Templated Synthesis of Cu2S Hollow Structures for Highly Active Ozone Decomposition
Abstract
:1. Introduction
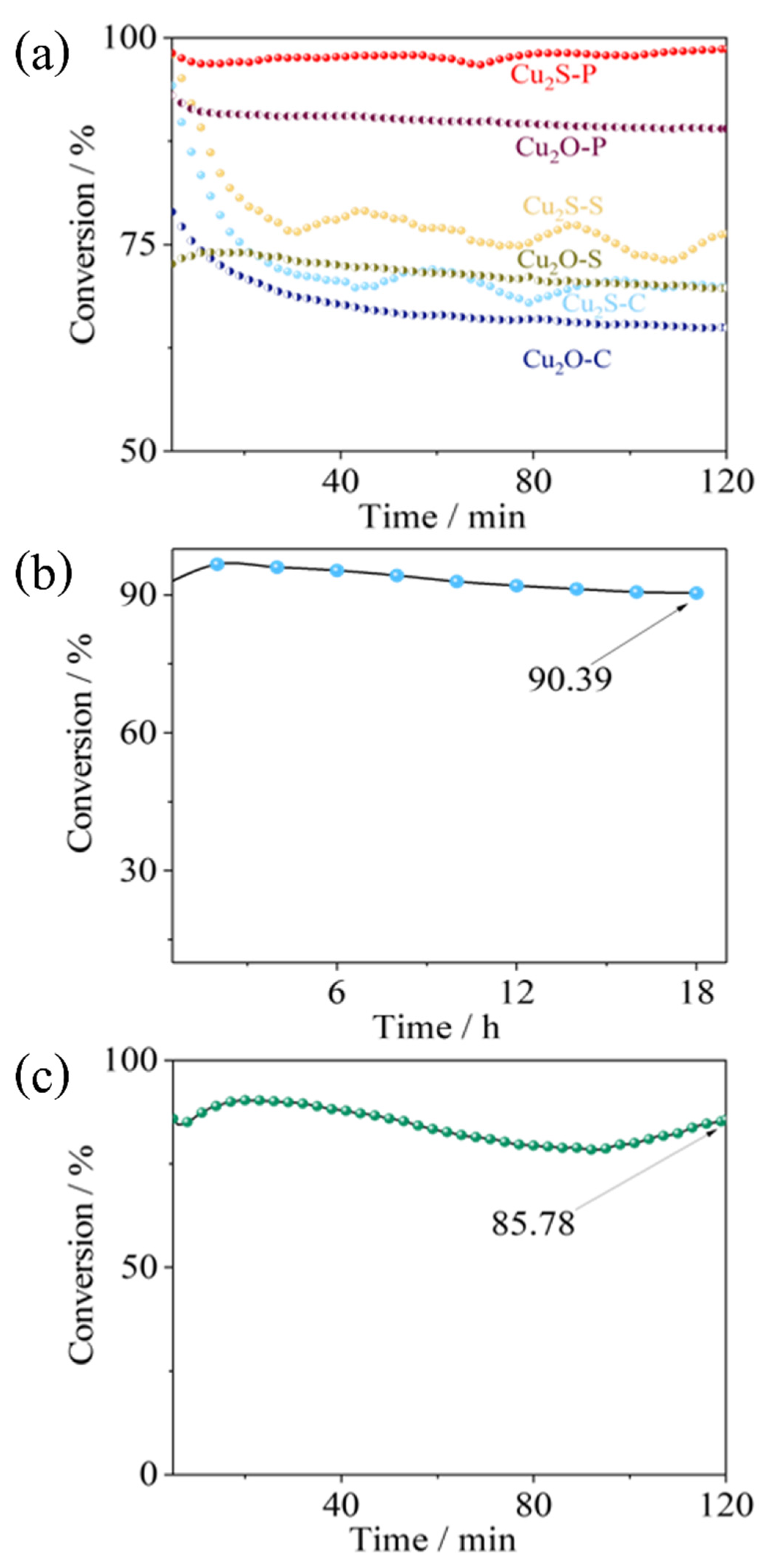
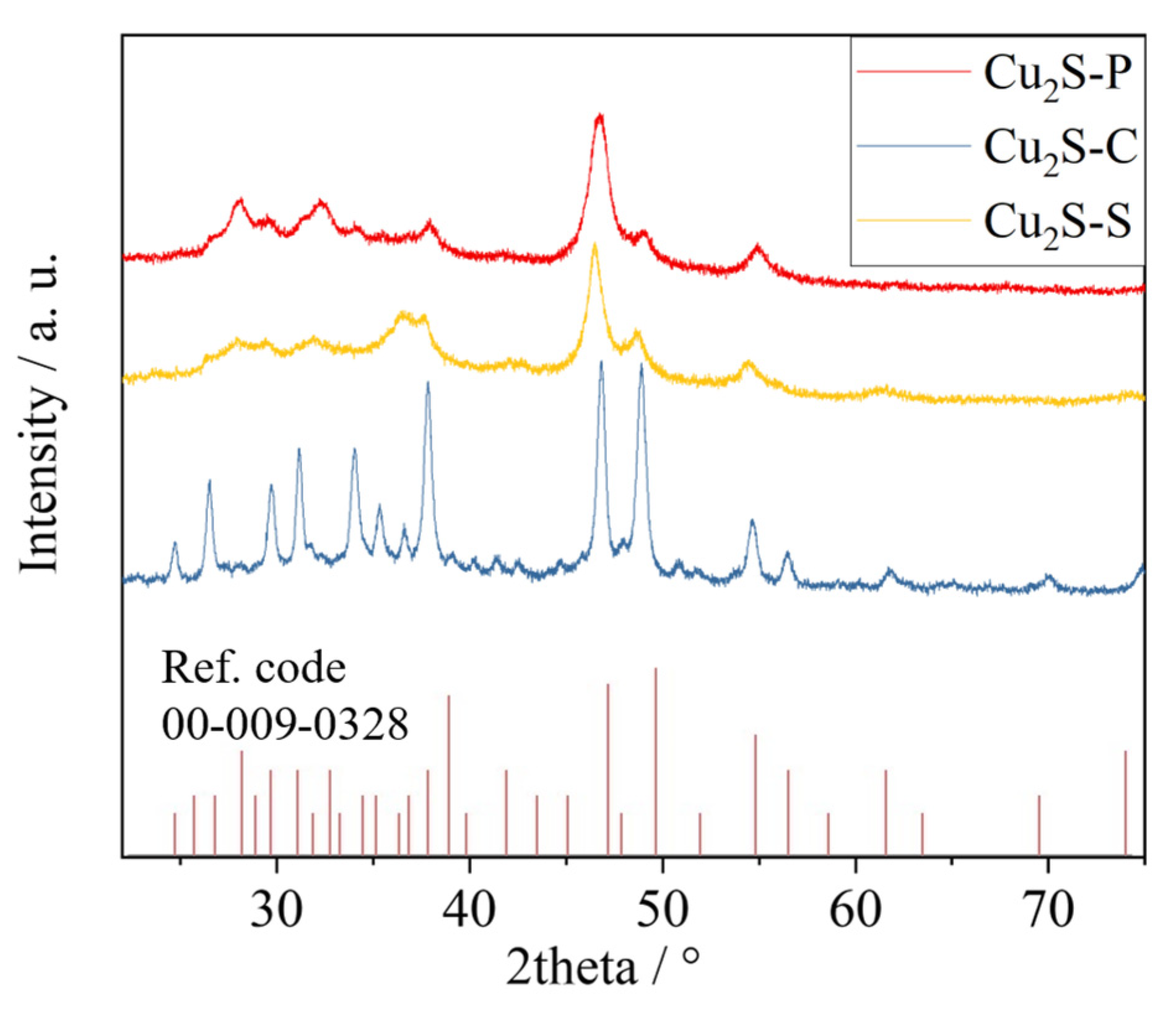
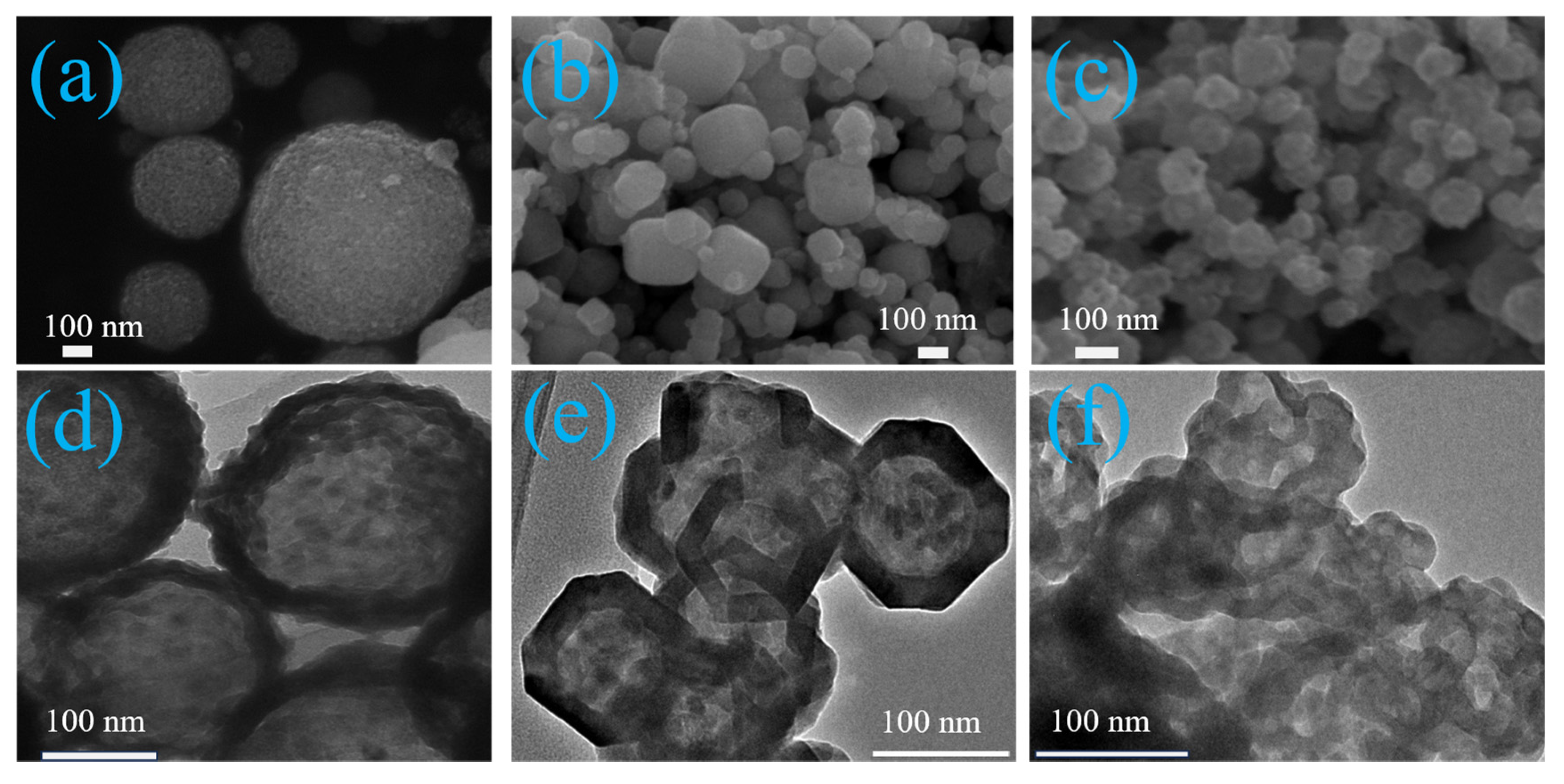
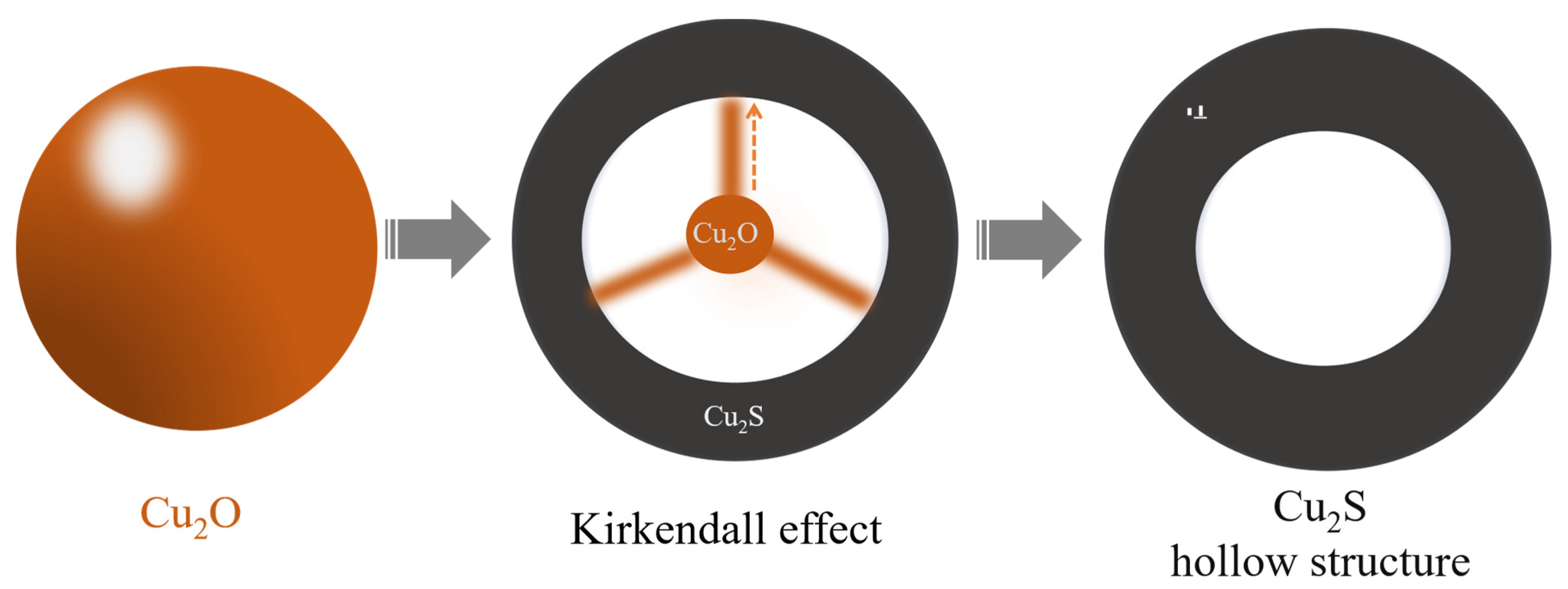
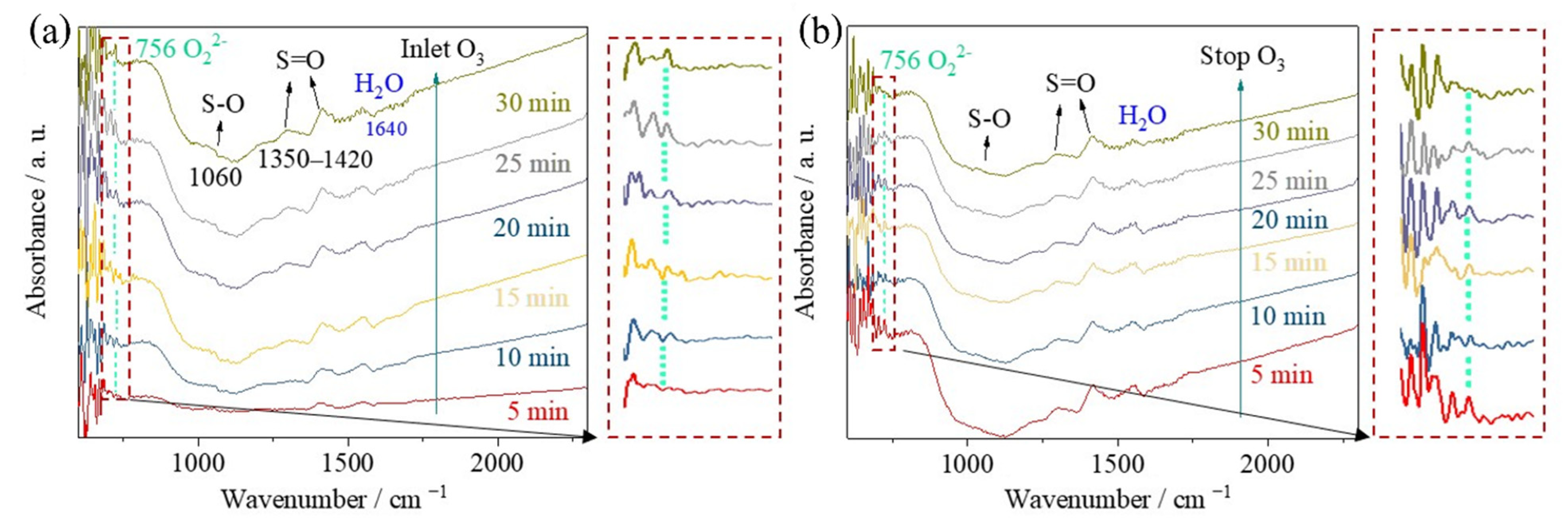
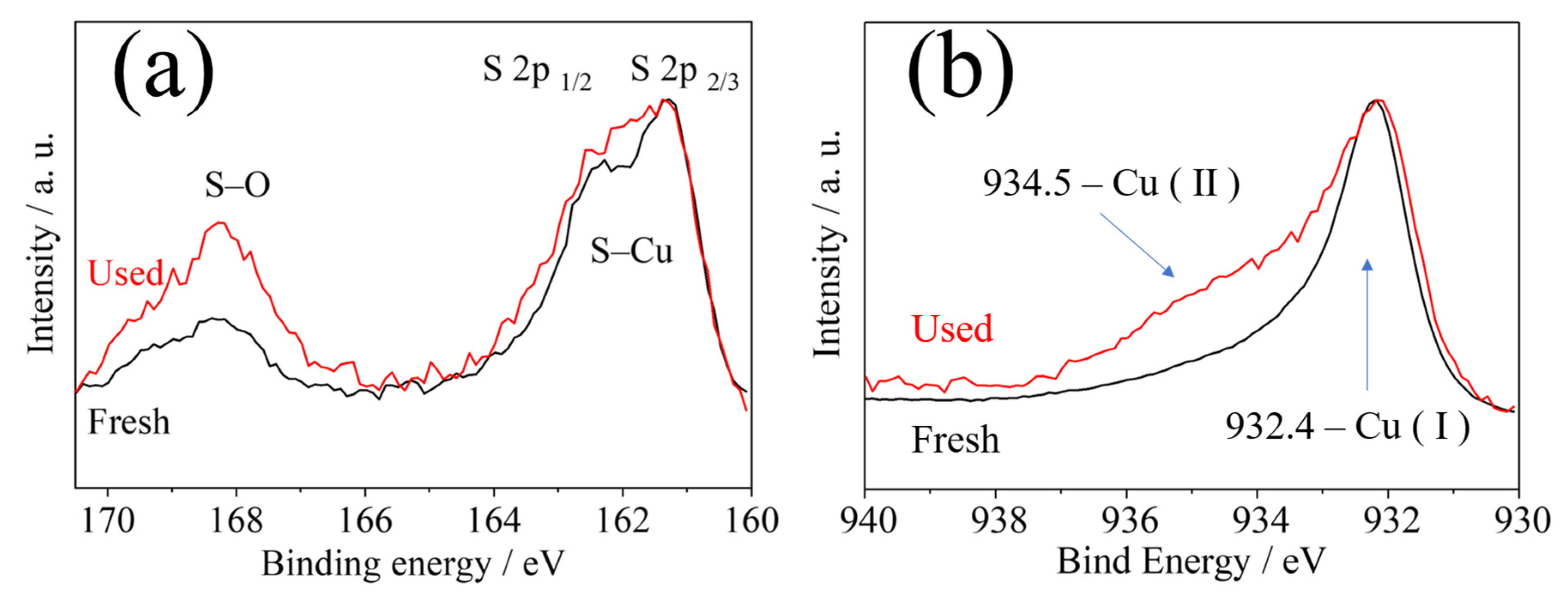
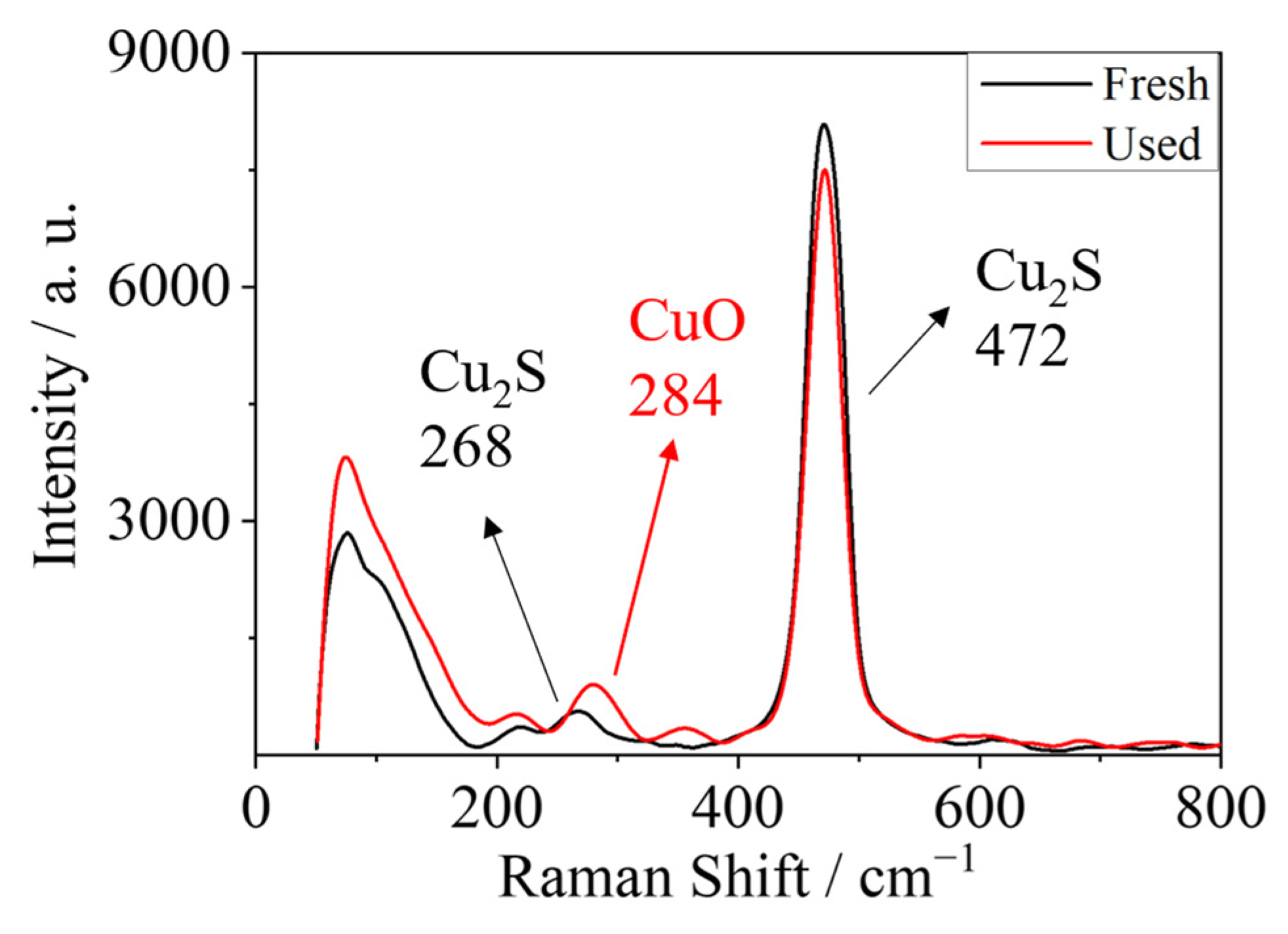
2. Results and Discussion
3. Experimental
3.1. Synthesis of Catalysts
3.2. Characterization of the Catalyst
3.3. Ozone Decomposition Test
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Brunekreef, B.; Holgate, S.T. Air pollution and health. Lancet 2002, 360, 1233–1242. [Google Scholar] [CrossRef]
- Taylan, O.; Alkabaa, A.S.; Alamoudi, M.; Basahel, A.; Balubaid, M.; Andejany, M.; Alidrisi, H. Air Quality Modeling for Sustainable Clean Environment Using ANFIS and Machine Learning Approaches. Atmosphere 2021, 12, 713. [Google Scholar] [CrossRef]
- Li, Y.; Yin, S.; Yu, S.; Bai, L.; Wang, X.; Lu, X.; Ma, S. Characteristics of ozone pollution and the sensitivity to precursors during early summer in central plain, China. J. Environ. Sci. 2021, 99, 354–368. [Google Scholar] [CrossRef] [PubMed]
- Touati, H.; Mehri, A.; Karouia, F.; Richard, F.; Batiot-Dupeyrat, C.; Daniele, S.; Clacens, J.-M. Low-Temperature O3 Decomposition over Pd-TiO2 Hybrid Catalysts. Catalysts 2022, 12, 448. [Google Scholar] [CrossRef]
- Hao, Z.; Cheng, D.; Yun, G.; Liang, Y. Supported gold catalysts used for ozone decomposition and simultaneous elimination of ozone and carbon monoxide at ambient temperature. Appl. Catal. B-Environ. 2001, 33, 217–222. [Google Scholar] [CrossRef]
