvents: Properties, Applications, and Perspectives. J. Nat. Prod. 2018, 81, 679–690. [Google Scholar] [CrossRef] [PubMed]
- van Osch, D.J.G.P.; Dietz, C.H.J.T.; van Spronsen, J.; Kroon, M.C.; Gallucci, F.; van Sint Annaland, M.; Tuinier, R. A Search for Natural Hydrophobic Deep Eutectic Solvents Based on Natural Components. ACS Sustain. Chem. Eng. 2019, 7, 2933–2942. [Google Scholar] [CrossRef]
- Liu, Y.; Wu, Y.; Liu, J.; Wang, W.; Yang, Q.; Yang, G. Deep eutectic solvents: Recent advances in fabrication approaches and pharmaceutical applications. Int. J. Pharm. 2022, 622, 121811. [Google Scholar] [CrossRef] [PubMed]
- Cicco, L.; Dilauro, G.; Perna, F.M.; Vitale, P.; Capriati, V. Advances in deep eutectic solvents and water: Applications in metal- and biocatalyzed processes, in the synthesis of APIs, and other biologically active compounds. Org. Biomol. Chem. 2021, 19, 2558–2577. [Google Scholar] [CrossRef] [PubMed]
- Bazzo, G.C.; Pezzini, B.R.; Stulzer, H.K. Eutectic mixtures as an approach to enhance solubility, dissolution rate and oral bioavailability of poorly water-soluble drugs. Int. J. Pharm. 2020, 588, 119741. [Google Scholar] [CrossRef]
- Abdelquader, M.M.; Li, S.; Andrews, G.P.; Jones, D.S. Therapeutic deep eutectic solvents: A comprehensive review of their thermodynamics, microstructure and drug delivery applications. Eur. J. Pharm. Biopharm. 2023, 186, 85–104. [Google Scholar] [CrossRef]
- Unlü, A.E.; Arikaya, A.; Takac, S. Use of deep eutectic solvents as catalyst: A mini-review. Green Proc. Synth. 2019, 8, 355–372. [Google Scholar] [CrossRef]
- Shahiri-Haghayegh, M.; Azizi, N. DES as catalyst. In Deep Eutectic Solvents: Synthesis, Properties and Applications; Ramón, D.J., Guillena, G., Eds.; Wiley-VCH: Weinheim, Germany, 2020; pp. 135–170. [Google Scholar]
- Amoroso, R.; Hollmann, F.; Maccallini, C. Choline Chloride-Based DES as Solvents/Catalysts/Chemical Donors in Pharmaceutical Synthesis. Molecules 2021, 26, 6286. [Google Scholar] [CrossRef]
- Hooshmand, S.E.; Kumar, S.; Bahadur, I.; Singh, T.; Varma, R.S. Deep eutectic solvents as reusable catalysts and promoter for the greener syntheses of small molecules: Recent advances. J. Mol. Liq. 2023, 271, 121013. [Google Scholar] [CrossRef]
- Shah, P.A.; Chavda, V.; Hirpara, D.; Sharma, V.S.; Shrivastav, P.S.; Kumar, S. Exploring the potential of deep eutectic solvents in pharmaceuticals: Challenges and opportunities. J. Mol. Liq. 2023, 390, 123171. [Google Scholar] [CrossRef]
