Boosting Solvent-Free Aerobic Oxidation of Benzylic Compounds into Ketones over Au-Pd Nanoparticles Supported by Porous Carbon
Abstract
:1. Introduction
2. Results
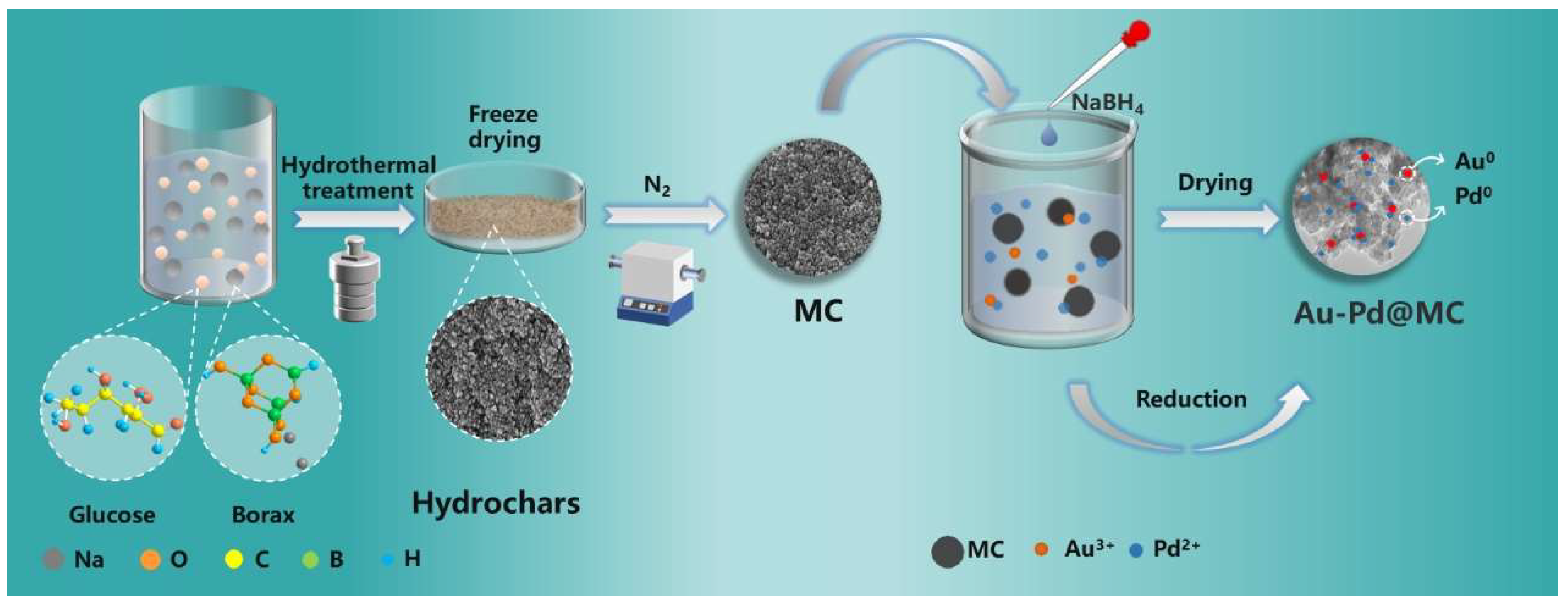
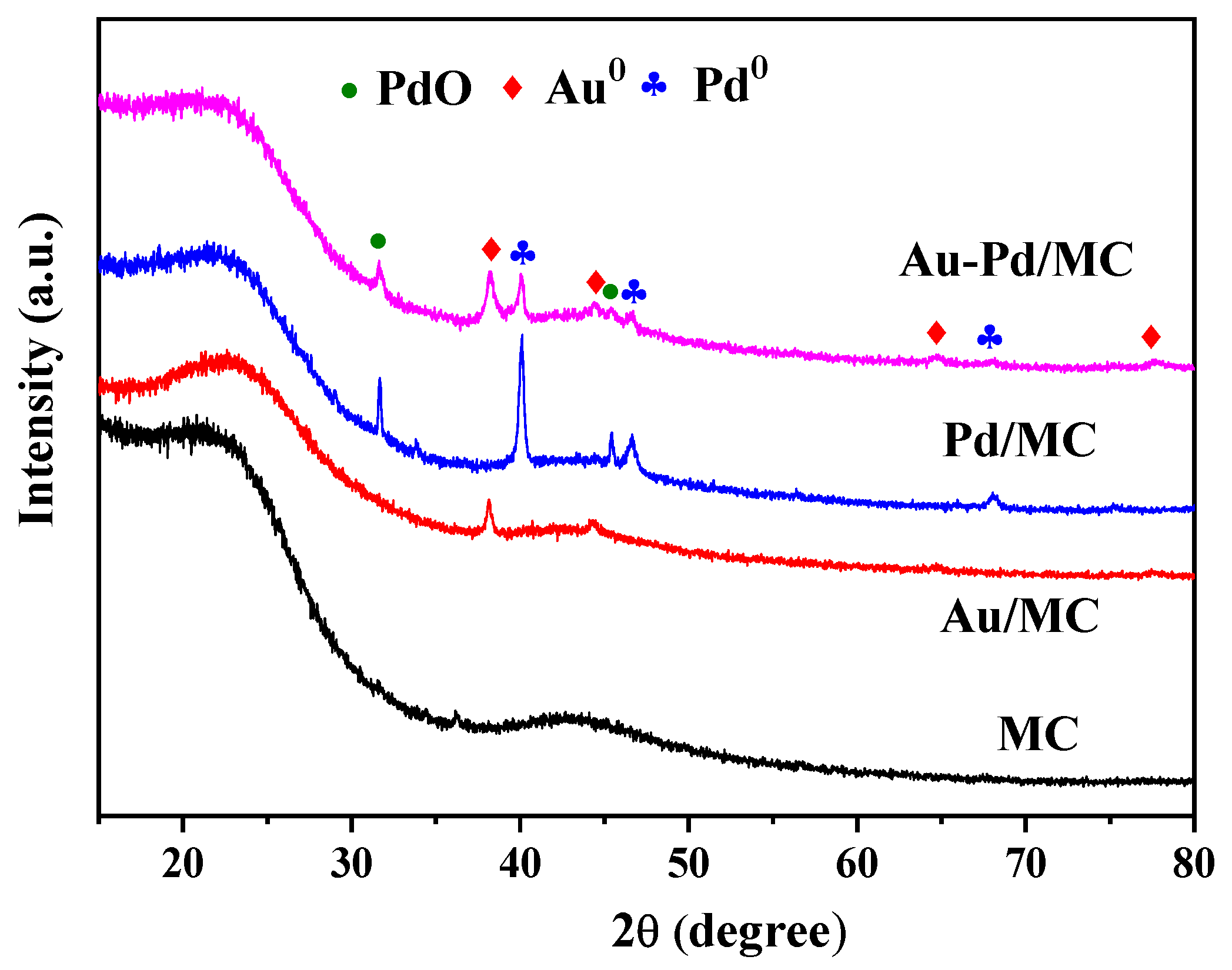
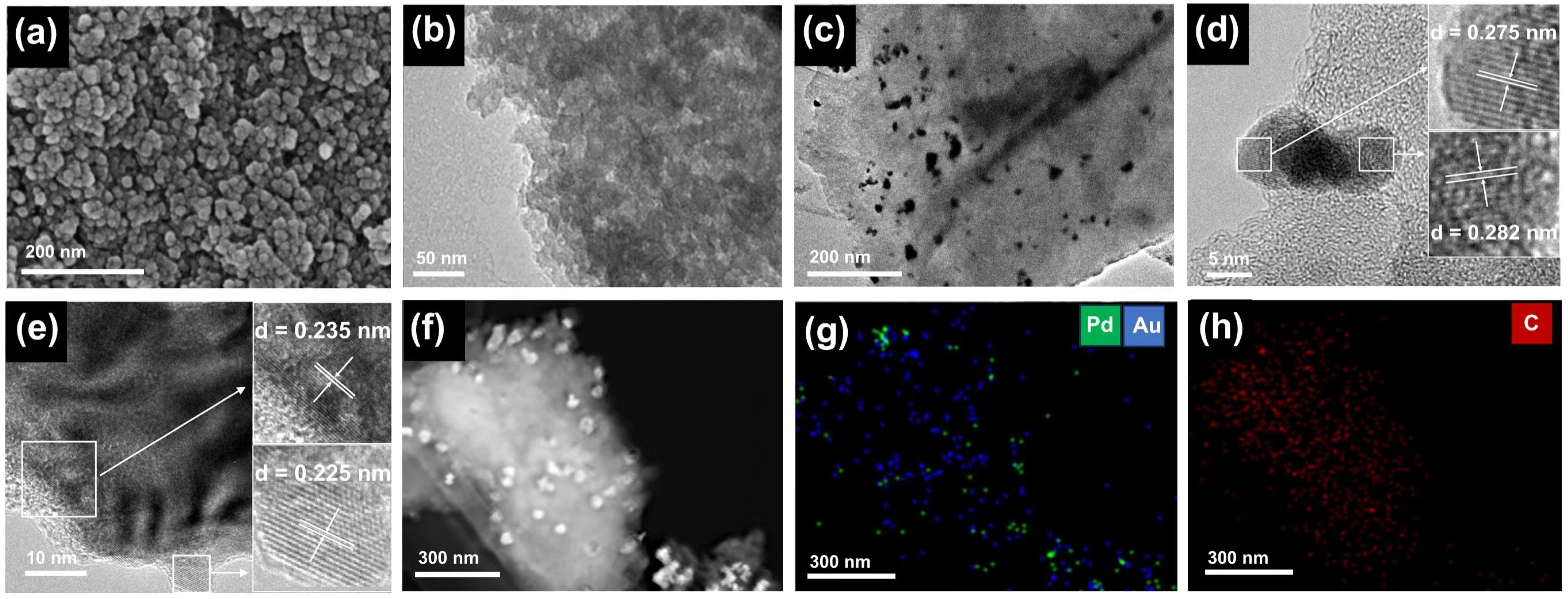
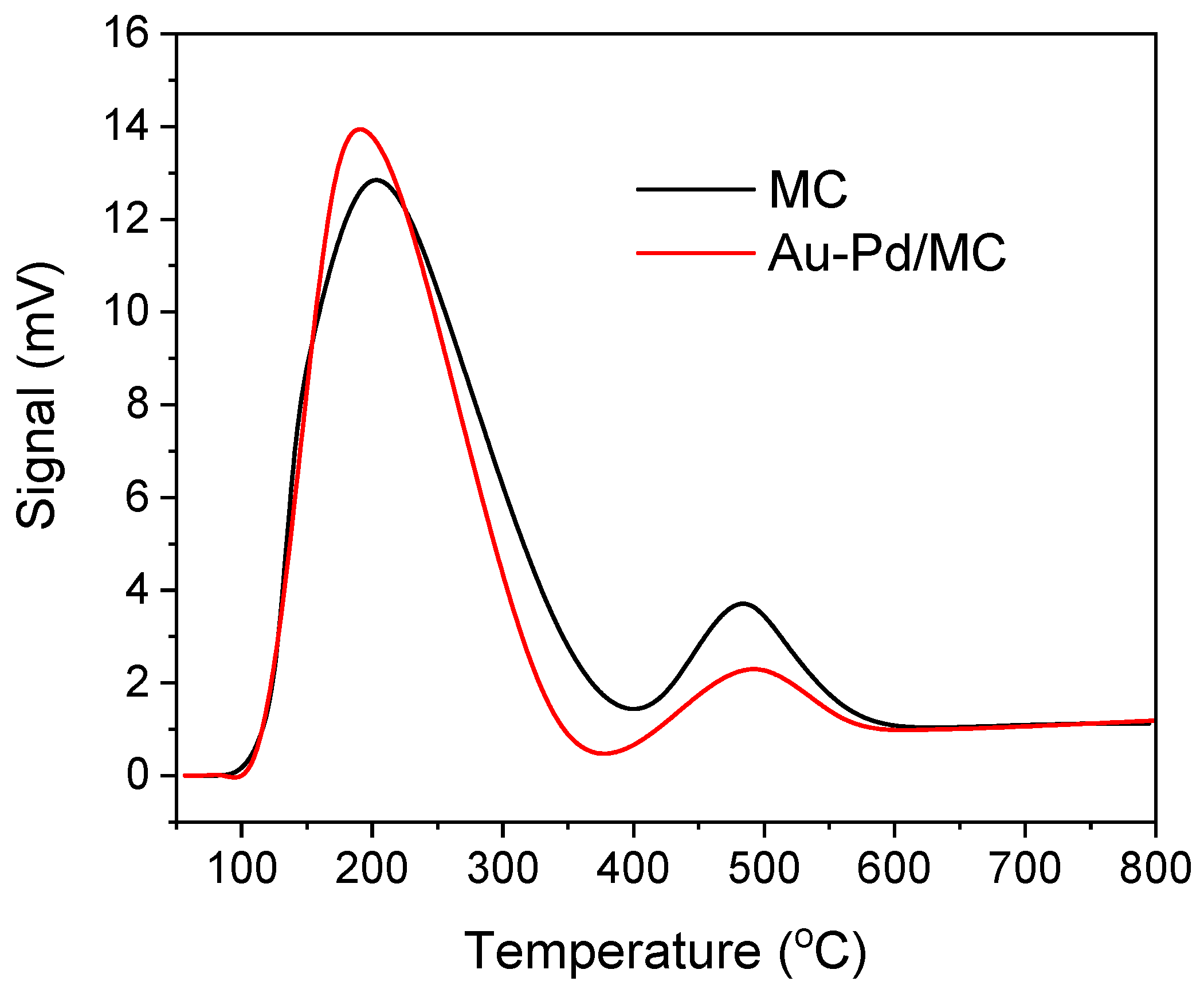
2.1. Characterization of the Catalyst
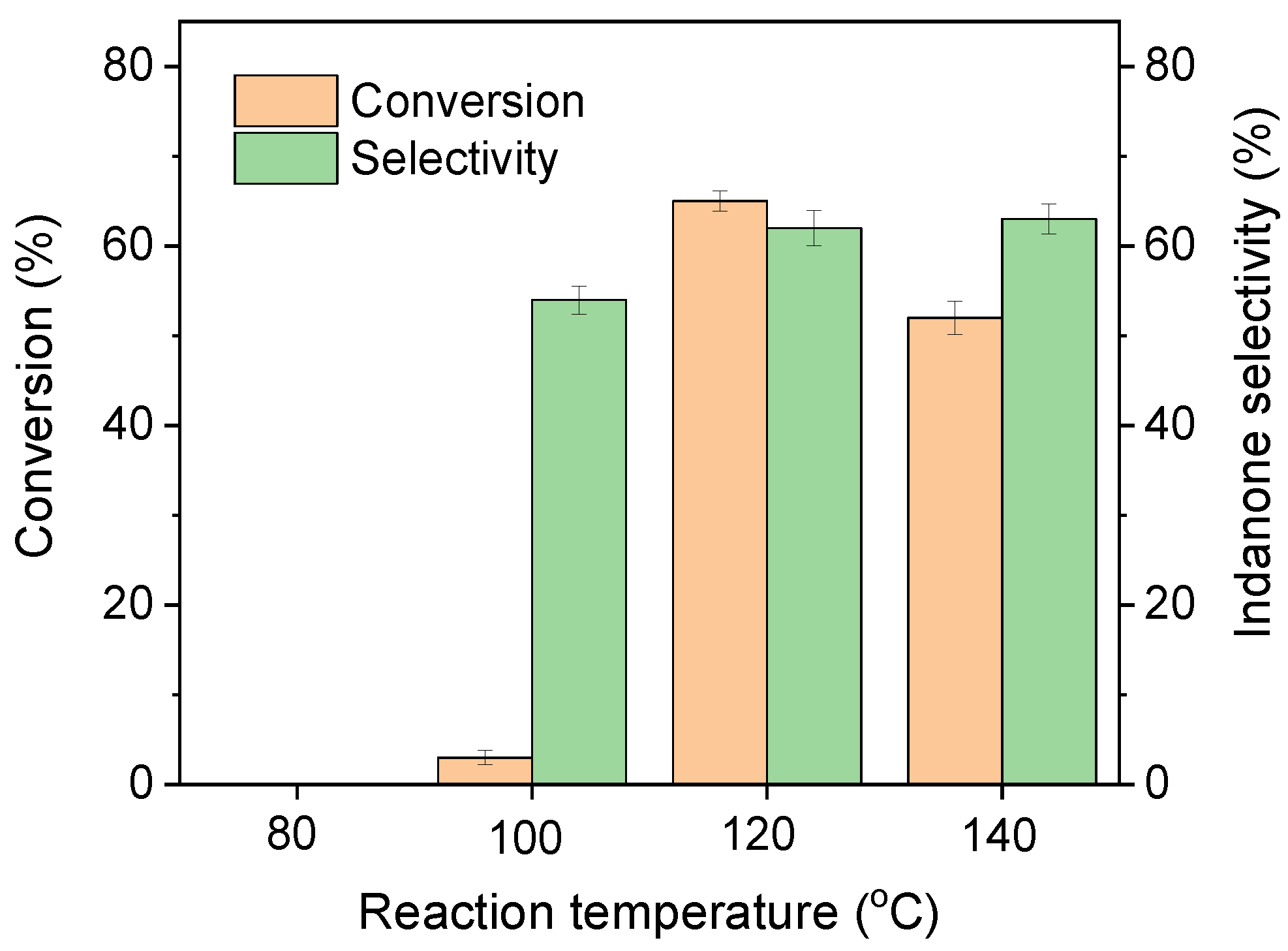
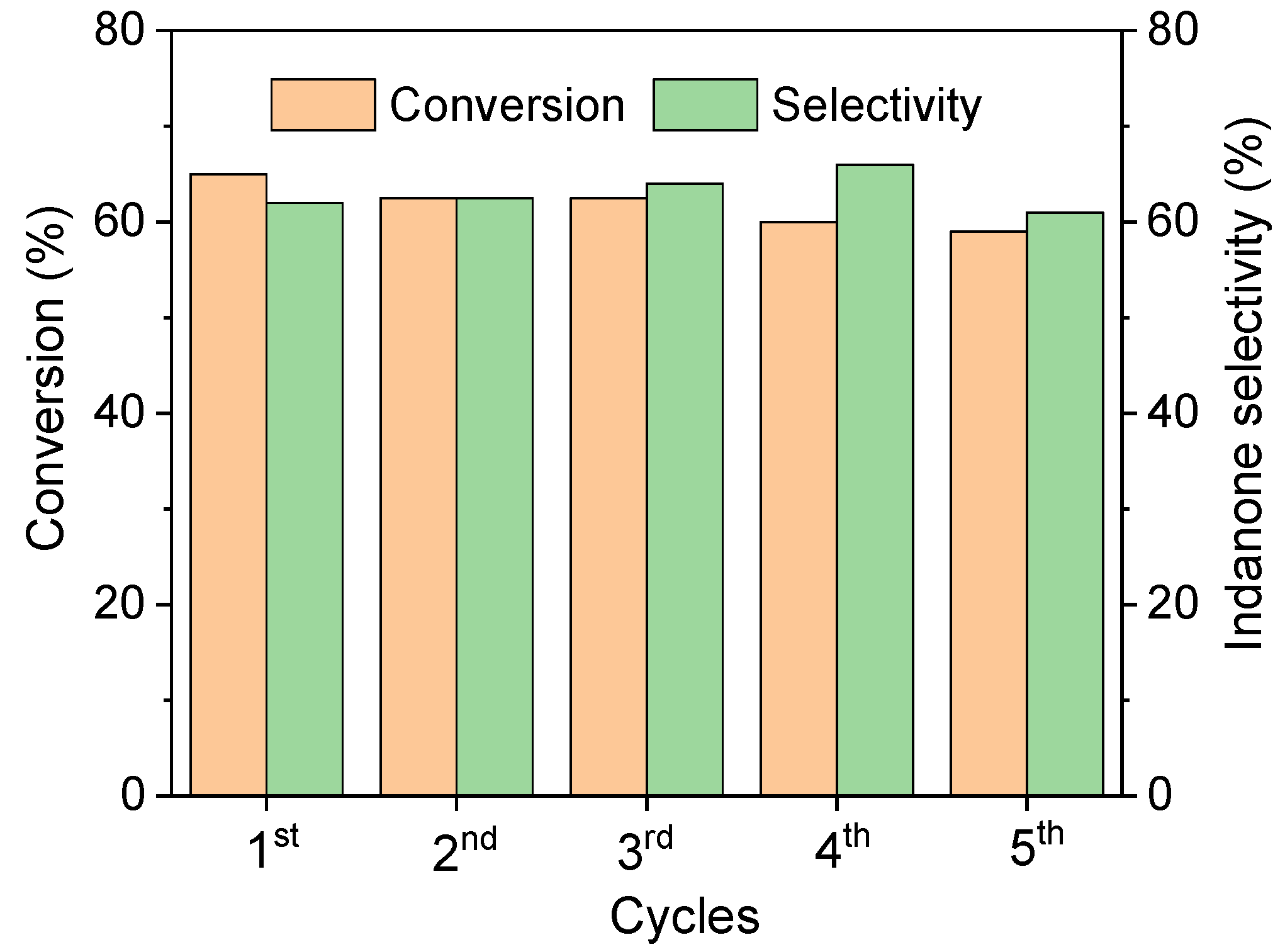
2.2. Catalytic Performance of the Catalyst
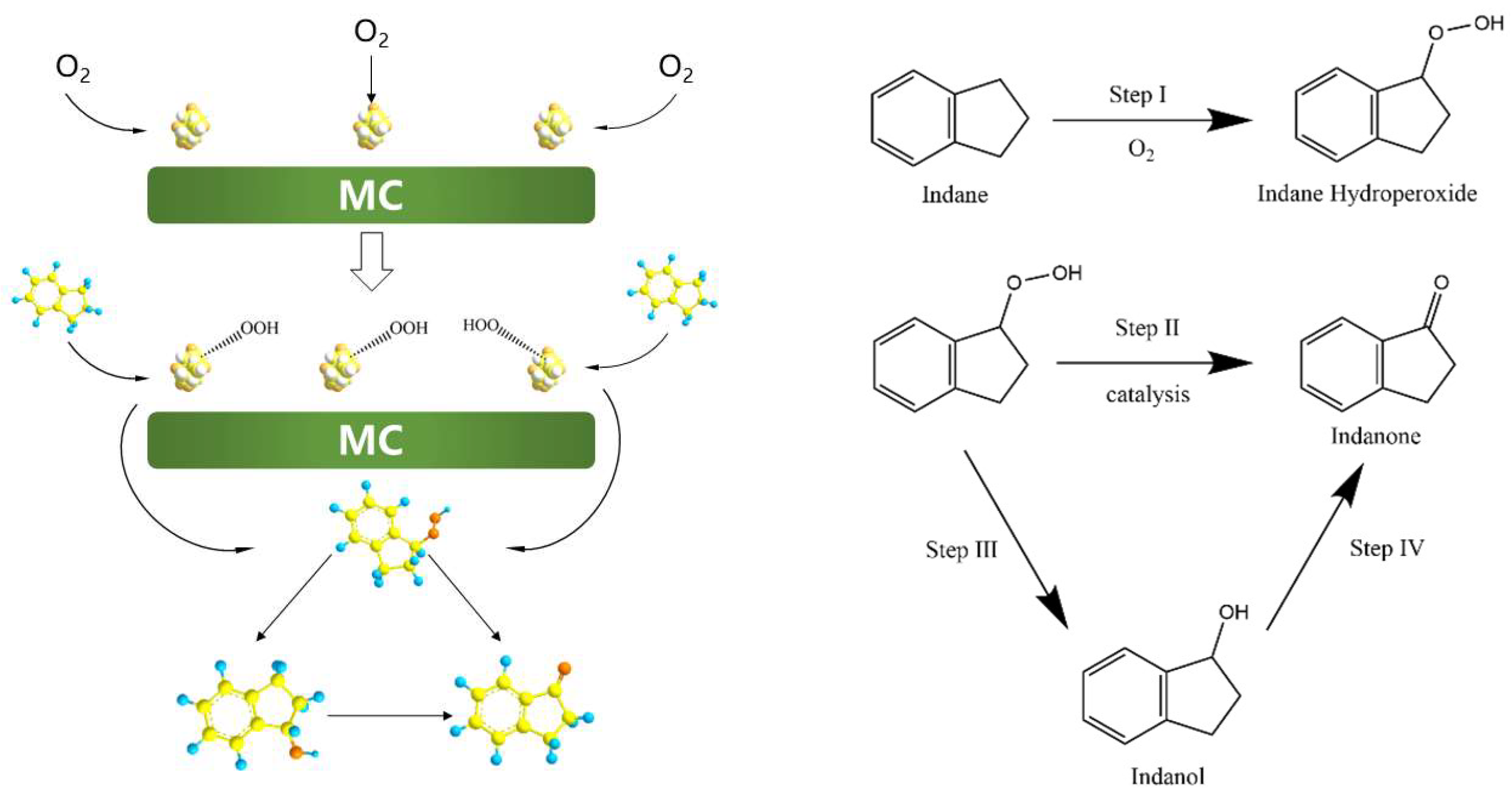
2.3. Reaction Pathway
2.4. Solvent-Free Oxidation of Other Benzylic Compounds
3. Experimental Section
3.1. Chemicals
3.2. Preparation of Porous Carbon (MC)
3.3. Preparation of Metal-Supported MC Catalysts
3.4. Characterization
3.5. Catalytic Experiments
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zhang, Q.; Chen, C.; Ma, H.; Miao, H.; Zhang, W.; Sun, Z.; Xu, J. Efficient Metal-free Aerobic Oxidation of Aromatic Hydrocarbons Utilizing Aryl-tetrahalogenated N-hydroxyphthalimides and 1,4-Diamino-2,3-dichloroanthraquinone. J. Chem. Technol. Biot. 2008, 83, 1364–1369. [Google Scholar] [CrossRef]
- Shen, H.M.; Hu, M.Y.; Liu, L.; Qi, B.; Ye, H.L.; She, Y.B. Efficient and Selective Oxidation of Tertiary Benzylic C–H Bonds with O2 Catalyzed by Metalloporphyrins under Mild and Solvent-free Conditions. Appl. Catal. A General 2020, 599, 117599. [Google Scholar] [CrossRef]
- Shi, H.; Cheng, L.; Pan, Y.; Mak, C.K.; Lau, K.C.; Lau, T.C. Synergistic Effects of CH3CO2H and Ca2+ on C−H bond activation by MnO4−. Chem. Sci. 2022, 13, 11600–11606. [Google Scholar] [CrossRef] [PubMed]
- Trammell, R.; D’Amore, L.; Cordova, A.; Polunin, P.; Xie, N.; Siegler, M.A.; Belanzoni, P.; Swart, M.; Garcia-Bosch, I. Directed Hydroxylation of sp2 and sp3 C–H Bonds Using Stoichiometric Amounts of Cu and H2O2. Inorg. Chem. 2019, 58, 7584–7592. [Google Scholar] [CrossRef]