- Gong, S.; Wu, X.; Zhang, J.; Han, N.; Chen, Y. Facile solution synthesis of Cu2O–CuO–Cu(OH)2 hierarchical nanostructures for effective catalytic ozone decomposition. Cryst. Eng. Comm. 2018, 20, 3096–3104. [Google Scholar] [CrossRef]
- Deng, H.; Kang, S.; Ma, J.; Wang, L.; Zhang, C.; He, H. Role of Structural Defects in MnOx Promoted by Ag Doping in the Catalytic Combustion of Volatile Organic Compounds and Ambient Decomposition of O3. Environ. Sci. Technol. 2019, 53, 10871–10879. [Google Scholar] [CrossRef]
- Wang, A.; Zhang, L.; Guan, J.; Wang, X.; Ma, G.; Fan, G.; Wang, H.; Han, N.; Chen, Y. Highly efficient ozone elimination by metal doped ultra-fine Cu2O nanoparticles. J. Environ. Sci. 2023, 134, 108–116. [Google Scholar] [CrossRef]
- Chu, B.; Ma, Q.; Liu, J.; Ma, J.; Zhang, P.; Chen, T.; Feng, Q.; Wang, C.; Yang, N.; Ma, H.; et al. Air Pollutant Correlations in China: Secondary Air Pollutant Responses to NOx and SO2 Control. Environ. Sci. Technol. Lett. 2020, 7, 695–700. [Google Scholar] [CrossRef]
- Mukta, A.; Hoque, M.; Sarker, E.; Hossain, N.; Biswas, K. Seasonal variations of gaseous air pollutants (SO2, NO2, O3, CO) and particulates (PM2.5, PM10) in Gazipur: An industrial city in Bangladesh. Adv. Environ. Technol. 2020, 6, 195–209. [Google Scholar] [CrossRef]
- Stevens, C.J.; Bell, J.N.B.; Brimblecombe, P.; Clark, C.M.; Dise, N.B.; Fowler, D.; Lovett, G.M.; Wolseley, P.A. The impact of air pollution on terrestrial managed and natural vegetation. Philos. T R Soc. A. 2020, 378, 20190317. [Google Scholar] [CrossRef]
- Ranjith, K.S.; Ranjith Kumar, D.; Huh, Y.S.; Han, Y.-K.; Uyar, T.; Rajendra Kumar, R.T. Promotional Effect of Cu2S–ZnS Nanograins as a Shell Layer on ZnO Nanorod Arrays for Boosting Visible Light Photocatalytic H2 Evolution. J. Phys. Chem. C. 2020, 124, 3610–3620. [Google Scholar] [CrossRef]