- Taylor, M.S.; Jacobsen, E.N. Asymmetric Catalysis by Chiral Hydrogen-Bond Donors. Angew. Chem. Int. Ed. 2006, 45, 1520–1543. [Google Scholar] [CrossRef] [PubMed]
- Pihko, P.P. (Ed.) Hydrogen Bonding in Organic Synthesis; Wiley-VCH: Weinheim, Germany, 2009. [Google Scholar]
- Saá, J.M.; Lillo, V.J.; Mansilla, J. Catalysis by Networks of Cooperative Hydrogen Bonds. In Noncovalent Interactions in Catalysis; Mahmudov, K.T., Kopylovich, M.N., Guedes da Silva, M.F.C., Pombeiro, A.J.L., Eds.; Royal Society of Chemistry: Cambridge, UK, 2019; pp. 66–93. [Google Scholar]
- Sasai, H.; Arai, S.; Tahara, Y.; Shibasaki, M. Catalytic Asymmetric Synthesis of α-Amino Phosphonates Using Lanthanoid-Potassium-BINOL Complexes. J. Org. Chem. 1995, 60, 6656–6657. [Google Scholar] [CrossRef]
- Kisanga, P.B.; Verkade, J.G. P(RNCH2CH2)3N: An efficient promoter for the nitroaldol (Henry) reaction. J. Org. Chem. 1999, 64, 4298–4303. [Google Scholar] [CrossRef]
- Milner, S.E.; Moody, T.S.; Maguir, A.R. Biocatalytic approaches to the Henry (nitroaldol) reaction. Eur. J. Org. Chem. 2012, 16, 3059–3067. [Google Scholar] [CrossRef]
- Luzzio, F.A. The Henry reaction: Recent examples. Tetrahedron 2001, 57, 915–945. [Google Scholar] [CrossRef]
- Singh, B.S.; Lobo, H.R.; Shankarling, G.S. Choline chloride based eutectic solvents: Magical catalytic system for carbon-carbon bond formation in the rapid synthesis of β-hydroxy functionalized derivatives. Catal. Commun. 2012, 24, 70–74. [Google Scholar] [CrossRef]
- Dong, S.; Liu, X.; Feng, X. Asymmetric Catalytic Rearrangements with α-Diazocarbonyl Compounds. Acc. Chem. Res. 2022, 55, 415–428. [Google Scholar] [CrossRef] [PubMed]
- Ford, A.; Miel, H.; Ring, A.; Slattery, C.N.; Maguire, A.R.; McKervey, M.A. Modern Organic Synthesis with α-diazocarbonyl compounds. Chem. Rev. 2015, 115, 9981–10080. [Google Scholar] [CrossRef]
- Miraki, M.K.; Mehraban, J.A.; Yazdani, E.; Heydari, A. Deep eutectic solvent (DES) as dual solvent/catalyst for synthesis of α-diazocarbonyl compounds using aldol-type coupling. J. Mol. Liq. 2017, 234, 129–132. [Google Scholar] [CrossRef]
- Liu, Z.; Yang, G.; Qin, X. Syntheses and biological activities of novel diheterocyclic compounds containing 1,2,4-triazolo [1,5-a]pyrimidine and 1,3,4-oxadiazole. J. Chem. Technol. Biotechnol. 2001, 76, 1154–1158. [Google Scholar] [CrossRef]