- Urgoitia, G.; SanMartin, R.; Herrero, M.T.; Esther Domínguez, E. Recent Advances in Homogeneous Metal-Catalyzed Aerobic C–H Oxidation of Benzylic Compounds. Catalysts 2018, 8, 640. [Google Scholar] [CrossRef]
- Boller, T.M.; Murphy, J.M.; Hapke, M.; Ishiyama, T.; Miyaura, N.; Hartwig, J.F. Mechanism of the Mild Functionalization of Arenes by Diboron Reagents Catalyzed by Iridium Complexes. Intermediacy and Chemistry of Bipyridine-Ligated Iridium Trisboryl Complexes. J. Am. Chem. Soc. 2005, 127, 14263–14278. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Chen, X.F.; Xu, G.Q.; Guan, J.; Cao, Q.; Dong, B.; Qi, Y.F.; Li, C.H.; Mu, X.D. Iridium Nanoparticles Supported on Hierarchical Porous N-doped Carbon: An Efficient Water-tolerant Catalyst for Bio-alcohol Condensation in Water. Sci. Rep. 2016, 6, 21365. [Google Scholar] [CrossRef]
- Ma, X.M.; Chen, F.F.; Zhang, X.; Wang, T.T.; Yuan, S.R.; Wang, X.S.; Li, T.J.; Gao, J.K. Hierarchical Co@C-N Synthesized by the Confined Pyrolysis of Ionic Liquid@ metal-organic Frameworks for the Aerobic Oxidation of Alcohols. New J. Chem. 2022, 46, 7528–7536. [Google Scholar] [CrossRef]
- Lounisa, Z.; Riahi, A.; Djafri, F.; Muzart, J. Chromium-exchanged Zeolite (CrE-ZSM-5) as Catalyst for Alcohol Oxidation and Benzylic Oxidation with t-BuOOH. Appl. Catal. A Gen. 2006, 309, 270–272. [Google Scholar] [CrossRef]
- Pandey, A.M.; Agalave, S.G.; Vinod, C.P.; Gnanaprakasam, B.G. MnO2@Fe3O4 Magnetic Nanoparticles as Efficient and Recyclable Heterogeneous Catalyst for Benzylic sp3 C−H Oxidation. Chem. Asian J. 2019, 14, 3414–3423. [Google Scholar] [CrossRef]
- Wu, H.; Song, J.; Xie, C.; Hu, Y.; Liu, S.; Han, B. Preparation of Copper Phosphate from Naturally Occurring Phytic Acid as an Advanced Catalyst for Oxidation of Aromatic Benzyl Compounds. ACS Sustain. Chem. Eng. 2018, 6, 13670–13675. [Google Scholar] [CrossRef]
- Singh, S.J.; Jayaram, R.V. Oxidation of Alkylaromatics to Benzylic Ketones using TBHP as an Oxidant over LaMO3 (M = Cr, Co, Fe, Mn, Ni) Perovskites. Catal. Commun. 2009, 10, 2004–2007. [Google Scholar] [CrossRef]