- Ansari, M.Z.; Faraz, M.; Munjal, S.; Kumar, V.; Khare, N. Highly dispersible and uniform size Cu2ZnSnS4 nanoparticles for photocatalytic application. Adv. Powder. Technol. 2017, 28, 2402–2409. [Google Scholar] [CrossRef]
- Wang, S.; Huang, Q.; Wen, X.; Li, X.-y.; Yang, S. Thermal oxidation of Cu2S nanowires: A template method for the fabrication of mesoscopic CuxO (x = 1,2) wires. Phys. Chem. Chem. Phys. 2002, 4, 3425–3429. [Google Scholar] [CrossRef]
- Zhu, S.; Wu, W.; Li, Z.; Luo, J. First-order transition in LK-99 containing Cu2S. Matter 2023, 6, 4401–4407. [Google Scholar] [CrossRef]
- Ahmed, H.S.; Mohammed, R.Y. The Effect of Deposition Parameters on Morphological and Optical Properties of Cu2S Thin Films Grown by Chemical Bath Deposition Technique. Photonics 2022, 9, 161. [Google Scholar] [CrossRef]
- Chen, T.-W.; Rajaji, U.; Chen, S.-M.; Govindasamy, M.; Paul Selvin, S.S.; Manavalan, S.; Arumugam, R. Sonochemical synthesis of graphene oxide sheets supported Cu2S nanodots for high sensitive electrochemical determination of caffeic acid in red wine and soft drinks. Compos. Part B Eng. 2019, 158, 419–427. [Google Scholar] [CrossRef]
- Honarnezhad, R.; Fathinia, M.; Khataee, A. Mechanical production and sonocatalytic application of Cu2S nanoparticles for degradation of isopropylxanthic acid: Kinetic modeling via white and black box methods. J. Mol. Liq. 2019, 287, 110899. [Google Scholar] [CrossRef]
- Kuo, H.; Chu, T.; Song, F.; Huang, H. Cu2O Nanocrystal-Templated Growth of Cu2S Nanocages with Encapsulated Au Nanoparticles and In-Situ Transmission X-ray Microscopy Study. Adv. Funct. Mater. 2011, 21, 792–797. [Google Scholar] [CrossRef]
- Ran, L.; Yin, L. Double-walled heterostructured Cu2−xSe/Cu7S4 nanoboxes with enhanced electrocatalytic activity for quantum dot sensitized solar cells. Cryst. Eng. Comm. 2017, 19, 5640–5652. [Google Scholar] [CrossRef]