- Martins, M.A.P.; Paveglio, G.C.; Munchen, T.S.; Meyer, A.R.; Moreira, D.N.; Rodrigues, L.V.; Frizzo, C.P.; Zanatta, N.; Bonacorso, H.G.; Melo, P.A.; et al. Deep eutectic solvent mediated synthesis of thiomethyltriazolo[1,5-a]pyrimidines. J. Mol. Liq. 2016, 223, 934–938. [Google Scholar] [CrossRef]
- Ganem, B. Strategies for innovation in multicomponent reaction design. Acc. Chem. Res. 2009, 42, 463–472. [Google Scholar] [CrossRef] [PubMed]
- Neto, B.A.D.; Rocha, R.O.; Rodrigues, M.O. Catalytic Approaches to Multicomponent Reactions: A Critical Review and Perspectives on the Roles of Catalysis. Molecules 2022, 27, 132. [Google Scholar] [CrossRef] [PubMed]
- Dos Santos, J.A.; de Castro, P.P.; de Oliveira, K.T.; Brocksom, T.J.; Amarante, G.W. Multicomponent Reactions Applied to Total Synthesis of Biologically Active Molecules: A Short Review. Curr. Top. Med. Chem. 2023, 23, 990–1003. [Google Scholar] [CrossRef] [PubMed]
- Selvam, T.P.; Kumar, P.V. Quinazoline Marketed drugs—A Review. Res. Pharm. 2011, 1, 1–21. [Google Scholar]
- Chinigo, G.M.; Paige, M.; Grindrod, S.; Hamel, E.; Daksanarmurthy, S.; Chruszcz, M.; Minor, W.; Brown, M.L. Asymmetric synthesis of 2,3-dihydro-2-arylquinazolin-4-ones: Methodology and application to a potent fluorescent tubulin inhibitor with anticancer activity. J. Med. Chem. 2008, 51, 4620–4631. [Google Scholar] [CrossRef]
- Lobo, H.R.; Singh, B.S.; Shankarling, G.S. Bio-compatible eutectic mixture for multi-component synthesis: A valuable acidic catalyst for synthesis of novel 2,3-dihydroquinazolin-4(1H)-one derivatives. Catal. Commun. 2012, 27, 179–183. [Google Scholar] [CrossRef]
- Peña-Solórzano, D.; González Guilombo, C.E.; Ochoa-Puentes, C. Rapid and eco-friendly high yield synthesis of dihydroquinazolinones mediated by urea/zinc chloride eutectic mixture. Sustain. Chem. Pharm. 2019, 14, 100167. [Google Scholar] [CrossRef]
- Wang, L.; Zhou, M.; Chen, Q.; He, M.-Y. Brønsted acidic deep eutectic solvent catalysed the one-pot synthesis of 2H-indazolo[2,1-b]phthalazine-triones. J. Chem. Res. 2013, 37, 598–600. [Google Scholar] [CrossRef]
- Asif, M. Some recent approaches of biologically active substituted pyridazine and phthalazine drugs. Curr. Med. Chem. 2012, 19, 2984–2991. [Google Scholar] [CrossRef]
- Devi, N.; Rawal, R.K.; Singh, V. Diversity-oriented synthesis of fused-imidazole derivatives via Groebke–Blackburn–Bienayme reaction: A review. Tetrahedron 2015, 71, 183–232. [Google Scholar] [CrossRef]