- Das, T.K.; Chaudhari, K.; Nandanan, E.; Chandwadkar, A.J.; Sudalai, A.; Ravindranathan, T.; Sivasanker, S. Cr-MCM-41-catalyzed Selective Oxidation of Alkylarenes with TBHP. Tetrahedron Lett. 1997, 38, 3631–3634. [Google Scholar] [CrossRef]
- Serra, S. MnO2/TBHP: A Versatile and User-Friendly Combination of Reagents for the Oxidation of Allylic and Benzylic Methylene Functional Groups. Eur. J. Org. Chem. 2015, 2015, 6472–6478. [Google Scholar] [CrossRef]
- Estrada, A.C.; Simões, M.M.Q.; Santos, I.C.M.S.; Neves, M.G.P.M.S.; Silva, A.M.S.; Cavaleiro, J.A.S.; Cavaleiro, A.M.V. Iron-substituted Polyoxotungstates as Catalysts in the Oxidation of Indane and Tetralin with Hydrogen Peroxide. Appl. Catal. A Gen. 2009, 366, 275–281. [Google Scholar] [CrossRef]
- Bo, C.B.; Li, M.; Chen, F.; Liu, J.C.; Dai, B.; Liu, N. Visible-Light-Initiated Air-Oxygenation of Alkylarenes to Carbonyls Mediated by Carbon Tetrabromide in Water. Chemsuschem 2023, 17, e202301015. [Google Scholar] [CrossRef] [PubMed]
- Song, J.L.; Hua, M.L.; Huang, X.; Ma, J.; Xie, C.; Han, B.X. Robust Bio-derived Polyoxometalate Hybrid for Selective Aerobic Oxidation of Benzylic C(sp3)-H Bonds. ACS Catal. 2023, 13, 4142–4154. [Google Scholar] [CrossRef]
- Fosshat, S.; Siddhiaratchi, S.D.M.; Baumberger, C.L.; Ortiz, V.R.; Fronczek, F.R.; Chambers, M.B. Light-Initiated C–H Activation via Net Hydrogen Atom Transfer to a Molybdenum(VI) Dioxo. J. Am. Chem. Soc. 2022, 144, 20472–20483. [Google Scholar] [CrossRef] [PubMed]
- Singha, A.; Kothari, A.C.; Bal, R.; Chowdhury, B. Dioxygen-triggered Oxidation of Benzylic C–H Bonds: Insight on the Synergistic Effect of Cu-Fe Bimetallic Oxide. React. Chem. Eng. 2023, 8, 2353–2364. [Google Scholar] [CrossRef]
- Guo, Z.; Liu, B.; Zhang, Q.; Deng, W.; Wang, Y.; Yang, Y. Recent Advances in Heterogeneous Selective Oxidation Catalysis for Sustainable Chemistry. Chem. Soc. Rev. 2014, 43, 3480–3524. [Google Scholar] [CrossRef]