- Yang, D.; Cao, L.; Huang, J.; Liu, Q.; Li, G.; He, D.; Wang, J.; Feng, L. Vanadium-doped hierarchical Cu2S nanowall arrays assembled by nanowires on copper foam as an efficient electrocatalyst for hydrogen evolution reaction. Scripta Mater. 2021, 196, 113756. [Google Scholar] [CrossRef]
- Yu, X.; Pan, L.; Son, K.; Mayer, T.; Zhang, D.; Hagfeldt, A.; Luo, J.; Grätzel, M. Solution-Processed Cu2S Photocathodes for Photoelectrochemical Water Splitting. ACS Energy Lett. 2018, 3, 760–766. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, Z. Tetrafunctional Cu2S thin layers on Cu2O nanowires for efficient photoelectrochemical water splitting. Nano Res. 2018, 11, 1530–1540. [Google Scholar] [CrossRef]
- Dhandapani, B.; Oyama, S.T. Gas phase ozone decomposition catalysts. Appl. Catal. B Environ. 1997, 11, 129–166. [Google Scholar] [CrossRef]
- Mousavi-Kamazani, M.; Zarghami, Z.; Salavati-Niasari, M. Facile and Novel Chemical Synthesis, Characterization, and Formation Mechanism of Copper Sulfide (Cu2S, Cu2S/CuS, CuS) Nanostructures for Increasing the Efficiency of Solar Cells. J. Phys. Chem. C 2016, 120, 2096–2108. [Google Scholar] [CrossRef]
- Mosali, V.S.S.; Puxty, G.; Horne, M.D.; Bond, A.M.; Zhang, J. Selective electrochemical methanation of carbon dioxide using a sulphide derived CuZn catalyst. Electrochim. Acta 2024, 475, 143628. [Google Scholar] [CrossRef]
- Mosali, V.S.S.; Zhang, X.; Liang, Y.; Li, L.; Puxty, G.; Horne, M.D.; Brajter-Toth, A.; Bond, A.M.; Zhang, J. CdS-Enhanced Ethanol Selectivity in Electrocatalytic CO2 Reduction at Sulfide-Derived Cu−Cd. ChemSusChem 2021, 14, 2924–2934. [Google Scholar] [CrossRef] [PubMed]