- Langer, S.; Arbilla, S.; Benavides, J.; Scatton, B. Zolpidem and alpidem: Two imidazopyridines with selectivity for omega 1- and omega 3-receptor subtypes. Adv. Biochem. Psychopharmacol. 1990, 46, 61. [Google Scholar]
- Shaabani, A.; Hooshmand, S.E. Choline chloride/urea as a deep eutectic solvent/organocatalyst promoted three-component synthesis of 3-aminoimidazo-fused heterocycles via Groebke–Blackburn–Bienayme process. Tetrahedron Lett. 2016, 57, 310–313. [Google Scholar] [CrossRef]
- Cho, H.; Ueda, M.; Shima, K.; Mizuno, A.; Hayashimatsu, M.; Ohnaka, Y. Takeuchi, Y.; Hamaguchi, M.; Aisaka, K.; Hidaka, T.; et al. Dihydropyrimidines: Novel calcium antagonists with potent and long-lasting vasodilative and anti-hypertensive activity. J. Med. Chem. 1989, 32, 2399–2406. [Google Scholar] [CrossRef] [PubMed]
- Biginelli, P. Synthesis of 3,4-dihydropyrimidin-2(1H)-ones. Gazz. Chim. Ital. 1893, 23, 360–413. [Google Scholar]
- Chandravarkar, A.; Aneeja, T.; Anilkumar, G. Advances in Biginelli reaction: A comprehensive review. J. Heterocycl. Chem. 2023, 61, 5–28. [Google Scholar] [CrossRef]
- Cui, Y.; Li, C.; Bao, M. Deep eutectic solvents (DESs) as powerful and recyclable catalysts and solvents for the synthesis of 3,4-dihydropyrimidin-2(1H)-ones/thiones. Green Process Synth. 2019, 8, 568–576. [Google Scholar] [CrossRef]
- Salem, M.S.; Ali, M.A.M. Novel pyrazolo[3,4-b]pyridine derivatives: Synthesis, characterization, antimicrobial and antiproliferative profile. Biol. Pharm. Bull. 2016, 39, 473–483. [Google Scholar] [CrossRef]
- Zhao, B.; Li, Y.; Xu, P.; Dai, Y.; Luo, C.; Sun, Y.; Ai, J.; Geng, M.; Duan, W. Discovery of substituted 1H-Pyrazolo[3,4-b]pyridine derivatives as potent and selective FGFR kinase inhibitors. ACS Med. Chem. Lett. 2016, 7, 629–634. [Google Scholar] [CrossRef]
- Santos, M.M.M. Recent advances in the synthesis of biologically active spirooxindoles. Tetrahedron 2014, 70, 9735–9757. [Google Scholar] [CrossRef]
- Zhang, W.-H.; Chen, M.-N.; Hao, Y.; Jiang, X.; Zhou, X.-L.; Zhang, Z.-H. Choline chloride and lactic acid: A natural deep eutectic solvent for one-pot rapid construction of spiro[indoline-3,4′-pyrazolo[3,4-b]pyridines]. J. Mol. Liq. 2019, 278, 124–129. [Google Scholar] [CrossRef]
- Bremner, J.B.; Samosorn, S. Azepines and their Fused-ring Derivatives. In Comprehensive Heterocyclic Chemistry III; Newkome, G.R., Ed.; Elsevier: Amsterdam, The Netherlands, 2008; pp. 1–43. [Google Scholar]