- Zhang, P.; Lu, H.; Zhou, Y.; Zhang, L.; Wu, Z.; Yang, S.; Shi, H.; Zhu, Q.; Chen, Y.; Dai, S. Mesoporous MnCeOx Solid Solutions for Low Temperature and Selective Oxidation of Hydrocarbons. Nat. Commun. 2015, 6, 8446. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Gao, T.N.; Chen, X.; Li, K.Q.; Ma, Y.L.; Xiong, H.L.; Qiao, Z.A. Mesoporous Metal Oxide Encapsulated Gold Nanocatalysts: Enhanced Activity for Catalyst Application to Solvent-free Aerobic Oxidation of Hydrocarbons. Inorg. Chem. 2018, 57, 12953–12960. [Google Scholar] [CrossRef] [PubMed]
- Zhong, H.; Wang, Y.; Cui, C.; Zhou, F.; Hu, S.; Wang, R. Facile Fabrication of Cu-based Alloy Nanoparticles Encapsulated within Hollow Octahedral N-doped Porous Carbon for Selective Oxidation of Hydrocarbons. Chem. Sci. 2018, 9, 8703–8710. [Google Scholar] [CrossRef] [PubMed]
- Vallés-García, C.; Montero-Lanzuela, E.; Navalon, S.; Álvaro, M.; Dhakshinamoorthy, A.; Garcia, H. Tuning the Active Sites in Reduced Graphene Oxide by Hydroquinone Functionalization for the Aerobic Oxidations of Thiophenol and Indane. Mol. Catal. 2020, 493, 111093. [Google Scholar] [CrossRef]
- Mączka, W.; Wińska, K.; Grabarczyk, M.; Galek, R. Plant-Mediated Enantioselective Transformation of Indan-1-One and Indan-1-ol. Catalysts 2019, 9, 844. [Google Scholar] [CrossRef]
- Dapurkar, S.E.; Shervani, Z.; Yokoyama, T.; Ikushima, Y.; Kawanami, H. Supported Gold Nanoparticles Catalysts for Solvent-free Selective Oxidation of Benzylic Compounds into Ketones at 1 atm O2. Catal. Lett. 2009, 130, 42–47. [Google Scholar] [CrossRef]
- Zhang, P.; Gong, Y.; Li, H.; Chen, Z.; Wang, Y. Solvent-free Aerobic Oxidation of Hydrocarbons and Alcohols with Pd@N-doped Carbon from Glucose. Nat. Commun. 2013, 4, 1593. [Google Scholar] [CrossRef]
- Tosun, R.B.; Hamaloğlu, K.Ö.; Tuncel, A.J.C. Bimetallic Pd-Au Nanoparticles Supported Monodisperse Porous Silica Microspheres as an Efficient Heterogenous Catalyst for Fast Oxidation of Benzyl Alcohol. ChemistrySelect 2022, 7, e2022016. [Google Scholar] [CrossRef]