- Van Thang, B.; Tung, H.T.; Phuc, D.H.; Nguyen, T.P.; Van Man, T.; Vinh, L.Q. High-efficiency quantum dot sensitized solar cells based on flexible rGO-Cu2S electrodes compared with PbS, CuS, Cu2S CEs. Sol. Energy Mater. Sol. Cells 2023, 250, 112042. [Google Scholar] [CrossRef]
- Ma, R.; Stegemeier, J.; Levard, C.; Dale, J.G.; Noack, C.W.; Yang, T.; Brown, G.E.; Lowry, G.V. Sulfidation of copper oxide nanoparticles and properties of resulting copper sulfide. Environ. Sci. Nano 2014, 1, 347–357. [Google Scholar] [CrossRef]
- Minguez-Bacho, I.; Courté, M.; Fan, H.J.; Fichou, D. Conformal Cu2S-coated Cu2O nanostructures grown by ion exchange reaction and their photoelectrochemical properties. Nanotechnology 2015, 26, 185401. [Google Scholar] [CrossRef]
- Gusak, A.M.; Tu, K.N. Interaction between the Kirkendall effect and the inverse Kirkendall effect in nanoscale particles. Acta Mater. 2009, 57, 3367–3373. [Google Scholar] [CrossRef]
- Rice, K.P.; Paterson, A.S.; Stoykovich, M.P. Nanoscale Kirkendall Effect and Oxidation Kinetics in Copper Nanocrystals Characterized by Real-Time, In Situ Optical Spectroscopy. Part. Part. Syst. Char. 2015, 32, 373–380. [Google Scholar] [CrossRef]
- Gong, S.; Wang, A.; Zhang, J.; Guan, J.; Han, N.; Chen, Y. Gram-scale synthesis of ultra-fine Cu2O for highly efficient ozone decomposition. RSC Adv. 2020, 10, 5212–5219. [Google Scholar] [CrossRef] [PubMed]
- Gong, S.Y.; Chen, J.Y.; Wu, X.F.; Han, N.; Chen, Y.F. In-situ synthesis of Cu2O/reduced graphene oxide composite as effective catalyst for ozone decomposition. Catal. Commun. 2018, 106, 25–29. [Google Scholar] [CrossRef]
- Zhang, H.; Deng, L.; Chen, J. How MoS2 assisted sulfur vacancies featured Cu2S in hollow Cu2S@MoS2 nanoboxes to activate H2O2 for efficient sulfadiazine degradation? Chem. Eng. J. 2022, 9, 10370. [Google Scholar] [CrossRef]