- Miki, T.; Kori, M.; Mabuchi, H.; Tozawa, R.-I.; Nishimoto, T.; Sugiyama, Y.; Teshima, K.; Yukimasa, H. Synthesis of novel 4, 1-benzoxazepine derivatives as squalene synthase inhibitors and their inhibition of cholesterol synthesis. J. Med. Chem. 2002, 45, 4571–4580. [Google Scholar] [CrossRef] [PubMed]
- Shaabani, A.; Hooshmand, S.E.; Nazeri, M.T.; Afshari, R.; Ghasemi, S. Deep eutectic solvent as highly efficient reaction media for the one-pot synthesis of benzo-fused seven-membered heterocycles. Tetrahedron Lett. 2016, 57, 3727–3730. [Google Scholar] [CrossRef]
- Dervan, P.B.; Edelson, B.S. Recognition of the DNA minor groove by pyrrole-imidazole polyamides. Curr. Opin. Struct. Biol. 2003, 13, 284–299. [Google Scholar] [CrossRef]
- He, G.; Bashkin, J.K. What is the antiviral potential of pyrrole-imidazole polyamides? Future Med. Chem. 2015, 7, 1953–1955. [Google Scholar] [CrossRef]
- Rushell, E.; Tailor, Y.K.; Khadewal, S.; Verma, K.; Agarwal, M.; Kumar, M. Deep eutectic solvent promoted synthesis of structurally divers hybrid molecules with privileged heterocyclic structures. New J. Chem. 2019, 43, 12462–12467. [Google Scholar] [CrossRef]
- Hu, H.-C.; Liu, Y.-H.; Li, B.-L.; Cui, Z.-S.; Zhang, Z.-H. Deep eutectic solvent based on choline chloride and malonic acid an efficient and reusable catalytic system for one-pot synthesis of functionalized pyrroles. RSC Adv. 2015, 5, 7720–7728. [Google Scholar] [CrossRef]
- Al-Mourabit, A.; Zancanella, M.A.; Tilvi, S.; Romo, D. Biosynthesis, asymmetric synthesis, and pharmacology, including cellular targets, of the pyrrole-2-aminoimidazole marine alkaloids. Nat. Prod. Rep. 2011, 28, 1229–1260. [Google Scholar] [CrossRef]
- Maiti, S.; Biswas, S.; Jana, U. Iron(III)-Catalyzed Four-Component Coupling Reaction of 1,3-Dicarbonyl Compounds, Amines, Aldehydes, and Nitroalkanes: A Simple and Direct Synthesis of Functionalized Pyrroles. J. Org. Chem. 2010, 75, 1674–1683. [Google Scholar] [CrossRef]
- Li, B.L.; Zhang, M.; Hu, H.C.; Du, X.; Zhang, Z.H. Nano-CoFe2O4 supported molybdenum as an efficient and magnetically recoverable catalyst for a one-pot, four-component synthesis of functionalized pyrroles. New J. Chem. 2014, 38, 2435–2442. [Google Scholar] [CrossRef]
- Murthi, P.R.K.; Rambabu, D.; Rao, M.V.B.; Pal, M. Synthesis of substituted pyrroles via Amberlyst-15 mediated MCR under ultrasound. Tetrahedron Lett. 2014, 55, 507–509. [Google Scholar] [CrossRef]