- Du, X.B.W.; Tan, M.W.; Wei, T.; Kobayashi, H.; Song, J.J.; Peng, Z.X.; Zhu, H.L.; Jin, Z.K.; Li, R.H.; Liu, W. Highly Efficient and Robust Nickel-iron Bifunctional Catalyst Coupling Selective Methanol Oxidation and Freshwater/seawater Hydrogen Evolution via CO-free Pathway. Chem. Eng. J. 2023, 452, 139404. [Google Scholar] [CrossRef]
- Long, J.L.; Liu, H.L.; Wu, S.J.; Liao, S.J.; Li, Y.W. Selective Oxidation of Saturated Hydrocarbons Using Au−Pd Alloy Nanoparticles Supported on Metal−Organic Frameworks. ACS Catal. 2013, 3, 647–654. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, B.; Fei, B.; Chen, X.; Zhang, J.; Mu, X. Tunable and Selective Hydrogenation of Furfural to Furfuryl alcohol and Cyclopentanone over Pt Supported on Biomass-derived Porous Heteroatom Doped Carbon. Faraday Discuss. 2017, 202, 79–98. [Google Scholar] [CrossRef]
- Zhang, L.; Cheng, L.; Hu, Y.; Xiao, Q.; Chen, X.; Lu, W. Robust Co3O4 Nanocatalysts Supported on Biomass-derived Porous N-doped Carbon Toward Low-Pressure Hydrogenation of Furfural. Front. Mater. Sci. 2023, 17, 230645. [Google Scholar] [CrossRef]
- Tang, T.T.; Ren, G.Y.; Wen, Y.; Lu, M.X.; Yao, Z.J.; Liu, T.C.; Shen, S.H.; Xie, H.J.; Xia, X.H.; Yang, Y.F. Spatially Confined Fe7S8 Nanoparticles Anchored on a Porous Nitrogen-Doped Carbon Nanosheet Skeleton for High-Rate and Durable Sodium Storage. ACS Appl. Mater. Interfaces 2023, 15, 30249–30261. [Google Scholar] [CrossRef]
- Chen, X.; Cheng, L.L.; Yang, Y.G.; Chen, X.F.; Chen, F.T.; Lu, W.Y. Construction of High-Density Fe Clusters Embedded in a Porous Carbon Nitride Catalyst with Effectively Selective Transformation of Benzene. ACS Sustain. Chem. Eng. 2023, 11, 1518–1526. [Google Scholar] [CrossRef]
- Zhao, L.L.; Chen, X.F.; Liu, X.Y.; Xu, G.Q.; Guo, X.C.; Yang, Y. Highly Efficient Synthesis of C5/C6 Sugar Alcohols from Bamboo Enabled by Mechanocatalytic Depolymerization. ACS Sustain. Chem. Eng. 2021, 9, 6697–6706. [Google Scholar] [CrossRef]