- Zhang, H.; Zeng, C.; Wu, H.; Yang, H.; Deng, L.; Zhou, L. ZIF-8 assisted synthesis of magnetic core-shell Fe3O4@CuS nanoparticles for efficient sulfadiazine degradation via H2O2 activation: Performance and mechanism. J. Colloid Interface Sci. 2021, 594, 502–512. [Google Scholar] [CrossRef]
- Zhang, H.; Zhou, C.; Zeng, H.; Deng, L.; Shi, Z. Can Cu2ZnSnS4 nanoparticles be used as heterogeneous catalysts for sulfadiazine degradation? J. Hazard. Mater. 2020, 395, 122613. [Google Scholar] [CrossRef]
- Wójcik, S.; Grzybek, G.; Stelmachowski, P.; Sojka, Z.; Kotarba, A. Bulk, Surface and Interface Promotion of Co3O4 for the Low-Temperature N2O Decomposition Catalysis. Catalysts 2020, 10, 41. [Google Scholar] [CrossRef]
- Cho, H.; Joo, H.; Kim, H.; Kim, J.-E.; Kang, K.-S.; Jung, H.; Yoon, J. Enhanced Photoelectrochemical Activity of TiO2 Nanotubes Decorated with Lanthanide Ions for Hydrogen Production. Catalysts 2022, 12, 866. [Google Scholar] [CrossRef]
- Zhang, L.; Huo, F.; Wang, A.; Chai, S.; Guan, J.; Fan, G.; Yang, W.; Ma, G.; Han, N.; Chen, Y. Coordination-Controlled Catalytic Activity of Cobalt Oxides for Ozone Decomposition. Inorg. Chem. 2023, 62, 9178–9189. [Google Scholar] [CrossRef]
- Li, W.; Gibbs, G.V.; Oyama, S.T. Mechanism of Ozone Decomposition on a Manganese Oxide Catalyst. 1. In Situ Raman Spectroscopy and Ab Initio Molecular Orbital Calculations. J. Am. Chem. Soc. 1998, 120, 9041–9046. [Google Scholar] [CrossRef]
- Li, X.; Ma, J.; Zhang, C.; Zhang, R.; He, H. Detrimental role of residual surface acid ions on ozone decomposition over Ce-modified γ-MnO2 under humid conditions. J. Environ. Sci. 2020, 91, 43–53. [Google Scholar] [CrossRef] [PubMed]