- Kamdar, N.R.; Haveliwala, D.D.; Mistry, P.T.; Patel, S.K. Design, synthesis and in vitro evaluation of antitubercular and anti-microbial activity of some novel pyrano-pyrimidines. Eur. J. Med. Chem. 2010, 45, 5056–5063. [Google Scholar] [CrossRef]
- Valderrama, J.A.; Colonelli, P.; Vásques, D.; González, M.F.; Rodríguez, J.A.; Theoduloz, C. Studies on quinones Part 44: Novel angucyclinone N-heterocyclic analogues endowed with antitumoral activity. Bioorg. Med. Chem. 2008, 16, 10172–10181. [Google Scholar] [CrossRef] [PubMed]
- Alizadeh, A.; Ghanbaripour, R.; Ng, S.W. An efficient synthesis of chromeno[2,3-d]- pyrimidine-2,4-diones with a nitroketene-aminal moiety at C(5) by a one-pot four-component reaction. Helv. Chim. Acta 2015, 98, 663–667. [Google Scholar] [CrossRef]
- Esmaeilia, A.A.; Salehan, F.; Habibi, A.; Fakhari, A.R. Efficient synthesis of novel pyrano[2,3-d]pyrido[1,2-a]pyrimidines via isocyanide-based three-component reaction. Tetrahedron Lett. 2016, 57, 100–102. [Google Scholar] [CrossRef]
- Monem, A.; Habibi, D.; Goudarzi, H. An acid-based DES as a novel catalyst for the synthesis of pyranopyrimidines. Sci. Rep. 2023, 13, 18009. [Google Scholar] [CrossRef] [PubMed]
- Bentley, R. Role of sulfur chirality in the chemical processes of biology. Chem. Soc. Rev. 2005, 34, 609–624. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Lu, G.P.; Cai, C. Facile aromatic nucleophilic substitution (SNAr) reactions in ionic liquids: An electrophile-nucleophile dual activation by [Omim]Br for the reaction. Green Chem. 2016, 18, 5580–5585. [Google Scholar] [CrossRef]
- Argüello, J.E.; Schmidt, L.C.; Peñéñory, A.B. One-Pot’ Two-Step Synthesis of Aryl Sulfur Compounds by Photoinduced Reactions of Thiourea Anion with Aryl Halides. Org. Lett. 2003, 5, 4133–4136. [Google Scholar] [CrossRef]
- Pant, P.L.; Shankarling, G.S. Deep Eutectic Solvent: An Efficient and Recyclable Catalyst for Synthesis of Thioethers. Chem. Select 2017, 2, 7645–7650. [Google Scholar] [CrossRef]
- Nugent, T.C. (Ed.) Chiral Amine Synthesis: Methods, Developments and Applications; Wiley-VCH: Weinheim, Germany, 2010; pp. 1–479. [Google Scholar]
- Singh, B.; Lobo, H.; Shankarling, G. Selective N-alkylation of aromatic primary amines catalysed by bio-catalyst of Deep Eutectic Solvent. Catal. Lett. 2011, 141, 178–182. [Google Scholar] [CrossRef]
- Carey, J.S.; Laffan, D.; Thomson, C.; Williams, M.T. Analysis of the reactions used for the preparation of drug candidate molecules. Org. Biomol. Chem. 2006, 4, 2337–2347. [Google Scholar] [CrossRef] [PubMed]