- Pan, Y.D.; Gao, J.K.; Li, Y.W.; Lv, E.J.; Khan, U.; Yang, X.G.; Yao, J.M.; Nairan, A.; Zhang, Q.C. Constructing Nitrogen-Doped Carbon Hierarchy Structure Derived from Metal-Organic Framework as High-Performance ORR Cathode Material for Zn-Air Battery. Small 2023, 20, 2304594. [Google Scholar] [CrossRef]
- Ge, N.Q.; Hu, X.M.; Pan, Z.P.; Cai, S.J.; Fu, F.Y.; Wang, Z.Q.; Yao, Y.M.; Liu, X.D. Sustainable Fabrication of Cellulose Aerogel Embedded with ZnO@noble metal (Ag, Au, Ag-Au) NPs for Sensitive and Reusable SERS Application. Colloids Surf. A 2023, 671, 131650. [Google Scholar] [CrossRef]
- Huang, M.H.; Li, Z.; Du, L.L.; Jin, Z.K.; Li, R.H. CuPd/MgO for Efficient Catalytic Hydrogen Production from Formaldehyde Solution at Room Temperature. Chin. J. Org. Chem. 2022, 38, 2452–2458. [Google Scholar]
- Velinova, R.; Naydenov, A.; Kichukova, D.; Tumbalev, V.; Atanasova, G.; Kovacheva, D.; Spassova, I. Highly Efficient RGO-Supported Pd Catalyst for Low Temperature Hydrocarbon Oxidation. Catalysts 2023, 13, 1224. [Google Scholar] [CrossRef]
- Guo, X.; Dong, H.; Li, B.; Dong, L.; Mu, X.; Chen, X. Influence of the Functional Groups of Multiwalled Carbon Nanotubes on Performance of Ru Catalysts in Sorbitol Hydrogenolysis to Glycols. J. Mol. Catal. A Chem. 2017, 426, 79–87. [Google Scholar] [CrossRef]
- Zhao, Z.P.; Wang, J.H.; Yu, X.; Li, H.J.; Han, L.T.; Wang, Z.Q.; Qiao, D.; Zhong, M.Q.; Chen, X.F. Preparation of Three-dimensional Mesoporous Carbon Electrode Materials as Electrocatalysts for Hydrogen Evolution Reaction. Int. J. Electrochem. Sci. 2021, 16, 21043. [Google Scholar] [CrossRef]
- Tang, C.; He, Z.; Liu, Y.; He, X.; Chen, G.; Xie, C.; Huang, J. AuPd Nanoporous Dendrites: High Electrocatalytic Activity and Surface Plasmon-enhanced Stability for Ethanol Electrooxidation. Chem. Eng. J. 2023, 453, 139962. [Google Scholar] [CrossRef]
- Meduri, K.; Stauffer, C.; O’Brien Johnson, G. Unique Structural Characteristics of Catalytic Palladium/gold Nanoparticles on Graphene. Microsc. Microanal. 2019, 25, 80–91. [Google Scholar] [CrossRef]