- Pietrogiacomi, D.; Sannino, D.; Magliano, A.; Ciambelli, P.; Tuti, S.; Indovina, V. The catalytic activity of CuSO4/ZrO2 for the selective catalytic reduction of NOx with NH3 in the presence of excess O2. Appl. Catal. B Environ. 2002, 36, 217–230. [Google Scholar] [CrossRef]
- Yang, S.; Chen, S.; Mosconi, E.; Fang, Y.; Xiao, X.; Wang, C.; Zhou, Y.; Yu, Z.; Zhao, J.; Gao, Y.; et al. Stabilizing halide perovskite surfaces for solar cell operation with wide-bandgap lead oxysalts. Science 2019, 365, 473–478. [Google Scholar] [CrossRef] [PubMed]
- Wahlqvist, M.; Shchukarev, A. XPS spectra and electronic structure of Group IA sulfates. J. Electron. Spectrosc. 2007, 156–158, 310–314. [Google Scholar] [CrossRef]
- Smirnov, M.Y.; Kalinkin, A.V.; Pashis, A.V.; Sorokin, A.M.; Noskov, A.S.; Kharas, K.C.; Bukhtiyarov, V.I. Interaction of Al2O3 and CeO2 Surfaces with SO2 and SO2 + O2 Studied by X-ray Photoelectron Spectroscopy. J. Phys. Chem. B. 2005, 109, 11712–11719. [Google Scholar] [CrossRef]
- Ma, G.; Tang, W.; Wang, A.; Zhang, L.; Guan, J.; Han, N.; Chen, Y. Heterojunctioned CuO/Cu2O catalyst for highly efficient ozone removal. J. Environ. Sci. 2023, 125, 340–348. [Google Scholar] [CrossRef]
- Huang, H.; Li, F.; Wang, H.; Zheng, X. The size controlled synthesis of Cu2S/P25 hetero junction solar-energy-materials and their applications in photocatalytic degradation of dyes. RSC Adv. 2017, 7, 50056–50063. [Google Scholar] [CrossRef]
- Fu, S.-Y.; Chang, H.-H.; Hsu, Y.-K.; Lin, Y.-G. Facile synthesis of Cu2S nanoarchitectures in application of surface enhanced Raman scattering. In Nanophotonic Materials XI; SPIE: Bellingham, WA, USA, 2014; pp. 25–28. [Google Scholar]