- Montalbetti, C.A.G.N.; Falque, V. Amide bond formation and peptide coupling. Tetrahedron 2005, 61, 10827–10852. [Google Scholar] [CrossRef]
- Kukushkin, V.Y.; Pombeiro, A.J.L. Metal-mediated and metal-catalyzed hydrolysis of nitriles. Inorg. Chim. Acta 2005, 358, 1–21. [Google Scholar] [CrossRef]
- Patil, U.B.; Singh, A.S.; Nagarkar, J.M. Choline chloride based eutectic solvent: An efficient and reusable solvent system for the synthesis of primary amides from aldehydes and from nitriles. RSC Adv. 2014, 4, 1102–1106. [Google Scholar] [CrossRef]
- Ghosh, A.K.; Brindisi, M. Organic Carbamates in Drug Design and Medicinal Chemistry. J. Med. Chem. 2015, 58, 2895–2940. [Google Scholar] [CrossRef] [PubMed]
- Carafa, M.; Iannone, F.; Mele, V.; Quaranta, E. Solventless selective phosgene-free N-carbonylation of N-heteroaromatics (pyrrole, indole, carbazole) under mild conditions. Green Chem. 2012, 14, 3377–3385. [Google Scholar] [CrossRef]
- Riemer, D.; Hirapara, P.; Das, S. Chemoselective synthesis of carbamates using CO2 as carbon source. ChemSusChem 2016, 9, 1916–1920. [Google Scholar] [CrossRef]
- Dindarloo Inaloo, I.; Majnooni, S. Ureas as safe carbonyl sources for the synthesis of carbamates with deep eutectic solvents (DESs) as efficient and recyclable solvent/catalyst systems. New J. Chem. 2018, 42, 13249–13255. [Google Scholar] [CrossRef]
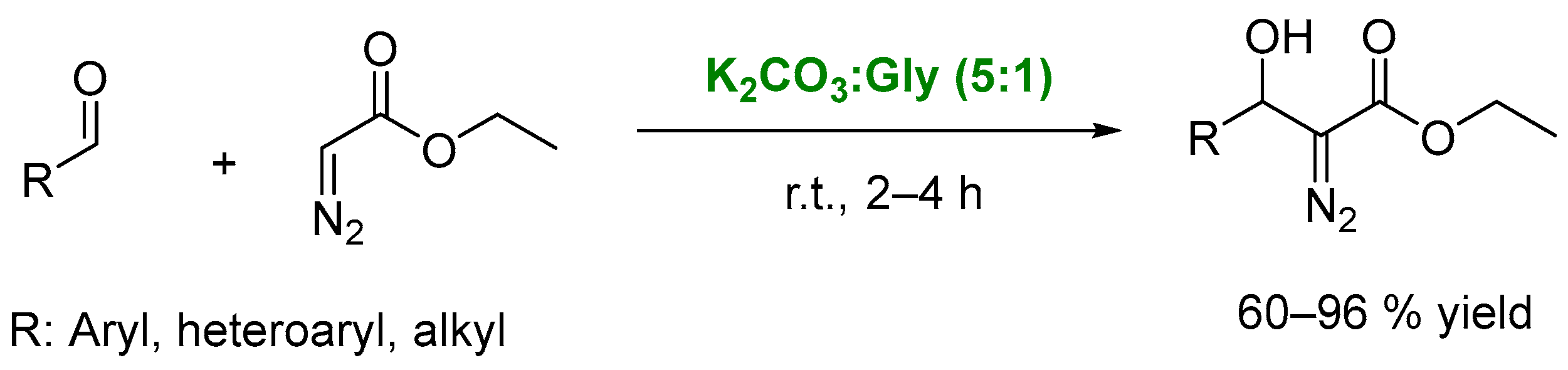



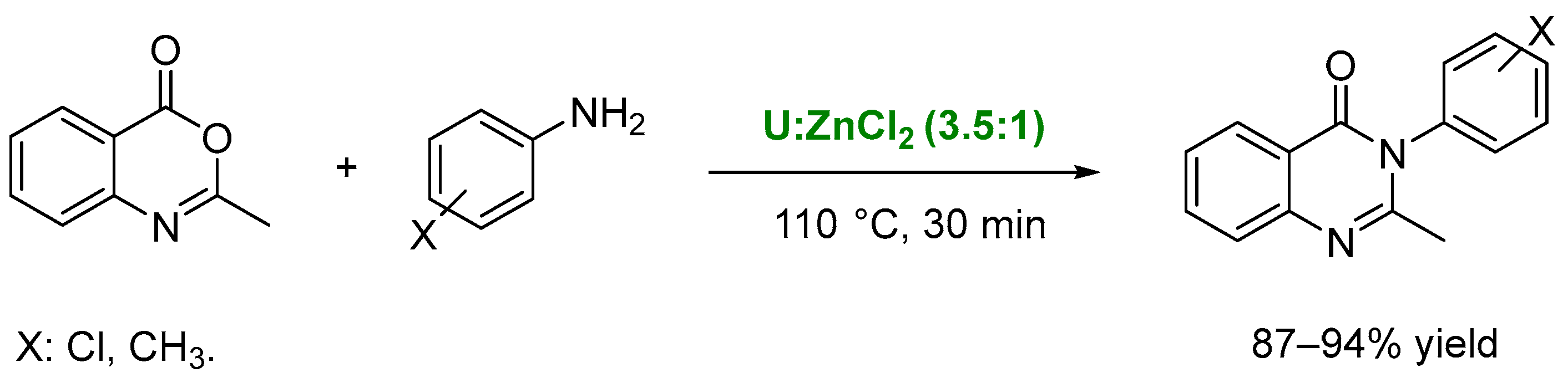
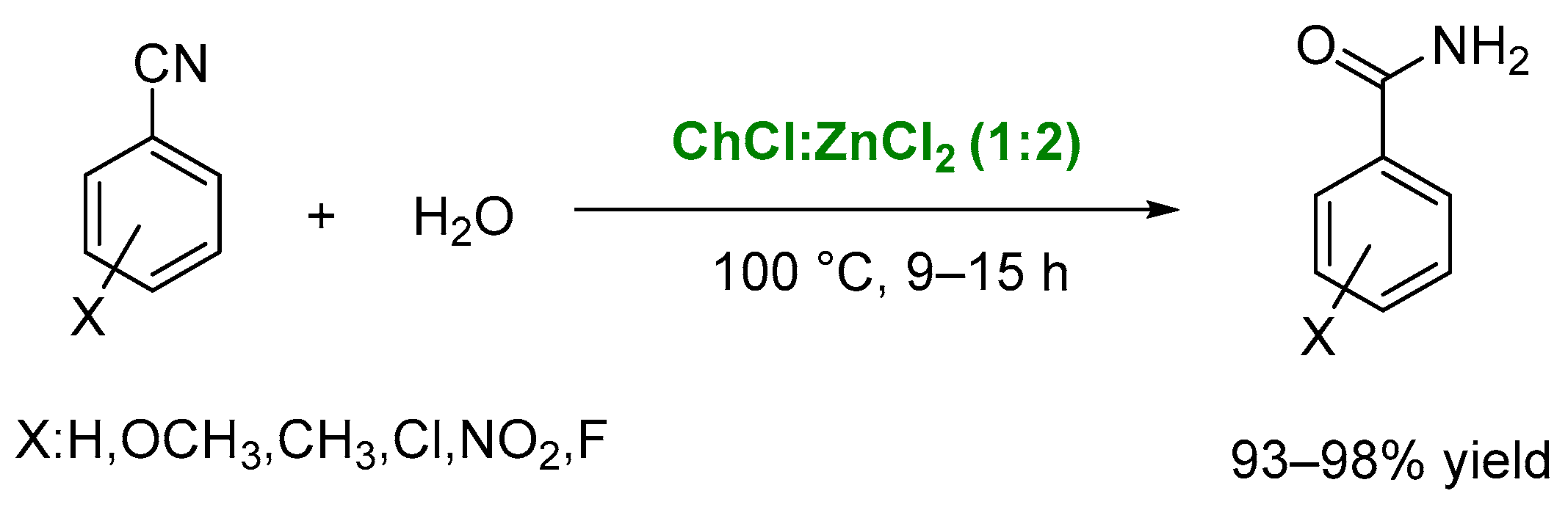
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Falcini, C.; Gonzalo, G.d. Deep Eutectic Solvents as Catalysts in the Synthesis of Active Pharmaceutical Ingredients and Precursors. Catalysts 2024, 14, 120. https://doi.org/10.3390/catal14020120
Falcini C, Gonzalo Gd. Deep Eutectic Solvents as Catalysts in the Synthesis of Active Pharmaceutical Ingredients and Precursors. Catalysts. 2024; 14(2):120. https://doi.org/10.3390/catal14020120
Chicago/Turabian StyleFalcini, Chiara, and Gonzalo de Gonzalo. 2024. "Deep Eutectic Solvents as Catalysts in the Synthesis of Active Pharmaceutical Ingredients and Precursors" Catalysts 14, no. 2: 120. https://doi.org/10.3390/catal14020120
APA StyleFalcini, C., & Gonzalo, G. d. (2024). Deep Eutectic Solvents as Catalysts in the Synthesis of Active Pharmaceutical Ingredients and Precursors. Catalysts, 14(2), 120. https://doi.org/10.3390/catal14020120