- Wang, H.Y.; Zhang, D.X.; Zhang, R.Y.; Ma, H.; Zhang, H.M.; Yao, R.J.; Liang, M.M.; Zhao, Y.Z.; Miao, Z.C. Dealloying Synthesis of Bimetallic (Au−Pd)/CeO2 Catalysts for CO Oxidation. ACS Omega 2023, 8, 11889–11896. [Google Scholar] [CrossRef]
- Guan, J.B.; Fang, Y.N.; Zhang, T.; Wang, L.; Zhu, H.; Du, M.L.; Zhang, M. Kelp-Derived Activated Porous Carbon for the Detection of Heavy Metal Ions via Square Wave Anodic Stripping Voltammetry. Electrocatalysis 2020, 11, 59–67. [Google Scholar] [CrossRef]
- Cheng, L.; Sun, S.; Chen, X.; Chen, F.; Chen, X.; Lu, W. Convenient Fabrication of Ultrafine VOx Decorated on Porous g-C3N4 for Boosting Photocatalytic Degradation of Pharmaceuticals with Peroxymonosulfate. Surf. Interfaces 2023, 42, 103300. [Google Scholar] [CrossRef]
- Zhang, X.F.; Lin, X.Y.; Huang, X.Y.; Chen, Y.Q.; Lin, S.; Huang, X.; Xie, Z.L. The Role Identification of Nitrogen Dopant in Nanocarboncatalysis. Carbon Future 2024, 1, 9200008. [Google Scholar]
- Ji, G.J.; Duan, Y.N.; Zhang, S.C.; Fei, B.H.; Chen, X.F.; Yang, Y. Selective Semihydrogenation of Alkynes Catalyzed by Pd Nanoparticles Immobilized on Heteroatom-doped Hierarchical Porous Carbon Derived from Bamboo Shoots. ChemSusChem 2017, 10, 3427–3434. [Google Scholar] [CrossRef] [PubMed]
- Fajín, J.L.C.; Cordeiro, M.N.D.S.; Gomes, J.R.B. Catalytic Reactions on Model Gold Surfaces: Effect of Surface Steps and of Surface Doping. Catalysts 2011, 1, 40–51. [Google Scholar] [CrossRef]
- Santiago-Portillo, A.; Navalón, S.; Cirujano, F.G.; Xamena, F.X.L.; Alvaro, M.; Garcia, H. MIL-101 as Reusable Solid Catalyst for Autoxidation of Benzylic Hydrocarbons in the Absence of Additional Oxidizing Reagents. ACS Catal. 2015, 5, 3216–3224. [Google Scholar] [CrossRef]



| Samples | SBET (m2 g−1) | Vtotal (cm3 g−1) | Vmicro (cm3 g−1) | Vmeso (cm3 g−1) |
|---|---|---|---|---|
| MC | 485 | 0.25 | 0.16 | 0.07 |
| 1%Au/MC | 415 | 0.20 | 0.14 | 0.04 |