- Mai, H.E.; Fang, P.; Xie, G.Q.; Xie, Y.L.; Luo, M.F. Characterization of CuO Species in CuO/CeO2-Al2O3 Catalysts by In-situ XRD, Raman Spectroscopy and TPR. Acta Phys. Chim. Sin. 2005, 21, 997–1000. [Google Scholar] [CrossRef]
- Liu, J.; Gao, Z.; Han, H.; Wu, D.; Xu, F.; Wang, H.; Jiang, K. Mesoporous Cu2O submicro-spheres, facile synthesis and the selective adsorption properties. Chem. Eng. J. 2012, 185–186, 151–159. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, D.; Zhang, X.; Qu, F. Template-Free Synthesis of Porous Cu2O Nanospheres at Room Temperature and Investigation on Their Adsorption Property. J. Nanomater. 2013, 2013, 378919. [Google Scholar] [CrossRef]


| Sample | BET Surface Area (m2/g) | Pore Size (Å) | Pore Volume (cm3/g) |
|---|---|---|---|
| Cu2S-C | 11.48 | 71.31 | 0.02047 |
| Cu2S-S | 7.31 | 111.75 | 0.02043 |
| Cu2S-P | 14.67 | 59.25 | 0.02174 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, Y.; Xu, Y.; Zhang, Q.; Zhao, X.; Xiao, F.; Wang, X.; Ma, G. Templated Synthesis of Cu2S Hollow Structures for Highly Active Ozone Decomposition. Catalysts 2024, 14, 153. https://doi.org/10.3390/catal14020153
Jiang Y, Xu Y, Zhang Q, Zhao X, Xiao F, Wang X, Ma G. Templated Synthesis of Cu2S Hollow Structures for Highly Active Ozone Decomposition. Catalysts. 2024; 14(2):153. https://doi.org/10.3390/catal14020153
Chicago/Turabian StyleJiang, Yishan, Ying Xu, Qichao Zhang, Xin Zhao, Feng Xiao, Xinbo Wang, and Guojun Ma. 2024. "Templated Synthesis of Cu2S Hollow Structures for Highly Active Ozone Decomposition" Catalysts 14, no. 2: 153. https://doi.org/10.3390/catal14020153
APA StyleJiang, Y., Xu, Y., Zhang, Q., Zhao, X., Xiao, F., Wang, X., & Ma, G. (2024). Templated Synthesis of Cu2S Hollow Structures for Highly Active Ozone Decomposition. Catalysts, 14(2), 153. https://doi.org/10.3390/catal14020153