| 1%Pd/MC | 413 | 0.18 | 0.15 | 0.05 |
| 1%Au-Pd/MC | 419 | 0.19 | 0.15 | 0.04 |
| Entry | Catalyst | t (h) | T (℃) | Conversion (%) | Selectivity (%) | |
|---|---|---|---|---|---|---|
 |  | |||||
| 1 | without catalyst | 4 | 100 | 0 | - | - |
| 2 | MC | 4 | 100 | 0 | - | - |
| 3 | 1%Pd/MC | 4 | 100 | 1.5 | 60 | 40 |
| 4 | 1%Au/MC | 4 | 100 | 1.9 | 55 | 45 |
| 5 | Au-Pd(1:3)/MC | 4 | 100 | 3 | 46 | 54 |
| 6 | 1%Pd/MC | 4 | 120 | 30 | 46 | 54 |
| 7 | 1%Au/MC | 4 | 120 | 55 | 42 | 58 |
| 8 | 1%Au/MC | 8 | 120 | 60 | 59 | 41 |
| 9 | 1%Au/MC | 12 | 120 | 64 | 72 | 28 |
| 10 | 5%Pd/C | 4 | 120 | 28 | 56 | 44 |
| 11 | Au-Pd(1:3)/MC | 4 | 120 | 65 | 38 | 62 |
| 12 | Au-Pd(1:3)/MC | 8 | 120 | 71 | 8 | 92 |
| 13 | Au-Pd(1:3)/MC | 24 | 120 | 76 | 0 | >99 |
| Entry | Substrate | t (h) | Conversion (%) | Product Selectivity (%) | |
|---|---|---|---|---|---|
| 1 |  | 4 | 11 | 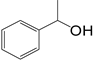 77 77 | 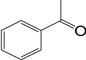 23 23 |
| 2 | 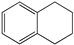 | 8 | 52 |  72 72 |  27 27 |
| 3 | 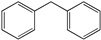 | 8 | 12 | 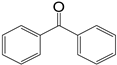 >99 >99 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, S.; Peng, X.; Guo, X.; Chen, X.; Liu, D. Boosting Solvent-Free Aerobic Oxidation of Benzylic Compounds into Ketones over Au-Pd Nanoparticles Supported by Porous Carbon. Catalysts 2024, 14, 158. https://doi.org/10.3390/catal14030158
Sun S, Peng X, Guo X, Chen X, Liu D. Boosting Solvent-Free Aerobic Oxidation of Benzylic Compounds into Ketones over Au-Pd Nanoparticles Supported by Porous Carbon. Catalysts. 2024; 14(3):158. https://doi.org/10.3390/catal14030158
Chicago/Turabian StyleSun, Shanshan, Xiaoyu Peng, Xingcui Guo, Xiufang Chen, and Di Liu. 2024. "Boosting Solvent-Free Aerobic Oxidation of Benzylic Compounds into Ketones over Au-Pd Nanoparticles Supported by Porous Carbon" Catalysts 14, no. 3: 158. https://doi.org/10.3390/catal14030158






